Abstract
Introduction
Isoprenoids are amongst the most abundant and diverse biological molecules and are involved in a broad range of biological functions. Functional understanding of their biosynthesis is thus key in many fundamental and applicative fields, including systems biology, medicine and biotechnology. However, available methods do not yet allow accurate quantification and tracing of stable isotopes incorporation for all the isoprenoids precursors.
Objectives
We developed and validated a complete methodology for quantitative metabolomics and isotopologue profiling of isoprenoid precursors in the yeast Saccharomyces cerevisiae.
Methods
This workflow covers all the experimental and computational steps from sample collection and preparation to data acquisition and processing. It also includes a novel quantification method based on liquid chromatography coupled to high-resolution mass spectrometry. Method validation followed the Metabolomics Standards Initiative guidelines.
Results
This workflow ensures accurate absolute quantification (RSD < 20%) of all mevalonate and prenyl pyrophosphates intermediates with a high sensitivity over a large linear range (from 0.1 to 50 pmol). In addition, we demonstrate that this workflow brings crucial information to design more efficient phytoene producers. Results indicate stable turnover rates of prenyl pyrophosphate intermediates in the constructed strains and provide quantitative information on the change of the biosynthetic flux of phytoene precursors.
Conclusion
This methodology fills one of the last technical gaps for functional studies of isoprenoids biosynthesis and should be applicable to other eukaryotic and prokaryotic (micro)organisms after adaptation of some organism-dependent steps. This methodology also opens the way to 13C-metabolic flux analysis of isoprenoid biosynthesis.
Electronic supplementary material
The online version of this article (10.1007/s11306-019-1580-8) contains supplementary material, which is available to authorized users.
Keywords: Systems biology, Mass spectrometry, Isoprenoids, Isotope labeling experiments
Introduction
Isoprenoids form one of the most abundant and diverse family of biological molecules on Earth (Buckingham 1993). They are produced by all organisms, from prokaryotes to eukaryotes, and are involved in a wide range of biological activities, including maintenance of membrane fluidity, defense against fungi and other pathogens, signaling, growth regulators, attractants for pollinators, or precursors for the synthesis of hormones, bile acids and sterols (Goldstein and Brown 1990; Liao et al. 2016). Moreover, isoprenoids possess strong biological activities against human diseases and are now used as anti-cancers, anti-inflammatories, antioxidants, antibacterial and health supplements (Barbieri et al. 2017; Cho et al. 2017; Gill et al. 2016; Rodriguez-Concepcion et al. 2018; Wang et al. 2005). Furthermore, an alteration of the isoprenoid levels in human cells has been related to several human diseases (Mullen et al. 2016; Pelleieux et al. 2018). The broad range of commercial applications of isoprenoids in agriculture, cosmetics and health has driven intense efforts to develop cost-effective and sustainable manufacturing processes in biotechnology (Ko et al. 2018; Lauersen 2018; Withers and Keasling 2007; Zhang et al. 2017). Comprehensive understanding of the operation and regulation of isoprenoids biosynthesis is thus key in a broad range of fundamental and applicative fields, including systems biology, medicine and biotechnology.
In Saccharomyces cerevisiae, as in many other eukaryotes and prokaryotes, isoprenoids biosynthesis starts with the condensation of two molecules of acetyl-CoA via the mevalonate pathway, which comprises six enzymatic steps to produce isopentenyl diphosphate (IPP), the common precursor of all isoprenoids (Katsuki and Bloch 1967; Lange et al. 2000). IPP is isomerized into dimethylallyl diphosphate (DMAPP), which is then condensed with IPP to generate geranyl pyrophosphate (GPP). Longer prenyl pyrophosphates are built from the condensation of IPP onto each intermediate, giving farnesyl pyrophosphate (FPP) from GPP and geranylgeranyl pyrophosphate (GGPP) from FPP. Evidently, accurate quantification of the mevalonate and prenyl pyrophosphates intermediates in biological samples is necessary to characterize the operation of isoprenoids biosynthesis, and ultimately understand its control and regulation. Only a few methods have been reported for the analysis of isoprenoids precursors (Supporting Table S-1). Virtually all available methods are limited to the quantification of only a few specific intermediates, and none of these studies aimed at measuring their labeling patterns. This would open the way towards isotope labeling experiments (ILEs) for metabolic flux analyses of isoprenoids biosynthesis. The most advanced method developed by Henneman et al. allows the quantification of all the intermediates in a single run by liquid chromatography coupled to mass spectrometry (LC–MS) (Henneman et al. 2008). Analysis of isoprenoids precursors could be improved further by reducing time and cost constrictions (e.g. caused by the preparation of internal standards by enzymatic or chemical synthesis for absolute quantification), as well as reducing the risk of instrument damage due to trimethylamine (e.g. it makes virtually impossible future analyses in positive mode without very extensive cleaning) (Rütters et al. 2000). Finally, sampling and sample preparation procedures have never been investigated nor optimized in detail despite being of utmost importance to ensure accurate measurement of metabolite concentrations and isotopic tracer incorporation (Bolten et al. 2007; Millard et al. 2014).
In this work, we present a complete framework for functional analysis of isoprenoids biosynthesis in yeast. This workflow covers all the experimental and computational steps, with in-depth evaluation of sampling, sample preparation, data acquisition and data processing procedures. We demonstrate that our workflow ensures accurate absolute quantification of all the intermediates in a single analysis and can also measure their incorporation of tracer—i.e. isotopologue distributions—in isotopic labeling experiments.
Experimental section
Strains and cultivations
All strains used in this study were derived from Saccharomyces cerevisiae CEN-PK2.1C (www.uni-frankfurt.de/fb15/mikro/euroscarf). To test the performance of the analytical method, we constructed two strains (S023 and S037) with higher levels of isoprenoid precursors than the wild-type strain. Both had integrated one extra copy of HMG1t and ERG20 under TDH3 and PGK promoters respectively. Furthermore, in S023 and S037 the GGPP synthase from Xanthophyllomyces dendrorhous (CrtE) was expressed constitutively (TEF1p) in a centromeric plasmid. Additionally, the strain S023 also bears one integrated copy of the phytoene synthase (converts GGPP to phytoene) from Pantoea ananatis (CrtB) under the transcriptional control of the PDC1p. This strain was used to test if the GGPP pool was consumed. The genotypes of the different strains are detailed in Supporting Table S-2.
All strains were grown in a modified Verduyn synthetic complete media containing glucose (111 mM), NH4Cl (75 mM), KH2PO4 (22 mM), MgSO4 (0.4 mM) and CSM Leu− (ForMedium LTD, Hunstaton, England) at pH 5.0 (Verduyn et al. 1992).
For metabolomics experiments, an initial OD600nm of 0.007 was used to inoculate 50 mL of Verduyn medium Leu− and cells were grown at 28 °C with an agitation of 220 rpm (MaxQ, ThermoFisher) until harvest at mid-exponential phase. To prepare the 13C internal standard used throughout this study, strain S037 was pre-cultured in 20 mL of Verduyn medium without any amino acids and 55 mM U-13C-glucose. This preculture was used to inoculate 600 mL of the same medium at an initial OD of 0.05. Cells were harvested at mid-exponential phase.
For isotope labeling experiments (ILEs), 200 mL of the same medium was inoculated at an initial OD of 0.007. When the OD was around 3, 10 mL of culture were harvested (time zero) and centrifuged (3000×g, 28 °C, 5 min), and cells were resuspended in the same medium with 55 mM of U-13C-glucose (99% 13C-purity, Eurisotop, Saint-Aubin, France) instead of unlabeled glucose. Samples were harvested at times 0, 1, 2, 5, 10, 15, 30, 45, 60, 90 and 120 min after the switch of label input.
Sampling and sample preparation
Intracellular metabolites were sampled by fast filtration (Kiefer et al. 2007; Millard et al. 2014). Briefly, 10 mL of broth were filtered through 0.45 μm Sartolon polyamide (Sartorius, Goettingen, Germany) and washed with 5 mL of fresh culture medium (without glucose). The filters were rapidly plunged into liquid nitrogen and then stored at −80 °C until extraction. Intracellular metabolites were extracted by incubating filters in closed glass tubes containing 5 mL of an isopropanol/H20 NH4HCO3 100 mM (50/50) mixture at 70 °C for 10 min. For absolute metabolite quantification 50 µL of 13C internal standard were added to each extract. Cellular extracts were cooled on ice and sonicated during 1 min. Cell debris was removed by centrifugation (5000×g, 4 °C, 5 min). Supernatants were evaporated overnight (SC110A SpeedVac Plus, ThermoFisher, Waltham, MA, USA), resuspended in 200 μL of methanol: NH4OH 10 mM (7:3) at pH 9.5 and stored at − 80 °C until analysis.
LC–MS analyses of metabolic intermediates
Analyses were carried out on a LC–MS platform composed of a Thermo Scientific™ Vanquish™ Focused UHPLC Plus system with DAD, coupled to a Thermo Scientific™ Q Exactive™ Plus hybrid quadrupole-Orbitrap™ mass spectrometer (ThermoFisher).
Analysis of isoprenoids precursors was performed on a Thermo Scientific™ Hypersil C18 GOLD™, 3 µm, and 2.1 × 100 mm column. The column was kept at 25 °C and the flow rate was set to 0.3 mL/min during first 2 min and 0.4 mL/min for the rest chromatographic run. The solvent system consisted of (A) 20 mM NH4FA, pH 9.5, in water and (B) 20 mM NH4FA, pH 9.5, in 9:1 (v/v) acetonitrile–water, with the following gradient: 0–12 min from 100% A to 100% B, 5.5 min kept with 100% B and within 0.5 min the return to the initial condition and 4 min equilibration of the column. The injection volume was 10 µL.
Mass detection was carried out in a negative electrospray ionization (ESI) mode. The settings of the mass spectrometer were as follows: spray voltage 3.2 kV, capillary and desolvation temperature were 350 and 400 °C respectively, maximum injection time 200 ms. Nitrogen was used as sheath gas (50 a.u.) and auxiliary gas (15 a.u.). The automatic gain control (AGC) was set at 106 and resolution at 70,000 from m/z 100 to 700. MS analyses were performed by targeted selected ion monitoring (tSIM) mode with 0.5 m/z isolation window for SIM and ± 10 ppm for inclusion tolerances. tSIM MS scans the targeted masses in different time segments selected based on the retention times of the analytes. Data acquisition was performed using Thermo Scientific Xcalibur software.
Internal standards obtained from uniformly 13C-labeled extracts from yeast were used to enable isotope-dilution mass spectrometry (IDMS) (Wu et al. 2005) for the identification and quantification of following compounds: MEV (CAS number 150-97-0), M5P (CAS number 1189-94-2), M5PP (CAS number 4872-34-8), IPP (CAS number 358-71-4) and DMAPP (CAS number 358-72-5) (isobaric compound not separated by the chromatographic method), GPP (CAS number 763-10-0), FPP (CAS number 13058-04-3), and GGPP (CAS number 6699-20-3). All metabolites were identified following minimum standards for level 1 identification (Sumner et al. 2007). Calibration mixtures (prepared at concentrations from 0.08 nM to 10 µM of each isoprenoid precursor) were used to construct calibration curves enabling to determine the concentration of each compound in the samples to be measured.
Quantification of phytoene
10 mL of yeast culture were harvested, centrifuged, and washed with 1 mL of MilliQ water. Cell pellets were freeze-dried and stored at − 80 °C until extracted. 0.11 nmol/mg DCW of β-apocarotenal was added to the dried cells, and phytoene was extracted with glass beads and 500 µL of acetone by three rounds of agitation of 20 s at 0.05 m/s with FastPrep FP120 (ThermoFisher). The acetone phase was transferred to a new tube and the extraction was repeated twice. Acetone extracts were pooled, centrifuged, dried under nitrogen flux and resuspended in 100 μL of acetone for HPLC analysis.
Analyses were carried out on a Thermo Scientific™ Vanquish™ Focused UHPLC Plus system with DAD. 5 µL of the extraction was injected in a column YMC carotenoid (100 × 2.0 mm and 3 µm particle size) equipped with a precolumn (100 × 2.0 mm and 3 µm particle size). The mobile phases used to separate and quantify phytoene and β-apocarotenal from the ergosterol and derivatives consisted in a mixture of A methanol/water (95:5) and B dichloromethane. The flow was 0.25 mL/min with the following gradient: 0–0.1 min 5% B, 0.1–0.5 min 20% B, 0.5–2 min 60% B, 2–5 min 80% B, 5–8 min 80% B and 8–11 min 5% B. Absorbance from 210 to 600 nm was followed during all the run with a data collection rate of 2 Hz and response time of 2 s. Phytoene was quantified by its absorbance at 286 nm and β-apocarotenal at 478 nm. Reference wavelength (600 nm) was subtracted for each of the wavelengths used for metabolite quantification.
Data processing and statistical analysis
MS data were processed using TraceFinder v3.2 (ThermoFisher), with a tolerance of 5 ppm to extract exact masses. In 13C-ILEs, isotopologue distributions were quantified from mass fractions after correction for the presence of all naturally occurring isotopes and isotopic purity of the tracer (99%) using IsoCor v2.0.4 (Millard et al. 2019), which ensures accurate correction of high-resolution isotopic data. IsoCor also calculates the mean 13C-enrichment (E) from the isotopologue distributions using the following formula:
| 1 |
where Mi is the proportion of the isotopologue with i 13C atoms for a metabolite containing n carbon atoms. IsoCor is freely available at https://github.com/MetaSys-LISBP/IsoCor and its documentation at https://isocor.readthedocs.io.
Labeling dynamics between metabolites and strains were compared as detailed in (Kiefer et al. 2015). Briefly, time-course 13C-enrichments were first fitted using the following logistic model:
| 2 |
Half time T50, which corresponding to the time needed to exchange half of 12C atoms of a metabolite pool by 13C atoms, were then calculated using the following equation:
| 3 |
For metabolomics experiments, standard deviations were calculated from three independent biological replicates, each with two technical replicates. For isotopic profiling, standard deviations were calculated from two independent biological experiments, with one technical replicate. For each intermediate, a two-sided Welch’s t test was used to assess the significance of differences of concentrations and labeling dynamics observed between the strains.
Results and discussion
LC–HRMS analysis of isoprenoids precursors
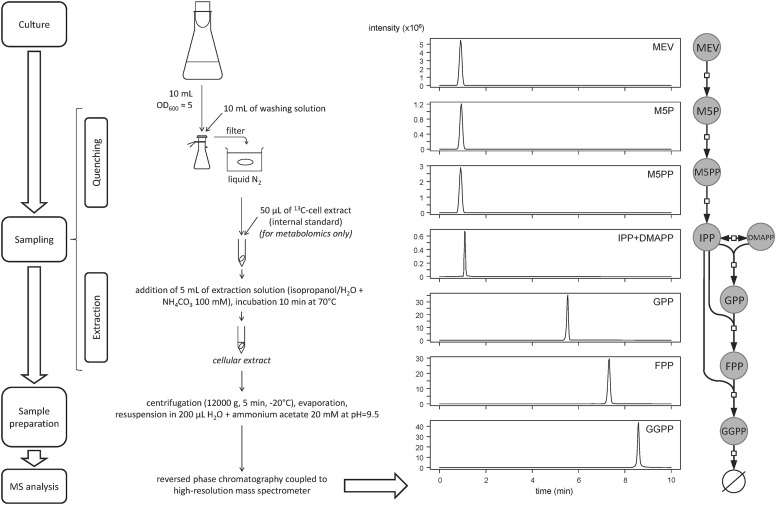
Metabolic intermediates of the isoprenoid biosynthetic pathway (MEV, M5P, M5PP, IPP, DMAPP, GPP, FPP and GGPP) were analyzed by reverse phase chromatography and detected by high-resolution mass spectrometry (HRMS) operating in negative mode. The MS acquisition method was optimized to enhance sensitivity and selectivity by using the tSIM mode. Selectivity was enhanced further by setting the resolution of the Orbitrap cell to 70,000, which was sufficient to avoid mass interference with the analytes of interest, even in complex cellular extracts. Finally, the mass scan range was limited to 600 m/z (from 100 to 700 m/z) to avoid systematic under representation of heavier isotopologues (Su et al. 2017) and ensure accurate metabolomics and isotopic results. Chromatographic separation of phosphorylated intermediates was improved by replacing metal tubing of the LC system by more inert peek tubing (Tuytten et al. 2006). Peaks tailing was reduced further by increasing the pH of the eluent and of the resuspension buffer to 9.5 to reduce adsorption on the stainless steel injector (Tuytten et al. 2006).
Solutions of commercial standards at 5 µM were used to determine the retention time of each compound (Table 1). A chromatogram demonstrating the separation of the isoprenoids precursors in a biological matrix is shown in Fig. 1.
Table 1.
Method validation for quantitative metabolomics of isoprenoids precursors
| Metabolite | Retention time (min) | Exact mass (m/z) | LOD (pmol) | Linear range (pmol) |
|---|---|---|---|---|
| MEV | 1.2 | 147.06628 | 0.1 | 1.5–50 |
| M5P | 1.2 | 227.03261 | 0.02 | 0.1–50 |
| M5PP | 0.9 | 306.99894 | 0.02 | 0.1–50 |
| IPP | 1.2 | 244.99855 | 0.01 | 0.2–50 |
| DMAPP | 1.2 | 244.99855 | 0.05 | 0.1–50 |
| GPP | 5.7 | 313.06115 | 0.01 | 0.5–50 |
| FPP | 7.4 | 381.12375 | 0.01 | 0.2–50 |
| GGPP | 8.7 | 449.18635 | 0.1 | 0.8–50 |
LOD limit of detection, expressed in pmol of compound injected on the column
Fig. 1.
Experimental workflow for quantification and isotopologue profiling of isoprenoids precursors in yeast. Cells are quenched in liquid nitrogen after fast filtration, and intracellular metabolites are extracted by incubating the filters during 10 min in 5 mL of isopropanol/H2O 100 mM NH4HCO3 (1:1) at 70 °C. The extract is centrifuged, dried, resuspended in 200 µL of methanol/NH4OH 10 mM (7:3) at pH 9.5, and analyzed by LC–HRMS. Chromatograms of commercial standards (at a concentration of 5 µM) prepared in a biological matrix are shown on the right
Except IPP and DMAPP which are isobaric compounds and could not be separated by the chromatographic method, all compounds could be detected with a high selectivity. We verified that M5PP does not contribute to the signal of M5P (e.g. due to in-source fragmentation and loss of a phosphate group) and observed a low interference (< 10%). Similarly, M5P does not interfere with measurement of MEV (< 10%).
Workflow for functional analysis of isoprenoids biosynthesis
With this LC–MS method in hand, we then focused on optimizing the sampling procedure, which is of utmost importance to obtain a reliable picture of the metabolome (Bolten et al. 2007; Millard et al. 2014). Intracellular metabolites of S. cerevisiae were collected by fast filtration to ensure rapid quenching of metabolism while reducing the concentrations of glucose, amino acids and salts that would impair both chromatographic separation and MS ionization. The extraction procedure was optimized to maximize metabolite recovery (amount recovered and reproducibility). Four different extraction procedures were tested. The first one was a cold extraction, acetonitrile/methanol/water (4:4:2) at − 20 °C during 1 h. This method has been described to be very efficient and specially for triphosphate compounds (Rabinowitz and Kimball 2007). Secondly, we tested three different protocols already used for isoprenoid extractions in different organisms. All of them use extraction solutions with basic pH and the extractions are performed at high temperature. The first one has already been used for the fission yeast Schizosaccharomyces pombe. This protocol consists in an extraction with isopropanol/H2O + 100 mM NH4HCO3 (1:1) at 70 °C during 15 min (Takami et al. 2012). We also tested if the extraction could be enhanced for S. cererevisiae using other organic solvents: ethanol/H2O + 100 mM NH4HCO3 (3:1) at 70 °C during 15 min (Tong et al. 2008), or butanol/ethanol/H2O + 100 mM NH4HCO3 (4:5:11) extraction at 70 °C during 15 min (Tong et al. 2005). The most efficient extraction solutions were isopropanol/H2O + 100 mM NH4HCO3 (1:1) and butanol/ethanol/H2O + 100 mM NH4HCO3 (4:5:11), but the first showed a better reproducibility (Supporting Figure S-1). We also determined the optimal incubation time. Different incubation times (5, 10, 15, 20, 30 min at 70 °C) were tested to maximize the intensity of the MS signals and evaluate metabolite degradation (Supporting Figure S-2). The signals were stable for all compounds and extraction times, indicating no significant degradation occurred in our conditions. Based on these results, we selected an extraction time of 10 min.
Finally, we implemented the isotope dilution mass spectrometry (IDMS) approach to ensure accurate absolute quantification of the different metabolites (Wu et al. 2005). The fully 13C-labeled cellular extract used as internal standard was produced from a S. cerevisiae strain with high pools of isoprenoids precursors (strain S037) grown on U-13C-glucose as sole carbon source, and using the optimized sampling and extraction procedures. All intermediates showed a high 13C-incorporation (mean molecular 13C-enrichment > 98%).
Method validation in cellular extracts
We evaluated the LC–HRMS method by preparing and analyzing in triplicate a mixture of commercial standards (at concentrations from 0.08 nM to 10 µM) prepared in a biological matrix (a uniformly 13C-labeled extract used as internal standard), and following the Metabolomics Standards Initiative guidelines (Sumner et al. 2007). The method showed an excellent stability in retention time (within ± 5%) for all metabolites, and the instrument remained stable without the need for extra cleaning or maintenance. We estimated the limit of detection (LOD), limit of quantification (LOQ) and linearity according to the Eurachem guidelines (Örnemark and Magnussonm 2014), with an acceptable accuracy and precision threshold fixed at ± 20%. Results (Table 1) show a high repeatability and reproducibility for all concentrations, with an excellent overall correlation coefficient (R2 > 0.99) for all metabolites. LOD was below 0.1 pmol injected on column for most compounds, and LOQ was about 0.1–1.5 pmol over a range of concentration spanning two to three orders of magnitude. Intra- and inter-day assay reproducibility was below 20% for all compounds. Overall, the method was found to be highly sensitive and reproducible for all compounds tested.
Determination of concentration and isotopic profiles of isoprenoids precursors
To demonstrate the applicability of the proposed workflow, we carried out functional investigations to guide rational design of phytoene production in S. cerevisiae. We first optimized the pool of phytoene precursors in the wild-type (WT) metabolic chassis by constructing the strain S037, which overexpresses HMG-CoA reductase (HMG1t), FPP synthase (ERG20) and GGPP synthase (CrtE). We then constructed the strain S023 by expressing a heterologous phytoene synthase (CrtB from Pantoea ananatis) which converts GGPP into phytoene. We performed metabolomics experiments to quantify the concentration of each intermediate in the three strains, and stable isotope labeling experiments which are of greatest interest to measure metabolic fluxes and infer flux control in living cells.
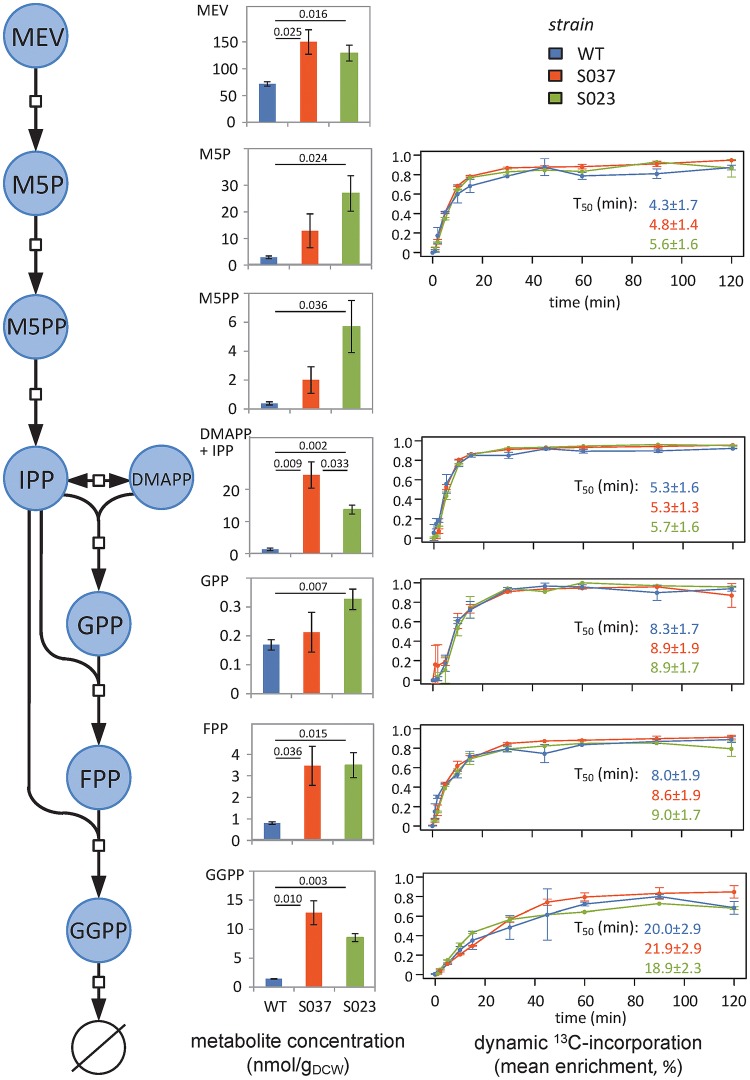
Metabolomics results showed very different concentration profiles in the three strains, with a good biological reproducibility for all intermediates (average RSD of 20%) (Fig. 2). GGPP concentration was increased tenfold in strain S037 (12.8 ± 2.0 nmol/g DCW) compared to the WT strain (1.4 ± 0.1 nmol/g DCW) (p = 0.010). Other pools of intermediates were also significantly increased in S037 (MEV, p = 0.024; IPP/DMAPP, 0.009; FPP, p = 0.036). This confirms the relevance of overexpressing HMG1t, ERG20 and CrtE to optimize the metabolic chassis. Additional expression of CrtB in strain S023 resulted in a slight decrease of GGPP pool (8.5 ± 0.7 nmol/g DCW, p = 0.056) compared to S037 to support phytoene production (with a total phytoene production of 1.2 ± 0.1 µmol/g DCW).
Fig. 2.
Absolute intracellular concentrations of isoprenoids precursors and dynamic 13C-incorporation following a switch from unlabeled to U-13C-glucose. A two-sided Welch’s t-test was performed to assess the significance of differences observed between strains. For metabolomics experiments, p-values are given when lower than 0.05. For isotope labeling experiments, differences in T50 between strains were not significant (p > 0.10)
Fluxes in linear pathways such as the isoprenoids biosynthetic pathway cannot be resolved using well-established stationary 13C-isotope labeling approaches. We thus carried out more complex instationary 13C-ILEs, where the transient label incorporation—i.e. isotopologue distribution—was measured into the different intermediates during 120 min following a switch of the cells from a cultivation medium with unlabeled glucose to a medium containing uniformly 13C-labeled glucose. Compared to metabolomics experiments, the sensitivity and selectivity of MS analyses tend to decrease in ILEs because a single metabolite gives rise to several MS peaks, and their intensity depend on the incorporation of isotopic tracers (e.g. a metabolite with n carbon atoms may give rise to n + 1 MS peaks in a 13C-ILE) (Heuillet et al. 2018). This was observed for mevalonate and mevalonate-5-pyrophosphate which showed low and variable signals. However, the method was sensitive enough to accurately quantify the isotopologue distributions of all the other intermediates (Figure S-3), including GGPP which contains a high number (20) of carbon atoms and hence gives a very high number of MS peaks. A total of 671 isotopologues were quantified for each strain (61 isotopologues from 6 metabolic pools × 11 time points). The reproducibility of the method was evaluated from the spread of measured isotopologue abundances and was expressed as the mean standard deviation over all the measured isotopologues (Heuillet et al. 2018). The mean precision on isotopologue quantification was 0.027, 0.013, and 0.013 for the wild type, S037 and S023 strains, respectively. Isotopologue distributions were used to calculate the dynamics of 13C-molecular enrichments (Fig. 2), which provide a direct readout of the propagation of tracer isotopes through the isoprenoids precursors and facilitate data interpretation. For each metabolite and each strain, the time needed to achieve 50% of final enrichment (T50) was estimated by fitting a logistic model (Kiefer et al. 2015). As expected, label incorporation followed the order of intermediates in the pathway, with a T50 ranging from 5 min for M5P to 20 min for GGPP (Fig. 2). As also expected, the final 13C-enrichments did not significantly differ between intermediates (~ 90%, p > 0.05). More surprisingly, the dynamic 13C-enrichments profiles and T50 values of all intermediates were also very similar for the three strains (p > 0.05) despite significant changes in pool sizes.
Integration of metabolomics and isotopic datasets may provide quantitative information on the biosynthetic flux of isoprenoid precursors. The labeling dynamics of a given metabolite reflects its turnover rate, which is roughly equivalent to the ratio of the metabolite pool size and the flux through that metabolite pool. The strong increase of pools in S037 and S023 strains compared to the WT strain are expected to slow down the transient 13C-incorporation (Buescher et al. 2015). However, the 13C-enrichment profiles and turnover rates were very similar in the three strains, which suggest that the flux increases roughly proportionally with the pools. The relative increase in GGPP biosynthesis in strains S037 and S023 compared to the wild-type strain could thus be estimated from the corresponding change in GGPP concentration. Assuming a constant turnover of GGPP, its biosynthetic flux increased by a factor 9.0 ± 2.6 in strain S037 and 7.0 ± 1.6 in S023 compared to the wild-type strain (p < 0.01), which support the relevance of the proposed strategy to enhance phytoene production.
Inferring absolute flux values from these data would require more sophisticated mathematical models of isoprenoids biosynthesis, such as non-stationary 13C-flux models. Still, these results already demonstrate the applicability of the proposed workflow to infer flux information on this pathway in yeast. Concentrations and isotopic labeling of the prenyl pyrophosphate intermediates could also complement other biochemical datasets (e.g. proteomics or enzyme activities) to infer quantitative information on the control exerted by each reaction step on the pathway flux, and thereby drive further strain optimization strategies.
Conclusion
We presented a workflow for functional analysis of isoprenoids biosynthesis in yeast. Procedures to quench metabolism and extract metabolic intermediates were optimized and a novel method was developed to measure concentrations and isotopic profiles of isoprenoids precursors by high pressure liquid chromatography coupled to high-resolution mass spectrometry. This workflow provides accurate quantification (RSD < 20%) over a large linear range (from 1 to 50 pmol) and showed a high sensitivity (LOD < 0.1 pmol) for all compounds tested. Integration of metabolomics data with dynamic 13C-incorporation provides quantitative information on the biosynthetic flux of phytoene precursors in S. cerevisiae. Overall, these results illustrate the value of the present workflow during iterative construction of a phytoene producing strain in biotechnology.
This methodology closes one of the remaining gaps for comprehensive, quantitative understanding of isoprenoids biosynthesis. Access to absolute intracellular concentrations of all intermediates in combination with the ability to quantify their isotopologue distribution will be a valuable tool for future investigations. It may also support the development of kinetic models of isoprenoids metabolism, thus opening the way to comprehensive, mechanistic understanding of the control and regulation of their biosynthesis. This workflow was designed and validated for yeast but should be applicable to other eukaryotic and prokaryotic organisms, including bacteria, plants, and mammals, after adaptation of organism-dependent steps (in particular quenching and extraction).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
MetaToul (Metabolomics & Fluxomics Facilities, Toulouse, France, www.metatoul.fr) and its staff members are gratefully acknowledged for technical support and access to mass spectrometry facilities. MetaToul is part of the national infrastructure MetaboHUB-ANR-11-INBS-0010 (www.metabohub.fr).
Author contributions
All authors designed the study. HBK and FB optimized and validated the LC–MS method. SCC constructed the yeast strains. SCC and PM carried out cultivation experiments, performed the isoprenoid extractions and prepared the IDMS. SCC, HKB and PMI analyzed and interpreted the data. GT, SH, JCP and FB supervised the project. All authors read and approved the manuscript.
Funding
This work was supported by the French National Research Agency project ENZINVIVO (ANR-16-CE11-0022).
Data availability
The datasets generated during the current study are available in the MetaboLights repository with identifier MTBLS923 (https://www.ebi.ac.uk/metabolights/MTBLS923).
Compliance with ethical standards
Conflict of interest
Sara Castaño-Cerezo, Hanna Kulyk-Barbier, Pierre Millard, Jean-Charles Portais, Stéphanie Heux, Gilles Truan, Floriant Bellvert declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sara Castaño-Cerezo, Hanna Kulyk-Barbier, and Pierre Millard contributed equally to this work.
References
- Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sánchez E, Nabavi SF, Nabavi SM. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiological Research. 2017;196:44–68. doi: 10.1016/J.MICRES.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Bolten CJ, Kiefer P, Letisse F, Portais J-C, Wittmann C. Sampling for metabolome analysis of microorganisms. Analytical Chemistry. 2007;75(10):3843–3849. doi: 10.1021/AC0623888. [DOI] [PubMed] [Google Scholar]
- Buckingham J. Dictionary of natural products. 1. London: Chapman & Hall; 1993. [Google Scholar]
- Buescher JM, Antoniewicz MR, Boros LG, Burgess SC, Brunengraber H, Clish CB, et al. A roadmap for interpreting 13 C metabolite labeling patterns from cells. Current Opinion in Biotechnology. 2015;34:189–201. doi: 10.1016/j.copbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KS, Lim Y-R, Lee K, Lee J, Lee JH, Lee I-S. Terpenes from forests and human health. Toxicological Research. 2017;33(2):97–106. doi: 10.5487/TR.2017.33.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill BS, Kumar S, Navgeet Triterpenes in cancer: significance and their influence. Molecular Biology Reports. 2016;43(9):881–896. doi: 10.1007/s11033-016-4032-9. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Henneman L, van Cruchten AG, Denis SW, Amolins MW, Placzek AT, Gibbs RA, et al. Detection of nonsterol isoprenoids by HPLC–MS/MS. Analytical Biochemistry. 2008;383(1):18–24. doi: 10.1016/j.ab.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuillet M, Bellvert F, Cahoreau E, Letisse F, Millard P, Portais J-C. Methodology for the validation of isotopic analyses by mass spectrometry in stable-isotope labeling experiments. Analytical Chemistry. 2018;90(3):1852–1860. doi: 10.1021/acs.analchem.7b03886. [DOI] [PubMed] [Google Scholar]
- Katsuki, H., & Bloch, K. (1967). Studies on the biosynthesis of ergosterol in yeast formation of methylated intermediates. Journal of Biological Chemistry, 242(2), 222–227. http://www.jbc.org/content/242/2/222.abstract. [PubMed]
- Kiefer P, Nicolas C, Letisse F, Portais J-C. Determination of carbon labeling distribution of intracellular metabolites from single fragment ions by ion chromatography tandem mass spectrometry. Analytical Biochemistry. 2007;360(2):182–188. doi: 10.1016/J.AB.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Kiefer P, Schmitt U, Müller JEN, Hartl J, Meyer F, Ryffel F, Vorholt JA. DynaMet: A fully automated pipeline for dynamic LC–MS data. Analytical Chemistry. 2015;87(19):9679–9686. doi: 10.1021/acs.analchem.5b01660. [DOI] [PubMed] [Google Scholar]
- Ko SC, Lee HJ, Choi SY, Choi J, Woo HM. Bio-solar cell factories for photosynthetic isoprenoids production. Planta. 2018 doi: 10.1007/s00425-018-2969-8. [DOI] [PubMed] [Google Scholar]
- Lange BM, Rujan T, Martin W, Croteau R. Isoprenoid biosynthesis: The evolution of two ancient and distinct pathways across genomes. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(24):13172–13177. doi: 10.1073/pnas.240454797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauersen KJ. Eukaryotic microalgae as hosts for light-driven heterologous isoprenoid production. Planta. 2018 doi: 10.1007/s00425-018-3048-x. [DOI] [PubMed] [Google Scholar]
- Liao P, Hemmerlin A, Bach TJ, Chye M-L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnology Advances. 2016;34(5):697–713. doi: 10.1016/j.biotechadv.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Millard P, Delépine B, Guionnet M, Heuillet M, Bellvert F, Létisse F. IsoCor: Isotope correction for high-resolution MS labeling experiments. Bioinformatics. 2019 doi: 10.1093/bioinformatics/btz209. [DOI] [PubMed] [Google Scholar]
- Millard, P., Massou, S., Wittmann, C., Portais, J.-C., & Létisse, F. (2014). Sampling of intracellular metabolites for stationary and non-stationary 13C metabolic flux analysis in Escherichia coli. Analytical Biochemistry, 465, 38–49. https://www.sciencedirect.com/science/article/pii/S0003269714003285?via%3Dihub. [DOI] [PubMed]
- Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nature Reviews Cancer. 2016;16(11):718–731. doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- Örnemark, B., & Magnussonm, U. (2014). Eurachem Guide: The fitness for purpose of analytical methods—A laboratory guide to method validation and related topics. https://www.eurachem.org/index.php/publications/guides/mv.
- Pelleieux S, Picard C, Lamarre-Théroux L, Dea D, Leduc V, Tsantrizos YS, Poirier J. Isoprenoids and tau pathology in sporadic Alzheimer’s disease. Neurobiology of Aging. 2018;65:132–139. doi: 10.1016/j.neurobiolaging.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JD, Kimball E. Acidic acetonitrile for cellular metabolome extraction from Escherichia coli. Analytical Chemistry. 2007;79(16):6167–6173. doi: 10.1021/ac070470c. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M, Avalos J, Bonet ML, Boronat A, Gomez-Gomez L, Hornero-Mendez D, et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Progress in Lipid Research. 2018;70:62–93. doi: 10.1016/J.PLIPRES.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Rütters H, Möhring T, Rullkötter J, Griep-Raming J, Metzger JO. The persistent memory effect of triethylamine in the analysis of phospholipids by liquid chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry. 2000;14(2):122–123. doi: 10.1002/(SICI)1097-0231(20000130)14:2<122::AID-RCM844>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Su X, Lu W, Rabinowitz JD. Metabolite spectral accuracy on orbitraps. Analytical Chemistry. 2017;89(11):5940–5948. doi: 10.1021/acs.analchem.7b00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI) Metabolomics. 2007;3(3):211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami T, Fang Y, Zhou X, Jaiseng W, Ma Y, Kuno T. A genetic and pharmacological analysis of isoprenoid pathway by LC-MS/MS in fission yeast. PLoS ONE. 2012;7(11):e49004. doi: 10.1371/journal.pone.0049004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Holstein SA, Hohl RJ. Simultaneous determination of farnesyl and geranylgeranyl pyrophosphate levels in cultured cells. Analytical Biochemistry. 2005;336(1):51–59. doi: 10.1016/j.ab.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Tong H, Wiemer AJ, Neighbors JD, Hohl RJ. Quantitative determination of farnesyl and geranylgeranyl diphosphate levels in mammalian tissue. Analytical Biochemistry. 2008;378(2):138–143. doi: 10.1016/j.ab.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Tuytten R, Lemière F, Witters E, Van Dongen W, Slegers H, Newton RP, et al. Stainless steel electrospray probe: A dead end for phosphorylated organic compounds? Journal of Chromatography A. 2006;1104(1–2):209–221. doi: 10.1016/j.chroma.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Verduyn C, Postma E, Scheffers WA, Van Dijken JP. Effect of benzoic acid on metabolic fluxes in yeasts: A continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8(7):501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- Wang GG, Tang WW, Bidigare RR. Terpenoids as therapeutic drugs and pharmaceutical agents. Totowa, NJ: Humana Press; 2005. pp. 197–227. [Google Scholar]
- Withers ST, Keasling JD. Biosynthesis and engineering of isoprenoid small molecules. Applied Microbiology and Biotechnology. 2007;75(5):980–990. doi: 10.1007/s00253-006-0593-1. [DOI] [PubMed] [Google Scholar]
- Wu L, Mashego MR, van Dam JC, Proell AM, Vinke JL, Ras C, et al. Quantitative analysis of the microbial metabolome by isotope dilution mass spectrometry using uniformly 13C-labeled cell extracts as internal standards. Analytical Biochemistry. 2005;336(2):164–171. doi: 10.1016/J.AB.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nielsen J, Liu Z. Engineering yeast metabolism for production of terpenoids for use as perfume ingredients, pharmaceuticals and biofuels. FEMS Yeast Research. 2017 doi: 10.1093/femsyr/fox080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available in the MetaboLights repository with identifier MTBLS923 (https://www.ebi.ac.uk/metabolights/MTBLS923).