Abstract
The main targets for neutralizing anti-hepatitis C virus (HCV) antibodies (HCV-nAbs) are the E1 and E2 envelope glycoproteins. We have studied the characteristics of HCV-nAbs through a retrospective study involving 29 HIV/HCV-coinfected patients who achieved sustained virological response (SVR) with peg-IFNα + ribavirin anti-HCV therapy. Plasma samples at baseline and week 24 after SVR were used to perform neutralization assays against five JFH1-based HCV recombinant viruses coding for E1 and E2 from genotypes 1a (H77), 1b (J4), 2a (JFH1), 3a (S52) and 4a (ED43). At baseline, the majority of plasma samples neutralized 1a, 1b, 2a, and 4a, but not 3a, genotypes. Twenty-four weeks following SVR, most neutralizing titers declined substantially. Furthermore, titers against 3a and 2a were not detected in many patients. Plasma samples with high HCV-nAb titers neutralized all genotypes, and the highest titers at the starting point correlated with the highest titers at week 24 after SVR. In conclusion, high titers of broad-spectrum HCV-nAbs were detected in HIV/HCV-coinfected individuals, however, those titers declined soon after SVR.
Subject terms: Hepatitis C, Antibodies, Hepatitis C virus
Introduction
Hepatitis C virus (HCV) infection is a serious global health problem. According to a recent WHO report, 71 million people were living with chronic HCV infection worldwide in 20151. These individuals are at risk of developing cirrhosis, end-stage liver disease, and hepatocellular carcinoma over time2,3. Despite the impressive efficacy of the direct-acting antivirals (DAAs) against HCV infection (>90% achieved sustained virological response [SVR]), there are still some problems facing the hope of controlling HCV infection with DAAs alone4. Since hepatitis C is largely asymptomatic in the initial stages, less than 20% of HCV-infected individuals worldwide are aware that they are infected and only a minority of them have access to anti-HCV treatment and thus, remain at risk of transmitting the HCV infection1. Furthermore, those that receive successful DAAs therapy are not protected against reinfection with HCV5. For these reasons, the development of an effective preventive vaccine will provide a useful, cost-effective tool at attempts to control and eradicate HCV infection6.
HCV is a positive-strand RNA virus with a nucleocapsid containing the HCV genome and an envelope crowned by the E1and E2 envelope glycoproteins, which are the main targets for neutralizing anti-HCV antibodies (HCV-nAbs)7. Several lines of evidence point to the involvement of HCV-nAbs in protecting from HCV infection: a rapid and potent HCV-nAbs response correlates with spontaneous HCV clearance8–12, and the passive administration of anti-HCV antibodies prevents HCV infection in experimental animals13–17. Therefore, studies monitoring titers and dynamics of HCV-nAbs are pertinent for designing strategies aimed to protect people from HCV infection, particularly in at-risk populations (injecting drug users [IDU] and men who have sex with men [MSM], among others).
Both primary HCV infection and HCV reinfections are common among human immunodeficiency virus (HIV)-infected individuals within the IDU and MSM groups18,19. However, little is known about the dynamics of HCV-nAbs in HIV-infected patients who eliminate HCV infection after HCV therapy.
In this study, we have analyzed the titers, breadth, and dynamic of HCV-nAbs in HIV/HCV-coinfected individuals before HCV treatment and after achieving SVR with HCV therapy.
Results
Patient characteristics at baseline
Table 1 shows the characteristics of each of the 29 HIV/HCV-coinfected patients at baseline. Overall, the median age was 49 years, 69% were male, 75.9% were IDU, and 27.6% had prior acquired immune deficiency syndrome (AIDS)-defining conditions. All patients were on combined antiretroviral therapy (cART), and all had undetectable HIV viral load (<50 copies/ml). The median count of CD4+ T-cells was 695 cells/mm3 and only eight patients had CD4+ T-cells < 500 cells/mm3. Regarding chronic hepatitis C, two patients were F0-F1 (<7.1 Kpa), five were F2 (7.1–9.5 Kpa), eight were F3 (9.5–12.5 Kpa), and 14 were F4 (≥12.5 Kpa). The median of log10 HCV-RNA was 6.62 IU/ml; 19 patients had log10 HCV-RNA values ≥ 6 IU/ml; 14 had had previous HCV therapy (median of 92 months before the baseline); and 13 patients were coinfected with HCV-GT1a, three with HCV-GT1b, eight with HCV-GT3 and five with HCV mixed infection.
Table 1.
Epidemiological and clinical characteristics at baseline of the HIV/HCV-coinfected patients included in the study.
| # | Age (years) | Gender | Previous HCV therapy | Time to baseline (months) | Nadir CD4+ T (cells/mm3) | Prior AIDS | IDU | CD4+/CD8+ | CD4+ T cells/mm3 | HIV-RNA < 50 cp/mL | HCV genotype | Log10 HCV-RNA (IU/mL) | LSM (kPa) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 29 | 45 | Male | — | — | 214 | Yes | No | 1.26 | 814 | Yes | 1a/2 | 6.39 | 6.7 |
| 37 | 55 | Male | Peg-IFNα/RBV | 106 | 41 | Yes | Yes | 2.32 | 518 | Yes | 1a | 7.10 | 9.8 |
| 38 | 42 | Male | Peg-IFNα/RBV | 14 | 24 | Yes | Yes | 1.00 | 1053 | Yes | 1a/4 | 5.73 | 17.8 |
| 48 | 49 | Female | — | — | 174 | Yes | No | 1.69 | 916 | Yes | 3 | 6.13 | 35.8 |
| 49 | 53 | Male | — | — | 60 | Yes | Yes | 0.83 | 523 | Yes | 1a | 5.87 | 10.8 |
| 95 | 48 | Female | — | — | 60 | No | Yes | 0.54 | 280 | Yes | 1a/1b | 6.04 | 20.6 |
| 96 | 53 | Male | — | — | 260 | No | Yes | 0.93 | 530 | Yes | 3 | 7.18 | 9.5 |
| 97 | 52 | Male | Peg-IFNα/RBV | 120 | 675 | No | Yes | 1.09 | 810 | Yes | 1a | 6.76 | 11.9 |
| 103 | 54 | Male | Peg-IFNα/RBV | 28 | 90 | No | No | 0.98 | 348 | Yes | 1b | 4.54 | 21.3 |
| 109 | 46 | Female | Peg-IFNα/RBV | 99 | 150 | No | Yes | 1.35 | 896 | Yes | 1a | 6.69 | 24.8 |
| 114 | 36 | Male | — | — | 314 | No | No | 0.87 | 887 | Yes | 1a | 6.49 | 8.8 |
| 115 | 52 | Male | — | — | 277 | No | No | 0.99 | 706 | Yes | 3 | 7.17 | 10.1 |
| 133 | 45 | Male | — | — | 421 | No | Yes | 1.38 | 695 | Yes | 3 | 7.00 | 4.9 |
| 139 | 57 | Male | — | — | 48 | Yes | Yes | 0.70 | 447 | Yes | 1a | 4.36 | 7.9 |
| 148 | 53 | Male | IFNα/RBV | 86 | 234 | No | Yes | 0.81 | 372 | Yes | 1a | 5.83 | 7.8 |
| 174 | 40 | Female | — | — | 122 | No | Yes | 0.84 | 662 | Yes | 4/1a | 6.46 | 13.8 |
| 213 | 50 | Male | — | — | 323 | No | Yes | 0.79 | 1210 | Yes | 3 | 6.47 | 25.4 |
| 216 | 50 | Female | — | — | 117 | No | Yes | 2.12 | 1008 | Yes | 3/4 | 6.23 | 10.4 |
| 432 | 49 | Female | — | — | 154 | Yes | Yes | 1.40 | 925 | Yes | 3 | 5.74 | 7.6 |
| 515 | 47 | Male | Peg-IFNα/RBV | 23 | 84 | No | Yes | 0.44 | 390 | Yes | 3 | 6.38 | 39.1 |
| 711 | 47 | Male | — | — | 46 | No | Yes | 2.09 | 200 | Yes | 1a | 6.00 | 12.8 |
| 718 | 44 | Female | Peg-IFNα/RBV | 47 | 196 | No | No | 0.87 | 578 | Yes | 3 | 6.11 | 13.4 |
| 719 | 46 | Male | — | — | 383 | No | Yes | 0.96 | 631 | Yes | 1a | 4.76 | 11.4 |
| 721 | 53 | Female | IFNα/RBV | 17 | 21 | No | Yes | 1.00 | 210 | Yes | 1a | 6.62 | 11.8 |
| 722 | 51 | Male | Peg-IFNα/RBV | 22 | 162 | No | No | 0.96 | 724 | Yes | 1b | 6.67 | 18.0 |
| 724 | 53 | Male | IFNα/RBV | 93 | 214 | No | Yes | 1.96 | 446 | Yes | 1a | 5.54 | 27.7 |
| 725 | 47 | Male | Peg-IFNα/RBV | 101 | 225 | No | Yes | 1.28 | 1274 | Yes | 1a | 6.46 | 14.5 |
| 726 | 43 | Female | Peg-IFNα/RBV | 67 | 303 | No | Yes | 1.26 | 762 | Yes | 1a | 5.67 | 10.2 |
| 729 | 47 | Male | IFNα/RBV | 68 | 261 | Yes | Yes | 1.33 | 1218 | Yes | 1b | 6.46 | 13.2 |
Abbreviations: AIDS, acquired immune deficiency syndrome; IDU, injecting drug user; HCV, hepatitis C virus; HCV-RNA, HCV plasma viral load; HIV, human immunodeficiency virus; HIV-RNA, HIV plasma viral load; LSM, liver stiffness measure; kPa, kilopascals; IFNα, interferon-alpha; peg-IFNα, PEGylated interferon-alpha; RBV, ribavirin.
Table 2 shows the characteristics of antiviral therapy in HIV/HCV-coinfected patients during the follow-up. All patients were treated with peg-IFNα + ribavirin (RBV), and DAAs were administered to 11 patients (Telaprevir to seven patients and Sofosbuvir to four patients). The majority of patients received anti-HCV treatment for 48 weeks. In addition, all patients were treated against HIV infection. At the end of follow-up, the median of CD4+ T-cells was 827 cells/mm3, and only seven patients had HIV viral load >50 copies/ml.
Table 2.
Characteristics of the antiviral therapy in HIV/HCV-coinfected patients included in the study during the follow-up.
| HCV therapy | Antiretroviral therapy | HIV biomarkers at the end of study | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient # | IFNα therapy | DAAs | Time on HCV therapy (wks.) | cART | Drug #1 | Drug #2 | Drug #3 | CD4+ T cells/mm3 | HIV-RNA (copies/mL) |
| 29 | Peg-IFNα/RBV | — | 48 | 2PI + II | Atazanavir/r | Raltegravir | 1177 | 224 | |
| 37 | Peg-IFNα/RBV | — | 48 | 2NRTI + NNRTI | Abacavir | Lamivudine | Etravirine | 827 | <50 |
| 38 | Peg-IFNα/RBV | Telaprevir | 48 | 2PI + II | Atazanavir/r | Raltegravir | 955 | <50 | |
| 48 | Peg-IFNα/RBV | — | 48 | NNRTI + NRTI + II | Tenofovir | Raltegravir | Etravirine | 1398 | <50 |
| 49 | Peg-IFNα/RBV | Telaprevir | 24 | 2NRTI + II | Tenofovir | Emtricitabine | Raltegravir | 609 | <50 |
| 95 | Peg-IFNα/RBV | Telaprevir | 48 | 2NRTI + NNRTI | Tenofovir | Emtricitabine | Etravirine | 260 | 78 |
| 96 | Peg-IFNα/RBV | — | 48 | 2NRTI + 2PI | Tenofovir | Emtricitabine | Atazanavir/r | 830 | 367 |
| 97 | Peg-IFNα/RBV | Telaprevir | 48 | 2NRTI + II | Tenofovir | Emtricitabine | Raltegravir | 580 | 523 |
| 103 | Peg-IFNα/RBV | Telaprevir | 48 | 2NRTI + NNRTI | Emtricitabine | Tenofovir | Etravirine | 304 | <50 |
| 109 | Peg-IFNα/RBV | Telaprevir | 48 | 2NRTI + NNRTI | Lamivudine | Abacavir | Etravirine | 1039 | <50 |
| 114 | Peg-IFNα/RBV | — | 48 | 2NRTI + NNRTI | Tenofovir | Emtricitabine | Rilpivirine | 1163 | <50 |
| 115 | Peg-IFNα/RBV | — | 48 | 2NRTI + NNRTI | Tenofovir | Emtricitabine | Efavirenz | 637 | <50 |
| 133 | Peg-IFNα/RBV | — | 48 | 2NRTI + PI | Tenofovir | Emtricitabine | Atazanavir/r | 597 | <50 |
| 139 | Peg-IFNα/RBV | Telaprevir | 48 | 2NRTI + II | Raltegravir | Lamivudine | Abacavir | 603 | <50 |
| 148 | Peg-IFNα/RBV | — | 36 | 2NRTI + II | Tenofovir | Emtricitabine | Raltegravir | 726 | <50 |
| 174 | Peg-IFNα/RBV | — | 48 | PI | Darunavir/r | 840 | <50 | ||
| 213 | Peg-IFNα/RBV | — | 48 | 2NRTI + PI | Lamivudine | Abacavir | Atazanavir/r | 1118 | <50 |
| 216 | Peg-IFNα/RBV | — | 48 | 2NRTI + NNRTI | Emtricitabine | Efavirenz | Tenofovir | 1144 | <50 |
| 432 | Peg-IFNα/RBV | Sofosbuvir | 24 | PI | Darunavir/r | 1150 | <50 | ||
| 515 | Peg-IFNα/RBV | Sofosbuvir | 24 | 2NRTI + II | Tenofovir | Emtricitabine | Raltegravir | 488 | <50 |
| 711 | Peg-IFNα/RBV | — | 48 | 2NRTI + II | Abacavir | Lamivudine | Raltegravir | 120 | <50 |
| 718 | Peg-IFNα/RBV | Sofosbuvir | 24 | PI | Darunavir/r | 790 | <50 | ||
| 719 | Peg-IFNα/RBV | Sofosbuvir | 24 | PI | Darunavir/r | 870 | <50 | ||
| 721 | Peg-IFNα/RBV | — | 36 | 2NRTI + II | Lamivudine | Abacavir | Raltegravir | 210 | 99000 |
| 722 | Peg-IFNα/RBV | — | 48 | 2NRTI + II | Tenofovir | Emtricitabine | Raltegravir | 660 | 190 |
| 724 | Peg-IFNα/RBV | — | 48 | 2NRTI + II | Raltegravir | Emtricitabine | Tenofovir | 350 | <50 |
| 725 | Peg-IFNα/RBV | — | 48 | 2NRTI + II | Tenofovir | Emtricitabine | Raltegravir | 1330 | <50 |
| 726 | Peg-IFNα/RBV | — | 48 | 2NRTI + II | Lamivudine | Abacavir | Raltegravir | 870 | <50 |
| 729 | Peg-IFNα/RBV | — | 48 | 2NRTI + II | Tenofovir | Emtricitabine | Raltegravir | 1150 | <50 |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; HIV-RNA, HIV plasma viral load; peg-IFNα, PEGylated interferon-alpha; RBV, ribavirin; DAAs, direct-acting antivirals; cART, combined antiretroviral therapy; NRTI, nucleoside analogue HIV reverse; NNRTI, non-nucleoside analogue HIV reverse transcriptase inhibitor; PI, protease inhibitor; II, integrase inhibitor.
Potency and breadth of neutralizing antibodies against HCV chimeric viruses
Plasma samples from 29 HIV/HCV-coinfected patients were screened for neutralization potency and breadth against a panel of five JFH1-based chimeric HCVs expressing E1and E2 glycoproteins from 1a (H77), 1b (J4), 2a (JFH1), 3a (S52) and 4a (ED43) genotypes (see Supplementary Fig. 1). Most plasma samples were able to neutralize 1a (H77), 1b (J4), 2a (JFH1), and 4a (ED43) viruses with variable potency, both at baseline and 24 weeks after SVR, while S52 (3a) was only minimally neutralized, in accordance with previous reports20. Furthermore, patients infected with a particular HCV genotype did not show higher neutralization titers against the chimeric virus containing glycoproteins from the same genotype compared to the other chimeric viruses carrying glycoproteins from a different genotype (see Supplementary Fig. 1). In other words, similar patterns of neutralization were observed in most of the patients, regardless of the infecting genotype. To confirm that the genetic distance between the viruses did not correlate with the neutralization pattern of the corresponding plasma samples, the E1E2 genes from eight patient-derived 1a viruses were amplified and sequenced. All eight sequences clustered with H77 (1a) and other 1a viruses (Supplementary Fig. 2). However, the corresponding plasma samples had higher HCV-nAb titers against J4 (1b) and ED43 (4a) than against H77 (1a) (Supplementary Fig. 1), confirming that there was no correlation between the genetic distance between the infecting viruses and the neutralizing capacity of the antibodies from the infected individuals.
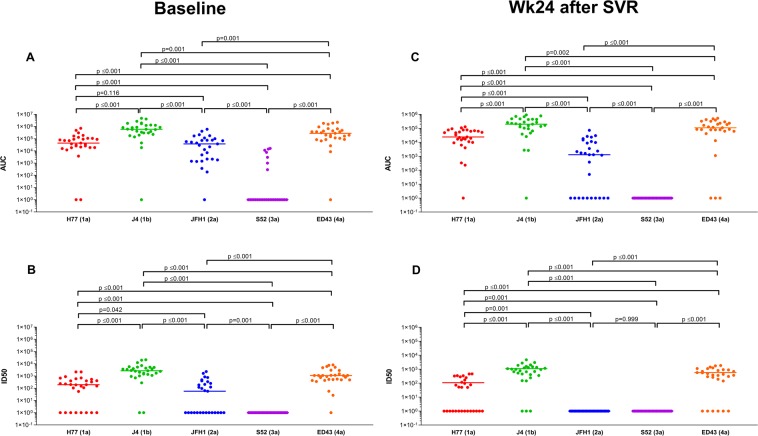
The neutralization potency against recombinant viruses was quantified by the AUC (see Supplementary Table 1) and the ID50 (see Supplementary Table 2). At baseline, median values for both AUC (Fig. 1A) and ID50 (Fig. 1B) had the following decreasing order of potency: J4 (1b) > ED43 (4a) > H77 (1a) > JFH1 (2a) > S52 (3a). At week 24 after SVR, the same decreasing order for the AUC (Fig. 1C) and the ID50 (Fig. 1D) was observed. However, it is important to note that HCV-nAbs against S52 (3a) were not detected in any of the patients at week 24 after SVR (Fig. 1C,D). Similarly, HCV-nAbs against JFH1 (2a) were also not detected in many patient samples from week 24 after SVR (Fig. 1C,D).
Figure 1.
Summary of the neutralization potency of HCV antibodies against recombinant viruses in HIV/HCV-coinfected patients, at baseline and 24 weeks after sustained virological response. Individual (circles) and median values (short horizontal bars) are represented. Abbreviations: AUC, area under the curve (arbitrary units); ID50, 50% inhibitory dose; SVR, sustained virological response; Wk, week. Statistics: p-values were calculated by the Wilcoxon test.
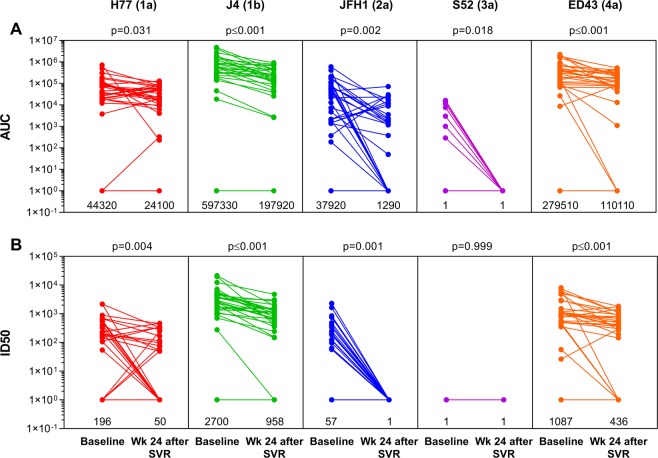
The impact of HCV clearance on the HCV-nAb titers was evaluated through an analysis of repeated measures (Fig. 2). We found significant decreases in AUC (p ≤ 0.05; Fig. 2A) and ID50 (p ≤ 0.05; Fig. 2B) values for all HCV recombinant viruses at week 24 after SVR, except for ID50 values from the S52 (3a) virus because all values were null.
Figure 2.
Impact of HCV clearance on HCV-nAb titers in HIV/HCV-coinfected patients who achieved sustained virological response. Abbreviations: AUC, area under the curve (arbitrary units); ID50, 50% inhibitory dose; SVR, sustained virological response; Wk, week. Statistics: p-values were calculated by the Wilcoxon test. Median values of AUC and ID50 are shown under each aligned dot plot.
To confirm that anti-HCV antibodies declined shortly after anti-HCV treatment, the reactivity of the 17 plasma samples positive for HCV-GT1a was tested at baseline and week 24 after SVR against the purified E2 glycoprotein from H77 (HCV-GT1a) in an enzyme-linked immunosorbent assay. Like HCV-nAbs, total antibodies against E2 were substantially reduced at week 24 after SVR (Supplementary Fig. 3).
Correlation analysis among HCV-nAb titers against chimeric viruses
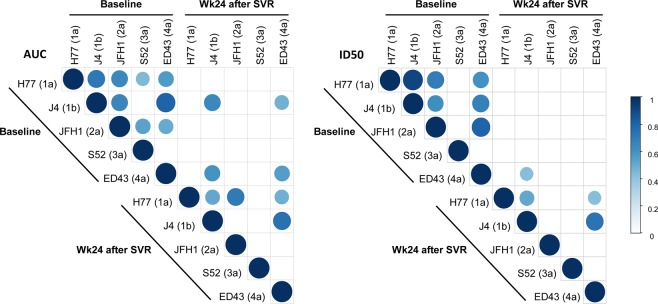
At baseline, the AUC values for a particular chimeric virus correlated positively with the corresponding values from the other chimeric viruses (Fig. 3). That is, patients with high HCV-nAb titers against a specific chimeric virus also had high titers against the other chimeric viruses (r > 0.4; p ≤ 0.05). The lowest correlations were found between S52 (3a) and J4 (1b), and between S52 (3a) and ED43 (4a) (Fig. 3). Similarly, a positive correlation was also observed among HCV-nAb titers (ID50) against H77 (1a), J4 (1B), JFH1 (2a), and ED43 (4a) (r > 0.4; p ≤ 0.05); while S52 (3a) did not show any correlation with the other chimeric viruses (Fig. 3).
Figure 3.
Summary of relevant correlation coefficients (r > 0.4; p < 0.05) among HCV-nAb titers against recombinant viruses in HIV/HCV-coinfected patients at baseline and 24 weeks after sustained virological response. The correlation coefficients are denoted by the intensity of the color and the size of the circle. Abbreviations: AUC, area under the curve (arbitrary units); ID50, 50% inhibitory dose; SVR, sustained virological response; Wk, week. Statistics: Correlation coefficients and p-values were calculated by the test of Spearman’s correlation coefficient.
At week 24 after SVR, HCV-nAb titers (AUC) against J4 (1b) and ED43 (4a) correlated positively with baseline values for J4 (1b) and ED43 (4a) (r > 0.4; p ≤ 0.05), indistinctly (Fig. 3). This means that patients with the highest HCV-nAb titers at the end of follow-up were those who also had the highest HCV-nAb titers at baseline. Furthermore, at week 24 after SVR, HCV-nAb titers against H77 (1a) correlated with HCV-nAb titers against J4 (1b), JFH1 (2a), and ED43 (4a); and HCV-nAb titers against J4 (1b) also correlated with HCV-nAb titers against ED43 (4a) (Fig. 3). When we analyzed the correlations among ID50 values, a similar pattern as that observed for the AUC values was found, but the number of relevant correlations was lower (Fig. 3).
Correlation among clinical data and HCV-nAb titers
At baseline, we found a significant positive relationship between the CD4/CD8 ratio and the HCV-nAb titers against H77 (1a) (AUC [r = 0.454; p = 0.013] and ID50 [r = 0.450; p = 0.014]). However, we did not find any correlation between the CD4/CD8 ratio and the HCV-nAb titers at the end of the study (see Supplementary Table 3). We also looked for correlations with other clinical variables (age, nadir CD4+ T cells/mm3, CD4+ T cells/mm3, log10 HCV-RNA, and LSM), but no significant association was found (data not shown).
Discussion
HCV co-infection has a high impact on the morbidity and mortality among HIV infected patients as a result of a more aggressive progression of liver fibrosis and cirrhosis21–24. In this population, successful anti-HCV treatment with peg-IFN and ribavirin had a positive impact on these conditions25–27, and treatment with DAAs improved patient-reported outcomes28–30. However, other relevant outcomes associated with HCV elimination in HIV/HCV-coinfected patients remain to be investigated.
Since HCV-nAbs correlate with protection and virus clearance31, it is important to know how treatment-mediated HCV elimination may influence HCV-nAb titers after therapy. In the present study, we found several things to be true. First, most of the HIV/HCV-coinfected patients had high titers of broad HCV-nAbs at baseline, but these titers declined significantly 24 weeks after SVR, particularly for JFH1 (2a) and S52 (3a) viruses, against which HCV-nAbs were generally not detected at that time. Second, patients with high HCV-nAb titers against a particular genotype also had high titers against the other genotypes and retained moderately high HCV-nAb titers at the end of follow-up. HCV-nAb titers against S52 (3a) showed the worst correlation with HCV-nAb titers against the other genotypes. Third, the CD4/CD8 ratio correlated positively with HCV-nAb titers against H77 (1a). Overall, by measuring potency and breadth of HCV-nAbs, our study provides functionally relevant results, as these antibodies are correlated with HCV protection13–17 and HCV clearance8–12. To our knowledge, this is the first study that analyzes the evolution of HCV-nAbs in a cohort of HIV/HCV-coinfected patients who eliminated HCV infection after HCV therapy.
The high degree of genetic diversity of HCV is a major impediment to vaccine development7. This genetic heterogeneity is particularly relevant in the envelope glycoproteins (E1 and E2), where amino acid sequences vary up to 30% between HCV genotypes, 20% within genotypes, and 10% within subtypes31. Defining the neutralizing breadth of cross-reactive anti-HCV antibodies is critical to understanding the protective capacity against different HCV genotypes. In this study, most of the patients showed high levels of broad-spectrum HCV-nAbs at baseline. Overall, the HCV-nAb titers (both AUC and ID50) were as follows: J4 (1b) > ED43 (4a) > H77 (1a) > JFH1 (2a) > S52 (3a). This response pattern has been previously described in patients monoinfected by HCV, possibly due to strain-specific, rather than genotype-specific effects on the induction of HCV-nAbs31. Thus, the neutralizing activity of a patient’s plasma was not dictated primarily by his infecting HCV genotype, since, for example, plasma from patients infected with HCV genotype 1a or 1b neutralized HCV chimeric virus for genotype 4 quite efficiently. However, it should be noted that chimeric viruses from HCV genotypes 2a and 3a were neutralized with less efficiency, regardless of the HCV genotype of the patient. Although a correlation between the genetic distance of the infecting viruses and the neutralizing capacity of the anti-HCV antibodies from the infected patients was not found, an important point to consider is that more than one infecting genotype has been detected in five individuals. This strongly suggests that different HCV genotypes might have infected most patients along the course of their chronic infections. This probably has contributed to the development of broad-spectrum HCV-nAbs.
Another relevant finding of our work was a significant decrease in HCV-nAb titers at week 24 after SVR, suggesting that continuous exposition to HCV antigens is necessary to maintain high HCV-nAb titers. In our study, the number of patients with very low HCV-nAb titers increased at the end of follow-up, particularly for JFH1 (2a) and S52 (3a) viruses. However, although the drop in HCV-nAb titers was more pronounced in the case of H77 (1a), J4 (1b), and ED43 (4a), titers against these viruses remained relatively high at week 24 after SVR. This was because titers against these viruses were usually higher than against the other viruses at baseline. There is not much information on the dynamics of antibodies against HCV in patients who eliminated HCV infection. Wiegand et al. found a rapid decrease of anti-HCV antibodies in patients with acute hepatitis C who were treated with IFN-α-2b and eliminated the HCV infection32. However, patients chronically infected with HCV who eliminated HCV infection after IFN therapy had a slow decrease, which may be prolonged for several years32–38. A rapid decrease of anti-HCV antibodies was also reported in HIV/HCV-coinfected individuals following peg-IFN-α/ribavirin treatment during acute, but not chronic, HCV infection39,40. In contrast, our study shows a quick drop in HCV-nAbs following peg-IFN-α/ribavirin treatment of chronic HCV-infected patients coinfected with HIV. Note that an important difference between our study and previous reports is that all these preceding studies evaluated total antibodies against HCV by commercial semiquantitative immunoassays. In contrast, we evaluated HCV-nAbs or anti-E2 antibodies by quantitative methods in which serial dilutions of plasma samples were tested. Titers and breadth of HCV-nAbs might more accurately reflect the efficacy of the humoral immune response and the capacity of the patient to be protected from HCV reinfections. Thus, the decline of HCV-nAbs observed in our study after anti-HCV therapy in HIV/HCV-coinfected individuals may be one of the reasons for the very high HCV reinfection rates observed in this population, highlighting the impact of being HIV positive on the probability of HCV reinfection41.
HIV infection leads to the destruction and functional impairment of CD4+ T-cells, which may lead to dysfunction of the HCV-specific humoral immune response42. The CD4/CD8 ratio describes the overall immune dysfunction in HIV-infected patients since a low or inverted CD4/CD8 ratio is associated with altered immune function, chronic inflammation, and immune senescence43,44. In our study, a positive correlation between the CD4/CD8 ratio and the HCV-nAb titers was observed for H77 (1a) at baseline, but not at the end of follow-up. In agreement with this, a CD4+ T-cell-dependent reduction of HCV-nAbs has been reported in patients previously infected with HCV following incident HIV infection45. Additionally, Lee et al. found that HCV-nAbs against JFH-1 correlated positively with baseline CD4+ T-cell counts and increased after cART in HIV/HCV-coinfected patients, regardless of the baseline CD4+ T-cell counts46. In addition to the T-cell, HIV infection induces dysregulation of B cells and damage of lymphoid organs, which endangers the humoral immune response47–50. Loss of memory B cells in HIV-infected patients has been amply documented51–53, and it has been associated with impaired immunization responses and reduced long-term serologic immunity to measles and Streptococcus pneumoniae54,55. Importantly, antiretroviral therapy did not restore serologic memory in primary or in chronic infection55. These results are in line with our observation that HCV-nAbs decrease short after HCV therapy in HIV-infected patients undergoing ART, in contrast to what has been observed in patients infected only with HCV32–38. Immunoglobulin class switching also seems to be impaired in HIV infections56,57, which might result in the production of antibodies with reduced affinity maturation and limited neutralizing breadth58,59. Conversely, in the present study, a broadly neutralizing antibody response against HCV has been observed in HIV/HCV-coinfected patients. However, additional neutralization experiments with a larger panel of HCVs and with plasma samples from HCV-monoinfected patients are necessary to clarify this point.
Limitations of the study
Firstly, this study has a retrospective design, data were collected retrospectively, and patients included in this study met a set of criteria for starting HCV treatment (see Patients and Methods), which may have introduced a selection bias. Secondly, this study has a limited number of patients, although the design with repeated measures considerably improves the statistical power of the study. Thirdly, a group of HCV-monoinfected patients was not used as a comparator to HIV/HCV-coinfected patients. Although it was not possible to include this group in our study, we would like to emphasize that several studies have reported a slow decrease of anti-HCV antibody titers following the anti-HCV treatment that may take several years32–38. However, these studies used semiquantitative assays and did not measure neutralizing antibodies, as it has been done in the present work. Fourthly, since peg-IFN-α + ribavirin therapy may have immunomodulatory effects affecting HCV-nAb titers, further studies using DAAs alone are needed. Fifthly, we did not evaluate the level of protection offered by the neutralizing antibodies measured in this study. This is an important issue, and the answer to this question will be highly relevant for the development of an antibody-based vaccine against HCV and to understand viral reinfections. Sixthly, although it has been shown that antibody titers decline after SVR, no information on memory B cell population nor residual liver immunity has been analyzed. Finally, we have used a reduced panel of recombinant HCVs for neutralization tests. The use of a larger and diverse panel of viruses could have added additional information on some aspects of the results.
Conclusions
In conclusion, high titers of broad-spectrum HCV-nAbs were observed in HIV/HCV-coinfected individuals. These titers, however, declined soon after SVR. Our results suggest that those individuals may not be able to maintain protecting anti-HCV antibodies after elimination of HCV by treatment. This should be taken into account in the development of potential anti-HCV vaccines.
Patients and Methods
Study subjects
We performed a retrospective study (before-and-after design) in 29 HIV/HCV-coinfected patients from the cohort of “Grupo de Estudio del SIDA” (GESIDA 3603b study, Spain; see Appendix), which has been previously described60. All patients were treated with pegylated interferon-alpha plus ribavirin (peg-IFNα + ribavirin) or peg-IFN-α/ribavirin/DAAs between February 2012 and February 2014 and had an SVR assessment at 24 weeks after the end of HCV treatment.
Clinical, epidemiological, and virological characteristics of each patient were collected prospectively60. This study was performed according to the Declaration of Helsinki and was approved by the Research Ethics Committee of the Instituto de Salud Carlos III (CEI PI 23_2011) and participating centers. The participants gave their informed consent before enrollment.
We used plasma samples from baseline (between one week before starting the anti-HCV treatment and the same day at which the treatment was initiated) and the end of follow-up (week 24 after SVR, which corresponds to week 48 after the end of HCV treatment) for each patient.
HCV genotyping
All samples were processed using the Abbott-Real Time-HCV genotype II (Abbott Laboratories, Illinois, USA) according to manufacturer instructions. First, viral RNA was extracted from 200µl of plasma sample using the QIAsymphony instrument, the commercial mini kit DSP Virus/Pathogen, and the protocol Cellfree 200 v.7. (Qiagen, Hilden, Germany). Internal, positive and negative controls from Abbott-RT-HCV II kit were incorporated into the extraction process. Then, multiplexed RT-PCR reactions were performed using the kit amplification reagent packs and the Abbott m2000rt instrument.
E1 and E2 sequencing and phylogenetic analysis
The viral RNA extracted for HCV genotyping was used to amplify the E1E2 from eight 1a patient-derived viruses. A nested RT-PCR was carried out using the Qiagen OneStep RT-PCR Kit (Qiagen, Hilden, Germany) following manufacturer’s instructions with the following pairs of primers (numbering reference to GenBank accession AF011751)61: 5′GGACGGGGTAAACTATGCAACAGG3′ (nucleotide position 818–841), 5′GGGATGCTGCATTGAGTA3′ (nucleotide position 2599–2616), 5′CACCATGGGTTGCTCTTTTTCTATC3′ (nucleotide position 843–869) and 5′TTACGCCTCCGCTTGGGATATGAGTAACATCAT3′ (nucleotide position 2550–2582). The PCR products were sequenced by the Sanger method with the previous inner forward and reverse primers and two additional primers: 5′AGSGTAYTWYTCCATGG3′ (nucleotide position 1418–1434) and 5′CARCCRAACCARTTGCCC3′ (nucleotide position 1996–1979).
Phylogenetic analyses were performed using a total of 34 E1/E2 sequences corresponding to different HCV 1 obtained from GenBank public database (KP098533.1, AF009606, M62321, M67463, HQ850279, EF407457, D90208, M58335, EU781827, EU781828, D14853, AY051292, AY651061, KJ439768, KC248194, AM910652, KC248198, KC248199, KJ439772, KJ439773, KC248193, KC248196, KJ439778, KJ439782, KJ439775, KJ439781, KJ439774, HQ537007, AJ851228, KJ439780, KJ439779, KJ439776, KJ439777, NC 004102.1) and 8 E1/E2 sequences from HCV 1a infected patients. The evolutionary history was inferred using the Neighbor-Joining method62, a bootstrap test with 1000 replicates63 and the Maximum Composite Likelihood method64. Evolutionary analyses were conducted in MEGA765. Moreover, the same analyses for these infected patients were performed with E1/E2 sequences from chimeric HCV (AB047639.1, EU204645.1, EU363760.1, EU363761.1, FJ230881.1) and a reference HCV 1a (isolate H77) (NC 004102.1).
Cells and viruses
Human hepatoma Huh7.5 cells were obtained from Apath LLC (760 Parkside Ave. Brooklyn, NY 11226, USA) and Huh7.5.1 clone 2 from F Chisari (The Scripps Research Institute, La Jolla, USA). Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 4 mM L-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin (DMEM10) at 37 °C in a 5% CO2 atmosphere. Plasmids encoding JFH1-based chimeric HCV genotypes 1a (H77/JFH1)66, 1b (J4/JFH1)67, 3a (S52/JFH1)68, and 4a (ED43/JFH1)66 were gifts from Jens Bukh (University of Copenhagen, Copenhagen, Denmark). The plasmid encoding HCV JFH1 (genotype 2a)69 was obtained from Apath LLC. Plasmids were linearized with Xba I, transcribed in vitro with the MEGAscript T7 kit (Invitrogen, Thermo Fisher, Rockford, IL, USA), and viruses were produced by electroporation of the resulting genomic RNA into Huh7.5.1 clone 2 cells.
HCV neutralization assay
A total of 12,000 Huh7.5 cells per well were plated in flat-bottom 96-well tissue culture plates and incubated overnight at 37 °C. The following day, 150 focus-forming units of chimeric HCVs were mixed with an equal volume of 1:2 serial dilutions (starting at a 1:50 dilution) of the plasma sample or a control plasma from a healthy donor in DMEM10, incubated at 37 °C for 1 hour and then added to cells. After three days of infection, the cells were fixed with cold methanol for 10 minutes, followed by a one-hour incubation with the anti-NS5A 9E10 antibody (a gift from Charles Rice, The Rockefeller University, New York, USA). Foci were visualized after one-hour incubation with an anti-mouse IgG horseradish peroxidase-linked whole secondary antibody (Abcam, Cambridge, UK) and 3-amino-9-ethylcarbazole (Sigma, St. Louis, MO, USA) and counted under a light microscope. Percentage of neutralization at each antibody dilution was calculated as [1- (foci in the presence of plasmatest/foci in the presence of plasmacontrol)] × 100%. With these data, titration curves for each type of virus were made using GraphPad Prism 7.00 for Windows (GraphPad Software, La Jolla, California USA). The 50% inhibitory dose (ID50) was calculated as the dilution of plasma that resulted in a 50% reduction in the number of foci. The area under the curve (AUC) was also calculated to quantify neutralization potency70 by using GraphPad Prims 7.00 and the following parameters: Baseline Y = 0; ignore peaks that are less than 10% of the distance from minimum to maximum Y; all peaks must go above the baseline. The AUC is expressed as X units times the Y units.
Production of HCV-E2 (H77) and quantitative enzyme-linked immunoassay
The ectodomain of E2 glycoprotein from genotype 1a (H77) was produced using the baculovirus/insect cell system according to a previously described procedure71 with slight modifications. Briefly, the DNA encoding the ectodomain of E2 (H77) protein, residues 384–661 (E2661) with the addition at the 5′ end of a six-histidine tag coding sequence, was inserted into the baculovirus transfer vector pAcGP67A (Pharmingen, San Diego, CA, USA) to create the recombinant plasmid pAcGP67A-E2661-H77. Insect Spodoptera frugiperda (Sf9) cells were cotransfected with flashBAC GOLDTM DNA (Oxford Expression Technologies, Oxford, UK) and the recombinant transfer vector pAcGP67A–E2661-H77 as indicated by the manufacturer. The protein was expressed by infecting High Five cells in Insect X-Press serum-free media with high titter virus (>108 pfu/ml) at MOI of 5–10. Medium with the secreted recombinant glycoprotein was collected approximately 120 h postinfection, dialyzed against 50 mM Tris-HCl pH 8.0, 50 mM NaCl, and loaded onto a Ni2+ -nitrilotriacetic acid-agarose column (Qiagen, Hilden, Germany). The recombinant E2661 protein was eluted with 200 mM imidazole in dialysis buffer.
Ninety-six-well microtiter plates were coated with 500 ng Galanthus nivalis lectin (GNA, Sigma, St. Louis, MO, USA) at 4 °C overnight, followed by blocking with 2% porcine serum in PBS. Then 40 ng of purified E2 were added to the wells for 2 h at room temperature, followed by incubation with plasma sample dilutions in blocking solution (dilution factor 2, initial dilution 1:50), anti-human IgG-Peroxidase (GE, Healthcare, Buckinghamshire, UK) and substrate (OPD, Sigma, St. Louis, MO, USA). Extensive washing with water was done after each step. Optical density was read at 492 nm.
Statistical analysis
The statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) 23.0 software (IBM Corp., Chicago, USA). Statistical significance was defined as p < 0.05. All p-values were two-tailed. The Wilcoxon test was used to analyze the paired samples within each group. The correlation was analyzed using the test of Spearman’s correlation coefficient.
Supplementary information
Acknowledgements
We want to acknowledge the patients participating in this study particularly, and to the Spanish HIV HGM BioBank integrated into the Spanish AIDS Research Network (RIS) and collaborating Centers for the generous gifts of clinical samples used in this work. The HIV BioBank, integrated in the Spanish AIDS Research Network, is supported by the Institute of Health Carlos III, ISCIII, Spanish Health Ministry (Grant nº RD06/0006/0035 and RD12/0017/0037) as part of the State Plan for Scientific and Technical Research and Innovation and co-financed by ISCIII- Sub-Directorate General for Research Assessment and Promotion and European Regional Development Fund (ERDF) and Foundation for Research and Prevention of AIDS in Spain (FIPSE). This study would not have been possible without the collaboration of all the patients, medical and nursing staff, and data managers who have taken part in the project (See Appendix, which show all collaborators). The RIS Cohort (CoRIS) is funded by the ISCIII through the Spanish AIDS Research Network (RIS C03/173 and RD12/0017/0018) as part of the State Plan for Scientific and Technical Research and Innovation and co-financed by ISCIII- Sub-Directorate General for Research Assessment and Promotion and European Regional Development Fund (ERDF). This study was supported by grants from Instituto de Salud Carlos III (ISCIII; grant numbers PI14/01094 and PI17/00657 to JB, PI17/00903 to JGG, PI14CIII/00011 and PI17CIII/00003 to SR) and Ministerio de Sanidad, Servicios Sociales e Igualdad (grant number EC11-241). The study was also funded by the RD16CIII/0002/0002, RD16/0025/0018, and RD16/0025/0017 projects as part of the Plan Nacional R + D + I and co-funded by ISCIII- Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER). JB is an investigator from the Programa de Intensificación de la Actividad Investigadora en el Sistema Nacional de Salud (I3SNS), Refs INT15/00079 and INT16/00100. Part of this work has been presented in the international Liver Congress 2019: Vigón L, Vázquez-Morón S, Berenguer J, Gonzalez-García J, Jiménez-Sousa MA, Guardiola JM, Crespo M, De los Santos I, Von Wichmann MA, Carrero A, Resino S, Martínez I. Rapid decrease in titer and breath of neutralizing anti-HCV antibodies in HIV/HCV-coinfected patients who achieved sustained virological response. The International Liver Congress 2019 - EASL (ILC 2019- EASL). Vienna, Austria. April 9–14, 2019.
Author Contributions
Conceptualization: S.R. and I.M. Data curation: J.B., J.G.G., J.M.G., M.C., I.L.S., M.A.V.W. and A.C. Formal analysis: L.V., S.R. and I.M. Funding acquisition: J.B., J.G.G. and S.R. Investigation and methodology: L.V., M.B.Y., J.G., S.V. and I.M. Project Administration: J.B. Supervision and visualization: S.R. and I.M. Writing – original draft preparation: L.V., S.V., S.R. and I.M. Writing – Review & Editing: M.A.J.S.
Data Availability
All relevant data are within the paper and Supporting Information files. For additional information, interested readers can contact Dr. Isidoro Martinez at imago@isciii.es.
Competing Interests
The authors declare no competing interests.
Footnotes
A comprehensive list of consortium members appears at the end of the paper.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Salvador Resino and Isidoro Martínez contributed equally.
Contributor Information
Salvador Resino, Email: sresino@isciii.es.
Isidoro Martínez, Email: imago@isciii.es.
The GESIDA 3603b Cohort Study Group:
P. Miralles, J. C. López, F. Parras, B. Padilla, T. Aldamiz-Echevarría, F. Tejerina, C. Díez, L. Pérez-Latorre, C. Fanciulli, I. Gutiérrez, M. Ramírez, S. Carretero, J. M. Bellón, J. Bermejo, V. Hontañón, J. R. Arribas, M. L. Montes, I. Bernardino, J. F. Pascual, F. Zamora, J. M. Peña, F. Arnalich, M. Díaz, P. Domingo, J. Sanz, M. J. Bustinduy, J. A. Iribarren, F. Rodríguez-Arrondo, E Van den Eynde, M. Pérez, E. Ribera, J. L. Casado, F. Dronda, A. Moreno, M. J. Pérez-Elías, M. A. Sanfrutos, S. Moreno, C. Quereda, A. Arranz, E. Casas, J. de Miguel, S. Schroeder, J. Vergas, M. J. Téllez, D. Vinuesa, L. Muñoz, J. Hernández-Quero, A. Ferrer, M. J. Galindo, L. Ortiz, E. Ortega, M. Montero, M. Blanes, S. Cuellar, J. Lacruz, M. Salavert, J. López-Aldeguer, G. Pérez, G. Gaspar, M. Yllescas, P. Crespo, E. Aznar, and H. Esteban
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-48592-5.
References
- 1.WHO. Global Hepatitis Report. World Health Organization (2017).
- 2.Lagging LM, et al. Progression of fibrosis in untreated patients with hepatitis C virus infection. Liver. 2002;22:136–144. doi: 10.1034/j.1600-0676.2002.01623.x. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 4.Cox AL. MEDICINE. Global control of hepatitis C virus. Science. 2015;349:790–791. doi: 10.1126/science.aad1302. [DOI] [PubMed] [Google Scholar]
- 5.Sulkowski MS, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. The New England journal of medicine. 2014;370:211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed KT, Almashhrawi AA, Ibdah JA, Tahan V. Is the 25-year hepatitis C marathon coming to an end to declare victory? World journal of hepatology. 2017;9:921–929. doi: 10.4254/wjh.v9.i21.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzarum N, Wilson IA, Law M. The Neutralizing Face of Hepatitis C Virus E2 Envelope Glycoprotein. Frontiers in immunology. 2018;9:1315. doi: 10.3389/fimmu.2018.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136:2377–2386. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavillette D, et al. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J Virol. 2005;79:6023–6034. doi: 10.1128/JVI.79.10.6023-6034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osburn WO, et al. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology. 2014;59:2140–2151. doi: 10.1002/hep.27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pestka JM, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci USA. 2007;104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito I, et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukh J, et al. Immunoglobulin with High-Titer In Vitro Cross-Neutralizing Hepatitis C Virus Antibodies Passively Protects Chimpanzees from Homologous, but Not Heterologous, Challenge. J Virol. 2015;89:9128–9132. doi: 10.1128/JVI.01194-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorner M, et al. A genetically humanized mouse model for hepatitis C virus infection. Nature. 2011;474:208–211. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farci P, et al. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci USA. 1996;93:15394–15399. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law M, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 17.Morin TJ, et al. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS Pathog. 2012;8:e1002895. doi: 10.1371/journal.ppat.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradat P, et al. Incidence of new hepatitis C virus infection is still increasing in French MSM living with. HIV. AIDS. 2018;32:1077–1082. doi: 10.1097/QAD.0000000000001789. [DOI] [PubMed] [Google Scholar]
- 19.Virlogeux V, et al. Modeling HIV-HCV coinfection epidemiology in the direct-acting antiviral era: the road to elimination. BMC Med. 2017;15:217. doi: 10.1186/s12916-017-0979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey, J. R. et al. Broadly neutralizing antibodies with few somatic mutations and hepatitis C virus clearance. JCI Insight2, 10.1172/jci.insight.92872 (2017). [DOI] [PMC free article] [PubMed]
- 21.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-Infected Person. Annals of internal medicine. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- 22.Benhamou Y, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 23.Graham CS, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 24.Koziel MJ, Peters MG. Viral hepatitis in HIV infection. The New England journal of medicine. 2007;356:1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berenguer J, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–413. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 26.Limketkai BN, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308:370–378. doi: 10.1001/jama.2012.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mira JA, et al. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56:1646–1653. doi: 10.1093/cid/cit103. [DOI] [PubMed] [Google Scholar]
- 28.Younossi ZM, et al. Patient-reported outcomes in patients co-infected with hepatitis C virus and human immunodeficiency virus treated with sofosbuvir and velpatasvir: The ASTRAL-5 study. Liver international: official journal of the International Association for the Study of the Liver. 2017;37:1796–1804. doi: 10.1111/liv.13462. [DOI] [PubMed] [Google Scholar]
- 29.Younossi ZM, et al. Sofosbuvir and ledipasvir improve patient-reported outcomes in patients co-infected with hepatitis C and human immunodeficiency virus. Journal of viral hepatitis. 2016;23:857–865. doi: 10.1111/jvh.12554. [DOI] [PubMed] [Google Scholar]
- 30.Marcellin F, et al. Patient-reported outcomes with direct-acting antivirals for the treatment of chronic hepatitis C: current knowledge and outstanding issues. Expert review of gastroenterology & hepatology. 2017;11:259–268. doi: 10.1080/17474124.2017.1285227. [DOI] [PubMed] [Google Scholar]
- 31.Kinchen VJ, Bailey JR. Defining Breadth of Hepatitis C Virus Neutralization. Frontiers in immunology. 2018;9:1703. doi: 10.3389/fimmu.2018.01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiegand J, et al. Long-term follow-up after successful interferon therapy of acute hepatitis C. Hepatology. 2004;40:98–107. doi: 10.1002/hep.20291. [DOI] [PubMed] [Google Scholar]
- 33.Kee KM, et al. Decreased anti-hepatitis C virus titer and associated factors in chronic hepatitis C patients after sustained virological response: a prospective study. J Gastroenterol Hepatol. 2012;27:1106–1111. doi: 10.1111/j.1440-1746.2011.06946.x. [DOI] [PubMed] [Google Scholar]
- 34.Lefrere JJ, et al. Full or partial seroreversion in patients infected by hepatitis C virus. The Journal of infectious diseases. 1997;175:316–322. doi: 10.1093/infdis/175.2.316. [DOI] [PubMed] [Google Scholar]
- 35.Toyoda H, et al. Changes in hepatitis C virus (HCV) antibody status in patients with chronic hepatitis C after eradication of HCV infection by interferon therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2005;40:e49–54. doi: 10.1086/428128. [DOI] [PubMed] [Google Scholar]
- 36.Takaki A, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 37.Kondili LA, et al. Infection rate and spontaneous seroreversion of anti-hepatitis C virus during the natural course of hepatitis C virus infection in the general population. Gut. 2002;50:693–696. doi: 10.1136/gut.50.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanotte P, et al. The kinetics of antibodies against hepatitis C virus may predict viral clearance in exposed hemophiliacs. The Journal of infectious diseases. 1998;178:556–559. doi: 10.1086/517473. [DOI] [PubMed] [Google Scholar]
- 39.Aebi-Popp K, et al. Rapid decline of anti-hepatitis C virus (HCV) antibodies following early treatment of incident HCV infections in HIV-infected men who have sex with men. HIV medicine. 2018;19:420–425. doi: 10.1111/hiv.12602. [DOI] [PubMed] [Google Scholar]
- 40.Vanhommerig JW, et al. Hepatitis C virus (HCV) antibody dynamics following acute HCV infection and reinfection among HIV-infected men who have sex with men. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;59:1678–1685. doi: 10.1093/cid/ciu695. [DOI] [PubMed] [Google Scholar]
- 41.Ingiliz P, et al. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. Journal of hepatology. 2017;66:282–287. doi: 10.1016/j.jhep.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Alimonti JB, Ball TB, Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol. 2003;84:1649–1661. doi: 10.1099/vir.0.19110-0. [DOI] [PubMed] [Google Scholar]
- 43.McBride JA, Striker R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017;13:e1006624. doi: 10.1371/journal.ppat.1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruno G, Saracino A, Monno L, Angarano G. The Revival of an “Old” Marker: CD4/CD8 Ratio. AIDS Rev. 2017;19:81–88. [PubMed] [Google Scholar]
- 45.Bailey JR, et al. CD4+ T-Cell-Dependent Reduction in Hepatitis C Virus-Specific Neutralizing Antibody Responses After Coinfection With Human Immunodeficiency Virus. The Journal of infectious diseases. 2015;212:914–923. doi: 10.1093/infdis/jiv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S, et al. The dynamics of HCV-specific antibody responses in HIV/HCV patients on long-term antiretroviral therapy. Clin Immunol. 2017;179:54–63. doi: 10.1016/j.clim.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Moir S, Fauci AS. B-cell responses to HIV infection. Immunol Rev. 2017;275:33–48. doi: 10.1111/imr.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amu S, Ruffin N, Rethi B, Chiodi F. Impairment of B-cell functions during HIV-1 infection. AIDS. 2013;27:2323–2334. doi: 10.1097/QAD.0b013e328361a427. [DOI] [PubMed] [Google Scholar]
- 49.Cagigi A, Nilsson A, De Milito A, Chiodi F. B cell immunopathology during HIV-1 infection: lessons to learn for HIV-1 vaccine design. Vaccine. 2008;26:3016–3025. doi: 10.1016/j.vaccine.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 50.Carrillo J, et al. Memory B cell dysregulation in HIV-1-infected individuals. AIDS. 2018;32:149–160. doi: 10.1097/QAD.0000000000001686. [DOI] [PubMed] [Google Scholar]
- 51.De Milito A, Morch C, Sonnerborg A, Chiodi F. Loss of memory (CD27) B lymphocytes in HIV-1 infection. AIDS. 2001;15:957–964. doi: 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- 52.Moir S, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrow M, Valentin A, Little R, Yarchoan R, Pavlakis GN. A splenic marginal zone-like peripheral blood CD27+B220- B cell population is preferentially depleted in HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2008;24:621–633. doi: 10.1089/aid.2007.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hart M, et al. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol. 2007;178:8212–8220. doi: 10.4049/jimmunol.178.12.8212. [DOI] [PubMed] [Google Scholar]
- 55.Titanji K, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. [DOI] [PubMed] [Google Scholar]
- 56.Qiao X, et al. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol. 2006;7:302–310. doi: 10.1038/ni1302. [DOI] [PubMed] [Google Scholar]
- 57.Xu W, et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol. 2009;10:1008–1017. doi: 10.1038/ni.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.West AP, Jr., et al. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fecteau JF, Cote G, Neron S. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol. 2006;177:3728–3736. doi: 10.4049/jimmunol.177.6.3728. [DOI] [PubMed] [Google Scholar]
- 60.Medrano LM, et al. Elevated liver stiffness is linked to increased biomarkers of inflammation and immune activation in HIV/hepatitis C virus-coinfected patients. AIDS. 2018;32:1095–1105. doi: 10.1097/QAD.0000000000001787. [DOI] [PubMed] [Google Scholar]
- 61.Urbanowicz RA, Ball JK, Tarr AW. Cloning and Analysis of Authentic Patient-Derived HCV E1/E2 Glycoproteins. Methods Mol Biol. 2019;1911:275–294. doi: 10.1007/978-1-4939-8976-8_19. [DOI] [PubMed] [Google Scholar]
- 62.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 63.Felsenstein J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 64.Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheel TK, et al. Development of JFH1-based cell culture systems for hepatitis C virus genotype 4a and evidence for cross-genotype neutralization. Proc Natl Acad Sci USA. 2008;105:997–1002. doi: 10.1073/pnas.0711044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gottwein JM, et al. Development and characterization of hepatitis C virus genotype 1-7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology. 2009;49:364–377. doi: 10.1002/hep.22673. [DOI] [PubMed] [Google Scholar]
- 68.Gottwein JM, et al. Robust hepatitis C genotype 3a cell culture releasing adapted intergenotypic 3a/2a (S52/JFH1) viruses. Gastroenterology. 2007;133:1614–1626. doi: 10.1053/j.gastro.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 69.Wakita T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu X, Gilbert PB, Hioe CE, Zolla-Pazner S, Self SG. Statistical approaches to analyzing HIV-1 neutralizing antibody assay data. Stat Biopharm Res. 2012;4:1–13. doi: 10.1080/19466315.2011.633860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez-Rodriguez M, et al. Structural properties of the ectodomain of hepatitis C virus E2 envelope protein. Virus Res. 2009;139:91–99. doi: 10.1016/j.virusres.2008.10.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and Supporting Information files. For additional information, interested readers can contact Dr. Isidoro Martinez at imago@isciii.es.