Abstract
In nematode Caenorhabditis elegans, some microRNAs (miRNAs) could be dysregulated by multi-walled carbon nanotubes (MWCNTs), suggesting their involvement in regulating the response of nematodes to MWCNTs. Among these dysregulated miRNAs induced by MWCNT exposure, prolonged exposure to MWCNTs increased mir-35 expression. mir-35 further acted in the intestine to regulate the response to MWCNTs. In the intestine, a transcription factor MAB-3 was identified as its target in regulating the response to MWCNTs. Moreover, during the control of response to MWCNTs, MAB-3 acted upstream of DAF-16, a fork head transcriptional factor in insulin signaling pathway. Therefore, MWCNTs exposure potentially dysregulates intestinal mir-35 and its direct target MAB-3, which may activate a protective intestinal response of nematodes against the MWCNTs toxicity.
Subject terms: Environmental impact, Biomarkers
Introduction
During the last decades, carbon nanotubes (CNTs) have attracted the great interest for some of their unique properties, such as stability, rigidity, extraordinary tensile strength, and efficient heat conduction1–3. Multi-walled CNTs (MWCNTs) consisting of concentric layers of single-walled CNTs have the potential in biomedical areas, including drug delivery, biosensors, medical imaging, and targeted therapeutic4–6. Meanwhile, safety and exposure concerns of MWCNT have aroused with its wide application. Some works have been performed with the aim to elucidate the cellular and the molecular mechanisms of MWCNTs toxicity in organism7–10. Nevertheless, the underlying molecular mechanisms for the response of organisms to MWCNTs are still largely unclear.
MicroRNAs (miRNAs), small noncoding regulatory molecules, regulate the expression and the functions of their target messenger RNAs (mRNAs) by binding to certain sites in 3′ untranslated region (UTR) and affecting their translation into proteins11. It has been well known that one certain miRNA can simultaneously target several or many mRNAs12. Some miRNAs have been shown to play pivotal roles in regulating various biological processes, such as growth and development13,14. The increasing evidence has further suggested that some miRNAs may function in the control of stress response15,16.
Nematode Caenorhabditis elegans is an important model animal for the identification and the functional analysis of miRNAs13,15. Meanwhile, C. elegans is very sensitive to the toxicity of environmental toxicants, and suitable for the elucidation of molecular mechanism for the observed toxicity of environmental toxicants at the whole animal level17. The previous studies have demonstrated that some miRNAs could be dysregulated by carbon-based nanomaterials, such as MWCNTs and graphene oxide (GO), and some miRNAs were further shown to be required for the control of toxicity induction of MWCNTs or GO18,19.
Among the dysregulated miRNAs by MWCNTs exposure, mir-35 could be increased by prolonged exposure to MWCNTs at concentrations ≥100 μg/L18. In addition, mutation of mir-35 could induce a susceptibility to MWCNTs toxicity at various aspects, such as induction of intestinal reactive oxygen species (ROS) and decrease in locomotion behavior18. However, the underlying mechanism for the role of mir-35 in regulating the response to MWCNTs is still unknown in nematodes. In this study, we first examined the tissue-specific activity of mir-35 in regulating the response to MWCNTs. Moreover, we identified the target of intestinal mir-35 and the underlying mechanism for intestinal mir-35 in regulating the response to MWCNTs. Our results demonstrated that the increase in mir-35 expression mediated a protective intestinal response to MWCNTs by suppressing function of MAB-3-DAF-16 signaling cascade. Our data provides an important molecular basis for intestinal response to MWCNTs in organisms.
Results
Alteration in mir-35 expression in MWCNTs exposed nematodes
After prolonged exposure from L1-larvae to adult day-1, MWCNTs at concentrations more than 0.1 μg/L significantly increased the mir-35 expression (Fig. S1). Meanwhile, in the isolated intestine, MWCNTs (≥0.1 μg/L) also significantly increased the mir-35 expression (data not shown). Additionally, the mir-35 expression was concentration-dependent in MWCNTs exposed nematodes (Fig. S1).
Tissue-specific activity of mir-35 in regulating the response to MWCNTs
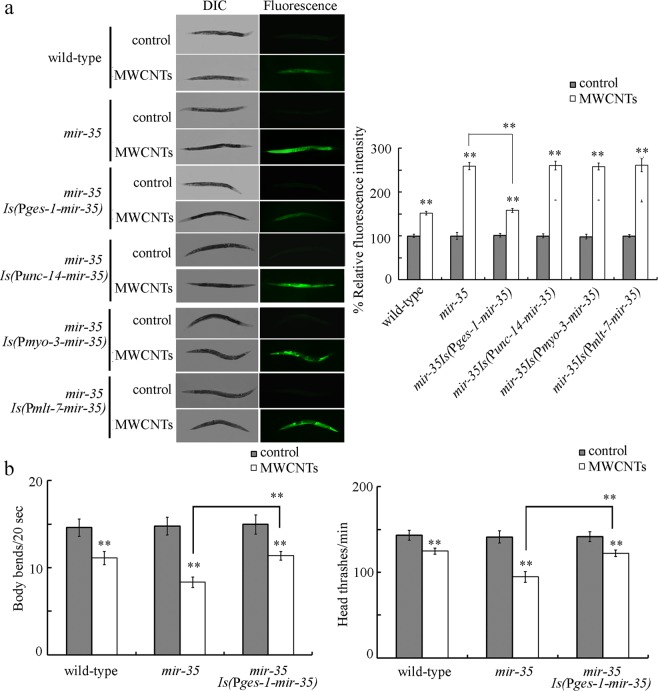
In nematodes, mir-35 is expressed in some tissues, including intestine, muscle, neurons, and epidermis20. Mutation of mir-35 induced a susceptibility to the toxicity of MWCNTs (0.1 μg/L) in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 1). Based on the rescue assays, we found that transgenic expression of neuronal, muscle, or epidermal mir-35 did not obviously affect the susceptibility of mir-35 mutant nematodes to the toxicity of MWCNTs (0.1 μg/L) in inducing intestinal ROS production (Fig. 1). In contrast, intestinal expression of mir-35 could effectively suppress the susceptibility of mir-35 mutant nematodes to the toxicity of MWCNTs (0.1 μg/L) in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 1). Therefore, mir-35 can act in the intestine to regulate the response of nematodes to MWCNTs.
Figure 1.
Tissue-specific activity of mir-35 in regulating the response to MWCNTs. (a) Tissue-specific activity of mir-35 in regulating the toxicity of MWCNTs in inducing intestinal ROS production. (b) Intestine-specific activity of mir-35 in regulating the toxicity of MWCNTs in decreasing locomotion behavior. Prolonged exposure was performed from L1-larvae to adult day 1. MWCNT exposure concentration was 0.1 μg/L. Bars represent means ± SD. **P < 0.01 vs control (if not specially indicated).
Transcriptomic changes induced by MWCNTs (0.1 μg/L)
To identify the direct potential target(s) of mir-35 in regulating the response to MWCNTs, we next determined the dysregulated gene profiling by MWCNTs (0.1 μg/L) using Illumina HiSeqTM 2000 sequencing technique. Totally 342 differentially expressed mRNAs were identified in MWCNTs (0.1 μg/L) exposed nematodes compared with control (Fig. 2, Table S1). Among these 342 candidate mRNAs, 149 mRNAs were up-regulated and 193 mRNAs were down-regulated in MWCNTs (0.1 μg/L) exposed nematodes (Fig. 2, Table S1).
Figure 2.
Dysregulated mRNAs induced by MWCNTs. (a) Heatmap showing the expression of mRNAs obtained from control and MWCNTs exposed nematodes. Relatively low expression levels are represented as blue, and relatively high expression levels are represented in red. (b) Scatter diagram of relationship between mRNA coverage of the control group and the MWCNTs exposure group. Prolonged exposure was performed from L1-larvae to adult day 1. MWCNT exposure concentration was 0.1 μg/L.
Prediction of potential targets of mir-35 in regulating the response to MWCNTs (0.1 μg/L)
We next sought to identify the potential targets of mir-35 during the control of response to MWCNTs. The corresponding targeted genes for mir-35 were predicted using TargetScan by searching for the presence of conserved sites that match seed region of mir-35 (version 6.2, http://www.targetscan.org/worm_52/) (Table S2). Among these predicted targeted genes, mca-3, T28D6.5, and mab-3 could also be dysregulated by MWCNTs (0.1 μg/L) (Fig. 3a, Table S1).
Figure 3.
Identificatio of potential targets of mir-35 in regulating the response to MWCNTs. (a) Search for the candidate targets for mir-35 in regulating the response to MWCNTs. (b) Effect of intestine-specific RNAi knockdown of mab-3 or mca-3 on toxicity of MWCNTs in inducing intestinal ROS production. Prolonged exposure was performed from L1-larvae to adult day 1. MWCNT exposure concentration was 0.1 μg/L. Bars represent means ± SD. **P < 0.01 vs control (if not specially indicated).
Exposure to MWCNTs (0.1 μg/L) could decrease the expressions of mab-3 and mca-3, and increase the expression of T28D6.5 (Table S1). Considering the fact that exposure to MWCNTs (0.1 μg/L) could increase the mir-35 expression (Fig. S1), we next focused on MAB-3 and MCA-3 to examine their role in regulating the response to MWCNTs. In nematodes, both MAB-3 and MCA-3 can be expressed in the intestine (https://wormbase.org). Using VP303 as a genetic tool, we found that intestine-specific RNA interference (RNAi) knockdown of mca-3 did not obviously affect the toxicity of MWCNTs (0.1 μg/L) in inducing intestinal ROS production (Fig. 3b). Different from this, intestine-specific RNAi knockdown of mab-3 significantly inhibited the toxicity of MWCNTs (0.1 μg/L) in inducing intestinal ROS production (Fig. 3b). That is, intestine-specific RNAi knockdown of mab-3 induced a resistance to the MWCNT toxicity, implying that MAB-3 may act as a target for mir-35 in regulating the response to MWCNTs. MAB-3 is a transcription factor in nematodes.
In vivo 3′-untranslated region (3′-UTR) binding assay of mir-35 with MAB-3
In nematodes, we observed that loss-of-function mutation of mir-35 could significantly increase the mab-3 expression (Fig. 4a). To confirm whether MAB-3 is a direct target of mir-35 during the regulation of response to MWCNTs, we constructed GFP vector driven by ges-1 promoter, which contained 3′-UTR of mab-3 (Pges-1::GFP-3′-UTR (mab-3 wt) or Pges-1::GFP-3′-UTR (mab-3mutant). The mir-35 binding site in mab-3 3′ UTR was mutated from CCCGGUG to CCTTGAG in order to prevent the binding of mir-35 (Fig. 4b). The related vector contruction information was outlined in Fig. 4c. Considering the fact that the mir-35 can not bind to unc-54 3′-UTR, a Pges-1::mCherry-3′-UTR (unc-54) was employed as an internal control. After MWCMT (0.1 μg/L) exposure, the GFP expression was significantly decreased in wild-type nematodes (Fig. 4d). In contrast, mutagenesis of binding site for mir-35 in mab-3 3′-UTR abolished this GFP expression decrease in wild-type nematodes (Fig. 4d). After MWCNT (0.1 μg/L) exposure, a higher GFP expression was observed in mir-35 mutant nematodes than that in wild-type nematodes (Fig. 4d). These observations support the role of MAB-3 as the direct target of intestinal mir-35 during the control of response to MWCMTs in nematodes.
Figure 4.
In vivo 3′-UTR binding assay of mab-3. (a) Effect of mir-35 mutation on mab-3 expression. Bars represent means ± SD. **P < 0.01 vs wild-type. (b) Predicted binding site on mab-3 3′ UTR by Targetscan. mir-35 seed sequence is shown in blue. (c) DNA construct outline. (d) Fluorescence images of the mab-3 3′-UTR GFP reporter in nematodes exposed to MWCNTs. wt, wild-type. MWCNT exposure concentration was 0.1 μg/L. Bars represent means ± SD. **P < 0.01 vs wt/control (if not specially indicated).
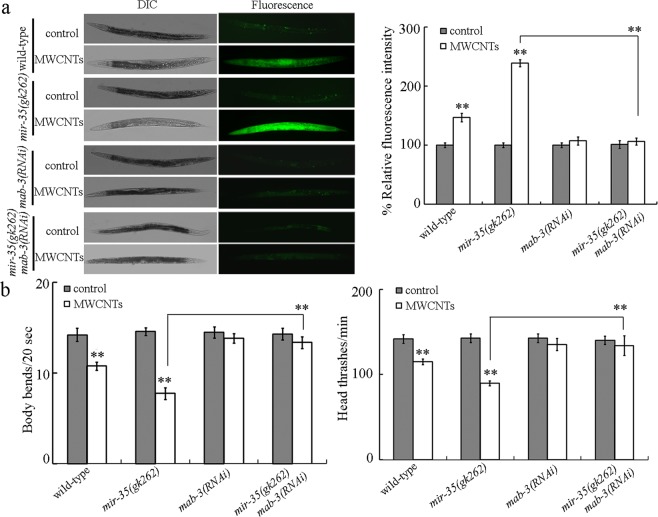
Genetic interaction between mir-35 and MAB-3 in regulating the response to MWCNTs
To further confirm the role of MAB-3 as the target of mir-35 in regulating the response to MWCNTs, we investigated the genetic interaction between mir-35 and MAB-3. We observed that both the intestinal ROS production and the locomotion behavior in MWCNTs exposed mir-35(gk262)mab-3(RNAi) nematodes were similar to those in MWCNTs exposed mab-3(RNAi) nematodes (Fig. 5). That is, RNAi knockdown of mab-3 could effectively suppress the susceptibility of mir-35 mutant nematodes to the MWCNTs toxicity.
Figure 5.
Genetic interaction between mir-35 and MAB-3 in regulating the response to MWCNTs. (a) Genetic interaction between mir-35 and MAB-3 in regulating the MWCNTs toxicity in inducing intestinal ROS production. (b) Genetic interaction between mir-35 and MAB-3 in regulating the MWCNTs toxicity in decreasing locomotion behavior. MWCNT exposure concentration was 0.1 μg/L. Bars represent means ± SD. **P < 0.01 vs control (if not specially indicated).
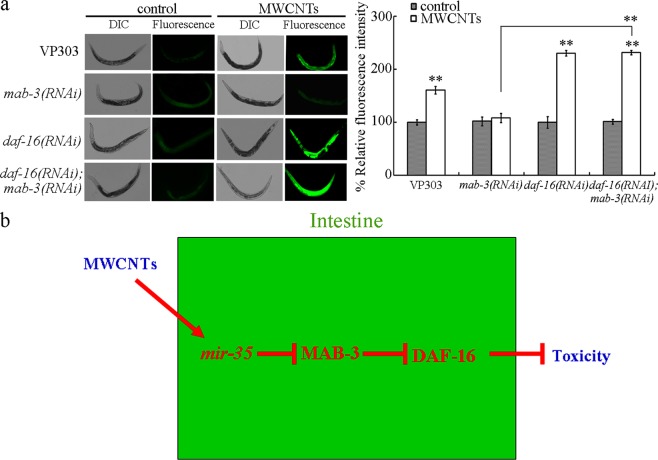
Genetic interaction between DAF-16 and MAB-3 in regulating the response to MWCNTs
Our previous study has indicated that the insulin signaling pathway regulates the toxicity of MWCNTs in nematodes21. In the insulin signaling pathway, daf-16 encodes a fork head transcriptional factor. Intestinal RNAi knockdown of daf-16 induced a susceptibility to the MWCNTs toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 6a). Moreover, we found that intestinal RNAi knockdown of daf-16 could further suppress the resistance of mab-3(RNAi) nematodes to the MWCNTs toxicity in inducing intestinal ROS production and in decreasing locomotion behavior (Fig. 6a). Therefore, MAB-3 may acts upstream of DAF-16 to regulate the response to MWCNTs in nematodes.
Figure 6.
Genetic interaction between DAF-16 and MAB-3 in the intestine to regulate the response to MWCNTs. (a) Genetic interaction between DAF-16 and MAB-3 in the intestine to regulate the MWCNTs toxicity in inducing intestinal ROS production. MWCNT exposure concentration was 0.1 μg/L. Bars represent means ± SD. **P < 0.01 vs control (if not specially indicated). (b) A diagram showing the mechanism for intestinal mir-35 in regulating the response to MWCNTs in nematodes.
Discussion
In this study, we observed that prolonged exposure (from L1-larvae to adult day-1) to MWCNTs (≥100 ng/L) could significantly increase the expression of mir-35 (Fig. S1). Early in 2009, it was predicted that the environmentally relevant concentrations for CNTs are 6.6–31.5 ng/L for sewage treatment plant effluent22. With the rapid increase in production and in application of CNTs23, 100 ng/L can be or will be considered as the environmentally relevant concentration. Thus, long-term exposure to MWCNTs at environmentally relevant concentration may induce a mir-35-mediated response in nematodes.
In nematodes, mir-35 is expressed in many tissues20. Meanwhile, we observed that the increase in mir-35 mediated a protective response to MWCNTs18. Among the examined tissues, we found that mir-35 only acted in the intestine to regulate the response to MWCNTs (Fig. 1). Therefore, the increase in mir-35 only mediated an intestinal response of nematodes to MWCNTs. In the intestine, it was reported that the mir-35 may also regulate the intestinal cell G1/S transition, since loss of mir-35 leaded to a decrease of nuclei numbers in intestine of nematodes24. Besides this, it was also found that the mir-35 could further regulate the germ cell proliferation or apoptosis by antagonizing certain molecular signal pathways, such as MAPK and core apoptosis pathways24,25, which suggests the germline activity of mir-35 in regulating the other aspects of biological processes in nematodes.
To understanding the molecular mechanism for intestinal mir-35 in regulating the response to MWCNTs, we tried to identify the potential target of intestinal mir-35 during the control of response to MWCNT exposure. We raised several lines of evidence to prove the role of a DM domain transcription factor MAB-3 as the target of intestinal mir-35 in regulating the response to MWCNTs. Firstly, loss-of-function of mir-35 significantly increased the mab-3 expression (Fig. 4a). Secondly, the phenotype of MWCNTs exposed mab-3(RNAi) nematodes was opposite to that in mir-35 mutant nematodes (Fig. 3b). Previous study also suggested that RNAi knockdown of mab-3 induced a resistance to oxidative stress26. Thirdly, 3′-UTR binding assay suggested the potential binding of intestinal mir-35 with 3′-UTR of mab-3 (Fig. 4d). Finally, functional analysis indicated that RNAi knockdown of mab-3 could suppress the susceptibility of mir-35 mutant nematodes to the MWCNTs toxicity (Fig. 5).
Insulin signaling pathway plays a crucial role in regulating the response of nematodes to various environmental toxicants or stresses27. In the insulin signaling pathway, the DAF-16 is a FOXO transcription factor, and DAF-16 usually act in the intestine to regulate the response of nematodes to various environmental toxicants or stresses by activating or inhibiting some of its downstream targets27,28. For the underlying of intestinal MAB-3 in regulating the response to MWCNTs, we found that intestinal RNAi knockdown of daf-16 could inhibit the resistance of mab-3(RNAi) nematodes to MWCNTs toxicity (Fig. 6a). Therefore, intestinal MAB-3 may regulate the response to MWCNTs by further suppressing the function of DAF-16 and its downstream targeted genes in the insulin signaling pathway. So far, the downstream targeted genes for intestinal DAF-16 in regulating the response to MWCNTs are still unknown.
In this study, we employed C. elegans to determine the molecular basis for the increase in mir-35 expression-mediated protective response to MWCNTs. The intestine-specific activity in regulating the response to MWCNTs was found in nematodes. In the intestine, a DM domain transcription factor MAB-3 acted as a target of mir-35 during the control of response to MWCNTs (Fig. 6b). For the underlying mechanism, we found that intestinal MAB-3 regulated the response to MWCNTs by suppressing the function of DAF-16 in the insulin signaling pathway (Fig. 6b). The identified signaling cascade of mir-35-MAB-3-DAF-16 provides an important basis for intestinal response to environmental toxicants in nematodes.
Methods
MWCNTs properties
MWCNTs were from Shenzhen Nanotech Port Co. Ltd (Shenzhen, China). Working concentrations of MWCNTs were prepared by diluting the stock solution (1 mg/mL) with K-medium (50 mM NaCl, 30 mM KCl, and 10 mM NaOAc, pH 6.0). Before the use, the working solutions were sonicated for 30 min (40 kHz, 100 W). Based on the analysis of transmission electron microscopy (TEM) (JEM-200CX, JEOL, Japan), the diameter of MWCNTs was 10~20 nm, and the length of MWCNTs was 0.4~4 μm (Fig. S2). The zeta potential of MWCNTs was −32.4 ± 2.2 mV18. In the used MWCNTs, we detected the presence of 0.077% Ni and 0.017% Fe using elemental inductively coupled plasma mass spectrometry (ICP-MS) (Thermo Elemental X7, USA). Exposure from L1-larvae to adult day-1 to 0.077% Ni or 0.017% Fe did not induce the obvious ROS production and alteration in locomotion behavior (data not shown), suggesting the observed MWCNTs toxicity was not due to the impurity.
Strain maintenance and exposure
Some of the used strains in the present study were originally obtained from Caenorhabditis Genetics Center. The used strains contain wild-type N2, mutant of mir-35(gk262), and transgenic strains of mir-35(gk262)Is(Pges-1-mir-35), mir-35(gk262)Is(Punc-14-mir-35), mir-35(gk262)Is(Pmyo-3-mir-35), mir-35(gk262)Is(Pmlt-7-mir-35) and VP303/rde-1(ne219); kbIs7. VP303 is a genetic tool for intestine-specific RNAi knockdown of certain gene(s)29. Nematodes were maintained on nematode growth medium (NGM) plates seeded with Escherichia coli OP50 as food as described30. The collected gravid animals were first lysed using bleaching mixture solution (0.45 M NaOH, 2% HOCl). After that, the released eggs were used to prepare age synchronous L1-larvae.
Prolonged exposure to MWCNTs was performed from L1-larvae to adult day-1 in liquid solutions with the addition of OP50 (~4 × 106 colony-forming units (CFUs)). The MWCNTs solutions were refreshed daily.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The animals were spun down in an eppendorf tube, and the total RNA extraction was performed with Trizol (Invitrogen, Carlsbad, CA). The cDNAs were synthesized by reverse transcription with the oligo-dT primer on total RNA. Quantitative PCR of target genes was carried out using SYBR® Green FastMix® according to manufacturer instruction with the ABI Prism7000a platform (Applied BioSystems, Warrington, UK) and normalized with the reference gene tba-1 encoding a Tubulin protein. Primers used for qRT-PCR are listed in Table S3. The mir-35 expression was expressed as relative expression ratio between mir-35 and F35C11.9 encoding a small nuclear RNA U6. Primer for reverse transcription of mir-35 is GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACTGCTA. Primer for real-time PCR of mir-35 is ATAATCTCACCGGGTGGAAACT, and common reward primer is GTGCAGGGTCCGAGGT. Forward primer F35C11.9 is GAAGATTAGCATGAACCC, and reverse primer F35C11.9 is TTGGAACGCTTTATGAAT. All reactions were performed in triplicate.
Toxicity assessment
ROS production was used to reflect the activation of oxidative stress31. The method for detecting intestinal ROS production was performed as described32. The test nematodes were washed off the plates with K buffer, and incubated with freshly prepared 1 µM CM-H2DCFDA for 3 h in the dark. After that, the nematodes were mounted on agar pads for examination with a laser scanning confocal microscope (Ex: 480 nm; Em: 510 nm). The fluorescence intensities were examined by Image J (NIH), and the semi-quantified ROS was expressed as relative fluorescent units (RFU). For each treatment, fifty nematodes were examined.
Locomotion behavior was used to reflect the functional state of motor neurons33. Head thrash and body bend were used to reflect the locomotion behavior as described34. After MWCNTs exposure, the nematodes were transferred onto freshly made NGM plate without food. A change for bending direction at body mid-region of nematodes is considered as a head thrash, and a change of posterior bulb direction is considered as a body bend. For each treatment, fifty nematodes were examined.
RNA-seq library preparation and HiSeq 2000 sequencing
HiSeq 2000 sequencing was performed as described previously21. MWCNT exposure concentration was 0.1 μg/L. After quality determination of RNA isolated using Nano Photometer P-Class, mRNA libraries were prepared with RNA-seq Sample Preparation kit (Illumina, Inc., San Diego, CA, USA) for the next Illumina HiSeqTM 2000 sequencing. Quality of reads was checked using Fast QC, and the total read numbers of control or MWCNTs exposure group data sets were normalized to equal levels. We determined dysregulated mRNAs in MWCNT (0.1 μg/L) exposed nematodes with fold change analysis together with the analysis based on statistical significance and use of a 2.0-fold change cutoff.
RNAi assay
RNAi was performed by feeding animals with E. coli HT115 expressing double-stranded RNA corresponding to certain gene(s) as described35. The prepared L1-larvae were grown on RNAi plates. When they developed into gravid, the adult nematodes were transferred onto a fresh plate to obtain the second generation for the toxicity assessment. HT115 bacteria harboring empty vector L4440 containing two T7 promoters flanking a polylinker was used as a control. RNAi efficiency was confirmed by qRT-PCR (data not shown).
DNA constructs and transformation
The promoter of ges-1 (expressed in intestine), unc-14 (expressed in neurons), myo-3 (expressed in muscle), or mlt-7 (expressed in epidermis) was amplified by PCR from wild-type genomic DNA. PCR amplified mir-35 was inserted into vector pPD_95_77 carrying ges-1, unc-14, myo-3, or mlt-7 promoter. Germline transformation was conducted by coinjecting a testing DNA (10–40 μg/mL) and a marker DNA of Pdop-1::rfp (60 μg/mL) into gonad of animals36. Primer information for vector constructions is shown in Table S4.
3′-UTR reporters and microscopy
The 3′-UTR (wt) of mab-3 was PCR amplified from genomic DNA. A mab-3 3′-UTR (mutant) reporter was constructed by replacing mir-35 binding site with an oligonucleotide containing complementary sequence of mir-35. The 3′ UTR reporter construct and mCherry internal control (Pges-1::mCherry-3′UTR (unc-54)) plasmid were coinjected into the gonad of nematodes as described36. GFP and mCherry expressions were analyzed under a fluorescence microscope. Related primer information for vector constructions is shown in Table S4.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 software (SPSS Inc., Chicago, USA). Differences between two groups were analyzed by student t test. Differences among more than two groups were analyzed by analysis of variance (ANOVA) and Dunnet’s test. Probability levels of 0.05 (*) and 0.01 (**) were considered to be statistically significant.
Supplementary information
Acknowledgements
This work was supported by National Natural Science Foundation of China (21707002), Excellent Young Talents Fund Program of Higher Education Institutions of Anhui Province (gxyqZD2016162), and Natural Science Foundation for Colleges and Universities of Anhui Province (KJ2017A227).
Author Contributions
Conceived and designed the experiments: Y.Z. and D.W. Performed the experiments and analyzed the data: L.J., Y.W. and Y.K. Wrote the paper: D.W.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yunli Zhao, Email: yunli201@126.com.
Dayong Wang, Email: dayongw@seu.edu.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-48646-8.
References
- 1.Xu Z, et al. Hydroxyl multi-walled carbon nanotube-modified nanocrystalline PbO2 anode for removal of pyridine from wastewater. J. Hazard. Mater. 2017;327:144–152. doi: 10.1016/j.jhazmat.2016.12.056. [DOI] [PubMed] [Google Scholar]
- 2.Zhao W, et al. Thin-film nanocomposite forward-osmosis membranes on hydrophilic microfiltration support with an intermediate layer of graphene oxide and multiwall carbon nanotube. ACS Appl. Mater. Interfaces. 2018;10:34464–34474. doi: 10.1021/acsami.8b10550. [DOI] [PubMed] [Google Scholar]
- 3.Seo Y, et al. Engineering copper nanoparticles synthesized on the surface of carbon nanotubes for anti-microbial and anti-biofilm applications. Nanoscale. 2018;10:15529–15544. doi: 10.1039/C8NR02768D. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, et al. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gulotty R, Castellino M, Jagdale P, Tagliaferro A, Balandin AA. Effects of functionalization on thermal properties of single-wall and multi-wall carbon nanotube-polymer nanocomposites. ACS Nano. 2013;7:5114–5121. doi: 10.1021/nn400726g. [DOI] [PubMed] [Google Scholar]
- 6.Hosnedlova B, et al. Carbon nanomaterials for targeted cancer therapy drugs: A critical review. Chem Rec. 2018;19:3253. doi: 10.1002/tcr.201800038. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, et al. Comparison of cytotoxic and inflammatory responses of pristine and functionalized multi-walled carbon nanotubes in RAW 264.7 mouse macrophages. J. Hazard. Mater. 2012;219–220:203–212. doi: 10.1016/j.jhazmat.2012.03.079. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L, Wan H-X, Liu Q-Z, Wang D-Y. Multi-walled carbon nanotubes-induced alterations in microRNA let-7 and its targets activate a protection mechanism by conferring a developmental timing control. Part. Fibre Toxicol. 2017;14:27. doi: 10.1186/s12989-017-0208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh M, et al. Changes in DNA methylation induced by multi-walled carbon nanotube exposure in the workplace. Nanotoxicology. 2017;11:1195–1210. doi: 10.1080/17435390.2017.1406169. [DOI] [PubMed] [Google Scholar]
- 10.Harik VM. Geometry of carbon nanotubes and mechanisms of phagocytosis and toxic effects. Toxicol. Lett. 2017;273:69–85. doi: 10.1016/j.toxlet.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki HI, Miyazono K. Dynamics of microRNA biogenesis: crosstalk between p53 network and microRNA processing pathway. J. Mol. Med. 2010;88:1085–1094. doi: 10.1007/s00109-010-0650-1. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.de Lencastre A, et al. MicroRNAs both promote and antagonize longevity in C. elegans. Curr. Biol. 2010;20:2159–2168. doi: 10.1016/j.cub.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori MA, et al. Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 2012;16:336–347. doi: 10.1016/j.cmet.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner JL, Jasiewicz KL, Fahley AF, Kemp BJ, Abbott AL. Loss of individual microRNAs causes mutant phenotypes in sensitized genetic backgrounds in C. elegans. Curr. Biol. 2010;20:1321–1325. doi: 10.1016/j.cub.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhi L-T, Yu Y-L, Li X-Y, Wang D-Y, Wang D-Y. Molecular control of innate immune response to Pseudomonas aeruginosa infection by intestinal let-7 in Caenorhabditis elegans. PLoS Pathog. 2017;13:e1006152. doi: 10.1371/journal.ppat.1006152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, D.-Y. Nanotoxicology in Caenorhabditis elegans. Springer Nature Singapore Pte Ltd. (2018).
- 18.Zhao Y-L, et al. In vivo translocation and toxicity of multi-walled carbon nanotubes are regulated by microRNAs. Nanoscale. 2014;6:4275–4284. doi: 10.1039/c3nr06784j. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, et al. Dysregulation of let-7 by PEG modified graphene oxide in nematodes with deficit in epidermal barrier. Ecotoxicol. Environ. Safety. 2019;169:1–7. doi: 10.1016/j.ecoenv.2018.10.106. [DOI] [PubMed] [Google Scholar]
- 20.Martinez NJ, et al. Genome-scale spatiotemporal analysis of Caenorhabditis elegans microRNA promoter activity. Genome Res. 2008;18:2005–2015. doi: 10.1101/gr.083055.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y-L, Yang J-N, Wang D-Y. A microRNA-mediated insulin signaling pathway regulates the toxicity of multi-walled carbon nanotubes in nematode Caenorhabditis elegans. Sci. Rep. 2016;6:23234. doi: 10.1038/srep23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottschalk F, Sonderer T, Scholz RW, Nowack E. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009;43:9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- 23.Mottier A, Mouchet F, Pinelli E, Gauthier L, Flahaut E. Environmental impact of engineered carbon nanoparticles: from releases to effects on the aquatic biota. Curr. Opin. Biotechnol. 2017;46:1–6. doi: 10.1016/j.copbio.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 24.Liu M, et al. Mir-35 is involved in intestine cell G1/S transition and germ cell proliferation in C. elegans. Cell Res. 2011;21:1605–1618. doi: 10.1038/cr.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran, A. T. et al. MiR-35 buffers apoptosis thresholds in the C. elegans germline by antagonizing both MAPK and core apoptosis pathways. Cell Death Differ., 10.1038/s41418-019-0325-6 (2019). [DOI] [PMC free article] [PubMed]
- 26.Inoue H, Nishida E. The DM domain transcription factor MAB-3 regulates male hypersensitivity to oxidative stress in Caenorhabditis elegans. Mol. Cell. Biol. 2010;30:3453–3459. doi: 10.1128/MCB.01459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, D.-Y. Molecular Toxicology in Caenorhabditis elegans. Springer Nature Singapore Pte Ltd. (2019).
- 28.Shao H-M, Han Z-Y, Krasteva N, Wang D-Y. Identification of signaling cascade in the insulin signaling pathway in response to nanopolystyrene particles. Nanotoxicology. 2019;13:174–188. doi: 10.1080/17435390.2018.1530395. [DOI] [PubMed] [Google Scholar]
- 29.Espelt MV, Estevez AY, Yin X, Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: Role of the inositol-1,4,5-trisphosphate receptor and phospholipases C β and γ. J. General Physiol. 2005;126:379–392. doi: 10.1085/jgp.200509355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L-F, et al. A circular RNA circ_0000115 in response to graphene oxide in nematodes. RSC Adv. 2019;9:13722–13735. doi: 10.1039/C9RA00997C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu P-D, et al. Dysregulation of neuronal Gαo signaling by graphene oxide in nematode Caenorhabditis elegans. Sci. Rep. 2019;9:6026. doi: 10.1038/s41598-019-42603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu Man, Kong Yan, Yuan Yujie, Wang Dayong. Neuronal damage induced by nanopolystyrene particles in nematode Caenorhabditis elegans. Environmental Science: Nano. 2019;6(8):2591–2601. [Google Scholar]
- 34.Qu M, Liu Y-Q, Xu K-N, Wang D-Y. Activation of p38 MAPK signaling-mediated endoplasmic reticulum unfolded protein response by nanopolystyrene particles. Adv. Biosys. 2019;3:1800325. doi: 10.1002/adbi.201800325. [DOI] [PubMed] [Google Scholar]
- 35.Ding X-C, et al. Toxicity of graphene oxide in nematodes with deficit in epidermal barrier caused by RNA interference knockdown of unc-52. Environ. Sci. Technol. Lett. 2018;5:622–628. doi: 10.1021/acs.estlett.8b00473. [DOI] [Google Scholar]
- 36.Mello C, Fire A. DNA transformation. Methods Cell. Biol. 1995;48:451–482. doi: 10.1016/S0091-679X(08)61399-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.