Abstract
Cardiac magnetic resonance (CMR) is becoming the imaging modality of choice in multicenter studies where highly reproducible measurements are necessary. The purpose of this study was to assess the effect of comprehensive initial training on reproducibility of quantitative left ventricular (LV) parameters estimated using strain-encoded (SENC) imaging. Thirty participants (10 patients with heart failure (HF) and preserved LV ejection fraction (HFpEF), 10 patients with HF and reduced LV ejection fraction (HFrEF) and 10 healthy volunteers) were examined using fast-SENC imaging. Four observers with different experience in non-invasive cardiac imaging completed comprehensive initial training course and were invited to perform CMR data analysis. To assess agreement between observers, LV volumes, mass, ejection fraction (LVEF), global longitudinal strain (GLS) and global circumferential strain (GCS) were estimated using dedicated software (MyoStrain, USA). To test intraobserver agreement data analysis was repeated after 4 weeks. SENC imaging and analysis were fast and were completed in less than 5 minutes. LV end-diastolic volume index (LVEDVi), LVEF and strain were significantly lower in HFpEF patients than in healthy volunteers (p = 0.019 for LVEDVi; p = 0.023 for LVEF; p = 0.004 for GLS and p < 0.001 for GCS). All LV functional parameters were further reduced in HFrEF. Excellent interobserver agreement was found for all LV parameters independently of the level of experience. The reproducibility of LV mass was lower, especially at the intraobserver level (ICC 0.91; 95% CI 0.74–0.96). LV volumetric and functional parameters derived using fast-SENC imaging, are highly reproducible. The appropriate initial training is relevant and allows to achieve highest concordance in fast-SENC measurements.
Subject terms: Cardiology, Diagnostic markers
Introduction
In addition to clinical signs and symptoms, a detailed assessment of structural and functional cardiac parameters is considered to be essential and provides important diagnostic information in patients with heart failure (HF)1,2. Over the past decade cardiac magnetic resonance (CMR) has evolved into the reference standard to assess cardiac anatomy and function3,4. Because of its excellent endocardial border definition, cine CMR imaging is the accepted gold standard for quantification of ventricular volumes, mass and ejection fraction5,6. While important achievements in CMR techniques have reduced total scan time, quantitative volumetric analysis has not changed significantly and requires time and human resources7,8.
Growing evidence suggests that conventional functional parameters such as left ventricular ejection fraction (LVEF) may not be sensitive enough to detect subtle changes in ventricular function9. Tissue-tracking techniques have enabled the non-invasive assessment of myocardial deformation and it appears that myocardial strain might be a more robust marker of the failing myocardium10–12. Strain-encoded magnetic resonance (SENC) imaging was developed on the concepts of myocardial tagging and first described in 200113. In line with other tissue tracking techniques, SENC provides quantitative information about myocardial mechanics and has been validated and applied in multiple experimental and clinical settings14–16. Fast-SENC technique is a real-time version of SENC that has shortened the scan duration to a single heartbeat17. The absence of contrast agent and free breathing during data acquisition are important advantages that make the technique desirable in daily routine. Moreover, recent achievements in fast-SENC data analysis tools have enabled quantification of conventional left ventricular (LV) volumetric and functional parameters with excellent accuracy and minimal educational efforts18.
More and more clinical decision making relies on CMR derived data19–21; hence, interobserver and intraobserver variability may become an important source of bias. Although recommendations exist for CMR acquisition22 and data post-processing23,24, the lack of proper initial training of the observers may lead to significant measurement variance which becomes more apparent in multicenter studies. Indeed, appropriate education25,26 as well as repeated measurements27 might improve interobserver reproducibility for volumetric and functional measures of the left ventricle.
We set up this study to investigate the effect of comprehensive initial training on reproducibility of LV volumes, mass, ejection fraction and strain derived using fast-SENC imaging. The main hypothesis was that appropriate initial training has an important impact in terms of cardiac imaging of the readers on the concordance of measurements.
Materials and Methods
Study population
The study population comprised patients with heart failure and preserved LVEF (HFpEF, n = 10), reduced LVEF (HFrEF, n = 10) and healthy volunteers (n = 10). The study complies with the Declaration of Helsinki and was approved by the ethics committee board of the Charité-Universitätsmedizin Berlin. All participants provided written informed consent before entering the study.
CMR acquisition
All CMR studies were performed on a 1.5 T MRI scanner (Achieva, Philips Healthcare, Best, The Netherlands) using a five-element phased array cardiac coil in supine position.
A previously described17 real-time free breathing SENC imaging technique (Myocardial Solutions, Inc., Morrisville, North Carolina, USA) based on the acquisition of two images with different frequency modulation was employed. Images were taken in three different LV long-axis (two-, three- and four-chamber) and three LV short-axis (at basal, mid-ventricular and apical level) views. The slices of different LV short-axis levels were identified as follows: basal level slice was considered and used for quantitative analysis if complete LV myocardium was visible throughout the entire cardiac cycle; mid-ventricular level slice was selected at the level of both papillary muscles; and apical level slice was considered if blood pool was still visible throughout the entire cardiac cycle (no obliteration of the LV cavity at end-systolic phase). Typical fast-SENC parameters were as follows: field-of-view = 256 × 256 mm2, slice thickness = 10 mm, voxel size = 4.0 × 4.0 × 10 mm3, single-shot spiral readout (3 interleaves) with acquisition time (TA) = 10 ms, flip angle = 30°, effective echo time (TE) = 0.7 ms, repetition time (TR) = 12 ms, temporal resolution = 36 ms, typical number of acquired phases = 22, spectrally selective fat suppression (SPIR), total acquisition time per slice < 1 s.
Study observers
The four observers with different knowledge and experience in CMR imaging were invited to perform analysis of acquired CMR data: (1) CMR expert (TL) (level 3 certified, performing routine clinical CMR scanning and data post-processing for >5 years); (2) CMR beginner (VZ) (with basic knowledge and <3 months of experience in CMR imaging); (3) Echo expert (HHC) (level 3 certified, performing advanced echocardiography studies including speckle tracking echocardiography in high-volume cardiovascular unit) and (4) Non-cardiac technician (JE) (fully-trained radiographer without any experience in data post-processing).
Training protocol
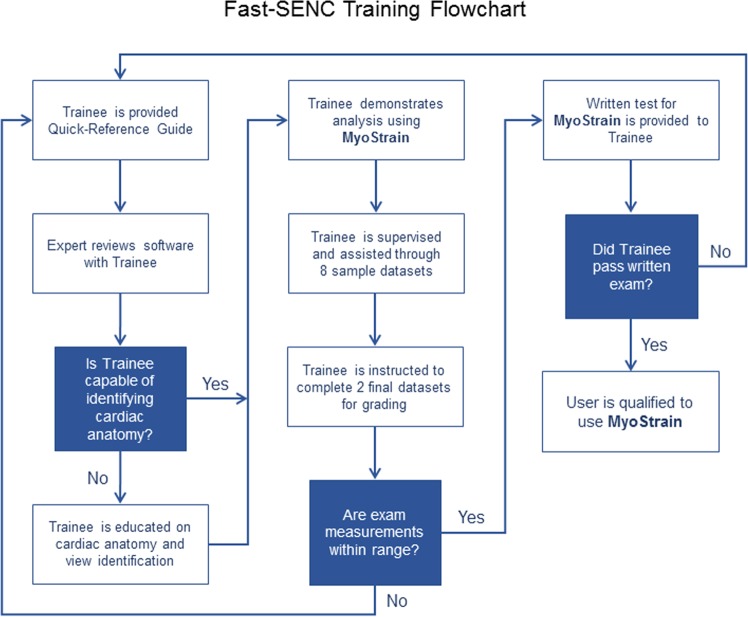
Before starting the CMR data analysis, all observers were trained similarly by a representative of the software company with an emphasis on possible sources of error. A Quick-Reference Guide was given to each trainee before starting the training. The training consisted of a 2 to 4-hour training course designed to provide observers with the skills necessary to correctly use the dedicated MyoStrain software (Fig. 1). A set of 8 cases was used during the training including different cardiac conditions (healthy, cardiomyopathies, ischemic heart disease) and possible image quality issues (suboptimal field-of-view, patient movement or image artifacts) which may arise when using the software. Each trainee had a hands-on analysis session in a blinded manner with personal feedback from the expert. Observers were instructed not to analyze if they thought image quality was inadequate. After completion of the 8 cases, 2 additional datasets were provided and analyzed independently from the expert. The analyses were collected and reviewed by the training site to ensure that measurements were performed correctly. Estimates collected from these analyses had to fall within acceptable ranges. A written test was also mandatory and comprised 50 questions covering cardiac anatomy, view identification and image quality as well as identification of systolic and diastolic cardiac phases. A score of 80% or above on the written exam was considered “passed”. After completion of the training course and positive feedback from the training site, observers were allowed to start study data analysis.
Figure 1.
Fast-SENC training flowchart. Before starting the analysis of CMR data, observers acquired similar comprehensive expert-guided training. The training consisted of a 2 to 4-hour training course designed to provide the skills necessary to correctly use the software. After successful completion of the training course and examination observers were allowed to start study data analysis.
Image analysis
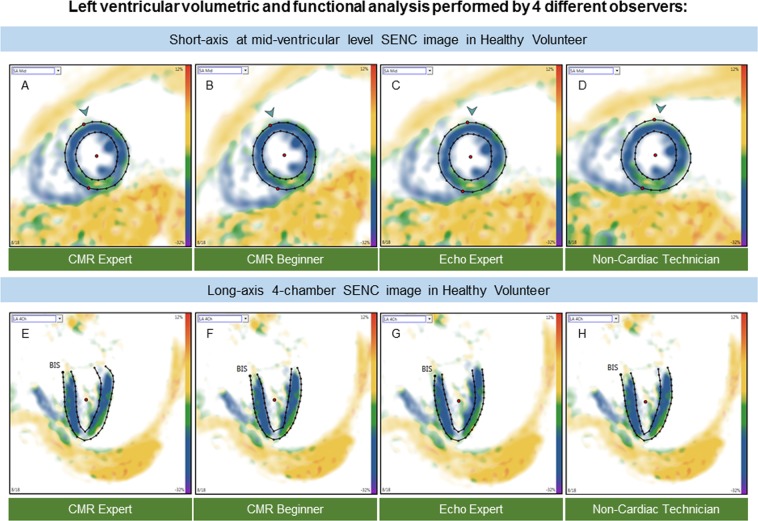
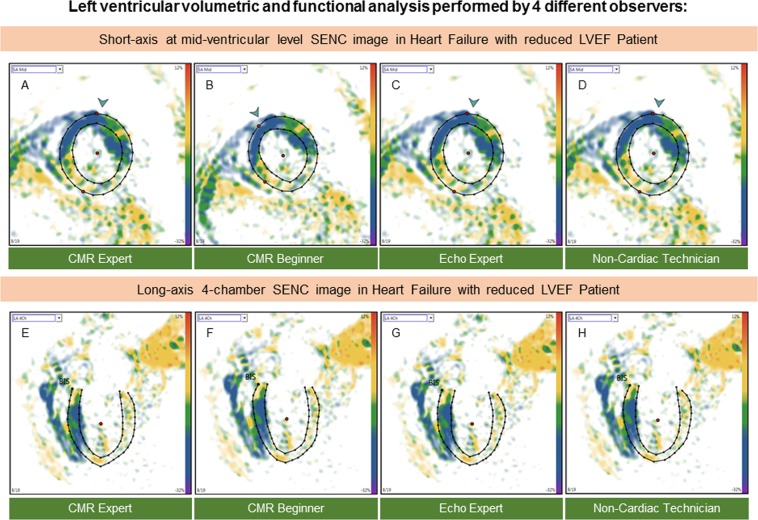
All fast-SENC images were uploaded from the MRI scanner and analyzed using dedicated MyoStrain, version 4.2 software (Morrisville, NC, USA). LV end-diastolic (LVEDV), LV end-systolic volumes (LVESV) and LV mass (LVM) were quantified using manual planimetry of the endocardial and epicardial surface from three long-axis fast-SENC images and LVEF was calculated (Figs 2 and 3). The quality of the contouring was evaluated by visually comparing the tracking process with the underlying myocardial motion. Papillary muscles were considered part of the blood pool. LV volumes and mass were adjusted to body surface area. The LV longitudinal and circumferential strain was extracted from three LV short-axis and three LV long-axis fast-SENC images, respectively. The global strain values were calculated by averaging measurements obtained from 16 segments for global longitudinal strain (GLS) and 17 segments for global circumferential strain (GCS).
Figure 2.
Example of fast-SENC images acquired in a healthy volunteer and uploaded into a dedicated MyoStrain software. CMR images were derived in three long-axis and three short-axis views. Endocardial and epicardial borders were traced at end-diastolic and end-systolic cardiac phases by four observers: CMR expert (A,E) CMR beginner (B,F) echocardiography expert (C,G) and non-cardiac technician (D,H). CMR = cardiac magnetic resonance; SENC = strain-encoded imaging.
Figure 3.
Example of fast-SENC images acquired in HFrEF patients and uploaded into a dedicated MyoStrain software. Endocardial and epicardial borders were traced at end-diastolic and end-systolic cardiac phases by four observers: CMR expert (A,E) CMR beginner (B,F) echocardiography expert (C,G) and non-cardiac technician (D,H). CMR = cardiac magnetic resonance; SENC = strain-encoded imaging; HFrEF = heart failure with reduced left ventricular ejection fraction.
Statistical analysis
Data were analyzed using IBM SPSS Statistics, version 21.0 software (SPSS Inc., Chicago, IL, USA) for Windows. Continuous variables were expressed as mean ± standard deviation. The distribution of continuous variables was assessed using the Shapiro-Wilk test and comparisons between groups were performed with the 2-sample t test and the Mann-Whitney U test where appropriate. Interobserver and intraobserver variability was assessed by intraclass correlation (ICC) (2-way mixed model, absolute agreement between single measurements) and Bland-Altman analysis28. Agreement was considered excellent for ICC >0.74, good for ICC 0.60–0.74, fair for ICC 0.40–0.59, and poor for ICC <0.4029. A p value of <0.05 was considered statistically significant.
Ethics approval and consent to participate
The local ethics committee (Charité-Universitätsmedizin Berlin) approved the research and consent was obtained for all study participants.
Results
All study participants were able to complete the entire study protocol. SENC imaging and analysis was fast with a 15 second scan time and a 3 to 5 minute post-processing time for complete quantitative assessment including LV volumes, mass, ejection fraction and global longitudinal and circumferential strain.
There was no significant difference in LVESV and LVM indices between healthy volunteers and HFpEF patients (p = 0.579 for LVESVi and p = 0.315 for LVMi), while LVEDVi, LVEF and strain values were significantly lower in HFpEF (87.80 ± 7.65 ml/m2 vs. 75.20 ± 11.66 ml/m2, p = 0.019 for LVEDVi; 60.41 ± 5.57% vs. 55.96 ± 3.40%, p = 0.023 for LVEF; −20.45 ± 1.46% vs. −18.92 ± 0.84%, p = 0.004 for GLS and −21.25 ± 1.19% vs. −17.35 ± 1.89%, p < 0.001 for GCS). All LV functional parameters were further reduced in HFrEF patients compared with healthy volunteers or HFpEF. LV volumes and mass were significantly larger in HFrEF than in other study subjects. Table 1 demonstrates fast-SENC derived parameters in the study population.
Table 1.
Comparison of LV volumes, mass, ejection fraction and strain parameters among healthy volunteers and patients with heart failure with preserved (HFpEF) and reduced LV ejection fraction (HFrEF).
| Parameter | Volunteers (n = 10) |
HFpEF (n = 10) |
HFrEF (n = 10) |
P value | ||
|---|---|---|---|---|---|---|
| Volunteers vs. HFpEF |
Volunteers vs. HFrEF |
HFpEF vs. HFrEF |
||||
| LVEDVi (ml/m2) | 87.80 ± 7.65 | 75.20 ± 11.66 | 133.74 ± 22.00 | 0.019 | <0.001 | <0.001 |
| LVESVi (ml/m2) | 34.71 ± 5.35 | 33.42 ± 7.38 | 100.31 ± 26.51 | 0.579 | <0.001 | <0.001 |
| LVEF (%) | 60.41 ± 5.57 | 55.96 ± 3.40 | 25.64 ± 10.45 | 0.023 | <0.001 | <0.001 |
| LVMi (g/m2) | 55.36 ± 7.35 | 59.89 ± 9.25 | 88.60 ± 17.22 | 0.315 | <0.001 | <0.001 |
| GLS (%) | −20.45 ± 1.46 | −18.92 ± 0.84 | −10.92 ± 4.33 | 0.004 | <0.001 | <0.001 |
| GCS (%) | −21.25 ± 1.19 | −17.35 ± 1.89 | −11.89 ± 3.07 | <0.001 | <0.001 | <0.001 |
Results are reported as mean ± standard deviation. LV = left ventricular; LVEDVi – left ventricular end-diastolic volume index; LVESVi = left ventricular end-systolic volume index; LVEF = left ventricular ejection fraction; LVMi = left ventricular mass index; GLS = global longitudinal strain; GCS = global circumferential strain; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
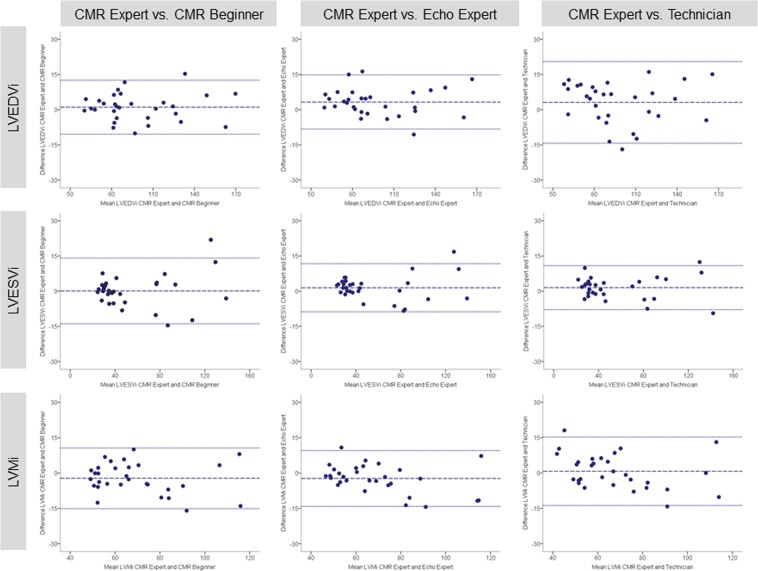
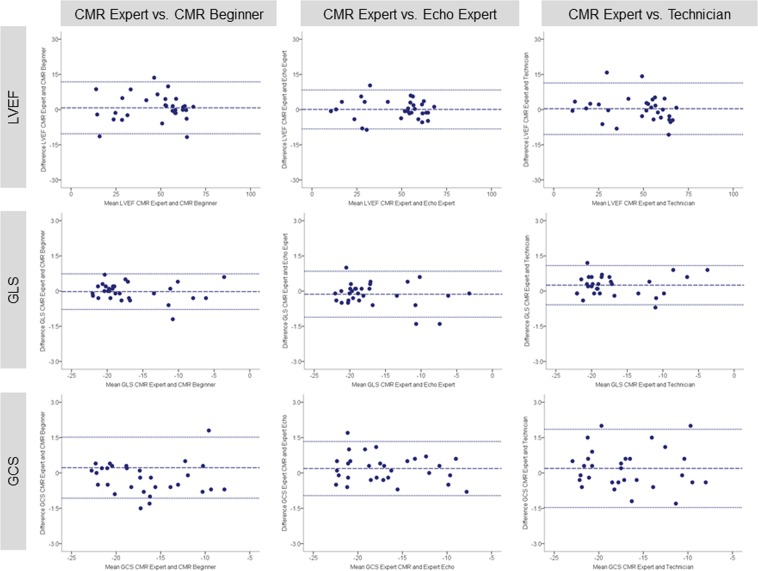
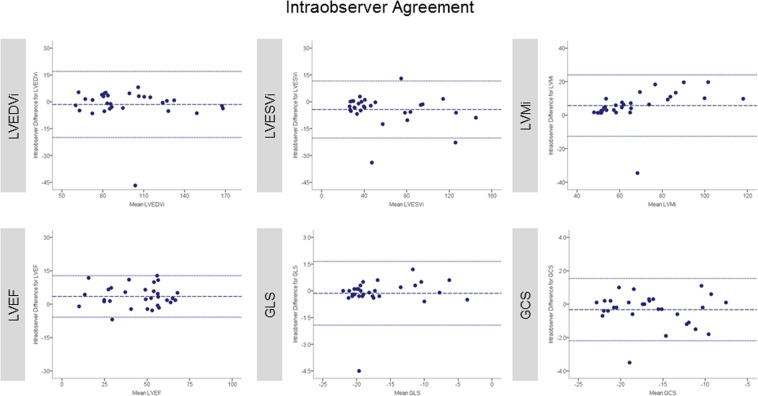
Excellent interobserver reproducibility was found for volumetric and functional LV parameters independently of the previous reader’s experience. The least reproducible measure was LVMi with lowest agreement at intraobserver level (ICC 0.91; 95% confidence interval 0.74–0.96) (intraobserver analysis was performed by CMR beginner). Tables 2 and 3 summarize values for mean difference ± SD, limit of agreement and ICC between study observers for LV volumes, mass and function. Correspondingly, Bland-Altman plots for LVEF and strain are displayed in Figs 4 and 5. Bland-Altman plots for intraobserver reproducibility are depicted in Fig. 6.
Table 2.
Bland-Altman analysis and ICC of pairwise comparison between study observers for LV volumes, mass, ejection fraction and strain parameters (analysis of entire study population, n = 30).
| Parameter | Mean difference ± SD | Limits of agreement | ICC (95% CI) |
|---|---|---|---|
| CMR Expert vs. CMR Beginner | |||
| LVEDVi (ml/m2) | 1.07 ± 5.86 | −10.42 to 12.56 | 0.99 (0.98 to 1.00) |
| LVESVi (ml/m2) | 0.10 ± 7.14 | −13.89 to 14.09 | 0.99 (0.98 to 1.00) |
| LVMi (g/m2) | −2.24 ± 6.57 | −15.13 to 10.64 | 0.97 (0.93 to 0.99) |
| LVEF (%) | 0.76 ± 5.64 | −10.30 to 11.82 | 0.97 (0.94 to 0.99) |
| GLS (%) | −0.02 ± 0.39 | −0.78 to 0.73 | 1.00 (1.00 to 1.00) |
| GCS (%) | 0.22 ± 0.66 | −1.07 to 1.52 | 0.99 (0.99 to 1.00) |
| CMR Expert vs. Echo Expert | |||
| LVEDVi (ml/m2) | 3.29 ± 5.89 | −8.25 to 14.83 | 0.99 (0.96 to 1.00) |
| LVESVi (ml/m2) | 1.45 ± 5.24 | −8.81 to 11.72 | 0.99 (0.99 to 1.00) |
| LVMi (g/m2) | −2.32 ± 6.04 | −14.16 to 9.51 | 0.98 (0.94 to 0.99) |
| LVEF (%) | 0.07 ± 4.23 | −8.22 to 8.36 | 0.99 (0.97 to 0.99) |
| GLS (%) | −0.13 ± 0.50 | −1.11 to 0.85 | 1.00 (1.00 to 1.00) |
| GCS (%) | 0.19 ± 0.59 | −0.96 to 1.33 | 1.00 (0.99 to 1.00) |
| CMR Expert vs. Non-Cardiac Technician | |||
| LVEDVi (ml/m2) | 3.12 ± 8.87 | −14.27 to 20.51 | 0.98 (0.95 to 0.99) |
| LVESVi (ml/m2) | 1.51 ± 4.79 | −7.88 to 10.90 | 1.00 (0.99 to 1.00) |
| LVMi (g/m2) | 0.75 ± 7.40 | −13.76 to 15.25 | 0.97 (0.93 to 0.98) |
| LVEF (%) | 0.38 ± 5.60 | −10.60 to 11.35 | 0.98 (0.95 to 0.99) |
| GLS (%) | 0.25 ± 0.43 | −0.58 to 1.09 | 1.00 (0.99 to 1.00) |
| GCS (%) | 0.19 ± 0.85 | −1.47 to 1.85 | 0.99 (0.98 to 1.00) |
Results are reported as mean ± standard deviation. ICC = intraclass correlation coefficient; CI = confidence interval; CMR = cardiac magnetic resonance. Other abbreviations as in Table 1.
Table 3.
Bland-Altman analysis and ICC of pairwise comparison to assess intraobserver agreement for LV volumes, mass, ejection fraction and strain parameters (analysis of entire study population, n = 30).
| Parameter | First measurement |
Second measurement |
Mean difference |
Limits of agreement |
ICC (95% CI) |
|---|---|---|---|---|---|
| LVEDVi (ml/m2) | 97.84 ± 29.26 | 99.36 ± 30.31 | −1.52 ± 9.42 | −19.98 to 16.95 | 0.98 (0.95 to 0.99) |
| LVESVi (ml/m2) | 56.05 ± 34.16 | 60.38 ± 35.90 | −4.33 ± 8.13 | −20.27 to 11.60 | 0.98 (0.95 to 0.99) |
| LVMi (g/m2) | 70.19 ± 20.20 | 64.45 ± 16.45 | 5.75 ± 9.34 | −12.57 to 24.06 | 0.91 (0.74 to 0.96) |
| LVEF (%) | 46.58 ± 17.02 | 43.10 ± 17.01 | 3.48 ± 4.76 | −5.85 to 12.81 | 0.97 (0.88 to 0.99) |
| GLS (%) | −16.74 ± 5.03 | −16.60 ± 4.87 | −0.14 ± 9.11 | −1.93 to 1.65 | 0.99 (0.98 to 1.00) |
| GCS (%) | −16.61 ± 4.47 | −16.28 ± 4.58 | −0.33 ± 0.95 | −2.19 to 1.54 | 0.99 (0.97 to 0.99) |
Results are reported as mean ± standard deviation. Abbreviations as in Table 1.
Figure 4.
Bland-Altman plots with limits of agreement (1.96 standard deviations) demonstrate the interobserver agreement of fast-SENC for LVEDVi, LVESVi and LVMi. The middle-dashed line is the mean of difference of measures. The upper and lower dotted lines are ±1.96 standard deviation. SENC = strain-encoded imaging; LVEDVi = left ventricular end-diastolic volume index; LVESVi = left ventricular end-systolic volume index; LVMi = left ventricular mass index.
Figure 5.
Bland-Altman plots with limits of agreement (1.96 standard deviations) demonstrate the interobserver agreement of fast-SENC for LVEF, GLS and GCS. LVEF = left ventricular ejection fraction; GLS = global longitudinal strain; GCS = global circumferential strain.
Figure 6.
Bland-Altman plots with limits of agreement show the intraobserver agreement of fast-SENC for LVEDVi, LVESVi, LVMi, LVEF, GLS and GCS. The middle-dashed line is the mean of difference of measures. The upper and lower dotted lines are ±1.96 standard deviation. SENC = strain-encoded imaging; LVEDVi = left ventricular end-diastolic volume index; LVESVi = left ventricular end-systolic volume index; LVMi = left ventricular mass index; LVEF = left ventricular ejection fraction; GLS = global longitudinal strain; GCS = global circumferential strain.
Discussion
The current study was designed to test whether comprehensive initial training has more relevant impact on the reproducibility of LV volumetric and functional parameters estimated using fast-SENC technique than observers experience. Our data analysis demonstrated that:
LV volumetric and functional parameters can be precisely derived from fast-SENC images in a single short data analysis session.
Appropriate initial training is important and has impact on the concordance in measurements among observers independently of their previous experience.
Excellent interobserver and intraobserver agreement is present for all quantitative LV parameters, especially for GLS and GCS, whereas measures of LV mass appear less robust.
The assessment of LV function is probably the most important part of every cardiac imaging study. The majority of clinical decision making algorithms largely rely on quantitative variables such as LVEF30–32. It has been shown that this single measure is critical for diagnosis of HF and selection of optimal medical or device therapy33. However, there is evidence that advanced measures of myocardial performance, such as strain or torsion, are better predictors of outcome than LVEF or wall motion score index34,35. Tissue-tracking techniques such as speckle tracking echocardiography, CMR tagging, displacement encoded with stimulated echoes (DENSE) imaging or feature tracking appear very promising and have shown the ability to detect early changes in myocardial motion36. Historically, CMR tagging was the first technique implemented for the analysis of myocardial deformation37. However, time-consuming data acquisition and analysis remain important limitations of this standard of reference technique38,39.
In 2001 Osman et al., proposed a new method for measuring the myocardial strain orthogonal to the imaging plane, called SENC-MRI13. The method required the acquisition of two images and allowed straightforward and fast computation of longitudinal strain. Recent achievements in SENC technique have shortened the scan duration to a single heartbeat and eliminated the demand of multiple breath-holds40. Fast-SENC was validated against the conventional CMR tagging and excellent correlation between the methods was shown41,42. The ability to obtain accurate measurements in a short time is highly desirable, especially in severely ill patients and children. We successfully completed image acquisition and data analysis in less than five minutes, while participants were still in the MRI scanner. Such achievements make the implementation of this technique in the clinical realm very promising.
The reliability and reproducibility of LV functional measures are of great importance for patient management, therapy monitoring and outcome studies43. A lower level of variability permits detection of smaller changes and may reduce the necessary sample size for clinical trials44,45. Neizel et al., evaluated interobserver agreement in healthy volunteers and found very high reproducibility in SENC strain measurements (r = 0.87), which was superior to CMR tagging (0.81)42. Hamdan et al., reported similar findings in healthy volunteers scanned on a 3.0 T MRI system with ICC between observers and repeated studies ranging from 0.92 to 0.9846. Our findings are in line with the results of previous studies. We found excellent interobserver and intraobserver agreement of LV global longitudinal and circumferential strain. Moreover, for the first time we demonstrated excellent interobserver and intraobserver reproducibility of LV volumes and ejection fraction estimated using fast-SENC imaging. As reported previously, the agreement of LV mass measurements was lower, especially at the intraobserver level (ICC 0.91; 95% confidence interval 0.74–0.96).
At present, CMR is becoming the imaging modality of choice in multicentric cardiovascular trials47; therefore, inter-institutional agreement of derived measures must be recognized as a relevant source of error. Sample size calculation is an important aspect of study design and depends on the concordance of measurements. Much attention has been attributed to CMR scanning and data analysis guidelines22–24. However, little is known on whether knowledge, experience or appropriate initial training has more impact on the precision of measurements. Beerbaum et al., investigated the impact of interobserver variance between the institutions for volumetric and flow CMR data. Images were analyzed by experienced readers only. Inter-institutional agreement was assessed before and after a dedicated training course. Interestingly, in patients, on transverse planes, variation coefficient for LV volumes was significantly decreased by training (p < 0.007). For short-axis volumetry training also resulted in narrower limits of agreement. The reproducibility did not improve significantly with training in healthy volunteers. However, the highest variability after training in volunteers was found for LV mass (transverse acquisition: 12–15%, short-axis acquisition: 9–12%)48.
In a recent study, Negishi et al. evaluated the role of experience in the precision and validity of strain measurements derived using speckle tracking echocardiography. Their study revealed that although the group with the highest level of experience achieved better agreement than those with no experience, the ICC of the inexperienced observers was still very high (0.996 vs. 0.975; p = 0.0002)49. To examine the importance of initial training we selected four study observers with different knowledge and experience background but provided comprehensive and expert-guided training. Data analysis demonstrated that interobserver agreement was excellent independently of readers’ expertise. Nevertheless, it should be noted that LV mass measurements were more variable, especially at the intraobserver level (analysis performed by a CMR beginner), confirming that degree of experience might be also important and should not be underestimated. Despite higher variability, the concordance of LV mass measurements is still clinically acceptable (ICC 0.91).
Our very recent study was conducted to evaluate the impact of proper training on the variability of myocardial strain measurements derived using different commercially available CMR feature tracking software packages. Study results demonstrated that dedicated training of the observer significantly improves reproducibility of LV GLS and GCS50. Findings of this study are in line with previous studies and confirm that appropriate initial training might be more important to achieve highest concordance in CMR measurements. In the light of inadequate experience in CMR imaging fast-SENC technique would be highly desirable in non-expert CMR centers where precise quantitative LV analysis could be performed rapidly even by unexperienced readers.
Limitations
Several limitations of the current study should be noted. First, the population of this study was relatively small. Second, it was single center, single vendor, single software and single MRI lab protocol. Third, only fast-SENC imaging was used to investigate the performance of observers without reflection of other CMR tissue tracking techniques such as tagging, DENSE or feature tracking. We also did not compare fast-SENC images acquired by MRI scanners of different vendors. Lastly, we did not assess the variability of measurements before the training of the observers.
Conclusion
Excellent reproducibility of LV volumetric and functional parameters makes fast-SENC a reliable imaging modality for future studies. Although level of experience is important, it appears that appropriate initial training has much more impact on the agreement of derived measurements. However, larger multicenter studies using MRI scanners and software packages from different vendors are necessary to confirm our findings.
Acknowledgements
We thank Anne Wölffel-Gale for editorial assistance. S.K. was supported by an unrestricted research grant from Philips Healthcare and Myocardial Solutions. C.S. is an employee of Philips Healthcare. The study was supported by the German Centre for Cardiovascular Research (DZHK).
Author Contributions
All authors made relevant contributions to the study and reviewed the manuscript. T.L., V.Z., J.E. and H.H. analyzed and interpreted data. T.L. performed statistical analysis and drafted the manuscript. C.S. participated in the design of the study and coordinated CMR data acquisition. R.G., S.G. and S.J.B. helped with the interpretation of data. R.Z., G.K., B.P. and A.S. made substantial contributions to the concept of the study and revised the manuscript for important intellectual content. S.K. designed and coordinated the study, helped to interpret the data and to draft the manuscript. All authors have read and approved the final manuscript.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
S.K. received an unrestricted research grant by Philips Healthcare and a research grant from Myocardial Solutions. C.S. is an employees of Philips Healthcare. T.L., S.K., A.S. and B.P. received support from the DZHK (German Centre for Cardiovascular Research). The remaining authors declare that they have no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pennell DJ. Ventricular volume and mass by CMR. J Cardiovasc Magn Reson. 2002;4:507–513. doi: 10.1081/JCMR-120016389. [DOI] [PubMed] [Google Scholar]
- 2.Solomon SD, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 3.Attili AK, Schuster A, Nagel E, Reiber JHC, Van Der Geest RJ. Quantification in cardiac MRI: Advances in image acquisition and processing. International Journal of Cardiovascular Imaging. 2010;26:27–40. doi: 10.1007/s10554-009-9571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flachskampf FA, et al. Cardiac Imaging to Evaluate Left Ventricular Diastolic Function. JACC Cardiovasc Imaging. 2015;8:1071–1093. doi: 10.1016/j.jcmg.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Chuang ML, et al. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardiol. 2000;35:477–484. doi: 10.1016/S0735-1097(99)00551-3. [DOI] [PubMed] [Google Scholar]
- 6.Lapinskas T. Ischemic heart disease: a comprehensive evaluation using cardiovascular magnetic resonance. Medicina (Kaunas). 2013;49:97–110. [PubMed] [Google Scholar]
- 7.Butler SP, et al. Reproducibility study of left ventricular measurements with breath-hold cine MRI using a semiautomated volumetric image analysis program. J Magn Reson Imaging. 1998;8:467–472. doi: 10.1002/jmri.1880080230. [DOI] [PubMed] [Google Scholar]
- 8.Thiele H, et al. Improved accuracy of quantitative assessment of left ventricular volume and ejection fraction by geometric models with steady-state free precession. J Cardiovasc Magn Reson. 2002;4:327–339. doi: 10.1081/JCMR-120013298. [DOI] [PubMed] [Google Scholar]
- 9.Cikes M, Solomon SD. Beyond ejection fraction: An integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J. 2016;37:1642–1650. doi: 10.1093/eurheartj/ehv510. [DOI] [PubMed] [Google Scholar]
- 10.Sachdev V, et al. Myocardial strain decreases with increasing transmurality of infarction: A doppler echocardiographic and magnetic resonance correlation study. J Am Soc Echocardiogr. 2006;19:34–39. doi: 10.1016/j.echo.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Pedrizzetti G, Mangual J, Tonti G. On the geometrical relationship between global longitudinal strain and ejection fraction in the evaluation of cardiac contraction. J Biomech. 2014;47:746–749. doi: 10.1016/j.jbiomech.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Buss SJ, et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Hear J Cardiovasc Imaging. 2015;16:307–315. doi: 10.1093/ehjci/jeu181. [DOI] [PubMed] [Google Scholar]
- 13.Osman NF, Sampath S, Atalar E, Prince JL. Imaging longitudinal cardiac strain on short-axis images using strain-encoded MRI. Magn Reson Med. 2001;46:324–334. doi: 10.1002/mrm.1195. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka A, et al. In situ constructive myocardial remodeling of extracellular matrix patch enhanced with controlled growth factor release. J Thorac Cardiovasc Surg. 2015;150:1280–1290. doi: 10.1016/j.jtcvs.2015.07.073. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim ESH, et al. Real-time MR imaging of myocardial regional function using strain-encoding (SENC) with tissue through-plane motion tracking. J Magn Reson Imaging. 2007;26:1461–1470. doi: 10.1002/jmri.21125. [DOI] [PubMed] [Google Scholar]
- 16.Korosoglou, G. et al. Strain‐encoded magnetic resonance: a method for the assessment of myocardial deformation. ESC Heart Fail. ehf2.12442 (2019). [DOI] [PMC free article] [PubMed]
- 17.Pan L, et al. Real-time imaging of regional myocardial function using fast-SENC. Magn Reson Med. 2006;55:386–395. doi: 10.1002/mrm.20770. [DOI] [PubMed] [Google Scholar]
- 18.Lapinskas T, et al. Strain-encoded cardiac magnetic resonance imaging: a new approach for fast estimation of left ventricular function. BMC Cardiovasc Disord. 2019;19:1–7. doi: 10.1186/s12872-019-1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mordi IR, et al. Comprehensive Echocardiographic and Cardiac Magnetic Resonance Evaluation Differentiates Among Heart Failure With Preserved Ejection Fraction Patients, Hypertensive Patients, and Healthy Control Subjects. JACC Cardiovasc Imaging. 2018;11:577–585. doi: 10.1016/j.jcmg.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Vincenti G, et al. Stress Perfusion CMR in Patients With Known and Suspected CAD: Prognostic Value and Optimal Ischemic Threshold for Revascularization. JACC Cardiovasc Imaging. 2017;10:526–537. doi: 10.1016/j.jcmg.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Bucciarelli-Ducci C, et al. CMR Guidance for Recanalization of Coronary Chronic Total Occlusion. JACC Cardiovasc Imaging. 2016;9:547–556. doi: 10.1016/j.jcmg.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. doi: 10.1186/1532-429X-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz-Menger J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valsangiacomo Buechel ER, et al. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: an expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Eur Hear J Cardiovasc Imaging. 2015;16:281–297. doi: 10.1093/ehjci/jeu129. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann R, et al. Refinements in stress echocardiographic techniques improve inter-institutional agreement in interpretation of dobutamine stress echocardiograms. Eur Heart J. 2002;23:821–829. doi: 10.1053/euhj.2001.2968. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann R, et al. Standardized guidelines for the interpretation of dobufamine echocardiography reduce interinstitutional variance in interpretation. Am J Cardiol. 1998;82:1520–1524. doi: 10.1016/S0002-9149(98)00697-3. [DOI] [PubMed] [Google Scholar]
- 27.Gertz RJ, et al. Inter-vendor reproducibility of left and right ventricular cardiovascular magnetic resonance myocardial feature-tracking. PLoS One. 2018;13:e0193746. doi: 10.1371/journal.pone.0193746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. Statistical Methods for Assessing Agreement Between Two Methods of Clinical Measurement. Lancet. 1986;327:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 29.Oppo K, Leen E, Angerson WJ, Cooke TG, McArdle CS. Doppler perfusion index: an interobserver and intraobserver reproducibility study. Radiology. 1998;208:453–457. doi: 10.1148/radiology.208.2.9680575. [DOI] [PubMed] [Google Scholar]
- 30.Harjola V-P, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur J Heart Fail. 2017;19:821–836. doi: 10.1002/ejhf.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamorano JL, et al. ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: The task force for cancer treatments and cardiovascular toxicity of the european society of cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 32.Baumgartner H, et al. ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 33.Ponikowski P, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 34.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: Comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 35.Gavara J, et al. Prognostic Value of Strain by Tissue Tracking Cardiac Magnetic Resonance After ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Imaging. 2017;11:1448–1457. doi: 10.1016/j.jcmg.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 2016;18:51. doi: 10.1186/s12968-016-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging–a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59–63. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- 38.Yeon SB, et al. Validation of in vivo myocardial strain measurement by magnetic resonance tagging with sonomicrometry. J Am Coll Cardiol. 2001;38:555–561. doi: 10.1016/S0735-1097(01)01397-3. [DOI] [PubMed] [Google Scholar]
- 39.Ibrahim E-SH. Myocardial tagging by Cardiovascular Magnetic Resonance: evolution of techniques-pulse sequences, analysis algorithms, and applications. J Cardiovasc Magn Reson. 2011;13:36. doi: 10.1186/1532-429X-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shehata ML, et al. Real-time single-heartbeat fast strain-encoded imaging of right ventricular regional function: Normal versus chronic pulmonary hypertension. Magn Reson Med. 2010;64:98–106. doi: 10.1002/mrm.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korosoglou G, et al. Real-time fast strain-encoded magnetic resonance imaging to evaluate regional myocardial function at 3.0 Tesla: Comparison to conventional tagging. J Magn Reson Imaging. 2008;27:1012–1018. doi: 10.1002/jmri.21315. [DOI] [PubMed] [Google Scholar]
- 42.Neizel M, et al. Strain-encoded (SENC) magnetic resonance imaging to evaluate regional heterogeneity of myocardial strain in healthy volunteers: Comparison with conventional tagging. J Magn Reson Imaging. 2009;29:99–105. doi: 10.1002/jmri.21612. [DOI] [PubMed] [Google Scholar]
- 43.Mirea O, et al. Variability and Reproducibility of Segmental Longitudinal Strain Measurement: A Report From the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging. 2018;11:15–24. doi: 10.1016/j.jcmg.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 44.Morton G, et al. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson. 2012;14:43. doi: 10.1186/1532-429X-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lapinskas T, et al. Cardiovascular magnetic resonance feature tracking in small animals – a preliminary study on reproducibility and sample size calculation. BMC Med Imaging. 2017;17:51. doi: 10.1186/s12880-017-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamdan A, et al. Strain-encoded MRI to evaluate normal left ventricular function and timing of contraction at 3.0 tesla. J Magn Reson Imaging. 2009;29:799–808. doi: 10.1002/jmri.21684. [DOI] [PubMed] [Google Scholar]
- 47.Symons R, et al. Long-Term Incremental Prognostic Value of Cardiovascular Magnetic Resonance After ST-Segment Elevation Myocardial Infarction. A Study of the Collaborative Registry on CMR in STEMI. JACC Cardiovasc Imaging. 2017;11:813–825. doi: 10.1016/j.jcmg.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 48.Beerbaum P, et al. Cardiac function by MRI in congenital heart disease: Impact of consensus training on interinstitutional variance. J Magn Reson Imaging. 2009;30:956–966. doi: 10.1002/jmri.21948. [DOI] [PubMed] [Google Scholar]
- 49.Negishi T, et al. Effect of Experience and Training on the Concordance and Precision of Strain Measurements. JACC Cardiovasc Imaging. 2017;10:518–522. doi: 10.1016/j.jcmg.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Backhaus SJ, et al. Cardiovascular magnetic resonance imaging feature tracking: Impact of training on observer performance and reproducibility. PLoS One. 2019;14:e0210127. doi: 10.1371/journal.pone.0210127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.