Abstract
Introduction
Autoantibody testing is helpful to predict the risk of progression to clinical arthritis in subjects at risk. Previous longitudinal studies have mainly selected autoantibody-positive arthralgia patients and, consequently, the predictive values of autoantibodies were evaluated relative to each other. This study assessed risks for arthritis development of anti-citrullinated protein antibodies (ACPA), rheumatoid factor (RF) and/or anti-carbamylated protein antibodies (anti-CarP) in arthralgia patients considered at risk for RA by rheumatologists based on clinical characteristics (Clinically Suspect Arthralgia, CSA).
Methods
Baseline ACPA, RF and anti-CarP autoantibody-status of 241 patients, consecutively included in the CSA-cohort, was studied for risk of developing clinical arthritis during a median follow-up of 103 (IQR 81-114) weeks.
Results
Univariable associations for arthritis development were observed for ACPA, RF and anti-CarP antibodies; Hazard Ratios (95%CI) were 8.5 (4.7-15.5), 5.1 (2.8-9.3) and 3.9 (1.9-7.7) respectively. In multivariable analysis, only ACPA was independently associated (HR 5.1; 2.0-13.2). Relative to autoantibody-negative CSA-patients, ACPA-negative/RF-positive patients had HRs of 2.6 (1.04-6.6), ACPA-positive/RF-negative patients 8.0 (2.4-27.4), and ACPA-positive/RF-positive patients 10.5 (5.4-20.6). Positive predictive values (PPV) for development of clinical arthritis within two years were: 38% for ACPA-negative/RF-positive, 50% for ACPA-positive/RF-negative and 67% for ACPA-positive/RF-positive patients. Higher ACPA-levels were not significantly associated with increased progression to clinical arthritis, in contrast to higher RF-levels. Autoantibody levels were stable during follow-up.
Conclusion
ACPA conferred the highest risk for arthritis development and had an additive value to RF. However, >30% of ACPA-positive/RF-positive CSA-patients did not develop arthritis during two-year follow-up. Thus, CSA and information on autoantibodies is insufficient to accurately identify imminent autoantibody-positive RA.
Keywords: Rheumatoid arthritis, Autoantigens and Autoantibodies, Biomarkers, Inflammation, Epidemiology
Introduction
Anti-Citrullinated Protein Antibodies (ACPA), Rheumatoid Factor (RF) and antibodies against carbamylated proteins (anti-CarP) can be present years before the first onset of symptoms of rheumatoid arthritis (RA)[1–3]. Initial observations on the association between autoantibodies and progression to clinical arthritis were largely done in nested case-control studies[2,3]. Results of these studies cannot be directly used for risk assessment in clinical practice; longitudinal studies performed in daily rheumatologic practice are needed to this end[4–6].
Most published longitudinal studies in arthralgia determined predictive values of ACPA and RF in persons that were selected for the presence of these autoantibodies[4,5,7,8]. Consequently, as a reference group of arthralgia patients without autoantibodies was not available, predictive values of the different autoantibodies were evaluated relative to each other[4,5]. RF-positive patients were often used as reference group, as presence of RF yielded the lowest risk of progression to clinical arthritis[5]. In addition, some of the patients in these studies had musculoskeletal symptoms but were not referred to secondary care because of these symptoms[7]. The selection method and reference group used in these studies may affect generalizability for arthralgia patients presenting to rheumatology outpatients clinics. Therefore, the risks provided by (combinations of) different autoantibodies in patients presenting with arthralgia at risk for RA is still undetermined.
The present study evaluated patients with clinically suspect arthralgia (CSA); these are patients without clinical arthritis that are considered at risk of progression to RA by their rheumatologists based on the clinical presentation. Identification of patients at risk based on clinical expertise is to some extent subjective and to allow inclusion of a more homogeneous group of patients in studies, a EULAR-definition for arthralgia suspicious for progression to rheumatoid arthritis was recently developed[9]. This definition is intended for use in arthralgia patients without clinical arthritis in whom imminent RA is considered more likely than other explanations. This will generate a more homogenous set of arthralgia patients at risk for RA and may facilitate generalizability of findings to arthralgia patients in other outpatient clinic settings.
To determine the value of RA-related autoantibodies in patients with CSA, this study aimed to: 1) Determine progression to clinical arthritis and the absolute risks provided by ACPA, RF and anti-CarP antibodies. 2) Determine the risk provided by combinations of the commercially available autoantibody-tests: ACPA and RF. 3) Evaluate if higher ACPA- and RF-levels conferred higher rates of progression to clinical arthritis. In addition, sub-analyses were performed in which we aimed to 4) Investigate differences in baseline characteristics of ACPA-positive/RF-positive patients that did and that did not progress to clinical arthritis and 5) Assess ACPA- and RF-levels over time, both in patients that progressed from CSA to arthritis and in patients that did not progress.
Methods
Patients
Two hundred and forty-one patients were consecutively included in the Leiden Clinically Suspect Arthralgia (CSA) cohort between April 2012 and March 2015, an inception cohort at the rheumatology outpatient clinic of the Leiden University Medical Centre, the Netherlands. Per definition, CSA-patients had no clinical arthritis, but recent-onset (<1 year) arthralgia of hand or feet joints and were considered at risk for RA based on the clinical expertise of the rheumatologists, as described previously[10]. Hence, patients were indicated as having CSA based on the first clinical presentation. As general practitioners in the region are discouraged to perform autoantibody testing before referral[11,12], information on ACPA- and RF-status were generally unknown at secondary care presentation. After inclusion, questionnaires were filled by patients and rheumatologists, joint counts performed, blood samples taken, and a unilateral contrast-enhanced MRI was made of 2nd-5th metacarpophalangeal, wrist and 1st-5th metatarsophalangeal joints of the most painful side (or dominant side in case of equally severe symptoms at both sides) using an MSK-extremity 1.5T MRI-scanner as described elsewhere[10,11] and in the Supplementary Methods. Regular follow-up visits were scheduled at 4,12 and 24 months and additional visits occurred in between if indicated (either if felt necessary by rheumatologists or at request of patients because of an increase in symptom severity). Treatment with Disease-Modifying Antirheumatic Drugs (DMARDs) was not allowed during the CSA study, NSAIDs were allowed. The CSA-cohort has been approved by the local medical ethical committee (named “Commissie Medische Ethiek”). All participants provided written informed consent according to the declaration of Helsinki.
Autoantibody determination
At baseline visit, Immunglobulin-G ACPA (EliA CCP (anti-CCP2), Phadia, Nieuwegein, the Netherlands), Immunoglobulin-M RF (as described previously, in-house ELISA[13]), and Immunoglobulin-G anti-CarP antibodies were determined. The cut-off for ACPA-positivity was >7 U/mL; for RF-positivity it was >3.5 IU/mL, according to the manufacturer’s instructions. ACPA- and RF-status were repeated after two years, or at the time of conversion to clinical arthritis. Anti-CarP was determined as described previously[14]. As no commercial kit is available for anti-CarP antibodies, we have used our in-house developed anti-CarP assay based on carbamylated Fetal Calf Serum (FCS) and as a control the non-modified FCS as the coating antigens in ELISA[14]. The cut-off was equivalent to 2 Standard Deviations (SD) above the mean in a group of healthy controls. The controls consisted of a group of 197 healthy blood donors. Mean age of the controls was 44.4 years (range 20-70 years, SD 14). 50.8% of controls were female. Controls were not allowed to have a rheumatic disease. 65.5% of controls had never smoked, 26.9% had previously smoked, 6.6% were current smokers and in two controls data on smoking status was missing.
Outcome
All patients were followed for ≥56 weeks. Median follow-up duration was 103 weeks, interquartile range 81 to 114 weeks. None of the patients were treated with DMARDs or corticosteroids in the phase of CSA. Primary endpoint was development of arthritis detected at physical examination (66 joints assessed) by the rheumatologist. Medical records of all patients were studied for established clinical arthritis until April 22nd 2016. Persistent arthritis was studied as secondary endpoint (through study of the medical record), which was defined as clinical arthritis that persisted at two subsequent visits or when DMARDs were prescribed when clinical arthritis was identified. The 2010 classification criteria for RA[15] were considered less suitable as secondary outcome, as autoantibody-negative patients require >10 involved joints to fulfil these criteria[16]. DMARDs were generally started shortly after patients had developed clinically evident arthritis and this may have prevented progression from unclassified arthritis to RA, particularly for autoantibody-negative patients.
Statistical analyses
Univariable and multivariable Cox proportional hazards regression analyses were performed with clinical arthritis as outcome. Time to clinical arthritis was defined as time from inclusion date in the cohort to the date of first detection of clinical arthritis. Patients who did not develop arthritis were censored at either the date that all medical files were studied on arthritis development or at the date of the 24-month follow-up visit. When evaluating hazard ratios and absolute risks for combinations of autoantibodies, we mainly restricted ourselves to the two commercially available autoantibodies (ACPA and RF), because otherwise small subgroups would be obtained (Supplementary Figure 1). To determine the association for arthritis development with autoantibody level, patients were categorized into tertiles based on ACPA-levels of ACPA-positive patients in our cohort or RF-levels in RF-positive patients in our cohort (hence creating three groups of similar size). For ACPA, these categories were 7-95 U/ml (with N=10), 96-325 U/ml (N=11) and ≥326 U/ml (N=11). For RF, the categories were 3.5-10 IU/ml (N=17), 11-40 IU/ml (N=17) and ≥41 IU/ml (N=17). Test characteristics and predictive values with 95% confidence intervals were calculated. Patient characteristics were compared using Mann-Whitney U tests, t-tests and χ2 tests as appropriate.
In addition to the analyses on all CSA-patients, the most important analyses were repeated in the subgroup of patients that also fulfilled the EULAR-definition of arthralgia suspicious for progression to RA (3/7 items present)[9]. Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS, version 23.0). P-values <0.05 were considered significant.
Results
Patients with CSA
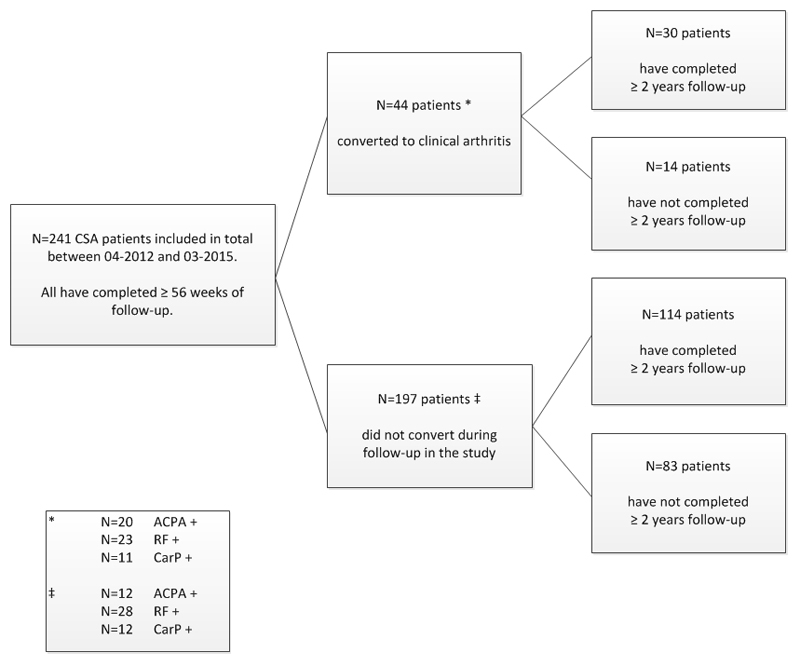
Baseline characteristics of the 241 CSA-patients are shown in Table 1. During a median follow-up period of 103 (IQR 81-114) weeks, 44 patients progressed to clinical arthritis (Figure 1). The secondary endpoint was obtained in 41 patients: 3 patients had clinical arthritis on only one occasion that resolved spontaneously (without DMARD treatment) before the next visit; one patient had clinical arthritis in a wrist joint and two patients in the elbow joint.
Table 1. Baseline characteristics of the CSA-patients (N=241).
| Patient characteristic | |
|---|---|
| Age in years, mean (SD) | 44 (13) |
| Female sex, n (%) | 187 (78) |
| Caucasian, n (%) | 224 (93) |
| Family history of RA, n (%) | 71 (30) |
| Symptom duration in weeks, median (IQR) | 18 (10 – 48) |
| Presence of morning stiffness ≥60 minutes, n (%) | 80 (33) |
| Current smoker, n (%) | 54 (22) |
| BMI in kg/m2, median (IQR) | 26 (24 – 30) |
| Baseline HAQ-score, median (IQR) | 0.50 (0.20 – 0.88) |
| 68-TJC, median (IQR) | 6 (3 – 10) |
| Increased CRP (>10 mg/L), n (%) | 53 (22) |
| Positive for EULAR-definition for arthralgia suspicious for progression to rheumatoid arthritis[9], n (%) | 178 (74) |
| Autoantibody status | |
| IgM-RF-positive (>3.5 IU/mL), n (%) | 51 (21) |
| ACPA-positive (>7 U/mL), n (%) | 32 (13) |
| Anti-CarP positive (>2 SD), n (%) | 23 (10) |
Glossary:
ACPA = anti-citrullinated peptide antibody; BMI = body mass index; CRP = C-reactive protein; HAQ = Health Assessment Questionnaire; IgM-RF = immunoglobulin M rheumatoid factor; IQR = interquartile range; RA = rheumatoid arthritis; SD = standard deviation; TJC = tender joint count.
Figure 1. Flowchart of the patient flow and development of clinical arthritis during two-year follow-up period of the present study.
Flowchart of the patient flow and development of clinical arthritis during two-year follow-up period of the present study.
Presence of autoantibodies and hazard ratio for progression to clinical arthritis
In univariable Cox regression, presence of ACPA was associated with arthritis development (Hazard Ratio 8.5; 95%CI 4.7-15.4). A similar observation was made for presence of RF (HR 5.1; 95%CI 2.8-9.3) or anti-CarP antibodies (HR 3.9; 95%CI 1.9-7.7). Multivariable analysis including all three autoantibodies – to correct for simultaneous presence of the autoantibodies – revealed an independent significant association for ACPA only (HR 5.1; 95%CI 2.0-13.2); the HR for RF and anti-CarP antibodies were 2.0 (95%CI 0.81-4.9) and 1.04 (95%CI 0.46-2.4) respectively. When age, gender, smoking and positive family history for RA were also included in the multivariable model, only ACPA was significantly associated with progression to RA (HR 5.3; 95%CI 2.0-14.2).
Combinations of ACPA and RF and associated hazards
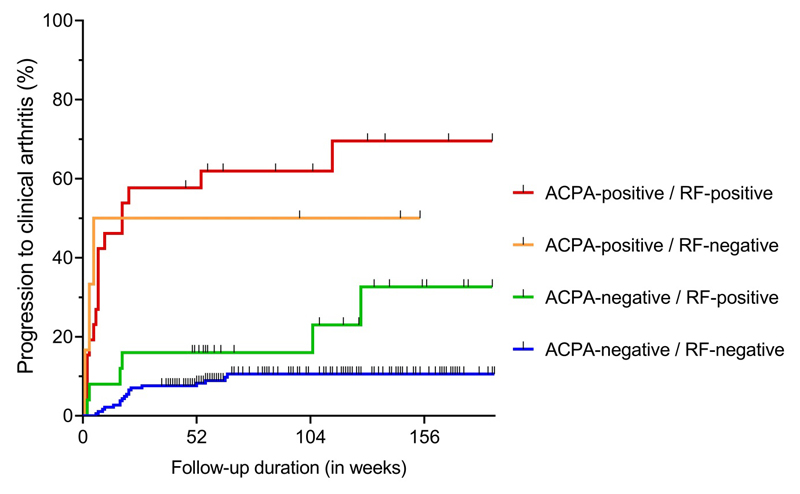
Combinations of ACPA and RF were studied next, as these are the commercially available tests and are most commonly used in daily rheumatologic care. With autoantibody-negative CSA patients as reference, ACPA-negative/RF-positive patients had a HR of 2.6 (95%CI 1.04-6.6) for developing clinical arthritis. ACPA-positive/RF-negative had a HR of 8.0 (95%CI 2.4-27.4), and ACPA-positive/RF-positive patients had a HR of 10.5 (95%CI 5.4-20.6), see Figure 2. The hazard was not significantly different between ACPA-positive/RF-negative and ACPA-positive/RF-positive patients (p=0.78), but there was a significantly different hazard ratio between ACPA-negative/RF-positive and ACPA-positive/RF-positive patients (p=0.005).
Figure 2. Kaplan-Meier One Minus Survival plots with combinations of Anti-Citrullinated Protein antibodies (ACPA) and Rheumatoid Factor (RF) and associated risks for progression to clinical arthritis over time.
With autoantibody-negative CSA-patients as reference (N=184), ACPA-negative/RF-positive patients (N=25) had a HR of 2.6 (1.04-6.6), ACPA-positive/RF-negative (N=6) a HR of 8.0 (2.4-27.4) and ACPA-positive/RF-positive patients (N=26) a HR of 10.5 (5.4-20.6) for progression to clinical arthritis.
Although subgroups became small when anti-CarP was also considered (Supplementary Figure 1), there were no significant associations of anti-CarP with arthritis development within ACPA-negative/RF-negative or within ACPA-positive/RF-positive patients (HR 2.7; 95%CI 0.62–11.9 and HR 1.0; 95%CI 0.37–2.7 respectively).
Association of autoantibody levels and arthritis development
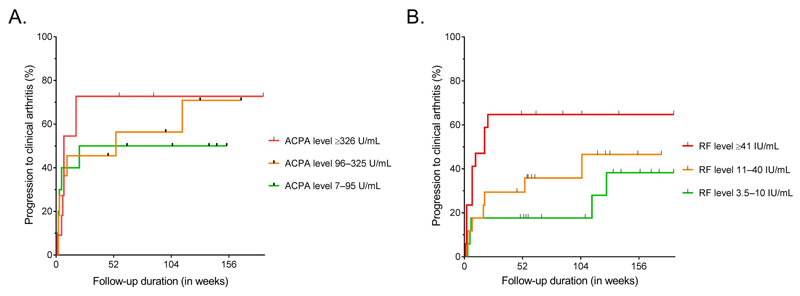
In RA, presence of multiple autoantibodies is associated with higher autoantibody levels[17,18]. In CSA-patients, higher ACPA-levels were observed in ACPA-positive/RF-positive patients than in ACPA-positive/RF-negative patients (median 237.5 U/mL versus 94 U/mL, p=0.17). Within ACPA-positive patients, ACPA-levels were not associated with higher hazards for progression to clinical arthritis (Figure 3A). RF-levels were significantly higher in ACPA-positive/RF-positive patients compared to ACPA-negative/RF-positive patients (median 36 IU/mL versus 12.5 IU/mL respectively, p=0.007). In addition, patients with RF-levels ≥41 IU/ml (highest tertile) had significantly increased hazard to progress to clinical arthritis (HR 3.3; 95%CI 1.1-9.6) compared to patients with RF-levels 3.5-10 IU/ml (lowest tertile, Figure 3B).
Figure 3. Kaplan-Meier One Minus Survival plots with (A) ACPA-levels in ACPA-positive CSA patients and (B) RF-levels in RF-positive CSA-patients and associated risks for progression to clinical arthritis over time.
A. The ACPA-positive patients in the second tertile (levels 96–325 U/ml, N=11) had a HR of 1.2 for progression to clinical arthritis (95%CI=0.39–3.9) compared to the patients in the lowest tertile (N=10). The ACPA-positive patients in the third and highest tertile (levels ≥326 U/ml, N=11) had a HR of 1.6 for progression to clinical arthritis (95%CI=0.50–4.8) compared to the patients in the lowest ACPA-level tertile.
B. The RF-positive patients in the second tertile (levels 11-40 IU/ml, N=17) had a HR of 1.6 for progression to clinical arthritis (95%CI=0.50–5.0) compared to the patients in the lowest tertile (N=17). The RF-positive patients in the third and highest tertile (levels ≥41 IU/ml, N=17) had a HR of 3.3 for progression to clinical arthritis (95%CI=1.1–9.6) compared to the patients in the lowest RF-level tertile.
Absolute risks and test characteristics for arthritis development at 2 years follow-up
In order to arrive at absolute risks for developing clinical arthritis of individual autoantibodies and combinations of ACPA and RF, patients that completed two-year follow-up were studied (n=144). Positive predictive values (PPV) for arthritis development within two years were: 63% for ACPA, 53% for RF and 50% for anti-CarP antibodies. Considering combinations of ACPA and RF, the PPV for ACPA-negative/RF-positive patients was 38%. For ACPA-positive/RF-negative patients, PPV was 50% and for ACPA-positive/RF-positive patients 67% (Table 2). Thus, of the ACPA-positive/RF-positive patients, 33% did not develop arthritis within two years. Sub-analyses with the secondary endpoint (persistent clinical arthritis) showed almost similar results (Supplementary Table 1).
Table 2. Test characteristics for Anti-Citrullinated Protein antibodies, Rheumatoid Factor and Anti-Carbamylated protein antibodies and conversion to clinical arthritis within two years as outcome (N=144).
| Sensitivity (95%CI) |
Specificity (95%CI) |
PPV (95%CI) |
NPV (95%CI) |
LR + (95%CI) |
LR – (95%CI) |
|
|---|---|---|---|---|---|---|
| Evaluating antibodies individually | ||||||
| ACPA + | 50% (32%–68%) |
92% (85%–96%) |
63% (41%–80%) |
88% (80%–93%) |
6.3 (3.1–13.0) |
0.54 (0.38–0.78) |
| IgM-RF + | 60% (41%–77%) |
86% (78%–92%) |
53% (35%–70%) |
89% (81%–94%) |
4.3 (2.5–7.3) |
0.47 (0.30–0.72) |
| Anti-CarP + | 24% (11%–44%) |
94% (87%–97%) |
50% (24%–76%) |
82% (74%–88%) |
3.8 (1.4–9.9) |
0.81 (0.66–1.0) |
| Evaluating combinations of ACPA and RF (ACPA- RF- as reference) | ||||||
| ACPA + IgM-RF + |
57% (34%–77%) |
94% (87%–98%) |
67% (41%–86%) |
91% (84%–96%) |
9.6 (4.1–22.7) |
0.46 (0.28–0.75) |
| ACPA+ IgM-RF – |
25% (6.7%–57%) |
97% (91%–99%) |
50% (14%–86%) |
91% (84%–96%) |
8.2 (1.9–36.0) |
0.77 (0.56–1.1) |
| ACPA – IgM-RF + |
40% (17%–67%) |
90% (83%–95%) |
38% (16%–64%) |
91% (84%–96%) |
4.2 (1.8–9.9) |
0.66 (0.44–1.0) |
Glossary:
IgM-RF = IgM rheumatoid factor; ACPA = anti-citrullinated protein antibodies; CarP = anti-carbamylated protein antibodies; 95%CI = 95% confidence interval; PPV = positive predictive value; NPV = negative predictive value; LR + = positive likelihood ratio; LR – = negative likelihood ratio.
Similar findings in patients that fulfilled the EULAR-definition of arthralgia suspicious for progression to RA
178 of the 241 patients (74%) that were identified as CSA by their rheumatologists also fulfilled the EULAR-definition. The HRs for progression to arthritis within 2-years were: 2.4 (95%CI 0.89-6.5) for ACPA-negative/RF-positive patients, 5.9 (95%CI 1.4-25.8) for ACPA-positive/RF-negative patients, and 9.7 (95%CI 4.7-20.2) for ACPA-positive/RF-positive patients (Supplementary Figure 2). Predictive values and test characteristics are presented in Supplementary Table 2. Of the ACPA-positive/RF-positive patients that fulfilled the EULAR-definition, 31% did not progress to RA.
Baseline characteristics of ACPA-positive/RF-positive CSA-patients that progressed to arthritis versus those that did not
We hypothesized that patients progressing to clinical arthritis had either higher autoantibody levels or more extended (systemic or local subclinical) inflammation than patients that did not progress. Therefore, we then explored if ACPA-positive/RF-positive CSA-patients that did not progress to arthritis during 2 years follow-up differed in baseline characteristics from those that progressed. Although the number of patients in both groups was small, no statistically significant or clinically relevant differences were observed (Table 3).
Table 3. Baseline characteristics of ACPA-positive/Rheumatoid Factor-positive patients that progressed to RA and did not progress to RA during 2 year follow-up.
| Patient characteristic | Convertors (N=12) |
Non-convertors (N=6) |
p-value | ||
|---|---|---|---|---|---|
| Age in years, mean (SD) | 45 | (15) | 49.8 | (11) | 0.50 |
| Female sex, n (%) | 9 / 12 | (75) | 5 / 6 | (83) | 0.69 |
| Family history of RA, n (%) | 4 / 12 | (33) | 0 / 6 | (0) | 0.11 |
| 68-TJC, median (IQR) | 5.0 | (3–8) | 5.5 | (2–9) | 0.87 |
| CRP | |||||
| Elevated CRP (>10 mg/L), n (%) | 5 / 12 | (42) | 2 / 6 | (33) | 0.73 |
| CRP-level, median (IQR) | 4.7 | (3–12) | 4.2 | (3–6) | 0.32 |
| IgM-RF level (IU/mL), mean (SD) | 76.6 | (77) | 79.2 | (64) | 0.93 |
| ACPA-level (U/mL), mean (SD) | 222.8 | (125) | 211.0 | (141) | 0.87 |
| MRI positive for inflammation | |||||
| Any inflammation present, n (%) | 9 / 10 | (90) | 5 / 6 | (83) | 0.70 |
| Total RAMRIS score, median (IQR) | 5.8 | (3–19) | 5.8 | (4–11) | 0.36 |
Symptoms were noted by rheumatologists as reported by the patients.
Glossary: ACPA = anti-citrullinated peptide antibody; CRP = c-reactive protein; IgM-RF = immunoglobulin M rheumatoid factor; IQR = interquartile range; MRI = magnetic resonance imaging; RA = rheumatoid arthritis; RAMRIS = Rheumatoid Arthritis MRI scoring system; SD = standard deviation; TJC = tender joint count.
Serum levels of ACPA and RF over time
Of the 44 CSA-patients that progressed to clinical arthritis, 20 were ACPA-positive with a median ACPA-level at CSA-inclusion of 266 U/ml (IQR 130-340) and 200 U/ml (IQR 91.75-340) at arthritis development (p=0.39). Similarly, of the 44 patients that progressed, 23 were RF-positive with a median RF-level of 29 IU/ml at CSA-inclusion and 39.5 IU/ml at arthritis development (p=0.99).
Autoantibody status and autoantibody levels were also assessed in patients who had completed two-year follow-up and did not progress to clinical arthritis (N=114). Of these patients, 10 were ACPA-positive at inclusion and none of these patients changed in ACPA-status during follow-up. The median ACPA-level in these non-converting patients was 304 U/ml at baseline and 340 U/ml after 2-years. Similarly, 16 patients not progressing to clinical arthritis were RF-positive at baseline; during follow-up, one RF-positive patient became RF-negative (levels 4.3 IU/mL and 3.0 IU/mL respectively) and one RF-negative patient became RF-positive after 2 years (levels <0.4 IU/mL and 12.0 IU/mL respectively). The median RF-level in non-converting patients was 16.5 IU/ml at baseline and 11 IU/ml after 2 years. Overall, status and levels of ACPA and RF were rather stable during two year follow-up, both in patients that progressed to clinical arthritis and in patients that did not progress to clinical arthritis.
Discussion
Early recognition of patients with imminent RA is an important but challenging topic. Autoantibodies have proven to be the most powerful predictors for development of clinical arthritis currently available. This study thoroughly determined the risks of individual autoantibodies, combinations of autoantibodies and autoantibody-levels in patients that were considered at risk for RA based on their clinical presentation. The absolute risks for progression to arthritis may be useful for daily clinical practice at places where patients present with arthralgia to rheumatology outpatient clinics. We observed that ACPA, RF and anti-CarP antibodies were associated with increased risks, but that only ACPA was independently associated with development of RA in multivariable analysis. Furthermore, although ACPA was clearly additive to RF in predicting risks, vice versa, RF was less additive to ACPA.
A previous study by Van Steenbergen evaluated the risk of ACPA, but not the other autoantibodies in CSA[11]. The current study explored different characteristics of several different autoantibodies in CSA, in a larger study population and during a longer duration of follow-up. As previously described, the absolute risk of ACPA for arthritis development within two years was 63%. Previous studies in other at-risk populations found lower positive predictive values. A study in ACPA-positive patients with non-specific musculoskeletal complaints showed progression to clinical arthritis of 47% within 12 months[7]. A study in ACPA-positive and/or RF-positive arthralgia patients found a PPV of 35% during the first year[8]. Positive predictive values are dependent on enrichment (i.e. prevalence) of cases in cohort studies, meaning that the same test may yield different results depending on the setting. Patients that are identified as having CSA by rheumatologists comprise a small group of all patients presenting with arthralgia to secondary care (<6%)[19]. This yielded higher prior chances for RA-development in CSA-patients than patients with non-specific arthralgia in secondary or primary care. Presumably, this explains the higher post-test chances of ACPA in this setting.
CSA is defined by the clinical expertise of rheumatologists and is therefore subjective. A EULAR-taskforce has recently derived a definition of arthralgia suspicious for progression to RA, in order to strip CSA from its subjectivity and to allow evaluation of a more homogeneous group of patients. Although further longitudinal studies on the accuracy of the EULAR-definition are required, the present data suggest that the clinical expertise of the rheumatologists was often in line with the EULAR-definition.
In the present data, despite small numbers, higher RF-levels were associated with an increased risk of progression to clinical arthritis. Furthermore, patients with higher RF-levels were also more often ACPA-positive. RF-positive/ACPA-positive patients had a higher risk of developing clinical arthritis than RF-positive/ACPA-negative patients. Hence, these findings are compatible with each other. ACPA-levels were not associated with a significantly increased risk of developing arthritis. This finding is in line with that of non-significant differences in the risk of developing arthritis between ACPA-positive/RF-positive and ACPA-positive/RF-negative patients, as both groups also had no significant differences in ACPA-level. However, it should be noted that subgroups of patients with different autoantibody levels were small.
This is the first longitudinal study evaluating the effect of anti-CarP antibodies in relation to RF and ACPA in CSA. A previous study observed an association of anti-CarP antibodies with arthritis development in non-specific autoantibody-positive arthralgia, but did not perform multivariable analysis including all three autoantibodies with an autoantibody-negative group as reference[6]. In our study, anti-CarP antibodies were not independently associated with arthritis development and a significant effect of anti-CarP, additive to ACPA and RF, could not be shown. However, the current anti-CarP antibody test is not commercially available which allows further optimization and afterwards evaluation in larger studies.
ACPA- and RF-levels were rather stable over time, both in CSA-patients that developed clinical arthritis and in patients that did not progress. Seroconversion during the study was rarely observed for RF and absent for ACPA. The finding of stable ACPA-levels in the phase of CSA and during progression to clinical (persistent) arthritis suggests that the broadening of the autoantibody response may already have occurred in an earlier, and perhaps asymptomatic, pre-arthritis phase.
A large limitation of this study is the sample size of subgroup analyses; especially the ACPA-positive/RF-negative subgroup was small. Validation of the presented findings in other cohorts of arthralgia suspicious for progression to RA is needed. The primary outcome used was clinically apparent arthritis. As arthritis can be subtle in early stages and variation in the sensitivity to detect clinical arthritis between rheumatologists exists, sub-analyses were performed with clinical arthritis that was persistent at two subsequent visits or that was treated with DMARDs as outcome (both outcomes reflect chronic disease). These analyses provided similar results.
Based on results of case-control studies, revealing that simultaneous presence of ACPA and RF almost does not occur in healthy controls[20,21], it is sometimes suggested that presence of both ACPA and RF in arthralgia is a guarantee for progression to clinical arthritis and RA. However, this was not observed in the present study and our findings are in line with results of other longitudinal studies. Bos et al[5] showed that 60% of ACPA-positive/RF-positive patients with non-specific arthralgia did not progress to clinical arthritis. Another study showed that 42% of ACPA-positive/anti-CarP-positive patients did not progress to arthritis[6]. Thus, previous studies also showed that presence of several autoantibodies in arthralgia was not always associated with arthritis development.
We hypothesized that ACPA-positive/RF-positive patients that did or did not progress would have lower autoantibody levels or less severe subclinical inflammation. However, no apparent differences were observed. An explanation for patients not progressing to clinical arthritis might be that the remaining ACPA-positive/RF-positive CSA patients will progress to clinical arthritis later on. Although we cannot exclude this, the Kaplan-Meier curves indicate that ACPA-positive/RF-positive patients mostly progressed in the first year and few converted in the second year. This makes the hypothesis that many subjects will progress after additional follow-up less likely. Other explanations are that patients that are truly pre-RA have differences in molecular characteristics of the autoantibodies themselves, or that another trigger (on top of the presence of autoantibodies) is required to develop clinically evident arthritis. This is a subject of further research.
In summary, presence of autoantibodies in CSA conferred increased absolute risks to develop clinical arthritis. Furthermore, positive predictive values in CSA were higher than that reported in non-specific arthralgia. However, also within CSA, presence of ACPA alone or a combination of ACPA and RF is insufficient to identify patients with imminent ACPA-positive RA with high accuracy (e.g. with PPVs >80%). Thus, in addition to clinical characteristics and autoantibodies, other biomarkers are needed for optimal prognostication.
Supplementary Material
Key Messages.
The presence of autoantibodies in CSA conferred increased absolute risks for developing clinical arthritis.
Although ACPA was additive to RF in predicting risks, RF was less additive to ACPA.
Within CSA, presence of ACPA (even at high level) and RF is insufficient to accurately identify imminent autoantibody-positive RA.
Acknowledgments
None.
Funding Statement
This work was supported by the Dutch Arthritis Foundation and a Vidi grant by the Netherlands Organisation of Health Research and Development. The Dutch Arthritis Foundation had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The Netherlands Organisation of Health Research and Development had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Competing Interests
All authors declare no conflicts of interest relevant to the presented manuscript.
References
- 1.Gan RW, Trouw LA, Shi J, et al. Anti-carbamylated Protein Antibodies Are Present Prior to Rheumatoid Arthritis and Are Associated with Its Future Diagnosis. J Rheumatol. 2015 Apr;42(4):572–9. doi: 10.3899/jrheum.140767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielen MMJ, Van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 2004 Feb;50(2):380–6. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 3.Rantapää-Dahlqvist S, De Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003 Oct;48(10):2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 4.Rakieh C, Nam JL, Hunt L, et al. Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis. 2015 Sep;74(9):1659–66. doi: 10.1136/annrheumdis-2014-205227. [DOI] [PubMed] [Google Scholar]
- 5.Bos WH, Wolbink GJ, Boers M, et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis. 2010 Mar;69(3):490–4. doi: 10.1136/ard.2008.105759. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, van de Stadt LA, Levarht EW, et al. Anti-Carbamylated Protein Antibodies Are Present in Arthralgia Patients and Predict the Development of Rheumatoid Arthritis. Arthritis Rheum. 2013 Apr;65(4):911–5. doi: 10.1002/art.37830. [DOI] [PubMed] [Google Scholar]
- 7.Nam JL, Hunt L, Hensor EM, et al. Enriching case selection for imminent RA: the use of anti-CCP antibodies in individuals with new non-specific musculoskeletal symptoms – a cohort study. Ann Rheum Dis. 2015 Sep 22; doi: 10.1136/annrheumdis-2015-207871. pii: annrheumdis-2015-207871. [DOI] [PubMed] [Google Scholar]
- 8.Van de Stadt LA, Witte BI, Bos WH, et al. A prediction rule for the development of arthritis in seropositive arthralgia patients. Ann Rheum Dis. 2013 Dec;72(12):1920–6. doi: 10.1136/annrheumdis-2012-202127. [DOI] [PubMed] [Google Scholar]
- 9.Van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJJ, et al. EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Ann Rheum Dis. 2016 Oct; doi: 10.1136/annrheumdis-2016-209846. [DOI] [PubMed] [Google Scholar]
- 10.Van Steenbergen HW, Van Nies JAB, Huizinga TWJ, et al. Characterising arthralgia in the preclinical phase of rheumatoid arthritis using MRI. Ann Rheum Dis. 2015 Jun;74(6):1225–32. doi: 10.1136/annrheumdis-2014-205522. [DOI] [PubMed] [Google Scholar]
- 11.Van Steenbergen HW, Mangnus L, Reijnierse M, et al. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann Rheum Dis. 2016 Oct;75(10):1824–30. doi: 10.1136/annrheumdis-2015-208138. [DOI] [PubMed] [Google Scholar]
- 12.Newsum EC, De Waal MWM, Van Steenbergen HW, et al. How do general practitioners identify inflammatory arthritis? A cohort analysis of Dutch general practitioner electronic medical records. Rheumatology (Oxford) 2016 May;55(5):848–53. doi: 10.1093/rheumatology/kev432. [DOI] [PubMed] [Google Scholar]
- 13.Van der Linden MPM, Batstra MR, Bakker-Jonges LE, et al. Toward a data-driven evaluation of the 2010 American College of Rheumatology/European League Against Rheumatism criteria for rheumatoid arthritis: Is it sensible to look at levels of rheumatoid factor? Arthritis Rheum. 2011 May;63(5):1190–9. doi: 10.1002/art.30200. [DOI] [PubMed] [Google Scholar]
- 14.Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci USA. 2011 Oct 18;108(42):17372–7. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010 Sep;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 16.Nordberg LB, Lillegraven S, Lie E, et al. Patients with seronegative RA have more inflammatory activity compared with patients with seropositive RA in an inception cohort of DMARD-naïve patients classified according to the 2010 ACR/EULAR criteria. Ann Rheum Dis. 2017 Feb;76(2):341–345. doi: 10.1136/annrheumdis-2015-208873. [DOI] [PubMed] [Google Scholar]
- 17.Figueiredo CP, Bang H, Cobra JF, et al. Antimodified protein antibody response pattern influences the risk for disease relapse in patients with rheumatoid arthritis tapering disease modifying antirheumatic drugs. Ann Rheum Dis. 2017 Feb;76(2):399–407. doi: 10.1136/annrheumdis-2016-209297. [DOI] [PubMed] [Google Scholar]
- 18.Van Heemst J, Trouw LA, Nogueira L, et al. An investigation of the added value of an ACPA multiplex assay in an early rheumatoid arthritis setting. Arthritis Res Ther. 2015 Oct 5;17:276. doi: 10.1186/s13075-015-0786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Steenbergen HW, Van der Helm-van Mil AHM. Clinical expertise and its accuracy in differentiating arthralgia patients at risk for rheumatoid arthritis from other patients presenting with joint symptoms. Rheumatology (Oxford) 2016 Jun;55(6):1140–1141. doi: 10.1093/rheumatology/kev431. [DOI] [PubMed] [Google Scholar]
- 20.Brink M, Hansson M, Mathsson-Alm L, et al. Rheumatoid factor isotypes in relation to antibodies against citrullinated peptides and carbamylated proteins before the onset of rheumatoid arthritis. Arthritis Res Ther. 2016 Feb 9;18:43. doi: 10.1186/s13075-016-0940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terao C, Ohmura K, Ikari K, et al. Effects of smoking and shared epitope on the production of anti-citrullinated peptide antibody in a Japanese adult population. Arthritis Care Res (Hoboken) 2014 Dec;66(12):1818–27. doi: 10.1002/acr.22385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.