Abstract
Background
Eosinophilic inflammation is a major phenotype associated with poorly controlled disease in nasal polyp patients. The difference between systemic and local eosinophilia in relation to disease control is poorly understood.
Objective
To explore whether blood and polyp tissue eosinophil numbers are independent risk factors for poor disease control in patients with nasal polyp.
Methods
By using the electronic medical records database and manual evaluation, 183 nasal polyp patients who had undergone endoscopic sinus surgery at least one year prior to the study with complete data of tissue specimens, baseline blood routine test, nasal endoscopy and sinus computed tomography, were identified and recruited to assess disease control based on the criteria of a European position paper on rhinosinusitis and nasal polyps 2012 (EPOS 2012). Multiple logistic regression model was used to determine the association between blood and tissue eosinophil numbers and risk of poor disease control by adjusting for demographics and comorbidities.
Results
We broke down the cohort into 4 groups according to blood (0.3 × 109/L) and tissue (10%) eosinophils. The patients without eosinophilic inflammation represented the largest group (41.5%). The group with concordant blood and tissue eosinophilia represented the second largest (31.2%), and the patients with isolated tissue (15.3%) or blood (12.0%) eosinophilia were relatively rare. Multiple logistic regression models found blood eosinophil count and tissue eosinophil percentage were independently associated with increased risk for poor disease control after adjustments for covariates related to poor treatment outcome. Furthermore, subjects with concordant blood and tissue eosinophilia had a higher risk for poor disease control than those with isolated blood or tissue eosinophilia.
Conclusion
Concordant blood and tissue eosinophilia relates to a higher likelihood of poor disease control than isolated blood or tissue eosinophilia after adjustment of potential confounders in nasal polyp patients.
Keywords: Eosinophilia, Disease control, Nasal polyps
Introduction
Chronic rhinosinusitis (CRS) is characterized by chronic mucosal inflammation and estimated to affect 14% of adults in the United States and 8% in China.1, 2 Although only ∼25–30% of patients with CRS develop nasal polyps, non-cancerous outgrowths of the epithelial lining of the sinonasal mucosa, patients with nasal polyps usually have greater severity of clinical disease and impairment of quality of life as well as cost burden compared to those without nasal polyps,3, 4, 5, 6 making it more clinically challenging to rhinology clinicians. Current treatment modalities for nasal polyps include comprehensive medical therapy and endoscopic sinus surgery, and the primary goal of treatment is to achieve and maintain clinical control.7 However, studies reported that over 30% of patients with nasal polyps remain uncontrolled despite current standard-of-care treatment.8, 9 Emerging evidence has suggested that this might be caused by an underlying variation of endotypes resulting in a discrepancy of clinical phenotype and disease prognosis.10, 11, 12 Thus, the need for finding relevant biomarkers in patients with poor disease control is generally acknowledged.
It has been recognized that eosinophilic inflammation is a major phenotype associated with poorly controlled disease and polyp recurrence in patients with nasal polyps.8, 13, 14 Studies using cluster analysis also have shown that cluster with eosinophilic nasal polyps is characterized by worse disease severity and poorer disease control and could be separated from other clusters.10, 15, 16 However, most studies were focused on the correlation of local eosinophils with disease phenotype, and little is known about the relationship between systemic and local eosinophilia in patients with nasal polyps. Although evidence has shown that with blood eosinophilia correlated with tissue eosinophilia in patients with nasal polyp,17, 18, 19 the correlation was only moderate, suggesting there are patients who have discordance between systemic and local eosinophilic inflammation.
In this study, we sought to investigate whether blood and polyp tissue eosinophil numbers have an additive value in relation to disease control after at least one year of endoscopic sinus surgery in a cohort of patients with nasal polyps. To the best of our knowledge, this is the first detailed investigation of the difference between blood and tissue eosinophilia in relation to treatment outcome in patients with nasal polyps.
Materials and methods
Study cohort
This study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. Written informed consent was obtained from all participants.
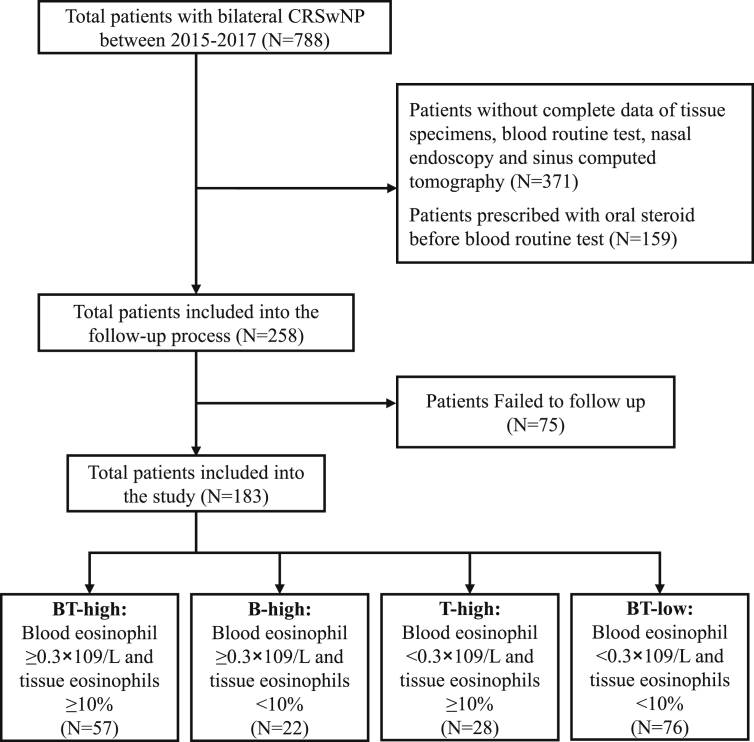
The study flow chart is shown in Fig. 1. Patients with a diagnosis of bilateral CRS with nasal polyps (CRSwNP) evaluated by a rhinologist were identified from the electronic medical records database of the First Affiliated Hospital of Sun Yat-sen University between January 2015 and December 2017. Patient data were cross-referenced to avoid duplication of individuals studied. Then the selected patients were further evaluated manually and enrolled according to the following entry criteria: (1) CRSwNP was diagnosed based on the European position paper on rhinosinusitis and nasal polyps (EPOS 2012).7 (2) Patients were performed bilateral endoscopic sinus surgery. In addition, patients with following criteria were excluded: (1) Patients without complete data of tissue specimens, baseline blood routine test, nasal endoscopy and sinus computed tomography. (2) Patients prescribed with systemic or intranasal corticosteroids within three months and one month before blood routine test, respectively, by cross-referencing patient's medication history with the electronic prescription record system of the First Affiliated Hospital of Sun Yat-sen University. (3) Patients were younger than 16 years of age. (4) Patients with a history of allergic dermatitis, food allergies or helminth infection. (5) Patients with fungal rhinosinusitis, cystic fibrosis, gastroesophageal reflux disease or sinonasal malignancies. Data were retrieved and exported in a standardized Microsoft Excel file, including identification number, age, gender, smoking habits, comorbidities (allergic rhinitis and asthma), endoscopic sinus surgery date, prior sinus surgery history, baseline blood eosinophil numbers, preoperative Lund-Kennedy endoscopic score (LKS), preoperative Lund-Mackay score (LMS), revisit times to clinic as well as medication treatment length after endoscopic sinus surgery. Allergic rhinitis was diagnosed based on the Allergic Rhinitis and its Impact on Asthma guideline.20 The diagnosis of asthma was performed by a specialist physician and was established according to the Global Initiative for Asthma 2006 guideline.21 The diagnosis of aspirin sensitivity was also performed by a specialist physician based on the documented history of intake of aspirin or non-steroid inciting worsening respiratory symptoms. LKS was evaluated and scored by two independent otolaryngologists who were blinded to each other. LMS was performed based on review and scoring of computed tomography (CT) scan images by a trained researcher.22
Fig. 1.
Flow diagram showing patient identification and classification of the retrospective cohort. CRSwNP, Chronic rhinosinusitis with nasal polyps.
Patients' follow-up and clinical assessment of disease control
Patients enrolled in the retrospective cohort study were followed up for the evaluation of treatment outcome, including total nasal symptom score (TNSS),7, 23 nasal endoscopy and clinical disease control level.7 The TNSS was calculated (range: 0–12) by adding up the individual nasal scores including nasal congestion, anterior rhinorrhea, postnasal drip, and loss of smell, each evaluated using a scale of 0 = None, 1 = Mild, 2 = Moderate, or 3 = Severe. The disease control of CRS was evaluated by two senior authors (Y. S. and J. S.) based on the classification criteria of EPOS 2012 (Table E1)7, which has recently been validated by our group and other groups.8, 9 Briefly, the disease control of CRS was divided into 3 levels: controlled, partly controlled, and uncontrolled. Controlled CRS was defined as no bothersome symptoms, with healthy or almost healthy mucosa and no need for systemic medicine to control disease. Partly controlled patients experienced less than 2 of the following items: persistent nasal blockage, mucopurulent rhinorrhea/postnasal drip, facial pain, impaired smell, sleep disturbance/fatigue, disease mucosa under nasal endoscopy, and the need for a course of antibiotics/systemic corticosteroids in the last months. Uncontrolled CRS was defined as 3 or more features of partly controlled CRS.
Histopathologic evaluation
Nasal polyp tissues of all participants were obtained during sinus surgery, fixed in 10% formalin and embedded in paraffin. Samples were cut in 4-μm sections and stained with hematoxylin-eosin. The stained sections were observed under a microscope (Leica DM4 B; Leica, Wetzlar, Germany) in a blinded fashion with regard to all clinical data by 2 independent observers (K.W. and M.Y.). To ensure that patients were consistently classified on the basis of the area of greatest inflammation, the top 5 densest cellular infiltrate areas of the subepithelial layer were chosen under low power field (100×) in each specimen. Then eosinophils and total inflammatory cells were quantified in the focus of each area under high power field (400×, 0.072mm2/frame, HPF). Eosinophil and total inflammatory cell count were recorded in each focus field at 400 power and reported as absolute number per HPF. The ratio of eosinophils to total inflammatory cells was calculated.
Statistical analysis
Statistical analyses were performed with SPSS version 20.0 statistical software (IBM SPSS, Armonk, NY). Group-wise differences between subjects with isolated blood or tissue eosinophilia or concordant blood and polyp tissue eosinophilia and subjects without eosinophilia were primarily studied. t Tests were used for continuous variables with normal distribution. Mann-Whitney U tests were used for continuous non-normally distributed variables. χ2 Tests were used to assess differences in categorical variables, such as gender or smoking prevalence. Pearson's correlation coefficient was used to calculate and to test the linear correlation between blood and tissue eosinophil numbers. Multiple logistic regression models with the variable allergic rhinitis, asthma, gender, number of weeks after surgery, smoking status, prior sinus surgery, LMS, LKS, and treatment outcome of the disease were used to estimate the likelihood of these events in relation to blood and tissue eosinophil categories. Moreover, a logistic regression model with increased blood eosinophil count and increased tissue eosinophil percentage as independent predictors was also performed to evaluate the independent effects of having increased blood and tissue eosinophil level on the studied treatment outcome. These models were adjusted for age, gender, smoking, prior sinus surgery, allergic rhinitis, and asthma. A P value of less than 0.05 was considered statistically significant. A significance level of P less than 0.008 was employed as the criterion for statistical significance to adjust for multiple comparisons when we compared the ratio of uncontrolled and partly controlled to controlled cases in subjects with isolated blood or tissue eosinophilia or concordant blood and tissue eosinophilia versus subjects without eosinophilia.
Results
General characteristics of the cohort
The general characteristics of the cohort are presented in Table E2. A total of 183 patients followed for a median time of 79.3 weeks were finally included in this retrospective analysis. 67.2% were men and the mean age was 39.5 years old. About 10.9% of patients had known allergic rhinitis, 12.6% had asthma, and 9.8% were current smokers. About 31.1% of patients had 2 or more sinus surgeries and 54.1% had post-surgery medication longer than 6 months.
Characterization of patients by disease control status
To characterize the patients with uncontrolled disease, 183 patients were subdivided into 3 groups based on the current EPOS 2012 control criteria for defining the level of control (Table 1). There were 40.4% of patients who had controlled disease, 30.6% with partly controlled disease, and 29.0% with uncontrolled disease. Comparing to the controlled group, the uncontrolled group had a higher percentage of allergic rhinitis and asthma, higher LMS, blood and tissue eosinophil numbers, post-operative TNSS and percentage of medication length >6 months after surgery. In addition, the partly controlled group had a higher percentage of prior sinus surgery, higher blood eosinophil numbers, post-operative TNSS, and percentage of medication length >6 months after surgery.
Table 1.
Comparison of demographics, comorbidities, clinical features at baseline and follow-up after at least one year of endoscopic sinus surgery among patients with different level of disease control according to the EPOS 2012 classification system.
| Controlled# | Partly controlled | Uncontrolled | |
|---|---|---|---|
| Subject, n (%) | 74 (40.4) | 56 (30.6) | 53 (29.0) |
| Male n, (%) | 48 (64.9) | 40 (71.4) | 35 (66.0) |
| Age, mean (SD) | 39.7 (14.6) | 37.7 (13.7) | 40.3 (11.7) |
| Weeks after surgery, median (IQR) | 91.4 (80.5) | 72.6 (44.3) | 70.6 (79.1) |
| Current smoker, n (%) | 9 (12.2) | 6 (10.7) | 3 (5.7) |
| Allergic rhinitis, n (%) | 7 (9.5) | 2 (3.6) | 11(20.8)$ |
| Asthma, n (%) | 6 (8.1) | 7 (12.5) | 10(18.9)$ |
| Prior sinus surgery, n (%) | 17 (23.0) | 23(41.1)$ | 17 (32.1) |
| Preoperative L-K endoscopic score, median (IQR) | 8.0 (4.0) | 9.0 (3.0) | 9.0 (3.0) |
| Preoperative L-M CT score, median (IQR) | 14.0 (7.0) | 15.0 (9.0) | 17.0(8.0)$ |
| Blood eosinophil count, median (IQR) ( × 109/L) | 0.17 (0.21) | 0.29(0.32)$ | 0.33(0.37)* |
| Blood eosinophil percentage, median (IQR) (%) | 2.1 (3.0) | 3.65(5.35)$ | 4.8(6.3)* |
| Tissue eosinophil count, median (IQR) (/HPF) | 22.0 (40.25) | 27.5 (69.25) | 63.0(139.5)€ |
| Tissue eosinophil percentage, median (IQR) (%) | 5.2 (19.8) | 9.3 (32.6) | 19.4(37.9)* |
| Post-operative TNSS, median (IQR) | 0.0 (1.0) | 2.0(1.0)€ | 4.0(2.0)€ |
| Medication length >6 months after surgery, n (%) | 28 (37.8) | 38(67.9)€ | 33(62.3)* |
#Comparator group; Results in boldface indicate a P value of less than 0.05. $P < 0.05; *P < 0.01; €P < 0.001.
SD, standard deviation; IQR, interquartile range; L-K, Lund-Kennedy; L-M, Lund-Mackay; CT, computed tomography; HPF, high power field; TNSS, total nasal symptom score.
Characteristics of the cohort by blood and tissue eosinophil count
To confirm the relationship between blood and tissue eosinophils, correlation analysis was performed between blood and tissue eosinophil numbers. In line with previous studies,17, 18, 24 we found that blood and tissue eosinophil counts and percentages were significantly correlated with each other (Figure E1). However, the correlation coefficients between blood and tissue eosinophil numbers were moderate (r = 0.407 and 0.311 for blood eosinophil count vs. tissue eosinophil percentage and count, respectively, r = 0.32 and 0.408 for blood eosinophil percentage vs. tissue eosinophil count and percentage, respectively). These results indicate there is discordance between systemic and local eosinophilia in patients with nasal polyps.
To further address this, we broke down the population into 4 groups according to blood and polyp tissue eosinophils (Fig. 1). Group 1 is patients without blood and polyp tissue eosinophilia (BT-low: Blood eosinophil count <0.3 × 109/L and tissue eosinophil percentage <10%), group 2 with isolated polyp tissue eosinophilia (T-high: Blood eosinophil count <0.3 × 109/L and tissue eosinophil percentage ≥10%), group 3 with isolated blood eosinophilia (B-high: blood eosinophil count ≥0.3 × 109/L and tissue eosinophil percentage <10%) and group 4 with concordant blood and polyp tissue eosinophilia (BT-high: blood eosinophil count ≥0.3 × 109/L and tissue eosinophil percentage ≥10%). As a result, we found that the BT-low patients represented the largest group, accounting for 41.5% of the cohort. The BT-high group was the second largest, accounting for 31.2% of the patients. Patients with T-high accounted for 15.3% of the cohort, whereas with B-high accounted for 12.0% (Table 2).
Table 2.
Baseline demographics and outcome characteristics of the retrospective cohort by blood and tissue eosinophil number.
| BT-low# | T-high | B-high | BT-high | |
|---|---|---|---|---|
| Subject, n (%) | 76 (41.5) | 28 (15.3) | 22 (12.0) | 57 (31.2) |
| Male, n (%) | 50 (65.8) | 17 (60.7) | 19 (86.4) | 37 (64.9) |
| Age, mean (SD) | 35.1 (14.5) | 43.9(12.0)* | 45.5(13.2)€ | 40.2(11.1)$ |
| Weeks after surgery, median (IQR) | 84.9 (77.2) | 78.1 (50.6) | 94.3 (77.5) | 69.6 (78.9) |
| Prior sinus surgery, n (%) | 27 (35.5) | 10 (35.7) | 7 (31.8) | 13 (22.8) |
| Current smoker, n (%) | 7 (9.2) | 0 (0) | 5(22.7)$ | 6 (10.5) |
| Allergic rhinitis, n (%) | 2 (2.6) | 3 (10.7) | 2 (9.1) | 13(22.8)* |
| Asthma, n (%) | 3 (3.9) | 4 (14.3) | 2 (9.1) | 14(24.6)* |
| Preoperative L-K endoscopic score, median (IQR) | 9.0 (3.0) | 9.0 (3.25) | 8.0 (3.25) | 8.0 (4.0) |
| Preoperative L-M CT score, median (IQR) | 14.0 (6.0) | 16.0 (20.75) | 15.0 (9.0) | 17.0(9.0)$ |
| Blood eosinophil count, median (IQR) ( × 109/L) | 0.1 (0.11) | 0.18 (0.13) | 0.43(0.19)€ | 0.47(0.21)€ |
| Blood eosinophil percentage, median (IQR) (%) | 1.3 (1.6) | 1.6 (2.3) | 5.9(2.7)€ | 6.7(4.5)€ |
| Tissue eosinophil count, median (IQR) (/HPF) | 7.5 (20.75) | 77.5(164.5)€ | 8.5 (16.0) | 110.0(137.5)€ |
| Tissue eosinophil percentage, median (IQR) (%) | 2.15 (3.9) | 24.4(25.3)€ | 2.9 (4.7) | 37.2(32.8)€ |
| Post-operative TNSS, median (IQR) | 1.0 (3.0) | 1.0 (2.8) | 2.5(4.3)$ | 2.0(2.0)$ |
| Medication length>6 months after surgery, n (%) | 43 (56.6) | 12 (42.9) | 13 (59.1) | 31 (54.4) |
| Treatment outcome* | ||||
| Controlled, n (%) | 43 (56.6) | 10 (35.7) | 6 (27.3) | 15 (26.3) |
| Partly Controlled, n (%) | 21 (27.6) | 9 (32.1) | 9 (40.9) | 17 (29.8) |
| Uncontrolled, n (%) | 12 (15.8) | 9 (32.1) | 7 (31.8) | 25 (43.9) |
#Comparator group; Results in boldface indicate a P value of less than 0.05. $P < 0.05; *P < 0.01; €P < 0.001.
SD, standard deviation; IQR, interquartile range; L-K, Lund-Kennedy; L-M, Lund-Mackay; CT, computed tomography; HPF, high power field; TNSS, total nasal symptom score.
Compared with the BT-low group, the BT-high patients were older and had a higher percentage of allergic rhinitis and asthma, as well as preoperative LMS. Interestingly, the percentage of current smokers was higher in B-high group versus the BT-low group. In addition, we did not find significant difference in the percentages of allergic rhinitis and asthma in T-high and B-high group versus BT-low group. There were no significant differences in weeks after surgery, percentage of prior sinus surgery and preoperative LKS in T-high, B-high and BT-high group versus BT-low group (Table 2).
As expected, the blood eosinophil numbers in B-high and BT-high groups and the tissue eosinophil numbers in T-high and BT-high groups were significantly higher than those in BT-low group. Moreover, we found that the tissue eosinophil percentage (P = 0.036), but not count (P = 0.25), was significantly higher in BT-high group than that in T-high group, and there were no significant differences in blood eosinophil count (P = 0.789) and percentage (P = 0.201) between BT-high and B-high patients (Table 2).
Blood and tissue eosinophil level in relation to disease control
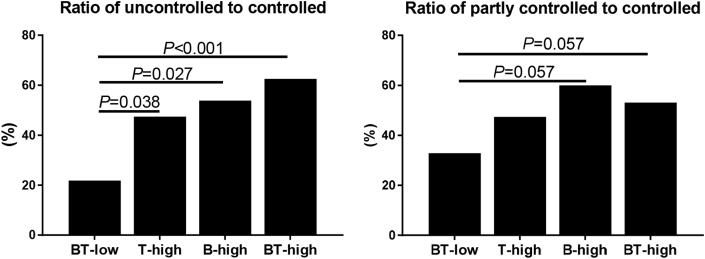
Compared with the BT-low group, the B-high and BT-high groups had higher post-operative TNSS. There was no significant difference in the percentage of medication length>6 months after surgery in T-high, B-high and BT-high groups versus the BT-low group. The partly controlled patients accounted for 27.6%, 32.1%, 40.9% and 29.8%, and the uncontrolled patients 15.8%, 32.1%, 31.8% and 43.9% of patients in BT-low, T-high, B-high and BT-high group, respectively (Table 2). The percentages of patients in the 3 categories of control were significantly different among these 4 groups. Moreover, the ratio of uncontrolled to controlled subjects was significantly higher in BT-high group compared with BT-low group, and there was a trend toward a higher ratio of uncontrolled to controlled subjects in T-high and B-high group than BT-low group (P = 0.038 and 0.027, Fig. 2). We also observed a similar trend toward a higher ratio of partly controlled to controlled subjects in B-high and BT-high group compared with BT-low group (both P = 0.057, Fig. 2).
Fig. 2.
Combination of normal or increased blood eosinophil counts and tissue eosinophil percentages in relation to the ratio of uncontrolled (A) and partial controlled (B) to controlled subjects.
We next performed a multiple logistic regression analysis to further determine the association of systemic and local eosinophilia with treatment outcome. The T-high, B-high and BT-high patients had an increasingly higher likelihood of uncontrolled disease versus the BT-low subjects (Table 3, P for trend < 0.001). This was also true after adjustment for age, gender, smoking, prior sinus surgery, allergic rhinitis, and asthma (Table 3, P for trend < 0.001). In addition, the B-high and BT-high subjects showed a higher likelihood of partly controlled disease after the adjustment (Table 3).
Table 3.
Association between poor disease control and blood and tissue eosinophilia.
| Unadjusted odds ratio (95% CI) |
Adjusteda odds ratio (95% CI) |
|||
|---|---|---|---|---|
| Uncontrolled | Partly controlled | Uncontrolled | Partly controlled | |
| BT-low | 1 | 1 | 1 | 1 |
| T-high | 3.23(1.07–9.73)$ | 1.83 (0.65–5.22) | 3.45(1.07–11.09)$ | 2.73 (0.88–8.49) |
| B-high | 4.18(1.181–14.8)$ | 3.07 (0.97–9.71) | 4.82(1.24–18.69)$ | 4.96(1.36–18.07)$ |
| BT-high | 5.97(2.42–14.76)€ | 2.32 (0.97–5.53) | 6.17(2.25–16.93)€ | 3.95(1.45–10.79)* |
| Ptrend value | 0.000 | 0.029 | 0.000 | 0.011 |
Multiple logistic regression analyses were performed. Unadjusted and adjusted odds ratios and 95% confidence interval (CI) are presented. All strata are compared with the BT-low group.
Results in boldface indicate a P value of less than 0.05. $P < 0.05; *P < 0.01; €P < 0.001.
Results were adjusted for age, gender, smoking, prior sinus surgery, allergic rhinitis and asthma.
Effect of various blood and tissue eosinophil cutpoints on disease control
We next determined the relationship of the blood and tissue eosinophil numbers at various selected cutpoints to the rate of uncontrolled and partly controlled disease. In unadjusted analyses, high blood tissue eosinophil numbers were associated with an increased rate of uncontrolled disease, starting at 0.2 × 109/L and 5% respectively, and the association was strongest at the 0.3 × 109/L blood eosinophil cutpoint and the 10% tissue eosinophil cutpoint (Table 4). We also observed the 0.2 × 109/L and 0.3 × 109/L blood eosinophil cutpoints were associated with an increased rate of partly controlled disease.
Table 4.
Unadjusted and adjusted effects of various blood and tissue eosinophil cutpoints on disease control.
| Eosinophil cutpoints n = 183 (% of patients) |
Unadjusted odds ratio (95% CI) |
Adjusteda odds ratio (95% CI) |
||
|---|---|---|---|---|
| Blood (×109/L) | Uncontrolled | Partly controlled | Uncontrolled | Partly controlled |
| ≥0.1 (79.8%) VS. < 0.1 (20.2%) | 1.14 (0.48–2.68) | 1.56 (0.64–3.82) | 1.08 (0.44–2.66) | 1.78 (0.69–4.58) |
| ≥0.2 (55.2%) VS. < 0.2 (44.8%) | 3.48(1.65–7.32)€ | 3.20(1.55–6.61)* | 3.22(1.46–7.12)* | 3.60(1.67–7.75)€ |
| ≥0.3 (42.6%) VS. < 0.3 (57.4%) | 3.56(1.69–7.49)€ | 2.19(1.06–4.55)$ | 3.30(1.45–7.49)* | 2.64(1.20–5.84)$ |
| ≥0.4 (27.3%) VS. < 0.4 (72.7%) | 3.39(1.48–7.76)* | 2.25 (0.97–5.23) | 3.09(1.30–7.34)$ | 2.55(1.06–6.12)$ |
| ≥0.5 (18.6%) VS. < 0.5 (81.4%) | 2.96(1.14–7.69)$ | 2.25 (0.85–5.95) | 2.65 (0.97–7.26) | 2.69 (0.97–7.42) |
| Tissue (%) | ||||
| ≥5 (59.0%) VS. <5 (41.0%) | 2.12(1.02–4.41)$ | 1.67 (0.82–3.38) | 1.81 (0.83–3.92) | 1.79 (0.85–3.78) |
| ≥10 (46.4%) VS. < 10 (53.6%) | 3.51(1.67–7.35)€ | 1.70 (0.83–3.46) | 3.08(1.39–6.82)* | 1.93 (0.90–4.16) |
| ≥15 (41.0%) VS. < 15 (59.0%) | 3.33(1.59–6.98)€ | 1.53 (0.74–3.18) | 2.94(1.36–6.38)* | 1.65 (0.77–3.56) |
| ≥20 (35.5%) VS. < 20 (64.5%) | 2.79(1.32–5.90)* | 1.61 (0.84–3.42) | 2.43(1.11–5.30)$ | 1.62 (0.74–3.55) |
Results in boldface indicate a P value of less than 0.05. $P < 0.05; *P < 0.01; €P < 0.001.
CI, confidence interval; VS, versus.
Results were adjusted for age, gender, smoking, prior sinus surgery, allergic rhinitis and asthma.
In multivariate analyses, after adjusting for age, gender, smoking, prior sinus surgery, allergic rhinitis and asthma, three blood eosinophil cutpoints (0.2 × 109/L, 0.3 × 109/L and 0.4 × 109/L) and three tissue eosinophil cutpoints (10%, 15% and 20%) were associated with an increased rate of uncontrolled disease, and the strongest association was also at the 0.3 × 109/L blood eosinophil cutpoint and the 10% tissue eosinophil cutpoint (Table 4). In addition, those three blood eosinophil cutpoints were also associated with an increased rate of partly controlled disease.
Independent effect of blood and tissue eosinophil count on disease control
We further analyzed the independent effects of blood and tissue eosinophilia by a multiple logistic regression model in which both these predictors were introduced simultaneously and adjusted for age, gender, smoking, prior sinus surgery, allergic rhinitis and asthma (Table 5). Both increased blood (≥0.3 × 109/L) and tissue (≥10%) eosinophil levels related independently to having uncontrolled disease, whereas increased blood, but not tissue, eosinophil level related to having partly controlled disease (Table 5).
Table 5.
Adjusteda odds ratios (95% CIs) for having uncontrolled and partly controlled CRSwNP if having increased blood or tissue eosinophil levels as independent predictors in the same model.
| Uncontrolled | Partly controlled | |
|---|---|---|
| Blood eosinophils≥0.3 × 109/L | 2.57(1.06–6.21)$ | 2.71(1.12–6.58)$ |
| Tissue eosinophils≥10% | 2.38(1.01–5.61)$ | 1.61 (1.47–3.79) |
Results in boldface indicate a P value of less than 0.05. $P < 0.05.
CI, confidence interval.
Results were adjusted for age, gender, smoking, prior sinus surgery, allergic rhinitis and asthma.
Discussion
Polyp tissue eosinophilia is an important biologic inflammatory marker that links nasal polyps to a specific endotype and responsiveness to corticosteroid therapy.25, 26 The novel finding in the present cohort study is that, although blood and tissue eosinophil numbers were positively correlated with each other, the blood eosinophil count and the tissue eosinophil percentage were independently associated with increased risk for poor disease control in nasal polyp patients receiving current standard-of-care therapy after adjustments for covariates previously shown to relate to poor treatment outcome. Importantly, subjects with concordant blood and tissue eosinophilia had a higher risk for poor disease control than those with isolated blood or tissue eosinophilia. These results corroborate the findings of previous studies,13, 14, 27 and they suggest that measurement of eosinophilic inflammation both in blood and polyp tissues could add predictive value to a disease control assessment using the EPOS 2012 criteria.7
The present study also provides figures on the proportion of patients with nasal polyps classified based on the site of eosinophilic inflammation defined by a blood eosinophil count cutpoint of 0.3 × 109/L and a tissue eosinophil percentage cutpoint of 10%. Overall, subjects without any sign of eosinophilia (BT-low) account for about 40% of the patients while 30% were subjects with concordant blood and tissue eosinophilia. Subjects with selective tissue and blood eosinophilia accounted for 15% and 12% of the patients, respectively. Although these proportions need to be confirmed prospectively in an independent, larger patient cohort, and can be varied depending on which cutpoint was used to define eosinophilia, we believe this classification based on the site of eosinophilic inflammation is diagnostically and therapeutically useful and goes in line with the need to endotype nasal polyps as advocated by the recent high-profile papers.26, 28 The reason for why there were approximately one-third of subjects displaying discordant eosinophilic inflammation between blood and polyp tissue remains unknown. However, we could speculate it might be due to the differential expression of chemokines in polyp tissues because of different types of epithelial injury, resulting in different degree of systemic and local attraction of eosinophils.29, 30, 31
Previous studies have revealed the association between tissue eosinophilic inflammation and different clinical outcomes, such as burden of CRS symptomatology, quality of life, and polyp recurrence. Soler et al. have shown that tissue eosinophilia correlated with worse disease severity on CT, endoscopic findings, and smell identification test,32 and it was associated with less improvement in both disease-specific and general quality of life.33 Nakayama et al.34 and Lou et al.13 have presented a strong association between tissue eosinophil numbers and polyp recurrence. However, few studies have looked at the relationship between blood eosinophilic inflammation and treatment outcomes. Interestingly, we found that the combination of increased blood tissue eosinophil levels strongly increased the odds ratio for uncontrolled and partly controlled disease in our study, which, to our knowledge, has not been shown before. This finding indicates that local and systemic eosinophilic inflammation may interact during the pathogenesis of CRSwNP. Indeed, a recent study has shown that increased circulating eosinophils in CRSwNP patients were in an activation state reflected by increased oxidative metabolism, more sensitive to IL-5 and upregulation of CD49d, CCR3, and CD25, suggesting the systemic, and not just local, nature of the eosinophilic inflammation seen in CRSwNP.35
The reason for the additive effect of blood and tissue eosinophilic inflammation remains unclear. However, it is important to note that patients in the BT-high group had much higher tissue eosinophil percentages than those in the T-high group (P = 0.036). By contrast, the blood eosinophil count and percentage of patients in the BT-high group were comparable to those in the B-high group (P = 0.789 and 0.201, respectively). Beyond the categorical analysis, the extent of local eosinophilic inflammation needs be taken into consideration. This finding reinforces the role of tissue eosinophilia in the loss of disease control after endoscopic sinus surgery as previously reported by our study and other studies,8, 13 and it suggests that blood eosinophils might be important in providing a pool of cells that can be recruited to the nasal mucosa, thus serving an additive role in the pathogenesis of nasal polyps.
A notable point in the present study was the cutpoints for eosinophilia. We purposely used 0.3 × 109/L as a cutpoint for blood eosinophil counts and 10% for polyp tissue eosinophil percentages to classify the cohort subjects. The cutpoint of 10% for tissue eosinophils has been extensively used for discriminating eosinophilic CRSwNP.36, 37, 38, 39, 40 Although there is no consensus on the definition of blood eosinophilia in CRSwNP, recent reports have revealed that blood eosinophil cutpoints of 0.21 × 109/L,18 0.24 × 109/L19 and 0.3 × 109/L,41 which were closed to the cutpoint of 0.3 × 109/L used in the present study, yielded maximal sensitivity and specificity for the diagnosis of tissue eosinophilia in CRSwNP. In addition, our analysis with various cutpoints for blood eosinophil counts showed that using the higher cutpoints did not increase the maximal odds ratios for uncontrolled disease in our cohort, but the contrary was true. This suggests that 0.3 × 109/L might be a more appropriate cutoff value in studies on treatment outcome in patients with CRSwNP. Indeed, this assumption was further supported by studies with anti-IL-5 showing that a better response to anti-IL-5 or anti-IL-5 receptor α monoclonal therapies when the blood eosinophil cutoff value was 0.3 × 109/L in patients with eosinophilic severe asthma.42, 43
A strength of our study was that we strictly excluded those prescribed systemic corticosteroids within 3 months before the baseline blood routine test, which could have substantially reduced the blood eosinophil counts. There were 159 patients excluded during the study entry evaluation because of oral steroid before baseline blood routine test, including 14 patients with asthma and 3 patients with aspirin-exacerbated respiratory disease. Although there might be inhaled corticosteroid (ICS)-related reduction in blood eosinophils44, we did not exclude subjects with asthma who had ICS before blood test because their average daily ICS dose prescribed was low.
There are several limitations in this study. The major one is that the preoperative data were not collected prospectively. Moreover, the analyses were based on a single time-point measurement of blood eosinophil count per patient, which does not take into account the possible fluctuation of blood eosinophil counts over time. In addition, we had to rely on preoperative sinus CT images to assess the degree of sinonasal inflammation because we were not able to assess disease severity using a validated 22-item Sinonasal Outcome Test score from database records. We were also restricted to the available data; for example, concentration of specific IgE in serum and pack-years of smoking are not available in the databases. Finally, the patients in this study were all recruited from a tertiary academic hospital and may not be representative of other medical care settings.
In summary, we have shown that systemic and local eosinophilia are differential risk factors for poor disease control after adjusting for many demographic, comorbidities, and characteristics previously shown to relate to poor treatment outcome in a large cohort of patients with nasal polyps receiving standard-of-care therapy. Importantly, we identified that concordant blood and tissue eosinophilia has a strong and independent additive effect in predicting the risk of poor disease control after at least 1 year of endoscopic sinus surgery. These findings suggest there could be benefit in doing blood eosinophil count together with polyp tissue eosinophil number for a more comprehensive assessment of EPOS-based disease control in patients with nasal polyps.
Conflict of interest statement
All authors declare no competing financial interests.
Funding statement
This study was supported by grants from the National Natural Science Foundation of China (81873691, 81770975 and 81800884), the China Postdoctoral Science Foundation Grant (2018T110914 and 2018M633245), and the Science and Technology Program of Guangzhou, China (201803010110).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
All authors were involved in the study. Study conception and design: J.S and Y.S. Patient identification, evaluation and follow-up: K.W, J.D, Y.C, F.C, W.X.G, Y.L, J.S and Y.S. Histology analysis: K.W, Y.C and M.Y. Aacquisitions of data or analysis and interpretation of data: K.W, J.D, Y.S and J.S. Quality control of the study: Y.S and J.S. Drafting the article: Y.S and J.D. Revising the article critically for important intellectual content and final approval of the version to be published: all authors.
Acknowledgments
We are extremely grateful to the whole Rhinology team of the First Affiliated Hospital of Sun Yet-Sen University, which includes graduate students, interviewers, computer technicians, volunteers and nurses for their assistance in follow-up visits. In particular, we would like to thank Prof. Lin Xu in the School of public health, Sun Yet-Sen University, for reviewing the manuscript and statistical advice. We also thank the Pathology department, the First Affiliated Hospital of Sun Yet-Sen University for its technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2019.100052.
Contributor Information
Jianbo Shi, Email: tsjbent@163.com, shijb@mail.sysu.edu.cn.
Yueqi Sun, Email: aqi1733@163.com, sunyq7@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Figure E1. Correlation between blood and tissue eosinophil numbers.
References
- 1.Fokkens W.J., Lund V.J., Mullol J. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50(1):1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 2.Shi J.B., Fu Q.L., Zhang H. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70(5):533–539. doi: 10.1111/all.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens W.W., Schleimer R.P., Kern R.C. Chronic Rhinosinusitis with Nasal Polyps. J Allergy Clin Immunol Pract. 2016;4(4):565–572. doi: 10.1016/j.jaip.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerji A., Piccirillo J.F., Thawley S.E. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol. 2007;21(1):19–26. doi: 10.2500/ajr.2007.21.2979. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya N. Influence of polyps on outcomes after endoscopic sinus surgery. Laryngoscope. 2007;117(10):1834–1838. doi: 10.1097/MLG.0b013e3180caa19d. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya N., Villeneuve S., Joish V.N. Cost burden and resource utilization in patients with chronic rhinosinusitis and nasal polyps. Laryngoscope. 2019 Feb 5 doi: 10.1002/lary.27852. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fokkens W.J., Lund V.J., Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;23:1–298. 3 p preceding table of contents. [PubMed] [Google Scholar]
- 8.Tao X., Chen F., Sun Y. Prediction models for postoperative uncontrolled chronic rhinosinusitis in daily practice. Laryngoscope. 2018;128(12):2673–2680. doi: 10.1002/lary.27267. [DOI] [PubMed] [Google Scholar]
- 9.van der Veen J., Seys S.F., Timmermans M. Real-life study showing uncontrolled rhinosinusitis after sinus surgery in a tertiary referral centre. Allergy. 2017;72(2):282–290. doi: 10.1111/all.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao B., Liu J.X., Li Z.Y. Multidimensional endotypes of chronic rhinosinusitis and their association with treatment outcomes. Allergy. 2018;73(7):1459–1469. doi: 10.1111/all.13411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomassen P., Vandeplas G., Van Zele T. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449–1456. doi: 10.1016/j.jaci.2015.12.1324. e1444. [DOI] [PubMed] [Google Scholar]
- 12.Akdis C.A., Bachert C., Cingi C. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European academy of allergy and clinical immunology and the american academy of allergy, asthma & immunology. J Allergy Clin Immunol. 2013;131(6):1479–1490. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lou H., Meng Y., Piao Y., Wang C., Zhang L., Bachert C. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. Am J Rhinol Allergy. 2015;29(5):350–356. doi: 10.2500/ajra.2015.29.4231. [DOI] [PubMed] [Google Scholar]
- 14.Tokunaga T., Sakashita M., Haruna T. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70(8):995–1003. doi: 10.1111/all.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner J.H., Chandra R.K., Li P., Bonnet K., Schlundt D.G. Identification of clinically relevant chronic rhinosinusitis endotypes using cluster analysis of mucus cytokines. J Allergy Clin Immunol. 2018;141(5):1895–1897. doi: 10.1016/j.jaci.2018.02.002. e1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lou H., Meng Y., Piao Y. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology. 2016;54(2):150–159. doi: 10.4193/Rhino15.271. [DOI] [PubMed] [Google Scholar]
- 17.Ishitoya J., Sakuma Y., Tsukuda M. Eosinophilic chronic rhinosinusitis in Japan. Allergol Int. 2010;59(3):239–245. doi: 10.2332/allergolint.10-RAI-0231. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y., Cao P.P., Liang G.T., Cui Y.H., Liu Z. Diagnostic significance of blood eosinophil count in eosinophilic chronic rhinosinusitis with nasal polyps in Chinese adults. Laryngoscope. 2012;122(3):498–503. doi: 10.1002/lary.22507. [DOI] [PubMed] [Google Scholar]
- 19.Ho J., Hamizan A.W., Alvarado R., Rimmer J., Sewell W.A., Harvey R.J. Systemic predictors of eosinophilic chronic rhinosinusitis. Am J Rhinol Allergy. 2018;32(4):252–257. doi: 10.1177/1945892418779451. [DOI] [PubMed] [Google Scholar]
- 20.Bousquet J., Khaltaev N., Cruz A.A. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 21.Bateman E.D., Hurd S.S., Barnes P.J. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 22.Lund V.J., Kennedy D.W. Quantification for staging sinusitis. The staging and therapy group. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 23.Kaiser H.B., Naclerio R.M., Given J., Toler T.N., Ellsworth A., Philpot E.E. Fluticasone furoate nasal spray: a single treatment option for the symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;119(6):1430–1437. doi: 10.1016/j.jaci.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Kountakis S.E., Arango P., Bradley D., Wade Z.K., Borish L. Molecular and cellular staging for the severity of chronic rhinosinusitis. Laryngoscope. 2004;114(11):1895–1905. doi: 10.1097/01.mlg.0000147917.43615.c0. [DOI] [PubMed] [Google Scholar]
- 25.Wen W., Liu W., Zhang L. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012;129(6):1522–1528. doi: 10.1016/j.jaci.2012.01.079. e1525. [DOI] [PubMed] [Google Scholar]
- 26.Gurrola J., 2nd, Borish L. Chronic rhinosinusitis: Endotypes, biomarkers, and treatment response. J Allergy Clin Immunol. 2017;140(6):1499–1508. doi: 10.1016/j.jaci.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Vlaminck S., Vauterin T., Hellings P.W. The importance of local eosinophilia in the surgical outcome of chronic rhinosinusitis: a 3-year prospective observational study. Am J Rhinol Allergy. 2014;28(3):260–264. doi: 10.2500/ajra.2014.28.4024. [DOI] [PubMed] [Google Scholar]
- 28.Bachert C., Zhang L., Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: Focus on nasal polyposis. J Allergy Clin Immunol. 2015;136(6):1431–1440. doi: 10.1016/j.jaci.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Poposki J.A., Uzzaman A., Nagarkar D.R. Increased expression of the chemokine CCL23 in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128(1):73–81. doi: 10.1016/j.jaci.2011.03.017. e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson S., Poposki J.A., Nagarkar D.R. Increased expression of CC chemokine ligand 18 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;129(1):119–127. doi: 10.1016/j.jaci.2011.08.021. e111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens W.W., Ocampo C.J., Berdnikovs S. Cytokines in Chronic Rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med. 2015;192(6):682–694. doi: 10.1164/rccm.201412-2278OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soler Z.M., Sauer D.A., Mace J., Smith T.L. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009;141(4):454–461. doi: 10.1016/j.otohns.2009.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soler Z.M., Sauer D., Mace J., Smith T.L. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010;142(1):64–71. doi: 10.1016/j.otohns.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama T., Yoshikawa M., Asaka D. Mucosal eosinophilia and recurrence of nasal polyps - new classification of chronic rhinosinusitis. Rhinology. 2011;49(4):392–396. doi: 10.4193/Rhino10.261. [DOI] [PubMed] [Google Scholar]
- 35.Dupuch V., Tridon A., Ughetto S. Activation state of circulating eosinophils in nasal polyposis. Int Forum Allergy Rhinol. 2018;8(5):584–591. doi: 10.1002/alr.22079. [DOI] [PubMed] [Google Scholar]
- 36.Cao P.P., Li H.B., Wang B.F. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124(3):478–484. doi: 10.1016/j.jaci.2009.05.017. 484 e471–472. [DOI] [PubMed] [Google Scholar]
- 37.Mahdavinia M., Suh L.A., Carter R.G. Increased noneosinophilic nasal polyps in chronic rhinosinusitis in US second-generation Asians suggest genetic regulation of eosinophilia. J Allergy Clin Immunol. 2015;135(2):576–579. doi: 10.1016/j.jaci.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jankowski R., Bouchoua F., Coffinet L., Vignaud J.M. Clinical factors influencing the eosinophil infiltration of nasal polyps. Rhinology. 2002;40(4):173–178. [PubMed] [Google Scholar]
- 39.Jeong W.J., Lee C.H., Cho S.H., Rhee C.S. Eosinophilic allergic polyp: a clinically oriented concept of nasal polyp. Otolaryngol Head Neck Surg. 2011;144(2):241–246. doi: 10.1177/0194599810391738. [DOI] [PubMed] [Google Scholar]
- 40.Deng H., Sun Y., Wang W. The hippo pathway effector Yes-associated protein promotes epithelial proliferation and remodeling in chronic rhinosinusitis with nasal polyps. Allergy. 2019;74(4):731–742. doi: 10.1111/all.13647. [DOI] [PubMed] [Google Scholar]
- 41.Snidvongs K., Lam M., Sacks R. Structured histopathology profiling of chronic rhinosinusitis in routine practice. Int Forum Allergy Rhinol. 2012;2(5):376–385. doi: 10.1002/alr.21032. [DOI] [PubMed] [Google Scholar]
- 42.FitzGerald J.M., Bleecker E.R., Nair P. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 43.Ortega H.G., Liu M.C., Pavord I.D. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 44.Currie G.P., Syme-Grant N.J., McFarlane L.C., Carey F.A., Lipworth B.J. Effects of low dose fluticasone/salmeterol combination on surrogate inflammatory markers in moderate persistent asthma. Allergy. 2003;58(7):602–607. doi: 10.1034/j.1398-9995.2003.00188.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1. Correlation between blood and tissue eosinophil numbers.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.