Abstract
Over the past few years extensive body of research was produced investigating the effects of repetitive transcranial magnetic stimulation (rTMS) for the treatment of chronic tinnitus with heterogeneous results. This heterogeneity is exemplified by two recently published large-scale clinical trials reporting different outcomes. Technical aspects of rTMS were suspected as a potential source for this incongruency. The aim of this systematic review is to examine the overall efficacy as well as to identify possible technical factors relevant for the effectiveness of rTMS tinnitus trials. Via a literature search appropriate original research papers were identified and rTMS parameters were extracted from each study arm for subsequent statistical analysis with respect to observed effects (significant vs. not significant pre-post rTMS effects). Our findings indicate that verum rTMS is superior to sham rTMS as demonstrated by the proportion of significant pre-post contrasts. Some relevant rTMS parameters (e.g., pulse waveform) are not reported. Lower rTMS stimulation intensity was associated with significant effects in verum rTMS arms. An additional stimulation of the DLPFC to the temporal cortex was not found to promote efficacy. Future research should consider differential effects of rTMS induced by technical parameters and strive for an exhaustive reporting of relevant rTMS parameters.
Subject terms: Neuroscience, Neuroscience, Neurology, Neurology
Introduction
Chronic subjective tinnitus is defined as the perception of a sound, such as ringing or buzzing, without the presence of an external or internal source1 with a duration of at least three months2. Approximately 10–15% of people living in industrial countries are affected by such persistent sounds and up to now, there is no available cure3. Etiology of tinnitus seems to be very heterogeneous, though in most cases it occurs after cochlear damages following noise trauma or hearing loss in general4. It is assumed, that as a consequence of diminished or missing acoustic input and the ensuing deprivation of neural input in the auditory pathways, pathological brain changes occur and the “phantom sound”, called tinnitus is generated5,6. From a neurophysiological perspective subjective tinnitus is therefore associated with altered neural activity along the auditory pathway7 and hyperactivity in auditory brain areas8,9 as well as non-auditory brain areas10. As noted by Theodoroff and Folmer11, these given pathological neural circumstances represent a significant leverage point for the application of recent neuromodulation techniques, in particular repetitive transcranial magnetic stimulation (rTMS).
15 years ago, low-frequency rTMS of the left auditory cortex was introduced as a new possibility to treat tinnitus based on the rationale to reduce the over-activated left temporal cortex12,13. Since that time a bulk of trials and also several reviews were published with heterogeneous evaluation of the putative efficacy of rTMS for the treatment of tinnitus. The findings and conclusions of clinical trials with rTMS in tinnitus manifest to be diverse and are particularly denoted with e.g. high interindividual variability, a lack of sham-controlled trials and small effect sizes14–16. An early review from Langguth et al. in 2008 resumed a “promising potential of rTMS for therapeutic management of tinnitus”17. This conclusion is supported by other reviews, which report rTMS as a new therapeutic tool for tinnitus15, with potential efficacy18, some given evidence19 or even significant medium to large effect sizes as shown by a meta-analysis20. Furthermore, left temporal low frequency rTMS was declared with a Level C recommendation (possible efficacy) in a consensus statement21. Other reviews indicated “very limited support for the use of low-frequency rTMS for the treatment of patients with chronic tinnitus”22 or a general tendency to not recommend rTMS for tinnitus23.
Beside heterogeneity in the evaluation of the efficacy of rTMS one aspect past reviews have in common, is a demand for the implementation of randomized, sham-controlled clinical trials with an appropriate sample size. To the best of our knowledge, two such trials were conducted and published. The ongoing discourse regarding rTMS in tinnitus proceeds by these two clinical trials with an almost identical methodological design and different reported results. Folmer and colleagues24 were able to show a significant effect of a sham-controlled 1 Hz rTMS protocol over the auditory cortex on the improvement of tinnitus severity in a study with 64 patients. In contrast, a recent published multi-center study from Landgrebe et al.25 involving 146 patients could not report any improvements as a consequence of rTMS even by investigating a larger sample. It was discussed in subsequent letters to the editors, that differences in samples (e.g., sample size), used trial design (e.g., outcome measures), but also technical parameters of rTMS (e.g., TMS devices) might be responsible for the conflicting results26,27. In the case of used TMS devices the direction of current flow differs by default28, which was shown to be critical for the induction of neuroplasticity21,29. Parameter space of technical aspects of rTMS is very large and all of them seem to be relevant for neurophysiological effects of rTMS30. As early findings indicate and thus as a rule of thumb, low stimulation frequency decreases (≤1 Hz) and high stimulation frequency increases cortical excitability (≥5 Hz)31,32. In the same manner, the number of pulses delivered per session or the stimulation intensity used, might be essential for the effectiveness of rTMS33,34.
Even if there are plenty of previous reviews, they remain narrow in focus dealing only with effectiveness in general or just patient characteristics, but do not take the rTMS parameters into account. Hence, the aim of this systematic review is to examine previous research concerning daily rTMS in tinnitus to present a statistical overview of general effectiveness of rTMS as indicated by verum-sham contrast and to investigate the influence of rTMS parameters on the effect of verum rTMS in tinnitus.
Materials and Methods
Protocol and registration
The review for this paper was conducted according to the guidelines for “Preferred Reporting Items for Systematic Reviews and Meta-Analysis” (PRISMA35; see Supplemental Material 1). Moreover, the details of the protocol for this review were registered in the International Prospective Register of Systematic Reviews, PROSPERO (CRD42018099744).
Search strategy, study selection and data collection
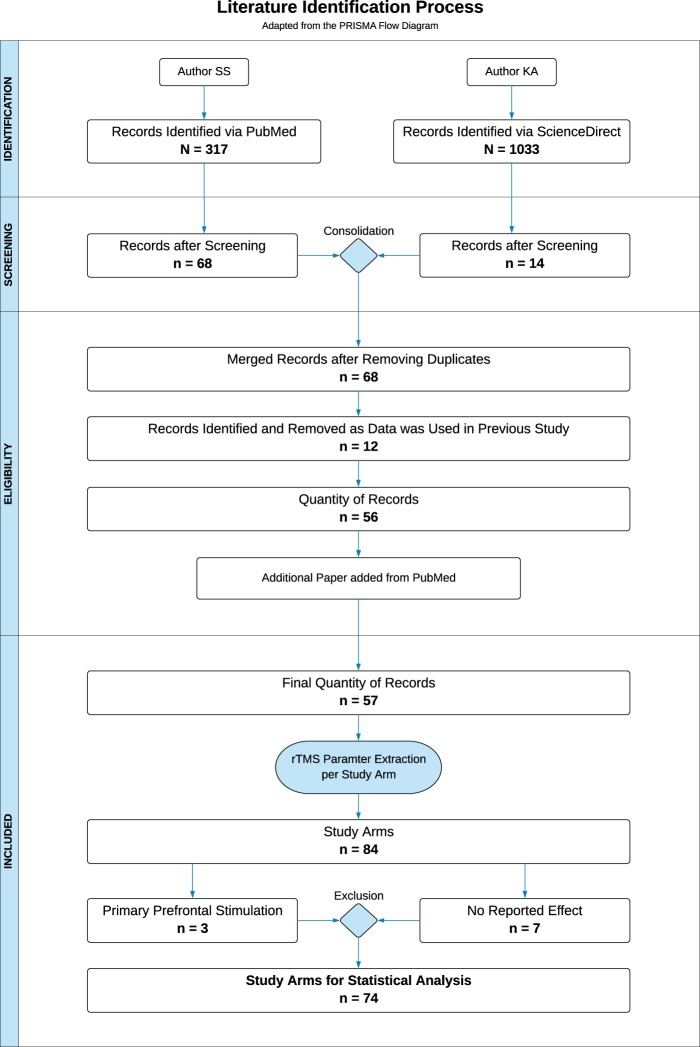
A systematic literature search was conducted in May 2018 by two independent individuals using the electronic research databases “PubMed” and “ScienceDirect” with the keywords “tinnitus” and “transcranial”. Figure 1 provides an overview of the literature identification process by the means of an adapted flow diagram originally provided by the PRISMA guidelines35. In order to identify a maximum quantity of research papers, the keywords were applied to all possible search fields. The utilization of “PubMed” resulted in N = 317 potential papers, the search with the database “ScienceDirect” was able to find N = 1.033 articles. An initial screening of the search outcomes was executed by examining the title as well as the corresponding abstracts with respect to previously defined inclusion criteria: original research data; application of rTMS; repeated sessions; focus on chronic tinnitus. N = 68 from PubMed and n = 14 from ScienceDirect appropriate research papers could be identified. Hereinafter, the results of both search engines were checked for duplicates and merged to a consolidated table with n = 68 articles with all publications of the ScienceDirect search included in the PubMed search. In a next step, the papers were resurveyed in full text, to ascertain, if the studies used data which was already published. As a result, n = 12 papers had to be excluded from our review. Towards the end of the literature identification process, an additional paper was added as detected by regular PubMed searches. Consequently, the final quantity of records for this review and subsequent analysis consisted of n = 57 research papers. In order to deploy statistical analysis, important rTMS parameters were extracted from the records for each verum study arm separately. Thus, the parameter extraction of one single paper could eventuate in multiple study arms for our analysis. Sham arms were not included as we were interested in the effects of rTMS parameters on treatment efficacy. Number of verum and sham arms were counted for estimation of overall rTMS efficacy (see statistics).
Figure 1.
Review Procedure. The procedure of this systematic review from literature identification to the final number of verum rTMS study arms for statistical analysis is depicted as a flow diagram adapted from the PRISMA guidelines35.
Parameters of interest were: manufacturer of the TMS device; type of TMS device; pulse waveform; coil type; coil orientation; stimulation position; stimulation laterality of auditory cortex stimulation (unilateral or bilateral); unilateral stimulation index (extracted by a calculated ratio for the stimulated hemisphere (left = 0, right = 1)); the definition of the stimulated hemisphere with respect to tinnitus laterality; stimulation frequency (Hz) (condensed to inhibitory or excitatory frequency protocols for analysis); stimulation intensity (% of motor threshold); mean motor threshold (%); motor threshold determination method (electromyography (EMG)/visual); number of sessions; overall pulses per session over auditory and prefrontal cortical areas; pulses per session over auditory cortical areas; overall total pulses over auditory and prefrontal cortical areas (calculated as the number of pulses per session * number of sessions); total pulses over auditory cortical (defined as the number of auditory pulses per session * number of sessions); use of a neuronavigation system (yes/no); additional stimulation (e.g., frontal stimulation additionally to auditory cortex stimulation; stimulation parameters were extracted equally; cf. Supplemental Material 2). Further study-specific information like type of additional treatment, study design or used outcome measurements were also extracted from the records, simply to provide an overview and were not considered for statistical analysis.
As no study arm reported the used pulse waveform, we decided to perform statistical analysis for this purpose with the default waveform of the used TMS devices. The information was gathered from user manuals and by contacting the manufacturers of the devices or the authors of the papers, respectively. Our dependent variable of interest was the reported effect of each of the study arms dichotomized to “significant” and “not significant”, as most papers did not report effect sizes. Since only N = 38 study arms provided information about the definition of a primary outcome instrument, a study arm was declared as significant, if 50% or more of the used outcome measurements were reported as “significant”. In case of a lack of information concerning relevant parameters or outcomes, the term “not reported” was used and was not considered in the definition of the reported effect.
Data analysis
Statistical analysis was performed with the statistic software R (R version 3.4.3; R Foundation for Statistical Computing, Austria; packages “tidyverse and “gmodels”) and focused on the change of symptoms from pre to post treatment of each extracted study arm. For all analyses, we concentrated on trials using stimulation of the temporal or temporoparietal cortex as most studies primarily stimulated these areas (for details see results section) and thus stimulation positions restricted solely to areas outside the temporal region were excluded (three study arms including prefrontal cortex). For seven study arms no information was provided about whether pre to post rTMS changes were significant or not. Therefore, n = 10 study arms were excluded, resulting in 74 study arms included in statistical analyses (cf. Fig. 1). Missing values, “not-reported” effects or information only provided in certain ranges (e.g., pulses per session 1800–3000) were excluded for each parameter analysis individually. Parameters were not analyzed, if 30% of the investigated arms did not provide data. Associations of categorical data with the reported effects (significant vs. not significant) were calculated with χ2-tests and Fisher’s exact tests in the case of cell frequencies below 5. To evaluate differences in parametric variables regarding the given effect, Mann-Whitney U-tests for independent samples were computed. Significance level was defined as p ≤ 0.05 and reported uncorrected for multiple comparisons.
Results
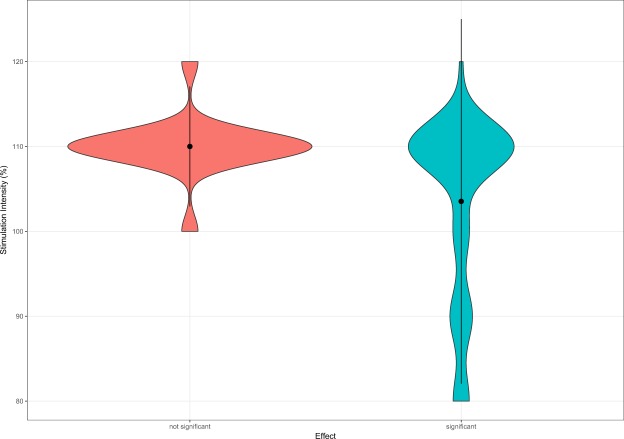
The extraction of relevant parameters from N = 57 research papers resulted in overall N = 74 study arms for which statistical comparisons from pre to post rTMS treatment were done. A detailed overview of the descriptive statistics of rTMS parameters of the single study arms can be found in Supplemental Material 2. Out of 74 study arms used for statistics, 56 arms reported significant effects (76%). In order to ascertain whether the efficacy of verum rTMS is higher in contrast to sham rTMS, we statistically compared the quantity of reported significant and not significant results of verum study arms with those of available 22 sham arms (5 significant; 23%). A χ2-test indicated a significant association of the type of study arm (verum or sham) and rTMS efficacy (pre-post change significant or not) showing a superiority of verum in contrast to sham rTMS (p < 0.05). Table 1 shows the results of the association of technical rTMS parameters with efficacy as indicated by pre-post changes in verum study arms. Analyses of parametric data revealed that the group of significant study arms, showed lower stimulation intensity (about 6.5% stimulator output) in contrast to not significant arms (cf. Fig. 2). Out of 18 study arms utilizing a stimulation intensity lower than 110%, 94.44% reported significant results. Whereas, in case of ≥110% stimulation intensity (two study arms with 120% stimulation intensity; one significant, one not significant), 68.00% of 50 study arms state significant findings.” To exclude a potential confounder caused by studies applying continuous theta burst stimulation (cTBS) using commonly lower stimulation intensities, we investigated a possible association of cTBS or rTMS with reported effects. Our results show no significant association (proportion of significant studies did not differ between cTBS and rTMS studies; cf. Table 1), indicating an exclusion of this potential bias in our stimulation intensity results.
Table 1.
Descriptive and statistical data of 74 study arms.
| rTMS Parameter | Significant Study Arms | Not significant Study Arms | p |
|---|---|---|---|
| n = 56 (75.68%) | n = 18 (24.32%) | ||
| N (%) | N (%) | ||
| Manufacturer TMS device | |||
| Magstim | 14 (77.78) | 4 (22.22) | |
| MagVenture/Medtronic | 34 (72.37) | 13 (27.66) | 0.76 |
| Default waveform | |||
| Biphasic cosine | 16 (80.00) | 4 (20.00) | |
| Biphasic sine | 36 (72.00) | 14 (28.00) | 0.56 |
| Stimulation position | |||
| Temporal cortex | 32 (78.05) | 9 (21.95) | |
| Temporo-parietal cortex | 23 (71.88) | 9 (28.12) | 0.54 |
| Auditory cortex stimulation laterality | |||
| Bilateral | 5 (66.67) | 1 (16.67) | |
| Unilateral | 50 (74.73) | 17 (25.37) | >0.99 |
| Auditory cortex stimulation hemisphere | |||
| Left | 36 (70.59) | 15 (29.41) | |
| Other (contralateral, ipsilateral, bilateral, left or right) | 19 (86.36) | 3 (13.64) | 0.24 |
| Stimulation frequency | |||
| Inhibitory (1 Hz, cTBS) | 49 (73.13) | 18 (26.87) | |
| Excitatory (10 Hz, 25 Hz) | 6 (100.00) | 0 (0.00) | 0.33 |
| Stimulation type | |||
| cTBS | 4 (80.00) | 1 (20.00) | |
| rTMS (1 Hz, 10 Hz, 25 Hz) | 50 (73.53) | 18 (26.47) | 0.33 |
| Motor threshold determination method | |||
| EMG | 36 (75.00) | 12 (25.00) | |
| Visual | 10 (76.92) | 3 (23.08) | >0.99 |
| Neuronavigation | |||
| Yes | 17 (73.91) | 6 (26.09) | |
| No | 38 (76.00) | 12 (24.00) | 0.85 |
| Prefrontal stimulation in addition to auditory cortex stimulation | |||
| Additional prefrontal stimulation | 9 (56.25) | 7 (43.75) | |
| No additional prefrontal stimulation | 46 (80.70) | 11 (19.30) | 0.04* |
| M ± SD | M ± SD | p | |
| Stimulation hemisphere - auditory cortical areas (left = 0; right = 1) |
0.12 ± 0.22 (n = 49) |
0.07 ± 0.21 (n = 17) |
0.66 |
| Stimulation intensity (%) |
103.53 ± 10.7 (n = 51) |
110.00 ± 3.54 (n = 17) |
0.02* |
| Number of sessions |
8.47 ± 3.38 (n = 55) |
9.22 ± 4.76 (n = 18) |
0.80 |
| Overall pulses per session − auditory and prefrontal cortical areas |
1807.64 ± 838.04 (n = 55) |
2488.89 ± 1095.92 (n = 18) |
0.03* |
| Pulses per session − auditory cortical areas |
1624.00 ± 671.85 (n = 55) |
1877.78 ± 662.04 (n = 18) |
0.21 |
| Overall total pulses − auditory and prefrontal cortical areas (pulses per session × number of sessions) |
16003.64 ± 11016.50 (n = 55) |
22333.33 ± 13266.50 (n = 18) |
0.07 |
| Total pulses − auditory cortical areas (pulses per session × number of sessions) |
14485.45 ± 9962.53 (n = 55) |
17277.78 ± 9730.52 (n = 18) |
0.20 |
*p ≤ 0.05.
Figure 2.
Reported Effect & Stimulation Intensity. The distribution of the stimulation intensity used by study arms separated for the reported results (significant effects N = 51; not significant effects N = 17).
With respect to number of pulses, significant study arms used less pulses. Since the number of pulses was only significant for overall pulses per session, we compared the reported effects of study arms which used an additional DLPFC stimulation (n = 16) with those free of any additional stimulation, as DLPFC stimulation in addition to auditory cortex stimulation might result more applied pulses. Indeed, a significant difference was found in the used number of pulses between arms with additional prefrontal stimulation (3056.25 ± 871.78) and those with only temporal rTMS (1672.28 ± 723.44), U = 108.50, p < 0.01. As pointed out in Table 1, a χ2-test found a significant association between the use of an additional DLPFC stimulation and whether or not the effect of rTMS was significant. Since 80.70% of the study arms without an additional stimulation of the DLPFC report significant results, whereas only 56.25% of the study arms with an additional stimulation show significant effects, our findings suggest no benefit of an additional DLPFC stimulation, rather the opposite seems to pertain.
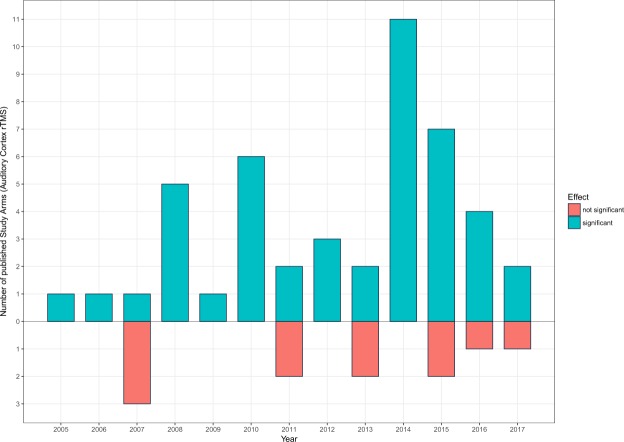
In order to preclude a potential publication bias caused by a possible high quantity of significant or not significant results published in certain years (e.g., more significant studies in early years and more not significant studies in late years36), publication years of 57 study arms with solely auditory cortex rTMS were considered for statistical analysis to check if there is a mean difference between significant and not significant effects. No significant difference was found, perpending an exclusion of a publication bias, U = 241.50, p = 0.82. Figure 3 presents a summary of the quantity of published auditory cortex rTMS trial arms for each year subdivided by the reported effect.
Figure 3.
Year & Number of published Study Arms. For each year, the number of published study arms with an exclusively auditory cortex rTMS grouped for significant and not significant reported effects is illustrated.
All other parametric parameters were not significant. Mean motor threshold was not analyzed due to more than 30% of missing data (n = 65).
Analysis of categorical data showed no significant associations e.g. between the manufacturer of the TMS device (Magstim vs. MagVenture/Medtronic; number of other manufacturers were too low to include in analysis) or the default waveform of the system (biphasic cosine vs. biphasic sine). Coil orientation was not analyzed, because it was not reported in 34 study arms. Likewise, the type of coil was not included in statistical analysis, since 93.2% of examined arms used a figure-of-eight coil. Table 2 provides an overview over the missing information for all parameters separated for significant and not significant study arms.
Table 2.
Missing data for rTMS parameters of 74 study arms.
| rTMS Parameter | Significant Study Arms | Not Significant Study Arms |
|---|---|---|
| N | N | |
| Manufacturer TMS device | — | — |
| Pulse waveform | 56 | 18 |
| Default waveform | — | — |
| Coil type | — | 1 |
| Coil orientation | 22 | 12 |
| Stimulation position | 1 | — |
| Stimulation hemisphere | — | 1 |
| Stimulation frequency (%) | 1 | — |
| Stimulation intensity (%) | 5 | 1 |
| Mean motor threshold (%) | 50 | 15 |
| Motor threshold determination method | 10 | 3 |
| Number of sessions | 1 | — |
| Overall pulses per session over auditory and non—auditory cortical areas | 1 | — |
| Neuronavigation | — | — |
Discussion
Due to ongoing discussions about the effectiveness of rTMS in chronic tinnitus and recently initiated considerations if beside methodological also technical parameters of rTMS affect treatment efficacy, we conducted a systematic review of previous research concerning daily rTMS in tinnitus with the aim to present a statistical overview of general effectiveness of rTMS and to identify the influence of rTMS parameters on the consequences of verum rTMS in tinnitus.
With respect to the question if rTMS is generally effective as a treatment in tinnitus, we demonstrated that the proportion of significant pre-post comparisons is significantly higher for verum in contrast to sham arms. The chosen statistical strategy with observed effects dichotomized to “significant” and “not significant” is limited. Meta-analyses are rather suitable as a statistical approach to resolve this question. Furthermore, the quantity of eligible studies might be too low for valid analysis at this stage. Despite two recently published large trials with contradictory findings24,25, the debate on recommendation of auditory cortex rTMS for the treatment of chronic tinnitus is still not completed.
We explored two main associations of technical parameters on rTMS efficacy. First, we found that a lower stimulation intensity was associated with significance in the investigated study arms. This finding is in contrast to earlier work in depression and basic research concerning the motor cortex, which suggested a linear dose-response relationship, e.g. better treatment response34,37 or an increased influence on motor evoked potentials (MEPs)33,38 is associated with higher stimulation intensity. A clarification of the detected reversed impact appears to be difficult. One feasible explanation for this could be, that the stimulation intensity generates the intended consequences only up to a certain extent and then the effect either disappears or inverts. Similar changes are observed by the use of cTBS, which is also known to generate inhibitory effects such as 1 Hz rTMS. Applied at higher stimulation intensities, the inhibitory effects shift to excitatory39,40. These observations somewhat corroborate our findings, although with our collected data and statistical analysis we are not able to make a statement about possible excitatory effects of high stimulation intensities.
A further possible explanation could be related to skull thickness. Compared to the rest of the cranium, the average bone thickness over temporal parts is described as the thinnest41. Due to the location of the primary motor cortex under thicker bones, namely the interface of the frontal and parietal osseous, the motor threshold at this position might be an insufficient point of reference for the determination of the stimulation intensity for other stimulation positions. With respect to our results, a high intensity rTMS over thinner temporal bones might result in some kind of hyperstimulation which may induce contrary effects as argued above. Higher stimulation intensity potentially caused by visual determination of the motor threshold42 as well as a lower intensities caused by cTBS were excluded as shown by our analysis (cf. results section).
Secondly, the present systematic review indicates, that the lower number of pulses, including auditory and prefrontal cortical areas (per session and also for the whole trial), the more significant study arms were found. With regard to TBS, past research already investigated the effects of longer stimulation protocols with the insight that a prolongation of the stimulation per se does not lead to an improvement. A doubling of the stimulation length induced reversed after-effects e.g. inhibitory became excitatory43. For rTMS, a meta-analysis reported similar results, indicating a smaller number of pulses per session related to antidepressant mechanism of action44. The authors of this meta-analysis refer to other conducted meta-analyses with no such association – the key role of the quantity of pulses remains to be clarified. The same applies for the field of tinnitus. Former studies observed a substantial improvement in tinnitus-related outcome measurements with the usage of a higher number of pulses45,46. In contrast our investigation suggests the complete opposite.
It is very probable, that the effect is conceivably caused by an additional stimulation of the DLPFC, which features significant more pulses per session (cf. results section). Based on the rationale that via prefrontal rTMS anti-depressant effects take place21, an assumed interplay of tinnitus and depression47,48 and the involvement of prefrontal areas in auditory gating and tinnitus, combined frontal and temporal rTMS was proposed for more efficient suppression of tinnitus symptoms49–51. In contrast, our results are not in accordance with these findings. Addition of prefrontal rTMS eventuated in a significantly lower percentage of significant effects. In tinnitus, not only prefrontal areas, rather several cortical regions are involved, suggesting a widely spread network and also interindividual network profiles52–55. This postulation of several involved regions offers a putative approach to explain the unfavorable effect of additional prefrontal rTMS as only a “prefrontal” subtype would best benefit from this treatment and in other subtypes it might be contraindicative. Our finding of better effects of merely temporal stimulation provides support for the notion, that the final common pathway of tinnitus related pathophysiological alterations might still be the auditory cortex56.
One might argue that initial positive findings of solely auditory cortex stimulation motivated the field to concentrate on this stimulation protocol and might have induced a publication bias in the sense that initial clinical trials are often reported as promising with large effect sizes followed by years of increasing frustration showing a decrease of effect sizes and an increase of negative trials. Our analysis indicates that there is no change in the number of significant studies published per year (see Fig. 3) not revealing a potential publication bias with a time trend.
Due to the limited amount of data we were only able to analyze the effects of single rTMS parameters alone. However, we are aware of the possibility of specific interactions of several parameters, as demonstrated by pulse-quantity-dependent after-effects of excitatory intermittent theta burst stimulation (iTBS) on MEPS to be more distinct after lower stimulation intensities57. Likewise, it may be possible, that the inequality in the number of significant (n = 56) and not significant (n = 18) study arms bears an influence on our results.
Howsoever, the role of stimulation intensities and pulses per session for the effectiveness of rTMS needs to be systematically examined to clarify the outstanding issues of dose-dependent effects in tinnitus trials, especially as these findings are contradictory to the experiences of rTMS for the treatment of depression58. One of the initial intentions of this review was to investigate the effect of different waveforms on the effectiveness of rTMS. Unfortunately, not a single study reported the used pulse waveform for the stimulation. We have therefore decided to statistically analyze the default waveform settings of the device. We found no significant association between the type of biphasic waveform and the significance level of the effect. It was stated, that the coil orientation and the related induced direction of the currents are crucial in rTMS59–61. Biphasic pulses appear to be stronger, when passing the area of interest in anterior-posterior direction28. Due to many missing in the reported data, it was not possible to analyze the critical parameter current direction.
With the emerge of new technical innovations in the field of brain stimulation, exact stimulation positions via TMS integrated neuronavigation systems became a standard procedure. This review intended to determine the benefits of TMS neuronavigation systems in tinnitus. No such benefit is implied by our results. Either the local precision of stimulation does not play such a big role as presumed, or the targets were not optimally selected. Technical parameters like intensity or pulses, with neural mechanism not entirely understood, seem to be more important.
Several considerable methodological disadvantages were observed in the course of this review. A very important parameter in order to compare outcomes of different studies and even specify the effectiveness of a specific intervention is the definition of a primary outcome. We identified a lack of this information in N = 36 study arms of interest, leading to an adapted definition of observed significance for our review. In the course of examining appropriate research papers, unexpected differences (e.g., breaks during stimulation) within the methodology of 1 Hz rTMS were observed in some of the studies62–67. Such conditions make a comparison of trials even more difficult and introduce noise to the data. A major insight of the present work is the lack of reported essential rTMS parameters in the literature. Not only the full information about the used waveform was missing, but also relevant data on coil orientation or mean motor threshold features many missing values (cf. Table 2), which restricted our analysis. Guidelines for reporting e.g. the interventional methods used in clinical trials68 or a checklist for reporting parameters when deploying TMS on the motor system69 already exist. A paper from Wilson & St George70 and a recent review focusing on rTMS in the context of depression71 already emphasize the need of fully reported methodological information. The latter even provides a checklist for reporting rTMS parameters. Since both checklists enumerate essential parameters and details for rTMS, we strongly recommend their usage in future studies, to prospectively ensure a more precise and fundamental comparison of non-invasive brain stimulation studies using rTMS.
Conclusion
The present systematic review demonstrates a higher efficacy of verum rTMS in contrast to sham rTMS. In verum arms, technical parameters such as stimulation intensity and number of pulses or restrictive stimulation of the auditory cortex were identified as relevant factors for clinical efficacy in a dose-dependent manner – less might be more. The impact of technical parameters in interaction with neurophysiological parameters (e.g., brain state before stimulation72–74) highlights the capability of rTMS in treating chronic tinnitus based on the premise to identify optimal stimulation protocols for single patients by means of personalized medical approaches75. In order to understand the consequences of considerable rTMS parameters in detail, standardized and sufficient reporting is highly required. As of yet, this is not the case – neither in tinnitus research nor in any other field utilizing rTMS24,25,45,49–51,62–67,75–119.
Supplementary information
Acknowledgements
This project was conducted as part of the European School for Interdisciplinary Tinnitus Research (ESIT) and received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement number 722046.
Author Contributions
M.S. and B.L. came up with the idea of the study. S.S. collected and analyzed the data. K.A. was involved in the literature identification process. M.S. and S.S. wrote the main manuscript. M.S. and B.L. supervised the review. Authors W.S., J.S., P.N. and B.L. contributed to and reviewed the manuscript.
Competing Interests
The authors declare that they have no conflict of interest associated with this publication and there has been no significant financial or non-financial support that could have influenced its outcome.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-48750-9.
References
- 1.Erlandsson S, Dauman N. Categorization of tinnitus in view of history and medical discourse. Int. J. Qual. Stud. Health Well-Being. 2013;8:23530. doi: 10.3402/qhw.v8i0.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall, D. A. Treatment options for subjective tinnitus: Self reports from a sample of general practitioners and ENT physicians within Europe and the USA. 15 (2011). [DOI] [PMC free article] [PubMed]
- 3.Heller AJ. Classification and epidemiology of tinnitus. Otolaryngol. Clin. North Am. 2003;36:239–248. doi: 10.1016/S0030-6665(02)00160-3. [DOI] [PubMed] [Google Scholar]
- 4.Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12:920–930. doi: 10.1016/S1474-4422(13)70160-1. [DOI] [PubMed] [Google Scholar]
- 5.Eggermont JJ. Pathophysiology of tinnitus. Prog. Brain Res. 2007;166:19–35. doi: 10.1016/S0079-6123(07)66002-6. [DOI] [PubMed] [Google Scholar]
- 6.Eggermont, J. J. & Tass, P. A. Maladaptive Neural Synchrony in Tinnitus: Origin and Restoration. Front. Neurol. 6 (2015). [DOI] [PMC free article] [PubMed]
- 7.Eggermont, J. J. & Roberts, L. E. The Neuroscience of Tinnitus: Understanding Abnormal and Normal Auditory Perception. Front. Syst. Neurosci. 6 (2012). [DOI] [PMC free article] [PubMed]
- 8.Farhadi M, et al. Functional brain abnormalities localized in 55 chronic tinnitus patients: fusion of SPECT coincidence imaging and MRI. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2010;30:864–870. doi: 10.1038/jcbfm.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folmer Robert L. Lateralization of neural activity associated with tinnitus. Neuroradiology. 2007;49(8):689–691. doi: 10.1007/s00234-007-0255-8. [DOI] [PubMed] [Google Scholar]
- 10.Vanneste S, De Ridder D. The auditory and non-auditory brain areas involved in tinnitus. An emergent property of multiple parallel overlapping subnetworks. Front. Syst. Neurosci. 2012;6:31. doi: 10.3389/fnsys.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theodoroff SM, Folmer RL. Repetitive Transcranial Magnetic Stimulation as a Treatment for Chronic Tinnitus. A Critical Review. 2013;34:10. doi: 10.1097/mao.0b013e31827b4d46. [DOI] [PubMed] [Google Scholar]
- 12.Eichhammer P, Langguth B, Marienhagen J, Kleinjung T, Hajak G. Neuronavigated repetitive transcranial magnetic stimulation in patients with tinnitus: a short case series. Biol. Psychiatry. 2003;54:862–865. doi: 10.1016/S0006-3223(02)01896-6. [DOI] [PubMed] [Google Scholar]
- 13.Langguth B, et al. Neuronavigated rTMS in a patient with chronic tinnitus. Effects of 4 weeks treatment. Neuroreport. 2003;14:977–980. doi: 10.1097/00001756-200305230-00014. [DOI] [PubMed] [Google Scholar]
- 14.Langguth B, De Ridder D. Tinnitus: therapeutic use of superficial brain stimulation. Handb. Clin. Neurol. 2013;116:441–467. doi: 10.1016/B978-0-444-53497-2.00036-X. [DOI] [PubMed] [Google Scholar]
- 15.Peng Z, Chen X-Q, Gong S-S. Effectiveness of Repetitive Transcranial Magnetic Stimulation for Chronic Tinnitus: A Systematic Review. Otolaryngol.-Head Neck Surg. 2012;147:817–825. doi: 10.1177/0194599812458771. [DOI] [PubMed] [Google Scholar]
- 16.Plewnia C. Brain Stimulation: New Vistas for the Exploration and Treatment of Tinnitus: Brain Stimulation. CNS Neurosci. Ther. 2011;17:449–461. doi: 10.1111/j.1755-5949.2010.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langguth B, et al. Controversy: Does repetitive transcranial magnetic stimulation/transcranial direct current stimulation show efficacy in treating tinnitus patients? Brain Stimulat. 2008;1:192–205. doi: 10.1016/j.brs.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Londero A, Bonfils P, Lefaucheur JP. Transcranial magnetic stimulation and subjective tinnitus. A review of the literature, 2014–2016. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018;135:51–58. doi: 10.1016/j.anorl.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson PH, Rinehart NJ, Enticott PG. Noninvasive stimulation of the temporoparietal junction: A systematic review. Neurosci. Biobehav. Rev. 2015;55:547–572. doi: 10.1016/j.neubiorev.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Soleimani R, Jalali MM, Hasandokht T. Therapeutic impact of repetitive transcranial magnetic stimulation (rTMS) on tinnitus: a systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2016;273:1663–1675. doi: 10.1007/s00405-015-3642-5. [DOI] [PubMed] [Google Scholar]
- 21.Lefaucheur J-P, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin. Neurophysiol. 2014;125:2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Meng Z, Liu S, Zheng Y, Phillips JS. Repetitive transcranial magnetic stimulation for tinnitus. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD007946.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Zenner H-P, et al. A multidisciplinary systematic review of the treatment for chronic idiopathic tinnitus. Eur. Arch. Otorhinolaryngol. 2017;274:2079–2091. doi: 10.1007/s00405-016-4401-y. [DOI] [PubMed] [Google Scholar]
- 24.Folmer RL, et al. Repetitive Transcranial Magnetic Stimulation Treatment for Chronic Tinnitus: A Randomized Clinical Trial. JAMA Otolaryngol.–Head Neck Surg. 2015;141:716–722. doi: 10.1001/jamaoto.2015.1219. [DOI] [PubMed] [Google Scholar]
- 25.Landgrebe M, et al. 1-Hz rTMS in the treatment of tinnitus: A sham-controlled, randomized multicenter trial. Brain Stimulat. 2017;10:1112–1120. doi: 10.1016/j.brs.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Folmer RL. Factors that contribute to the efficacy of repetitive transcranial magnetic stimulation (rTMS) for tinnitus treatment. Brain Stimulat. 2017;10:1121–1122. doi: 10.1016/j.brs.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Landgrebe M, Schecklmann M, Langguth B. Reply to the letter of Robert L. Folmer: Does treatment response depend on the type of stimulation device? Brain Stimulat. 2017;10:1123–1124. doi: 10.1016/j.brs.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin. Neurophysiol. 2001;112:250–258. doi: 10.1016/S1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- 29.Sommer M, Paulus W. TMS waveform and current direction. Oxf. Handb. Transcranial Stimul. 2008 doi: 10.1093/oxfordhb/9780198568926.013.0002. [DOI] [Google Scholar]
- 30.Rossini PM, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman RE, Cavus I. Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. Am. J. Psychiatry. 2002;159:1093–1102. doi: 10.1176/appi.ajp.159.7.1093. [DOI] [PubMed] [Google Scholar]
- 32.Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain J. Neurol. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald P. Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin. Neurophysiol. 2002;113:1136–1141. doi: 10.1016/S1388-2457(02)00145-1. [DOI] [PubMed] [Google Scholar]
- 34.Padberg F, et al. Repetitive Transcranial Magnetic Stimulation (rTMS) in Major Depression: Relation between Efficacy and Stimulation Intensity. Neuropsychopharmacology. 2002;27:638–645. doi: 10.1016/S0893-133X(02)00355-X. [DOI] [PubMed] [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:6. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jennions MD, Møller AP. Relationships fade with time: a meta-analysis of temporal trends in publication in ecology and evolution. Proc. R. Soc. B Biol. Sci. 2002;269:43–48. doi: 10.1098/rspb.2001.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzgerald PB, Hoy KE, Anderson RJ, Daskalakis ZJ. A study of the pattern of response to Rtms treatment in depression. Depress. Anxiety. 2016;33:746–753. doi: 10.1002/da.22503. [DOI] [PubMed] [Google Scholar]
- 38.Lang N, et al. Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clin. Neurophysiol. 2006;117:2292–2301. doi: 10.1016/j.clinph.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki T, et al. The intensity of continuous theta burst stimulation, but not the waveform used to elicit motor evoked potentials, influences its outcome in the human motor cortex. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation. 2018;11:400–410. doi: 10.1016/j.brs.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of Human Corticospinal Excitability Induced by Magnetic Theta-burst Stimulation: Evidence of Rapid Polarity-Reversing Metaplasticity. Cereb. Cortex. 2008;18:2046–2053. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- 41.Mahinda HAM, Murty OP. Variability in thickness of human skull bones and sternum – an autopsy experience. J. Forensic Med. 2009;26:7. [Google Scholar]
- 42.Westin GG, Bassi BD, Lisanby SH, Luber B. Determination of motor threshold using visual observation overestimates transcranial magnetic stimulation dosage: Safety implications. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2014;125:142–147. doi: 10.1016/j.clinph.2013.06.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gamboa OL, Antal A, Moliadze V, Paulus W. Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp. Brain Res. Exp. Hirnforsch. Exp. Cerebrale. 2010;204:181–187. doi: 10.1007/s00221-010-2293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kedzior, Azorina, V. & Reitz, S. More female patients and fewer stimuli per session are associated with the short-term antidepressant properties of repetitive transcranial magnetic stimulation (rTMS): a meta-analysis of 54 sham-controlled studies published between 1997–2013. Neuropsychiatr. Dis. Treat. 727, 10.2147/NDT.S58405 (2014). [DOI] [PMC free article] [PubMed]
- 45.Park JH, et al. Difference in Tinnitus Treatment Outcome According to the Pulse Number of Repetitive Transcranial Magnetic Stimulation. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2015;36:1450–1456. doi: 10.1097/MAO.0000000000000802. [DOI] [PubMed] [Google Scholar]
- 46.Plewnia C, et al. Dose-dependent attenuation of auditory phantom perception (tinnitus) by PET-guided repetitive transcranial magnetic stimulation. Hum. Brain Mapp. 2007;28:238–246. doi: 10.1002/hbm.20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geocze L, Mucci S, Abranches DC, Marco MA, Penido NO. Systematic review on the evidences of an association between tinnitus and depression. Braz. J. Otorhinolaryngol. 2013;79:106–111. doi: 10.5935/1808-8694.20130018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziai K, Moshtaghi O, Mahboubi H, Djalilian HR. Tinnitus Patients Suffering from Anxiety and Depression: A Review. Int. Tinnitus J. 2017;21:68–73. doi: 10.5935/0946-5448.20170013. [DOI] [PubMed] [Google Scholar]
- 49.Kleinjung T, et al. Combined temporal and prefrontal transcranial magnetic stimulation for tinnitus treatment: a pilot study. Otolaryngol.–Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2008;138:497–501. doi: 10.1016/j.otohns.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Langguth B, et al. Efficacy of different protocols of transcranial magnetic stimulation for the treatment of tinnitus: Pooled analysis of two randomized controlled studies. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry. 2014;15:276–285. doi: 10.3109/15622975.2012.708438. [DOI] [PubMed] [Google Scholar]
- 51.Lehner A, et al. Multisite rTMS for the treatment of chronic tinnitus: stimulation of the cortical tinnitus network–a pilot study. Brain Topogr. 2013;26:501–510. doi: 10.1007/s10548-012-0268-4. [DOI] [PubMed] [Google Scholar]
- 52.Adjamian P, Sereda M, Hall DA. The mechanisms of tinnitus: Perspectives from human functional neuroimaging. Hear. Res. 2009;253:15–31. doi: 10.1016/j.heares.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Elgoyhen AB, Langguth B, De Ridder D, Vanneste S. Tinnitus: perspectives from human neuroimaging. Nat. Rev. Neurosci. 2015;16:632–642. doi: 10.1038/nrn4003. [DOI] [PubMed] [Google Scholar]
- 54.Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear. Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 55.Schlee W, et al. Mapping cortical hubs in tinnitus. BMC Biol. 2009;7:80. doi: 10.1186/1741-7007-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schecklmann M, et al. Auditory cortex is implicated in tinnitus distress: a voxel-based morphometry study. Brain Struct. Funct. 2013;218:1061–1070. doi: 10.1007/s00429-013-0520-z. [DOI] [PubMed] [Google Scholar]
- 57.Nettekoven C, et al. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J. Neurosci. Off. J. Soc. Neurosci. 2014;34:6849–6859. doi: 10.1523/JNEUROSCI.4993-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am. J. Psychiatry. 2003;160:835–845. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- 59.Kammer T, Beck S, Erb M, Grodd W. The influence of current direction on phosphene thresholds evoked by transcranial magnetic stimulation. Clin. Neurophysiol. 2001;112:2015–2021. doi: 10.1016/S1388-2457(01)00673-3. [DOI] [PubMed] [Google Scholar]
- 60.Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-T. [DOI] [PubMed] [Google Scholar]
- 61.Sale MV, Lavender AP, Opie GM, Nordstrom MA, Semmler JG. Increased intracortical inhibition in elderly adults with anterior–posterior current flow: A TMS study. Clin. Neurophysiol. 2016;127:635–640. doi: 10.1016/j.clinph.2015.04.062. [DOI] [PubMed] [Google Scholar]
- 62.Kim BG, et al. Comparison of the outcomes of repetitive transcranial magnetic stimulation to the ipsilateral and contralateral auditory cortex in unilateral tinnitus. Electromagn. Biol. Med. 2014;33:211–215. doi: 10.3109/15368378.2013.801353. [DOI] [PubMed] [Google Scholar]
- 63.Kim HJ, et al. Long-term effects of repetitive transcranial magnetic stimulation in unilateral tinnitus. The Laryngoscope. 2014;124:2155–2160. doi: 10.1002/lary.24722. [DOI] [PubMed] [Google Scholar]
- 64.Piccirillo JF, et al. Low-frequency repetitive transcranial magnetic stimulation to the temporoparietal junction for tinnitus. Arch. Otolaryngol. Head Neck Surg. 2011;137:221–228. doi: 10.1001/archoto.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piccirillo JF, et al. Low-frequency repetitive transcranial magnetic stimulation to the temporoparietal junction for tinnitus: four-week stimulation trial. JAMA Otolaryngol.–Head Neck Surg. 2013;139:388–395. doi: 10.1001/jamaoto.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossi S, et al. Effects of repetitive transcranial magnetic stimulation on chronic tinnitus: a randomised, crossover, double blind, placebo controlled study. J. Neurol. Neurosurg. Psychiatry. 2007;78:857–863. doi: 10.1136/jnnp.2006.105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang H, et al. The Characteristic and Changes of the Event-Related Potentials (ERP) and Brain Topographic Maps before and after Treatment with rTMS in Subjective Tinnitus Patients. PLoS ONE. 2013;8:e70831. doi: 10.1371/journal.pone.0070831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J. Pharmacol. Pharmacother. 2010;1:100–107. doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chipchase L, et al. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: An international consensus study. Clin. Neurophysiol. 2012;123:1698–1704. doi: 10.1016/j.clinph.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson, M. T. & St George, L. Repetitive Transcranial Magnetic Stimulation: A Call for Better Data. Front. Neural Circuits10 (2016). [DOI] [PMC free article] [PubMed]
- 71.Chantebel Romain, Chesneau Adélise, Tavernier Elsa, El-Hage Wissam, Caille Agnès. Completeness of Descriptions of Repetitive Transcranial Magnetic Stimulation Intervention. The Journal of ECT. 2019;35(1):7–13. doi: 10.1097/YCT.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 72.Weisz N, Steidle L, Lorenz I. Formerly known as inhibitory: effects of 1-Hz rTMS on auditory cortex are state-dependent: Formerly known as inhibitory. Eur. J. Neurosci. 2012;36:2077–2087. doi: 10.1111/j.1460-9568.2012.08097.x. [DOI] [PubMed] [Google Scholar]
- 73.Siebner HR, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J. Neurosci. Off. J. Soc. Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silvanto J, Pascual-Leone A. State-Dependency of Transcranial Magnetic Stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kreuzer PM, et al. Combined rTMS treatment targeting the Anterior Cingulate and the Temporal Cortex for the Treatment of Chronic Tinnitus. Sci. Rep. 2015;5:18028. doi: 10.1038/srep18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vielsmeier V, et al. A Pilot Study of Peripheral Muscle Magnetic Stimulation as Add-on Treatment to Repetitive Transcranial Magnetic Stimulation in Chronic Tinnitus. Front. Neurosci. 2018;12:68. doi: 10.3389/fnins.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cacace AT, et al. Glutamate is down-regulated and tinnitus loudness-levels decreased following rTMS over auditory cortex of the left hemisphere: A prospective randomized single-blinded sham-controlled cross-over study. Hear. Res. 2017 doi: 10.1016/j.heares.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 78.James GA, et al. Neural activity during attentional conflict predicts reduction in tinnitus perception following rTMS. Brain Stimulat. 2017;10:934–943. doi: 10.1016/j.brs.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noh T-S, et al. Comparison of treatment outcomes between 10 and 20 EEG electrode location system-guided and neuronavigation-guided repetitive transcranial magnetic stimulation in chronic tinnitus patients and target localization in the Asian brain. Acta Otolaryngol. (Stockh.) 2017;137:945–951. doi: 10.1080/00016489.2017.1316870. [DOI] [PubMed] [Google Scholar]
- 80.Kreuzer PM, et al. Individualized Repetitive Transcranial Magnetic Stimulation Treatment in Chronic Tinnitus? Front. Neurol. 2017;8:126. doi: 10.3389/fneur.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noh T-S, et al. Comparison of Treatment Outcomes Following Either Prefrontal Cortical-only or Dual-site Repetitive Transcranial Magnetic Stimulation in Chronic Tinnitus Patients: A Double-blind Randomized Study. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2017;38:296–303. doi: 10.1097/MAO.0000000000001266. [DOI] [PubMed] [Google Scholar]
- 82.Wang H, et al. Factor Analysis of Low-Frequency Repetitive Transcranial Magnetic Stimulation to the Temporoparietal Junction for Tinnitus. Neural Plast. 2016;2016:2814056. doi: 10.1155/2016/2814056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Labar D, Labar AS, Edwards D. Long-Term Distributed Repetitive Transcranial Magnetic Stimulation for Tinnitus: A Feasibility Study. Neuromodulation J. Int. Neuromodulation Soc. 2016;19:249–253. doi: 10.1111/ner.12390. [DOI] [PubMed] [Google Scholar]
- 84.Wang H, Li B, Wu H, Shi H, Yin S. Combination of gaps in noise detection and visual analog scale for measuring tinnitus components in patients treated with repetitive transcranial magnetic stimulation. Auris. Nasus. Larynx. 2016;43:254–258. doi: 10.1016/j.anl.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 85.Lo YL, et al. A comparison study of repetitive transcranial magnetic stimulation for tinnitus treatment in an Asian population. Clin. Neurol. Neurosurg. 2014;119:96–99. doi: 10.1016/j.clineuro.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 86.Bilici S, Yigit O, Taskin U, Gor AP, Yilmaz ED. Medium-term results of combined treatment with transcranial magnetic stimulation and antidepressant drug for chronic tinnitus. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. EUFOS Affil. Ger. Soc. Oto-Rhino-Laryngol. - Head Neck Surg. 2015;272:337–343. doi: 10.1007/s00405-013-2851-z. [DOI] [PubMed] [Google Scholar]
- 87.Ting SKS, et al. Short duration repetitive transcranial magnetic stimulation for tinnitus treatment: a prospective Asian study. Clin. Neurol. Neurosurg. 2011;113:556–558. doi: 10.1016/j.clineuro.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 88.Anders M, et al. Efficacy of repetitive transcranial magnetic stimulation for the treatment of refractory chronic tinnitus: a randomized, placebo controlled study. Neuro Endocrinol. Lett. 2010;31:238–249. [PubMed] [Google Scholar]
- 89.Kleinjung T, et al. Which tinnitus patients benefit from transcranial magnetic stimulation? Otolaryngol.–Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2007;137:589–595. doi: 10.1016/j.otohns.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 90.Wang H, et al. A Pilot Study of EEG Source Analysis Based Repetitive Transcranial Magnetic Stimulation for the Treatment of Tinnitus. Plos One. 2015;10:e0139622. doi: 10.1371/journal.pone.0139622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Langguth B, et al. Transcranial Magnetic Stimulation for the Treatment of Tinnitus: A New Coil Positioning Method and First Results. Brain Topogr. 2006;18:241–247. doi: 10.1007/s10548-006-0002-1. [DOI] [PubMed] [Google Scholar]
- 92.Chung H-K, et al. Effectiveness of Theta-Burst Repetitive Transcranial Magnetic Stimulation for Treating Chronic Tinnitus. Audiol. Neurotol. 2012;17:112–120. doi: 10.1159/000330882. [DOI] [PubMed] [Google Scholar]
- 93.Barwood CHS, et al. The Effect of rTMS on Auditory Processing in Adults with Chronic, Bilateral Tinnitus: A Placebo-Controlled Pilot Study. Brain Stimulat. 2013;6:752–759. doi: 10.1016/j.brs.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 94.De Ridder D, Song J-J, Vanneste S. Frontal cortex TMS for tinnitus. Brain Stimulat. 2013;6:355–362. doi: 10.1016/j.brs.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 95.Forogh B, Yazdi-Bahri S-M, Ahadi T, Fereshtehnejad S-M, Raissi GR. Comparison of two protocols of transcranial magnetic stimulation for treatment of chronic tinnitus: a randomized controlled clinical trial of burst repetitive versus high-frequency repetitive Transcranial Magnetic Stimulation. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2014;35:227–232. doi: 10.1007/s10072-013-1487-5. [DOI] [PubMed] [Google Scholar]
- 96.Hoekstra CEL, Versnel H, Neggers SFW, Niesten MEF, van Zanten GA. Bilateral low-frequency repetitive transcranial magnetic stimulation of the auditory cortex in tinnitus patients is not effective: a randomised controlled trial. Audiol. Neurootol. 2013;18:362–373. doi: 10.1159/000354977. [DOI] [PubMed] [Google Scholar]
- 97.Khedr EM, et al. Contralateral versus ipsilateral rTMS of temporoparietal cortex for the treatment of chronic unilateral tinnitus: comparative study. Eur. J. Neurol. 2010;17:976–983. doi: 10.1111/j.1468-1331.2010.02965.x. [DOI] [PubMed] [Google Scholar]
- 98.Khedr EM, Rothwell JC, Ahmed MA, El-Atar A. Effect of daily repetitive transcranial magnetic stimulation for treatment of tinnitus: comparison of different stimulus frequencies. J. Neurol. Neurosurg. Psychiatry. 2008;79:212–215. doi: 10.1136/jnnp.2007.127712. [DOI] [PubMed] [Google Scholar]
- 99.Kleinjung T, et al. Repetitive transcranial magnetic stimulation for tinnitus treatment: no enhancement by the dopamine and noradrenaline reuptake inhibitor bupropion. Brain Stimulat. 2011;4:65–70. doi: 10.1016/j.brs.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 100.Kleinjung T, et al. Levodopa does not enhance the effect of low-frequency repetitive transcranial magnetic stimulation in tinnitus treatment. Otolaryngol.–Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2009;140:92–95. doi: 10.1016/j.otohns.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 101.Kleinjung T, et al. Long-term effects of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic tinnitus. Otolaryngol.–Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2005;132:566–569. doi: 10.1016/j.otohns.2004.09.134. [DOI] [PubMed] [Google Scholar]
- 102.Kreuzer PM, et al. A proof-of-concept study on the combination of repetitive transcranial magnetic stimulation and relaxation techniques in chronic tinnitus. J. Neural Transm. Vienna Austria. 2016;1996(123):1147–1157. doi: 10.1007/s00702-016-1588-4. [DOI] [PubMed] [Google Scholar]
- 103.Kreuzer PM, et al. Can Temporal Repetitive Transcranial Magnetic Stimulation be Enhanced by Targeting Affective Components of Tinnitus with Frontal rTMS? A Randomized Controlled Pilot Trial. Front. Syst. Neurosci. 2011;5:88. doi: 10.3389/fnsys.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Langguth B, et al. High-frequency priming stimulation does not enhance the effect of low-frequency rTMS in the treatment of tinnitus. Exp. Brain Res. 2008;184:587–591. doi: 10.1007/s00221-007-1228-1. [DOI] [PubMed] [Google Scholar]
- 105.Lehner A, Schecklmann M, Greenlee MW, Rupprecht R, Langguth B. Triple-site rTMS for the treatment of chronic tinnitus: a randomized controlled trial. Sci. Rep. 2016;6:22302. doi: 10.1038/srep22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marcondes RA, et al. Repetitive transcranial magnetic stimulation improve tinnitus in normal hearing patients: a double-blind controlled, clinical and neuroimaging outcome study. Eur. J. Neurol. 2010;17:38–44. doi: 10.1111/j.1468-1331.2009.02730.x. [DOI] [PubMed] [Google Scholar]
- 107.Mennemeier M, et al. Variable changes in PET activity before and after rTMS treatment for tinnitus. The Laryngoscope. 2011;121:815–822. doi: 10.1002/lary.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park S, et al. Combined rTMS to the auditory cortex and prefrontal cortex for tinnitus control in patients with depression: a pilot study. Acta Otolaryngol. (Stockh.) 2013;133:600–606. doi: 10.3109/00016489.2012.763181. [DOI] [PubMed] [Google Scholar]
- 109.Plewnia C, et al. Treatment of chronic tinnitus with theta burst stimulation: a randomized controlled trial. Neurology. 2012;78:1628–1634. doi: 10.1212/WNL.0b013e3182574ef9. [DOI] [PubMed] [Google Scholar]
- 110.Plewnia C, et al. Moderate therapeutic efficacy of positron emission tomography-navigated repetitive transcranial magnetic stimulation for chronic tinnitus: a randomised, controlled pilot study. J. Neurol. Neurosurg. Psychiatry. 2007;78:152–156. doi: 10.1136/jnnp.2006.095612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roland LT, Peelle JE, Kallogjeri D, Nicklaus J, Piccirillo JF. The effect of noninvasive brain stimulation on neural connectivity in Tinnitus: A randomized trial. The Laryngoscope. 2016;126:1201–1206. doi: 10.1002/lary.25650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sahlsten H, et al. Electric field-navigated transcranial magnetic stimulation for chronic tinnitus: a randomized, placebo-controlled study. Int. J. Audiol. 2017;56:692–700. doi: 10.1080/14992027.2017.1313461. [DOI] [PubMed] [Google Scholar]
- 113.Sahlsten H, et al. Electric field navigated transcranial magnetic stimulation for chronic tinnitus: A pilot study. Int. J. Audiol. 2015;54:899–909. doi: 10.3109/14992027.2015.1054041. [DOI] [PubMed] [Google Scholar]
- 114.Schecklmann M, et al. Neuronavigated left temporal continuous theta burst stimulation in chronic tinnitus. Restor. Neurol. Neurosci. 2016;34:165–175. doi: 10.3233/RNN-150518. [DOI] [PubMed] [Google Scholar]
- 115.Smith JA, et al. Repetitive transcranial magnetic stimulation for tinnitus: a pilot study. The Laryngoscope. 2007;117:529–534. doi: 10.1097/MLG.0b013e31802f4154. [DOI] [PubMed] [Google Scholar]
- 116.Vanneste S, De Ridder D. Differences between a single session and repeated sessions of 1 Hz TMS by double-cone coil prefrontal stimulation for the improvement of tinnitus. Brain Stimulat. 2013;6:155–159. doi: 10.1016/j.brs.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 117.Yilmaz M, Yener MH, Turgut NF, Aydin F, Altug T. Effectiveness of transcranial magnetic stimulation application in treatment of tinnitus. J. Craniofac. Surg. 2014;25:1315–1318. doi: 10.1097/SCS.0000000000000782. [DOI] [PubMed] [Google Scholar]
- 118.Thabit MN, Fouad N, Shahat B, Youssif M. Combined Central and Peripheral Stimulation for Treatment of Chronic Tinnitus: A Randomized Pilot Study. Neurorehabil. Neural Repair. 2015;29:224–233. doi: 10.1177/1545968314542616. [DOI] [PubMed] [Google Scholar]
- 119.Formánek M, et al. Combined transcranial magnetic stimulation in the treatment of chronic tinnitus. Ann. Clin. Transl. Neurol. 2018;5:857–864. doi: 10.1002/acn3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.