Abstract
Varicella-zoster virus (VZV) is a neurotropic alphaherpesvirus that causes
chickenpox and shingles. ORF7 is an important virulence determinant of VZV in both
human skin and nerve tissues, however, its specific function and involved molecular
mechanism in VZV pathogenesis remain largely elusive. Previous yeast two-hybrid
studies on intraviral protein-protein interaction network in herpesviruses have
revealed that VZV ORF7 may interact with ORF53, which is a virtually unstudied but
essential viral protein. The aim of this study is to identify and characterize VZV
ORF53, and to investigate its relationship with ORF7. For this purpose, we prepared
monoclonal antibodies against ORF53 and, for the first time, characterized it as a
~40 kDa viral protein predominantly localizing to the trans-Golgi network of the
infected host cell. Next, we further confirmed the interaction between ORF7 and
ORF53 by co-immunoprecipitation and co-localization studies in both
plasmid-transfected and VZV-infected cells. Moreover, interestingly, we found that
ORF53 lost its trans-Golgi network localization and became dispersed in the
cytoplasm of host cells infected with an ORF7-deleted recombinant VZV, and thus ORF7
seems to play a role in normal subcellular localization of ORF53. Collectively,
these results suggested that ORF7 and ORF53 may function as a complex during
infection, which may be implicated in VZV pathogenesis. 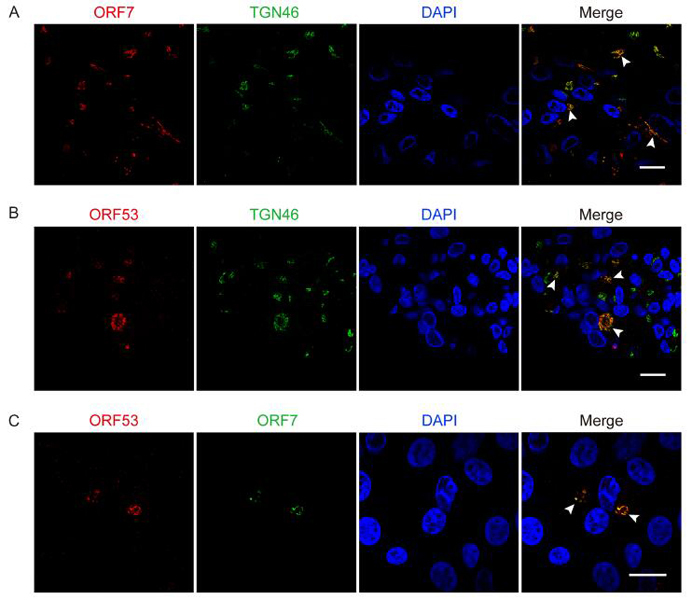
Keywords: varicella-zoster virus (VZV), ORF7, ORF53, protein-protein interaction, trans-Golgi network
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81601762), the National Science and Technology Major Project of Infectious Diseases (No. 2017ZX10304402), the National Science and Technology Major Projects for Major New Drugs Innovation and Development (No. 2017ZX09101005-005-003) and the Scientific Research Foundation of State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics (No. 2016ZY005).
Footnotes
These authors contributed equally to this work.
Contributor Information
Hua Zhu, Email: zhuhu@njms.rutgers.edu.
Tong Cheng, Email: tcheng@xmu.edu.cn.
References
- Albecka A, Owen DJ, Ivanova L, Brun J, Liman R, Davies L, Ahmed MF, Colaco S, Hollinshead M, Graham SC, Crump CM. Dual function of the pul7-pul51 tegument protein complex in herpes simplex virus 1 infection. J Virol. 2017;91(pii):e02196–16. doi: 10.1128/JVI.02196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AR, Kilmartin JV, Gergely F. Cdk5rap2 functions in centrosome to spindle pole attachment and DNA damage response. J Cell Biol. 2010;189:23–39. doi: 10.1083/jcb.200912163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AS, Chouljenko VN, Jambunathan N, Subramanian R, Mottram P, Kousoulas KG. Phenylalanine residues at the carboxyl terminus of the herpes simplex virus 1 ul20 membrane protein regulate cytoplasmic virion envelopment and infectious virus production. J Virol. 2014;88:7618–7627. doi: 10.1128/JVI.00657-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Straus SE, Arvin AM. Fields virology. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2773–2818. [Google Scholar]
- Donsante A, Yi L, Zerfas PM, Brinster LR, Sullivan P, Goldstein DS, Prohaska J, Centeno JA, Rushing E, Kaler SG. Atp7a gene addition to the choroid plexus results in long-term rescue of the lethal copper transport defect in a menkes disease mouse model. Mol Ther. 2011;19:2114–2123. doi: 10.1038/mt.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, Patrick D, Blanchette C, Mansi JA. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: A prospective study. CMAJ. 2010;182:1731–1736. doi: 10.1503/cmaj.091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- Grigoryan S, Kinchington PR, Yang IH, Selariu A, Zhu H, Yee M, Goldstein RS. Retrograde axonal transport of vzv: Kinetic studies in hesc-derived neurons. J Neurovirol. 2012;18:462–470. doi: 10.1007/s13365-012-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HF, Wang W, Jiang X, Zeng WB, Shen ZZ, Song YG, Yang H, Liu XJ, Dong X, Zhou J, Sun JY, Yu FL, Guo L, Cheng T, Rayner S, Zhao F, Zhu H, Luo MH. Orf7 of varicellazoster virus is required for viral cytoplasmic envelopment in differentiated neuronal cells. J Virol. 2017;91:e00127–17. doi: 10.1128/JVI.00127-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37. doi: 10.1186/1741-7015-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas K, Edte A, Bertrand I. The impact of herpes zoster and post-herpetic neuralgia on quality of life: Patient-reported outcomes in six european countries. Z Gesundh Wiss. 2012;20:441–451. doi: 10.1007/s10389-011-0481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo WX, Zhang J, Yang HJ, Li SW, Xie XY, Pang SQ, Li SJ, Xia NS. Construction and application of an escherichia coli high effective expression vector with an enhancer. Sheng Wu Gong Cheng Xue Bao. 2000;16:578–581. [PubMed] [Google Scholar]
- Nonnenmacher ME, Cintrat JC, Gillet D, Weber T. Syntaxin 5-dependent retrograde transport to the trans-golgi network is required for adeno-associated virus transduction. J Virol. 2015;89:1673–1687. doi: 10.1128/JVI.02520-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay S, Meyer NL, Puschnik AS, Davulcu O, Diep J, Ishikawa Y, Jae LT, Wosen JE, Nagamine CM, Chapman MS, Carette JE. An essential receptor for adeno-associated virus infection. Nature. 2016;530:108–112. doi: 10.1038/nature16465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller RJ, Fetters R. The herpes simplex virus 1 ul51 protein interacts with the ul7 protein and plays a role in its recruitment into the virion. J Virol. 2015;89:3112–3122. doi: 10.1128/JVI.02799-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selariu A, Cheng T, Tang Q, Silver B, Yang L, Liu C, Ye X, Markus A, Goldstein RS, Cruz-Cosme RS, Lin Y, Wen L, Qian H, Han J, Dulal K, Huang Y, Li Y, Xia N, Zhu H. Orf7 of varicella-zoster virus is a neurotropic factor. J Virol. 2012;86:8614–8624. doi: 10.1128/JVI.00128-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellberger T, Hauser R, Baiker A, Pothineni VR, Haas J, Uetz P. Improving the yeast two-hybrid system with permutated fusions proteins: The varicella zoster virus interactome. Proteome Sci. 2010;8:8. doi: 10.1186/1477-5956-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P, Dong YA, Zeretzke C, Atzler C, Baiker A, Berger B, Rajagopala SV, Roupelieva M, Rose D, Fossum E, Haas J. Herpesviral protein networks and their interaction with the human proteome. Science. 2006;311:239–242. doi: 10.1126/science.1116804. [DOI] [PubMed] [Google Scholar]
- Wang W, Cheng T, Zhu H, Xia NS. Insights into the function of tegument proteins from the varicella zoster virus. Science China-Life Sciences. 2015;58:739–749. doi: 10.1007/s11427-015-4887-3. [DOI] [PubMed] [Google Scholar]
- Weinke T, Edte A, Schmitt S, Lukas K. Impact of herpes zoster and post-herpetic neuralgia on patients’ quality of life: A patient-reported outcomes survey. Z Gesundh Wiss. 2010;18:367–374. doi: 10.1007/s10389-010-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12:197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Rowe J, Wang W, Sommer M, Arvin A, Moffat J, Zhu H. Genetic analysis of varicella-zoster virus orf0 to orf4 by use of a novel luciferase bacterial artificial chromosome system. J Virol. 2007;81:9024–9033. doi: 10.1128/JVI.02666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Selariu A, Warden C, Huang G, Huang Y, Zaccheus O, Cheng T, Xia N, Zhu H. Genome-wide mutagenesis reveals that orf7 is a novel vzv skin-tropic factor. PLoS Pathog. 2010;6:e1000971. doi: 10.1371/journal.ppat.1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang Y, Hu J, Zhang X, Zhang YW. Cuta divalent cation tolerance homolog (escherichia coli) (cuta) regulates beta-cleavage of beta-amyloid precursor protein (app) through interacting with beta-site app cleaving protein 1 (bace1) J Biol Chem. 2012;287:11141–11150. doi: 10.1074/jbc.M111.330209. [DOI] [PMC free article] [PubMed] [Google Scholar]


