Abstract
Activation of specific sets of protein kinases by intracellular signal molecules
has become more and more apparent in the past decade. Phosphorylation, one of key
posttranslational modification events, is activated by kinase or regulatory protein
and is vital for controlling many physiological functions of eukaryotic cells such
as cell proliferation, differentiation, malignant transformation, and signal
transduction mediated by external stimuli. Moreovers, the reversible modification of
phosphorylation and dephosphorylation can result in different features of the target
substrate molecules including DNA binding, protein-protein interaction, subcellular
location and enzymatic activity, and is often hijacked by viral infection.
Epstein-Barr virus (EBV) and Kaposi’s sarcomaassociated herpesvirus (KSHV), two
human oncogenic gamma-herpesviruses, are shown to tightly associate with many
malignancies. In this review, we summarize the recent progresses on understanding of
molecular properties and regulatory modes of cellular and viral proteins
phosphorylation influenced by these two tumor viruses, and highlight the potential
therapeutic targets and strategies against their related cancers. 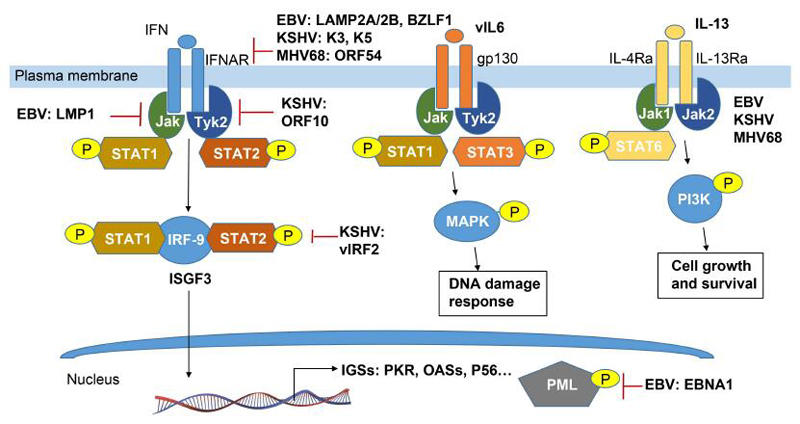
Keywords: Epstein-Barr Virus (EBV), Kaposi’s sarcoma-associated herpesvirus (KSHV), phosphorylation
Acknowledgments
The authors would like to apologize to the many researchers who have contributed to this area of research but have not been cited in this review due to space limitations. This work is supported by the National Natural Science Foundation of China (NO. 81471930, 81402542, 81672015, 81772 166), and National Key Research and Development Program of China (2016YFC1200400). FW is a scholar of Pujiang Talents in Shanghai. QC is a scholar of New Century Excellent Talents in University of China.
Footnotes
These authors contributed equally to this work.
Contributor Information
Shuvomoy Banerjee, Email: sbanerjee@amity.edu.
Fang Wei, Email: fangwei@sjtu.edu.cn.
Qiliang Cai, Email: qiliang@fudan.edu.cn.
References
- Adamson AL, Darr D, Holley-Guthrie E, Johnson RA, Mauser A, Swenson J, Kenney S. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J Virol. 2000;74:1224–1233. doi: 10.1128/jvi.74.3.1224-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–3114. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai R, Kato A, Kato K, Kanamori-Koyama M, Sugimoto K, Sairenji T, Nishiyama Y, Kawaguchi Y. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J Virol. 2006;80:5125–5134. doi: 10.1128/JVI.02674-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj BG, Verma SC, Lan K, Cotter MA, Woodman ZL, Robertson ES. KSHV encoded LANA upregulates Pim-1 and is a substrate for its kinase activity. Virology. 2006;351:18–28. doi: 10.1016/j.virol.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Lu J, Cai Q, Sun Z, Jha HC, Robertson ES. EBNA3C augments Pim-1 mediated phosphorylation and degradation of p21 to promote B-cell proliferation. PLoS Pathog. 2014;10:e1004304. doi: 10.1371/journal.ppat.1004304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz GL, Shackelford J, Pagano JS. Epstein-Barr virus latent membrane protein 1 regulates the function of interferon regulatory factor 7 by inducing its sumoylation. J Virol. 2012;86:12251–12261. doi: 10.1128/JVI.01407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzeno S, Lu F, Guo M, Barbash O, Zhang F, Herman JG, Klein PS, Rustgi A, Diehl JA. Identification of mutations that disrupt phosphorylation-dependent nuclear export of cyclin D1. Oncogene. 2006;25:6291–6303. doi: 10.1038/sj.onc.1209644. [DOI] [PubMed] [Google Scholar]
- Bisson SA, Page AL, Ganem D. A Kaposi’s sarcoma-associated herpesvirus protein that forms inhibitory complexes with type I interferon receptor subunits, Jak and STAT proteins, and blocks interferon-mediated signal transduction. J Virol. 2009;83:5056–5066. doi: 10.1128/JVI.02516-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottero V, Kerur N, Sadagopan S, Patel K, Sharma-Walia N, Chandran B. Phosphorylation and polyubiquitination of transforming growth factor beta-activated kinase 1 are necessary for activation of NF-kappaB by the Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor. J Virol. 2011;85:1980–1993. doi: 10.1128/JVI.01911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann MM, Schulz TF. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J Gen Virol. 2006;87:1047–1074. doi: 10.1099/vir.0.81598-0. [DOI] [PubMed] [Google Scholar]
- Burger M, Hartmann T, Burger JA, Schraufstatter I. KSHVGPCR and CXCR2 transforming capacity and angiogenic responses are mediated through a JAK2-STAT3-dependent pathway. Oncogene. 2005;24:2067–2075. doi: 10.1038/sj.onc.1208442. [DOI] [PubMed] [Google Scholar]
- Burkhardt AL, Bolen JB, Kieff E, Longnecker R. An Epstein-Barr virus transformation-associated membrane protein interacts with src family tyrosine kinases. J Virol. 1992;66:5161–5167. doi: 10.1128/jvi.66.8.5161-5167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burysek L, Pitha PM. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits doublestranded RNA-activated protein kinase. J Virol. 2001;75:2345–2352. doi: 10.1128/JVI.75.5.2345-2352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Verma SC, Choi JY, Ma M, Robertson ES. Kaposi’s sarcoma-associated herpesvirus inhibits interleukin-4-mediated STAT6 phosphorylation to regulate apoptosis and maintain latency. J Virol. 2010;84:11134–11144. doi: 10.1128/JVI.01293-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Verma SC, Lu J, Robertson ES. Molecular biology of Kaposi’s sarcoma-associated herpesvirus and related oncogenesis. Adv Virus Res. 2010;78:87–142. doi: 10.1016/B978-0-12-385032-4.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campistol JM, Gutierrez-Dalmau A, Torregrosa JV. Conversion to sirolimus: a successful treatment for posttransplantation Kaposi’s sarcoma. Transplantation. 2004;77:760–762. doi: 10.1097/01.tp.0000115344.18025.0b. [DOI] [PubMed] [Google Scholar]
- Chang LS, Wang JT, Doong SL, Lee CP, Chang CW, Tsai CH, Yeh SW, Hsieh CY, Chen MR. Epstein-Barr virus BGLF4 kinase downregulates NF-kappaB transactivation through phosphorylation of coactivator UXT. J Virol. 2012;86:12176–12186. doi: 10.1128/JVI.01918-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Campbell M, Robertson ES. Human Oncogenic Herpesvirus and Post-translational Modifications -Phosphorylation and SUMOylation. Front Microbiol. 2016;7:962. doi: 10.3389/fmicb.2016.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hutt-Fletcher L, Cao L, Hayward SD. A positive autoregulatory loop of LMP1 expression and STAT activation in epithelial cells latently infected with Epstein-Barr virus. J Virol. 2003;77:4139–4148. doi: 10.1128/JVI.77.7.4139-4148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yuan Z. Interplay between hepatitis B virus and the innate immune responses: implications for new therapeutic strategies. Virol Sin. 2014;29:17–24. doi: 10.1007/s12250-014-3412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Sun F, Han L, Qu Z. Kaposi’s sarcoma herpesvirus (KSHV) microRNA K12-1 functions as an oncogene by activating NF-kappaB/IL-6/STAT3 signaling. Oncotarget. 2016;7:33363–33373. doi: 10.18632/oncotarget.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MR, Chang SJ, Huang H, Chen JY. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J Virol. 2000;74:3093–3104. doi: 10.1128/jvi.74.7.3093-3104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Weidner-Glunde M, Varjosalo M, Rainio EM, Lehtonen A, Schulz TF, Koskinen PJ, Taipale J, Ojala PM. KSHV reactivation from latency requires Pim-1 and Pim-3 kinases to inactivate the latency-associated nuclear antigen LANA. PLoS Pathog. 2009;5:e1000324. doi: 10.1371/journal.ppat.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi LM, Yu JS, Chang YS. Identification of protein kinase CK2 as a potent kinase of Epstein-Barr virus latent membrane protein 1. Biochem Biophys Res Commun. 2002;294:586–591. doi: 10.1016/S0006-291X(02)00515-6. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur J Biochem. 2001;268:5001–5010. doi: 10.1046/j.0014-2956.2001.02473.x. [DOI] [PubMed] [Google Scholar]
- Cook ID, Shanahan F, Farrell PJ. Epstein-Barr virus SM protein. Virology. 1994;205:217–227. doi: 10.1006/viro.1994.1637. [DOI] [PubMed] [Google Scholar]
- Cousins E, Nicholas J. Role of human herpesvirus 8 interleukin-6-activated gp130 signal transducer in primary effusion lymphoma cell growth and viability. J Virol. 2013;87:10816–10827. doi: 10.1128/JVI.02047-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Davison AJ, Dolan A, Frame MC, McGeoch DJ, Meredith DM, et al. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J GenVirol. 1992;73:303–311. doi: 10.1099/0022-1317-73-2-303. [DOI] [PubMed] [Google Scholar]
- Ersing I, Bernhardt K, Gewurz BE. NF-kappaB and IRF7 pathway activation by Epstein-Barr virus Latent Membrane Protein 1. Viruses. 2013;5:1587–1606. doi: 10.3390/v5061587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban M, Garcia MA, Domingo-Gil E, Arroyo J, Nombela C, Rivas C. The latency protein LANA2 from Kaposi’s sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J Gen Virol. 2003;84:1463–1470. doi: 10.1099/vir.0.19014-0. [DOI] [PubMed] [Google Scholar]
- Feldman ER, Kara M, Coleman CB, Grau KR, Oko LM, Krueger BJ, Renne R v, Dyk LF, Tibbetts SA. Virus-encoded microRNAs facilitate gammaherpesvirus latency and pathogenesis in vivo. MBio. 2014;5:e00981–00914. doi: 10.1128/mBio.00981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger TR, Martin JM. The Epstein-Barr virus-encoded LMP-1 oncoprotein negatively affects Tyk2 phosphorylation and interferon signaling in human B cells. J Virol. 2006;80:11638–11650. doi: 10.1128/JVI.01570-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz BE, Mar JC, Padi M, Zhao B, Shinners NP, Takasaki K, Bedoya E, Zou JY, Cahir-McFarland E, Quackenbush J, Kieff E. Canonical NF-kappaB activation is essential for Epstein-Barr virus latent membrane protein 1 TES2/CTAR2 gene regulation. J Virol. 2011;85:6764–6773. doi: 10.1128/JVI.00422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffin L, Yan F B, Major M, Damania B. Modulation of Kaposi’s sarcoma-associated herpesvirus interleukin-6 function by hypoxia-upregulated protein 1. J Virol. 2014;88:9429–9441. doi: 10.1128/JVI.00511-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez CM, Wong EL, Bowser BS, Hong GK, Kenney S, Damania B. Identification and characterization of the Orf49 protein of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2006;80:3062–3070. doi: 10.1128/JVI.80.6.3062-3070.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havemeier A, Gramolelli S, Pietrek M, Jochmann R, Sturzl M, Schulz TF. Activation of NF-kappaB by the Kaposi’s sarcoma-associated herpesvirus K15 protein involves recruitment of the NF-kappaB-inducing kinase, IkappaB kinases, and phosphorylation of p65. J Virol. 2014;88:13161–13172. doi: 10.1128/JVI.01766-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, He YS, Kim Y, Chu L, Ohmstede C, Biron KK, et al. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J Virol. 1997;71:405–411. doi: 10.1128/jvi.71.1.405-411.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Zhao J, Zhang J, Jung JU, Feng P. NF-kappaB activation coordinated by IKKbeta and IKKepsilon enables latent infection of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2014;88:444–455. doi: 10.1128/JVI.01716-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Kim KS, Flano E, Wu TT, Tong LM, Park AN, Song MJ, Sanchez DJ, O’Connell RM, Cheng G, Sun R. Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response. Cell Host Microbe. 2009;5:166–178. doi: 10.1016/j.chom.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakiri D. Multifunctional non-coding Epstein-Barr virus encoded RNAs (EBERs) contribute to viral pathogenesis. Virus Res. 2016;212:30–38. doi: 10.1016/j.virusres.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Jakubiec A, Jupin I. Regulation of positive-strand RNA virus replication: the emerging role of phosphorylation. Virus Res. 2007;129:73–79. doi: 10.1016/j.virusres.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha HC, Banerjee S, Robertson ES. The Role of Gammaherpesviruses in Cancer Pathogenesis. Pathogens. 2016;5(pii):E18. doi: 10.3390/pathogens5010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha HC, Upadhyay S M A, Lu J, Cai Q, Saha A, Robertson ES. H2AX phosphorylation is important for LANA-mediated Kaposi’s sarcoma-associated herpesvirus episome persistence. J Virol. 2013;87:5255–5269. doi: 10.1128/JVI.03575-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Kawaguchi Y, Tanaka M, Igarashi M, Yokoyama A, Matsuda G, et al. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1delta (EF-1delta): EF-1delta is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J Gen Virol. 2001;82:1457–1463. doi: 10.1099/0022-1317-82-6-1457. [DOI] [PubMed] [Google Scholar]
- Kato K, Yokoyama A, Tohya Y, Akashi H, Nishiyama Y, Kawaguchi Y. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J Gen Virol. 2003;84:3381–3392. doi: 10.1099/vir.0.19454-0. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kato K. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev Med Virol. 2003;13:331–340. doi: 10.1002/rmv.402. [DOI] [PubMed] [Google Scholar]
- Keating JA, Striker R. Phosphorylation events during viral infections provide potential therapeutic targets. Rev Med Virol. 2012;22:166–181. doi: 10.1002/rmv.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim WS, Hong JY, Ryu KJ, Kim SJ, Park C. Epstein-Barr virus EBNA2 directs doxorubicin resistance of B cell lymphoma through CCL3 and CCL4-mediated activation of NF-kappaB and Btk. Oncotarget. 2016;8:5361–5370. doi: 10.18632/oncotarget.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim WS, Yun Y, Park C. Epstein-Barr virus latent membrane protein 1 increases chemo-resistance of cancer cells via cytoplasmic sequestration of Pim-1. Cell Signal. 2010;22:1858–1863. doi: 10.1016/j.cellsig.2010.07.013. [DOI] [PubMed] [Google Scholar]
- King CA. Kaposi’s sarcoma-associated herpesvirus kaposin B induces unique monophosphorylation of STAT3 at serine 727 and MK2-mediated inactivation of the STAT3 transcriptional repressor TRIM28. J Virol. 2013;87:8779–8791. doi: 10.1128/JVI.02976-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiriazis A, Vahakoski RL, Santio NM, Arnaudova R, Eerola SK, Rainio EM, Aumuller IB, Yli-Kauhaluoma J, Koskinen PJ. Tricyclic Benzo[cd]azulenes selectively inhibit activities of Pim kinases and restrict growth of Epstein-Barr virus-transformed cells. PLoS One. 2013;8:e55409. doi: 10.1371/journal.pone.0055409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kis LL, Gerasimcik N, Salamon D, Persson EK, Nagy N, Klein G, Severinson E, Klein E. STAT6 signaling pathway activated by the cytokines IL-4 and IL-13 induces expression of the Epstein-Barr virus-encoded protein LMP-1 in absence of EBNA-2: implications for the type II EBV latent gene expression in Hodgkin lymphoma. Blood. 2011;117:165–174. doi: 10.1182/blood-2010-01-265272. [DOI] [PubMed] [Google Scholar]
- Koon HB, Bubley GJ, Pantanowitz L, Masiello D, Smith B, Crosby K, Proper J, Weeden W, Miller TE, Chatis P, Egorin MJ, Tahan SR, Dezube BJ. Imatinib-induced regression of AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2005;23:982–989. doi: 10.1200/JCO.2005.06.079. [DOI] [PubMed] [Google Scholar]
- Leang RS, Wu TT, Hwang S, Liang LT, Tong L, Truong JT, Sun R. The anti-interferon activity of conserved viral dUTPase ORF54 is essential for an effective MHV-68 infection. PLoS Pathog. 2011;7:e1002292. doi: 10.1371/journal.ppat.1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Means R, Lang S, Jung JU. Downregulation of gamma interferon receptor 1 by Kaposi’s sarcoma-associated herpesvirus K3 and K5. J Virol. 2007;81:2117–2127. doi: 10.1128/JVI.01961-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bhaduri-McIntosh S. A Central Role for STAT3 in Gammaherpesvirus-Life Cycle and -Diseases. Front Microbiol. 2016;7:1052. doi: 10.3389/fmicb.2016.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Gao Y, Lin X, He Z, Zhao Q, Deng Q, Lan K. A human herpesvirus miRNA attenuates interferon signaling and contributes to maintenance of viral latency by targeting IKKepsilon. Cell Res. 2011;21:793–806. doi: 10.1038/cr.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Fu B, Wu F, Li X, Yuan Y, Zhu F. ORF45 of Kaposi’s sarcoma-associated herpesvirus inhibits phosphorylation of interferon regulatory factor 7 by IKKepsilon and TBK1 as an alternative substrate. J Virol. 2012;86:10162–10172. doi: 10.1128/JVI.05224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Begley M, Michowski W, Inuzuka H, Ginzberg M, Gao D, Tsou P, Gan W, Papa A, Kim BM, Wan L, Singh A, Zhai B, Yuan M, Wang Z, Gygi SP, Lee TH, Lu KP, Toker A, Pandolfi PP, Asara JM, Kirschner MW, Sicinski P, Cantley L, Wei W. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature. 2014;508:541–545. doi: 10.1038/nature13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kaneko S, Yang L, Feldman RI, Nicosia SV, Chen J, Cheng JQ. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J Biol Chem. 2004;279:52175–52182. doi: 10.1074/jbc.M406802200. [DOI] [PubMed] [Google Scholar]
- Ma Z, Jacobs SR, West JA, Stopford C, Zhang Z, Davis Z, Barber GN, Glaunsinger BA, Dittmer DP, Damania B. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc Natl Acad Sci U S A. 2015;112:E4306–E4315. doi: 10.1073/pnas.1503831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik P, Clements JB. Protein kinase CK2 phosphorylation regulates the interaction of Kaposi’s sarcoma-associated herpesvirus regulatory protein ORF57 with its multifunctional partner hnRNP K. Nucleic Acids Res. 2004;32:5553–5569. doi: 10.1093/nar/gkh876. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Matta H, Gopalakrishnan R, Graham C, Tolani B, Khanna A, Yi H, Suo Y, Chaudhary PM. Kaposi’s sarcoma associated herpesvirus encoded viral FLICE inhibitory protein K13 activates NF-kappaB pathway independent of TRAF6, TAK1 and LUBAC. PLoS One. 2012;7:e36601. doi: 10.1371/journal.pone.0036601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, Ganem D. Phosphorylation and function of the kaposin B direct repeats of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2006;80:6165–6170. doi: 10.1128/JVI.02331-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordasini V, Ueda S, Aslandogmus R, Berger C, Gysin C, Huhn D, Sartori AA, Bernasconi M, Nadal D. Activation of ATR-Chk1 pathway facilitates EBV-mediated transformation of primary tonsillar B-cells. Oncotarget. 2017;8:6461–6474. doi: 10.18632/oncotarget.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Morrison TE, Mauser A, Wong A, Ting JP, Kenney SC. Inhibition of IFN-gamma signaling by an Epstein-Barr virus immediate-early protein. Immunity. 2001;15:787–799. doi: 10.1016/s1074-7613(01)00226-6. [DOI] [PubMed] [Google Scholar]
- Munz C. EBV Infection of Mice with Reconstituted Human Immune System Components. Curr Top Microbiol Immunol. 2015;391:407–423. doi: 10.1007/978-3-319-22834-1_14. [DOI] [PubMed] [Google Scholar]
- Mutocheluh M, Hindle L, Areste C, Chanas SA, Butler LM, Lowry K, Shah K, Evans DJ, Blackbourn DJ. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor-2 inhibits type 1 interferon signalling by targeting interferon-stimulated gene factor-3. J Gen Virol. 2011;92:2394–2398. doi: 10.1099/vir.0.034322-0. [DOI] [PubMed] [Google Scholar]
- Najjar I, Baran-Marszak F L, Clorennec C, Laguillier C, Schischmanoff O, Youlyouz-Marfak I, Schlee M, Bornkamm GW, Raphael M, Feuillard J, Fagard R. Latent membrane protein 1 regulates STAT1 through NF-kappaB-dependent interferon secretion in Epstein-Barr virus-immortalized B cells. J Virol. 2005;79:4936–4943. doi: 10.1128/JVI.79.8.4936-4943.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin PA, Yan CM, Forte E, Bocedi A, Tourigny JP, White RE, Allday MJ, Patel A, Dave SS, Kim W, Hu K, Guo J, Tainter D, Rusyn E, Luftig MA. An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe. 2010;8:510–522. doi: 10.1016/j.chom.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning S, Campos AD, Darnay BG, Bentz GL, Pagano JS. TRAF6 and the three C-terminal lysine sites on IRF7 are required for its ubiquitination-mediated activation by the tumor necrosis factor receptor family member latent membrane protein 1. Mol Cell Biol. 2008;28:6536–6546. doi: 10.1128/MCB.00785-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning S, Pagano JS, Barber GN. IRF7: activation, regulation, modification and function. Genes Immun. 2011;12:399–414. doi: 10.1038/gene.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panousis CG, Rowe DT. Epstein-Barr virus latent membrane protein 2 associates with and is a substrate for mitogenactivated protein kinase. J Virol. 1997;71:4752–4760. doi: 10.1128/jvi.71.6.4752-4760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee D, Seo T, Chung J, Choe J. Kaposi’s sarcomaassociated herpesvirus (human herpesvirus-8) open reading frame 36 protein is a serine protein kinase. J Gen Virol. 2000;81:1067–1071. doi: 10.1099/0022-1317-81-4-1067. [DOI] [PubMed] [Google Scholar]
- Park J, Lee MS, Yoo SM, Seo T. A novel protein encoded by Kaposi’s sarcoma-associated herpesvirus open reading frame 36 inhibits cell spreading and focal adhesion kinase activation. Intervirology. 2007;50:426–432. doi: 10.1159/000112949. [DOI] [PubMed] [Google Scholar]
- Parravicini C, Chandran B, Corbellino M, Berti E, Paulli M, Moore PS, Chang Y. Differential viral protein expression in Kaposi’s sarcoma-associated herpesvirus-infected diseases: Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. Am J Pathol. 2000;156:743–749. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Pickin KA, Chaudhury S, Dancy BC, Gray JJ, Cole PA. Analysis of protein kinase autophosphorylation using expressed protein ligation and computational modeling. J Am Chem Soc. 2008;130:5667–5669. doi: 10.1021/ja711244h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher TJ, Zhao S, Geiger JN, Joneja B, Wojchowski DM. Pim-1 kinase protects hematopoietic FDC cells from genotoxininduced death. Oncogene. 2000;19:3684–3692. doi: 10.1038/sj.onc.1203684. [DOI] [PubMed] [Google Scholar]
- Polson AG, Huang L, Lukac DM, Blethrow JD, Morgan DO, Burlingame AL, Ganem D. Kaposi’s sarcoma-associated herpesvirus K-bZIP protein is phosphorylated by cyclin-dependent kinases. J Virol. 2001;75:3175–3184. doi: 10.1128/JVI.75.7.3175-3184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainio EM, Ahlfors H, Carter KL, Ruuska M, Matikainen S, Kieff E, Koskinen PJ. Pim kinases are upregulated during Epstein-Barr virus infection and enhance EBNA2 activity. Virology. 2005;333:201–206. doi: 10.1016/j.virol.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Reddy SS, Foreman HC, Sioux TO, Park GH, Poli V, Reich NC, Krug LT. Ablation of STAT3 in the B cell compartment restricts gammaherpesvirus latency in vivo. MBio. 2016;7(pii):e00723–16. doi: 10.1128/mBio.00723-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta M, Iwakiri D, Kanda T, Imaizumi T, Takada K. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 2006;25:4207–4214. doi: 10.1038/sj.emboj.7601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta M, Takada K. Modulation of innate immunity system by Epstein-Barr virus-encoded non-coding RNA and oncogenesis. Cancer Sci. 2010;101:29–35. doi: 10.1111/j.1349-7006.2009.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarek G, Ma L, Enback J, Jarviluoma A, Moreau P, Haas J, Gessain A, Koskinen PJ, Laakkonen P, Ojala PM. Kaposi’s sarcoma herpesvirus lytic replication compromises apoptotic response to p53 reactivation in virus-induced lymphomas. Oncogene. 2013;32:1091–1098. doi: 10.1038/onc.2012.118. [DOI] [PubMed] [Google Scholar]
- Schang LM. First demonstration of the effectiveness of inhibitors of cellular protein kinases in antiviral therapy. Expert Rev Anti Infect Ther. 2006;4:953–956. doi: 10.1586/14787210.4.6.953. [DOI] [PubMed] [Google Scholar]
- Scholle F, Longnecker R, Raab-Traub N. Epithelial cell adhesion to extracellular matrix proteins induces tyrosine phosphorylation of the Epstein-Barr virus latent membrane protein 2: a role for C-terminal Src kinase. J Virol. 1999;73:4767–4775. doi: 10.1128/jvi.73.6.4767-4775.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo T, Park J, Lim C, Choe J. Inhibition of nuclear factor kappaB activity by viral interferon regulatory factor 3 of Kaposi’s sarcoma-associated herpesvirus. Oncogene. 2004;23:6146–6155. doi: 10.1038/sj.onc.1207807. [DOI] [PubMed] [Google Scholar]
- Shah KM, Stewart SE, Wei W, Woodman CB, O’Neil JD, Dawson CW, Young LS. The EBV-encoded latent membrane proteins, LMP2A and LMP2B, limit the actions of interferon by targeting interferon receptors for degradation. Oncogene. 2009;28:3903–3914. doi: 10.1038/onc.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemi Z, Furukawa Y, Hosokawa K, Minami S, Matsuhiro J, Nakata S, Watanabe T, Kagawa H, Nakagawa K, Takeda H, Fujimuro M. Diallyl trisulfide induces apoptosis by suppressing NF-kappaB signaling through destabilization of TRAF6 in primary effusion lymphoma. Int J Oncol. 2016;48:293–304. doi: 10.3892/ijo.2015.3247. [DOI] [PubMed] [Google Scholar]
- Singh VV, Dutta D, Ansari MA, Dutta S, Chandran B. Kaposi’s sarcoma-associated herpesvirus induces the ATM and H2AX DNA damage response early during de novo infection of primary endothelial cells, which play roles in latency establishment. J Virol. 2014;88:2821–2834. doi: 10.1128/JVI.03126-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivachandran N, Cao JY, Frappier L. Epstein-Barr virus nuclear antigen 1 Hijacks the host kinase CK2 to disrupt PML nuclear bodies. J Virol. 2010;84:11113–11123. doi: 10.1128/JVI.01183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, Perl AE, Travers KJ, Wang S, Hunt JP, Zarrinkar PP, Schadt EE, Kasarskis A, Kuriyan J, Shah NP. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Tao YG, Deng XY, Jin X, Tan YN, Tang M, Wu Q, Lee LM, Cao Y. Heterodimer formation between c-Jun and Jun B proteins mediated by Epstein-Barr virus encoded latent membrane protein 1. Cell Signal. 2004;16:1153–1162. doi: 10.1016/j.cellsig.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Stallone G, Infante B, Grandaliano G, Schena FP, Gesualdo L. Kaposi’s sarcoma and mTOR: a crossroad between viral infection neoangiogenesis and immunosuppression. Transpl Int. 2008;21:825–832. doi: 10.1111/j.1432-2277.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- Stallone G, Schena A, Infante B D, Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- Stephens BJ, Han H, Gokhale V V, Hoff DD. PRL phosphatases as potential molecular targets in cancer. Mol Cancer Ther. 2005;4:1653–1661. doi: 10.1158/1535-7163.MCT-05-0248. [DOI] [PubMed] [Google Scholar]
- Tarakanova VL, Leung-Pineda V, Hwang S, Yang CW, Matatall K, Basson M, Sun R, Piwnica-Worms H, Sleckman B V HWt. Gamma-herpesvirus kinase actively initiates a DNA damage response by inducing phosphorylation of H2AX to foster viral replication. Cell Host Microbe. 2007;1:275–286. doi: 10.1016/j.chom.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KE, Mossman KL. Recent advances in understanding viral evasion of type I interferon. Immunology. 2013;138:190–197. doi: 10.1111/imm.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, Pucciarini A, Bigerna B, Pacini R, Wells VA, Sportoletti P, Pettirossi V, Mannucci R, Elliott O, Liso A, Ambrosetti A, Pulsoni A, Forconi F, Trentin L, Semenzato G, Inghirami G, Capponi M D, Raimondo F, Patti C, Arcaini L, Musto P, Pileri S, Haferlach C, Schnittger S, Pizzolo G, Foa R, Farinelli L, Haferlach T, Pasqualucci L, Rabadan R, Falini B. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305–2315. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R, Dawson CW, Hu C, Shah KM, Owen TJ, Date KL, Maia SP, Shao J, Arrand JR, Young LS, O’Neil JD. Epstein-Barr virus-encoded EBNA1 inhibits the canonical NFkappaB pathway in carcinoma cells by inhibiting IKK phosphorylation. Mol Cancer. 2010;9:1. doi: 10.1186/1476-4598-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang’ondu R, Teal S, Park R, Heston L, Delecluse H, Miller G. DNA Damage Signaling Is Induced in the Absence of Epstein-Barr Virus (EBV) Lytic DNA Replication and in Response to Expression of ZEBRA. PLoS One. 2015;10:e0126088. doi: 10.1371/journal.pone.0126088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhu C, Wei F, Gao S, Zhang L, Li Y, Feng Y, Tong Y, Xu J, Wang B, Yuan Z, Robertson ES, Cai Q. Nuclear Localization and Cleavage of STAT6 Is Induced by Kaposi’s Sarcoma-Associated Herpesvirus for Viral Latency. PLoS Pathog. 2017;13:e1006124. doi: 10.1371/journal.ppat.1006124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhu C, Wei F, Zhang L, Mo X, Feng Y, Xu J, Yuan Z, Robertson E, Cai Q. Constitutive Activation of Interleukin-13/STAT6 Contributes to Kaposi’s Sarcoma-Associated Herpesvirus-Related Primary Effusion Lymphoma Cell Proliferation and Survival. J Virol. 2015;89:10416–10426. doi: 10.1128/JVI.01525-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Doong SL, Teng SC, Lee CP, Tsai CH, Chen MR. Epstein-Barr virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J Virol. 2009;83:1856–1869. doi: 10.1128/JVI.01099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ren J, Li G, Moorman JP, Yao ZQ, Ning S. LMP1 signaling pathway activates IRF4 in latent EBV infection and a positive circuit between PI3K and Src is required. Oncogene. 2016;36:2265–2274. doi: 10.1038/onc.2016.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lu X, Zhu L, Shen Y, Chengedza S, Feng H, Wang L, Jung JU, Gutkind JS, Feng P. IKK epsilon kinase is crucial for viral G protein-coupled receptor tumorigenesis. Proc Natl Acad Sci U S A. 2013;110:11139–11144. doi: 10.1073/pnas.1219829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Wu JW. Autophosphorylation kinetics of protein kinases. Biochem J. 2002;368:947–952. doi: 10.1042/BJ20020557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasil LR, Wei L, Chang C, Lan L, Shair KH. Regulation of DNA Damage Signaling and Cell Death Responses by Epstein-Barr Virus Latent Membrane Protein 1 (LMP1) and LMP2A in Nasopharyngeal Carcinoma Cells. J Virol. 2015;89:7612–7624. doi: 10.1128/JVI.00958-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Zhu Q, Ding L, Liang Q, Cai Q. Manipulation of the host cell membrane by human gamma-herpesviruses EBV and KSHV for pathogenesis. Virol Sin. 2016;31:395–405. doi: 10.1007/s12250-016-3817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Li W, Shao Y, Avey D, Fu B, Gillen J, Hand T, Ma S, Liu X, Miley W, Konrad A, Neipel F, Sturzl M, Whitby D, Li H, Zhu F. Inhibition of cGAS DNA Sensing by a Herpesvirus Virion Protein. Cell Host Microbe. 2015;18:333–344. doi: 10.1016/j.chom.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A, Scully R. Hijacking the DNA damage response to enhance viral replication: gamma-herpesvirus 68 orf36 phosphorylates histone H2AX. Mol Cell. 2007;27:178–179. doi: 10.1016/j.molcel.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Gershburg E, Pagano JS. Hyperphosphorylation of EBNA2 by Epstein-Barr virus protein kinase suppresses transactivation of the LMP1 promoter. J Virol. 2005;79:5880–5885. doi: 10.1128/JVI.79.9.5880-5885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Shackelford J, Pagano JS. cdc2/cyclin B1-dependent phosphorylation of EBNA2 at Ser243 regulates its function in mitosis. J Virol. 2006;80:2045–2050. doi: 10.1128/JVI.80.4.2045-2050.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Chan B, Samarina N, Abere B, Weidner-Glunde M, Buch A, Pich A, Brinkmann MM, Schulz TF. Cytoplasmic isoforms of Kaposi sarcoma herpesvirus LANA recruit and antagonize the innate immune DNA sensor cGAS. Proc Natl Acad Sci U S A. 2016;113:E1034–E1043. doi: 10.1073/pnas.1516812113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Li LL, Hu DS, Deng XY, Cao Y. Role of Epstein-Barr virus encoded latent membrane protein 1 in the carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol. 2007;4:185–196. [PubMed] [Google Scholar]
- Zhu FX, King SM, Smith EJ, Levy DE, Yuan Y. A Kaposi’s sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc Natl Acad Sci U S A. 2002;99:5573–5578. doi: 10.1073/pnas.082420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkin S, Davidovich A, Don J. The PIM-2 kinase is an essential component of the ultraviolet damage response that acts upstream to E2F-1 and ATM. J Biol Chem. 2013;288:21770–21783. doi: 10.1074/jbc.M113.458851. [DOI] [PMC free article] [PubMed] [Google Scholar]


