Abstract
The past few years have witnessed a fast-growing research interest on the study of sulfonyl fluorides as reactive probes in chemical biology and molecular pharmacology, which raises an urgent need for the development of effective synthetic methods to expand the toolkit. Herein, we present the invention of a facile and general approach for the synthesis of aliphatic sulfonyl fluorides via visible-light-mediated decarboxylative fluorosulfonylethylation. The method is based on abundant carboxylic acid feed stock, applicable to various alkyl carboxylic acids including primary, secondary, and tertiary acids, and is also suitable for the modification of natural products like amino acids, peptides, as well as drugs, forging a rapid, metal-free approach to build sulfonyl fluoride compound libraries of considerable structural diversity. Further diversification of the SO2F-containing products is also demonstrated, which allows for access to a range of pharmaceutically important motifs such as sultam, sulfonate, and sulfonamide.
Subject terms: Chemical biology, Drug discovery, Chemistry
Sulfonyl fluorides are important probes in chemical biology and molecular pharmacology. Here, the authors report a mild visible light-mediated decarboxylative fluorosulfonylethylation for the synthesis of aliphatic sulfonyl fluorides from a wide range of carboxylic acids, including natural products and drug derivatives.
Introduction
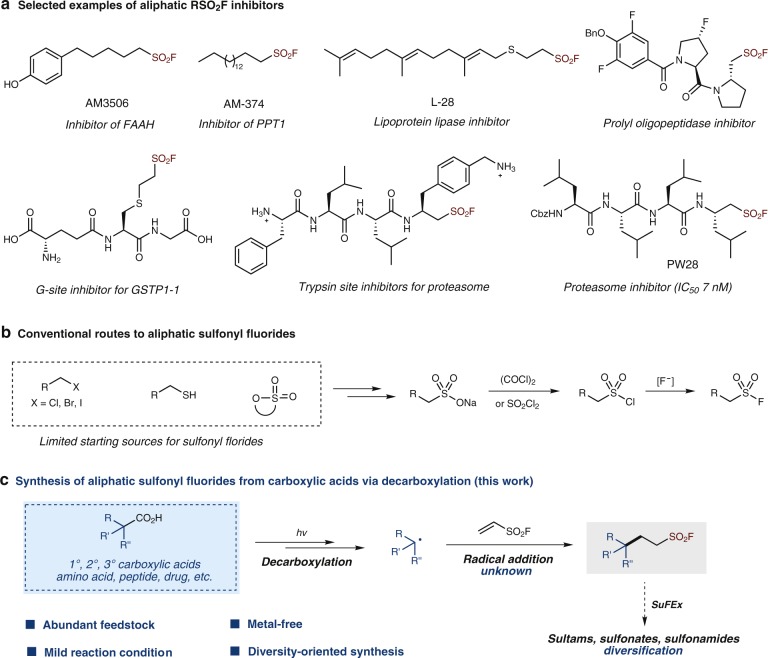
Rapid assembly of compound libraries with high efficiency and diversity is critical to drug discovery. By virtue of the combination of strong thermodynamic driving forces and well-controlled reaction pathways, click reactions afford bond construction in an operationally simple, modular, efficient, and reliable manner. Since the introduction of the conceptual framework by Sharpless and co-workers, click chemistry has gained a great impact on many areas in the last two decades, not only organic synthesis but also biology, medicine, and materials science1. Among various reactions identified for click chemistry, Sulfur(VI) Fluoride Exchange (SuFEx) represents the latest and also one of the most powerful click reactions2–5. Next to the recent significant progress in synthetic methodology development and polymer preparation6–11, another intriguing application of SuFEx chemistry could be the use of sulfonyl fluorides in chemical biology and molecular pharmacology as a privileged type of warheads12–21. The unique properties observed with sulfonyl fluorides could be ascribed to (i) the right balance of inherent electrophilic reactivity of SVI–F and stability under physiological aqueous conditions, and (ii) the proton (H+)-mediated reactivity-switch-on mechanism that makes its reactivity sensitive to the microenvironment of the binding site, and thus allows a site-specific targeting under various chemical and biological contexts2,12–14. The successful identification of their highly selective inhibition activity has boosted a fast-growing research interest in recent years to develop enzyme inhibitors or chemical probes based on sulfonyl fluorides12–26. However, the development in this area is significantly hampered by the limited availability of sulfonyl fluorides12–14. The potential observed with aliphatic sulfonyl fluorides, in particular the peptide-type inhibitors12,22–26 (Fig. 1a), has drawn our attention from a synthetic point of view. Typically, aliphatic sulfonyl fluorides are prepared via fluoride-chloride exchange with the corresponding sulfonyl chlorides, which can be prepared from thiols, halides, or sultones (Fig. 1b). However, these methods have very limited sources of starting compounds2. Accordingly, a general and facile synthetic route to expand the toolkit of sulfonyl fluorides on both dimensions of synthetic efficiency and structural diversity is in high demand12–14.
Fig. 1.
Aliphatic RSO2F inhibitors and the synthetic strategies. a Selected examples of aliphatic sulfonyl fluoride inhibitors. b Conventional synthetic routes to aliphatic sulfonyl fluorides. c Synthesis of aliphatic sulfonyl fluorides from carboxylic acids via visible-light-mediated decarboxylation (this work)
Carboxylic acid is ubiquitous in nature, and widely present in natural products, medicines, and materials. The merits such as stability, low toxicity, and commercial availability make carboxylic acids as an ideal type of building blocks in organic synthesis27. We conceived that the combination of carboxylic acid as a radical source with an appropriate SO2F-containing radical acceptor would forge a general approach to various sulfonyl fluorides (Fig. 1c). With this aim in mind, we found carboxylic acids are known to readily undergo decarboxylation to release alkyl radicals after activated in the form of N-hydroxyphthalimide (NHPI) esters28–30 via photocatalysis31–43 or low-valent transition metal catalysis44–48. While vinyl sulfonyl fluoride (VSF), a readily available SO2F-containing reagent49–54, could potentially be employed as the radical acceptor, though its utilization and compatibility in the radical reactions is unknown. Herein, we report our effort on this approach and the invention of a facile and general radical method for the synthesis of aliphatic sulfonyl fluorides based on naturally abundant carboxylic acid feed stock. This visible-light-mediated transformation features its high efficiency and a broad reaction scope. The wide availability of carboxylic acids, including amino acids, peptides, and many pharmaceuticals, allows for a fast construction of aliphatic sulfonyl fluoride libraries of considerable structural diversity. Further diversification of the SO2F-containing products is also demonstrated in the derivatization to pharmaceutically important motifs such as sultams, sulfonates, and sulfonamides.
Results
Reaction optimization
We commenced our study by using dihydrocinnamic acid-derived redox active ester (1) as the model substrate and diisopropylethylamine (DIPEA) as a reductant for photocatalyst screening. As shown in Table 1, with the commonly used transition metal photocatalysts, fac-Ir(ppy)3 gave the desired product in 29% yield (entry 1), while lower yield (24%) was obtained with [Ru(bpy)3]Cl2·6H2O (entry 2). To our delight, eosin Y, a cheap and metal-free organic dye could furnish a slightly better yield (35%, entry 3). Importantly, this metal-free system could avoid the transition metal contaminant which may bring about detrimental effect in biological and related applications55. Encouraged by this result, more organic dyes were screened (entries 4–7), such as fluorescein, rhodamine B, rhodamine 6G, riboflavin and isatin, but none of them could give a better yield. Further increasing the VSF loading to five equivalents and the catalyst loading to 10 mol% could slightly improve the yield from 35 to 38%, but the improvement was quite limited (entry 8 vs. entry 3). Nevertheless, the disodium salt of eosin Y was identified slightly more efficient than eosin Y, giving 37% yield with two equivalents of VSF only and a lower catalyst loading (5 mol%, entry 9).
Table 1.
Reaction optimization (Reaction conditions: 0.05 mmol scale, VSF (2 eq), photocatalyst (5 mol%), in MeCN (0.5 mL) at room temperature, under the irradiation of 18 W × 2 blue LED bulbs. Left entries: with DIPEA (2 eq) as the reductant. Right entries: with Eosin Y-Na2 (5 mol%) as the catalyst)
|
| |||||
|---|---|---|---|---|---|
| Entry | Photocatalyst | Yielda | Entry | Reductant | Yielda |
| 1 | Ir(ppy)3 | 29% | 10 | TEA | <2% |
| 2 | [Ru(bpy)3]Cl2·6H2O | 24% | 11 | DMA | N.P. |
| 3 | Eosin Y | 35% | 12 | TMEDA | <2% |
| 4 | Fluorescein | 26% | 13 | PMDETA | N.P. |
| 5 | Rhodamine B | 17% | 14 | HE | 89% |
| 6 | Rhodamine 6G | 26% | 15 | HE/DIPEA 2:1 | 73% |
| 7 | Isatin | 32% | 16 | HE/TEA 2:1 | 29% |
| 8b | Eosin Y | 38% | 17c | HE | 69% |
| 9 | Eosin Y-Na2 | 37% | 18d | HE | N.P. |
DMA N,Nʹ-dimethylaniline, TMEDA N,N,Nʹ,Nʹ-tetramethylethanediamine, PMDETA N,N,Nʹ,Nʺ,Nʺ-pentamethyldiethylenetriamine
aDetermined by 19F NMR with PhCF3 as an internal standard or by GC-MS analysis
bEosin Y (10 mol%), VSF (5 eq), in DCM, green LED, 18 h
cWith 1 eq of VSF
dIn dark, N.P. = no product was observed
Vinyl sulfonyl fluoride (VSF) is known about six orders of magnitude more reactive than vinyl phenyl sulfone as a Michael acceptor51; however, under this photoredox catalytic reaction condition, the yield (37%) was even lower than that of vinyl phenyl sulfone (45%), showing no correlation to the acceptor activity in this reaction. To our surprise, following the reaction by 19F NMR unveiled a range of mass peaks between 50 to 60 ppm, which suggested severe side reactions happened, probably due to the high reactivity of VSF and its poor compatibility with the photoredox conditions. Further reaction optimization (reductant amines, solvent, light source, etc.; see Supplementary Table 1–3) was thus performed. Surprisingly, when DIPEA was replaced with triethylamine (TEA), almost no product formed (entry 10). This dramatic effect of amine reductant was unexpected, which urged us to follow the reaction by NMR analysis and also carry out several control experiments (see Supplementary Discussion). In both cases of DIPEA and TEA, the redox active ester 1 were completely consumed after 12 h, and almost no VSF left, so the low yields did not result from low conversion. Notably, for control reactions without redox active ester 1, the 19F NMR also showed the disappearance of the peak of VSF, indicating that VSF may be unstable and prone to polymerize56 in the presence of DIPEA or TEA under the photoredox conditions. Therefore, more amines were tested, and finally, Hantzsch ester (HE, diethyl 1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate) was found to be uniquely beneficial to the reaction, able to shut down the undesired reaction pathways and afford 3 in a decent yield of 89% (entry 14), probably by a fast hydrogen atom transfer process to the radical adduct (for mechanistic study, isotope-labeling experiments and a proposed mechanism, please see Supplementary Discussion and Supplementary Figs. 3–5). NMR experiments showed that VSF was much more stable in the presence of HE, and even till the end of the reaction, more than 90% of the excessive one equivalent of VSF remained intact. Moreover, mixing VSF and HE without 1 under the same irradiation conditions for 12 h showed no detectable loss of VSF, in sharp contrast to the cases of DIPEA and TEA (for comparison, see Supplementary Figs. 1 and 2). The addition of DIPEA or TEA was even detrimental to the reaction, leading to a dramatic drop in yield (entries 15 and 16); further confirming this catalytic system (Eosin Y-Na2/HE) is superior to the known Eosin Y/DIPEA combination to ensure a high reaction yield. It is worth mentioning that the product could be obtained in 69% yield with one equivalent of VSF only (entry 17), while without light no reaction was observed (entry 18).
Substrate scope
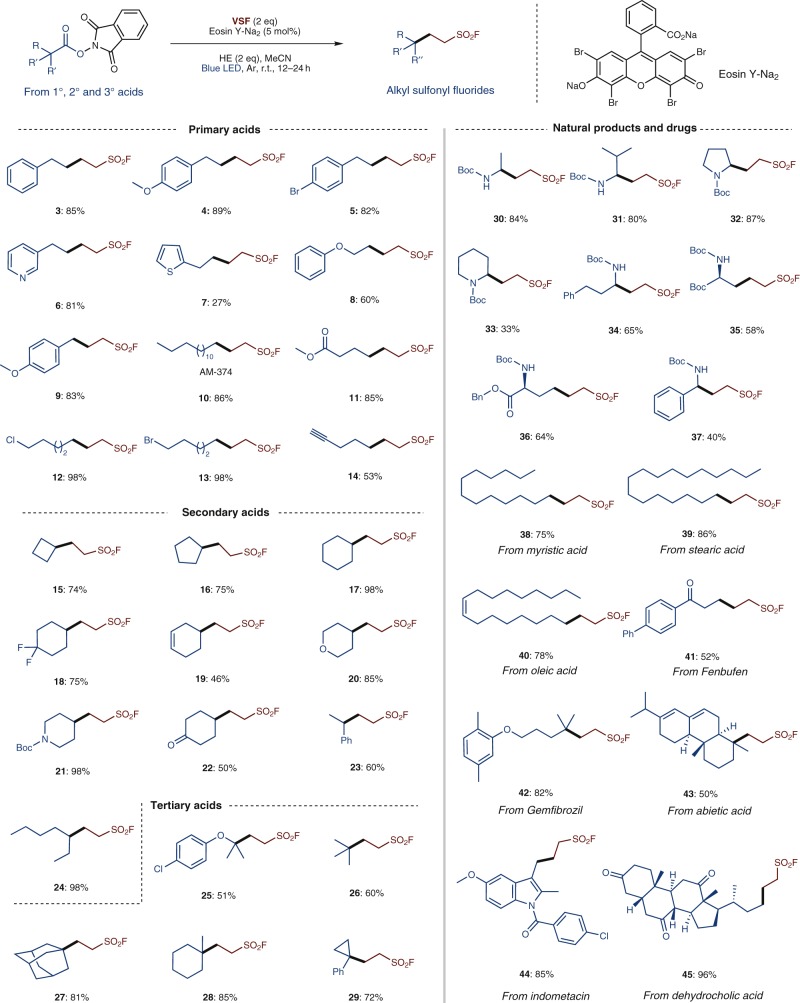
With the catalytic synthetic method (Eosin Y-Na2/HE) successfully established, we next examined the reaction scope with a variety of primary, secondary and tertiary acids (Fig. 2). In cases of primary acids, bromide (5 and 13) and chloride substituents (12) were well tolerated under the present photoredox conditions and excellent yields can be achieved. Acids containing a heteroarene group like pyridine (6) or thiophene (7) are suitable substrates, though lower yield was obtained in the latter case. Acid with an alkyne moiety could deliver the desired product (14) in a moderate yield. Secondary acids with different ring sizes, all reacted well and high yields were obtained (15–22). Acyclic ones were also transformed readily (23 and 24). Tertiary acids such as alpha-oxo-acid and simple pivalic acid delivered the products 25 and 26 in good yields, respectively. Reactions could also proceed well on the six- and three-membered ring site (28 and 29). To further examine the reaction scope, a number of naturally occurring carboxylic acids and several drugs were tested. High yields were in general achieved. The decarboxylative fluorosulfonylethylation of amino acids (30–34) allows the introduction of an SO2F group at the C-terminus of an amino acid chain, while the side-chain carboxylic acid group of aspartic acid and glutamic acid could also be utilized (35 and 36). The low isolated yield of product 33 is due to the difficult separation from the pyridine-byproduct of Hantzsch ester by chromatography, as the 19F NMR yield is over 90%. Furthermore, the carboxylic groups of drugs such as Fenbufen, Gemfibrozil, and Indometacin could be modified readily in good to high yields to introduce the sulfonyl fluoride function through this decarboxylative approach (41–42 and 44). As outlined in Fig. 2, under the current photoredox catalytic system, primary, secondary, and tertiary carbon radicals can all be readily generated from the corresponding redox active esters and trapped by VSF in high efficiency, allowing for a fast construction of aliphatic sulfonyl fluoride libraries of considerable structural diversity. And noteworthy, primary (9), secondary (23 and 37), and tertiary (29) benzylic radicals are also well accommodated.
Fig. 2.
Substrate scope. Reactions were performed on a 0.2 mmol scale, and all yields represent isolated yields. Substrates for 33, 34 and 37 are unnaturally occurring amino acids
Product diversification
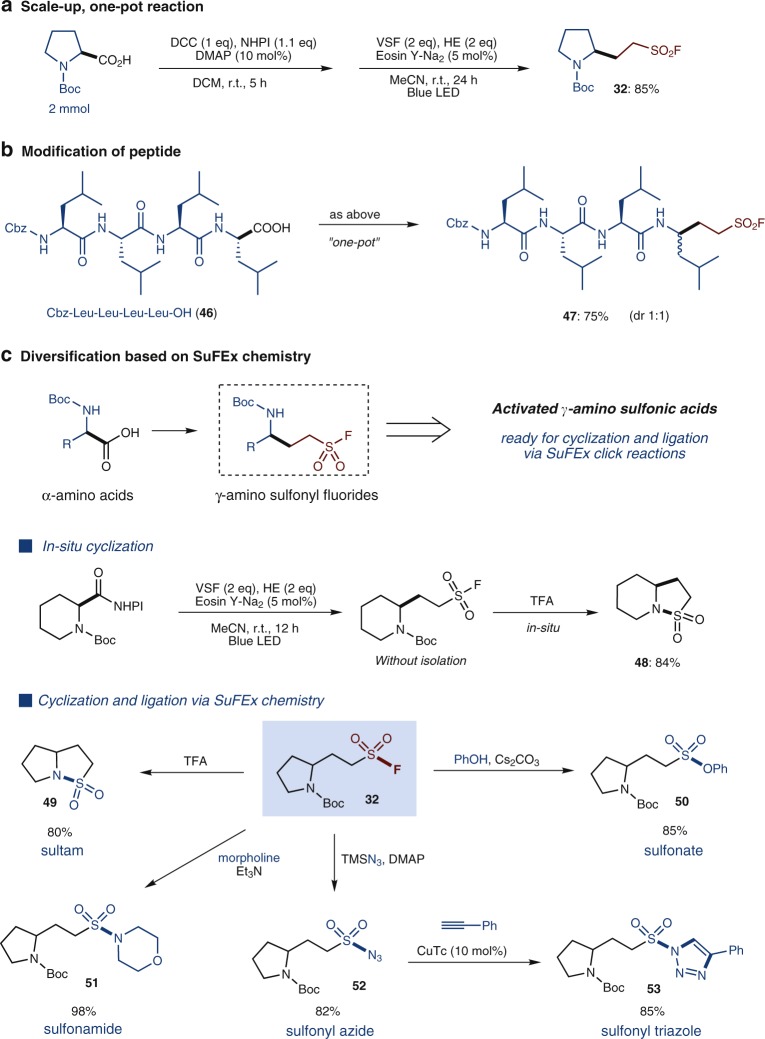
Regarding reaction practicality, the free acid can be directly used for this reaction by forming the redox active ester in situ, as exemplified in a scaled-up reaction with N-Boc-protected l-proline (Fig. 3a). The in situ procedure proved to be equally efficient and afforded the desired product 32 in 85% isolated yield. This convenient, one-pot procedure was later employed to modify the C-end of a tetrapeptide (46) (Fig. 3b). The SO2F-containing product, peptide 47 is a homolog of PW28 (Fig. 1a) which exhibited high inhibition activity to proteasome (IC50 7 nm)22,23. In addition, we tested the stability of the obtained product 3, 32 and tetrapeptide 47 in physiological buffers (PBS, pH 7.2), which unveiled that the three sulfonyl fluorides are quite stable under this aqueous conditions, without detectable loss or decomposition after 24 h by 19F NMR analysis (see Supplementary Note 1 and Supplementary Figs. 6–8). Moreover, the γ-amino sulfonyl fluoride products such as 30–34 derived from the α-amino acids are new compounds and to some extent can be regarded as a type of activated γ-amino sulfonic acids, which would be expected to undergo cyclization or ligation to other molecules to construct pharmaceutically important motifs such as sultam, sulfonate, sulfonamide etc.57–61.
Fig. 3.
Reaction practicability and diversification of products. a Scale-up, one-pot reaction. b Modification of peptide. c Diversification of products through SuFEx chemistry
As demonstrated in Fig. 3c, bicyclic sultams 48 and 49 could be obtained in high yields via a deprotection-intramolecular cyclization sequence, which represents a facile synthetic route to sultams58. On the other hand, proline-derived sulfonyl fluoride 32 could be further diversified through SuFEx click reactions with phenol, morpholine, and TMSN3, delivering the corresponding sulfonate 50, sulfonamide 51, and sulfonyl azide 52, respectively, in high yields. Notably, the introduction of an azide group (52) allows for a further ligation to alkynes through azide-alkyne click reactions, as exemplified by the synthesis of triazole compound 53.
Discussion
In conclusion, a facile catalytic system has been successfully developed for the synthesis of aliphatic sulfonyl fluorides based on abundant carboxylic acid feed stock. A variety of alkyl carboxylic acids including primary, secondary, and tertiary ones, as well as amino acids, peptides, and several drugs can be readily transformed into the corresponding alkyl sulfonyl fluorides with high reaction efficiency and structural diversity. Further diversification of the sulfonyl fluoride products has been also demonstrated through the intramolecular cyclizations and SuFEx reactions. The current method provides a general and rapid approach for the synthesis of aliphatic sulfonyl fluorides and derivatives, which expands the chemical biology toolkit and could benefit the related studies in the context of biology and drug discovery.
Methods
General procedure
Under argon, to an oven-dried Schlenk tube (10 mL) equipped with a stir bar, was added NHPI redox active ester (0.2 mmol, 1 equiv.), Eosin Y-Na2 (6.8 mg, 0.01 mmol, 0.05 equiv.), and HE (101.2 mg, 0.4 mmol, 2 equiv.), followed by the addition of dry MeCN (2 mL) and VSF (32 μL, 0.4 mmol, 2 equiv.). The reaction mixture was then degassed by three freeze-pump-thaw cycles. The Schlenk tube was then backfilled with argon. The reaction mixture was stirred at room temperature for 12−24 h under the irradiation of blue LED bulb (18 W × 2, at approximately 2 cm away from the light sources, ca. 25 °C). The product was purified by flash chromatography (SiO2, petroleum ether/ethyl acetate = 20:1 to 4:1) to give the corresponding pure product. Full experimental details and characterization of new compounds can be found in the Supplementary Methods and Supplementary Figs. 9–108.
Supplementary information
Acknowledgements
We gratefully acknowledge the Recruitment Program of Global Experts (1000plan), Natural Science Foundation of China (Nr. 21602028), and Fuzhou University for the financial support.
Author contributions
R.X. developed the reactions, and performed the reaction scope investigation, mechanistic study, and product derivatization. T.X. and T.C. participated in the synthesis of substrates, M.Y. helped the study of the reaction scope. S.L. conceived this concept and prepared this manuscript with feedback from R.X.
Data availability
The authors declare that all data supporting the findings of this study are available within the article and Supplementary Information files, and are also available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks Zhen-Yu Tang and other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11805-6.
References
- 1.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Dong J, Krasnova L, Finn MG, Sharpless KB. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 2014;53:9430–9448. doi: 10.1002/anie.201309399. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Wu P, Moses JE, Sharpless KB. Multidimensional SuFEx click chemistry: sequential sulfur(VI) fluoride exchange connections of diverse modules launched from an SOF4 hub. Angew. Chem. Int. Ed. 2017;56:2903–2908. doi: 10.1002/anie.201611048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao B, Li S, Wu P, Moses JE, Sharpless KB. SuFEx chemistry of thionyl tetrafluoride (SOF4) with organolithium nucleophiles: synthesis of sulfonimidoyl fluorides, sulfoximines, sulfonimidamides, and sulfonimidates. Angew. Chem. Int. Ed. 2018;57:1939–1943. doi: 10.1002/anie.201712145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T, et al. A new portal to sufex click chemistry: a stable fluorosulfuryl imidazolium salt emerging as an “F–SO2+” donor of unprecedented reactivity, selectivity, and scope. Angew. Chem. Int. Ed. 2018;57:2605–2610. doi: 10.1002/anie.201712429. [DOI] [PubMed] [Google Scholar]
- 6.Chinthakindi PK, Arvindsson PI. Sulfonyl fluorides (SFs): more than click reagents? Eur. J. Org. Chem. 2018;27-28:3648–3666. doi: 10.1002/ejoc.201800464. [DOI] [Google Scholar]
- 7.Zha GF, et al. SO2F2-mediated oxidative dehydrogenation and dehydration of alcohols to alkynes. J. Am. Chem. Soc. 2018;140:17666–17673. doi: 10.1021/jacs.8b10069. [DOI] [PubMed] [Google Scholar]
- 8.Hmissa T, et al. Autocatalytic synthesis of bifluoride ionic liquids by SuFEx click chemistry. Angew. Chem. Int. Ed. 2018;57:16005–16009. doi: 10.1002/anie.201808304. [DOI] [PubMed] [Google Scholar]
- 9.Gao B, et al. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 2017;9:1083–1088. doi: 10.1038/nchem.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C, Flynn JP, Niu J. Facile synthesis of sequence-regulated synthetic polymers using orthogonal sufex and CuAAC click reactions. Angew. Chem. Int. Ed. 2018;57:16194–16199. doi: 10.1002/anie.201811051. [DOI] [PubMed] [Google Scholar]
- 11.Brooks K, et al. SuFEx postpolymerization modification kinetics and reactivity in polymer brushes. Macromolecules. 2018;51:297–305. doi: 10.1021/acs.macromol.7b02372. [DOI] [Google Scholar]
- 12.Narayanan A, Jones LH. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 2015;6:2650–2659. doi: 10.1039/C5SC00408J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones LH. Emerging utility of fluorosulfate chemical probes. ACS Med. Chem. Lett. 2018;9:584–586. doi: 10.1021/acsmedchemlett.8b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martín-Gago P, Olsen CA. Arylfluorosulfate‐based electrophiles for covalent protein labeling: a new addition to the arsenal. Angew. Chem. Int. Ed. 2019;58:957–966. doi: 10.1002/anie.201806037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, et al. Arylfluorosulfates inactivate intracellular lipid binding protein(s) through chemoselective SuFEx reaction with a binding site tyr residue. J. Am. Chem. Soc. 2016;138:7353–7364. doi: 10.1021/jacs.6b02960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q, et al. Broad-spectrum kinase profiling in live cells with lysine-targeted sulfonyl fluoride probes. J. Am. Chem. Soc. 2017;139:680–685. doi: 10.1021/jacs.6b08536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang N, et al. Genetically encoding fluorosulfate-L-tyrosine to react with lysine, histidine, and tyrosine via SuFEx in proteins in vivo. J. Am. Chem. Soc. 2018;140:4995–4999. doi: 10.1021/jacs.8b01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uematsu S, Tabuchi Y, Ito Y, Taki M. Combinatorially screened peptide as targeted covalent binder: alteration of bait-conjugated peptide to reactive modifier. Bioconjugate Chem. 2018;29:1866–1871. doi: 10.1021/acs.bioconjchem.8b00301. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, et al. SuFEx click chemistry enabled late-stage drug functionalization. J. Am. Chem. Soc. 2018;140:2919–2925. doi: 10.1021/jacs.7b12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X, et al. An affinity-based probe for the human adenosine A2A receptor. J. Med. Chem. 2018;61:7892–7901. doi: 10.1021/acs.jmedchem.8b00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortenson DE, et al. “Inverse drug discovery” strategy to identify proteins that are targeted by latent electrophiles as exemplified by aryl fluorosulfates. J. Am. Chem. Soc. 2018;140:200–210. doi: 10.1021/jacs.7b08366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouwer AJ, et al. Peptido sulfonyl fluorides as new powerful proteasome inhibitors. J. Med. Chem. 2012;55:10995–11003. doi: 10.1021/jm301443r. [DOI] [PubMed] [Google Scholar]
- 23.Dubiella C, et al. Selective inhibition of the immunoproteasome by ligand‐induced crosslinking of the active site. Angew. Chem. Int. Ed. 2014;53:11969–11973. doi: 10.1002/anie.201406964. [DOI] [PubMed] [Google Scholar]
- 24.Fadeyi OO, et al. Covalent enzyme inhibition through fluorosulfate modification of a noncatalytic serine residue. ACS Chem. Biol. 2017;12:2015–2020. doi: 10.1021/acschembio.7b00403. [DOI] [PubMed] [Google Scholar]
- 25.Guardiola S, et al. Targeted covalent inhibition of prolyl oligopeptidase (POP): discovery of sulfonylfluoride peptidomimetics. Cell Chem. Biol. 2018;25:1031–1037. doi: 10.1016/j.chembiol.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Artschwager R, Ward DJ, Gannon S, Brouwer AJ, van de Langemheen H, Kowalski H, Liskamp RMJ. Potent and highly selective inhibitors of the proteasome trypsin-like site by incorporation of basic side chain containing amino acid derived sulfonyl fluorides. J. Med. Chem. 2018;61:5395–5411. doi: 10.1021/acs.jmedchem.8b00685. [DOI] [PubMed] [Google Scholar]
- 27.Gooßen LJ, Rodríguez N, Gooßen K. Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 2008;47:3100–3120. doi: 10.1002/anie.200704782. [DOI] [PubMed] [Google Scholar]
- 28.Xuan J, Zhang ZG, Xiao WJ. Visible-light-induced decarboxylative functionalization of carboxylic acids and their derivatives. Angew. Chem. Int. Ed. 2015;54:15632–1564. doi: 10.1002/anie.201505731. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Hu P, Zhang M, Su W. Metal-catalyzed decarboxylative C-H functionalization. Chem. Rev. 2017;117:8864–8907. doi: 10.1021/acs.chemrev.6b00516. [DOI] [PubMed] [Google Scholar]
- 30.Schwarz J, König B. Decarboxylative reactions with and without light-a comparison. Green. Chem. 2018;20:323–361. doi: 10.1039/C7GC02949G. [DOI] [Google Scholar]
- 31.Schwarz J, König B. Metal-free, visible-light-mediated, decarboxylative alkylation of biomass-derived compounds. Green. Chem. 2016;18:4743–4749. doi: 10.1039/C6GC01101B. [DOI] [Google Scholar]
- 32.Cheng WM, Shang R, Fu Y. Photoredox/Brønsted acid Co-catalysis enabling decarboxylative coupling of amino acid and peptide redox-active esters with N‑heteroarenes. ACS Catal. 2017;7:907–911. doi: 10.1021/acscatal.6b03215. [DOI] [Google Scholar]
- 33.Candish L, Teders M, Glorius F. Transition-metal-free, visible-light-enabled decarboxylative borylation of aryl N‑hydroxyphthalimide esters. J. Am. Chem. Soc. 2017;139:7440–7443. doi: 10.1021/jacs.7b03127. [DOI] [PubMed] [Google Scholar]
- 34.Fawcett A, et al. Photoinduced decarboxylative borylation of carboxylic acids. Science. 2017;357:283–286. doi: 10.1126/science.aan3679. [DOI] [PubMed] [Google Scholar]
- 35.Zhao W, Wurz RP, Peters JC, Fu GC. Photoinduced, copper-catalyzed decarboxylative C-N coupling to generate protected amines: an alternative to the curtius rearrangement. J. Am. Chem. Soc. 2017;139:12153–12156. doi: 10.1021/jacs.7b07546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao R, Frey A, Balon J, Hu X, Decarboxylative C. (sp3)-N cross coupling via synergetic photoredox and Copper catalysis. Nat. Catal. 2018;1:120–126. doi: 10.1038/s41929-017-0023-z. [DOI] [Google Scholar]
- 37.Wang C, et al. Visible-light-driven, copper-catalyzed decarboxylative C(sp3)-H alkylation of glycine and peptides. Angew. Chem. Int. Ed. 2018;57:15841–15846. doi: 10.1002/anie.201809400. [DOI] [PubMed] [Google Scholar]
- 38.Cheng W-M, Shang R, Fu Y. Irradiation- induced palladium-catalyzed decarboxylative desaturation enabled by a dual ligand system. Nat. Commun. 2018;9:1–9. doi: 10.1038/s41467-017-02088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patra T, Mukherjee S, Ma J, Strieth-Kalthoff F, Glorius F. Visible-light photosensitized aryl and alkyl decarboxylative functionalization reactions. Angew. Chem. Int. Ed. 2019;58:10514–10520. doi: 10.1002/anie.201904671. [DOI] [PubMed] [Google Scholar]
- 40.Fu M-C, Shang R, Zhao B, Wang B, Fu Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science. 2019;363:1429–1434. doi: 10.1126/science.aav3200. [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Zhu N, Chen P, Lin Z, Liu G. Enantioselective decarboxylative cyanation employing cooperative photoredox catalysis and Copper catalysis. J. Am. Chem. Soc. 2017;139:15632–15635. doi: 10.1021/jacs.7b09802. [DOI] [PubMed] [Google Scholar]
- 42.Proctor RSJ, Davis HJ, Phipps RJ. Catalytic enantioselective Minisci-type addition to heteroarenes. Science. 2018;360:419–422. doi: 10.1126/science.aar6376. [DOI] [PubMed] [Google Scholar]
- 43.Ma J, et al. Synthesis of β-substituted γ-aminobutyric acid derivatives through enantioselective photoredox catalysis. Angew. Chem. Int. Ed. 2018;57:11193–11197. doi: 10.1002/anie.201804040. [DOI] [PubMed] [Google Scholar]
- 44.Qin T, et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science. 2016;352:801–805. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu X, Xiao B, Liu L, Fu Y. Formation of C(sp3)–C(sp3) bonds through nickel-catalyzed decarboxylative olefin hydroalkylation reactions. Chem. Eur. J. 2016;22:11161–11164. doi: 10.1002/chem.201602486. [DOI] [PubMed] [Google Scholar]
- 46.Huihui KMM, et al. Decarboxylative cross-electrophile coupling of N‑hydroxyphthalimide esters with aryl iodides. J. Am. Chem. Soc. 2016;138:5016–5019. doi: 10.1021/jacs.6b01533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue W, Oestreich M. Copper-catalyzed decarboxylative radical silylation of redox-active aliphatic carboxylic acid derivatives. Angew. Chem. Int. Ed. 2017;56:11649–11652. doi: 10.1002/anie.201706611. [DOI] [PubMed] [Google Scholar]
- 48.Liu XG, et al. Decarboxylative Negishi coupling of redox-active aliphatic esters by cobalt catalysis. Angew. Chem. Int. Ed. 2018;57:13096–13100. doi: 10.1002/anie.201806799. [DOI] [PubMed] [Google Scholar]
- 49.Krutak JJ, Burpitt RD, Moore WH, Hyatt JA. Chemistry of ethenesulfonyl fluoride. fluorosulfonylethylation of organic compounds. J. Org. Chem. 1979;44:3847–3858. doi: 10.1021/jo01336a022. [DOI] [Google Scholar]
- 50.Ungureanu A, Levens A, Candish L, Lupton DW. N–heterocyclic carbene catalyzed synthesis of δ–sultones via α,β–unsaturated sulfonyl azolium intermediates. Angew. Chem. Int. Ed. 2015;54:11780–11784. doi: 10.1002/anie.201504633. [DOI] [PubMed] [Google Scholar]
- 51.Chen Q, Mayer P, Mayr H. Ethenesulfonyl fluoride: the most perfect Michael acceptor ever found? Angew. Chem. Int. Ed. 2016;55:12664–12667. doi: 10.1002/anie.201601875. [DOI] [PubMed] [Google Scholar]
- 52.Qin HL, Zheng Q, Bare GAL, Wu P, Sharpless KB. A Heck-Matsuda process for the synthesis of β-arylethenesulfonyl fluorides: selectively addressable Bis-electrophiles for SuFEx click chemistry. Angew. Chem. Int. Ed. 2016;55:14155–14158. doi: 10.1002/anie.201608807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zha GF, et al. Palladium catalyzed fluorosulfonylvinylation of organic iodides. Angew. Chem. Int. Ed. 2017;56:4849–4852. doi: 10.1002/anie.201701162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X-Y, Wu Y, Zhou J, Wang P, Yu J-Q. Synthesis of β-arylethenesulfonyl fluoride via Pd-catalyzed nondirected C–H alkenylation. Org. Lett. 2019;21:1426–1429. doi: 10.1021/acs.orglett.9b00165. [DOI] [PubMed] [Google Scholar]
- 55.Hari DP, Schroll P, König B. Metal-free, visible-light-mediated direct C-H arylation of heteroarenes with aryl diazonium salts. J. Am. Chem. Soc. 2012;134:2958–2961. doi: 10.1021/ja212099r. [DOI] [PubMed] [Google Scholar]
- 56.Fujigaya T, et al. New photoresist materials for 157-nm lithography. poly[vinylsulfonyl fluoride-co-4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropyl)-styrene] partially protected with tert-butoxycarbonyl. Chem. Mater. 2003;15:1512–1517. doi: 10.1021/cm020198h. [DOI] [Google Scholar]
- 57.Scott KA, Njardarson JT. Analysis of US FDA-approved drugs containing sulfur atoms. Top. Curr. Chem. 2018;376:1–34. doi: 10.1007/s41061-017-0179-7. [DOI] [PubMed] [Google Scholar]
- 58.Debnath S, Mondal S. Sultams: recent syntheses and applications. Eur. J. Org. Chem. 2018;2018:933–956. doi: 10.1002/ejoc.201701491. [DOI] [Google Scholar]
- 59.Zhersh SA, et al. Saturated heterocyclic aminosulfonyl fluorides: new scaffolds for protecting-group-free synthesis of sulfonamides. Chem. Eur. J. 2018;24:8343–8349. doi: 10.1002/chem.201801140. [DOI] [PubMed] [Google Scholar]
- 60.Gaulton A, et al. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012;40:D1100–D1107. doi: 10.1093/nar/gkr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aminov R. History of antimicrobial drug discovery: major classes and health impact. Biochem. Pharm. 2017;133:4–19. doi: 10.1016/j.bcp.2016.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article and Supplementary Information files, and are also available from the corresponding author upon reasonable request.