Abstract
We examined the influence of dysfunctional, non-lesional white matter on cognitive performance in multiple sclerosis (MS). Forty-six MS subjects were assessed using MRI-based myelin water imaging (MWI), and average myelin water fraction (MWF) values across 20 white matter regions of interest (ROIs) were determined. A data-fusion method, multiset canonical correlation analysis (MCCA), was used to investigate the multivariate, deterministic joint relations between MWF, executive function, and demographic and clinical characteristics. MCCA revealed one significant component (p = 0.009) which consisted of three linked profiles, with a pairwise correlation between the MWF and cognitive profiles of r = 0.37, a correlation between MWF and demographics profiles of r = 0.31, and between cognitive and demographics profiles r = 0.64. White matter ROIs representing long-range intra-hemispheric tracts and ROIs connecting the two hemispheres were positively related through their individual profiles to overall cognitive performance, education and female gender, while age, EDSS, and disease duration were related negatively. Surprisingly, lesions within the ROIs had a negligible effect on overall relations between imaging, cognitive, and demographic variables. These findings indicate that there is a strong association between a pattern of MWF values and cognitive performance in MS, which is modulated by age, education, and disease severity. Moreover, this consistent relation involves multiple white matter regions and is separate from the influence of lesions.
Keywords: Myelin water fraction, Cognitive performance, Multiset canonical correlation analysis, Multiple sclerosis, Brain
Highlights
-
•
White matter myelination, cognitive performance, and demographic and clinical variables are all consistently linked in MS.
-
•
Multivariate approach links imaging, cognitive, and clinical features in joint analysis.
-
•
Education promotes cognitive performance and myelin content.
-
•
Age and disease severity are negatively associated with myelination and cognitive performance.
-
•
Demyelination in lesions had minimal impact on overall relation between imaging, cognitive, and demographic characteristic.
1. Introduction
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system with a wide spectrum of motor and non-motor impairments such as fatigue, vision impairments, balance issues, and cognitive impairments that greatly affect quality of life. A hallmark of MS is the deterioration of the white matter (WM) microstructural integrity, due to inflammation, edema, axonal loss, and demyelination. Lesions, or focal plaques, typically seen as hyperintensities on proton density or T2 weighted magnetic resonance images, are often characterised by a high degree of demyelination. In addition to focal loss of myelin in lesions, a general decrease in overall myelin can be observed in non-lesional tissue, both in diffusely abnormal white matter (DAWM) and normal-appearing white matter (NAWM) (MacKay and Laule, 2016).
MS disease manifestations are highly variable in both symptom presentation and radiological MRI markers (Chard and Trip, 2017). Lesions appearing similar to each other on conventional MRI may not correspond to consistent patterns of clinical symptoms. Besides lesion location, the extent of lesioned tissue also provides limited information about behavioural consequences. This mismatch between radiological markers and disease manifestation has been termed the ‘clinical-radiological paradox’ and has hampered the ability to robustly infer disease status and course based on MRI lesion characteristics alone (Chard and Trip, 2017).
Despite much research, there is still limited understanding of the relations between cognitive impairments and WM lesions in MS, likely because higher cognitive functioning requires a distributed network of multiple brain regions acting in concert (McIntosh, 2000; McIntosh and Korostil, 2008), as opposed to independent activity in discrete loci. A crucial aspect of effective communication between distinct brain regions is the myelination of axons in the WM, as speed and coherence of signal transmission is a critical factor in complex motor and cognitive function. In previous in vivo research inferring relations between MRI white matter integrity measures and cognition, most relied on measures only partially associated with myelin content such as the diffusion tensor imaging (DTI) measures of fractional anisotropy (FA) and radial diffusivity (Kerchner et al., 2012; Rizio and Diaz, 2016; Roberts et al., 2013). Other studies have also utilized the magnetic transfer ratio (MTR) as a measure of WM integrity in disease (Faiss et al., 2014) and healthy aging (Seiler et al., 2014) While there is some degree of correspondence between these measures and myelination of underlying tissue, they do not directly quantify myelin content nor are they specific to it (Mädler et al., 2008; Vavasour et al., 2011). External factors such as direct axonal damage, or underlying architecture of WM fibre bundles can affect DTI measures (Bouhrara et al., 2018). Similarly, a change in myelination may not be quantitatively reflected in these measures, rendering them non-specific to myelin. In contrast, the myelin water fraction (MWF) gained from in vivo T2 relaxation studies has a very good correspondence to stained myelin content examined histopathologically, the gold standard of assessing myelination (Laule et al., 2006, Laule et al., 2008). While MWF has been extensively utilized in MS research, a direct link to cognitive performance is still lacking.
Previous studies exploring the association between WM microstructure and cognitive performance have also largely attempted to relate performance on a particular cognitive test to a measure in a specific WM region; however, it is far more likely that several brain regions jointly engage in a given task. Moreover, since it is difficult to design cognitive tests that selectively probe one particular aspect of cognition in isolation, changes in myelin markers will probably have widespread downstream effects across multiple cognitive tests and domains. Thus, methods that accommodate multivariate clinical and imaging data, to assess the joint relations between two or more sets of variables may be more advantageous.
In this study we have tried to address the aforementioned limitations by 1) using a myelin specific measure of WM integrity 2) using a data-driven multivariate approach suitable for fusion of cognitive performance, demographic and clinical data in a cohort of MS subjects. We use the multivariate, data-driven method of multiset canonical correlation analysis (MCCA) to examine the associations between overall myelin content and cognitive profiles, and clinical variables such as age, gender, years of education, and disease severity. We hypothesized that a significant association would exist between overall cognitive performance across multiple tests, overall MWF values, and demographic variables.
2. Materials & methods
This study received ethical approval from the University of British Columbia Clinical Research Ethics Board, and all subjects provided written, informed consent. We enrolled a total of 46 subjects (35F/11 M) diagnosed with relapsing-remitting multiple sclerosis (RRMS) based on the McDonald 2005 criteria (Polman et al., 2011), with an average (± standard deviation) age of 42.9 ± 10.9 years (Table 1). All imaging data were acquired on a Philips (Netherlands) Achieva 3 T MRI scanner with an 8 channel head coil. We acquired a full brain 3DT1-weighted scan for structural references with an inversion recovery MPRAGE sequence TI = 808 ms, TR = 1800 ms and an isotropic voxel size of 1mm3. T2 relaxation data were collected using a modified GRASE sequence with 32 echoes with 10 ms echo spacing and TR = 1000 ms. Twenty slices were acquired at 5 mm slice thickness and reconstructed to 40 slices at 2.5 mm. The in-plane voxel size was 1x1mm. A dual echo PDw/T2w scan with TE1 = 8.4 ms, TE2 = 80 ms, TR = 2800 ms and voxel size of 0.97 × 0.97x5mm3 was used for lesion identification.
Table 1.
Demographics, clinical, and cognitive measures. Displayed are averages and standard deviations. For clinical measures (EDSS and disease duration) the median and their respective ranges are shown.
| Demographics & clinical measures | Mean ± SD |
|---|---|
| Age (years) | 42.8 ± 10.8 |
| Gender | 36 F, 10 M |
| Education (years) | 14.8 ± 2.4 |
| Median, [range] | |
| EDSS | 2, [0, 6] |
| Disease duration (years) | 10, [0.3, 36] |
| Neuropsychological scores | Mean ± SD |
|---|---|
| Working Memory Index (WMI) | 93.73 ± 12.42 |
| Processing Speed Index (PSI) | 101.88 ± 16.01 |
| Verbal Letter Fluency Test (FAS) | 41.03 ± 10.74 |
| Trail Making Test A (TMT A) | 30.73 ± 12.25 |
| Trail Making Test B (TMT B) | 74.18 ± 61.03 |
The multi-echo GRASE sequence was analyzed using in-house MATLAB code which uses an NNLS fitting methods to approximate the multi-exponential decay curve with a number of basis functions, resulting in one whole cerebrum MWF map per subject. The algorithm includes correction for stimulated echoes as well as a regulariser to make the fit more robust against noise in the time domain (Prasloski et al., 2012). The calculation of MWF was performed as described in (Prasloski et al., 2012).
WM lesions were delineated semi-automatically utilizing the PDw/T2w images. A radiologist with extensive experience in MS lesion identification digitally marked all lesions with seed points using a custom-built software interface. T2 lesions were then segmented using a previously validated method (McAusland et al., 2010) that automatically computes the extent of each marked lesion using a customized Parzen window classifier to estimate the intensity distribution of the lesions.
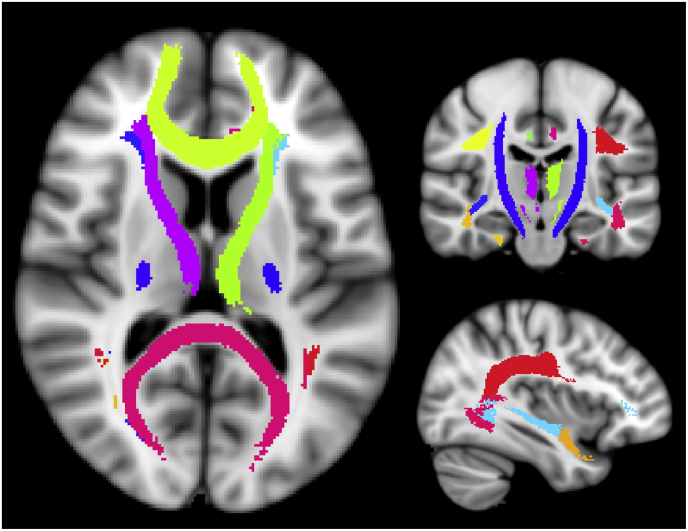
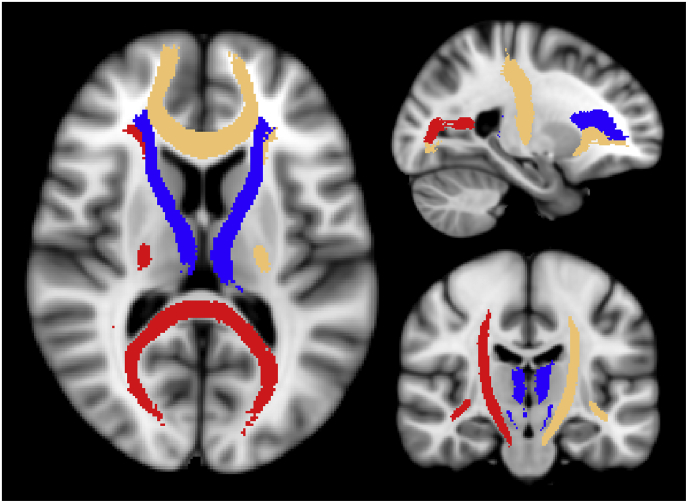
We used 20 WM ROIs, part of the FSL (FMRIB, Oxford) package, which cover the majority of the WM and delineate major WM tracts (Fig. 1). A full list of ROI names can be found in supplementary table T1. In order to extract the average MWF per ROI, the ROIs were non-linearly registered to each subjects' native space using the registration parameters obtained from registering the MNI template to the first echo of the multi-echo T2 data, and the FNIRT program of FSL (Jenkinson et al., 2012). All registrations were visually checked for accuracy and if necessary, registrations were re-performed with adjusted parameters to ensure an appropriate alignment between images. Once the ROIs were registered, a white matter mask (obtained from the 3DT1 image with FAST (Jenkinson et al., 2012) and registered to the multi-echo data with FLIRT (Jenkinson et al., 2012)), was applied to ensure only WM voxels were being considered for analysis. The MWF averages of all WM ROIs comprised the imaging set Xnxk with n = 46 subjects and k = 20 WM ROIs (features).
Fig. 1.
Visualisation of the WM ROIs used in this study. The ROIs were taken from the John's Hopkins University atlas provided in FSL. A complete list of ROI names is listed in supplementary Table T1.
All subjects were assessed with a cognitive battery evaluating performance in processing speed, working memory, executive function and attention domain. We administered the subtests of the Wechsler Adult Intelligence Scale-IV (WAIS IV) (Wechsler, 1939) that included digit span, arithmetic, letter number sequencing, symbol search, and coding. Composite index scores from the WAIS-IV were obtained for use in the analysis including Working Memory Index (WMI), which utilized scores from digit span, arithmetic, and letter number sequencing subtests. The WAIS-IV Processing Speed Index (PSI) was based on the symbol search and coding subtests. In addition to WAIS IV, we further assessed Verbal Letter Fluency Test (FAS) (Lezak, 2012), and Trail-Making Test, (TMT A and B) (Arnett and Labovitz, 1995) to evaluate executive function and attention. Detailed explanations of the evaluated abilities of each test can be found in our previous study (Lin et al., 2017). In the end, WMI, PSI, FAS, and the TMT A/B raw scores formed the cognitive set Ynxl with n = 46 subjects and l = 5 cognitive features.
The subjects age, gender, years of education, Kurtzke Expanded Disability Status Scale (EDSS), and disease duration (DD) in years were collated to form the demographic set Znxo with n = 46 subjects and o = 5 demographic and disease severity features.
2.1. Statistical analysis
2.1.1. Multivariate correlation analysis
One method to relate two sets of multivariate data is canonical correlation analysis (CCA) (Hotelling, 1936), with the goal of finding linear combinations of the original variables that are maximally correlated. In other words, CCA finds linear transformations (canonical vectors) for each set, such that the correlation between the projections of the original data (canonical variates) onto these canonical vectors are maximised. In the case of more than two groups of datasets, an extension of CCA, multiset CCA (MCCA) (Kettenring, 1971) can be employed. MCCA identifies a correlation structure among canonical variates of multiple datasets by a series of linear transformations so that they are maximally correlated. The projections are called canonical variates (Pi), with i = X, Y, Z for each respective set, and can be viewed as condensed representations, or profiles of each set, while sharing commonalities across sets. Further profiles/canonical variates can be extracted with new sets of canonical vectors such that they have maximum correlation among them but are uncorrelated to the prior canonical variates. Canonical loadings are often used to assess the contribution of the original variables to the canonical variates in a set, and are defined as the correlation between each variable and the canonical variate.
2.1.2. Methodological considerations
All three sets, X (MWF), Y (cognitive scores), and Z (demographics) served as the input to the MCCA model. To avoid overfitting, we utilized principal component analysis (PCA) to reduce the dimensions of each data set to a common dimensionality of five components prior to the MCCA step. The significance of MCCA components was assessed with a nonparametric permutation test in which the order of subjects was permuted and MCCA was performed again. This procedure was done 1000 times to generate a null distribution of pairwise correlation values and the original correlations were assessed against this distribution to define significance. To estimate the robustness of the loadings, we performed a leave-one-out cross validation.
2.1.3. Effect of lesions
In order to investigate the effects of lesion tissue in this methodology, we computed two measures of ‘lesion contribution’ per ROI. The first measure, ‘lesion percentage’ is the ratio of lesional voxels to total number of voxels for a given ROI. The second measure, ‘subject lesions’ is the count of subjects that had at least one lesion in a particular ROI. We performed multiple linear regression with the two lesion contribution measures as predictors and MCCA loadings on ROIs as outcome variables. As a second test, MCCA was performed twice. Once as described above, and repeated, but specifically excluding voxels that were contained within the lesions, by subtracting the lesion mask from the ROI mask prior to calculating the average MWF per ROI. We then compared the results from the MCCA when lesion tissue was included and when it was excluded.
2.1.4. Post-hoc tests
In order to determine the biological significance of the significant canonical variate, we performed different post-hoc analyses. First, we employed k-means clustering on the weighted values from the X, Y, Z (i.e. MWF, cognition and demographic) data sets constituting the significant canonical variate. As per design, these combinations were ones that resulted in the largest correlation between data sets. In the clustering we were looking for a differentiation between mildly and moderately affected subjects, thus we limited the number of clusters to two. We used the non-parametric Kruskal-Wallis test to compare the two groups with regards to their disease state.
For further validation of the clusters, and to demonstrate that the canonical variate had biological meaning, we used an independent measure, the average whole brain cortical thickness based on the high resolution 3DT1 sequence and obtained from Freesurfer (Fischl, 2012), to test for differences between the two clusters.
3. Results
3.1. Multiset canonical correlation analysis
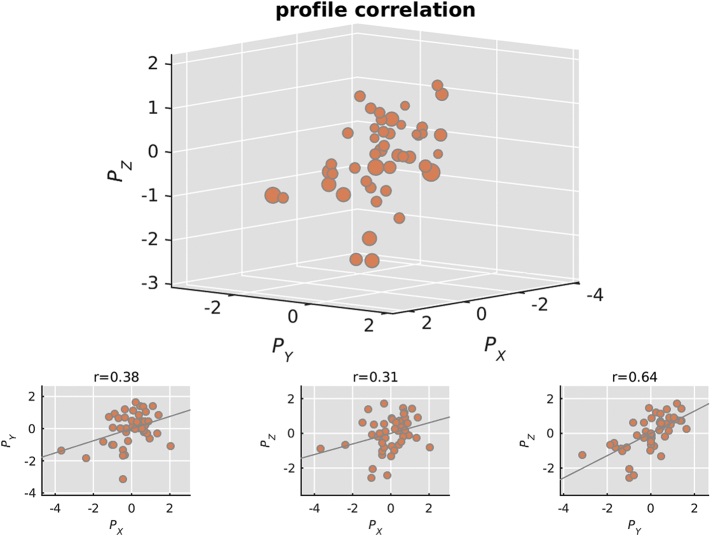
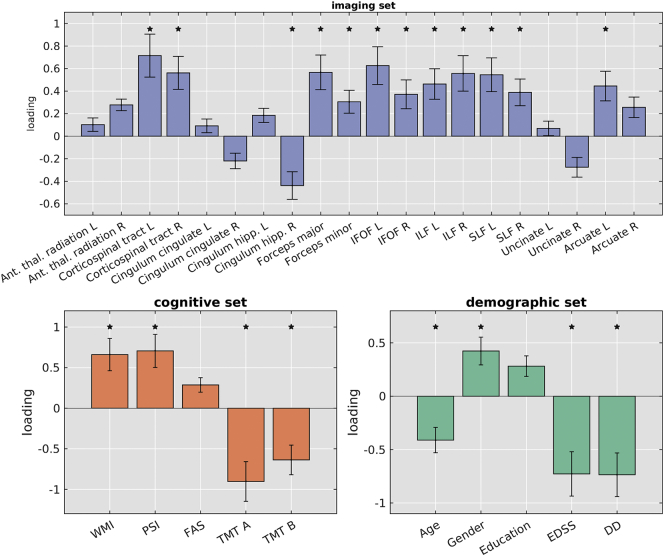
We found one significant MCCA component relating imaging, cognitive, and demographic variables (permutation test p = 0.009). The pairwise correlations between the profiles were rxy = 0.37, rxz = 0.31, and ryz = 0.64, with rij and i,j being the imaging, cognitive, or demographic profile (Fig. 2). Fig. 3 shows the canonical loadings, which are Pearson's correlations between the profiles and the original features in their respective set and reflect the shared variance between the original features and its profile. Error bars are 95% confidence intervals, determined with a leave-one-out cross validation.
Fig. 2.
Correlations of the canonical variates or profiles. Top: A 3D representation of the correlation between canonical variates. Bottom: The pairwise correlations between canonical variates P are: 0.38 between set X (MWF) and Y (cognitive), 0.31 between set X and set Z (demographics and disease severity), and 0.64 between set Y and set Z. The overall significance of the component was p = 0.009, assessed with a permutation test.
Fig. 3.
Loadings of individual features per set when lesions were included in the analysis. Top: displays loadings of the imaging set, lower left: shows the loadings of cognitive set, lower right: shows the loadings of demographic set.
ant. Thal. radiation = anterior thalamic radiation, cingulum hipp. = cingulum hippocampus, IFOF = inferior fronto-occipital fasciculus, ILF = inferior longitudinal fasciculus, SLF = superior longitudinal fasciculus; DD = disease duration
All features across all three sets contributed significantly to their respective profiles, as suggested by the confidence intervals not spanning zero. At the same time, there was considerable variability among the loadings, indicating a distinct hierarchy of contributions between features towards each profile. Three WM ROIs display a negative loading, while the remaining 17 ROIs showed a positive loading on the WM profile. In the cognitive profile, WMI, PSI, and FAS showed positive loadings whereas TMT A and B exhibited a negative loading. This opposite contribution of cognitive tests to the cognitive profile was expected since higher scores of the WMI, PSI, and FAS indicate better performance, while the opposite is true for the TMT A/B tests as higher scores signify worse performance. The variables of the demographic set demonstrated a mix of positive and negative loadings, with gender and education showing positive contributions to this profile. In contrast, age, EDSS, and disease duration loaded negatively onto this profile.
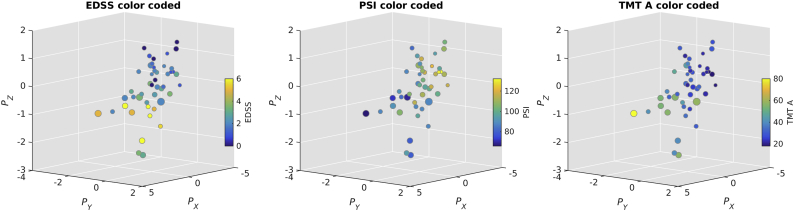
Fig. 4 illustrates the opposing contributions to their respective profiles from some of the variables showing some of the highest loadings from the cognitive and demographic sets. The figure displays the profiles with subjects color-coded according to their EDSS score (Fig. 4 left), their PSI score (Fig. 4 middle), and their TMT A score (Fig. 4 right).
Fig. 4.
Scatter plots of canonical variates with color coding of different variables from set Y and set Z. Left: shows subjects with higher EDSS are clustered along the negative axes. Middle: color coding according to PSI scores where strongly performing subjects are clustered along the positive axes displaying an inverse pattern than plot on the left. Right: performance in TMT A scores color coded where poorly performing subjects cluster along the negative axes, showing a similar pattern to that of left figure which means that subjects with higher EDSS perform worse on TMT A. Size of markers reflects the distance from the point furthest back in Px-Py plane
3.2. Effect of lesions on MCCA
A multiple regression model estimating the profile loadings of WM ROIs, as the dependent variable, with ‘lesion percentage’ and ‘subject lesions’, as the independent variables, was not significant (F(17,20) = 2.47, p = 0.114).
The repeat MCCA analysis with lesion tissue removed resulted in a very similar correlation pattern among the three profiles with one significant component (p = 0.005) and pairwise correlations of rxy = 0.37, rxz = 0.31, and ryz = 0.64. Visualisations and tables with more details about the two MCCA runs can be found in the Supplementary Figs. F1-F4 and Tables T2-T4. Fig. 5 shows the WM ROIs with a substantial lesion contribution (in this case measured by how many subjects presented a lesion in an ROI), that also had a significant loading on the WM profile.
Fig. 5.
Color coded ROIs that showed lesions in at least 50% of subjects (red) and 33% of subjects (orange) with some of the largest loadings onto the WM profile. The bilateral thalamic radiation in blue showed lesions in at least 50% of subjects but did not show large loadings. Even though there was a substantial lesion contribution in those ROIs, it had minimal effects on the loadings.
3.3. Post hoc results
After a k-means clustering looking for two clusters, cluster one was comprised of 36 subjects and cluster two included 10 subjects (Fig. 6). The clustering separated subjects into a mildly affected (average EDSS = 2.09, average DD = 9.32 years) and a moderately affected (average EDSS = 3.80, average DD = 19.10 years) group with p = 0.0065 for EDSS and p = 0.0093 for DD (Table 2). As expected, cluster 2, with greater EDSS and higher DD, exhibited greater cortical thinning (2.61 mm vs 2.51 mm p = 0.0143, for cluster 1 and cluster 2, respectively).
Fig. 6.
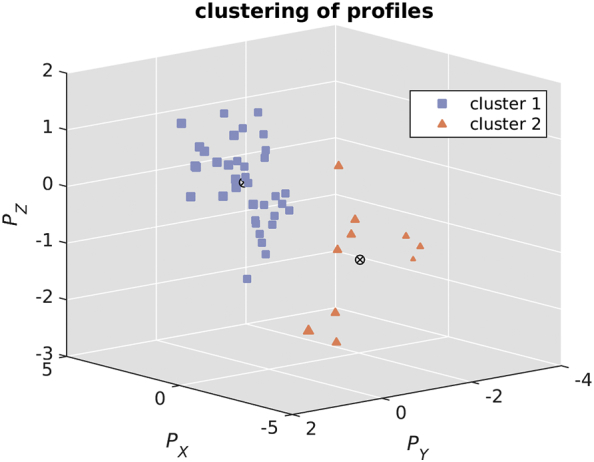
Using k-means on canonical variates of significant component looking for two clusters (one mildly (average EDSS = 2.09, average DD = 9.32 years) and one moderately (average EDSS = 3.80, average DD = 19.10 years) affected subgroup). The number of subjects per cluster are 36, and 10, respectively.
Table 2.
Comparison of clinical and cognitive variables between cluster. Clinical, cognitive and cortical thickness measures with boldface p-values are significant at the 0.05 significant level.
| Cluster 1 mean ± SD | Cluster 2 mean ± SD | Kruskal-Wallis p-value | |
|---|---|---|---|
| Age (years) | 41.5 ± 10.97 | 47.9 ± 9.36 | 0.1126 |
| Gender | 30f/6 m | 5f/5 m | |
| Education (years) | 14.94 ± 2.45 | 14.30 ± 2.58 | 0.2419 |
| Disease duration (years) | 9.32 ± 6.80 | 19.10 ± 10.90 | 0.0093 |
| EDSS | 2.09 ± 1.62 | 3.80 ± 1.60 | 0.0065 |
| WMI | 94.11 ± 13.46 | 83.50 ± 8.51 | 0.0230 |
| PSI | 102.31 ± 13.39 | 85.30 ± 14.91 | 0.0044 |
| FAS | 40.78 ± 11.40 | 37.10 ± 12.35 | 0.5938 |
| TMT A | 28.42 ± 7.29 | 54.00 ± 11.46 | <0.0001 |
| TMT B | 67.42 ± 38.092 | 144.40 ± 83.84 | <0.0001 |
| Cortical thickness (mm) | 2.61 ± 0.10 | 2.51 ± 0.09 | 0.0143 |
4. Discussion
We utilized a multivariate, data-driven approach, in order to reveal a joint pattern of covarying features consisting of profiles of WM myelin integrity, cognitive performance, and demographic and disease factors in a cohort of RRMS subjects. The data fusion performed here is completely data-driven and uses simple linear weights in order to decompose the data sets into latent, maximally cross-correlated profiles. The data-driven nature of the analysis minimises a priori assumptions about potential interactions within and across data domains, while its multivariate nature allows the modeling of shared features across data sets. This is in contrast to univariate approaches where only local or distinct features can be examined. The power of MCCA analysis approach lies in its ability to naturally reveal cross-modality relationships and it suggests some interesting conclusions: 1) factors such as disease duration, gender and age predict cognitive performance in early MS more than myelin features (correlation between profiles of cognition and demographics r = 0.64, MWF vs cognition r = 0.37, and MWF vs demographics r = 0.31), 2) relative independent of lesion presence and exact lesion location, there is a robust association between myelin content in long-range intrahemispheric connections and the corpus callosum and cognitive performance.
Many cognitive domains can be profoundly affected by altered microstructural integrity as seen in MS. Processing speed and tasks requiring the ability to focus attention, scan quickly, discriminate and order information in order to process it, and working memory, are all commonly impaired in MS (Chiaravalloti and DeLuca, 2008; Guimarães and Sá, 2012; Pujol et al., 2001). Complex attention involving alertness, selective/focused/divided attention, and vigilance rather than “simple” attention (e.g. repeating a series of digits) is also impaired (Chiaravalloti and DeLuca, 2008; Guimarães and Sá, 2012). Impairments in executive function, a set of abilities which facilitate goal-oriented behavior as well as adaption to environmental changes such as planning, shifting, and fluency, appears to impact the quality-of-life the most among the cognitive deficits seen in MS (Foong et al., 1997; Holland et al., 2014; Preston et al., 2013). Early research proposed that memory deficits in MS were caused by an inability to sustain or support effective information retrieval (Beatty, 1993), however difficulty in acquiring new knowledge (i.e. memory encoding) might be a greater problem than information retrieval (i.e. memory retrieval) (Chiaravalloti and DeLuca, 2008).
Several studies have attempted to establish the links between white matter changes and cognitive decline in MS. Demyelination, inferred by lesions in conventional MRI images in the medial frontal region, is associated with slow responses in an attention task (Pujol et al., 2001). Studies utilizing DTI have suggested that processing speed deficits were related to reduced FA in the corpus callosum and superior longitudinal fasciculus, two major tracts connecting the two hemispheres and frontal, temporal, and parietal lobes (Genova et al., 2013). Compared to cognitively-preserved MS patients, cognitively-impaired patients evaluated in spatial and verbal memory, information processing speed, working memory, and verbal fluency spheres exhibited reduced FA in the corpus callosum, superior and inferior longitudinal fasciculus, corticospinal tracts, forceps major, cingulum, and fornices (Hulst et al., 2013).
While here we have shown a significant relation between cognition and MWF measures in RRMS, relations between MWF imaging and cognition have also been explored in the non-MS literature. In children (n = 108 children ages: 2.5 months - 5.5 years), a significant positive association between myelination and cognitive and motor abilities can be shown (Dean et al., 2015; Deoni et al., 2016). Further, a relation was found between highly myelinated axons in the corpus callosum and the Wechsler Intelligence Scale for Children in five male children aged between 8 and 12 (Whitaker et al., 2008). A myelin water imaging study found positive relations between frontal lobe myelination and both age and years of education in controls (n = 27) but not in subjects with schizophrenia (n = 30) (Flynn et al., 2003). A more recent study of young adults with ages ranging from 15 to 38 years found a positive relation between frontal lobe myelination and age, North American Adult Reading Test (NAART) IQ, and years of education (Lang et al., 2014). In older patients with mild cognitive impairments (MCI), substantial decreased myelin integrity has been observed; however, associations between decreased myelination and poor cognitive performance were not documented (Bouhrara et al., 2018). Finally, a study of myelination in 61 healthy volunteers aged 18 to 84 years found a quadratic relation between MWF and age (Arshad et al., 2016) emphasizing that age affects myelin differently across lifespan. Overall, these studies highlight the importance of often widely spatially distributed myelin profile integrity to cognitive function. However, the link between myelin integrity and cognitive abilities in MS is lacking. This study potentially overcomes the research gap of myelin-cognition relations in MS as we discovered multivariate relations between myelin and cognitive performance. Perhaps, the multivariate approach used in this study is a key factor to study human brain and behaviour.
As executive function profoundly affects the quality-of-life of people with MS, the original study design was to investigate the relations between imaging features, clinical variables, and primarily executive performance in MS. Therefore, the test battery for this study was based on widely-used tasks in assessing executive functioning and processing speed rather than commonly-used cognition screening tests such as the Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS) (Langdon et al., 2012). Another neuropsychological battery that targets specific domains affected in MS is the Minimal Neuropsychological Assessment of MS Patients (MACFIMS) (Benedict et al., 2002), which provides comprehensive evaluation of cognitive function in MS. In fact, our test battery evaluates some domains that are also examined in MACFIMS such as working memory, processing speed, and executive function. Due to the fact that executive function and processing speed were our primary and secondary targets to evaluate, we specifically chose tests tailored to assess these domains, rather than the standardized test used in MS. Therefore, we decided to use sub-tests of WAIS IV to evaluate working memory and processing speed and executive function was also examined with TMT A/B and FAS. We note that the loadings across specific cognitive tests in the significant MCCA component were relatively uniform (note that for TMT A/B, longer times represent worsening performance, so the loadings in Fig. 3, lower left are reasonable since higher scores on TMT A/B are anticorrelated to the remaining cognitive tests where higher scores indicate better performance). We therefore do not believe that differing cognitive tests would alter our overall conclusions substantially.
We used MCCA to create profiles of WM myelin integrity, cognitive performance, and demographic and disease factors which were closely related to one another. Since WMI, PSI, and FAS together with gender and education all loaded positively on their respective profiles, it is implied that subjects with higher education and being female (females were coded 1, males were coded 0 in analysis) performed better in these cognitive tests. Most WM ROIs loaded positively on its profile, and the aforementioned cognitive tests loaded positively on their profile. This implies a positive relation between MWF and cognitive performance, education, and female gender. In contrast, the negative loadings of age, EDSS, and disease duration on the demographics profile, and the positive loadings of WMI, PSI, and FAS on the cognitive profile, suggest that age and disease progression have adverse effects on cognitive performance. Similarly, age, EDSS, and disease duration showed opposite loadings to that of WM ROIs on their respective profiles, indicating an inverse relation between MWF and age, and disease duration.
The cognitive tests TMT A/B loaded negatively on the cognitive profile, while in the demographics profile, age, EDSS (disease severity), and disease duration had negative loadings, and years of education had a positive loading. This indicates that age and a more severe disease state were associated with higher TMT A/B scores (i.e., slower times to complete the task, reflecting a worse performance), but years of education was not. Similarly, the mostly positive loadings of most WM ROIs in the myelin profile, suggest a worse performance in TMT A/B with low MWF, an imaging feature associated with more severe disease involvement.
Our results further support the notion that higher-order functions require multiple brain regions to coordinate together, especially the longitudinal fasciculus which connect frontal/parietal/occipital areas (i.e. long-range intrahemispheric connections) and the corpus callosum which links the two hemispheres (i.e. interhemispheric connections) (Schulte and Müller-Oehring, 2010). In the present study, white matter ROIs associated most with the imaging profile included the bilateral corticospinal tract, forceps major, forceps minor, bilateral inferior fronto-occipital fasciculus, bilateral inferior longitudinal fasciculus, bilateral superior longitudinal fasciculus, and left arcuate. These WM tracts connect geographically remote brain regions, as well as cortical and subcortical areas connecting regions for processing speed ability, higher-order cognitive functions, and motor function (Roberts et al., 2013). The forceps minor, forceps major, superior and inferior longitudinal fasciculus, and inferior fronto-occipital fasciculus have been reported to be involved in processing speed function, and influencing TMT performance in both older adults and MS subjects (Genova et al., 2013; Kerchner et al., 2012). The inferior and superior longitudinal fasciculi have been shown to play a role in higher-order cognitive function as they connect association cortices – key regions for higher-order functions (Jung et al., 2016). Indeed, the cognitive tests that are positively expressed in the cognitive profile are WMI, PSI, and FAS, tests that assess working memory, processing speed, and verbal fluency and are all impaired in MS (Chiaravalloti and DeLuca, 2008; Guimarães and Sá, 2012; Pujol et al., 2001). On the other hand, the timed TMT A/B tests, where higher scores signify worse performance, are negatively associated with the cognitive profile, indicating an inverse relation between the imaging features and these particular tests.
The fact that the corticospinal tract WM ROI was also loading positively on the imaging profile is perhaps surprising. A DTI study suggested that the corticospinal tract has been related to motor performance but not higher-order cognitive functions (Lövdén et al., 2014), as would be expected. While the quantitative measure of myelin and joint multivariate data analysis utilized here may be more sensitive, there may be other explanations. For example, the corticospinal tract is required to execute the processed information required in different cognitive tasks. One model parcellates executive function into three components – input (require long-range connections), core process (in the prefrontal cortex), and output (require coordination of motor and subcortical connections for taking actions) (Miller and Cohen, 2001). This model implies that the corticospinal tract might be involved in the output component to execute actions based on processed information. Therefore, as a resource to execute the information, it is reasonable that WM integrity in the corticospinal tract is involved in executive function. Further, there may be a correlative as opposed to a causal relation between corticospinal tract integrity and cognitive function: presumably people with worsening disease, and reduced WM integrity would be more likely to also have impairments in their motor system.
Some of our results describing factors preserving executive function in MS are perhaps unsurprising. We found positive loadings of WMI, PSI, FAS, as well as a positive loading of education onto their respective profiles, suggesting a positive relation between these features. In the theory of cognitive reserve (Stern, 2002; Sumowski and Leavitt, 2013; Tucker-Drob et al., 2011), higher levels of education may alter synaptic organization and/or neuronal networks so individuals can still sustain damage and maintain adequate cognitive function (Stern, 2002). Also, we found that age, EDSS and disease duration loaded negatively on the demographic profile indicating a negative association with cognitive tests that loaded positively on the cognitive profile. Of note, EDSS and disease duration had the highest loadings in the demographics profile, indicating a central role of these measures' influence on both imaging and cognition sets. We also observed that better cognitive performance was associated with female gender. Although we speculate that such a pattern may support the purported neuroprotective effects of estrogen (Jacobs and D'Esposito, 2011; Miller and Cronin-Golomb, 2010), more evidence is needed to draw such conclusion.
The cluster analysis produced a reasonable separation of mildly- and moderately-affected sub-groups across all three sets, further supporting a strong relation between MWF, cognitive abilities, and demographics. The additional comparison of an unrelated measure, namely cortical thickness, reinforces the utility of MCCA as well as strengthens our results of finding an association between MWF, cognitive performance, demographics and clinical variables. The groupings based on the MCCA results show a similar pattern of cortical thinning with the moderately affected sub-group having a decreased overall cortical thickness and are in line with previous research (Steenwijk et al., 2016).
Importantly, our results were largely independent of exact lesion location. A multiple linear regression model was unable to detect a relation between lesion contribution per ROI and the MWF loadings we found suggesting that MWF loadings are independent of lesion contribution. Furthermore, when the MCCA analysis was performed with and without lesion tissue included in WM ROIs the results were largely unchanged. There has been a long-standing debate on local vs distributed representation of brain function (Kaas, 1987). In MS there can be a very close association between lesion location and clinical effects, with optic neuritis being a prime example. However, with higher cognitive functions, such clinicopathological correlation between lesion location and behavioural effect is less clear, as distributed brain regions must be recruited to facilitate complex tasks. While we have shown a relationship between NAWM and some cognitive functions, we cannot discern, with the current analysis, whether or not this related to primary dysfunction in the NAWM, or secondary changes from focal lesions.
There are a number of limitations to our study. Since none of the MS subjects demonstrated overt cognitive impairment at the time of examination based on our previous report which investigated the same cohort (Lin et al., 2017), the results of this study reflect brain-behaviour associations at early stages of the disease. Further, the analyzed cohort was comprised solely from early stages of the RRMS subtype which was owed to the study design, extrapolations to different subtypes of MS should be taken with care. Due to the fact the analysis was based on a set of WM ROIs from a template, not all of the WM is necessarily covered, although we note that most major WM fibre bundles were included. This may have led to individual lesions not being accounted for in this analysis in case they did not overlap with any of the standard ROIs. In addition, the investigated cohort was in the relatively early stages of MS with low to moderate lesion burden such that the impact of focal demyelination of lesions within the ROIs was limited. Thus, the minor change in results of the MCCA when including and excluding lesions may be partly due to the fact that not all lesion had been considered. Finally, a complicating factor is that myelin as a function of age may follow a quadratic function (Arshad et al., 2016), so extrapolating to broader age ranges may pose a risk – our results may therefore only be interpretable for the age range of our cohort.
To conclude, with quantitative WM myelin measures and a joint multivariate analysis, we found individual profiles of myelin integrity, cognition, and demographical features in MS that where highly similar. Higher myelin integrity supported better cognitive function and was positively related to education as well as female gender; while disease severity and aging were associated with worsening cognitive performance. In the future, a multimodal approach including functional measures gained from fMRI may be used to study the interplay between structure and function and use their complementary information to further prognosticate cognitive deficits in MS.
Conflicts of interests
TRB, SJL, BK, IV, and AM have no conflicts of interest to declare. SK has received research funding from Sanofi Genzyme, F. Hoffmann La Roche. David Li has received research funding from the Canadian Institute of Health Research and Multiple Sclerosis Society of Canada. He is the Emeritus Director of the UBC MS/MRI Research Group which has been contracted to perform central analysis of MRI scans for therapeutic trials with Novartis, Perceptives, Roche and Sanofi-Aventis. The UBC MS/MRI Research Group has also received grant support for investigator-initiated independent studies from Genzyme, Merck-Serono, Novartis and Roche. He has acted as a consultant to Vertex Pharmaceuticals and served on the Data and Safety Advisory Board for Opexa Therapeutics and Scientific Advisory Boards for Adelphi Group, Celgene, Novartis and Roche. He has also given lectures which have been supported by non-restricted education grants from Academy of Health Care Learning, Biogen-Idec, Consortium of MS Centers, Novartis, Sanofi-Genzyme and Teva. Anthony Traboulsee has the following competing financial interests: Research funding from Chugai, Roche, Novartis, Genzyme, Biogen. Consultancy honoraria from Genzyme, Roche, Teva, Biogen, Serono. Martin McKeown has research funding from the Mottershead Foundation, the National Science and Engineering Research Council (NSERC) and has received honoraria from Abbvie Corporation and Allergan, Inc.
Funding
MS Society of Canada.
Acknowledgements
We thank all participants and their families for their cooperation as well as the MR technologists for the data collection. MJM is supported by the University of British Columbia / Pacific Parkinson's Research Centre Chair in Parkinson's research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101926.
Appendix A. Supplementary data
Supplementary material
References
- Arnett J.A., Labovitz S.S. Effect of physical layout in performance of the trail making test. Psychol. Assess. 1995;7:220–221. [Google Scholar]
- Arshad M., Stanley J.A., Raz N. Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. Neuroimage. 2016;143:26–39. doi: 10.1016/j.neuroimage.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty W.W. Memory and “frontal lobe” dysfunction in multiple sclerosis. J. Neurol. Sci. 1993;115:S38–S41. doi: 10.1016/0022-510x(93)90207-f. Suppl. [DOI] [PubMed] [Google Scholar]
- Benedict R.H.B., Fischer J.S., Beatty W.W., Bobholz J., Chelune G.J., Langdon D.W., Caruso L., Foley F., LaRocca N.G., Vowels L., Weinstein A., DeLuca J., Rao S.M., Munschauer F., Archibald C.J., Arnett P.A., Fisk J.D. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin. Neuropsychol. 2002;16:381–397. doi: 10.1076/clin.16.3.381.13859. (Neuropsychology, Dev. Cogn. Sect. D) [DOI] [PubMed] [Google Scholar]
- Bouhrara M., Reiter D.A., Bergeron C.M., Zukley L.M., Ferrucci L., Resnick S.M., Spencer R.G. Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimers Dement. 2018:1–7. doi: 10.1016/j.jalz.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard D., Trip S.A. Resolving the clinico-radiological paradox in multiple sclerosis. F1000Research. 2017;6:1828. doi: 10.12688/f1000research.11932.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti N.D., DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Dean D.C., O’Muircheartaigh J., Dirks H., Waskiewicz N., Walker L., Doernberg E., Piryatinsky I., Deoni S.C.L. Characterizing longitudinal white matter development during early childhood. Brain Struct. Funct. 2015;220:1921–1933. doi: 10.1007/s00429-014-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C.L., O’Muircheartaigh J., Elison J.T., Walker L., Doernberg E., Waskiewicz N., Dirks H., Piryatinsky I., Dean D.C., Jumbe N.L., Jumbe N.L. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct. Funct. 2016;221:1189–1203. doi: 10.1007/s00429-014-0947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiss J.H., Dähne D., Baum K., Deppe R., Hoffmann F., Köhler W., Kunkel A., Lux A., Matzke M., Penner I.K., Sailer M., Zettl U.K. Reduced magnetisation transfer ratio in cognitively impaired patients at the very early stage of multiple sclerosis: a prospective, multicenter, cross-sectional study. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2013-004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn S.W., Lang D.J., Mackay A.L., Goghari V., Vavasour I.M., Whittall K.P., Smith G.N., Arango V., Mann J.J., Dwork A.J., Falkai P., Honer W.G. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol. Psychiatry. 2003;8:811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- Foong J., Rozewicz L., Quaghebeur G., Davie C.A., Kartsounis L.D., Thompson A.J., Miller D.H., Ron M.A. Executive function in multiple sclerosis. The role of frontal lobe pathology. Brain. 1997;120:15–26. doi: 10.1093/brain/120.1.15. [DOI] [PubMed] [Google Scholar]
- Genova H.M., Deluca J., Chiaravalloti N., Wylie G. The relationship between executive functioning, processing speed, and white matter integrity in multiple sclerosis. J. Clin. Exp. Neuropsychol. 2013;35:631–641. doi: 10.1080/13803395.2013.806649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães J., Sá M.J. Cognitive dysfunction in multiple sclerosis. Front. Neurol. MAY. 2012:1–8. doi: 10.3389/fneur.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland A.A., Graves D., Greenberg B.M., Harder L.L. Fatigue, emotional functioning, and executive dysfunction in pediatric multiple sclerosis. Child Neuropsychol. 2014;20:71–85. doi: 10.1080/09297049.2012.748888. [DOI] [PubMed] [Google Scholar]
- Hotelling H. Relations between two sets of Variates. Biometrika. 1936;28:321. [Google Scholar]
- Hulst H.E., Steenwijk M.D., Versteeg A., Pouwels P.J.W., Vrenkan H., Uitdehaag B.M.J., Polman C.H., Geurts J.J.G., Barkhof F. Cognitive impairment in MS: Imapct of white matter integrity, gray matter volume, and lesions. Neurology. 2013;80:1025–1032. doi: 10.1212/WNL.0b013e31828726cc. [DOI] [PubMed] [Google Scholar]
- Jacobs E., D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for Women’s health. J. Neurosci. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jung J.Y., Cloutman L.L., Binney R.J., Lambon Ralph M.A. The structural connectivity of higher order association cortices reflects human functional brain networks. Cortex. 2016;97:221–239. doi: 10.1016/j.cortex.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J.H. The Organization of Neocortex in mammals: implications for theories of brain function. Annu. Rev. Psychol. 1987;38:129–151. doi: 10.1146/annurev.ps.38.020187.001021. [DOI] [PubMed] [Google Scholar]
- Kerchner G.A., Racine C.A., Hale S., Wilheim R., Laluz V., Miller B.L., Kramer J.H. Cognitive processing speed in older adults: relationship with white matter integrity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenring J.R. Canonical analysis of several sets of variables. Biometrika. 1971;58:433. [Google Scholar]
- Lang D.J.M., Yip E., MacKay A.L., Thornton A.E., Vila-Rodriguez F., MacEwan G.W., Kopala L.C., Smith G.N., Laule C., MacRae C.B., Honer W.G. 48 echo T₂ myelin imaging of white matter in first-episode schizophrenia: evidence for aberrant myelination. NeuroImage. Clin. 2014;6:408–414. doi: 10.1016/j.nicl.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon D., Amato M., Boringa J., Brochet B., Foley F., Fredrikson S., Hamalainen P., Hartung H.-P., Krupp L., Penner I., Reder A., Benedict R. Recommendations for a brief international cognitive assessment for multiple sclerosis (BICAMS) Mult. Scler. J. 2012;18:891–898. doi: 10.1177/1352458511431076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule C., Leung E., Lis D.K.B., Traboulsee A.L., Paty D.W., MacKay A.L., Moore G.R.W. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult. Scler. 2006;12:747–753. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- Laule C., Kozlowski P., Leung E., Li D.K.B., MacKay A.L., Moore G.R.W. Myelin water imaging of multiple sclerosis at 7??T: correlations with histopathology. Neuroimage. 2008;40:1575–1580. doi: 10.1016/j.neuroimage.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Lezak M.D. Oxford University Press; 2012. Neuropsychological Assessment. [Google Scholar]
- Lin S.-J., Lam J., Beveridge S., Vavasour I., Traboulsee A., Li D.K.B., MacKay A., McKeown M., Kosaka B. Cognitive performance in subjects with multiple sclerosis is robustly influenced by gender in canonical-correlation analysis. J. Neuropsychiatr. Clin. Neurosci. 2017;29:119–127. doi: 10.1176/appi.neuropsych.16040083. [DOI] [PubMed] [Google Scholar]
- Lövdén M., Köhncke Y., Laukka E.J., Kalpouzos G., Salami A., Li T.-Q., Fratiglioni L., Bäckman L. Changes in perceptual speed and white matter microstructure in the corticospinal tract are associated in very old age. Neuroimage. 2014;102:520–530. doi: 10.1016/j.neuroimage.2014.08.020. [DOI] [PubMed] [Google Scholar]
- MacKay A.L., Laule C. Magnetic resonance of myelin water: an in vivo marker for myelin. Brain Plast. 2016;2:71–91. doi: 10.3233/BPL-160033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mädler B., Drabycz S.A., Kolind S.H., Whittall K.P., MacKay A.L. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn. Reson. Imaging. 2008;26:874–888. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- McAusland J., Tam R.C., Wong E., Riddehough A., Li D.K.B. Optimizing the use of radiologist seed points for improved multiple sclerosis lesion segmentation. IEEE Trans. Biomed. Eng. 2010;57:2689–2698. doi: 10.1109/TBME.2010.2055865. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R. Towards a network theory of cognition. Neural Netw. 2000;13:861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Korostil M. Interpretation of neuroimaging data based on network concepts. Brain Imaging Behav. 2008;2:264–269. [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller I.N., Cronin-Golomb A. Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov. Disord. 2010;25:2695–2703. doi: 10.1002/mds.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M., Fujihara K., Havrdova E., Hutchinson M., Kappos L., Lublin F.D., Montalban X., O’Connor P., Sandberg-Wollheim M., Thompson A.J., Waubant E., Weinshenker B., Wolinsky J.S. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasloski T., Mädler B., Xiang Q.S., MacKay A.L., Jones C. Applications of stimulated echo correction to multicomponent T2 analysis. Magn. Reson. Med. 2012;67:1803–1814. doi: 10.1002/mrm.23157. [DOI] [PubMed] [Google Scholar]
- Preston J., Hammersley R., Gallagher H. The executive dysfunctions most commonly associated with multiple sclerosis and their impact on occupational performance. Br. J. Occup. Ther. 2013;76:225–233. [Google Scholar]
- Pujol J., Vendrell P., Deus J., Junqué C., Bello J., Martí-Vilalta J.L., Capdevila A. The effect of medial frontal and posterior parietal demyelinating lesions on Stroop interference. Neuroimage. 2001;13:68–75. doi: 10.1006/nimg.2000.0662. [DOI] [PubMed] [Google Scholar]
- Rizio A.A., Diaz M.T. Language, aging, and cognition: frontal aslant tract and superior longitudinal fasciculus contribute toward working memory performance in older adults. Neuroreport. 2016;27:689–693. doi: 10.1097/WNR.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R.E., Anderson E.J., Husain M. White matter microstructure and cognitive function. Neurosci. 2013;19:8–15. doi: 10.1177/1073858411421218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T., Müller-Oehring E.M. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychol. Rev. 2010;20:174–190. doi: 10.1007/s11065-010-9130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler S., Pirpamer L., Hofer E., Duering M., Jouvent E., Fazekas F., Mangin J.-F., Chabriat H., Dichgans M., Ropele S., Schmidt R. Magnetization transfer ratio relates to cognitive impairment in normal elderly. Front. Aging Neurosci. 2014;6:263. doi: 10.3389/fnagi.2014.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenwijk M.D., Geurts J.J.G., Daams M., Tijms B.M., Wink A.M., Balk L.J., Tewarie P.K., Uitdehaag B.M.J., Barkhof F., Vrenken H., Pouwels P.J.W. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain. 2016;139:115–126. doi: 10.1093/brain/awv337. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Sumowski J.F., Leavitt V.M. Cognitive reserve in multiple sclerosis. Mult. Scler. J. 2013;19:1122–1127. doi: 10.1177/1352458513498834. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob E., Johnson K., Jones R. The cognitive reserve hypothesis: a longitudinal examination of age- associated declines in reasoning and processing speed. Dev. Psychol. 2011;45:431–446. doi: 10.1037/a0014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavasour I.M., Laule C., Li D.K.B., Traboulsee A.L., MacKay A.L. Is the magnetization transfer ratio a marker for myelin in multiple sclerosis? J. Magn. Reson. Imaging. 2011;33:710–718. doi: 10.1002/jmri.22441. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Williams & Wilkins Co; Baltimore: 1939. The Measurement of Adult Intelligence. [Google Scholar]
- Whitaker K.J., Kolind S.H., MacKay A.L., Clark C.M. Quantifying development: investigating highly myelinated voxels in preadolescent corpus callosum. Neuroimage. 2008;43:731–735. doi: 10.1016/j.neuroimage.2008.07.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material