Abstract
Purpose
Primary hyperparathyroidism (PHPT) is a frequent endocrine pathology that has surgical treatment as its only decisive measure. High-Resolution Neck Ultrasonography with color-Doppler (CDHR-NUS) and 99mTechnetium-SestaMIBI Parathyroid Scintigraphy (99mTc-MIBI PS) are the two instrumental exams more commonly used in the preoperatory localization of pathologic parathyroids. The aim of this observational study was to outline—in accordance with the latest scientific literature—the precise role of CDHR-NUS in the environment of PHPT, comparing it with that of Parathyroid Scintigraphy.
Methods
136 patients operated on for PHPT and underwent CDHR-NUS and 99mTc-MIBI PS preoperatively. The CDHR-NUS was carried out by an expert medical sonographer. The results of the two methods were compared between each other and with the results of the operative act for the evaluation of accordance and diagnostic performances.
Results
PHPT is prevalently due to monoglandular pathology (SGD). The parallel use of CDHR-NUS and of 99mTc-MIBI PS does not determine a significant increase in diagnostic accuracy. The preoperative accordance evaluation between the two methods does not exclude the presence of multiglandular pathology (MGD) with certainty.
Conclusions
CDHR-NUS is an accurate as well as cost-effective method; its role as a main and eventual unique preoperative localization method in patients affected by PHPT is confirmed. In the presence of expert medical sonographers, the sequential use of the two methods is retained correct and their use in parallel is neither justified nor cost-effective. The preoperative accordance evaluation between the two methods is neither necessary nor indispensable.
Keywords: Primary hyperparathyroidism, Parathyroid adenoma, Localization, Ultrasonography
Riassunto
Scopo
L’iperparatiroidismo primitivo (PHPT) è una frequente patologia endocrina che ha come unico trattamento risolutivo quello chirurgico. L’ecografia del collo ad alta risoluzione con color-Doppler (CDHR-NUS) e la Scintigrafia Paratiroidea con 99mTecnezio-SestaMIBI (99mTc-MIBI PS) sono i due esami strumentali più comunemente utilizzati nella localizzazione preoperatoria delle paratiroidi patologiche. Scopo di questo studio osservazionale è di delineare —in conformità con la più recente letteratura scientifica—il preciso ruolo della CDHR-NUS nell’ambito del PHPT, confrontandolo con quello della scintigrafia paratiroidea.
Materiali
136 pazienti operati (PTX) per PHPT e sottoposti pre-operatoriamente a CDHR-NUS e 99mTc-MIBI PS. La CDHR-NUS è stata eseguita da un medico ecografista esperto. Gli esiti delle due metodiche sono stati confrontati fra loro e con le risultanze dell’atto operatorio per valutazione di concordanza e prestazioni diagnostiche.
Risultati
Il PHPT è prevalentemente dovuto a patologia monoghiandolare (SGD). L’uso pararallelo di CDHR-NUS e di 99mTc-MIBI PS non determina un significativo incremento dell’accuratezza diagnostica. La valutazione preoperatoria di concordanza fra le due metodiche non può escludere con certezza la presenza di patologia multighiandolare (MGD).
Conclusioni
La CDHR-NUS è una metodica accurata e cost-effective; si conferma il suo ruolo di principale ed eventualmente unica metodica di localizzazione preoperatoria nel paziente affetto da PHPT. In presenza di medici ecografisti esperti, si ritiene corretto l’uso sequenziale delle due metodiche e non giustificato né cost-effective il loro uso parallelo. La valutazione preoperatoria di concordanza fra le due metodiche non è necessaria né indispensabile.
Introduction
The parathyroids are endocrine glands that secrete parathormone (PTH), a polypeptide hormone that intervenes in the maintenance of normal serum calcium levels. The parathyroids are small anatomical structures (about 5 × 3 × 1 mm, weight between 25 and 50 mg) localized in two on each side of the postero-medial surface of the thyroid lobes. Normally there are four parathyroids, differentiated in two superior (P4 or PIV) and two inferior (P3 or PIII), called “orthotopic” if situated in typical sites and “ectopic” if located in atypical sites. Intrathyroidal ectopic localization isn’t rare (2–4% of all cases operated on) and more often involves the PIII [1].
Primary hyperparathyroidism (PHPT) is an endocrine pathology determined by an excessive secretion of parathormone (PTH) by one or more independently hyperfunctioning parathyroids.
PHTP occurs in a sporadic form (SPHTP, Sporadic Primary Hyperparathyroidism) in the majority of the cases (97–98%). SPHTP is currently the third most frequent endocrine disease diagnosed after diabetes and thyreopathies and the most common cause of hypercalcaemia in the general population. It affects the female sex more frequently (ratio F/M 3:1) and has a peak incidence in subjects aged 50–60; half of the patients affected by this pathology are post-menopausal aged woman. In 90% of the cases the cause of SPHTP is a single-adenoma (SA), benign neoplasm developed in a single parathyroid (single-gland disease, SGD); the inferior parathyroids are more frequently affected than the superior ones; the adenoma occurs on an ectopic parathyroid in 4–16% of the cases. The multiglandular pathology (multi-gland disease, MGD)—characterized by the involvement of two or more glands in the form of adenoma or hyperplasia or both (adenoma-hyperplasia)—are responsible for the remaining 9–10% of cases; the most frequent form of MGD is the double adenoma (DA) and may involve the two parathyroids on the same side (ipsilateral DA) or the parathyroids on two different sides (contralateral DA). Parathyroid carcinoma as a cause of PHPT is extremely rare (< 1% of the cases) [2].
The familial or inherited forms (FPHPT, Familial Primary Hyperparathyroidism) are rare (2–3% of cases) and arise predominantly in the context of a MEN (Multiple Endocrine Neoplasia) type 1 or 2A; they occur in younger subjects (mean age onset of the disease 20–25 years) and are more often supported by MGD [3].
The greater bone reabsorption secondary to the excess of PTH produced by one or more hyperfunctioning parathyroids determines a reduction in bone mineral density (BMD) of a variable degree from patient to patient, with situations ranging from mild osteopenia to severe osteoporosis with a high risk of bone fractures [4].
Hypercalcaemia—due to both increased calcium bone mobilization and increased renal and intestinal absorption—is present in most patients with PHPT in mild form (≤ 11.9 mg/dl). Hypercalciuria resulting from hypercalcaemia promotes the onset of nephrolithiasis and nephrocalcinosis.
In most cases (about 80%) PHPT is completely asymptomatic and diagnosed following the random finding of hypercalcaemia [5]. Nephrolithiasis still represents the most common clinical manifestation of the disease (renal colic). Asthenia, easy fatigability and widespread osteoarticular pains are relatively common non-specific symptoms in symptomatic patients. The forms characterized by severe hypercalcaemia (≥ 14 mg/dl), quite rare, are often characterized by more relevant manifestations (nausea, vomiting, acute pancreatitis, sleepiness, depression, mental confusion) and could also be responsible for hypercalcemic crisis, rare endocrinological emergency (found in ˂1% of patients with PHPT) but potentially lethal [6].
The diagnosis of PHPT is essentially done in laboratory and is based on the finding of persistent hypercalcaemia associated with elevated serum levels of PTH (sPTH—classical PHPT). In a minority of cases you can have a normal calcaemia in the presence of elevated sPTH (normocalcemic PHPT) [7] or hypercalcaemia associated with an inappropriately normal sPTH, that are included in the upper half of the normal range (normohormonal PHPT) [8]. In the presence of a coexisting vitamin D deficiency—now considered more common in patients affected by PHPT than in the general population [9]—the calcaemia values can be falsely normal, plasma levels of PTH may be more elevated and associated with larger parathyroid adenomas and bone manifestations of PHPT may be more evident with more severe situations of osteoporosis [10].
Parathyroidectomy surgery (PTX) is the only decisive treatment of PHPT and is indicated:
in election both in symptomatic and asymptomatic patients who present at least one of the conditions specified by the current guidelines [11];
in emergency in rare cases complicated by hypercalcemic crisis not responsive to medical therapy [12].
The main benefits of a resolutive PTX are represented, other than by normalization of calcaemia and sPTH, by a significant and permanent reduction in the incidence of nephrolithiasis recurrence [13] and by a rapid and protracted recovery of BMD, resulting in a significant reduction in the risk of fractures [14].
The traditional intervention of PTX is that of a bilateral neck exploration (BNE) and foresees the identification of all four of the parathyroids with the exeresis of those resulting macroscopically pathological and the eventual biopsy of those considered dubious. Today, it still represents the gold standard with which all alternative surgical techniques must be compared; if performed by experienced surgeons, it has a success rate (in terms of biological healing) of more than 95%, with a complication rate of 1–4% [15]. Until the first half of the 90s, the intervention was carried out without the aid of preoperative instrumental examinations, considered not necessary due to their relatively low diagnostic accuracy (in this regard, the American radiologist J.L. Doppman had stated in his famous aphorism: “The only localization study required by a patient undergoing initial parathyroid surgery is to locate an experienced parathyroid surgeon” [16]).
The evidence that PHPT is in most cases due to SGD, the improvement of diagnostic accuracy of imaging methods and the introduction into the clinical practice of the intraoperative measurement of PTH (IOPTHm) [17] have allowed the affirmation from the second half of the 1990s of monolateral surgical techniques known as LNE (Limited Neck Exploration) and represented mainly by UNE (Unilateral Neck Exploration; the two parathyroid sites are explored only on one side) [18] and MIP (Minimally Invasive Parathyroidectomy; a single parathyroid site is explored) [19]. The advantages of these new techniques, used today in the best part of cases to replace BNE, are essentially represented: by the reduction in operating time and hospital stay; by reducing the rate of complication both immediate and at a distance; from a better esthetic result, as the operation is carried out through a more limited cutaneous incision [20]. Their success rate may be equal to that of BNE, provided, however, that the operation is preceded by accurate instrumental examinations localizing the pathological parathyroid glands and is carried out by an expert surgeon.
The role of imaging methods is limited to the preoperative localization of pathological parathyroid glands in patients with PHPT laboratory diagnosis in which indication was given to surgical intervention. High Resolution Neck Ultrasonography with color-Doppler (CDHR-NUS) and Parathyroid Scintigraphy with 99mTechnetium-SestaMIBI (99mTc-MIBI PS) are currently the two most commonly used instrumental examinations in the preoperative localization of pathological parathyroid glands and are also known as “first line localization methods” [21, 22].
The CDHR-NUS is performed using linear probes regulated at higher frequencies (≥ 10 MHz), in order to obtain the best spatial resolution. The examination must include the entire anterior cervical region, paying particular attention to the thyroid region. The pathological parathyroid (schematically defined “adenoma”) appears on the ultrasonography as solid hypoechoic formation of various dimensions, with well-defined margins, oval or oblong and with a longer longitudinal axis, which contrasts with the greater echogenicity of the thyroid parenchyma. The adenoma of a superior parathyroid results in being localized in the retrothyroidal region, medium-superiorly or superiorly with respect to the posterior profile of the thyroid lobe (Figs. 1, 2). The adenoma of an inferior parathyroid is localized in close adjacency with the inferior pole of the thyroid lobe or inferior to it (with partially or totally retrosternal localization) [23] (Figs. 3, 4, 5). Larger adenomas may have a partially fluid ecostructure due to the presence of cystic involution areas [24] (Fig. 6).
Fig. 1.
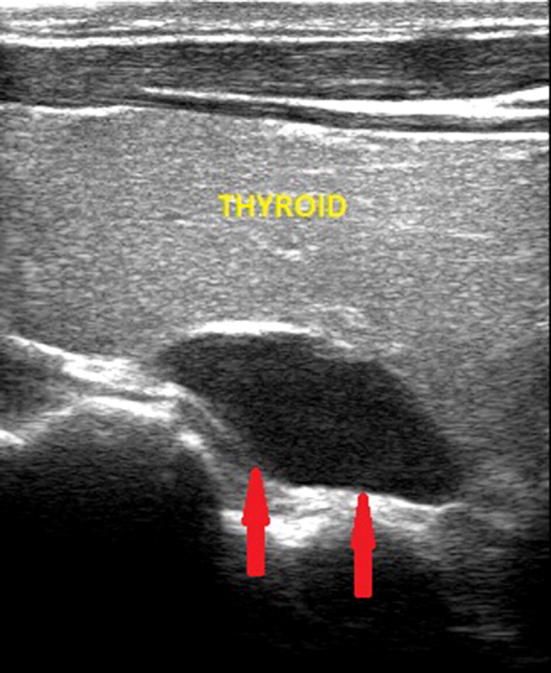
Ultrasound image of a right superior parathyroid adenoma (indicated by the red arrows), hypoechoic, median retrothyroidal and of the maximum (longitudinal) diameter of about 2.2 cm
Fig. 2.
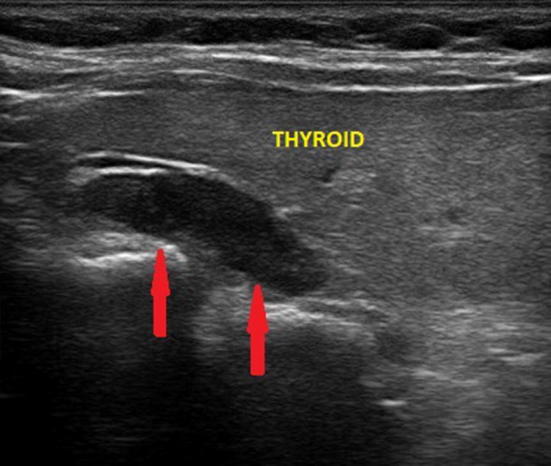
Ultrasound image of a left superior parathyroid adenoma (indicated by the red arrows), upper retrothyroidal and of the maximum (longitudinal) diameter of about 2.3 cm. The echogenicity of the thyroid is reduced due to autoimmune thyroid disease
Fig. 3.
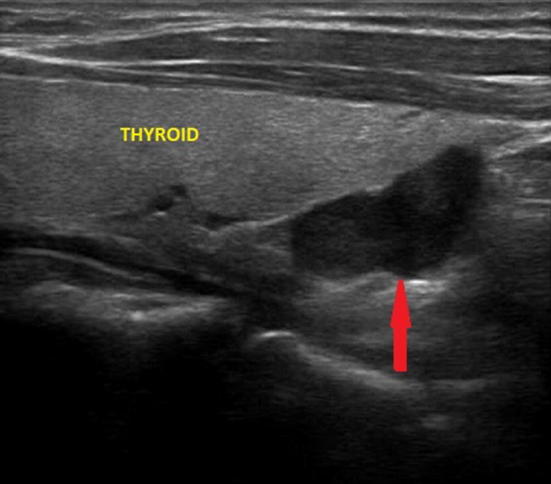
Ultrasound image of a right inferior parathyroid adenoma (indicated by the red arrow), lower retrothyroidal and of the maximum (longitudinal) diameter of about 2 cm
Fig. 4.
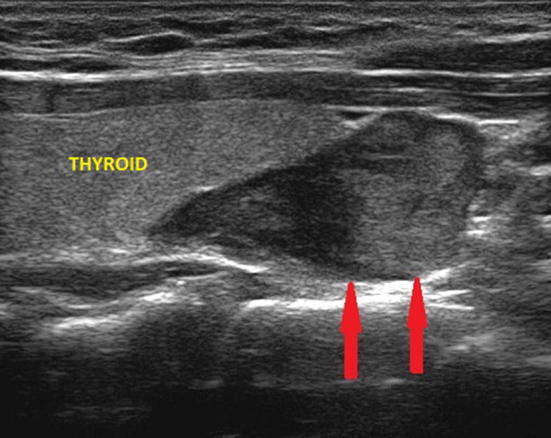
Ultrasound image of a right inferior parathyroid adenoma (indicated by the red arrows), adjacent to the pole of the thyroid lobe and of the maximum (longitudinal) diameter of about 2.8 cm
Fig. 5.
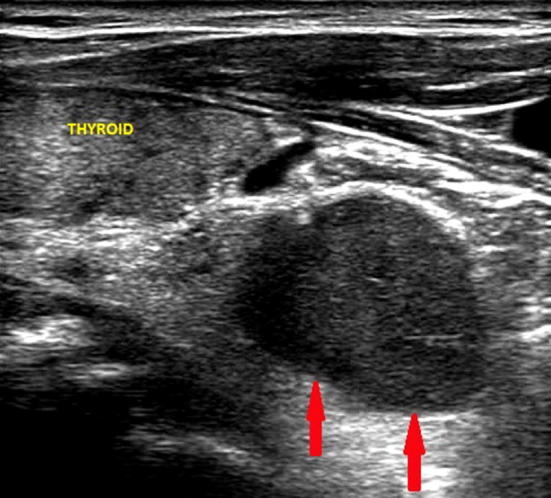
Ultrasound image of a left inferior parathyroid adenoma (indicated by the red arrows), located near the pole of the thyroid lobe with partial retrosternal localization and of the maximum (longitudinal) diameter of about 2.3 cm
Fig. 6.
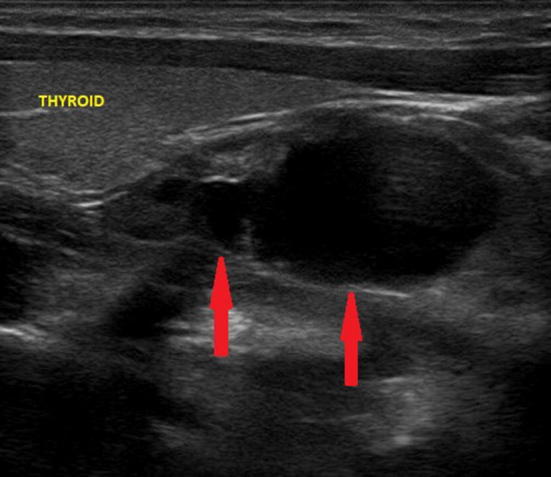
Ultrasound image of predominantly cystic adenoma (poor solid component) of the right inferior parathyroid gland (indicated by the red arrows), adjacent to the pole of the thyroid lobe and of the maximum (longitudinal) diameter of about 3.5 cm
The contextual use of the color-Doppler determines a significant increase in the sensitivity of the ultrasound [25, 26]. On color-Doppler examination, solid adenomas often appear as more vividly vascularized structures than the adjacent thyroid parenchyma; the vascular pattern is predominantly central and a polar afferent arterial vessel is also frequently identified (Fig. 7). This is useful both to distinguish the parathyroid adenoma from a lymphadenopathy or from an exophytic thyroid node [27] (Fig. 8) and to identify an intrathyroidal parathyroid adenoma (Fig. 9), differentiating it from a common thyroid node [28, 29]. A recent prospective study also shows that the use of the semi-quantitative US-elastography with Elastoscan (ECI) improves US accuracy in distinguishing between parathyroid lesions and ectopic thyroid nodules or lymph nodes [30].
Fig. 7.
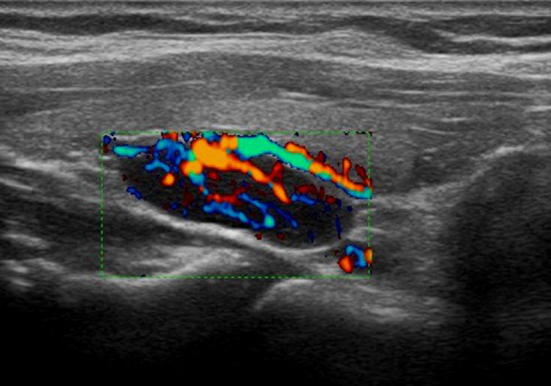
Ultrasound image with color-Doppler of parathyroid adenoma. The color-Doppler shows a vivid vascularization of a prevalently central type; an afferent polar vessel is also visible
Fig. 8.
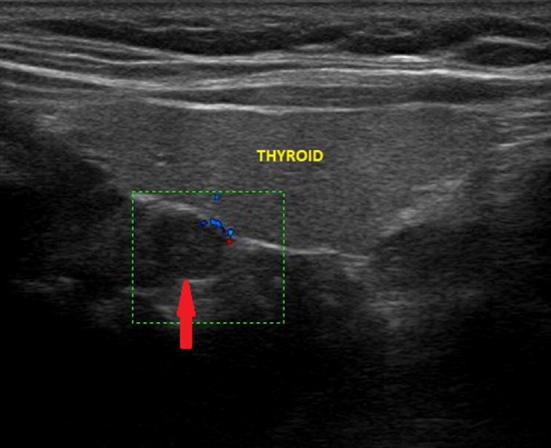
Ultrasound image of retrothyroidal exophytic thyroid node (indicated by the red arrow) with a maximum diameter of about 7 mm, which simulates a superior pathological parathyroid; the color-Doppler examination shows poor peripheral vascularization
Fig. 9.
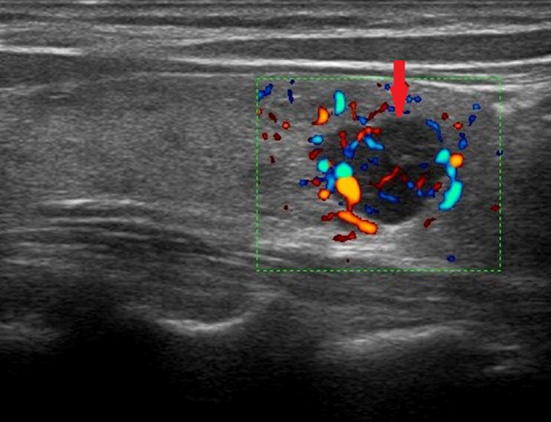
Ultrasound image with color-Doppler of intrathyroidal right inferior adenoma (indicated by the red arrow) of the maximum (longitudinal) diameter of about 12 mm; the vascularization appears lively and predominantly central
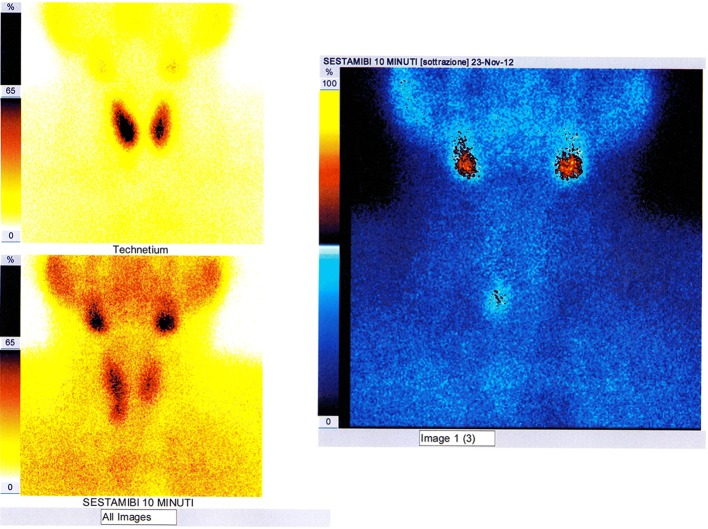
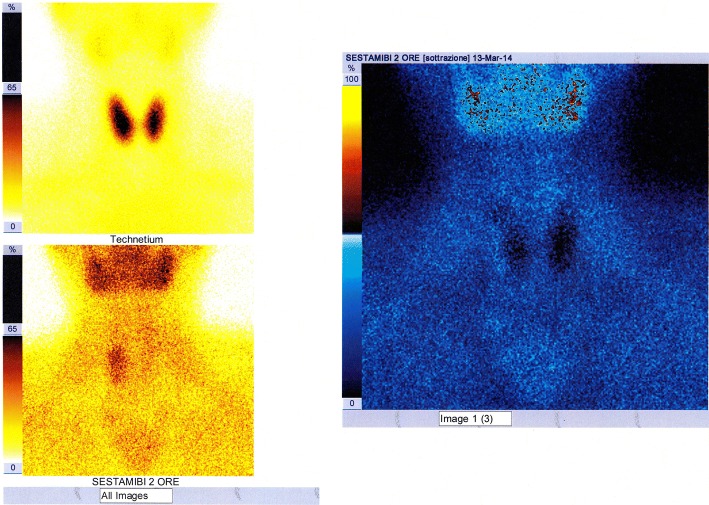
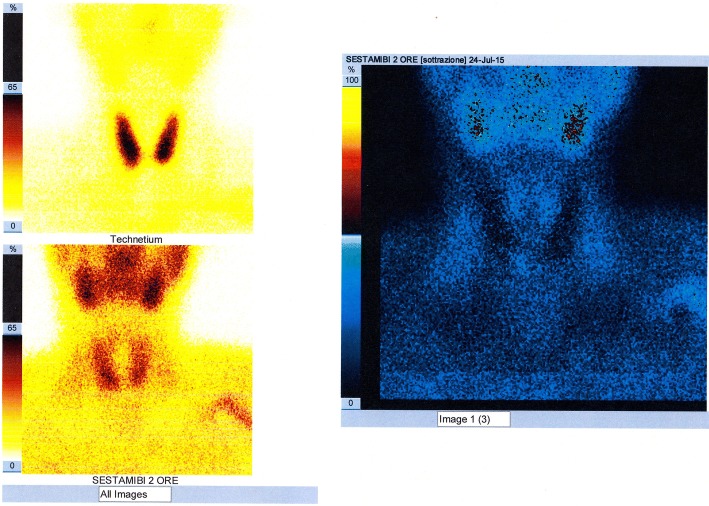
The 99mTc-MIBI PS [31] is a diagnostic method of nuclear medicine that uses intravenous administration of 99mTc-SestaMIBI (Technetium-99m-2-methoxyisobutyl-isonitrile), radioactive tracer captured by both the thyroid and the parathyroid with mitochondrial accumulation. The scintigraphic trace is a planar image obtained from detection—on an area comprising both the neck and the mediastinum and by means of an apparatus called gamma-camera—of the gamma radiation emitted by the tracer and its processing. The scintigraphic technique named “single-tracer double-phaseˮ is based on the differential washout phenomenon (the 99mTc-SestaMIBI is eliminated more rapidly from the thyroid than from the pathological parathyroid); acquisitions are performed at 10–15 min (early phase) and at 120–180 min (late phase) by intravenous bolus administration of the tracer. In the images obtained, the pathological parathyroid tissue corresponds to a fixation focus of the tracer which is more evident in the late phase; poor uptake or rapid wash-out of the 99mTc-SestaMIBI by some pathological parathyroid glands (partially cystic adenomas or with scarcity of oxyphilous cells) may result in a false negativity of the examination. The scintigraphic technique, called “double tracer subtractionˮ, more investigative, foresees bolus intravenous administration of both the 99mTc-SestaMIBI and (after the late phase) of the 99mTcO4− (technetium-99m pertechnetate), tracer captured only by the thyroid which allows to obtain a thyroid image that is “subtracted” digitally from the one previously obtained with the 99mTc-Sestamibi (Fig. 10); this second technique can be useful in the presence of thyroid nodular pathology, being able to reduce false positivities due to the slower disposal of 99mTc-SestaMIBI by some thyroid nodules.
Fig. 10.
Image of planar scintigraphic examination performed with either “single-tracer double-phase” technique or with “double tracer subtraction” technique, documenting uptake by a right inferior parathyroid adenoma, which had already been visualized by the CDHR-NUS (see Fig. 3)
The sensitivity of the two above mentioned scintigraphic techniques is superimposable [32] and significant advantages in the use of one technique rather than the other have not yet been shown in statistical terms (Figs. 11, 12).
Fig. 11.
Image of planar scintigraphic examination performed with either “single-tracer double-phase” technique or with “double tracer subtraction” technique, with false negative result (failure to visualize the pathological parathyroid); the parathyroid adenoma (right inferior, solid and with a maximum diameter of about 2.8 cm) had already been visualized by the CDHR-NUS (see Fig. 4)
Fig. 12.
Image of planar scintigraphic examination performed with either “single-tracer double-phase” “technique or with “double tracer subtraction” technique, with false negative result (failure to visualize the pathological parathyroid); the parathyroid adenoma (right inferior, mainly cystic and with a maximum diameter of about 3.5 cm) had already been visualized by the CDHR-NUS (see Fig. 6)
The traditional planar imaging of the 99mTc-MIBI PS has subsequently been enriched with the introduction into clinical practice of equipment able to provide also a 3D tomographic imaging. The 99mTc-MIBI PS SPECT/CT (Single Photon Emission Computed Tomography with CT scan)—introduced more recently and not yet available in all diagnostic centers even for the highest cost of equipment—provides images of fusion resulting from the integration of data acquired by the SPECT technique with the anatomical ones acquired with the use of X-rays emitted by a rotating radiogenic tube (“hybrid imaging”); the images obtained allow to assign a precise anatomical localization to accumulation area of the radiotracer [33] (Fig. 13).
Fig. 13.
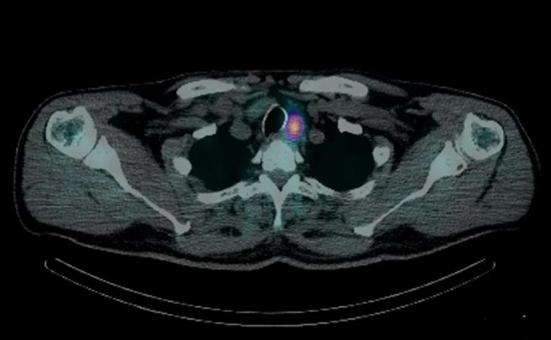
Image of scintigraphic examination SPECT/CT of a left inferior parathyroid adenoma, which had already been visualized by the CDHR-NUS (see Fig. 5). The tracer uptake area is located caudally at the inferior pole of the thyroid lobe from the tomographic image
CDHR-NUS and 99mTc-MIBI PS (also SPECT/CT) have similar diagnostic accuracy: at a recent meta-analysis [34]; the “pooled” values of sensibility and the positive predictive value are, respectively, equal to 76.1% and 93.2% for the CDHR-NUS and to 78.9% and 90.7% for the 99mTc-MIBI PS. The overall diagnostic sensitivity of both methods is, however, higher for SA (more than 85%) and considerably lower (less than 50%) for MGD, more than likely due to their limited ability to visualize pathological parathyroids of a smaller size [35, 36]. The diagnostic accuracy of both methods can also decrease in the presence of thyroid nodular disease [22, 37].
Compared to the 99mTc-MIBI PS, the CDHR-NUS:
is easier to carry out and less expensive;
can provide more details regarding localization of the pathological parathyroid glands and their relationship with the surrounding anatomical structures;
does not expose the patient to ionizing radiation;
is operator-dependent and its diagnostic yield is, therefore, strongly conditioned by the experience of the medical sonographer;
has a lower sensitivity in identifying the ectopic pathological parathyroids [38], in particular those located in the mediastinum or at the tracheo-esophageal groove.
In common clinical practice, parallel use of CDHR-NUS and 99mTc-MIBI PS (preoperative performance of both in all patients with PHPT) is the most widely used diagnostic protocol [39, 40] and is supported by the following:
numerous previous studies have shown that the joint use of the two methods increases the diagnostic accuracy in the localization of pathological parathyroid glands [37, 41–45], above all in the presence of thyroid nodular disease [46, 47];
the comparison of their outcomes (concordance/discordance), together with certain clinical-anamnestic data, conditions the choice of the type of surgical intervention [48]: full agreement of the two methods on a single site of localization, together with determined serum calcium levels (total calcaemia ≥ 12 mg/dl or ionized calcaemia > 1.4 mmol/l) and sPTH (≥ 140 pg/ml), predicts with high accuracy (> 95%) the presence of SA as a cause of PHPT and allow the surgeon to carry out a LNE (MIP or UNE) [49–51]; in case of discordance of the results (and, therefore, also of the negativity of one of the two methods), although the probability of MGD is significant [52], the surgeon can still decide for a LNE, which he will have to carry out with the indispensable aid of the IOPTHm [53–55]; in case both of the methods are negative, with the probability of MGD being even higher (especially in the presence of a MEN), the surgeon may use “second-line localization methods”[represented by Computed Tomography (CT) and Magnetic Resonance (MR)]—in order to be able to try a LNE or opt for the BNE without resorting to further instrumental examinations [20, 56–58].
Methods
An analytical observational study was performed that examined a series of 136 patients (102 females and 34 males, about 3:1 F/M ratio, aged between 17 and 83 years, average age about 59 years) with laboratory diagnosis of PHPT, submitted since 2001 at 2017 to PTX surgery. To the 61 patients from our previous studies [59] operated on in the period 2001–2011 and examined retrospectively, an additional 75 patients operated in the period 2012–2017 were added and examined with the same variables in a prospective manner. The present study was, therefore, carried out by examining a larger case study of a single medical sonographer expert in cervical pathology, with the aim of confirming the role of CDHR-NUS as the main and possibly unique method of preoperative localization of pathological parathyroid tissue.
PHPT was completely asymptomatic in 53 patients (39%) and the diagnosis was made following the random finding of hypercalcaemia. In 83 symptomatic patients (61%), nephrolithiasis was the most common manifestation, being present in 61 of them (73.5%); other clinical manifestations (more frequently represented by asthenia, easy fatigue, arthromyalgia, widespread bone pains) were present in 23 patients (27.7%).
The PHPT laboratory picture was classical (hypercalcaemia associated with high sPTH) in 131 patients (96.3%), normocalcemic in three patients (2.2%) and normohormonal in the remaining two patients (1.5%). Hypercalcaemia was present in most cases (133 patients, 97.8%) and was only ionized in two patients (2%), mild (10.3–11.9 mg/dl) in 105 patients (79%), moderate (12–13.9 mg/dl) in nineteen patients (14%) and severe (≥ 14 mg/dl) in seven patients (5%); in only three of these last seven patients severe hypercalcaemia manifested with important symptoms and led to hospitalization. sPTH was increased in most cases (134 patients, 98.5%), with values within twice the upper limit of normal (≤ 140 mg/dl) in 57 patients (42.5%) and greater than twice the same limit (> 140 mg/dl) in 77 patients (57.5%), in which it had values between 297 and 800 mg/dl (mean 150 mg/dl). sPTH was inappropriately normal (normohormonal PHTP) only in two patients (1.5%) (Table 1).
Table 1.
Characteristics of the sample under examination
| Total number of patients | 136 |
| Male | 34 |
| Female | 102 |
| F/M | 3:1 |
| Average diagnosis age | 59 |
| Asymptomatic patients | 53 (39%) |
| Symptomatic patients | 83 (61%) |
| Nephrolithiasis | 61 (73.5%) |
| Other symtomatology | 23 (27.7%) |
| PHPT laboratory picture | - |
| Classic | 131 (96.3%) |
| Normocalcemic | 3 (2.2%) |
| Normohormonal | 2 (1.5%) |
| Serum calcium levels hypercalcaemia | 133 (97.8%) |
| Only ionized | 2 (2%) |
| Mild (10.3–11.9 mg/dl) | 105 (79%) |
| Moderate (12–13.9 mg/dl) | 19 (14%) |
| Severe (≥ 14 mg/dl) | 7 (5%) |
| Normocalcaemia | 3 (2.2%) |
| Serum PTH levels increased | 134 (98.5%) |
| Within twice the upper limit of normality (≤ 140 pg/ml) | 57 (42.5%) |
| Over twice the upper limit of normalilty (> 140 pg/ml) | 77 (57.5%) |
| Inappropriately normal | 2 (1.5%) |
All patients were preoperatively submitted to both CDHR-NUS and 99mTc-MIBI PS, with the aim of obtaining the largest quantity of information possible about the exact location of pathological parathyroids. US-guided needle aspiration was not performed in any of the patients, as the information provided by CDHR-NUS and 99mTc-MIBI PS was considered sufficient. Second-line localization methods (CT or MR) were used in only six patients (4.4%).
The CDHR-NUS of all patients in the series was performed and reported by a single medical sonographer expert in cervical pathology; the examinations were performed by ultrasound machines equipped with multi-frequency linear probes and color-power-Doppler module (ATL HDI 3000, ESAOTE MyLab 70 XVG, ESAOTE MyLab Twice). In all patients, B-mode real-time ultrasonography was integrated with color-power-Doppler examination and the thyroid was also examined. A concomitant nodular thyroid disease was present in 77 patients (56.6%) and absent in the remaining 59 (43.4% of cases). Elastosonography was not performed in any of the patients, as it was not available in the equipments in use.
99mTc-MIBI PS, reported by several nuclear physicians, was performed in 80 patients (58.9%) with double tracer subtraction technique and in the remaining 56 patients (41.1%) with single-tracer double-phase technique. Tomographic imaging (SPECT and SPECT/CT) was only used on ten patients (7.3%); SPECT/CT was only recently acquired by one of the diagnostic centers of the present study.
To each of the two diagnostic methods were ideally assigned four potential sites for preoperative localization of pathological parathyroids (superior or median right/left for the two superior parathyroids, inferior right/left for the two inferior parathyroids). The localization site indicated in the report of CDHR-NUS and 99mTc-MIBI PS was compared with the results of the operative act, taken as gold standard for each patient. For both of the methods were considered true positive (TP) pathological parathyroids correctly localized and excised, false negative (FN) pathological parathyroids not localized and in any case removed, false positive (FP) the localization sites indicated but not corresponding at the time of intervention to any pathological parathyroid and true negatives (TN) localization sites not mentioned in the report and resulting surgical disease free (corresponding to normal parathyroid sites). The diagnostic performance indexes of the two methods—sensitivity, specificity, positive predictive value (PPV), negative predictive values (NPV), likelihood ratio (LR)—were determined using statistical software (SPSS ver 17, Chicago IL, USA) and were then compared to each other. Furthermore, an analysis of the reciprocal preoperative concordance/discordance of CDHR-NUS and 99mTc-MIBI PS was performed, distinguishing—on the basis of what was described in the reports—the following four possibilities: full concordance, when both agreed on the exact location of one or more pathological parathyroids; partial concordance, when both did not fully agree on the location of the pathological parathyroid (agreeing on the side but not on the location or indicating one of the two only the side and not the location or still both indicating the same site and only one of the two only a further localization site); discordance, when they indicated completely different localizations or were positive and the other negative by localization of pathological parathyroid; negativity of both methods for localization of pathological parathyroids. The 136 patient in the series were, therefore, divided into four groups (A, B, C, D) (Table 2).
Table 2.
Preoperative concordance/discordance/negativity of CDHR-NUS and 99mTc-MIBI PS
| Group A full concordance patients (%) |
Group B partial concordance patients (%) |
Group C discordance patients (%) |
Group D negativity of both methods patients (%) |
|---|---|---|---|
| 80 (58.8) | 17 (12.5) | 37 (27.2) | 2 (1.5) |
Surgical interventions were performed by endocrine-surgeons under general anesthesia using anterior basicervical cervicotomy. In 99 patients (72.8%) the intervention was only parathyroid (PTX) and in the remaining 37 patients (27.2%) also thyroid (partial or total thyroidectomy for concomitant thyroid pathology or by intratiroidal localization of the parathyroid adenoma). PTX was performed by UNE in 121 patients (89%) and by BNE in the remaining 15 patients (11%). The BNE, used as the first surgical approach only in unique patient effected by MEN1, was necessary in the other 14 cases due to an insufficient drop of IOPTH levels or to MGD reported by CDHR-NUS and/or 99mTc-MIBI PS or even (in only one case) for negativity of CDHR-NUS and 99mTc-MIBI PS. In all cases the surgeon used both the localization information provided by CDHR-NUS and 99mTc-MIBI PS and the IOPTHm as an immediate guide to the effectiveness of the intervention. The removed parathyroids were subjected both to extemporaneous histological examination of the freezer and to definitive histological examination.
In all the patients the intervention was decisive (complete remission of the PHPT laboratory picture both in the immediate post-operative and in a control visit carried out within one month of the intervention) and without major complications.
Results
In the context of the 136 interventions carried out, a total of 148 pathological parathyroids were removed, having a maximum diameter of between 0.5 and 7 cm (weight between 200 mg and 32 g); the histological examination identified 141 of them (95.2%) as “adenoma” and the remaining seven (4.8%) as “hyperplasia”. There were no cases of PHPT supported by parathyroid carcinoma. The pathology responsible for PHPT (Table 3) was represented in 126 patients (92.6%) by SGD (single adenoma, SA) and in ten patients (7.4%) by MGD. Of the 126 SAs, 38 (30%) involved the superior parathyroid and 88 (70%) involved the inferior parathyroid; the inferior 88 SAs, unlike what is described in Ref. [60], were localized more frequently on the right (49, 56%) than on the left (39, 44%). The three cases of normocalcemic PHPT and the two cases of normohormonal PHPT were also due to SGD. 22 SAs (17.4% of the 126 removed) were ectopic results: nine upper mediastinal, six intrathyroidal, five tracheo-esophageal, two retroesophageal/paratracheal. Also in our series the intrathyroidal localization was not infrequent (4.7% of all the removed parathyroids) and involved the inferior parathyroid in the majority of cases (five out of six, 83%). MGD was sustained in six patients (60%) by a double adenoma (DA, ipsilateral in three cases and contralateral in the other three cases), in three patients with adenoma + hyperplasia and in a patient with diffused hyperplasia in a MEN type I (all four affected glands); in three of these ten patients, one of the pathologic parathyroids was ectopic (13.6% of the 22 removed; one posterior paratracheal within an ipsilateral DA, one retroesophageal as part of a contralateral DA and one mediastinal thymic within a diffuse hyperplasia). Of all 148 pathological parathyroids removed, 25 (about 17%) were, therefore, ectopic (22 in the context of SGD and three in the field of MGD); the ectopic localization affected the inferior parathyroid more frequently (17 cases, 68%) than the superior parathyroid (eight cases, 32%).
Table 3.
Type of parathyroid pathology that determined PHPT in the case series
| Parathyroid pathologies | Patients (%) |
|---|---|
| SGD (single adenoma—SA) (126 parathyroids removed) | 126 (92.6) |
| Superior parathyroids | 38 (30) |
| Inferior parathyroids | 88 (70) |
| Right Inferior | 49 (56) |
| Left Inferior | 39 (44) |
| Ectopic parathyroids | 22 (17.4% of 126 removed SAs) |
| Superior mediastinal | 9 |
| Intrathyroidal | 6 |
| Tracheo-esophageal | 5 |
| Retro-esophageal/paratracheal | 2 |
| MGD (22 parathyroids removed) | 10 (7.4) |
| Double Adenoma (DA) | 6 |
| Adenoma + Hyperplasia | 3 |
| Diffused Hyperplasia (MEN1) | 1 |
| Ectopic parathyroids | 3 (13.6% of the 22 pathological parathyroids removed) |
| Paratracheal | 1 |
| Retroesophageal | 1 |
| Superior Mediastinal | 1 |
| Ectopic pathological parathyroids (EPP) (in the whole series) | 25 (17% of all 148 pathological parathyroids removed) |
| Inferior parathyroids | 17 (68) |
| Superior parathyroids | 8 (32) |
| Definitive histological examination (operating piece—148 removed pathological parathyroids) | |
| Adenoma | 141 (95.2) |
| Hyperplasia | 7 (4.8) |
For both the CDHR-NUS and the 99mTc-MIBI PS, the diagnostic performance indexes were calculated (Tables 4, 5):
for the whole series (SGD + MGD—136 patients);
for the SGD (126 patients);
for the MGD (10 patients);
for ectopic pathological parathyroids (EPP—25 patients in the whole series).
Table 4.
Diagnostic performance of CDHR-NUS
| CDHR-NUS | SGD + MGD (136 patients) | SGD (126 patients) | MGD (10 patients) | EPP (25 patients) | ||||
|---|---|---|---|---|---|---|---|---|
| (%) | CI 95% | (%) | CI 95% | (%) | CI 95% | (%) | CI 95% | |
| Sensibility | 89 | 84–94 | 94 | 90–98 | 59 | 39–80 | 80 | 66–94 |
| Specificity | 99 | 97–100 | 98 | 97–100 | 100 | 100–100 | 100 | 100–100 |
| PPV | 96 | 92–99 | 94 | 90–98 | 100 | 100–100 | 100 | 100–100 |
| NPV | 96 | 94–98 | 98 | 96–100 | 67 | 49–84 | 92 | 86–98 |
| LR+ | 59.91 | 27.0–132.8 | 56.8 | 27.2–118.5 | ||||
| LR− | 0.11 | 0.07–0.17 | 0.06 | 0.03–0.12 | 0.41 | 0.25–0.68 | 0.20 | 0.10–0.41 |
Table 5.
Diagnostic performance of 99mTc-MIBI PS
| 99mTc-MIBI PS | SGD + MGD (136 patients) | SGD (126 patients) | MGD (10 patients) | EPP (25 patients) | ||||
|---|---|---|---|---|---|---|---|---|
| (%) | CI 95% | (%) | CI 95% | (%) | CI 95% | (%) | CI 95% | |
| Sensibility | 71 | 64–78 | 74 | 67–81 | 35 | 14–56 | 53 | 35–71 |
| Specificity | 98 | 97–99 | 98 | 97–99 | 100 | 100–100 | 100 | 100–100 |
| PPV | 93 | 88–98 | 93 | 88–98 | 100 | 100–100 | 100 | 100–100 |
| NPV | 90 | 87–93 | 91 | 89–94 | 61 | 44–77 | 83 | 75–91 |
| LR+ | 35.1 | 17.5–70.2 | 37.3 | 18.6–74.6 | ||||
| LR− | 0.30 | 0.2–0.38 | 0.27 | 0.20–0.35 | 0.65 | 0.47–0.90 | 0.47 | 0.32–0.68 |
The diagnostic performance indexes obtained with the use of both methods for both the whole series (SGD + MGD—136 patients) (Table 6) and for the SGD alone (126 patients) (Table 7) were also determined.
Table 6.
Diagnostic performance of CDHR-NUS + 99mTc-MIBI PS for the whole series
| CDHR-NUS +99mTc-MIBI PS | SGD + MGD (136 patients) | CI 95% |
|---|---|---|
| Sensibility | 91% | 86–95 |
| Specificity | 99% | 98–100 |
| PPV | 97% | 95–100 |
| NPV | 99% | 97–100 |
| LR+ | 88.8 | 33.4–235.7 |
| LR− | 0.09 | 0.06–0.15 |
Table 7.
Diagnostic performance of the CDHR-NUS + 99mTc-MIBI PS for SGD
| CDHR-NUS +99mTc-MIBI PS | SGD (126 patients) | CI 95% |
|---|---|---|
| Sensibility | 95% | 92–99 |
| Specificity | 99% | 98–100 |
| PPV | 97% | 94–100 |
| NPV | 99% | 97–100 |
| LR+ | 98.5 | 37.1–201.4 |
| LR− | 0.05 | 0.02–0.10 |
In order to evaluate the effectiveness of the two diagnostic methods in predicting/excluding preoperatively the presence of SA/MGD, the concordance/discordance data reported in Tab. 2 were compared with the results of the operative act (finding of SGD vs MGD) (Table 8); MGD was distinguished in “reported” when preoperatively identified by one or both methods and “not reported” if identified only by surgery.
Table 8.
Preoperative concordance/discordance/negativity of CDHR-NUS and 99mTc-MIBI PS and findings of the operative act (SA vs MGD)
| Glandular pathology (operative response) | Group A full concordance patients (%) |
Group B partial concordance patients (%) |
Group C discordance patients (%) | Group D negativity of both methods patients (%) |
|---|---|---|---|---|
| 80 (58.8) | 17 (12.5) | 37 (27.2) | 2 (1.5) | |
| SA | 75 (94) | 17 (100) | 32 (86.5) | 2 (100) |
| MGD reported | 2 (2.5) | 0 | 2 (5) | 0 |
| MGD not reported | 3 (4)* | 0 | 3 (8.5)* | 0 |
| Total MGD | 5 (6) | 0 | 5 (13.5) | 0 |
*p = 0.523
From the analysis of the data processed, the following emerges:
the CDHR-NUS carried out by an expert medical sonographer has a high sensitivity and higher than that of the 99mTc-MIBI PS in all four processes performed (Tables 4, 5):
89% CDHR-NUS vs 71% 99mTc-MIBI PS for all the cases (SGD + MGD);
94% CDHR-NUS vs 74% 99mTc-MIBI PS for the SGD;
59% CDHR-NUS vs 35% 99mTc-MIBI PS for MGD;
80% CDHR-NUS vs 53% 99mTc-MIBI PS for EPP.
According to literature data, the sensitivity of both CDHR-NUS and 99mTc-MIBI PS for MGD was significantly lower (50% and 35%, respectively) and this explains why the sensitivity values of both methods were higher in 126 patients with SGD (94% CDHR-NUS and 99mTc-MIBI PS 74%) and lower in the whole series (CDHR-NUS 89% and 99mTc-MIBI PS 71%).
The simultaneous use of both methods does not determine a significant increase in total sensitivity, both in the case of the whole series (136 patients; 91% with CDHR-NUS + 99mTc-MIBI PS vs 89% with CDHR-NUS alone) than in the case of only SGD (126 patients; 95% with CDHR-NUS + 99mTc-MIBI PS vs 94% with only CDHR-NUS) (Tables 6, 7).
We think that the high values of specificity detected both for CDHR-NUS and for 99mTc-MIBI PS, not all substantially different, are due to the type of statistical analysis performed: since there were no healthy patients in the case series (all the patients examined had a certain diagnosis of PHPT), the true negatives were numerous because corresponding in each patient to the disease-free parathyroid sites.
As already observed in other studies [54, 61], the preoperative concordance of the two methods was obtained in only a part of the patients (full in 80, 58.8%, partial in seventeen, 12.5%). Three cases of MGD (corresponding, respectively, to adenoma + ipsilateral hyperplasia, adenoma + contralateral hyperplasia and diffuse hyperplasia within MEN1) were only operatively identified (“not reported”) among the 80 patients (group A, 58.8% of the cases) in which both methods agreed fully on an SA (incidence in this group of MGD not reported 4% and overall 6%). Three additional cases of MGD (corresponding to two contralateral DAs and one ipsilateral DA) were only operatively identified (“not reported”) among the 37 patients in which the methods were discordant (only one of the two methods positive for SGD; group C, 27.2% of the cases; incidence of MGD not reported 8.5% and overall 13.5%). The incidence of MGD not identified by CDHR-NUS nor by 99mTc-MIBI PS (“not reported”), although prevalent in group C (discordance, 8.5%), is, therefore, present to a non-negligible extent also in group A (full concordance, 4%). Of the remaining four cases of MGD, in two the pathology was already reported preoperatively by both methods (group A, corresponding, respectively, to an ipsilateral DA and a contralateral DA) and in the remaining two the same was already reported preoperatively only by the CDHR-NUS, resulting in the 99mTc-MIBI PS negative (group C, corresponding also in this case, respectively, to an ipsilateral DA and to a contralateral DA). There was no operative finding of MGD nor in the 17 cases of partial agreement (12.5%—group C) nor between the two cases of negativity of both methods (1.5%—group D) (Table 8).
Discussion and conclusions
Also in this study, according to data from literature, the PHPT is confirmed to be a prevalent pathology in the female sex (F/M 3:1) and sustained in the majority of cases (92.6%) by SGD (SA).
The high sensitivity values obtained by CDHR-NUS both in the overall series (136 patients, 89%) and in the SGD (126 patients, 94%), even in the presence of concomitant thyroid disease in a non negligible percentage (77 patients, 56.5%), confirm that the ultrasonography is an operator-dependent method and its diagnostic performance is considerably higher if performed by those with more experience [27]. This is further demonstrated by the greater sensitivity obtained from CDHR-NUS compared to 99mTc-MIBI PS even in the localization of ectopic adenomas. In our series the CDHR-NUS was able to identify most of the ectopic adenomas both mediastinal (eight out of ten, 80%) and—with the aid of color-Doppler—intrathyroidal (five out of six, 83%) and to locate all five tracheo-esophageal ectopic adenomas (100%).
It is believed that the lowest sensitivity values obtained from the 99mTc-MIBI PS compared to the CDHR-NUS are predominantly due to intrinsic limitations to the method (poor uptake or rapid wash-out of the 99mTc-SestaMIBI by some adenomas) and to the heterogeneous experience of the pool of nuclear physicians who reported the examinations of individual patients. We also believe that the use of tomographic imaging (SPECT and SPECT/CT) in only a few patients in the series has had little influence on the diagnostic accuracy of 99mTc-MIBI PS. We think, in accordance with what has already been stated by previous studies [62, 63] and also considering what has been observed up to now in clinical practice, that the use of SPECT/CT does not determine a significant increase in the diagnostic accuracy of the planar scintigraphic examination, being a complement of the latter and sharing with it the same limitations: the non-captive pathological parathyroid—possible occurrence in up to a third of patients [64]—is not visible at SPECT for the lack of emission of gamma radiation (cause of false negativity of the planar scintigraphic examination) or to the CT for the absence of the contrast medium and for the limited resolution of the obtained images (the examination is performed with a low dose protocol) (Fig. 14). We report that an overall sensitivity value of 99mTc-MIBI PS similar to that of our case series (about 71%) was obtained in a recent study [65] in which all scintigraphic tests were performed with SPECT/CT. We, therefore, think that the utility of the SPECT/CT is limited to a better planning of the surgical intervention in cases of negativity of the ultrasound examination.
Fig. 14.
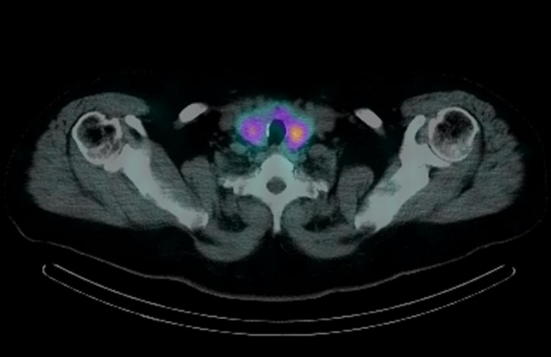
Image of scintigraphic examination SPECT/CT with false negative result (failure to visualize the pathological parathyroid). Parathyroid adenoma (left superior), not even visible at planar scintigraphy due to lack of uptake, had already been visualized by the CDR-HNUS (see Fig. 2)
The low overall increase in total sensitivity obtained with the use of both methods in the entire case series (91% with CDHR-NUS and 99mTc-MIBI PS vs 89% with CDHR-NUS alone) and in the SGD (95% with CDHR-NUS and 99mTc-MIBI PS vs 94% with CDHR-NUS alone) also corroborates what has already been stated by numerous previous studies [25, 66–69] in favor of the serial or sequential use of the two diagnostic methods: in the presence of expert medical sonographers, the CDHR-NUS assumes the role of main and possibly unique method for preoperative localization of pathological parathyroid glands. In these circumstances the execution of 99mTc-MIBI PS (even with SPECT/CT, if available) results justified and necessary only in the case of negative or non-diagnostic outcome of the ultrasonography. This is further confirmed by what we found in our series: in 30 of the 31 cases (96.7%) of positive CDHR-NUS and of contextual negative or non-diagnostic outcome of 99mTc-MIBI PS, the surgical intervention was performed with success based on the ultrasonography data only and with the help of the IOPTHm, without using any second-line localization method; the CT scan was performed in only one of these 31 cases, which was then due to a DA, but had no use, having located only one of the two pathological parathyroid glands (the one already reported by the CDHR-NUS); 99mTc-MIBI PS did not add any additional localization information in all cases in which CDHR-NUS was positive and was useful only in the six cases (4.4% of the total) in which the CDHR-NUS was negative or doubtful, localizing five SAs and one of the adenomas of a MGD (DA).
Therefore, in reiterating that in expert hands the CDHR-NUS is an accurate preoperative localization method, we should remember that it is also a “cost-effective” method [70, 71]. When carried out as the only preoperative localization method, it offers the following advantages:
it is harmless and easy to perform and also allows a contextual evaluation of the thyroid; the results obtained also confirm that any concomitant thyroid nodular pathology does not significantly influence the diagnostic sensitivity of the method [72];
it allows to obtain good details regarding the pathological parathyroids (dimensions, echostructure), their location and their relationship with the surrounding anatomical structures and allows the expert surgeon to carry out a targeted and decisive intervention, also with the help of the IOPTHm;
it is cheaper and allows to reduce the costs of preoperative imaging, without causing a significant reduction of diagnostic accuracy [69, 73]: based on the current reimbursement schedule of the Piedmont Region of Italy, healthcare facilities receive €28.4 for an ultrasonography of thyroid-parathyroid and €191.1 for a parathyroid scintigraphy (also SPECT/CT);
it avoids the patient the ionizing radiation dose of 99mTc-MIBI PS: from the literature data [74], it appears that the effective dose delivered by parathyroid scintigraphy is about 6 mSv with single-tracer double-phase technique, of about 7.5 mSv with double-tracer subtraction technique and 8–9 mSv with the simultaneous use of CT (SPECT/CT) and is, respectively, 300 times, 375 times and 400–450 times the dose delivered by a chest radiograph (0.02 mSv).
We also think—based on the resulting data (Table 8)—that the assessment of concordance/discordance between the two methods is neither a necessary nor indispensable datum: full agreement does not guarantee—as already demonstrated by previous studies [52, 75]—the absence of MGD, which has been identified only operatively in three patients in group A with the fundamental aid of IOPTHm [76]; the different incidence of MGD in groups A and C (4% and 8.5%) was not statistically significant (p = 0.523) and this shows that the finding of MGD in the two samples under examination is random and that concordance and discordance have a similar limited ability to exclude or confirm the presence of MGD preoperatively; it is also confirmed that the discordance between the two methods or the negativity of both does not correspond in most cases to the presence of MGD [77], considering that a SA was operatively detected in 34 of the 39 patients in the two groups (C and D, 87.2%).
It is now increasingly evident that MGD, whose real incidence would currently be underestimated by monolateral surgical techniques (UNE and MIP) [78], cannot be excluded preoperatively with absolute certainty with some of the diagnostic methods available today or with their joint use [79]. The current state of affairs is that to date no perfect preoperative localization method exists and that preoperative localization studies reliably identify the majority of patients with SGD and only a minority of those with MGD [57].
In accordance with what has already been stated in other studies, we, therefore, believe that, in the presence of expert medical sonographers, the parallel use of CDHR-NUS and 99mTc-MIBI PS may now be definitively abandoned because of the following:
“not cost-effective” [80], i.e. not justified in terms of cost/effectiveness, being more expensive and not determining a significant increase in diagnostic accuracy; the convenience of the parallel use of the two methods is still supported by studies in which the ultrasonography has a significantly lower sensitivity because it is not carried out by expert medical sonographers [81];
no longer justified by an unnecessary or indispensable request for preoperative agreement [82].
In the case of a negative result of both the CDHR-NUS and of 99mTc-MIBI PS, it could be “not cost-effective” and, therefore, the use of second-line localization methods (CT and MR) is not justified because:
in the face of higher costs (based on the current reimbursement schedule of the Piedmont Region of Italy, healthcare facilities receive €244.5 for a CT neck and thorax without and with contrast medium and €368.4 for a MR neck and thorax without and with contrast medium), they cannot guarantee—also considering the diagnostic accuracy values reported in literature and also in this case lower for MGD [83]—the localization of the pathological parathyroid in all cases that resulted negative to CDHR-NUS and 99mTc-MIBI PS;
both with CT and MR, the patient is exposed to potential damage by the contrast agent (nephrotoxicity, possibility of even serious adverse reactions); in relation to the CT protocol currently more used and publicized by scientific literature (4D-CT) [84]—we recall the further and significant exposure to ionizing radiation that it causes to the patient who has already performed the 99mTc-MIBI PS and which corresponds to an effective dose of at least 10 mSv (500 times the dose delivered by a chest radiography); moreover, its use is contraindicated in young patients (women aged ≤ 30 years and men aged ≤ 20 years), especially for the increased risk of thyroid cancer related to a dose to the thyroid organ of about 57 times higher than that of 99mTc-MIBI PS (92 mGy vs 1.6 mGy) [85]
Due to the above mentioned reasons, the use of second-line methods cannot be considered as routine and should be limited to selected cases.
Considering everything said, we do not agree with the diagnostic localization strategies proposed by recent studies based on cost-effectiveness criteria and which foresee the use of CDHR-NUS followed by that of 4D-CT (without performing the 99mTc-MIBI PS) in case of a negative ultrasonography [86] or the use in all cases of both the CDHR-NUS and the 99mTc-MIBI PS followed by that of the 4D-CT if the two methods of first line are discordant [87]; we can agree with the sequential use of CDHR-NUS and 99mTc-MIBI PS (also proposed by our study), followed by performance of the 4D-CT only in selected cases [88], but remembering the possible use of the MRI in cases in which 4D-CT is contraindicated. In accordance with previous studies [89], we believe that it is still of fundamental importance—for effective and resolutive PHPT surgical treatment—not only the availability of accurate preoperative imaging but also and above all the skill and experience of the endocrine-surgeon, who must be able to convert, if necessary (insufficient or failed drop of IOPTH levels), a monolateral intervention (UNE or MIP) into a bilateral intervention (BNE) and to carry out the latter even in the absence of valid preoperative indications of localization due to the negativity of the methods. The success of PTX still depends mainly on the surgeon’s experience rather than on the type of surgical technique used [90]; this is also shown by the fact that the lack of preoperative localization of pathological parathyroid glands due to the negative outcome of the imaging methods does not necessarily correspond to a lower success rate of the surgical intervention [58] and that the failure of the same is a more frequent occurrence in hospitals with a lower number of annual interventions [91].
At the conclusion of this study:
we think that the famous aphorism by J.L. Doppman is still current today but it can be rephrased as follows: “The only localization studies required by a patient undergoing initial parathyroid surgery is to locate an experienced parathyroid surgeon and an equally skilled parathyroid sonographer”;
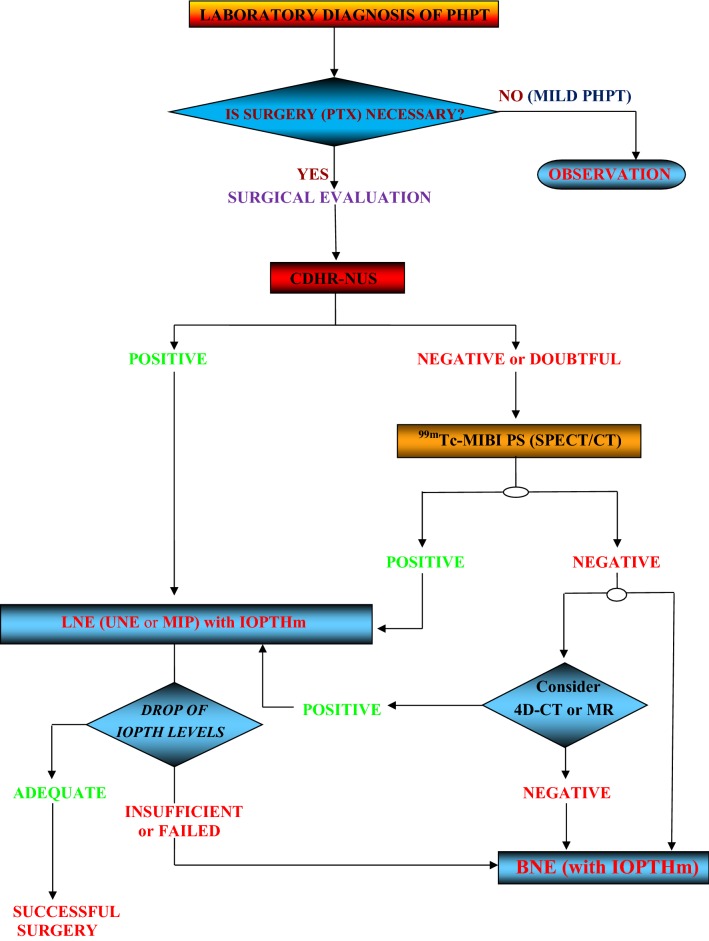
we propose a surgical treatment algorithm (Fig. 15), applicable to all patients affected by SPHPT not previously operated and based on sequential use of CDHR-NUS and 99mTc-MIBI PS with the aid of IOPTHm.
Fig. 15.
Surgical treatment algorithm for SPHPT
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. Am J Surg. 2006;191(3):418–423. doi: 10.1016/j.amjsurg.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 2.Fraser WD. Hyperparathyroidism. Lancet. 2009;374:145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 3.Marx SJ, Lourenco DM. Familial hyperparathyroidism—disorders of growth and secretion in hormone-secretory tissue. Horm Metab Res. 2017;49(11):805–815. doi: 10.1055/s-0043-120670. [DOI] [PubMed] [Google Scholar]
- 4.Marcocci C, Cianferotti L, Cetani F. Bone disease in primary hyperparathyrodism. Ther Adv Musculoskelet Dis. 2012;4:357–368. doi: 10.1177/1759720X12441869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg SJ, Walker MD, Bilezikian JP. Asymptomatic primary hyperparathyroidism. J Clin Densitom. 2013;16(1):14–21. doi: 10.1016/j.jocd.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad S, Kuraganti G, Steenkamp D. Hypercalcemic crisis: a clinical review. Am J Med. 2010;128:239–245. doi: 10.1016/j.amjmed.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Cusano NE, Silverberg SJ, Bilezikian JP. Normocalcemic Primary Hyperparathyroidism. J Clin Densitom. 2013;16(1):33–39. doi: 10.1016/j.jocd.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Applewhite MK, White MG, Tseng J, Mohammed MK, Mercier F, Kaplan EL, Angelos P, Vokes T, Grogan RH. Normohormonal primary hyperparathyroidism is a distinct form of primary hyperparathyroidism. Surgery. 2017;161(1):62–69. doi: 10.1016/j.surg.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Walker MD, Silverberg SJ. Primary hyperparathyroidism. Nat Rev Endocrinol. 2017;8:1–11. doi: 10.1038/nrendo.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverberg SJ. Vitamin D deficiency and primary hyperparathyroidism. J Bone Miner Res. 2007;22(S2):V100–V104. doi: 10.1359/jbmr.07s202. [DOI] [PubMed] [Google Scholar]
- 11.Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci M, Potts JT., Jr Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3561–3569. doi: 10.1210/jc.2014-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannon J, Lew JI, Solorzano CC. Parathyroidectomy for hypercalcemic crisis: 40 years’ experience and long-term outcomes. Surgery. 2010;148(4):807–812. doi: 10.1016/j.surg.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 13.Mollerup CL, Vestergaard P, Frøkjær VG, Mosekilde L, Christiansen P, Blichert-Toft M. Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow up study. BMJ. 2002;325:1–5. doi: 10.1136/bmj.325.7368.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolighed L, Vestergaard P, Heickendorff L, Sikjaer T, Rejnmark L, Mosekilde L, Christiansen P. BMD improvements after operation for primary hyperparathyroidism. Langenbecks Arch Surg. 2013;398(1):113–120. doi: 10.1007/s00423-012-1026-5. [DOI] [PubMed] [Google Scholar]
- 15.Allendorf J, DiGorgi M, Spanknebel K, Inabnet W, Chabot J. LoGerfo P (2007) 1112 consecutive bilateral neck explorations for primary hyperparathyroidism. World J Surg. 2007;31(11):2075–2080. doi: 10.1007/s00268-007-9068-5. [DOI] [PubMed] [Google Scholar]
- 16.Doppman JL. Reoperative parathyroid surgery; localization procedures. Prog Surg. 1986;18:117–132. [Google Scholar]
- 17.Patel KN, Caso R. Intraoperative Parathyroid Hormone Monitoring: optimal Utilization. Surg Oncol Clin N Am. 2016;25(1):91–101. doi: 10.1016/j.soc.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Westerdhal J, Berngelfelz A. Unilateral versus bilateral neck exploration for primary hyperparathyroidism: five-year follow-up of a randomized controlled trial. Ann Surg. 2007;246(6):976–980. doi: 10.1097/SLA.0b013e31815c3ffd. [DOI] [PubMed] [Google Scholar]
- 19.Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectoy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg. 2011;253(3):585–591. doi: 10.1097/SLA.0b013e318208fed9. [DOI] [PubMed] [Google Scholar]
- 20.Lew JI, Solorzano CC. Surgical management of primary hyperparathyroidism: state of the art. Surg Clin North Am. 2009;89(5):1205–1225. doi: 10.1016/j.suc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Mohebati A, Shaha AR. Imaging techniques in parathyroid surgery for primary hyperparathyroidism. Am J Otolaryngol. 2012;33(4):457–468. doi: 10.1016/j.amjoto.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelz RR, Fraker DL. Hyperparathyroidism: what preoperative imaging is necessary? Adv Surg. 2015;49:247–262. doi: 10.1016/j.yasu.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Wang C. The anatomic basis of parathyroid surgery. Ann Surg. 1976;183(3):271–275. doi: 10.1097/00000658-197603000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy KL, Yim JH, Zuckerbraun BS, Ogilvie JB, Peel RL, Carty SE. Cystic parathyroid lesions: functional and nonfunctional parathyroid cysts. Arch Surg. 2009;144(1):52–56. doi: 10.1001/archsurg.2008.531. [DOI] [PubMed] [Google Scholar]
- 25.Mohammadi A, Moloudi F, Ghasemi-rad M. The role of colour Doppler ultrasonography in the preoperative localization of parathyroid adenomas. Endocr J. 2012;59(5):375–382. doi: 10.1507/endocrj.ej11-0351. [DOI] [PubMed] [Google Scholar]
- 26.Rickes S, Sitzy J, Neye H, Ocran KW, Wermke W. High-resolution ultrasound in combination with colour-Doppler sonography for preoperative localization of parathyroid adenomas in patients with primary hyperparathyroidism. Ultraschall Med. 2003;24(2):85–89. doi: 10.1055/s-2003-38667. [DOI] [PubMed] [Google Scholar]
- 27.Reeder SB, Desser TS, Weigel RJ, Jeffrey RB. Sonography in primary hyperparathyroidism: review with emphasis on scanning technique. J Ultrasound Med. 2002;21(5):539–552. doi: 10.7863/jum.2002.21.5.539. [DOI] [PubMed] [Google Scholar]
- 28.Yabuta T, Tsushima Y, Masuoka H, Tomoda C, Fukushima M, Kihara M, Inoue H, Higashiyama T, Takamura Y, Ito Y, Kobayashi K, Miya A, Miyauchi A. Ultrasonographic features of intrathyroidal parathyroid adenoma causing primary hyperparathyroidism. Endocr J. 2011;58(11):989–994. doi: 10.1507/endocrj.ej11-0069. [DOI] [PubMed] [Google Scholar]
- 29.Abboud B, Sleilaty G, Ayoub S, Hachem K, Smayra T, Ghorra C, Abadjian G. Intrathyroid parathyroid adenoma in primary hyperparathyroidism: can it be predicted preoperatively? World J Surg. 2007;31(4):817–823. doi: 10.1007/s00268-006-0767-0. [DOI] [PubMed] [Google Scholar]
- 30.Isidori AM, Cantisani V, Giannetta Diacinti D, David E, Forte V, Elia D, De Vito C, Sbardella E, Gianfrilli D, Monteleone F, Pepe J, Minisola S, Ascenti G, D’Andrea V, Catalano C, D’Ambrosio F. Multiparametric ultrasonography and ultrasound elastography in the differentiation of parathyroid lesions from ectopic thyroid lesions or lymphadenopathies. Endocrine. 2017;57(2):335–343. doi: 10.1007/s12020-016-1116-1. [DOI] [PubMed] [Google Scholar]
- 31.Palestro CJ, Tomas MB, Tronco GG. Radionuclide imaging of the parathyroid gland. Semin Nucl Med. 2005;35(4):266–276. doi: 10.1053/j.semnuclmed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Shah S, Win Z, Al-Nahhas A. Multimodality imaging of parathyroid glands in primary hyperparathyroidism. Minerva Endocrinol. 2008;33(3):193–202. [PubMed] [Google Scholar]
- 33.Eslamy HK, Ziessman HA. Parathyroid scintigraphy in patients with primary hyperparathyroidism: 99 mTc SestaMIBI SPECT and SPECT/CT. Radiographics. 2008;28(5):1471–1476. doi: 10.1148/rg.285075055. [DOI] [PubMed] [Google Scholar]
- 34.Cheung K, Wang TS, Farrokhyar F, Roman SA, Sosa JA. A meta-analysis of preoperative localization techniques for patients with primary hyperparathyroidism. Ann Surg Oncol. 2012;19(2):577–583. doi: 10.1245/s10434-011-1870-5. [DOI] [PubMed] [Google Scholar]
- 35.Berber E, Parikh RT, Ballem N, Garner CN, Milas M, Siperstein AE. Factors contributing to negative parathyroid localization: an analysis of 1000 patients. Surgery. 2008;144(1):74–79. doi: 10.1016/j.surg.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Ruda JM, Hollenback CS, Stack BC. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132(3):359–372. doi: 10.1016/j.otohns.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Kebapci M, Entok E, Kebapci N, Adapinar B. Preoperative evaluation of parathyroid lesions in patients with concomitant thyroid disease: role of high resolution ultrasonography and dual phase technetium 99 m sestaMIBI scintigraphy. J Endocrinol Invest. 2004;27(1):24–30. doi: 10.1007/BF03350906. [DOI] [PubMed] [Google Scholar]
- 38.Uruno T, Kebebew E. How to localize parathyroid tumors in primary hyperparathyroidism? J Endocrinol Invest. 2006;29(9):840–847. doi: 10.1007/BF03347381. [DOI] [PubMed] [Google Scholar]
- 39.Kunstman JW, Kirsch JD, Mahajan A, Udelsman R. Clinical review: parathyroid localization and implications for clinical management. J Clin Endocrinol Metab. 2013;98(3):902–912. doi: 10.1210/jc.2012-3168. [DOI] [PubMed] [Google Scholar]
- 40.Phillips CD, Shatzkes DR. Imaging of the parathyroid glands. Semin Ultrasound CT MR. 2012;33(2):123–129. doi: 10.1053/j.sult.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Lumachi F, Ermani M, Basso S, Zucchetta P, Borsato N, Favia G. Localization of parathyroid tumors in the minimally invasive era: which tecnique should be chosen? Population based analysis of 253 patients undergoing parathyroidectomy and factors affecting parathyroid glands detection. Endocr Relat Cancer. 2001;8(1):63–69. doi: 10.1677/erc.0.0080063. [DOI] [PubMed] [Google Scholar]
- 42.Scheiner JD, Dupuy DE, Monchik JM, Noto RB, Cronan JJ. Pre-operative localization of parathyroid adenomas: a comparison of power and colour doppler ultrasonography with nuclear medicine scintigraphy. Clin Radiol. 2001;56(12):984–988. doi: 10.1053/crad.2001.0793. [DOI] [PubMed] [Google Scholar]
- 43.Lumachi F, Zucchetta P, Tregnaghi A, Marzola MC, Cecchin D, Marchesi P, Bui F, Iacobone M. Noninvasive parathyroid imaging in primary hyperparathyroidism. Ann Ital Chir. 2003;74(4):385–388. [PubMed] [Google Scholar]
- 44.Johnson NA, Tublin ME. Ogilvie JB (2007) Parathyroid imaging: technique and role in the preoperative evaluation of primary hyperparathyroidism. AJR Am J Roentgenol. 2007;188(6):1706–1715. doi: 10.2214/AJR.06.0938. [DOI] [PubMed] [Google Scholar]
- 45.Yuan LL, Kan Y, Ma DQ, Yang JG. Combined application of ultrasound and SPECT/CT has incremental value in detecting parathyroid tissue in SHPT patients. Diagn Interv Imaging. 2016;97(2):219–225. doi: 10.1016/j.diii.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Sukan A, Reyhan M, Aydin M, Yapar AF, Sert Y, Canpolat T, Aktas A. Preoperative evaluation of hyperparathyroidism: the role of dual-phase parathyroid scintigraphy and ultrasound imaging. Ann Nucl Med. 2008;22(2):123–131. doi: 10.1007/s12149-007-0086-z. [DOI] [PubMed] [Google Scholar]
- 47.Krausz Y, Lebensart PD, Klein M, Weininger J, Blachar A, Chisin R, Shiloni E. Preoperative localization of parathyroid adenoma in patients with concomitant thyroid nodular disease. World J Surg. 2000;24(12):1573–1578. doi: 10.1007/s002680010280. [DOI] [PubMed] [Google Scholar]
- 48.Ryan JA, Jr, Lee FT. Maximizing outcomes while minimizing exploration in hyperparathyroidism using localization tests. Arc Surg. 2004;139(8):838–843. doi: 10.1001/archsurg.139.8.838. [DOI] [PubMed] [Google Scholar]
- 49.Arici C, Cheah WK, Ituarte PH, Morita E, Lynch TC, Siperstein AE, Duh QY, Clark OH. Can localization studies be used to direct focused parathyroid operations? Surgery. 2001;129(6):720–729. doi: 10.1067/msy.2001.114556. [DOI] [PubMed] [Google Scholar]
- 50.Kebebew E, Hwang J, Reiff E, Duh QY, Clark OH. Predictors of single-gland vs multigland parathyroid disease in primary hyperparathyroidism: a simple and accurate scoring model. Arch Surg. 2006;141(8):777–782. doi: 10.1001/archsurg.141.8.777. [DOI] [PubMed] [Google Scholar]
- 51.Kavanagh DO, Fitzpatrick P, Myers E, Kennelly R, Skehan SJ, Gibney RG, Hill AD, Evoy D, McDermott EW. A predictive model of suitability for minimally invasive parathyroid surgery in the treatment of primary hyperparathyroidism. World J Surg. 2012;36(5):1175–1181. doi: 10.1007/s00268-011-1377-z. [DOI] [PubMed] [Google Scholar]
- 52.Siperstein A, Berber E, Mackey R, Alghoul M, Wagner K, Milas M. Prospective evaluation of sestaMIBI scan, ultrasonography and rapid PTH to predict the success of limited exploration for sporadic primary hyperparathyroidism. Surgery. 2004;136(4):872–880. doi: 10.1016/j.surg.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 53.Calò PG, Medas F, Loi G, Erdas E, Pisano G, Nicolosi A. Feasibility of unilateral parathyroidectomy in patients with primary hyperparathyroidism and negative or discordant localization studies. Updates Surg. 2016;68(2):155–161. doi: 10.1007/s13304-015-0342-z. [DOI] [PubMed] [Google Scholar]
- 54.Lew JI, Solorzano CC, Montano RE, Carneiro-Pla DM, Irvin GL., 3rd Role of intraoperative parathyroid hormone monitoring during parathyroidectomy in patients with discordant localization studies. Surgery. 2008;144(2):299–306. doi: 10.1016/j.surg.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 55.Barczynski M, Konturek A, Cichon S, Hubalewska-Dydejczyk A, Golkowski F, Huszno B. Intraoperative parathyroid hormone assay improves outcomes of minimally invasive parathyroidectomy mainly in patients with a presumed solitary parathyroid adenoma and missing concordance of preoperative imaging. Clin Endocrinol. 2007;66(6):878–885. doi: 10.1111/j.1365-2265.2007.02827.x. [DOI] [PubMed] [Google Scholar]
- 56.Sebag F, Hubbard JG, Maweja S, Misso C, Tardivet L, Henry JF. Negative preoperative localization studies are highly predictive of multiglandular disease in sporadic primary hyperparathyroidism. Surgery. 2003;134(6):1038–1042. doi: 10.1016/j.surg.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 57.Moalem J, Guerrero M, Kebebew E. Bilateral neck exploration in primary hyperparathyroidism—when is it selected and how is it performed? World J Surg. 2009;33(11):2282–2891. doi: 10.1007/s00268-009-9941-5. [DOI] [PubMed] [Google Scholar]
- 58.Wachtel H, Bartlett EK, Kelz RR, Cerullo I, Karakousis GC, Fraker DL. Primary hyperparathyroidism with negative imaging: a significant clinical problem. Ann Surg. 2014;260(3):474–482. doi: 10.1097/SLA.0000000000000875. [DOI] [PubMed] [Google Scholar]
- 59.Vitetta GM, Neri P, Chiecchio A, Carriero A, Cirillo S, Balbo Mussetto A, Codegone A. Role of ultrasonography in the management of patients with primary hyperparathyroidism: retrospective comparison with technetium-99 m sestaMIBI scintigraphy. J Ultrasound. 2014;17:1–12. doi: 10.1007/s40477-014-0067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marzouki HZ, Chavannes M, Tamilia M, Hier MP, Black MJ, Levental M, Payne RJ. Location of parathyroid adenomas: 7-year experience. J Otolaryngol Head Neck Surg. 2010;39(5):551–554. [PubMed] [Google Scholar]
- 61.Bachar G, Mizrachi A, Hadar T, Feinmesser R, Shpitzer T. Role of parathyroid hormone monitoring during parathyroidectomy. Head Neck. 2011;33(12):1754–1757. doi: 10.1002/hed.21666. [DOI] [PubMed] [Google Scholar]
- 62.Ruf J, Seehofer D, Denecke T, Stelter L, Rayes N, Felix R, Amthauer H. Impact of image fusion and attenuation correction by SPECT-CT on the scintigraphic detection of parathyroid adenomas. Nuklearmedizin. 2007;46(1):15–21. [PubMed] [Google Scholar]
- 63.Gayed IW, Kim EE, Broussard WF, Evans D, Lee J, Broemeling LD, Ochoa BB, Moxley DM, Erwin WD, Podoloff DA. The value of 99mTc-sestaMIBI SPECT/CT over conventional SPECT in the evaluation of parathyroid adenomas or hyperplasia. J Nucl Med. 2005;46(2):248–252. [PubMed] [Google Scholar]
- 64.Mihai R, Simon D, Hellman P. Imaging for primary hyperparathyroidism—an evidence-based analysis. Langenbecks Arch Surg. 2009;394(5):765–784. doi: 10.1007/s00423-009-0534-4. [DOI] [PubMed] [Google Scholar]
- 65.Berner AM, Haroon A, Nowosinska E, Offiah C, Luqman M, Newell M, Jan H. Localization of parathyroid disease with ‘sequential multiphase and dual-tracer’ technique and comparison with neck ultrasound. Nucl Med Commun. 2015;36(1):45–52. doi: 10.1097/MNM.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 66.Haber RS, Kim CK, Inabnet WB. Ultrasonography for preoperative localization of enlarged parathyroid glands in primary hyperparathyroidism: comparison with (99m) technetium sestaMIBI scintigraphy. Clin Endocrinol. 2002;57(2):241–249. doi: 10.1046/j.1365-2265.2002.01583.x. [DOI] [PubMed] [Google Scholar]
- 67.Grosso I, Sargiotto A, D’Amelio P, Tamone C, Gasparri G, De Filippi PG, Picciotto G, Isaia G. Preoperative localization of parathyroid adenoma with sonography and 99mTc SestaMIBI scintigraphy in primary hyperparathyroidism. J Clin Ultrasound. 2007;35(4):186–190. doi: 10.1002/jcu.20319. [DOI] [PubMed] [Google Scholar]
- 68.Tresoldi S, Pompili G, Maiolino R, Flor N, De Pasquale L, Bastagli A, Sardanelli F, Cornalba G. Primary hyperparathyroidism: can ultrasonography be the only preoperative diagnostic procedure? Radiol Med. 2009;114(7):1159–1172. doi: 10.1007/s11547-009-0447-x. [DOI] [PubMed] [Google Scholar]
- 69.Aliyev S, Agcaoglu O, Aksoy E, Birsen O, Milas M, Mitchell J, Siperstein A, Berber E. An analysis of whether surgeon-performed neck ultrasound can be used as the main localizing study in primary hyperparathyroidism. Surgery. 2014;156(5):1127–11131. doi: 10.1016/j.surg.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Gilat H, Cohen M, Feinmesser R, Benzion J, Shvero J, Segal K, Ulanovsky D, Shpitzer T. Minimally invasive procedure for resection of a parathyroid adenoma: the role of preoperative high-resolution ultrasonography. J Clin Ultrasound. 2005;33(6):283–287. doi: 10.1002/jcu.20131. [DOI] [PubMed] [Google Scholar]
- 71.Ulanovski D, Feinmesser R, Cohen M, Sulkes J, Dudkiew M. Shpitzer P (2002) Preoperative evaluation of patients with parathyroid adenoma: role of high-resolution ultrasonography. Head Neck. 2002;24(1):1–5. doi: 10.1002/hed.10043. [DOI] [PubMed] [Google Scholar]
- 72.Abboud B, Sleilaty G, Rabaa L, Daher R, Abou Zeid H, Jabbour H, Hachem K, Smayra T. Ultrasonography: highly accuracy technique for preoperative localization of parathyroid adenoma. Laryngoscope. 2008;118(9):1574–1578. doi: 10.1097/MLG.0b013e31817aecad. [DOI] [PubMed] [Google Scholar]
- 73.Solorzano CC, Carneiro-Pla DM, Irvin GL. Surgeon-performed ultrasonography as the initial and the only localizing study in sporadic and primary hyperparathyroidism. J Am Coll Surg. 2006;202(1):18–24. doi: 10.1016/j.jamcollsurg.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 74.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248(1):254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 75.Siperstein A, Berber E, Barbosa GF, Tsinberg M, Greene AB, Mitchell J, Milas M. Predicting the success of limited exploration for primary hyperparathyroidism using ultrasound, sestaMIBI, and intraoperative parathyroid hormone: analysis of 1158 cases. Ann Surg. 2008;248(3):420–428. doi: 10.1097/SLA.0b013e3181859f71. [DOI] [PubMed] [Google Scholar]
- 76.Bobanga ID, McHenry CR. Is intraoperative parathyroid hormone monitoring necessary for primary hyperparathyroidism with concordant preoperative imaging? Am J Surg. 2017;213(3):484–488. doi: 10.1016/j.amjsurg.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 77.Philippon M, Guerin C, Taieb D, Vaillant J, Morange I, Brue T, Conte-Devolx B, Henry JF, Slotema E, Sebag F, Castinetti F. Bilateral neck exploration in patients with primary hyperparathyroidism and discordantimaging results: a single-centre study. Eur J Endocrinol. 2014;170(5):719–725. doi: 10.1530/EJE-13-0796. [DOI] [PubMed] [Google Scholar]
- 78.Lee NC, Norton JA. Multiple-gland disease in primary hyperparathyroidism: a function of operative approach? Arch Surg. 2002;137(8):896–900. doi: 10.1001/archsurg.137.8.896. [DOI] [PubMed] [Google Scholar]
- 79.Vanderbulcke O, Delaere P, Vander Poorten V, Debruyne F. Incidence of multiglandular disease in sporadic primary hyperparathyroidism. B-ENT. 2014;10(1):1–6. [PubMed] [Google Scholar]
- 80.Coelho MC, de Oliveira E, Silva de Morais NA, Beuren AC, Lopes CB, Santos CV, Cantoni J, Neto LV, Lima MB. Role of imaging tests for preoperative location of pathologic parathyroid tissue in patients with primary hyperparathyroidism. Endocr Pract. 2016;22(9):1062–1067. doi: 10.4158/EP151137.OR. [DOI] [PubMed] [Google Scholar]
- 81.Patel CN, Salahudeen HM, Lansdownb M, Scarsbrook AF. Clinical utility of ultrasound and 99mTc sestaMIBI SPECT/CT for preoperative localization of parathyroid adenoma in patients with primary hyperparathyroidism. Clin Radiol. 2010;65(4):278–287. doi: 10.1016/j.crad.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 82.Gergel M, Brychta I, Vician M, Olejnik J. Primary hyperparathyreosis: is concordant sonography and scintigraphy really so important? Bratisl Lek Listy. 2014;115(10):649–652. doi: 10.4149/bll_2014_125. [DOI] [PubMed] [Google Scholar]
- 83.Lubitz CC, Hunter GJ, Hamberg LM, Parangi S, Ruan D, Gawande A, Gaz RD, Randolph GW, Moore FD, Jr, Hodin RA, Stephen AE. Accuracy of 4-dimensional computed tomography in poorly localized patients with primary hyperparathyroidism. Surgery. 2010;148(6):1129–1138. doi: 10.1016/j.surg.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Day KM, Elsayed M, Beland MD, Monchik JM. The utility of 4-dimensional computed tomography for preoperative localization of primary hyperparathyroidism in patients not localized by sestaMIBI or ultrasonography. Surgery. 2015;157(3):534–539. doi: 10.1016/j.surg.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 85.Mahajan A, Starker LF, Ghita M, Udelsman R, Brink JA, Carling T. Parathyroid four-dimensional computed tomography: evaluation of radiation dose exposure during preoperative localization of parathyroid tumors in primary hyperparathyroidism. World J Surg. 2012;36(6):1335–1339. doi: 10.1007/s00268-011-1365-3. [DOI] [PubMed] [Google Scholar]
- 86.Lubitz CC, Stephen AE, Hodin RA, Pandharipande P. Preoperative localization strategies for primary hyperparathyroidism: an economic analysis. Ann Surg Oncol. 2012;19(13):4202–4209. doi: 10.1245/s10434-012-2512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang TS, Cheung K, Farrokhyar F, Roman SA, Sosa JA. Would scan, but which scan? A cost-utility analysis to optimize preoperative imaging for primary hyperparathyroidism. Surgery. 2011;150(6):1286–1294. doi: 10.1016/j.surg.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 88.Solorzano CC, Carneiro-Pla D. Minimizing cost and maximizing success in the preoperative localization strategy for primary hyperparathyroidism. Surg Clin North Am. 2014;94(3):587–605. doi: 10.1016/j.suc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 89.Minisola S, Cipriani C, Diacinti D, Tartaglia F, Scillitani A, Pepe J, Scott-Coombes D. Imaging of the parathyroid glands in primary hyperparathyroidism. Eur J Endocrinol. 2016;174(1):D1–D8. doi: 10.1530/EJE-15-0565. [DOI] [PubMed] [Google Scholar]
- 90.Udelsman R, Åkerström G, Biagini C, Duh QY, Miccoli P, Niederle B, Tonelli F. The surgical management of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99(10):3595–3606. doi: 10.1210/jc.2014-2000. [DOI] [PubMed] [Google Scholar]
- 91.Chen H, Wang TS, Yen TW, Doffek K, Krzywda E, Schaefer S, Sippel RS, Wilson SD. Operative failures after parathyroidectomy for hyperparathyroidism: the influence of surgical volume. Ann Surg. 2010;252(4):691–695. doi: 10.1097/SLA.0b013e3181f698df. [DOI] [PubMed] [Google Scholar]