Abstract
People over the age of 50 are the fastest growing segment of the HIV-infected population in the United States. Although antiretroviral therapy has remarkable success controlling the systemic HIV infection, HIV-associated neurocognitive disorder (HAND) prevalence has increased or remained the same among this group, and cognitive deficits appear more severe in aged patients with HIV. The mechanisms of HAND in the aged population are not completely understood; a leading hypothesis is that aged individuals with HIV might be at higher risk of developing Alzheimer’s disease (AD) or one of the AD-related dementias (ADRD). There are a number of mechanisms through which chronic HIV disease alone or in combination with antiretroviral therapy and other co-morbidities (e.g: drug use, hepatitis C virus (HCV)) might be contributing to HAND in individuals over the age of 50 years, including: 1) overlapping pathogenic mechanisms between HIV and aging (eg: decreased proteostasis, DNA damage, chronic inflammation, epigenetics, vascular), which could lead to accelerated cellular aging and neurodegeneration and/or 2) by promoting pathways involved in AD/ADRD neuropathogenesis (e.g: triggering amyloid β, tau, or α-synuclein accumulation). In this manuscript, we will review some of the potential common mechanisms involved and evidence in favor and against a role of AD/ADRD in HAND.
Keywords: aging, Alzheimer’s disease, HIV associated cognitive impairment, neurodegeneration
Introduction
There is a global trend toward a rise of HIV among older adults. In 2013, the Center for Disease Control (CDC) estimated that 42% of Americans living with HIV were at least 50 years, 25% were at least 55 years old, and 6% were at least 65 years old (CDC, 2018). With longer life expectancy, individuals living with chronic HIV are at greater risk of developing age-related metabolic, cardiovascular, neoplastic and neurodegenerative disorders. Common neurodegenerative disorders of the aging population leading to severe neurological and psychiatric impairment include Alzheimer’s disease (AD), Lewy body disease (LBD), vascular cognitive impairment-dementia (VCID) and frontotemporal dementia (FTD). It is estimated that in the US alone approximately 5.3 million people age 65 or older live with AD and that by the year 2050 this number will triple (Hebert et al, 2013). Considerable overlap among these disorders is observed, in particular in those with advanced age (Boyle et al, 2018; Robinson et al, 2018).
Alzheimer’s disease is the 6th most common cause of death in the US and AD and related dementing disorders (ADRD) including LBD, FTD and VCID are the only top causes of death for which no prevention or cure is currently available (Alzheimer's Association, 2013). For this reason, the US and other countries, have developed National plans to address this public health emergency (International, 2016; SECRETARY and EVALUATION, 2016). In 2011, the US Congress signed into law the National Alzheimer's Project Act (NAPA) (Public Law 111-375). The Act called for the creation of a National Plan to Address AD/ADRD and one of its major goals is to accelerate the development of treatments that would prevent, halt, or reverse the course of AD/ADRD by the year 2025. In support of the National Plan, the US Congress has appropriated over 2 billion dollars to NIA/NIH to advance the research agenda to date.
The key to developing such treatments resides in better understanding the mechanisms of neurodegeneration and thereby developing disease-modifying therapeutics. The neurodegenerative process is characterized by the progressive accumulation of proteins (including the formation of inclusions and trans-cellular propagation), damage of selective synaptic circuitries leading to neuronal loss, neuroinflammation with astrogliosis and microgliosis, myelin degradation and vascular alterations. In view of the genetic and neuropathological evidence, research in AD/ADRD has focused on better understanding the abnormal accumulation of proteins with amyloid properties (beta pleated sheets) in the brain. However, there is growing evidence that age-related factors also play a key role. For example in AD, Aβ and tau accumulate in the neocortex and hippocampus (Crews and Masliah, 2010), while in LBD, which represents an heterogeneous group of disorders that includes Parkinson’s disease (PD), PD dementia (PDD) and dementia with Lewy bodies (DLB) (Donaghy and McKeith, 2014; McKeith et al, 2004; McKeith, 2000) the neuronal protein α-synuclein (α-syn) accumulates in the cortical striato-nigral system and other subcortical nuclei (Goedert et al, 2001; Spillantini, 1999).In FTD, either tau or TDP-43 aggregates are found in multiple cortical regions (Ferrari et at, 2011; Goedert et at, 2012). Moreover, in FTD cases with the GGGGCC expansion mutation located in intron 1 of the C9ORF72 gene, there is an accumulation of TDP43 and repeat-associated non-ATG (RAN) translation proteins (Zu et al, 2013) in the affected brain regions. In addition, it is now evident that α-syn accumulates in the amygdala in AD (Winslow et al, 2014) and TDP-43 aggregates are found in the limbic system in AD, DLB (Arai et al, 2009) and hippocampal sclerosis in older affected individuals (James et al, 2016; Murray et al, 2014). In VCID there is ischemic micro-vessel disease that can be accompanied by accumulation of amyloid (Vinters et al, 2018).
The mechanisms leading to proteinopathy in these disorders (progressive accumulation of Aβ, tau, α-syn, and TDP-43) is not completely understood, however evidence supports the hypothesis that genetic and environmental factors might result in an imbalance in the rates of synthesis, aggregation, and clearance of these proteins resulting in the generation of toxic oligomers, protofibrils, and fibrils with amyloid characteristics that could propagate from cell to cell in a prion-like fashion (Valera et al, 2016). The contribution of AD/ADRD to the neurocognitive impairment in older individuals with HIV is not fully understood. In this manuscript we will review some of the potential common mechanisms involved and evidence in favor and against a role of AD/ADRD in HIV associated neurocognitive disorders (HAND).
HIV neuropathogenesis in the anti-retroviral era
It is estimated that there are over 30 million people living with HIV worldwide and in the US, the aging population represents one of the fastest growing population with HIV (Scott et al, 2011). A considerable proportion of patients with HIV, especially those with advancing age, develop cognitive impairment that can range from mild to severe disruptions, which jointly are now denominated as HAND (Morgan et al, 2012). In the pre-highly active antiretroviral therapy (HAART) era, it was recognized that HIV can penetrate the blood-brain barrier (BBB) resulting in HIV encephalitis (HIVE) that could manifest clinically as HAND, which manifests as dementia at the extreme end of the spectrum (Crews et al, 2009; Ellis et al, 2007; Morgello, 2018; Sillman et al, 2018) (Figure 1A). At the time, neurological manifestations were also reported in patients with HIV, which were usually the result of opportunistic infections (Figure 1B).
Figure 1. Mechanisms of neurodegeneration in HIV associated neurocognitive disorders (HAND) in the pre and highly active antiretroviral (HAART) eras.
(A) Schematic representation of mechanisms leading to HIV encephalitis and neurodegeneration initiating with trafficking of HIV infected macrophages through an injured blood-brain barrier followed by activation and infection of microglial and astroglial cells resulting in neurotoxicity and inflammation with synapto-dendritic damage and selective neuronal damage. (B) In the pre-HAART era, HIV CNS pathology was characterized by the presence of microglial nodules, multinucleated giant cells, severe astrogliosis, myelin loss and neurodegenerative pathology accompanied by opportunistic infections. Modern treatments with HAART results in HIV suppression and immune recovery; the pathology has shifted to a more subtle, chronic neurodegenerative process with lower or undetectable HIV in the CNS and diffuse astrogliosis, some microglial nodules, white matter alterations and vascular changes accompanied by co-morbid conditions associated with aging.
In the central nervous system (CNS), macrophages and microglial cells have been identified as a primary reservoir for HIV infection (Gendelman et al, 1997; Gonzalez-Scarano and Martin-Garcia, 2005; Haas et al, 2000; Wiley et al, 1996) with productive infection also detected in astrocytes (Carroll-Anzinger and Al-Harthi, 2006). In the pre-HAART era, classical HIVE (Morgello, 2018; Sillman et al, 2018) was characterized by the presence of microglial nodules, multinucleated giant cells (MNGC), severe astrogliosis, myelin loss (Budka et al, 1991), neurodegenerative pathology with synaptic-dendritic damage (Everall et al, 1999), and selective loss of calbindin and excitatory neurons in the neocortex, hippocampus and striatum (Masliah et al, 1995) (Figure 1).
Modern treatments with HAART resulted in HIV suppression and immune recovery; consequently the pathology of HIVE has shifted from subcortical regions towards a cortical pattern (Brew, 2004; Soontornniyomkij et al, 2018) and from a subacute, rapidly progressive disorder to a more subtle, chronic neurodegenerative process (Everall et al, 2005; Xu and Ikezu, 2009)(Figure 1B). Viral levels in the CNS are lower or undetectable and neuropathologically these patients usually show diffuse astrogliosis, some microglial nodules, white matter alterations and vascular changes with peri-vascular lymphocytic infiltration; however, MNGC are not present (Desplats et al, 2013; Fields et al, 2013) (Figure 1B).
In spite of the successful use of HAART to control systemic HIV infections, the prevalence of mild to moderate HAND (Budka et al, 1987; Cherner et al, 2007; Gendelman et al, 1997; Heaton et al, 2010; Wiley and Achim, 1994) has remained the same or increased (Heaton et al, 2011a; Joska et al, 2010). In particular, among people over the age of 50, age and HIV synergize to worsen cognitive performance (Morgan et al, 2012). The mechanisms of neurodegeneration in HAND are not completely understood; however, a number of studies have proposed that HIV proteins such as gp120, tat, and nef activate neuro-inflammatory and apoptotic pathways (Kaul et al, 2001), dysregulate calcium homeostasis by abnormally activating glutamate receptors (Lipton, 1994; Nath et al, 2000), promote oxidative stress (Nath, 2002; Norman et al, 2008), deplete neurotrophic factors (Fields et al, 2014; Mocchetti et al, 2008) and cause vascular damage (Soontornniyomkij et al, 2014) (Figure 1A). Remarkably, recent studies have shown that regardless of effective CNS viral suppression with HAART, neurotoxic HIV proteins, such as Tat, are persistently expressed in the brain of HIV-infected individuals (Johnson et al, 2013). Moreover, we have shown that with HAART antiretroviral therapy, prolonged viral suppression might be associated with epigenetic alterations mediated by transcription factors that suppress HIV such as BCL11b potentially resulting in chronic neuroinflammation (Desplats et al, 2013).
In older individuals with HIV, additional mechanisms intrinsic to the aging process such as defective proteostasis, altered stress response, epigenetic alterations, macromolecular damage, cellular senescence, inflammation, and stem cell deficits might be also at play (Figure 2). Together, these mechanisms are thought to be the corner-stones of aging and are studied under the umbrella of geroscience (Kennedy et al, 2014; Sierra, 2016). Interestingly, brain aging and neuronal damage in individuals with chronic HIV infection are aggravated, but contrast, early HAART treatment ameliorates brain aging in well-controlled HIV Infection (Boban et al, 2018) (Figure 2). Thus, most of our understanding of HIV neuropathogenesis is largely based on clinical outcomes that were predominantly seen in the era preceding the development of antiretroviral therapy. Moreover, the majority of basic neuro-HIV research has focused on evaluating neuronal damage in the context of active viral replication and outcomes related to HIVE. However, it is critical to examine neuronal impairment caused by HIV in the context of viral suppression and antiretroviral therapies. There is an urgent need to bridge the gap between pathogenesis research and observed clinical outcomes. As a result of a major demographic shift in the HIV-infected population in the US, there is also a critical need to better define the underlying pathophysiology of neurodegenerative processes, neurological complications, and neurocognitive decline accompanying HIV during the course of aging.
Figure 2. Potential mechanisms of neurodegeneration in HIV and aging.
Common pathways involved in aging and chronic HIV disease in the CNS might interact leading to neurodegeneration. Such mechanisms include defective proteostasis (eg: altered proteasome, proteolysis, autophagy), mitochondrial abnormalities, epigenetic alterations, DNA damage, cell senescence, neuroinflammation, oxidative stress and stem cell defects. Together, these alterations might lead to abnormal protein accumulation (Aβ, Tau, α-synuclein) and hyperactivation of signaling pathways (eg: CDK5, JNK) involved in neuronal damage.
Common mechanisms of neurodegeneration in aging and HIV
With the advent of HAART, HIV disease has transitioned from a subacute, rapidly progressive condition to a slowly advancing, chronic disorder in which the aging processes synergize with the pathogenic mechanisms driven by HIV in the CNS (Figure 1B). Remarkably, cellular and molecular mechanisms involved in the aging process including defective proteostasis, mitochondrial abnormalities, epigenetic alterations, DNA damage, cellular senescence, and stem cell defects have also been also shown to be driven by HIV, leading to the concept that HIV might accelerate the aging process or hijack aging mechanisms (Figure 2). Increased effects of aging in the brain in HAART treated HIV cases can be predicted using neuroimaging. Recent studies have shown that age exacerbates HIV-associated white matter abnormalities and loss of fronto-subcortical white matter integrity (Seider et al, 2016). In contrast, a more recent study found that the extent of increased brain aging related to cognitive deficits. However, predicted brain age difference did not correlate with chronological age or duration of HIV infection, suggesting that HIV disease may accentuate rather than accelerate brain aging (Cole et al, 2017). Moreover, it has been proposed that some of the mechanisms of action of antiretroviral drugs, such as reverse transcription, protease inhibition and metabolic effects, may also contribute to this cascade (Soontornniyomkij et al, 2018).
In terms of epigenetic alterations, recent studies have investigated DNA methylation (Levine et al, 2016) using the Illumina Infinium Human Methylation 450K platform, to study biological aging in HIV+ brains. The purpose behind these studies was to compare biological age, determined via the epigenetic clock, with chronological age to obtain a measure of age acceleration, which was then compared between those with HAND and neurocognitively normal individuals. The normal control and HAND groups did not differ with regard to demographical, pathological, or virologic measures. HAND was associated with accelerated aging relative to neuro-cognitively normal individuals, with an average relative acceleration of 3.5 years. These results suggest that the increased risk of a neurocognitive disorder due to HIV might be mediated by an epigenetic aging mechanism (Levine et al, 2016).
The role of stem cell alterations in aging and neuro-HIV is not completely clear given that neurogenesis in the adult human CNS is a subject of debate (Kuhn et al, 2018). However, alterations in adult neurogenesis have been noted in the hippocampus of HIV-infected individuals and could be related to HAND (Ferrell and Giunta, 2014). Similarly, in vitro studies have shown that HIV proteins such as Tat and gp120 (Avraham et al, 2015) arrest proliferation and development of neuro-stem cells (Mishra et al, 2010; Yao et al, 2012). In vitro neuronal precursor cell (NPC)-derived neurosphere assays showed that Tat-containing conditioned media from astrocytes or recombinant Tat protein inhibited NPC proliferation and migration and altered NPC differentiation (Fan et al, 2016). In vivo studies in conditional tg mice have suggested similar results. For example, in the doxycycline-inducible and astrocyte-specific HIV-1 Tat tg mice (iTat), Tat expression in glial cells resulted in a reduction in the numbers and maturation of neuron progenitor cells (NPCs) in mouse hippocampus. This occurs via a mechanism involving Notch signaling since the Notch signaling inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) rescued the neurogenesis defects in the iTat mice (Fan et al, 2016); this may be a possible therapeutic approach for HAND in the aging population.
Another interesting process in which HIV and aging might overlap is disruption of proteostasis, a protein quality control mechanism involved in protein folding and clearance of protein aggregates (Figure 2). The protein clearance pathway includes mechanisms such as direct degradation of proteins by proteases, the proteasome, or via lysosomal pathways such as autophagy (Cuervo, 2004). Studies in the brains of patients with HIV, as well as in vitro work have shown that HIV proteins like Tat and gp120 could inhibit proteases such as neprilysin and IDE that degrade amyloid beta protein (Pulliam, 2009). These studies have also shown widespread alterations in components of the proteasomal machinery leading to synaptic alterations (Nguyen et al, 2010). Moreover, recent studies have shown that HIV proteins (eg: Tat, gp120, and nef) might interfere with protein quality control and clearance pathways such as autophagy (Alirezaei et al, 2008a; Alirezaei et al, 2008b; Fields et al, 2015a; Fields et al, 2013; Zhou et al, 2011) (Figure 2).
Autophagy is a complex process that involves nucleation, initiation, elongation and termination proteins. Initially, a phagophore forms and develops into the autophagosome, a double-membrane sac that delivers cytoplasmic material to the lysosomal compartment for degradation (Codogno et al, 2012). Deficits in autophagy have been described in AD (Nixon et al, 2005; Pickford et al, 2008), Parkinson’s disease (PD) (Crews et al, 2010; Cuervo et al, 2004) and other aging-related disorders (Cuervo, 2004). Specifically, the autophagy nucleation protein beclin-1 and closure protein light chain (LC) 3 have been implicated in human disease (Crews et al, 2010; Gozuacik and Kimchi, 2004; Jaeger and Wyss-Coray, 2010). Similarly, neurodegeneration has been linked to defects in autophagy in patients with HIV (Alirezaei et al, 2008a; Alirezaei et al, 2008b; Fields et al, 2015a; Fields et al, 2013; Zhou et al, 2011) (Figure 2).
Interestingly, in the brains of patients with HIVE under the age of 50 years and in young transgenic (tg) mice expressing HIV-gp120 protein (gp120 tg) (Toggas et al, 1994) autophagy was upregulated while in the brains of aged patients with HIVE or aged Tat and gp120 tg mice autophagy was down-regulated (Fields et al, 2015a; Fields et al, 2013). Moreover, activation of autophagy by beclin-1 gene transfer (Fields et al, 2013) or treatment with rapamycin ameliorated the neurodegenerative phenotype (Fields et al, 2015a). HIV might alter autophagy at various stages including vesicle formation, nucleation, and elongation (Campbell and Spector, 2013; Spector and Zhou, 2008). The alterations in the autophagy pathway mediated by Tat, gp120, and nef might depend on the cell cycle of HIV, with autophagy upregulation during the initial events and downregulation during the permissive stage of the infection (Campbell and Spector, 2013). Nef has been shown to bind beclin-1 blocking the formation of autophagic vacuoles (Kyei et al, 2009) and Tat has been shown to inhibit autophagosome maturation and acidification (Fields et al, 2015a). Likewise, in vivo studies in iTat tg mice showed increased autophagosome accumulation in neurons, altered LC3II levels, and neurodegeneration (Fields et al, 2015a). These effects were reversed by rapamycin treatment. Tat protein may induce autophagosome and lysosome fusion through interaction with LAMP2A leading to abnormal neuronal autophagy function and dysregulated degradation of critical intracellular (Fields et al, 2015a; Fields et al, 2017). Alterations in these protein clearance mechanisms might lead to abnormal accumulation of proteins associated with AD/ADRD, neuroinflammation, oxidative stress and mitochondrial alterations (Figure 2).
Neurodegenerative disorders in aged patients with HAND
With the growth of the aged HIV population in the US, which already includes over 1 million individuals, there is an urgent need to better understand the contribution of AD and related dementias (eg: LBD, FTD, and VCID) to HAND and to what extent the HIV associated neuropathology and AD/ADRD overlap. Moreover, we need to know if HIV in the older population augments the risk for AD/ADRD and to what extent chronic viral suppression, viral silencing, HAART and co-morbidities (eg: alcohol and drug abuse, HVC) might contribute to increased risk of HAND and AD/ADRD in these individuals. For instance, individuals with a history of alcohol abuse showed the most severe cognitive deficits and older age was also associated with poor cognitive performance (Cohen et al, 2018; Zahr, 2018) In this context, in 2018 the National Institute on Aging (NIA) in collaboration with the National Institute of Mental Health (NIMH) and the Office of AIDS Research (OAR) published a call for applications (RFA-AG-18-023) to help address these questions affecting the aging population with HIV in the US.
Previous studies have shown that HAND could be more persistent and severe in the older population (Becker et al, 2004; Heaton et al, 2011 b; Valcour and Paul, 2006). For example, it has been shown that subjective cognitive impairment (SCI) may be a predictor of more severe neurocognitive impairment. Among HIV+ individuals, SCI was associated with lower performance-based learning and delayed memory scores and worsening global everyday functioning, indicating an increased risk for developing major neurocognitive disorders as these HIV+ individuals age (Sheppard et al, 2018). Moreover, a recent study showed that HIV-infected individuals over the age of 60 years who have at least one ApoE ε4 allele displayed more severe HAND and evidence of brain atrophy by MRI suggesting an age-related exacerbation of HIV-related pathology (Wendelken et al, 2016). This is consistent with previous studies showing that the presence of ApoE ε4 allele predicted the presence of amyloid plaques and cognitive impairment in HIV patients (Soontornniyomkij et al, 2012). A recent study has reviewed the evidence for the influence of aging and ApoE status on HIV-associated neurocognitive impairment; they conclude that HIV-infected individuals are clearly living longer with HIV, and therefore factors related to aging need to be investigated in the context of effective HAART (Geffin and McCarthy, 2018a; Geffin and McCarthy, 2018b).
There are a number of mechanisms through which chronic HIV disease alone or in combination with antiretroviral therapy and other co-morbidities (eg: drug use, HCV) might be contributing to HAND in older individuals, including: 1) synergy between overlapping pathogenic mechanisms involving HIV and aging that could lead to accelerated cellular aging and neuronal cell death and/or 2) by promoting pathways involved in AD/ADRD neuropathogenesis (eg: triggering Aβ protein, tau of a-syn accumulation) (Figures 2 and 3). These two pathways can act independently or cooperatively leading to neurodegeneration of selective populations and HAND in the aging population. In the previous section, we described some of the common mechanisms involving chronic HIV disease and aging including decreased proteostasis, DNA damage, chronic inflammation and epigenetic dysregulation (Levine et al, 2016) that could lead to neurodegeneration (Figure 2). Moreover, abnormal functioning of clearance pathways including proteolysis (Pulliam, 2009), proteasome (Nguyen et al, 2010) and autophagy (Zhou et al, 2011) might result in accumulation of Aβ (Achim et al, 2009), α-syn (Ebrahimi-Fakhari et al, 2011; Everall et al, 2009; Khanlou et al, 2009) and tau proteins (Patrick et al, 2011a) in aged patients with HIV (Figure 2).
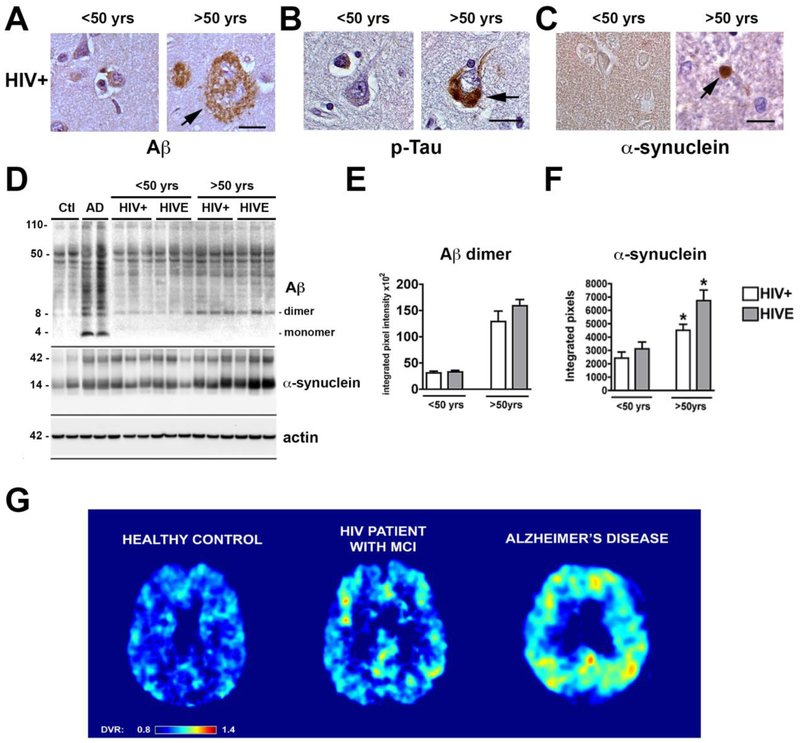
Figure 3. Brain Proteinopathy in aging and HIV.
With aging in an individual with chronic HIV disease protein aggregates involved in AD/ADRD might abnormally accumulate. (A) Representative images of the frontal cortex immunostained with an antibody against Aβ (4G8) from an HIV+ case under 50 yrs of age showing no amyloid deposition while an older case shows plaque-like lesion; (B) frontal cortex immunostained with an antibody against p-Tau (PHF-1) from HIV+ case under 50 yrs of age showing no neuronal alterations while an older case shows tangle-like lesion; (C) temporal cortex immunostained with an antibody against alpha-synuclein (SYN1) from HIV+ case under 50 yrs of age showing neuropil immunoreactivity while an older case shows Lewy-like dystrophic neurites (Bar=10 μm) . (D) Representative immunoblot analysis with the frontal cortex fractioned from control, AD and HIV+ cases under and over the age of 50 years. Samples from the frontal cortex were fractioned by ultracentrifugation and the membrane fractions ran in SDS-PAGE gels followed by blotting onto nitrocellulose membranes. Compared to controls in AD cases there was a considerable accumulation of monomers and multimers at various molecular weights, in older HIV cases Aβ dimers, as well as α-synuclein monomer and trimers appear to accumulate. (E, F) Image analysis corrected to actin for levels of Aβ dimers and α-synuclein (n=3 cases per group). (G) Representative FDDNP parametric PET images from normal control, HIV+ case with mild cognitive impairment (MCI) or Alzheimer’s disease. The analysis of FDDNP scans was performed for frames between 15 and 85 min using Logan graphical analysis with cerebellum as reference region. The resulting distribution volume ratios (DVR) were used to generate the DVR parametric images. The DVR values have been color-coded in such a way that all values below DVR 0.8 are shown as dark blue and all values above DVR 1.4 are shown as dark red. The DVR values between are shown in colors of a rainbow as shown on the color bar at the bottom of the image. The control shown is a 43-year-old female with MMSE score 30. The HIV patient with MCI is a 45-year-old male. The AD patient is an 81-year-old female with MMSE score 15. The images shown are transaxial cuts slightly above the basal ganglia so that posterior cingulate gyrus is visible. The frontal lobe is at the top and parietal lobe is at the bottom. The AD case shows high levels of FDDNP binding (high DVR values) throughout the neocortex, most prominently in parietal and frontal cortices and in posterior cingulate. The control case has a uniformly low level of FDDNP binding (low DVR) throughout the cortex. The HIV cases show a limited number of isolated areas with medium to high level of FDDNP binding in prefrontal areas, in posterior and anterior cingulate gyri, and asymmetrically in the parietal lobe.
For example, autopsy studies have shown accumulation of Aβ deposits forming diffuse plaques in the frontal cortex and hippocampus of aged HIV cases (Achim et al, 2009) (Figure 3A), increased intra-neuronal granular Aβ immunoreactivity associated with lysosomes and axons (Green et al, 2005), intra-neuronal phosphorylated tau aggregates mimicking pre-tangles (Patrick et al, 2011a) (Figure 3B) and α-syn accumulating in the striato-nigral system forming Lewy body- like inclusions and dystrophic neurites (Ebrahimi-Fakhari et al, 2011; Everall et al, 2009; Khanlou et al, 2009) (Figure 3C) in aged patients with HIV. Consistent with these findings, immunoblot analysis of frontal cortex samples from young (less than 50 yr/o) and older patients with HIV showed that Aβ accumulates as a dimer (Figure 3D, E) while α-syn accumulates as monomers and trimers (Figure 3D, F). Older HIV cases displaying Aβ, Tau or α-syn protein accumulation often display clinical features of HAND, as well as motor deficits, but no definitive correlation between the severity of the pathology and the neurological alterations have been found. The strongest microanatomical correlate to the cognitive impairment seen in aged patients with HIV is the extent of the damage to synapses and dendrites in the neocortex and hippocampus (Levine et al, 2016; Moore et al, 2006). In addition to the parenchymal diffuse amyloid deposits, congophilic amyloid angiopathy (CAA) has been reported in 6.7% of older HIV cases and in 4.5% of 22 controls, always in association with Aβ plaques and with ApoE ε4 predicting amyloid deposition and HAND (Soontornniyomkij et al, 2012). In terms of the amyloid deposits in these cases, it is worth mentioning that they resemble lesions found in early stages of AD but are not sufficient to meet criteria of AD/ADRD, with the exception of a few cases (Figure 3A). For instance, we recently reported the case of a 79-year-old man with HIV who presented with short-term memory deficits, increased challenges managing finances, an episode of getting lost, and motor slowing when writing and walking. HIV infection was diagnosed 14 months prior (Hellmuth et al, 2018). He was treated with HAART and had some initial memory improvements however his cognitive impairment re-appeared despite undetectable plasma HIV RNA levels. He developed worsening cognitive status consistent with dementia and parkinsonism and died 4 years after the initial diagnosis of HIV due to cardiovascular failure caused by aortic stenosis (Hellmuth et al, 2018). Neuropathologically, there was severe cerebral atrophy, neuronal loss, and gliosis in the neocortex, hippocampus and S. Nigra with abundant plaques and tangles to warrant the diagnosis of AD, consistent with Braak Stage VI. There were no Lewy bodies, glial cytoplasmic inclusions, TDP43 inclusions, microglial nodules or multinucleated giant cells (Hellmuth et al, 2018). Thus, a case like this raises the question as to what extent the AD neuropathology was the source of HAND and if this patient already had pre-clinical AD and the HIV infection merely accelerated or exacerbated AD. Moreover, one could argue that this was a classical AD case in an aged individual in which HIV had no contribution. The rapid progression, severity, and closeness of the onset of neurological alterations and HIV seropositivity argue against this possibility. In a more recent study from the Manhattan HIV Brain Bank, the autopsy results in two aged individuals treated with HAART and virally suppressed were reported. One showed AD neuropathology and age-related tau astrogliopathy (ARTAG). She was homozygous for APOE ε3/ε3. The second also displayed ARTAG and cerebral congophilic angiopathy. She was an APOE ε3/ε4 heterozygote. They conclude that in aged individuals in the setting of HIV, AD neuropathology may occur with or without symptomatic cognitive dysfunction (Morgello, 2018).
Other neuropathological features associated with HIV that might explain HAND in the older population includes neuro-inflammation, synaptic and dendritic degeneration, and vascular disease. Interestingly, in spite the fact that HIV levels in the brains of older individuals were lower compared to younger cases with HIVE, the levels of astrogliosis, microgliosis, and synaptic-dendritic degeneration were greater in HIV cases over 50 years of age (Fields et al, 2013). Patients with HIV and motor alterations have also been shown to display degeneration of the striato-nigral system, while those with HAND display degeneration of pyramidal (Crews et al, 2009) and inhibitory neurons in the neocortex and hippocampus (Everall et al, 1999), age might exacerbate this phenotype. Similarly, other studies have concluded that gliosis and cerebrovascular disease are common brain pathologies among older HIV+ patients in the late HAART era and that cerebrovascular disease, interferon responses, and neuroinflammation are likely factors contributing to brain aging and HAND in older HIV+ patients on current HAART regimens (Solomon et al, 2017). However, unlike other studies (Green et al, 2005; Rempel and Pulliam, 2005) the study by Solomon and colleagues did not find a correlation between aging and amyloid deposition in their autopsy series (Solomon et al, 2017).
In terms of potential interactions between HIV and AD/ADRD pathways (Figure 2), recent studies have shown that HIV proteins in addition to interacting with aging-related pathways might also interfere with the APP metabolism and Tau phosphorylation and aggregation. For example, Tat, nef, and gp120 interact with Lamp2 and Beclin-1 interfering with autophagy leading to Aβ, Tau and a-syn accumulation (Campbell et al, 2015; Fields et al, 2015a; Fields et al, 2013). Moreover, Tat blocks neprilysin- an Aβ degrading enzyme resulting in reduced clearance of Aβ and abnormal accumulation (Daily et al, 2006; Rempel and Pulliam, 2005). In addition, Tat interacts with Aβ, promoting Aβ fibrillation, misfolding and aggregation, with Aβ and Tat co-localizing in amyloid plaques of aged patients with HAND and in double transgenic Tat and APP mice (Hategan et al, 2017). Moreover, APP binds the HIV-1 Gag polyprotein, retains it in lipid rafts and blocks HIV-1 production. Gag could also enhance secretase- dependent cleavage of APP, leading to increased synthesis of toxic Aβ that promotes degeneration of cortical neurons, which can be prevented by γ-secretase inhibitor treatment (Chai et al, 2017). HIV proteins such as Tat and gp120 activate signaling pathways that lead to Tau phosphorylation and toxicity, among them most remarkably GSK3β and the cyclin-dependent kinase [CDK] 5, a member of the Ser/Thr CDK family involved in cell migration, angiogenesis, neurogenesis, and synaptic plasticity. Tat, via calcium dysregulation, promotes calpain-1 cleavage of p35 to p25, which in turn hyperactivates CDK5 resulting in abnormal phosphorylation of downstream targets such as Tau, collapsin response mediator protein-2 [CRMP2], doublecortin [DCX] (Fields et al, 2015b; Patrick et al, 2011 b) (Figure 2).
Together these clinicopathological and mechanistic studies illustrate the complexity of understanding both the relationship and the differences between HAND and AD/ADRD in the context of aging. In this regards, considerable efforts are underway to understand the natural history of HAND (Alakkas et al, 2018) and a potential relationship with AD/ADRD (Milanini and Valcour, 2017) by identifying novel biomarkers. In the AD field, with the support of the NIA and the Alzheimer’s Association efforts are underway to evaluate a biomarker-based framework for the diagnosis of AD (Jack et al, 2018). Biomarkers were grouped into Aβ, pathologic tau, and neurodegeneration [AT(N)]. This ATN system groups different biomarkers (imaging and biofluids) by the pathologic process each measure.
In the field of aging and HIV, there have been efforts to evaluate the usefulness of these ATN biomarkers to differentiate HAND and AD. Previous studies have analyzed levels of Aβ, Tau and neurofilament light protein (NF-L) in cerebrospinal fluid (CSF) and other fluids. Some studies have found that the CSF biomarker profile in aged HAND patients was similar to the profile typically found in patients with AD who are HIV-negative with increased t-tau and p-tau, a decreased level of Aβ42 and normal levels of NF-L, sAPPα, and sAPPβ (Makitalo et al, 2015). Reduced levels of Aβ42 have been confirmed by other independent studies in aged patients with HAND with a family history of dementia (Fazeli et al, 2016). In similarity with other studies, APOE ε4 genotype was not directly associated with HAND but correlated with CSF levels of Aβ1-42. However, in this study, the majority of participants increased CSF p-tau levels were associated with current neurocognitive impairment (Cysique et al, 2015). A more recent study also found that amyloid metabolism was influenced by HIV infection in a subtype-dependent manner. Aβ-42 levels were lower in HIV1-C than B, suggesting that there may be greater deposition of Aβ-42 in HIV1-C. These individuals also showed increase p-tau in CSF. The authors concluded that differences between HIV and AD in the patterns of Aβ and Tau biomarkers suggest that CNS HIV infection and AD may not share some of the same mechanisms of neuronal injury (de Almeida et al, 2018). Assessment of CSF biomarkers may be a valuable tool for clinicians to distinguish between HAND and AD.
The results of positron emission tomography (PET) imaging studies to detect Aβ in HIV cases with cognitive impartment have been controversial. For instance, in middle-aged HIV-positive participants, even with HAND, no evidence of increased (11)C-PiB uptake indicative of amyloid deposition similar to AD was detected, while typical AD cases showed extensive (11)C-PiB uptake. They concluded that similar studies in older participants with HAND and longitudinal studies are needed (Ances et al, 2012). In contrast, a more recent study in an older individual with HAND (Turner et al, 2016) showed abnormalities in the brain by MRI and PET-FDG, Aβ and p-Tau were found in the CSF and an amyloid PET/CT with [18F]florbetaben showed extensive cortical radiotracer deposition. We have more recently imaged with [18F]FDDN ((2-(1-{6-[(2-fluoroethyl(methyl)amino]-2-naphthyl}ethylidene)malononitrile) PET in a few patients with HIV who also have cognitive impairments and found increased tracer retention in the neocortex when compared to healthy control but to a lesser extent than what is typically seen in AD (Figure 3G). It has been proposed that while (11)C-PiB might detect more compact late stage deposits the FDDN might detect looser earlier stage deposits. In terms of Tau in HAND, currently no PET-Tau imaging studies have been performed; however, as indicated above, there are some studies that have shown accumulation of p-Tau in cortical neurons (Anthony et al, 2006; Patrick et al, 2011 b) in HAND and increased levels in CSF. Although some controversial results have been reported in CSF, a comprehensive review (Brown et al, 2014), concluded that those maintained on long-term HAART may develop tau pathology beyond the extent seen in the studies reviewed herein and overtime may then reach the threshold for clinical manifestation.
In summary, most of these studies have investigated the contribution of AD to HAND; however, it is likely that other disorders such as DLB and VICD might be contributing factors, and future studies will be needed to elucidate the extent to which each disorder contributes to AD.
Acknowledgments:
Drs. Jorge R. Barrio, Vladimir Kepe, and Harry V. Vinters at UCLA. Dr. Igor Grant and the HIV Neurobehavioral Research Program at UCSD MH72529 and MH105319 to UCLA.
References
- (2005). HIV/AIDS surveillance report. 1–63. [Google Scholar]
- (2007). HIV/AIDS surveillance report. 1–54. [Google Scholar]
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E, Neurobehavioral Research C (2009). Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol 4: 190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakkas A, Ellis RJ, Watson CW, Umlauf A, Heaton RK, Letendre S, Collier A, Marra C, Clifford DB, Gelman B, Sacktor N, Morgello S, Simpson D, McCutchan JA, Kallianpur A, Gianella S, Marcotte T, Grant I, Fennema-Notestine C, Group C (2018). White matter damage, neuroinflammation, and neuronal integrity in HAND. Journal of neurovirology: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS (2008a). Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PloS one 3: e2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Fox HS (2008b). Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy 4: 963–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association (2013). 2013 Alzheimer's Disease Facts and Figures. Alzheimer's Dementia 9: 1–71. [DOI] [PubMed] [Google Scholar]
- Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, Aldea P, Fagan AM, Holtzman DM, Morris JC, Clifford DB (2012). 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Archives of neurology 69: 72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE (2006). Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol 111: 529–38. [DOI] [PubMed] [Google Scholar]
- Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, Iritani S, Onaya M, Akiyama H (2009). Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol 117: 125–36. [DOI] [PubMed] [Google Scholar]
- Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Wood J, Wang L, Masliah E, Avraham S (2015). Impaired neurogenesis by HIV-1-Gp120 is rescued by genetic deletion of fatty acid amide hydrolase enzyme. Br J Pharmacol 172: 4603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Lopez OL, Dew MA, Aizenstein HJ (2004). Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 18 Suppl 1: S11–8. [PubMed] [Google Scholar]
- Boban JM, Kozic DB, Brkic SV, Lendak DF, Thurnher MM (2018). Early Introduction of cART Reverses Brain Aging Pattern in Well-Controlled HIV Infection: A Comparative MR Spectroscopy Study. Frontiers in aging neuroscience 10: 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Yu L, Leurgans SE, Wilson RS, Brookmeyer R, Schneider JA, Bennett DA (2018). Attributable risk of Alzheimer's dementia attributed to age-related neuropathologies. Ann Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ (2004). Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS 18 Suppl 1: S75–8. [PubMed] [Google Scholar]
- Brown LA, Scarola J, Smith AJ, Sanberg PR, Tan J, Giunta B (2014). The role of tau protein in HIV-associated neurocognitive disorders. Mol Neurodegener 9: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budka H, Costanzi G, Cristina S, Lechi A, Parravicini C, Trabattoni R, Vago L (1987). Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical, and electron microscopical study of 100 autopsy cases. Acta Neuropathol 75: 185–98. [DOI] [PubMed] [Google Scholar]
- Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES, Cornblath DR, Dal Canto MC, DeGirolami U, Dickson D, et al. (1991). HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain pathology 1: 143–52. [DOI] [PubMed] [Google Scholar]
- Campbell GR, Rawat P, Bruckman RS, Spector SA (2015). Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration. PLoS pathogens 11: e1005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GR, Spector SA (2013). Inhibition of human immunodeficiency virus type-1 through autophagy. Curr Opin Microbiol 16: 349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll-Anzinger D, Al-Harthi L (2006). Gamma interferon primes productive human immunodeficiency virus infection in astrocytes. J Virol 80: 541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2018). HIV Among People Aged 50 and Older. [Google Scholar]
- Chai Q, Jovasevic V, Malikov V, Sabo Y, Morham S, Walsh D, Naghavi MH (2017). HIV-1 counteracts an innate restriction by amyloid precursor protein resulting in neurodegeneration. Nature communications 8: 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Cysique L, Heaton RK, Marcotte TD, Ellis RJ, Masliah E, Grant I, Group H (2007). Neuropathologic confirmation of definitional criteria for human immunodeficiency virus-associated neurocognitive disorders. Journal of neurovirology 13: 23–8. [DOI] [PubMed] [Google Scholar]
- Codogno P, Mehrpour M, Proikas-Cezanne T (2012). Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol 13: 7–12. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Gullett JM, Porges EC, Woods AJ, Lamb DG, Bryant VE, McAdams M, Tashima K, Cook R, Bryant K, Monnig M, Kahler CW, Monti PM (2018). Heavy Alcohol Use and Age Effects on HIV-Associated Neurocognitive Function. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Underwood J, Caan MW, De Francesco D, van Zoest RA, Leech R, Wit FW, Portegies P, Geurtsen GJ, Schmand BA, Schim van der Loeff MF, Franceschi C, Sabin CA, Majoie CB, Winston A, Reiss P, Sharp DJ, collaboration C (2017). Increased brain-predicted aging in treated HIV disease. Neurology 88: 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Masliah E (2010). Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol Genet 19: R12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Patrick C, Achim CL, Everall IP, Masliah E (2009). Molecular pathology of neuro-AIDS (CNS-HIV). Int J Mol Sci 10: 1045–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, Hansen L, Adame A, Galasko D, Masliah E (2010). Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PloS one 5: e9313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cuervo AM (2004). Autophagy: in sickness and in health. Trends Cell Biol 14: 70–7. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004). Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305: 1292–5. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Hewitt T, Croitoru-Lamoury J, Taddei K, Martins RN, Chew CS, Davies NN, Price P, Brew BJ (2015). APOE epsilon4 moderates abnormal CSF-abeta-42 levels, while neurocognitive impairment is associated with abnormal CSF tau levels in HIV+ individuals - a cross-sectional observational study. BMC Neurol 15: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily A, Nath A, Hersh LB (2006). Tat peptides inhibit neprilysin. Journal of neurovirology 12: 153–60. [DOI] [PubMed] [Google Scholar]
- de Almeida SM, Ribeiro CE, Rotta I, Piovesan M, Tang B, Vaida F, Raboni SM, Letendre S, Potter M, Batistela Fernandes MS, Ellis RJ, Group HIVNRC (2018). Biomarkers of neuronal injury and amyloid metabolism in the cerebrospinal fluid of patients infected with HIV-1 subtypes B and C. Journal of neurovirology 24: 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Dumaop W, Smith D, Adame A, Everall I, Letendre S, Ellis R, Cherner M, Grant I, Masliah E (2013). Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology 80: 1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaghy PC, McKeith IG (2014). The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimer's research & therapy 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK (2011). Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. The Journal of neuroscience : the official journal of the Society for Neuroscience 31: 14508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E (2007). HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 8: 33–44. [DOI] [PubMed] [Google Scholar]
- Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, Moore D, Ellis R, Cherner M, Gelman B, Morgello S, Singer E, Grant I, Masliah E, National Neuro ATC (2009). Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. Journal of neurovirology 15: 360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E (2005). The shifting patterns of HIV encephalitis neuropathology. Neurotoxicity research 8: 51–61. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E (1999). Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain pathology 9: 209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Gao X, Chen J, Liu Y, He JJ (2016). HIV Tat Impairs Neurogenesis through Functioning As a Notch Ligand and Activation of Notch Signaling Pathway. J Neurosci 36: 11362–11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PL, Moore DJ, Franklin DR, Umlauf A, Heaton RK, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor NC, Morgello S, Simpson DM, McCutchan JA, Grant I, Letendre SL (2016). Lower CSF Abeta is Associated with HAND in HIV-Infected Adults with a Family History of Dementia. Curr HIV Res 14: 324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R, Kapogiannis D, Huey ED, Momeni P (2011). FTD and ALS: a tale of two diseases. Curr Alzheimer Res 8: 273–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell D, Giunta B (2014). The impact of HIV-1 on neurogenesis: implications for HAND. Cell Mol Life Sci 71: 4387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Eleuteri S, Campos S, Serger E, Trejo M, Kosberg K, Adame A, Spencer B,.Rockenstein E, He JJ, Masliah E (2015a). HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci 35: 1921–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Langford TD, Rockenstein E, Masliah E (2014). Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders. J Neuroimmune Pharmacol 9: 102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields J, Dumaop W, Rockenstein E, Mante M, Spencer B, Grant I, Ellis R, Letendre S, Patrick B, Adame A, Masliah E (2013). Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. Journal of neurovirology 19: 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields JA, Dumaop W, Crews L, Adame A, Spencer B, Metcalf J, He J, Rockenstein E, Masliah E (2015b). Mechanisms of HIV-1 Tat neurotoxicity via CDK5 translocation and hyperactivation: role in HIV-associated neurocognitive disorders. Curr HIV Res 13: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields JA, Metcalf J, Overk C, Adame A, Spencer B, Wrasidlo W, Florio J, Rockenstein E, He JJ, Masliah E (2017). The anticancer drug sunitinib promotes autophagyand protects from neurotoxicity in an HIV-1 Tat model of neurodegeneration. Journal of neurovirology 23: 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffin R, McCarthy M (2018a). Aging and Apolipoprotein E in HIV Infection. Journal of neurovirology 24: 529–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffin R, McCarthy M (2018b). Correction to: Aging and apolipoprotein E in HIV infection. Journal of neurovirology 24: 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R (1997). The neuropathogenesis of the AIDS dementia complex. AIDS 11 Suppl A: S35–45. [PubMed] [Google Scholar]
- Goedert M, Ghetti B, Spillantini MG (2012). Frontotemporal dementia: implications for understanding Alzheimer disease. Cold Spring Harb Perspect Med 2: a006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Anthony Crowther R, Grazia Spillantini M (2001). Parkinson's Disease, Dementia with Lewy Bodies, and Multiple System Atrophy as alpha-Synucleinopathies. Methods Mol Med 62: 33–59. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J (2005). The neuropathogenesis of AIDS. Nat Rev Immunol 5: 69–81. [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A (2004). Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23: 2891–906. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL (2005). Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19: 407–11. [DOI] [PubMed] [Google Scholar]
- Haas DW, Clough LA, Johnson BW, Harris VL, Spearman P, Wilkinson GR, Fletcher CV, Fiscus S, Raffanti S, Donlon R, McKinsey J, Nicotera J, Schmidt D, Shoup RE, Kates RE, Lloyd RM Jr., Larder B (2000). Evidence of a source of HIV type 1 within the central nervous system by ultraintensive sampling of cerebrospinal fluid and plasma. AIDS Res Hum Retroviruses 16: 1491–502. [DOI] [PubMed] [Google Scholar]
- Hategan A, Bianchet MA, Steiner J, Karnaukhova E, Masliah E, Fields A, Lee MH, Dickens AM, Haughey N, Dimitriadis EK, Nath A (2017). HIV Tat protein and amyloid-beta peptide form multifibrillar structures that cause neurotoxicity. Nat Struct Mol Biol 24: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75: 2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I (2011a). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H (2011b). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology 17: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA (2013). Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 80: 1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth J, Milanini B, Masliah E, Tartaglia MC, Dunlop MB, Moore DJ, Javandel S, DeVaughn S, Valcour V (2018). A neuropathologic diagnosis of Alzheimer's disease in an older adult with HIV-associated neurocognitive disorder. Neurocase 24: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International AsD (2016). National Alzheimer's and dementia plans. [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement 14: 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger PA, Wyss-Coray T (2010). Beclin 1 complex in autophagy and Alzheimer disease. Archives of neurology 67: 1181–4. [DOI] [PubMed] [Google Scholar]
- James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA (2016). TDP-43 stage, mixed pathologies, and clinical Alzheimer's-type dementia. Brain : a journal of neurology 139: 2983–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A (2013). Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A 110: 13588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ (2010). Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. Journal of neurovirology 16: 101–14. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA (2001). Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410: 988–94. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F (2014). Geroscience: linking aging to chronic disease. Cell 159: 709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanlou N, Moore DJ, Chana G, Cherner M, Lazzaretto D, Dawes S, Grant I, Masliah E, Everall IP, Group H (2009). Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. Journal of neurovirology 15: 131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Toda T, Gage FH (2018). Adult Hippocampal Neurogenesis: A Coming-of-Age Story. J Neurosci 38: 10401–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, Wu L, Kominami E, Ueno T, Yamamoto A, Federico M, Panganiban A, Vergne I, Deretic V (2009). Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol 186: 255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Quach A, Moore DJ, Achim CL, Soontornniyomkij V, Masliah E, Singer EJ, Gelman B, Nemanim N, Horvath S (2016). Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. Journal of neurovirology 22: 366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA (1994). AIDS-related dementia and calcium homeostasis. Ann N Y Acad Sci 747: 205–24. [DOI] [PubMed] [Google Scholar]
- Makitalo S, Mellgren A, Borgh E, Kilander L, Skillback T, Zetterberg H, Gisslen M (2015). The cerebrospinal fluid biomarker profile in an HIV-infected subject with Alzheimer's disease. AIDS Res Ther 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim CL, Wiley CA (1995). Differential vulnerability of calbindin-immunoreactive neurons in HIV encephalitis. J Neuropathol Exp Neurol 54: 350–7. [DOI] [PubMed] [Google Scholar]
- McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, Dickson D, Dubois B, Duda JE, Feldman H, Gauthier S, Halliday G, Lawlor B, Lippa C, Lopez OL, Carlos Machado J, O'Brien J, Playfer J, Reid W, International Psychogeriatric Association Expert Meeting on DLB (2004). Dementia with Lewy bodies. Lancet neurology 3: 19–28. [DOI] [PubMed] [Google Scholar]
- McKeith IG (2000). Spectrum of Parkinson's disease, Parkinson's dementia, and Lewy body dementia. Neurol Clin 18: 865–902. [DOI] [PubMed] [Google Scholar]
- Milanini B, Valcour V (2017). Differentiating HIV-Associated Neurocognitive Disorders From Alzheimer's Disease: an Emerging Issue in Geriatric NeuroHIV. Curr HIV/AIDS Rep 14: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Taneja M, Malik S, Khalique H, Seth P (2010). Human immunodeficiency virus type 1 Tat modulates proliferation and differentiation of human neural precursor cells: implication in NeuroAIDS. Journal of neurovirology 16: 355–67. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A, Masliah E (2008). Chemokine receptors and neurotrophic factors: potential therapy against aids dementia? Journal of neuroscience research 86: 243–55. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I, Group H (2006). Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 20: 879–87. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Iudicello JE, Weber E, Duarte NA, Riggs PK, Delano-Wood L, Ellis R, Grant I, Woods SP, Group HIVNRP (2012). Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr 61: 341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S (2018). HIV neuropathology. Handb Clin Neurol 152: 3–19. [DOI] [PubMed] [Google Scholar]
- Murray ME, Cannon A, Graff-Radford NR, Liesinger AM, Rutherford NJ, Ross OA, Duara R, Carrasquillo MM, Rademakers R, Dickson DW (2014). Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol 128: 411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A (2002). Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis 186 Suppl 2: S193–8. [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD (2000). Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol 47: 186–94. [PubMed] [Google Scholar]
- Nguyen TP, Soukup VM, Gelman BB (2010). Persistent hijacking of brain proteasomes in HIV-associated dementia. The American journal of pathology 176: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM (2005). Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 64: 113–22. [DOI] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Reynolds HM, Kiebala M, De Mesy Bentley KL, Trejo M, Volsky DJ, Maggirwar SB, Dewhurst S, Masliah E, Gelbard HA (2008). HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PloS one 3: e3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C, Crews L, Desplats P, Dumaop W, Rockenstein E, Achim CL, Everall IP, Masliah E (2011a). Increased CDK5 Expression in HIV Encephalitis Contributes to Neurodegeneration via Tau Phosphorylation and Is Reversed with Roscovitine. Am J Pathol 178: 1646–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C, Crews L, Desplats P, Dumaop W, Rockenstein E, Achim CL, Everall IP, Masliah E (2011b). Increased CDK5 expression in HIV encephalitis contributes to neurodegeneration via tau phosphorylation and is reversed with Roscovitine. The American journal of pathology 178: 1646–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T (2008). The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest 118: 2190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L (2009). HIV regulation of amyloid beta production. J Neuroimmune Pharmacol 4: 213–7. [DOI] [PubMed] [Google Scholar]
- Rempel HC, Pulliam L (2005). HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS 19: 127–35. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A, Hurtig HI, Siderowf A, Grossman M, McMillan CT, Miller B, Duda JE, Irwin DJ, Wolk D, Elman L, McCluskey L, Chen-Plotkin A, Weintraub D, Arnold SE, Brettschneider J, Lee VM, Trojanowski JQ (2018). Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain : a journal of neurology 141: 2181–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Carey CL, Weber E, Bondi MW, Grant I, Group HIVNRC (2011). Neurocognitive consequences of HIV infection in older adults: an evaluation of the "cortical" hypothesis. AIDS Behav 15: 1187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SECRETARY OOTA, EVALUATION FPA (2016). National Plan to Address Alzheimer's Disease. [Google Scholar]
- Seider TR, Gongvatana A, Woods AJ, Chen H, Porges EC, Cummings T, Correia S, Tashima K, Cohen RA (2016). Age exacerbates HIV-associated white matter abnormalities. Journal of neurovirology 22: 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DP, Woods SP, Massman PJ, Gilbert PE (2018). Frequency and Correlates of Subjective Cognitive Impairment in HIV Disease. AIDS Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F (2016). The Emergence of Geroscience as an Interdisciplinary Approach to the Enhancement of Health Span and Life Span. Cold Spring Harbor perspectives in medicine 6: a025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman B, Woldstad C, McMillan J, Gendelman HE (2018). Neuropathogenesis of human immunodeficiency virus infection. Handb Clin Neurol 152: 21–40. [DOI] [PubMed] [Google Scholar]
- Solomon IH, De Girolami U, Chettimada S, Misra V, Singer EJ, Gabuzda D (2017). Brain and liver pathology, amyloid deposition, and interferon responses among older HIV-positive patients in the late HAART era. BMC Infect Dis 17: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, Umlauf A, Masliah E, Levine AJ, Singer EJ, Vinters HV, Gelman BB, Morgello S, Cherner M, Grant I, Achim CL (2012). Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS 26: 2327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Umlauf A, Chung SA, Cochran ML, Soontornniyomkij B, Gouaux B, Toperoff W, Moore DJ, Masliah E, Ellis RJ, Grant I, Achim CL (2014). HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS 28: 1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Umlauf A, Soontornniyomkij B, Gouaux B, Ellis RJ, Levine AJ, Moore DJ, Letendre SL (2018). Association of antiretroviral therapy with brain aging changes among HIV-infected adults. AIDS 32: 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector SA, Zhou D (2008). Autophagy: an overlooked mechanism of HIV-1 pathogenesis and neuroAIDS? Autophagy 4: 704–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG (1999). Parkinson's disease, dementia with Lewy bodies and multiple system atrophy are alpha-synucleinopathies. Parkinsonism Relat Disord 5: 157–62. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L (1994). Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367: 188–93. [DOI] [PubMed] [Google Scholar]
- Turner RS, Chadwick M, Horton WA, Simon GL, Jiang X, Esposito G (2016). An individual with human immunodeficiency virus, dementia, and central nervous system amyloid deposition. Alzheimers Dement (Amst) 4: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Paul R (2006). HIV infection and dementia in older adults. Clin Infect Dis 42: 1449–54. [DOI] [PubMed] [Google Scholar]
- Valera E, Spencer B, Masliah E (2016). Immunotherapeutic Approaches Targeting Amyloid-beta, alpha-Synuclein, and Tau for the Treatment of Neurodegenerative Disorders. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 13: 179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinters HV, Zarow C, Borys E, Whitman JD, Tung S, Ellis WG, Zheng L, Chui HC (2018). Review: Vascular dementia: clinicopathologic and genetic considerations. Neuropathol Appl Neurobiol 44: 247–266. [DOI] [PubMed] [Google Scholar]
- Wendelken LA, Jahanshad N, Rosen HJ, Busovaca E, Allen I, Coppola G, Adams C, Rankin KP, Milanini B, Clifford K, Wojta K, Nir TM, Gutman BA, Thompson PM, Valcour V (2016). ApoE epsilon4 Is Associated With Cognition, Brain Integrity, and Atrophy in HIV Over Age 60. J Acquir Immune Defic Syndr 73: 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Achim C (1994). Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol 36: 673–6. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Baldwin M, Achim CL (1996). Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS 10: 843–7. [DOI] [PubMed] [Google Scholar]
- Winslow AR, Moussaud S, Zhu L, Post KL, Dickson DW, Berezovska O, McLean PJ (2014). Convergence of pathology in dementia with Lewy bodies and Alzheimer's disease: a role for the novel interaction of alpha-synuclein and presenilin 1 in disease. Brain 137: 1958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Ikezu T (2009). The comorbidity of HIV-associated neurocognitive disorders and Alzheimer's disease: a foreseeable medical challenge in post-HAART era. J Neuroimmune Pharmacol 4: 200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Yang L, Buch S (2012). Platelet-derived growth factor-BB restores human immunodeficiency virus Tat-cocaine-mediated impairment of neurogenesis: role of TRPC1 channels. J Neurosci 32: 9835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM (2018). The Aging Brain With HIV Infection: Effects of Alcoholism or Hepatitis C Comorbidity. Frontiers in aging neuroscience 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Masliah E, Spector SA (2011). Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. J Infect Dis 203: 1647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Liu Y, Banez-Coronel M, Reid T, Pletnikova O, Lewis J, Miller TM, Harms MB, Falchook AE, Subramony SH, Ostrow LW, Rothstein JD, Troncoso JC, Ranum LP (2013). RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci U S A 110: E4968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]