Abstract
Background
Physiological changes are induced by immersion, swimming and using diving equipment. Divers must be fit to dive. Using medication may impact the capacity to adapt to hyperbaric conditions. The aim of this systematic review is to assess the interaction of diving/hyperbaric conditions and medication and to provide basic heuristics to support decision making regarding fitness to dive in medicated divers.
Methods
This was a systematic review of human and animal studies of medications in the hyperbaric environment. Studies were subdivided into those describing a medication/hyperbaric environment interaction and those concerned with prevention of diving disorders. Studies without a relation to diving with compressed air, and those concerning oxygen toxicity, hyperbaric oxygen therapy or the treatment of decompression sickness were excluded.
Results
Forty-four studies matched the inclusion criteria. Animal studies revealed that diazepam and valproate gave limited protection against the onset of the high-pressure neurological syndrome. Lithium had a protective effect against nitrogen-narcosis and losartan reduced cardiac changes in repetitive diving. Human studies showed no beneficial or dangerous pressure-related interactions. In prevention of diving disorders, pseudoephedrine reduced otic barotrauma, vitamins C and E reduced endothelial dysfunction after bounce diving and hepatic oxidative stress in saturation diving.
Discussion and conclusions
Animal studies revealed that psycho-pharmaceuticals can limit the onset of neurologic symptoms and cardiovascular protective drugs might add a potential protective effect against decompression sickness. No evidence of significant risks due to changes in pharmacologic mechanisms were revealed and most medication is not a contraindication to diving. For improving decision making in prescribing medicine for recreational and occupational divers and to enhance safety by increasing our understanding of pharmacology in hyperbaric conditions, future research should focus on controlled human studies.
Keywords: Diving, Drugs, Pharmacokinetics, Decompression sickness, Fitness to dive, Review article
Introduction
Scuba diving is an increasingly popular sport with more than 15 million divers worldwide completing more than 250 million dives per year.[ 1] Certification for scuba diving can be obtained through diving certification organizations. These include, for example, the Professional Association of Diving Instructors (PADI) and Scuba Schools International (SSI). This certification typically involves online classes, classroom instruction, pool practice and open-water training. Since physiological changes are induced by immersion, swimming and using special equipment during diving, divers must be fit to dive.[ 2 , 3] The laws of physics that are important to take into consideration while diving are Boyle’s law, Henry’s law and Dalton’s law. These laws provide explanations for the possible occurrences of barotrauma, decompression sickness (DCS), nitrogen narcosis and oxygen toxicity, amongst other pathophysiological impacts of the underwater environment.[ 4] Although the hazards of diving are principally identical for sport, commercial and military divers, the risks may vary depending on the varying diving procedures and equipment used. Appropriate training, skills and equipment can aid in reducing the risk of diving and, depending on jurisdictions, regular medical assessment is required before diving.
Medical disorders or use of medication may have an impact on the capacity to adapt to hyperbaric conditions and could affect medical fitness to dive.[ 5] Illnesses, such as asthma or epilepsy, require a medical clearance. However, in most cases, evidence of causality is absent and it is not always straightforward to predict the effect of medication on cognitive and physical functioning in hyperbaric conditions. General health, specifics of the disorder, medication interaction and the hyperbaric conditions are all factors in this assessment process. Obviously, regulations concerning commercial divers are stricter, and illnesses prior to a diving career are a stronger contraindication than onset during a diving career. Many protocols have been written for selecting humans for work under hyperbaric conditions or (recreational) diving, however robust evidence to guide practice is limited.[ 6 - 7]
The primary aim of this systematic review of the current human and animal study literature was to assess the interactions between the hyperbaric environment and medications. The secondary aim was to provide a heuristic approach to support decision making regarding physical fitness for occupational health under hyperbaric conditions and (recreational) diving.
Methods
PROTOCOL
The protocol for objectives, literature search strategies, inclusion and exclusion criteria and outcome measurements was prepared a priori, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[ 8] statement and is described in this section.
LITERATURE SEARCH STRATEGY
This systematic review sought human and animal studies investigating pharmacodynamic/kinetic effects of the hyperbaric environment on medications or the effects of medications used while diving on the risk of diving disorders. An electronic database search of PubMed, Medline, Embase Science Citation Index Expanded, the Web of Science and World Wide Web search (key words '(scuba) diving', 'hyperbaric', 'medication', 'drugs') was performed up to 16 February 2018. These databases were searched for articles published using the medical subject headings (MeSH) or entry terms from Table 1. We focused on the disease entities and most common pharmacological agents. Studies pertaining to the treatment of decompression sickness (DCS), oxygen toxicity and hyperbaric oxygen therapy were excluded from this review because of the different objectives and subject inclusion methods of these studies.
Table 1. Search PUBMED .
| ('Diving' [MeSH] OR 'dive' [tw] OR 'diving' [tw] OR 'diver' [tw] OR 'divers' [tw] OR 'scuba' [tw] OR 'hyperbaric' [tw] OR 'deep sea' [tw] OR 'aquanaut' [tw] OR 'aquanauts' [tw] OR 'frogman' [tw] OR 'frogmen' [tw]) AND 'Drug Therapy' [MeSH] OR 'drug' [tw] OR 'drugs' [tw] OR 'medication' [tw] OR 'medications' [tw] OR 'Pharmacological Actions Category' [MeSH] OR 'Pharmaceutical Preparations' [Mesh] OR 'pharmaceuticals' [tw] OR 'medicament' [tw] OR 'medicaments' [tw] OR 'pharmacological' [tw]) |
The reference lists from the included studies were searched to identify additional studies. Two authors (TCFvD, EH) independently identified the studies for inclusion and exclusion and extracted the data. Any inconsistencies between the authors were discussed until consensus was reached. The accuracy of the extracted data was further confirmed by the senior author (RAvH).
QUALITY ASSESSMENT
Studies were rated on the level of evidence provided according to criteria by the Centre for Evidence Based Medicine in Oxford. The methodological quality of observational comparative studies was assessed by the modified Newcastle-Ottawa Scale.[ 9 - 10]
Results
PRISMA FLOWCHART
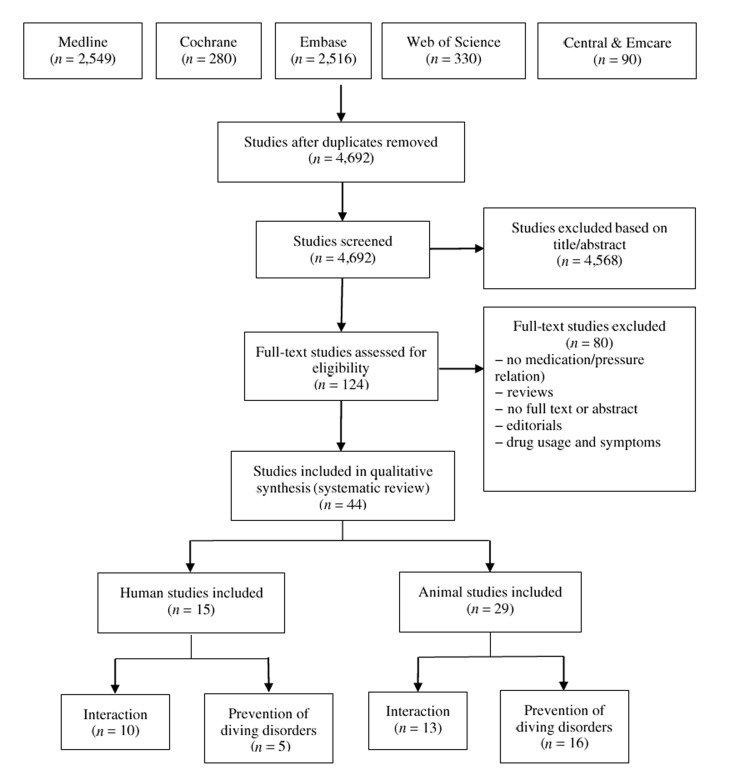
The PRISMA flowchart quantitatively illustrates the search through to the final studies included in this review (Figure 1). The 44 included studies were subdivided between human and animal studies and further subdivided into two topics, being (1) medication and hyperbaric interaction, and (2) prevention of diving disorders.
Figure 1.
Literature search flow chart on the effects of medications under hyperbaric conditions
MEDICATION AND HYPERBARIC INTERACTION
Table 2 shows the results of the human studies concerning interaction of drugs and hyperbaric pressure. Pharmacologic agents studied were aspirin, dipyridamole, scopolamine, clemastine, pseudoephedrine, dimenhydrinate, cyclizine, oral contraceptives, bleomycin and psychotropic drugs. Aspirin and dipyridamole accounted for some beneficial physiologic changes at a cellular level (preservation of platelet numbers) compared to a placebo following saturation dives.[ 11 - 12] No pressure-related interactions were found. No significant effects on diver performance from transdermal scopolamine were seen. Aggravation of mild symptoms of dry mouth and blurred vision compared to placebo was reported.[ 13 - 14] Dimenhydrinate adversely effected mental flexibility at depth.[ 15] Cyclizine had a potential small adverse effect on grammatical reasoning, which is increased at depth, but had no effect on a manual task.[ 16] These effects on various aspects of diver performance, especially at depth, could possibly contribute to cyclizine worsening the risks of diving.[ 17] For all other agents studied, no beneficial or dangerous direct pressure-related interactions were found.[ 18 - 21]
Table 2. Interaction of hyperbaric pressure and the use of pharmacologic agents in human studies; ATA − atmospheres absolute pressure; DCT − decompression time; LoE − level of evidence; MTC − mega thrombocyte; n − number; OW(HL) − open water (Hydrolab); PC − pressure chamber; RBC − red blood cell count; resp − respiratory; VK744 − analogue of dipyridamole .
| Reference | n | Drug | Control | Pressure (ATA) | Bottom time | Location | Blinded | Outcome | Results | LoE |
| 12 | 20 | Aspirin | Placebo | 2.4 | 60 h | OW(HL) | Yes | Platelet count | Reduced platelets in control group | Ib |
| 12 | 12 | VK744 (dipyridamole) | Placebo | 2.4 | 5 days (incl. DCT) | OW(HL) | Yes | Platelet count | Greater elevation of MTC in VK744; reduced platelets in control group | Ib |
| 11 | 24 | Aspirin; Dipyridamole | Placebo | 2.8 | 48 h | PC | Yes | Platelet count and function | Reduced platelets in dipyridamole and control groups | Ib |
| 13 | 10 | Scopolamine | Placebo | 2.8 | 5.5 days | PC | Yes | Cognitive | No significant effects | Ib |
| 14 | 24 | Scopolamine | Placebo | 4.8 | 11 min | PC | Yes | Cognitive | No significant effects | Ib |
| 21 | 102 | Clemastine fumarate | Placebo | 6.1 | 20 min | PC | Yes | Cognitive | No significant effects | Ib |
| 15 | 30 | Pseudoephedrine; Dimenhydrinate | Placebo | 3.0 | 30 min | PC | Yes | Cognitive | Pseudoephedrine nil effects; dimenhydrinate reduced performance | Ib |
| 17 | 24 | Pseudoephedrine; Cyclizine | Placebo | 4.0 | 5 min | PC | Yes | Cognitive | Pseudoephedrine nil effects; cyclizine reduced performance | Ib |
| 18 | 30 | Oral contraceptives | None | 3.5 | 25 min | OW | No | Bubble production | No differences | IV |
| 19 | 1 | Bleomycin | None | Multiple | Multiple | OW | No | Resp. distress or fatigue | No complications | V |
| 20 | 1,608 | Psychotropic drugs | None (survey) | Multiple | Unknown | OW | No | Narcosis (severe) | No significant effects | IIb |
Several animal studies showed significant beneficial effects of pharmacologic agents when animals were exposed to hyperbaric conditions (Table 3). Diazepam and valproate reduced the severity of the high-pressure nervous syndrome (HPNS) at depths beyond 150 metres’ sea water (msw) in rats and baboons.[ 22 , 23] Lithium provided some protection against nitrogen narcosis in rats.[ 24] Lastly, losartan prevented deleterious changes in cardiac function seen after repeated hyperbaric exposures in rats.[ 25]
Table 3. Interaction of hyperbaric pressure and the use of pharmacologic agents in animal studies; ATA − atmospheres absolute pressure; 1 ATA − control group had drug but remained at 1 ATA; * denotes an additional control group without drug but at test pressure; DCS − decompression sickness; EC − Escherichia coli; He − helium; HPNS − high-pressure nervous syndrome; LoE − level of evidence; MIC − minimum inhibitory concentration; MK-801 − dizocilpine; n − number; N2 − nitrogen; NA − not applicable; NR − not reported; PC − pressure chamber; PCP − phencyclidine; SA − Staphylococcus aureus; Salm − Salmonella sp; SKF − alazocine; U − unknown .
| Reference | n | Type | Drug | Control (ATA) | Pressure (ATA) | Bottom time (h) | Location | Blinded | Outcome | Results | LoE |
| 29 | 138 | Rats | Salicylate | 1* | 9 | 3.5 | PC | Yes | Anti-pyretic effect | No interaction | Ib |
| 28 | U | SA, EC | Antibiotics | 1 | Multiple | 16 | OW | N/A | Antibiotic efficacy | Increased MIC: penicillin for SA | IIb |
| 24 | 40 | Rats | Lithium | 1* | 19.2 | NR | PC | Yes | N2 narcosis HPNS | Reduced narcosis; potentiation of HPNS | Ib |
| 22 | 78 | Rats | Diazepam | 1 | 90 | 2.5 | PC | NR | Anesthetic and anticonvulsive effect; HPNS | Prevention of convulsion; reduction of HPNS | IIb |
| 30 | U | Dogs | Theophylline | 1 | 6 | U | PC | No | Pharmacokinetics | No changes | IIb |
| 31 | U | Dogs | Meperidine | 1 | 6 | U | PC | No | Pharmacokinetics | No changes | IIb |
| 33 | U | Dogs | Pentobarbital | 1 | 6 | U | PC | No | Pharmacokinetics | No changes | IIb |
| 32 | U | Dogs | Salicylate | 1 | 6 | U | PC | No | Pharmacokinetics | Increased clearance at 2.8 ATA and 100% O2 | IIb |
| 23 | 8 | Baboons | Sodium valproate | * only | 61 | 5 | PC | Yes | Effect on HPNS | Reduced severity | Ib |
| 34 | 25 | Rats | NMDA antagonists | 1* | till onset HPNS | several | PC | NR | Effect on HPNS | Little or no effect | Ib |
| 27 | U | SA, EC, Salm | Antibiotics | 1 | 36; 71 | 18 | PC | N/A | Antibiotic efficacy | Increased MIC: penicillin for SA; gentamycin and rifampicin for EC + Salm | IIb |
| 25 | 19 | Rats | Losartan | 1* | 5 | 30 min x 40 d | PC | NR | Cardiac function | Prevention of change | Ib |
| 26 | 67 | Mice | Sildenafil | * only | 10.2 | 45 min | PC | Yes | DCS | Increased incidence | Ib |
Conversely, some studies showed significant adverse effects. Sildenafil use showed an increased incidence of DCS combined with an exacerbated reduction in platelet numbers after hyperbaric exposure.[ 26] The antibiotics benzyl-penicillin, gentamycin and rifampicin were less effective at simulated depth.[ 27 , 28] Finally, lithium significantly potentiated the onset and severity of HPNS symptoms.[ 24] Studies of the use of salicylate, theophylline, meperidine, pentobarbital, and non-competitive N-methyl-D-aspartate receptor (NMDA) receptor antagonists did not show an interaction between the pharmacologic agents and the hyperbaric environment.[ 29 - 34]
PHARMACOLOGICAL AGENTS IN PREVENTION OF DIVING DISORDERS
In human studies investigating protective effects of medications on diving disorders, pseudoephedrine, the anti-oxidants vitamin C and E and statins were tested (Table 4). The use of pseudoephedrine resulted in fewer otologic barotrauma symptoms in comparison to placebo.[ 35] In one study vitamins C and E showed reversal of brachial artery endothelial dysfunction, potentially diminishing bubble formation.[ 36] Statins did not reduce post-dive bubble formation.[ 37] Antioxidants (vitamins C, E and tea catechins) reduced hepatic oxidative stress in saturation diving,[ 38] but the same vitamins did not improve eustachian tube function after oxygen dives.[ 39]
Table 4. Effects of pharmacologic agents in prevention of diving disorders in human studies; ATA − atmospheres absolute pressure; LoE − level of evidence; n − number; OW(HL) − open water (hydro lab); PC − pressure chamber; Vit − vitamin .
| Reference | n | Drug | Control | Pressure (ATA) | Bottom time | Location | Blinded | Outcome | Results | LoE |
| 35 | 116 | Pseudoephedrine | Placebo | 2.2 | Several dives | PC | Yes | Otologic symptoms | Lower TEED score, less discomfort and blockage | Ib |
| 39 | 15 | Vitamin C and E | Placebo | 1.4 | 30 min | OW | Yes | Eustachian tube function | No difference | Ib |
| 38 | 10 | Vitamin C and E tea catechins | None | 41 | 30 days | PC | No | Oxidative stress (liver function) | Less oxidative stress | IIb |
| 36 | 6 | Vitamin C and E | Placebo | 4.0 | 36 min | OW | Yes | Endothelial/cardiac function | Reversal of brachial artery endothelial dysfunction | Ib |
| 37 | 16 | Statins | Placebo | 2.8 | 80 min | PC | Yes | Bubble formation | No difference | Ib |
Table 5 shows the results of pharmacologic DCS prevention in animal studies. When used before hyperbaric exposure, clopidogrel,[ 40] cyproheptadine,[ 41] dimethothiazine,[ 42] aspirin and levodopa,[ 43] and escin[ 44] showed a significant reduction of mortality and incidence of DCS. Combined use of aspirin with levodopa had an added beneficial effect. A significantly decreased incidence of DCS and reduction of symptoms was seen with fluoxetine, abciximab, hydrogen enriched saline and simvastatin.[ 45 - 48] A beneficial effect on DCS symptoms was seen also with dibutyryl cAMP[ 49] and cyclohexanone (with decompression beyond normobaric pressure to an equivalent of 26,000 feet).[ 50] Pre-treatment with terbutaline, heparin, superoxide dismutase, catalase or amphetamine exhibited no relevant effects.[ 51 - 53] The anti-depressant spadin, a sortilin-derived peptide, was the only medication that showed a negative effect in a simulated diving study, with increased susceptibility to neurologic DCS symptoms.[ 54]
Table 5. Effects of pharmacologic agents on DCS prevention in animal studies; ATA − atmospheres absolute pressure; 1 ATA − control group had drug but remained at 1 ATA; * denotes an additional control group without drug but at test pressure; PC − pressure chamber; DCS − decompression sickness; fb – followed by; ft – feet; H2 − hydrogen; LoE − Level of Evidence; n − number; N/A − not applicable; NR − not reported; PC – pressure chamber; SOD − superoxide dismutase; RBC − red blood cell; SOD – superoxide dismutase; W/D ratio − wet/dry ratio .
| Reference | n | Type | Drug (group) | Control (ATA) | Pressure (ATA) | Bottom time | Location | Blinded | Results | LoE |
| 55 | 138 | Guinea pigs | Theophylline | * only | 7.4 | 60 min | PC | Yes | 50% mortality reduction (100% combined with 100% O2) | Ib |
| 50 | 110 | Mice | Cyclohexanone | 1 * | 6.3 fb 26,000 ft | 6 h | PC | NR | Protective effect | Ib |
| 42 | 200 | Mice | Dimetotiazine | * only | 6.3 | 6 h | PC | NR | Reduced mortality, manifestations and pathologic changes | Ib |
| 51 | 7 | Rabbits | Terbutaline | * only | 2 fb 39,000 ft | NR | PC | NR | Reduced incidence | IV |
| 41 | 500 | Mice | Cyproheptadine | * only | 6.3 | 6 h | PC | NR | Reduced mortality, manifestations and pathologic changes | Ib |
| 53 | 64 | Mice | Amphetamine + cyproheptadine | 1 * | 6.3 | 6 h | PC | NR | No additive effect of amphetamine combined with cyproheptadine | IIb |
| 43 | 202 | Rats | Levodopa + aspirin | * only | 7.0 | 30 min | PC | NR | Reduced incidence and mortality enhanced with combined therapy | Ib |
| 52 | 44 | Dogs | Heparin; SOD; catalase | 1 * | 10 | ≥ 10 min | PC | NR | No effect | Ib |
| 49 | 45 | Rats | Dibutyryl cAMP | 1 * | 6.3 / 7.0 | 120 / 60 min | PC | NR | Reduced inflammation and pulmonary oedema | Ib |
| 47 | 84 | Rats | H2-enriched saline | 1 * | 7.1 | 90 min | PC | NR | Reduced incidence | Ib |
| 45 | 91 | Mice | Fluoxetine | 1 * | 10.2 | 45 min | PC | Yes | Reduced incidence and better neurological recovery; reduced loss of platelets and RBCs | Ib |
| 40 | 111 | Rats | Clopidogrel | 1 * | 16.3 | 270 s | PC | NR | Reduced mortality and inflammatory lung injury | Ib |
| 46 | 80 | Mice | Antiplatelet drugs | 1 * | 9.2 | 45 min | PC | NR | Reduced incidence with abciximab. | Ib |
| 48 | NR | Rats | Simvastatin | * only | 7.1 | 100 min | PC | NR | Reduced incidence and inflammatory lung injury | Ib |
| 54 | 280 | Mice | Fluoxetine; spadin | 1 * | 9.0 | 45 min | PC | NR | Fluoxetine protective; spadin increased susceptibility | Ib |
| 44 | 90 | Rats | Escin | * only | 7 | 90 min | PC | Yes | Reduced incidence and mortality | Ib |
Discussion
GENERAL
Animal studies revealed that slight benefits might be expected from some psychopharmaceutic agents against the onset of HPNS symptoms.[ 22 - 24] Also, several cardiovascular drugs could add a potential protective effect against DCS.[ 40 , 43 , 46 , 48] However, these pharmacologic agents were tested on small mammals. Studies using dogs were specifically aimed at pharmacokinetics of drugs under hyperbaric conditions.[ 30 - 33] Apart from an increased clearance of salicylate at depth, these studies did not reveal significant pharmacokinetic changes due to hyperbaric conditions. In contrast to the protective effect of theophylline found in guinea pigs,[ 55] a study in dogs showed that the use of a bronchodilator (aminophylline) before venous infusion of microbubbles resulted in an increased passage of these microbubbles across the pulmonary microvasculature.[ 56]
Studies of effects of pharmacological agents in hyperbaric conditions in humans are scarce. This is not surprising given the ethical considerations. Available studies showed few major effects of pharmacological agents in hyperbaric conditions. Most evidence was gained through animal experiments with often extreme diving profiles (to provoke bubble formation). Extrapolation of animal studies to humans should be done with caution.[ 57]
Human studies are needed to examine whether the medications or vitamins used in the animal studies will have beneficial or harmful effects for healthy divers in wet circumstances. Besides the pharmacological effect, diving with medication affecting cardiovascular responses should be undertaken cautiously, lest there be unintended effects on life-threatening conditions such as immersion pulmonary oedema.[ 58 - 60] Immersion alters the balance between the sympathetic and parasympathetic nervous systems, thus affecting the responses to many physiological processes.[ 61 , 62] However, most research was conducted in recompression chambers, where subjects are exposed to hyperbaric pressure without immersion in water. These so called ‘dry-dives’ simulate hyperbaric conditions and accompanying hyperoxia or increased uptake of inert gases, but do not simulate immersion, hypothermia or the increased workload of fin swimming. Results from dry-dives should be interpreted cautiously when assessing the effects of pharmacologic interventions in scuba diving.
FITNESS TO DIVE
The primary aim of this systematic review was the assessment of available evidence on the effects of hyperbaric circumstances on use of medication, thereby enhancing heuristics supported by evidence-based medicine in the assessment of fitness to dive. Based on the outcomes of this study, being physically fit to dive remains the cornerstone of medical diving clearance. This judgment is often based on assumptions and risk analysis, when the risk is not easily assessed. In the medical examination of a diver, an understanding of the effects of medication, the disease being treated and the impact that the hyperbaric environment might have on both is essential. Any serious symptoms of illness or side effects of medication under normobaric circumstances are potentially a valid reason for rejection.
ENVIRONMENTAL AND HYPERBARIC CONDITIONS
Environmental conditions during a dive vary enormously, from relaxed scuba diving in calm, warm, shallow tropical waters, to professional divers at extreme depth for several weeks (i.e., saturation diving). Conditions can deteriorate rapidly, caused by change in the environment (waves, currents), technical apparatus malfunctions or problems with a diving buddy. In all these situations it becomes a challenge to adapt oneself physically and mentally to the new circumstances.
Apart from diving medical clearance, an individual diver should answer the following key questions before every dive:
Is my physical condition sufficient to cope with the required strain?
Am I mentally prepared to cope with the demanding situation?
Do I (and my buddy) possess the skills for this activity?
Thus, a tailored assessment that encompasses more than medical conditions and medications is required. A diving medical clearance which imposes depth limitations is unjustified as circumstances can change rapidly.
LIMITATIONS
To our knowledge, this is the first systematic review focusing on the effects of the hyperbaric environment on medication, and the effects of medication on diving disorders. However, any conclusions are substantially limited by the poor quality of the available evidence. According to the Quality Assessment there is a risk of bias in most of the included animal studies. Also, protocols vary between studies, making it harder to compare them in a meta-analysis. Different hyperbaric pressures or lengths of exposure might influence the outcome. Furthermore, most studies used dry dives, whilst immersion is essential for a proper judgement of the effects of medication on safety during diving.
Conclusion
This systematic review revealed no evidence of significant risks due to changes in pharmacologic mechanisms in the hyperbaric environment. However, it is unlikely that hyperbaric conditions diminish any risks of medication encountered in non-hyperbaric conditions. Regarding prevention or treatment of DCS, pharmacologic agents targeted at cardiovascular diseases like aspirin, losartan, clopidogrel or simvastatin could add a potential protective effect although evidence is limited. The anti-depressant fluoxetine may also warrant further investigation. For decision making in prescribing medicine for recreational and occupational divers and to enhance safety by increasing our understanding of pharmacy and diving, future research should focus on human studies in submersed circumstances.
Footnotes
Funding: nil
Conflict of interest
Rob van Hulst is a member of the DHM Editorial Board but played no part in the peer review for this paper.
Contributor Information
Erik Hoencamp, Institute of Psychology, Leiden University, Leiden, The Netherlands.
Thijs TCF van Dongen, Defense Healthcare Organization, Ministry of Defense, Utrecht, The Netherlands; Department of Surgery, Alrijne Hospital, Leiderdorp, The Netherlands.
Pieter-Jan AM van Ooij, Diving Medical Center, Royal Netherlands Navy, Den Helder, The Netherlands.
Thijs T Wingelaar, Diving Medical Center, Royal Netherlands Navy, Den Helder, The Netherlands.
Mees L Vervelde, Central Military Hospital, Ministry of Defense, Utrecht.
Dave AA Koch, Diving Medical Center, Royal Netherlands Navy, Den Helder, The Netherlands.
Rob A van Hulst, Diving Medical Center, Royal Netherlands Navy, Den Helder, The Netherlands; Department of Anesthesiology, Academic Medical Center, Amsterdam, The Netherlands.
Rigo Hoencamp, Defense Healthcare Organization, Ministry of Defense, Utrecht, The Netherlands; Department of Surgery, Alrijne Hospital, Leiderdorp, The Netherlands; Division of Surgery, Leiden University Medical Center, Leiden.
References
- Germonpré P. The medical risks of underwater diving and their control . International Sportmed Journal. 2006;7(1):1–15. [Google Scholar]
- Bosco G, Rizzato A, Moon RE, Camporesi EM. Environmental physiology and diving medicine . Front Psychol. 2018;9:72. doi: 10.3389/fpsyg.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling JED, Elliott DH, Nome T. Fitness to dive standards: guidelines for medical assessment of working divers . 2003. [cited 2018 March 1]. Available from: http://www.edtc.org/EDTC-Fitnesstodivestandard-2003.pdf .
- Vann RD, Butler FK, Mitchell SJ, Moon RE. Decompression illness . Lancet. 2011;377(9760):153–64. doi: 10.1016/S0140-6736(10)61085-9. [DOI] [PubMed] [Google Scholar]
- British Thoracic Society, Fitness to Dive Group, Subgroup of the British Thoracic Society Standards of Care Committee . Memory and metacognition in dangerous situations: investigating cognitive impairment from gas narcosis in undersea divers. Thorax. 2003; 58: 3- 13. 10.1136/thorax.58.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challenor J. Medical Assessment of Working Divers. Fitness to Dive Standards of European Diving Technology Committee. In Wendling J, Elliott D, Nome D, editors. . European Diving Technology Committee; 2004. p. 216; Occupational Medicine 2005; 55: 581. doi: 10.1093/occmed/kqi154. [DOI] [Google Scholar]
- Health and Safety Executive . The medical examination and assessment of commercial divers . London: Health and Safety Executive; 2015. [cited 2018 March 17]. Available from: http://www.hse.gov.uk/pubns/ma1.htm . [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre for Evidence-Based Medicine . Oxford Centre for Evidence-based Medicine – Levels of evidence ; 2009. [cited 2018 March 17] Available from: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. [Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. [cited 2018 March 17] Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Philp RB, Bennett PB, Andersen JC, Fields GN, McIntyre BA, Francey I, et al. Effects of aspirin and dipyridamole on platelet function, hematology, and blood chemistry of saturation divers . Undersea Biomed Res. 1979;6:127–46. [PubMed] [Google Scholar]
- Philp RB, Freeman D, Francey I, Bishop B. Hematology and blood chemistry in saturation diving: I. Hematology and blood chemistry in saturation diving: I. Antiplatelet drugs, aspirin, and VK744 . Undersea Biomed Res. 1975;2:233–49. [PubMed] [Google Scholar]
- Schwartz HJC, Curley MD. Transdermal scopolamine in the hyperbaric environment. Navy Experimental Diving Unit Report, 1986 (No. 2-86). cited 2018 March 19 Available from: http://archive.rubicon-foundation.org/xmlui/handle/123456789/3528.
- Williams TH, Wilkinson AR, Davis FM, Frampton CM. Effects of transcutaneous scopolamine and depth on diver performance . Undersea Biomed Res. 1988;15:89–98. [PubMed] [Google Scholar]
- Taylor D, O’Toole K, Auble T, Ryan C, Sherman D. The psychometric and cardiac effects of pseudoephedrine and antihistamines in the hyperbaric environment. SPUMS Journal. 2001; 31: 50- 7. Available from: http://archive.rubicon-foundation.org/xmlui/handle/123456789/7711. [cited 2018 March 17]. [Google Scholar]
- McGeoch G, Davis FM, Fletcher L. The effects on performance of cyclizine and pseudoephedrine during dry chamber dives breathing air to 30 metres’ depth . SPUMS Journal. 2005; 35: 178- 82. [cited 2018 March 19] Available from: http://archive.rubicon-foundation.org/xmlui/handle/123456789/9791. [Google Scholar]
- Clarke JE. Moving in extreme environments: inert gas narcosis and underwater activities . Extrem Physiol Med. 2015;4:1. doi: 10.1186/s13728-014-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussuges A, Retali G, Bodere-Melin M, Gardette B, Carturan D. Gender differences in circulating bubble production after SCUBA diving . Clin Physiol Funct Imag. 2009;29:400–5. doi: 10.1111/j.1475-097X.2009.00884.x. [DOI] [PubMed] [Google Scholar]
- Gray RN Jr. Scuba diving post-bleomycin therapy: a case report . Undersea Hyperb Med. 2010;37:455–7. [PubMed] [Google Scholar]
- Krummel T, Thiery A, Villain M, Schittly B, Brouant B. Psychotropic drug use in recreational scuba divers and its effect on severe narcosis . Int J Sports Med. 2017;38:322–8. doi: 10.1055/s-0042-122336. [DOI] [PubMed] [Google Scholar]
- Sipinen SA, Kulvik M, Leinio M, Viljanen A, Lindholm H. Neuropsychologic and cardiovascular effects of clemastine fumarate under pressure . Undersea Hyperb Med. 1995;22:401–6. [PubMed] [Google Scholar]
- Gran L, Coggin R, Bennett PB. Diazepam under hyperbaric conditions in rats . Acta Anaesthesiol Scand. 1980;24:407–11. doi: 10.1111/j.1399-6576.1980.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Pearce PC, Clarke D, Doré CJ, Halsey MJ, Luff NP, Maclean CJ. Sodium valproate interactions with the HPNS: EEG and behavioral observations . Undersea Biomed Res. 1989;16:99–113. [PubMed] [Google Scholar]
- Bennett PB, Leventhal BL, Coggin R, Roby J, Racanska L. Lithium effects: protection against nitrogen narcosis, potentiation of HPNS . Undersea Biomed Res. 1980;7:11–6. [PubMed] [Google Scholar]
- Stuhr LE, Maehle BO. The effect of Losartan, an angiotensin II antagonist, on cardiac function, mass and morphology in rats after repeated hyperbaric exposures . Scand J Clin Lab Invest. 1997;57:253–61. doi: 10.3109/00365519709060034. [DOI] [PubMed] [Google Scholar]
- Blatteau JE, Brubakk AO, Gempp E, Castagna O, Risso JJ, Vallée N. ROAD project: Robotics for assisted diving, 22nd Mediterranean Conference on Control and Automation. PLoS One. 2013;8:e60639. doi: 10.1371/journal.pone.0060639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind J, Attwell RW. The effect of antibiotics on bacteria under hyperbaric conditions . J Antimicrob Chemother. 1996;37:253–63. doi: 10.1093/jac/37.2.253. [DOI] [PubMed] [Google Scholar]
- Wild JR. Induction of staphylococcal beta-lactamase in response to low concentrations of methicillin under simulated diving environments . Can J Microbiol. 1977; 23: 116- 21. WOS:A1977CV60000016. Available from: https://www.nrcresearchpress.com/doi/pdf/10.1139/m77-01 [cited 2018 March 17]. [Google Scholar]
- Hart JL. The antipyretic effects of hyperbaric air and salicylate on rats . Undersea Biomed Res. 1974;1:83–9. [PubMed] [Google Scholar]
- Kramer WG, Gross DR, Fife WP, Chaikin BN. Theophylline pharmacokinetics during hyperbaria and hyperbaric hyperoxia in the dog . Res Commun Chem Pathol Pharmacol. 1981;34:381–8. [PubMed] [Google Scholar]
- Kramer WG, Gross DR, Moreau PM, Fife WP. Drug disposition under hyperbaric and hyperbaric hyperoxic conditions: meperidine in the dog . Aviat Space Environ Med. 1983;54:410–2. [PubMed] [Google Scholar]
- Kramer WG, Welch DW, Fife WP, Chaikin BN, Gross DR. Salicylate pharmacokinetics in the dog at 6 ATA in air and at 2.8 ATA in 100% oxygen . Aviat Space Environ Med. 1983;54:682–4. [PubMed] [Google Scholar]
- Kramer WG, Welch DW, Fife WP, Chaikin BN, Medlock C, Gross DR, et al. Pharmacokinetics of pentobarbital under hyperbaric and hyperbaric hyperoxic conditions in the dog . Aviat Space Environ Med. 1983;54:1005–8. [PubMed] [Google Scholar]
- Wardley-Smith B, Wann KT. The effects of non-competitive NMDA receptor antagonists on rats exposed to hyperbaric pressure . Eur J Pharmacol. 1989;165:107–12. doi: 10.1016/0014-2999(89)90775-9. [DOI] [PubMed] [Google Scholar]
- Brown M, Jones J, Krohmer J. Pseudoephedrine for the prevention of barotitis media: a controlled clinical trial in underwater divers . Ann Emerg Med. 1992;21:849–52. doi: 10.1016/s0196-0644(05)81033-9. [DOI] [PubMed] [Google Scholar]
- Obad A, Palada I, Valic Z, Ivancev V, Baković D, Wisløff U, et al. The effects of acute oral antioxidants on diving-induced alterations in human cardiovascular function . J Physiol. 2007;578(Pt 3):859–70. doi: 10.1113/jphysiol.2006.122218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplessis CA, Fothergill D, Schwaller D, Hughes L, Gertner J. Prophylactic statins as a possible method to decrease bubble formation in diving . Aviat Space Environ Med. 2007;78:430–4. [PubMed] [Google Scholar]
- Ikeda M, Nakabayashi K, Shinkai M, Hara Y, Kizaki T, Oh-ishi S, et al. Supplementation of antioxidants prevents oxidative stress during a deep saturation dive . Tohoku J Exp Med. 2004;203:353–7. doi: 10.1620/tjem.203.353. [DOI] [PubMed] [Google Scholar]
- Mutzbauer TS, Neubauer B, Sigg O, Tetzlaff K. Can eustachian tube ventilatory function impairment after oxygen diving be influenced by application of free radical scavenger vitamins C and E? . Laryngoscope. 2001;111:861–6. doi: 10.1097/00005537-200105000-00020. [DOI] [PubMed] [Google Scholar]
- Bao XC, Chen H, Fang YQ, Yuan HR, You P, Ma J, et al. Clopidogrel reduces the inflammatory response of lung in a rat model of decompression sickness . Respir Physiol Neurobiol. 2015;211:9–16. doi: 10.1016/j.resp.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Chryssanthou C, Rubin L, Graber B. Amelioration of decompression sickness in mice by pretreatment with cyproheptadine . Undersea Biomed Res. 1980;7:321–9. [PubMed] [Google Scholar]
- Chryssanthou C, Teichner F, Koutsoyiannis M. Studies on dysbarism V. Prevention of decompression sickness in mice by dimethothiazine . Aerosp Med. 1974;45:279–82. [PubMed] [Google Scholar]
- Popovic P, Popovic V, Honeycutt C. Levodopa and aspirin pretreatment beneficial in experimental decompression sickness . Proc Soc Exp Biol Med. 1982;169:140–3. doi: 10.3181/00379727-169-41322. [DOI] [PubMed] [Google Scholar]
- Zhang K, Jiang Z, Ning X, Yu X, Xu J, Buzzacott P, et al. Endothelia-targeting protection by escin in decompression sickness rats . Sci Rep. 2017;7:41288. doi: 10.1038/srep41288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatteau JE, Barre S, Pascual A, Castagna O, Abraini JH, Risso JJ, et al. Protective effects of fluoxetine on decompression sickness in mice . PLoS One. 2012;7(11):e49069. doi: 10.1371/journal.pone.0049069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts K, Pontier JM, Mazur A, Theron M, Buzzacott P, Wang Q, et al. Mechanism of action of antiplatelet drugs on decompression sickness in rats: a protective effect of anti-GPIIbIIIa therapy . J Appl Physiol (1985). 2015;118:1234–9. doi: 10.1152/japplphysiol.00125.2015. [DOI] [PubMed] [Google Scholar]
- Ni XX, Cai ZY, Fan DF, Liu Y, Zhang RJ, Liu SL, et al. Protective effect of hydrogen-rich saline on decompression sickness in rats . Aviat Space Environ Med. 2011;82:604–9. doi: 10.3357/asem.2964.2011. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wang D, Xu J, Li R, Cai Z, Liu K, et al. Simvastatin decreases incidence of decompression sickness in rats . Undersea Hyperb Med. 2015;42:115–23. [PubMed] [Google Scholar]
- Little TM, Butler BD. Dibutyryl cAMP effects on thromboxane and leukotriene production in decompression-induced lung injury . Undersea Hyperb Med. 1997;24:185–91. [PubMed] [Google Scholar]
- Chryssanthou C, Teichner F, Antopol W. Studies on dysbarism IV. Production and prevention of decompression sickness in “non-susceptible” animals . Aerosp Med. 1971;42:864–7. [PubMed] [Google Scholar]
- Balldin U, Linér M, Antopol W. Preventive effect of a vasodilator on the occurrence of decompression sickness in rabbits . Aviat Space Environ Med. 1978;49:759–62. [PubMed] [Google Scholar]
- Catron PW, Thomas LB, McDermott JJ, Holt MA, Harabin AL, Flynn ET. Failure of heparin, superoxide dismutase, and catalase to protect against decompression sickness . Undersea Biomed Res. 1987;14:319–30. [PubMed] [Google Scholar]
- Chryssanthou C, Rodrigues L, Branden P. Amelioration of decompression sickness by combined cyproheptadine-amphetamine treatment . In: Bachrach AJ, Matzen MM. . Underwater physiology VII: Proceedings of the seventh symposium on underwater physiology. Kensington (MD): Undersea and Hyperbaric Medical Society; 1981. p. 753- 63. Available from: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.852.3366&rep=rep1&type=pdf [cited 2018 March 19]. [Google Scholar]
- Vallee N, Lambrechts K, de Maistre S, Royal P, Mazella J, Borsotto M, et al. Fluoxetine protection in decompression sickness in mice is enhanced by blocking TREK-1 potassium channel with the “spadin” antidepressant . Front Physiol. 2016;7:42. doi: 10.3389/fphys.2016.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SD, Spencer MP. Pharmacologic agents in the prevention of decompression sickness . J Occup Med. 1969;11:252–6. [PubMed] [Google Scholar]
- Butler BD, Hills BA. The lung as a filter for microbubbles . J Appl Physiol Respir Environ Exerc Physiol. 1979;47:537–43. doi: 10.1152/jappl.1979.47.3.537. [DOI] [PubMed] [Google Scholar]
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke . Ann Neurol. 2006;59:467–77. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Ng A, Edmonds C. Immersion pulmonary oedema and Takotsubo cardiomyopathy . Diving Hyperb Med. 2015;45:255–7. [PubMed] [Google Scholar]
- Peacher DF, Martina SD, Otteni CE, Wester TE, Potter JF, Moon RE. Immersion pulmonary edema and comorbidities: case series and updated review . Med Sci Sports Exerc. 2015;47:1128–34. doi: 10.1249/MSS.0000000000000524. [DOI] [PubMed] [Google Scholar]
- Smart DR, Sage M, Davis FM. Two fatal cases of immersion pulmonary oedema - using dive accident investigation to assist the forensic pathologist . Diving Hyperb Med. 2014;44:97–100. [PubMed] [Google Scholar]
- Schipke JD, Pelzer M. Effect of immersion, submersion, and scuba diving on heart rate variability . Br J Sports Med. 2001;35:174–80. doi: 10.1136/bjsm.35.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shykoff BE. Pulmonary effects of six-hour dives:in-water or dry chamber exposure to an oxygen-partial pressure of 1.6 atm. Technical Report NEDU 05-19 . Panama City (FL): Navy Experimental Diving Unit; 2005. [cited 2018 March 19]. Available from: https://apps.dtic.mil/docs/citations/ADA443182 . [Google Scholar]