Abstract
Purpose
To compare the diagnostic accuracy of sonographic features with ultrasound-guided fine-needle aspiration (FNA) cytology in the detection of malignant thyroid nodules.
Methods
This analytical cross-sectional study was conducted on patients with the diagnosis of thyroid nodule, who underwent ultrasound-guided FNA in Shahid Beheshti teaching hospital, Babol, northern Iran, between 2015 and 2017. The characteristics of the nodules obtained from ultrasonography were recorded. Regression analysis was used to assess the relation between sonographic findings and malignancy. We also used a receiver operator characteristics analysis to estimate the ability of ultrasound to predict the characteristic features of malignancy, as estimated by the area under the curve.
Results
In total, 898 thyroid nodules were included in the study, of which 55 (6.1%) were malignant and 843 (93.9%) were benign. There were significant positive associations between malignancy and hypoechogenicity [odds ratio (OR) 3.577, 95% confidence interval (CI) 2.045–6.256], fine calcification (OR 5.849, 95% CI 2.642–12.949), irregular margin (OR 4.366, 95% CI 2.284–8.345) and taller-than-wide shape (OR 5.199, 95% CI 2.125–12.721). The overall accuracies of hypoechogenicity, irregular margin, taller-than-wide shape and fine calcification were 0.804, 0.729, 0.705 and 0.575, respectively.
Conclusion
According to the present study, the use of ultrasonography (along with ultrasound-guided FNA) is very effective in the diagnosis, follow-up, and indication for surgery of a thyroid nodule.
Keywords: Ultrasonography, Fine-needle aspiration, Biopsy, Cytology
Introduction
A thyroid nodule is an abnormal tissue growth that causes the formation of a mass in the thyroid gland [1]. Thyroid nodules are not rare. In non-endemic areas with adequate iodine intake levels, thyroid nodules are detected in 5% of women and 1% of men. However, thyroid nodules can be identified by high-resolution ultrasonography in 19–67% of individuals who have been randomly selected from the population (especially in women and the elderly) [2]. Some patients have multiple thyroid nodules, which may cause compression symptoms in surrounding organs, or hyperthyroidism. Every nodule should raise the suspicion of malignancy, but only one out of 20 clinically diagnosed nodules is malignant [3, 4]. This figure corresponds to approximately 2–4 cases per 100,000 individuals in a population per year, accounting for only 1% of all cancers and 0.5% of all deaths due to cancer [1].
Over the past 2 decades, the method of managing a thyroid nodule has changed due to the widespread use of modern diagnostic methods such as ultrasonography and fine-needle aspiration (FNA). Despite these advances, however, there is still no full agreement on the conventional and practical strategy for investigating and diagnosing a thyroid nodule [5, 6]. Several studies have reported the utility of ultrasonography alone for distinguishing benign nodules from malignant ones [7, 8]. Fine-needle aspiration is a common method for the preoperative diagnosis of a thyroid nodule [9]. The American Thyroid Association recommends the performance of fine-needle aspiration as the preliminary step in diagnosing every thyroid nodule larger than 1 cm in diameter [10].
In most regions of Iran, due to dietary iodine deficiency, in addition to endemic goiter, thyroid nodules are considered a relatively common disease of the thyroid gland. Hence, to understand the nature of a thyroid nodule, it is necessary to make an accurate diagnostic approach, which must exclude the possibility of malignancy. Considering that the literature related to diagnostic accuracy of ultrasound in differentiating benign from malignant nodules is not extensive, especially not in our region, the aim of this study was to compare the ultrasonographic characteristics of thyroid nodules with the results of ultrasound-guided FNA.
Materials and methods
Locations and patients
This analytical cross-sectional study was conducted on patients who were referred to the radiologist for ultrasound-guided fine-needle aspiration (FNA). The study population consisted of patients who visited the clinics of Shahid Beheshti teaching hospital or private offices in the city of Babol, northern Iran, from 2015 to 2017, and were then diagnosed as having thyroid nodules in a thyroid examination by an endocrinologist or showed evidence of nodules during thyroid ultrasonography by a radiologist. The subjects were selected by census method. Patients with purely cystic nodules without solid focus, patients unwilling to perform FNA, and those with suspected cytology results (atypical diagnosis) were excluded from the study.
Ultrasound imaging and FNA
A nodule was suspected to be malign if one or more of the following sonographic features were found: hypoechogenicity, fine calcification, irregular margins, or a taller-than-wide shape. These categorizations were based on previous studies [11–13] and our preliminary analyses in this study. Ultrasound-guided FNA was performed on all of the nodules using a Samsung H60 ultrasound machine, with a 3–14 MHz linear array transducer. The aspiration was performed with the freehand technique using a 23-gauge needle attached to a 5-cc syringe, by a senior radiologist with more than 10 years of experience in performing the procedure. In the case of solid-cystic nodules, ultrasound-guided FNA was performed from the solid area of the sample nodule.
FNA cytology
The specimen was then sent to the lab on smear glass slides after being dried in the open air and after fixation with 95% alcohol. Finally, the fixed slides were stained using the Papanicolaou, Giemsa and hematoxylin and eosin methods. To reduce interobserver error, the identical interpretation, as well as the identical qualitative control of cases such as slide preparation technique, their fixation and staining, and cytohistological examination of all samples were performed by a single pathologist with more than 15 years of experience in the procedure. To control bias, the pathologist was also blinded to the ultrasound diagnosis of thyroid nodule. Two pathologists examined some of the samples when the decision-making was difficult.
Data collection
After the ultrasound-guided FNA was performed, the following demographic information was collected for patients: age; cytological results (the results of ultrasound-guided FNA); and all of the characteristics of the nodules obtained from ultrasonography, including the size of the nodule (< 2 cm or > 2 cm), nodule echogenicity (hyper, hypo, iso), the presence of calcification (negative, fine + coarse, fine, coarse, and rim), the margins of the nodule (regular, irregular, ill-defined), taller-than-wide shape (yes/no), and lymph node involvement (yes/no). These data were recorded in checklist form. Patients with malignant cytology underwent surgery.
Statistical analysis
Data were analyzed using SPSS software. Descriptive statistics were used to analyze the data. If a thyroid nodule was established as malignant in both ultrasound and cytology, it was considered a true positive (TP), and if was determined to be benign, it was considered as true negative (TN). A false positive (FP) was considered when sonography was suggestive of malignancy but histopathology was not consistent, and a false negative (FN) was considered when ultrasound did not show malignancy but cytology suggested it. Sensitivity was calculated as the proportion of TP to TP + FN, specificity as the proportion of TN to TN + FP, positive predictive value (PPV) as the proportion of TP to TP + FP, negative predictive value (NPV) as the proportion of TN to TN + FN, and accuracy as proportion of TP + TN in all patients. Regression analysis was used to assess the relation between sonographic findings and malignancy. The data were presented as odds ratio (OR) and 95% confidence interval (CI). We also used a receiver operator characteristics (ROC) analysis to estimate the ability of ultrasound to predict characteristic features of malignancy, as estimated by the area under the curve (AUC). A p value of less than 0.05 was considered significant.
Ethical issues
Written informed consent was obtained from all subjects after a full explanation of the study. The ethics committee of Babol University of Medical Sciences (code: MUBABOL.HRI.REC.1396.40) approved this protocol. The information about each patient was kept confidential.
Results
In total, 718 patients with thyroid nodules underwent ultrasound-guided FNA. Of these, 566 had a single and others had multiple thyroid nodules. Overall, 927 thyroid nodules were initially assessed, of which 29 had atypical diagnosis on cytology and were excluded from further investigation. A total of 898 nodules were finally included in the study. The number of female patients was 802 (89.3%), and the remainder was male. The mean age of the patients was reported as 44.76 ± 13.55 years old, with a range of 14–83 years. The mean nodule size was 2.17 ± 1.30 cm, in the range of 0.5–9.5 cm. The frequency and percentage of the clinical information concerning the patients’ nodules are presented in Table 1. The results of cytology also demonstrated that 59.1% of the nodules were nodular goiter, 22.3% were colloid nodule plus cystic degeneration, 6.1% were papillary thyroid carcinoma, 5.5% were hyperplastic nodular goiter, 6.3% were nodular goiter (in background lymphocytic thyroiditis), and 0.7% were Hashimoto’s thyroiditis.
Table 1.
Characteristics of the thyroid nodules
| Variables | Frequency (total = 898) | Percent |
|---|---|---|
| Nodule size (cm) | ||
| < 2 | 514 | 57.2 |
| ≥ 2 | 384 | 42.8 |
| Echogenicity | ||
| Hyperechogenicity | 617 | 68.7 |
| Hypoechogenicity | 221 | 24.6 |
| Isoechogenicity | 60 | 6.7 |
| Calcification | ||
| Negative | 634 | 70.6 |
| Fine calcification | 47 | 5.2 |
| Coarse calcification | 67 | 7.5 |
| Fine + coarse calcification | 138 | 15.4 |
| Rim calcification | 12 | 1.3 |
| Margin of nodule | ||
| Regular | 779 | 86.7 |
| Irregular | 82 | 9.1 |
| Ill defined | 37 | 4.1 |
| Taller-than-wide shape | ||
| Negative | 868 | 96.7 |
| Positive | 30 | 3.3 |
| Cytology | ||
| Benign | 843 | 93.9 |
| Malignant | 55 | 6.1 |
Table 2 shows the association between the cytology results (benign or malignant) and the sonographic features of the thyroid nodules. There were significant positive associations between malignancy and hypoechogenicity (OR 3.577, 95% CI 2.045–6.256), fine calcification (OR 5.849, 95% CI 2.642–12.949), irregular margin (OR 4.366, 95% CI 2.284–8.345), and taller-than-wide shape (OR 5.199, 95% CI 2.125–12.721).
Table 2.
Association between sonographic features and cytology results of the nodules
| Sonographic features | Benign [n (%)] | Malignant [n (%)] | p value | Odds ratio (95% confidence interval) |
|---|---|---|---|---|
| Nodule size (cm) | ||||
| < 2 | 478 (93) | 36 (7) | 1 | |
| ≥ 2 | 365 (95.1) | 19 (4.9) | 0.206 | 0.691 (0.390–1.225) |
| Echogenicity | ||||
| Hyperechogenicity | 592 (95.9) | 25 (4.1) | 1 | |
| Hypoechogenicity | 192 (86.9) | 29 (13.1) | < 0.001 | 3.577 (2.045–6.256) |
| Isoechogenicity | 59 (98.3) | 1 (1.7) | 0.375 | 0.401 (0.053–3.015) |
| Calcification | ||||
| Negative | 606 (95.6) | 28 (4.4) | 1 | |
| Fine calcification | 37 (78.7) | 10 (21.3) | < 0.001 | 5.849 (2.642–12.949) |
| Coarse calcification | 62 (92.5) | 5 (7.5) | 0.269 | 1.745 (0.651–4.682) |
| Fine + coarse calcification | 127 (92) | 11 (8) | 0.089 | 1.875 (0.910–3.864) |
| Rim calcification | 11 (91.7) | 1 (8.3) | 0.524 | 1.968 (0.245–15.779) |
| Margin of nodule | ||||
| Regular | 741 (95.1) | 38 (4.9) | 1 | |
| Irregular | 67 (81.7) | 15 (18.3) | < 0.001 | 4.366 (2.284–8.345) |
| Ill defined | 35 (94.6) | 2 (5.4) | 0.885 | 1.114 (0.258–4.807) |
| Taller-than-wide shape | ||||
| Negative | 820 (94.5) | 48 (5.5) | 1 | |
| Positive | 23 (93.9) | 7 (23.3) | < 0.001 | 5.199 (2.125–12.721) |
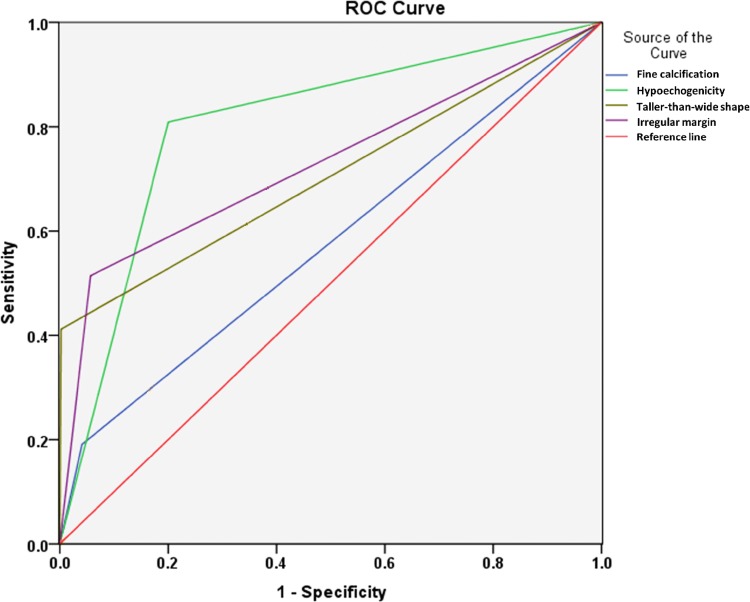
Figure 1 indicates the ROC curve for the ability of hypoechogenicity, fine calcification, irregular margin and taller-than-wide shape in predicting malignant thyroid nodule. Hypoechogenicity had the greatest predictive ability (AUC = 0.804), followed by irregular margin (AUC = 0.729), taller-than-wide shape (AUC = 0.705) and fine calcification (AUC = 0.575). The computed sensitivity, specificity, PPV, NPV and accuracy for each of these sonographic features are also shown in Table 3. Figures 2 and 3 show the ultrasound-guided FNA of a benign and a malignant nodule, respectively.
Fig. 1.
Receiver operating characteristic (ROC) curve of different sonographic features for predicting thyroid nodule malignancy
Table 3.
Diagnostic value of sonographic characteristics for malignant thyroid nodules
| Characteristics of ultrasound features | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Accuracy |
|---|---|---|---|---|---|
| Hypoechogenicity | 52.7 | 77.2 | 13.1 | 96.2 | 75.7 |
| Fine calcification | 18.2 | 95.6 | 21.3 | 94.7 | 90.9 |
| Irregular margin | 27.3 | 92.1 | 18.3 | 95.1 | 88.1 |
| Taller-than-wide shape | 12.7 | 97.3 | 23.3 | 94.5 | 92.1 |
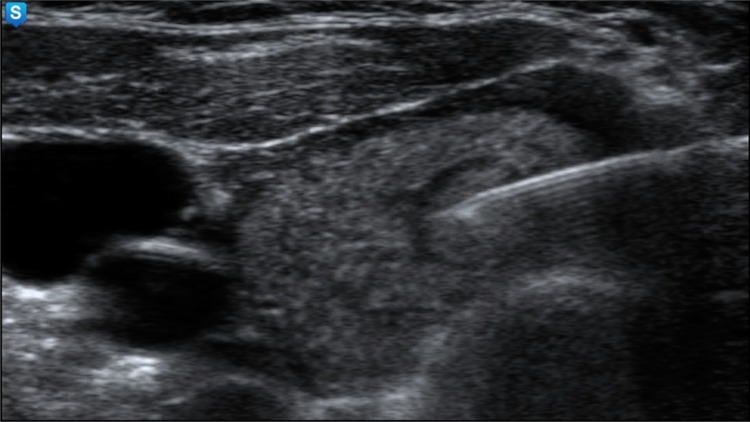
Fig. 2.
The ultrasound-guided fine-needle aspiration from an isoechoic nodule with regular margin and hypoechoic rim with 8 mm diameter in the right thyroid lobe, which was proved by cytology to be a colloid nodule
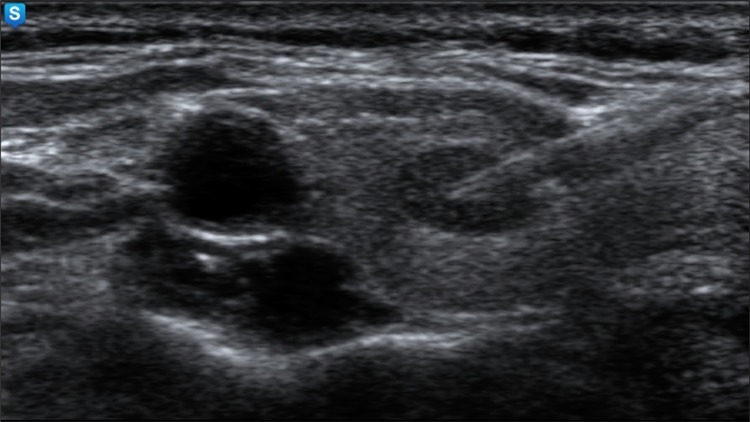
Fig. 3.
The ultrasound-guided fine-needle aspiration from a hypoechoic solid nodule without evidence of calcification, which was proved by cytology to be a papillary carcinoma
Discussion
In this study, we assessed the diagnostic accuracy of different sonographic characteristics in differentiating benign and malignant thyroid nodules. Multiple studies in recent years have shown the capability of ultrasonography as the first useful tool in diagnosing and determining the nature and location of thyroid nodules [14, 15]. Over the past three decades, the prevalence of thyroid malignancy has increased [16], but the use of FNA has led to a significant decrease in the removal of the thyroid for benign lesions. Using its results in the case of a malignancy has enabled the accurate design of initial surgery [17].
According to the findings of this study, the size of the thyroid nodules was not useful in distinguishing between benign and malignant nodules. The impact of size on thyroid cancer risk is unclear. Some researchers have suggested a positive relation between size and risk of malignancy [13, 18], but others have reported that the size of the nodule was not predictive of malignancy [19–21]. A study by Kamran et al. [13] showed a direct relation between size and malignancy, and a threshold was detected at 2 cm. Overall, thyroid nodules with size of ≥ 4 cm frequently undergo surgical removal because of concern about cancer, even those with non-malignant features [22, 23]. Accordingly, it can be argued that larger nodules are not necessarily worrying, but there is a need for additional evidence to confirm the presence of malignancy in these nodules.
In our study, hypoechogenicity was the most valuable predictive measure for malignancy, with 53% sensitivity, 77% specificity and 76% accuracy. In another study by Iran by Alam et al. [12], these rates were reported as 42, 86 and 75%, respectively, which were close to our results. Malignancy was observed in only 4% of the solid hyperechoic nodules [24]. Despite some conflicting results, most studies, similar to ours, state that a decrease in echogenicity increases the risk for thyroid nodule malignancy [24, 25].
According to our results, fine calcification was associated with high specificity but low sensitivity, which was in agreement with the previously published data [12, 26, 27]. Calcifications have been related to both benign and malignant thyroid nodules. Fine calcification or microcalcification (< 1 mm diameter) were associated with malignancy, but there were conflicting data about coarse calcification or macrocalcification (> 1 mm diameter) [28–31]. Microcalcification has been stated to be highly specific (89–97%) but not sensitive (about 20–33%) for papillary thyroid carcinoma [12, 32], which is in agreement with our results. However, some other results showed a higher rate of sensitivity [33].
Microcalcifcations correspond pathologically to papillary cancer with calcified psammoma bodies [34, 35]. On the other hand, peripheral rim or eggshell calcification and macrocalcified thyroid nodules are considered indicators of a benign nodule [36, 37]. Altogether, the studies mostly consider fine calcification a predictor for malignancy, but additional studies are needed to confirm the relationship between coarse calcification and malignancy.
Analyses also showed that irregular margin and taller-than-wide shape were other ultrasonographic features that predicted thyroid malignancy with high specificity but low sensitivity. These findings were inconsistent with other studies [8, 25, 38]. Irregular spiculated or microlobulated margins are stated to be predictors of malignancy, but an ill-defined margin can be associated with both benign and malignant nodules [24, 39]. Nodules with a taller-than-wide shape have also been shown to be highly associated with malignancy, as in our study. The specificity of this association was 97% in our study and around 90% in previous studies [39, 40]. When the anteroposterior diameter is more than the transverse diameter, the nodule is described as “taller-than-wide” in shape. Ren et al. [41] reported that a taller-than-wide shape is a very useful predictor of papillary thyroid carcinoma in small nodules.
A limitation of our study was the lack of access to the results of repeat FNA in some patients with the atypical diagnosis. Further, the pathological results of malignant thyroids of patients who underwent surgery were not collected. Therefore, we suggest designing new studies to compare the sonographic and FNA results with pathological findings. In addition, we propose considering elastosonography results and other characteristics of malignancy, such as growth of the nodule with capsule overcoming, lymph nodes with altered structure (e.g., rounded, without hilum, with multiple vascular poles and with suspect secondary microcalcifications) in further surveys. Multicenter studies are recommended to enable more generalizable results. Further studies also need to be done to assess the association between the number of microcalcifications found in a thyroid nodule and the malignancy.
Conclusion
The results of this study showed that ultrasound features such as hypoechogenicity, fine calcification, irregular margin and taller-than-wide shape are the potential indicators of malignant thyroid nodules and useful in their diagnosis. It can be stated that the use of ultrasonography, along with ultrasound-guided FNA, is very effective in the diagnosis of, follow-up regarding, and planning of surgery for thyroid nodules.
Acknowledgements
We would like to thank the Vice Chancellor for the Research of Babol University of Medical Sciences for financial support. We are also thankful to Dr. Kourosh Movagharnejad for his help in cytological assessments.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This study was funded by the Vice Chancellor for the Research of Babol University of Medical Sciences (grant number 9603612).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am. 2010;43(2):229–238. doi: 10.1016/j.otc.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351(17):1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 3.Schiro AJ, Pinchot SN, Chen H, Sippel RS. Clinical efficacy of fine-needle aspiration biopsy of thyroid nodules in males. J Surg Res. 2010;159(2):645–650. doi: 10.1016/j.jss.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association AT, Hyperthyroidism AAoCETo. Thyrotoxicosis OCo. Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21(6):593–646. doi: 10.1089/thy.2010.0417. [DOI] [PubMed] [Google Scholar]
- 5.Sakorafas GH. Thyroid nodules; interpretation and importance of fine-needle aspiration (FNA) for the clinician–practical considerations. Surg Oncol. 2010;19(4):e130–e139. doi: 10.1016/j.suronc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Stanek-Widera A, Biskup-Frużyńska M, Zembala-Nożyńska E, Śnietura M. Lange D (2016) The diagnosis of cancer in thyroid fine needle aspiration biopsy.: surgery, repeat biopsy or specimen consultation? Pol J Pathol. 2016;67(1):19–23. doi: 10.5114/pjp.2016.59225. [DOI] [PubMed] [Google Scholar]
- 7.Lee YH, Baek JH, Jung SL, Kwak JY, Kim J-H, Shin JH, et al. Ultrasound-guided fine needle aspiration of thyroid nodules: a consensus statement by the Korean Society of Thyroid Radiology. Korean J Radiol. 2015;16(2):391–401. doi: 10.3348/kjr.2015.16.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ram N, Hafeez S, Qamar S, Hussain SZ, Asghar A, Anwar Z, et al. Diagnostic validity of ultrasonography in thyroid nodules. J Pak Med Assoc. 2015;65(8):875–878. [PubMed] [Google Scholar]
- 9.Feldkamp J, Führer D, Luster M, Musholt TJ, Spitzweg C, Schott M. Fine needle aspiration in the investigation of thyroid nodules: indications, procedures and interpretation. Dtsch Arztebl Int. 2016;113(20):353. doi: 10.3238/arztebl.2016.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HaugenBryan R, AlexanderErik K, BibleKeith C, DohertyGerard M, MandelSusan J, NikiforovYuri E, et al. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith-Bindman R, Lebda P, Feldstein VA, Sellami D, Goldstein RB, Brasic N, et al. Risk of thyroid cancer based on thyroid ultrasound imaging characteristics: results of a population-based study. JAMA Intern Med. 2013;173(19):1788–1795. doi: 10.1001/jamainternmed.2013.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alam T, Khattak YJ, Beg M, Raouf A, Azeemuddin M, Khan AA. Diagnostic accuracy of ultrasonography in differentiating benign and malignant thyroid nodules using fine needle aspiration cytology as the reference standard. Asian Pac J Cancer Prev. 2014;15(22):10039–10043. doi: 10.7314/apjcp.2014.15.22.10039. [DOI] [PubMed] [Google Scholar]
- 13.Kamran SC, Marqusee E, Kim MI, Frates MC, Ritner J, Peters H, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98(2):564–570. doi: 10.1210/jc.2012-2968. [DOI] [PubMed] [Google Scholar]
- 14.Xie C, Cox P, Taylor N, LaPorte S. Ultrasonography of thyroid nodules: a pictorial review. Insights Imaging. 2016;7(1):77–86. doi: 10.1007/s13244-015-0446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito Y, Amino N, Yokozawa T, Ota H, Ohshita M, Murata N, et al. Ultrasonographic evaluation of thyroid nodules in 900 patients: comparison among ultrasonographic, cytological, and histological findings. Thyroid. 2007;17(12):1269–1276. doi: 10.1089/thy.2007.0014. [DOI] [PubMed] [Google Scholar]
- 16.Konturek A, Barczyński M, Stopa M, Nowak W. Trends in prevalence of thyroid cancer over three decades: a retrospective cohort study of 17,526 surgical patients. World J Surg. 2016;40(3):538–544. doi: 10.1007/s00268-015-3322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams BA, Bullock MJ, Trites JR, Taylor SM, Hart RD. Rates of thyroid malignancy by FNA diagnostic category. J Otolaryngol Head Neck Surg. 2013;42(1):61. doi: 10.1186/1916-0216-42-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raparia K, Min SK, Mody DR, Anton R, Amrikachi M. Clinical outcomes for “suspicious” category in thyroid fine-needle aspiration biopsy: patient’s sex and nodule size are possible predictors of malignancy. Arch Pathol Lab Med. 2009;133(5):787–790. doi: 10.5858/133.5.787. [DOI] [PubMed] [Google Scholar]
- 19.Mendelson AA, Tamilia M, Rivera J, Hier MP, Sherman M, Garfield N, et al. Predictors of malignancy in preoperative nondiagnostic biopsies of the thyroid. J Otolaryngol Head Neck Surg. 2009;38(3):395–400. [PubMed] [Google Scholar]
- 20.Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91(9):3411–3417. doi: 10.1210/jc.2006-0690. [DOI] [PubMed] [Google Scholar]
- 21.Rahimi M, Farshchian N, Rezaee E, Shahebrahimi K, Madani H. To differentiate benign from malignant thyroid nodule comparison of sonography with FNAC findings. Pak J Med Sci. 2013;29(1):77–80. doi: 10.12669/pjms.291.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wharry LI, McCoy KL, Stang MT, Armstrong MJ, LeBeau SO, Tublin ME, et al. Thyroid nodules (≥ 4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014;38(3):614–621. doi: 10.1007/s00268-013-2261-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Kim NK, Oh YL, Kim HJ, Kim SY, Chung JH, et al. The validity of ultrasonography-guided fine needle aspiration biopsy in thyroid nodules 4 cm or larger depends on ultrasonography characteristics. Endocrinol Metab (Seoul). 2014;29(4):545–552. doi: 10.3803/EnM.2014.29.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anil G, Hegde A, Chong FHV. Thyroid nodules: risk stratification for malignancy with ultrasound and guided biopsy. Cancer Imaging. 2011;11(1):209–223. doi: 10.1102/1470-7330.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Lei K-R, He Y-P, Li X-L, Ren W-W, Zhao C-K, et al. Malignancy risk stratiication of thyroid nodules: comparisons of four ultrasound thyroid imaging reporting and data systems in surgically resected nodules. Sci Rep. 2017;7(1):11560. doi: 10.1038/s41598-017-11863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim E-K, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. Am J Roentgenol. 2002;178(3):687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 27.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 28.Kim BK, Choi YS, Kwon HJ, Lee JS, Heo JJ, Han YJ, et al. Relationship between patterns of calcification in thyroid nodules and histopathologic findings. Endocr J. 2013;60(2):155–160. doi: 10.1507/endocrj.ej12-0294. [DOI] [PubMed] [Google Scholar]
- 29.Berker D, Isik S, Ozuguz U, Tutuncu YA, Kucukler K, Akbaba G, et al. Prevalence of incidental thyroid cancer and its ultrasonographic features in subcentimeter thyroid nodules of patients with hyperthyroidism. Endocrine. 2011;39(1):13–20. doi: 10.1007/s12020-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 30.Park YJ, Kim J-A, Son EJ, Youk JH, Kim E-K, Kwak JY, et al. Thyroid nodules with macrocalcification: sonographic findings predictive of malignancy. Yonsei Med J. 2014;55(2):339–344. doi: 10.3349/ymj.2014.55.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrone L, Mannucci E, De MF, Parenti G, Biagini C, Panconesi R, et al. A simple ultrasound score for the identification of candidates to fine needle aspiration of thyroid nodules. J Endocrinol Invest. 2012;35(8):720–724. doi: 10.3275/7978. [DOI] [PubMed] [Google Scholar]
- 32.Wang N, Xu Y, Ge C, Guo R, Guo K. Association of sonographically detected calcification with thyroid carcinoma. Head Neck. 2006;28(12):1077–1083. doi: 10.1002/hed.20481. [DOI] [PubMed] [Google Scholar]
- 33.Gu W-J, Yan H-X, Luo Y-K, Wang F-L, Yang G-Q, Guo Q-H, et al. Characterization of papillary thyroid microcarcinomas using sonographic features in malignant papillary thyroid cancer: a retrospective analysis. Med (Baltim) 2015;94(21):e841-e. doi: 10.1097/MD.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyo J-S, Kang G, Kim D-H, Park C, Kim JH, Sohn JH. The prognostic relevance of psammoma bodies and ultrasonographic intratumoral calcifications in papillary thyroid carcinoma. World J Surg. 2013;37(10):2330–2335. doi: 10.1007/s00268-013-2107-5. [DOI] [PubMed] [Google Scholar]
- 35.Pusztaszeri MP, Sadow PM, Faquin WC. Images in endocrine pathology: psammomatoid calcifications in oncocytic neoplasms of the thyroid, a potential pitfall for papillary carcinoma. Endocr Pathol. 2013;24(4):246–247. doi: 10.1007/s12022-013-9242-2. [DOI] [PubMed] [Google Scholar]
- 36.Chen C-Y, Tseng H-S, Lee C-H, Chan W. Primary squamous cell carcinoma of the thyroid gland with eggshell calcification: sonographic and computed tomographic findings. J Ultrasound Med. 2010;29(11):1667–1670. doi: 10.7863/jum.2010.29.11.1667. [DOI] [PubMed] [Google Scholar]
- 37.Yaturu S, Rainer L. Thyroid nodule with eggshell calcification and oncocytic thyroid cancer. Med Sci Monit. 2010;16(3):CS25–CS28. [PubMed] [Google Scholar]
- 38.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260(3):892–899. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- 39.Moon W-J, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011;12(1):1–14. doi: 10.3348/kjr.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon SJ, Yoon DY, Chang SK, Seo YL, Yun EJ, Choi CS, et al. “Taller-than-wide sign” of thyroid malignancy: comparison between ultrasound and CT. AJR Am J Roentgenol. 2010;194(5):W420–W424. doi: 10.2214/AJR.09.3376. [DOI] [PubMed] [Google Scholar]
- 41.Ren J, Liu B, Zhang L-L, Li H-Y, Zhang F, Li S, et al. A taller-than-wide shape is a good predictor of papillary thyroid carcinoma in small solid nodules. J Ultrasound Med. 2015;34(1):19–26. doi: 10.7863/ultra.34.1.19. [DOI] [PubMed] [Google Scholar]