Abstract
Introduction
Keloids are a prevalent chronic skin disorder with significant psychosocial morbidity. Intralesional corticosteroid injections are the first-line treatment but are painful and require repeated injections by medical professionals. Dissolving microneedles are a novel method of cutaneous drug delivery that induces minimal/no pain and can be self-administered. The objective of the study was to evaluate the efficacy and safety of triamcinolone-embedded dissolving microneedles in treatment of keloids.
Methods
This was a single-blind, intra-individual controlled two-phase clinical trial of 8-week duration each. Two keloids per subject were selected for (1) once-daily 2-min application with microneedles for 4 weeks, followed by no treatment for the next 4 weeks, or (2) non-intervention as control. Primary outcome was change in keloid volume as assessed by a high-resolution 3D scanner.
Results
There was significant reduction in keloid volume compared with controls after 4 weeks of treatment. This reduction was greater with a higher dosage of triamcinolone used.
Conclusions
Once-daily application of dissolving triamcinolone-embedded microneedles significantly reduced the volume of keloids. The treatment was safe, can be self-administered and can serve as an alternative for patients unsuitable for conventional treatments.
Trial Registration
Trial Registry: Health Science Authority (Singapore) Clinical Trials Register Registration number: 2015/00440.
Keywords: Keloids, Microneedles, Scars
Introduction
Keloids are a common skin disorder in a younger age group [1] and in certain racial groups, in particular those of Afro-Caribbean [2, 3] or Asian [4] descent. The pathophysiology is poorly understood and is likely multifactorial [2]. Itch and pain are common symptoms that affect up to 80% of patients [5]. The undesirable, disfiguring appearance of keloids can also lead to emotional distress and psychosocial burden [6, 7].
Current therapeutic options are limited and pose significant problems for patients. The first-line option is multiple repeated intralesional corticosteroid injections by a health professional. The treatment is operator-dependent, painful (because of keloid hypersensitivity [5] and the need to push the triamcinolone suspension into a dense nodule) and inconvenient, thereby precluding therapy in many patients [8]. In a previous study, the mean pain score of conventional triamcinolone injection was 7.9/10 with 33% of patients giving up treatment within ten injections because of pain and lack of immediate improvement [9, 10]. Moreover, there is a high recurrence rate of up to 50% when treatment ceases [11]. Other commonly employed therapies include cryotherapy, radiotherapy and occlusive dressings, which have poor efficacy rates, prominent side effects or a high rate of recurrence [11–14].
Microneedles are micron-sized needles typically ranging from 150 to 800 μ in length. Different types of microneedles exist, such as solid, coated, hollow and dissolving microneedles, each with distinct advantages and disadvantages [15, 16]. Microneedles are able to effectively penetrate the stratum corneum without strongly stimulating the pain-transmitting nerve fibres residing in the deeper dermis. Microneedles are increasingly being used in the fields of drug and vaccine delivery, interstitial fluid extraction and cosmetic applications such as skin rejuvenation and scars [17, 18] as they are minimally painful, safe and can even be self-administered.
We hypothesize that dissolving triamcinolone-embedded microneedles can be an effective and safe alternative for treatment of keloids. We embedded triamcinolone acetonide, the corticosteroid frequently used for intralesional keloid injections, into microneedles composed of hyaluronic acid, a naturally occurring ground substance in the dermis. We performed preclinical studies in cell cultures, mice, guinea pigs and rabbits, comprising sterility, cytotoxicity, systemic toxicity, skin irritation, delay contact sensitization and phototoxicity tests, which demonstrated safety of this pharmaceutical composition. In this clinical trial, we aim to evaluate the efficacy and safety of triamcinolone-embedded dissolving microneedles in reducing the volume of keloids. Secondarily, we aim to determine whether keloidal pain and itch scores vary with the treatment.
Methods
This was a single-blind intra-individual controlled two-phase clinical trial of 8-week duration each, conducted at a national dermatology institution. This study was approved by the Health Sciences Authority, Singapore, and the National Healthcare Group’s Ethics Review Board. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Subjects
The subjects were patients who fulfilled the inclusion and exclusion criteria set out in the protocol. In each subject, two similar keloids approximately 1–2 cm in size (corresponding to the size of the microneedle patch) were identified and were allocated to either microneedle treatment or control arms. The keloid that was located in an anatomical region within easier reach by the subject (either on the chest, arms or shoulders) was allocated for self-treatment using microneedles. A non-interventional control arm was allocated as keloids are known to spontaneously change in size over time [19]. All patients except one had received prior treatment for keloids with intra-lesional triamcinolone with a washout period of at least 6 months.
Intervention
Subjects were taught to perform self-application of the microneedles at a dosage of one patch per day for 30 days. A spring applicator delivering the microneedles into the skin at a speed of 2 m/s and a force of 1.0–1.6 N was used, such that the administration of the microneedles was repeatable and consistent throughout the trial (Fig. 1). The skin surface was sterilized with 70% ethanol/isopropyl alcohol solution and the triamcinolone-embedded microneedles were applied for 2 min. This duration was used as we had previously determined that application for 2 min in the skin allows full delivery of the drug.
Fig. 1.
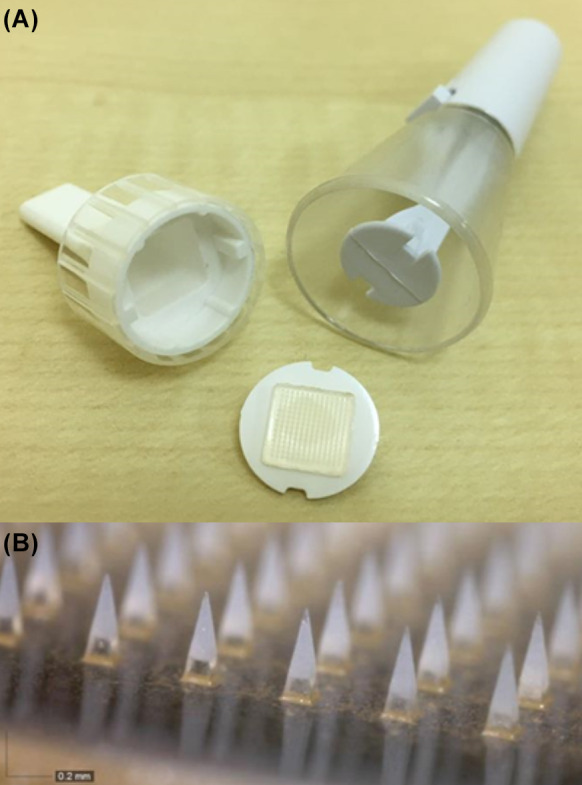
a Dissolving triamcinolone-embedded hyaluronic acid microneedle patch (bottom). A holder (left) is used to affix the patch to a spring applicator (right). The latter is used so that the force delivering the microneedles into the skin is constant throughout the trial. b Close-up view of hyaluronic acid microneedles with triamcinolone acetonide embedded at the sharp ends
Drug-Eluding Dissolving Microneedles
The hyaluronic acid microneedles (Micropoint, Singapore) were 600 µm in height arranged in 14 × 14 arrays. Triamcinolone acetonide (Yung Shin Pharm., Taiwan) was embedded into 50% of the microneedles from the sharp end. In the first phase of the study, 0.025 mg of triamcinolone was loaded per microneedle patch, which amounts to a cumulative dose of 0.750 mg over 30 days. In the second phase, a fourfold increase in dosage was used: 0.1 mg of triamcinolone was loaded per patch, which amounts to a cumulative dose of 3.0 mg over 30 days. This dosage of 3.0 mg per month matches the expected dosage of conventional monthly intralesional corticosteroid injection.
Outcome Measures
Assessments were performed at baseline (visit 1), after 4 weeks of treatment (visit 2) and after another 4 weeks of non-treatment (visit 3). The primary outcome measure was an objective volumetric assessment using a high-resolution three-dimensional scanner, which has a 3D resolution of 0.1 mm (Space Spider, Artec 3D, Luxembourg). Three measurements were taken for each keloid at each reading and the mean volume was used in the analysis.
The secondary outcome measures were subjective assessments, comprising the average pain and itch scores the subjects experienced in their keloids over the preceding 1 week, and the Vancouver Scar Scale (VSS) administered by a blinded evaluator. The pain and itch scores were assessed using numerical rating scales ranging 0–10. The VSS is a clinical scoring scale assessing the severity of scars using the parameters of height, pliability, vascularity and pigmentation [20, 21]. Throughout this study, the VSS was evaluated by a single investigator to avoid inter-individual variability in scoring.
At the end of the trial, subjects were asked to compare the convenience of use, perceived efficacy and overall preference between the microneedles and their previous treatment using intralesional corticosteroid injections.
Monitoring
To monitor for compliance and proper administration of microneedles, subjects were to return the used microneedles and we checked that dissolution of the sharp ends of microneedles had occurred.
At baseline and after 4 weeks of treatment, we used high-definition optical coherence tomography (HD-OCT, Skintell, Agfa, Belgium) to image each of the keloids. At baseline, the mean 3D thickness of the epidermis was measured: over a scanned surface area of 1.5 mm2, the 3D image volume was segmented into the epidermis and dermis using an algorithm, and the mean thickness of the undulating epidermal layer was calculated. At 4 weeks, the depths of microneedle penetration into the skin were determined using the en face images in HD-OCT, and the mean at two sites of microneedle-penetration per keloid was calculated.
Safety Evaluation
Safety and tolerability to the microneedles was monitored throughout the study. At each follow-up, subjects were evaluated for side effects. The keloids were clinically screened by a dermatologist for signs of infection and contact dermatitis.
Statistical Analysis
We calculated that 25 patients were required to achieve ≥ 90% power in detecting a 30% change in keloid size. Multi-level mixed effect models were used to test the difference in keloid volume, pain score, itch score and VSS score across the visits and between the treatment groups, adjusting for baseline characteristics including age, gender, race and size of keloid at baseline. p < 0.05 was considered statistically significant. Statistics was generated using STATA version 14.0. All subjects who started on the intervention were included in this intention-to-treat analysis.
Results
Phase One
A total of 28 patients, 24 (85.7%) males and four (14.3%) females, were recruited into the study. Twenty-seven subjects completed the study and one defaulted without starting treatment. The mean duration of keloids was 4.77 years. The median baseline volume of the control keloids (49.3 mm3, range 2.5–855.0 mm3) was higher than that of the microneedle-treated keloids (30.7 mm3, range 1.4–1467.3 mm3). Imaging and measurement using HD-OCT revealed the mean depth of microneedle penetration to be 96.2 µm (range 64.6–154.2 µm) from the skin surface, at the dermal layer. In comparison, the mean 3D epidermal thickness of the keloids at baseline was 29.0 µm (range 15.4–43.4 µm).
After 4 weeks of microneedle treatment, the mean keloid volume reduced significantly from 117.6 mm3 at visit 1 to 108.5 mm3 at visit 2 (mean change: − 9.1 ± 15.4 mm3, p = 0.001) (Table 1). This change was significantly greater compared with the change in the control keloids (p = 0.019). After the subsequent 4 weeks without treatment (visit 3), the mean volume of treated keloids significantly increased in size (p = 0.017), which was not significantly different from the size at baseline (p = 0.312).
Table 1.
Keloid size (mm3) comparing control (non-intervention) and microneedle-treated keloids at baseline (visit 1), after 4 weeks of treatment (visit 2) and a subsequent 4 weeks of no treatment (visit 3)
| Keloid size (mm3) | Visit 1 | Visit 2 | Visit 3 | Change from visit 1 to 2 | Change from visit 2 to 3 | Change from visit 1 to 3 |
|---|---|---|---|---|---|---|
| Phase one (n = 27) | ||||||
| Control keloids | ||||||
| Mean ± SD | 148.6 ± 204.4 | 155.7 ± 221.9 | 155.8 ± 223.9 | 7.2 ± 25.7 | 0.05 ± 11.7 | 7.2 ± 29.0 |
| Median (min, max) | 49.3 (2.5, 855.0) | 44.0 (1.5, 979.0) | 48.0 (2.5, 1001.0) | 0.7 (− 23.7, 124) | − 0.33 (− 29.7, 24.7) | 0.3 (− 11.3, 146) |
| p value for comparing visits within the control group | 0.104 | 0.983 | 0.101 | |||
| Microneedle-treated keloids | ||||||
| Mean ± SD | 117.6 ± 280.8 | 108.5 ± 271.9 | 114.9 ± 285.3 | − 9.1 ± 15.4 | 6.4 ± 14.2 | − 2.7 ± 12.4 |
| Median (min, max) | 30.7 (1.4, 1467.3) | 25.7 (1.6, 1419.3) | 34.3 (1.1, 1488.0) | − 5.0 (− 56.7, 10.3) | 2.2 (− 4.3, 68.7) | − 1.7 (− 43.7, 20.7) |
| p value for comparing visits within the microneedle group | 0.001 | 0.017 | 0.312 | |||
| Phase two (n = 17) | ||||||
| Control keloids | ||||||
| Mean ± SD | 182.0 ± 330.1 | 188.6 ± 376.1 | 186.8 ± 368.4 | 6.7 ± 58.3 | − 1.9 ± 11.7 | 4.8 ± 55.1 |
| Median (min, max) | 66.3 (7.0, 1341.2) | 58.2 (5.4, 1563.2) | 57.0 (7.5, 1537.5) | − 1.0 (− 67.7, 222.0) | 1.3 (− 27.9, 14.1) | 0.5 (− 95.5, 196.3) |
| p value for comparing visits within the control group | 0.545 | 0.498 | 0.663 | |||
| Microneedle-treated keloids | ||||||
| Mean ± SD | 171.6 ± 432.6 | 149.6 ± 418.2 | 172.3 ± 448.8 | − 22.1 ± 28.0 | 22.8 ± 38.5 | 0.7 ± 25.1 |
| Median (min, max) | 35.7 (8.4, 1826.9) | 28.2 (3.0, 1761.2) | 38.8 (7.2, 1892.3) | − 7.2 (− 75.7, 19.8) | 5.9 (− 1.9, 131.2) | 0.6 (− 50.2, 65.4) |
| p value for comparing visits within the microneedle group | 0.001 | 0.006 | 0.917 | |||
| p value for comparing visits between control and microneedle groups | ||||||
| Phase one control versus phase one microneedle group | 0.019 | 0.125 | 0.090 | |||
| Phase two control versus phase two microneedle group | 0.035 | 0.001 | 0.766 | |||
| p value for comparing microneedle groups between phase one and phase two | ||||||
| Phase one microneedle versus phase two microneedle group | 0.029 | 0.014 | 0.400 | |||
Significance level of p ≤ 0.05 are shown in underline
Multi-level mixed effect models were used to test the difference in the keloid size across the visits and between the treatment groups, adjusting for baseline characteristics such as age, gender, race and size of keloid at baseline (visit 1)
The mean baseline pain and itch scores were low for both the microneedle-treated (pain 0.9, itch 1.8) and control (pain 1.2, itch 2.2) keloids. There was a significant reduction in pain scores in the microneedle-treated keloids after 4 weeks of treatment (p = 0.007) and this effect persisted at 8 weeks from baseline (p = 0.035) (Table 2). There were no significant differences in itch scores and the investigator-graded VSS scores between the microneedle-treated and control keloids (Table 3).
Table 2.
Pain score comparing control (non-intervention) and microneedle-treated keloids at baseline (visit 1), after 4 weeks of treatment (visit 2) and a subsequent 4 weeks of no treatment (visit 3)
| Pain score | Visit 1 | Visit 2 | Visit 3 | Change from visit 1 to 2 | Change from visit 2 to 3 | Change from visit 1 to 3 |
|---|---|---|---|---|---|---|
| Phase one (n = 27) | ||||||
| Control keloids | ||||||
| Mean ± SD | 1.2 ± 2.0 | 1.0 ± 1.8 | 1.0 ± 1.9 | − 0.2 ± 1.0 | − 0.04 ± 0.8 | − 0.2 ± 1.3 |
| Median (min, max) | 0.0 (0.0, 6.0) | 0.0 (0.0, 5.0) | 0.0 (0.0, 7.0) | 0.0 (− 5.0, 1.0) | 0.0 (− 2.0, 3.0) | 0.0 (− 5.0, 3.0) |
| p value for comparing visits within the control group | 0.344 | 0.796 | 0.256 | |||
| Microneedle-treated keloids | ||||||
| Mean ± SD | 0.9 ± 1.5 | 0.3 ± 0.8 | 0.4 ± 1.1 | − 0.5 ± 1.1 | − 0.1 ± 0.9 | − 0.4 ± 1.1 |
| Median (min, max) | 0.0 (0.0, 5.0) | 0.0 (0.0, 3.0) | 0.0 (0.0, 4.0) | 0.0 (− 4.0, 0.0) | 0.0 (− 2.0, 4.0) | 0.0 (− 4.0, 1.0) |
| p value for comparing visits within the microneedle group | 0.007 | 0.509 | 0.035 | |||
| Phase two (n = 17) | ||||||
| Control keloids | ||||||
| Mean ± SD | 0.6 ± 1.8 | 0.7 ± 1.8 | 0.4 ± 1.2 | 0.1 ± 0.5 | − 0.3 ± 0.8 | − 0.2 ± 0.7 |
| Median (min, max) | 0.0 (0.0, 7.0) | 0.0 (0.0, 7.0) | 0.0 (0.0, 4.0) | 0.0 (0.0, 2.0) | 0.0 (− 3.0, 0.0) | 0.0 (− 3.0, 0.0) |
| p value for comparing visits within the control group | 0.477 | 0.141 | 0.286 | |||
| Microneedle-treated keloids | ||||||
| Mean ± SD | 0.4 ± 1.7 | 0.1 ± 0.5 | 0.5 ± 1.9 | − 0.3 ± 1.2 | 0.4 ± 1.5 | 0.1 ± 0.2 |
| Median (min, max) | 0.0 (0.0, 7.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 8.0) | 0.0 (− 5.0, 0.0) | 0.0 (0.0, 6.0) | 0.0 (0.0, 1.0) |
| p value for comparing visits within the microneedle group | 0.187 | 0.157 | 0.792 | |||
| p value for comparing visits between control and microneedle groups | ||||||
| Phase one control versus phase one microneedle group | 0.230 | 0.551 | 0.551 | |||
| Phase two control versus phase two microneedle group | 0.058 | 0.025 | 0.161 | |||
| p value for comparing microneedle groups between phase one and phase two | ||||||
| Phase one microneedle versus phase two microneedle group | 0.515 | 0.105 | 0.055 | |||
Significance level of p ≤ 0.05 are shown in underline
Table 3.
Itch score comparing control (non-intervention) and microneedle-treated keloids at baseline (visit 1), after 4 weeks of treatment (visit 2), and a subsequent 4 weeks of no treatment (visit 3)
| Itch score | Visit 1 | Visit 2 | Visit 3 | Change from visit 1 to 2 | Change from visit 2 to 3 | Change from visit 1 to 3 |
|---|---|---|---|---|---|---|
| Phase one (n = 27) | ||||||
| Control keloids | ||||||
| Mean ± SD | 2.2 ± 2.8 | 1.9 ± 2.7 | 1.8 ± 2.5 | − 0.3 ± 1.2 | − 0.1 ± 1.2 | − 0.4 ± 1.7 |
| Median (min, max) | 0.0 (0.0, 8.0) | 0.0 (0.0, 8.0) | 0.0 (0.0, 8.0) | 0.0 (− 5.0, 1.5) | 0.0 (− 4.0, 3.0) | 0.0 (− 5.0, 3.0) |
| p value for comparing visits within the control group | 0.236 | 0.620 | 0.109 | |||
| Microneedle-treated keloids | ||||||
| Mean ± SD | 1.8 ± 2.3 | 1.2 ± 1.7 | 1.2 ± 1.7 | − 0.6 ± 1.6 | 0.0 ± 2.2 | − 0.6 ± 1.7 |
| Median (min, max) | 1.0 (0.0, 8.0) | 0.0 (0.0, 6.0) | 0.0 (0.0, 7.5) | 0.0 (− 6.0, 2.0) | 0.0 (− 6.0, 7.5) | 0.0 (− 6.5, 2.0) |
| p value for comparing visits within the microneedle group | 0.073 | 1.000 | 0.073 | |||
| Phase two (n = 17) | ||||||
| Control keloids | ||||||
| Mean ± SD | 1.8 ± 2.3 | 2.2 ± 2.7 | 1.4 ± 2.2 | 0.4 ± 2.0 | − 0.8 ± 1.5 | − 0.4 ± 1.8 |
| Median (min, max) | 0.0 (0.0, 7.0) | 1.0 (0.0, 8.0) | 0.0 (0.0, 7.0) | 0.0 (− 2.0, 8.0) | 0.0 (− 4.0, 2.0) | 0.0 (− 3.5, 4.0) |
| p value for comparing visits within the control group | 0.329 | 0.036 | 0.402 | |||
| Microneedle-treated keloids | ||||||
| Mean ± SD | 1.7 ± 2.3 | 1.0 ± 1.9 | 0.9 ± 1.9 | − 0.7 ± 1.1 | − 0.1 ± 0.6 | − 0.8 ± 1.3 |
| Median (min, max) | 0.0 (0.0, 7.5) | 0.0 (0.0, 7.5) | 0.0 (0.0, 7.5) | 0.0 (− 3.5, 1.0) | 0.0 (− 1.0, 1.5) | 0.0 (− 3.5, 1.5) |
| p value for comparing visits within the microneedle group | 0.005 | 0.507 | 0.002 | |||
| p value for comparing visits between control and microneedle groups | ||||||
| Phase one control versus phase one microneedle group | 0.394 | 0.761 | 0.683 | |||
| Phase two control and phase two microneedle group | 0.005 | 0.050 | 0.275 | |||
| p value for comparing microneedle groups between phase one and phase two | ||||||
| Phase one microneedle versus phase two microneedle group | 0.489 | 0.395 | 0.811 | |||
Significance level of p ≤ 0.05 are shown in underline
Phase Two
Phase two was conducted with a 6-month washout period after completion of phase one. Seventeen subjects from phase one continued participation in phase two, and they comprised 15 (88.2%) males and 2 (11.8%) females. All 17 subjects completed the study. Similar to phase one, the median baseline volume of the control keloids (66.3 mm3, range 7.0–1341.2) was higher than that of the microneedle-treated keloids (35.7 mm3, range 8.4–1826.9).
After 4 weeks of microneedle treatment, the mean keloid volume reduced significantly from 171.6 mm3 at visit 1 to 149.6 mm3 at visit 2 (mean change: − 22.1 ± 28.0 mm3, p = 0.001) (Table 1). This change was significantly greater than the change in the control keloids (p = 0.035) (Figs. 2, 3). After the subsequent 4 weeks without treatment (visit 3), the mean keloid volume significantly increased in size (p = 0.006), which was not significantly different from the size at baseline (p = 0.917).
Fig. 2.
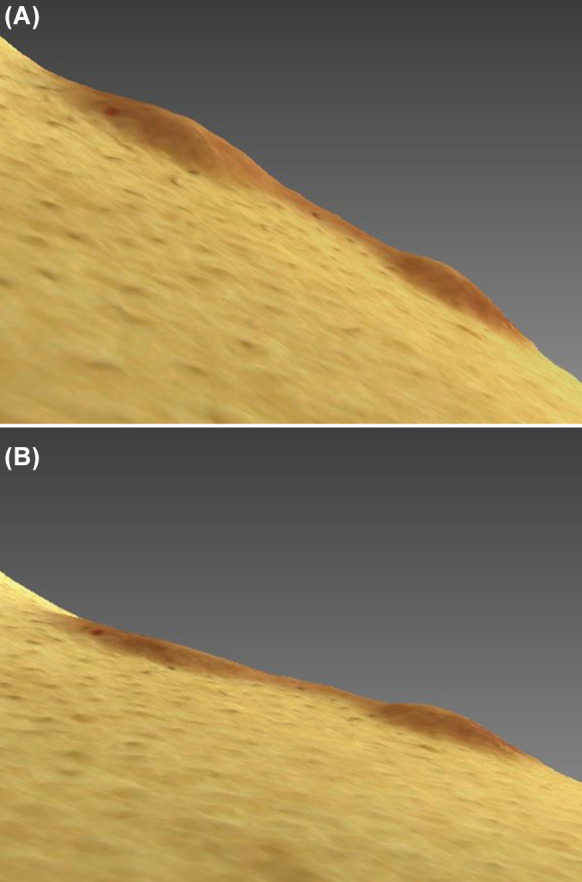
Scanning photos with a three-dimensional camera of a microneedle-treated keloid (left) and a control keloid (right) in a subject with the two keloids in close proximity. Compared with baseline (a), the volume of the microneedle-treated keloid was significantly lower after 4 weeks of treatment (b)
Fig. 3.
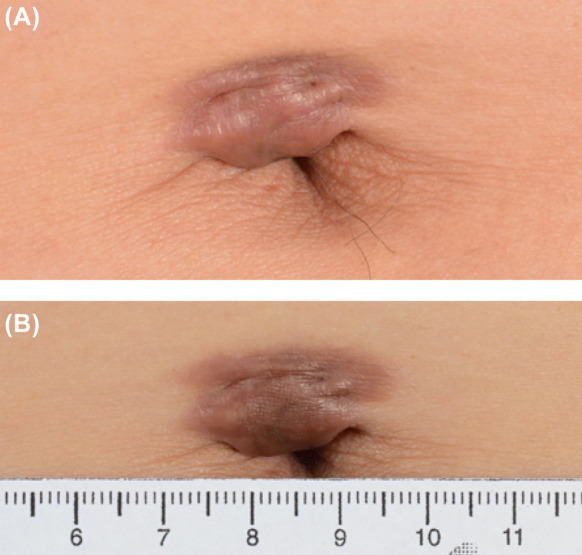
Representative clinical photos of a keloid at the umbilicus at baseline (a) and after 4 weeks of treatment with triamcinolone-embedded dissolving microneedles (b). Erythaema and volume of the keloid were reduced, associated with wrinkling on the skin surface and enlargement of the umbilical opening
The mean baseline pain and itch scores were low for both the microneedle-treated (pain 0.4, itch 1.7) and control (pain 0.6, itch 1.8) keloids. There was a significant reduction in itch scores in the microneedle-treated keloids after 4 weeks of treatment (p = 0.005) and this effect persisted at 8 weeks from baseline (p = 0.002) (Table 3). This reduction in itch score after treatment for 4 weeks was also significantly more than for the controls (p = 0.005).
There was no significant change in the pain score for either the microneedle-treated or control keloids. For the investigator-graded VSS score, a significant reduction in the microneedle-treated keloids after 4 weeks of treatment was observed (p = 0.002).
Comparisons Between Phase One and Two
The dosage of triamcinolone used in phase two was four times higher than that in phase one. The mean reduction in keloid volume after 4 weeks of treatment in phase two was significantly greater than in phase one (p = 0.029) (Table 1). Correspondingly, the reduction in investigator-graded VSS score after 4 weeks of treatment was significantly greater in phase two than in phase one (p = 0.017). The main parameters within the VSS scoring that resulted in this difference were the pliability, height and pigmentation scores.
Subjective Assessment
The subjects tolerated the microneedles well and there was no trial withdrawal. There were no incidences of infection or contact dermatitis from the microneedles. There was also no reported aggravation or worsening of keloids after microneedle application. Patients experienced occasional mild itch and/or pain with application of the microneedles and only two patients stopped applying the microneedles earlier because of moderate itch (at day 23 and day 26 respectively).
At the end of both phases of the trial, the subjects were asked to compare the microneedle treatment with the conventional intralesional corticosteroid injections. Seventeen subjects had participated in both phases of the trial and all of them had prior experience of receiving intralesional corticosteroid injections for their keloids. All felt that the microneedle treatment was not painful and 15 felt that the microneedle treatment was more convenient. Six subjects felt that microneedle treatment was more effective, three felt that it was equally effective, while eight felt that it was less effective. Overall, 13 of these 17 subjects preferred microneedle treatment to intralesional injections.
Discussion
To circumvent the problems of intralesional steroid injections, we used dissolving microneedles as a novel method of drug delivery in the treatment of keloids. Preliminary studies in animals and humans demonstrated dissolution of the drug-embedded portion of the microneedles in the skin after 2 min [22]. The main advantages of the microneedles are that they are not painful and can be self-administered by patients. The dosage of triamcinolone used in phase two corresponds with the monthly dosage of intralesional triamcinolone administered in clinical practice—0.1 ml of 20 mg/ml triamcinolone for a 1–2-cm-diameter keloid, averaging 3 mg per month.
The triamcinolone delivered by dissolving microneedles is deposited at the upper dermis over a wide area. In both phases of the study, there was a significant reduction in keloid volume compared with control and this reduction was significantly higher when a higher dose of triamcinolone was used. After a reduction in keloid volume with 4 weeks of treatment, the keloids increased back in size after the end of treatment, approaching the size at baseline. These findings indicate that the microneedle treatment is efficacious. In conventional intralesional treatment, such occurrences are similarly observed, and keloids frequently regrow after initial treatment; multiple monthly injections are thus performed, such that recurrence may be reduced. Similarly, we expect multiple months of treatment with microneedles will be required to reduce the regrowth of keloids.
There was no consistent significant improvement in itch and pain scores across the two dosages of triamcinolone. This is likely due to the low baseline pain and itch scores of the keloids in this study. Overall, almost half of the subjects (8/17) felt that conventional intralesional corticosteroid injection was more effective than microneedle treatment. However, despite this perception, most subjects (13/17) preferred microneedle treatment over intralesional injections. This was accounted for by the markedly less pain experienced (17/17) and convenience (15/17) of microneedle treatment compared with intralesional injections. On average, 20–30 triamcinolone injections are required over 3 to 5 years for effective treatment of keloids [9]. Given the long duration of treatment, self-administration with dissolving microneedles is preferable. These advantages can potentially translate into improved patient compliance and persistence with treatment, which may possibly result in a lower rate of recurrence over a longer period of time.
The limitations of this study include 63% (17/27) of the subjects in phase one participated in phase two of the study, which was conducted after a 6-month interval. Treatment was allocated based on keloid location (in an easy-to-reach anatomical location) instead of being randomized from all keloids present on the patient. The keloid volumes were determined objectively using a high-resolution 3D scanner; however, manual delineation of the borders of the keloids in a software is still required. Keloid thickness and volume varied at baseline and may have influenced the depth of penetration and hence effectiveness of treatment. Although the investigator performing this work was blinded, the element of human inconsistency in keloid volume assessment cannot be totally eliminated. With respect to subjective scorings of pain, itch and VSS scores, the margins of inaccuracies are likely high because of the low baseline levels of pain and itch and sizes of the keloids. Comparison of the experience of microneedle treatment with previous conventional intralesional injection was also subjected to recall bias.
Conclusions
In summary, results of this study demonstrate that once-daily application of dissolving triamcinolone-embedded microneedles significantly reduces the volume of keloids and this reduction was greater with a higher dose of triamcinolone used. The treatment was safe and can serve as an alternative for patients unsuitable for conventional treatments.
Acknowledgements
We thank all participants of the study.
Funding
This study is funded by the National Medical Research Council, Singapore (NMRC/TA/0032/2015). Article processing charges were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
The authors Colin WX Tan, Wei Ding Tan, Ruchir Srivastava, Ai Ping Yow, Damon WK Wong and Hong Liang Tey have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by the Health Sciences Authority, Singapore, and the National Healthcare Group’s Ethics Review Board. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.9009647.
References
- 1.Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg. 1989;84:827–837. doi: 10.1097/00006534-198911000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–125. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halim AS, Emami A, Salahshourifar I, et al. Keloid scarring: understanding the genetic basis, advances, and prospects. Arch Plast Surg. 2012;39:184–189. doi: 10.5999/aps.2012.39.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhady SM, Sivanantharajah K. Keloids in various races. A review of 175 cases. Plast Reconstr Surg. 1969;44(6):564–566. doi: 10.1097/00006534-196912000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Lee SS, Yosipovitch G, Chan YH, et al. Pruritus, pain, and small nerve fiber function in keloids: a controlled study. J Am Acad Dermatol. 2004;51:1002–1006. doi: 10.1016/j.jaad.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 6.Bijlard E, Kouwenberg CA, Timman R, et al. Burden of keloid disease: a cross-sectional health-related quality of life assessment. Acta Derm Venereol. 2017;97(2):225–9. doi: 10.2340/00015555-2498. [DOI] [PubMed] [Google Scholar]
- 7.Bock O, Schmid-Ott G, Malewski P, et al. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297(10):433–438. doi: 10.1007/s00403-006-0651-7. [DOI] [PubMed] [Google Scholar]
- 8.Gold MH, McGuire M, Mustoe TA, et al. International advisory panel on scar management. Updated international clinical recommendations on scar management: part 2—algorithms for scar prevention and treatment. Dermatol Surg. 2014;40(8):825–831. doi: 10.1111/dsu.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 9.Muneuchi G, Suzuki S, Onodera M, et al. Long-term outcome of intralesional injection of triamcinolone acetonide for the treatment of keloids scars in Asian patients. Scand J Plast Reconstr Hand Surg. 2006;40(2):111–116. doi: 10.1080/02844310500430003. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Wu X, Liu K, et al. Topical cryoanesthesia for the relief of pain caused by steroid injections used to treat hypertrophic scars and keloids. Medicine. 2017;96(43):e8353. doi: 10.1097/MD.0000000000008353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly AP. Medical and surgical therapies for keloids. Dermatol Ther. 2004;17(2):212–218. doi: 10.1111/j.1396-0296.2004.04022.x. [DOI] [PubMed] [Google Scholar]
- 12.Arno AI, Gauglitz GG, Barret JP, et al. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns. 2014;40(7):1255–1266. doi: 10.1016/j.burns.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenna S, Aveta A, Filoni A, et al. A new carbon dioxide laser combined with cyanoacrylate glue to treat earlobe keloids. Plast Reconstr Surg. 2012;129(5):843e–844e. doi: 10.1097/PRS.0b013e31824a6207. [DOI] [PubMed] [Google Scholar]
- 14.Ud-Din S, Bayat A. New insights on keloids, hypertrophic scars and striae. Dermatol Clin. 2014;32(2):193–209. doi: 10.1016/j.det.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Kolli CS. Microneedles: bench to bedside. Ther Deliv. 2015;6(9):1081–1088. doi: 10.4155/tde.15.67. [DOI] [PubMed] [Google Scholar]
- 16.Larrañeta E, McCrudden MT, Courtenay AJ, et al. Microneedles: a new frontier in nanomedicine delivery. Pharm Res. 2016;33(5):1055–1073. doi: 10.1007/s11095-016-1885-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, Yeo DC, Wiraja C, et al. Peptide delivery with poly (ethylene glycol) diacrylate microneedles through swelling effect. Bioeng Transl Med. 2017;2(3):258–67. doi: 10.1002/btm2.10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao Chang, Mengjia Zheng, Xiaojun Yu, et al. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv Mater. 2017;29(37):1702243. doi: 10.1002/adma.201702243. [DOI] [PubMed] [Google Scholar]
- 19.Smith OJ, McGrouther DA. The natural history and spontaneous resolution of keloid scars. J Plast Reconstr Aesthet Surg. 2014;67(1):87–92. doi: 10.1016/j.bjps.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Nedelec B, Shankowsky A, Tredgett EE. Rating the resolving hypertrophic scar: comparison of the Vancouver Scar Scale and scar volume. J Burn Care Rehabil. 2000;21:205–212. doi: 10.1097/00004630-200021030-00005. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan T, Smith J, Kermode J, et al. Rating the burn scar. J Burn Care Rehabil. 1990;11:256–260. doi: 10.1097/00004630-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Z, Luo H, Lu W, et al. Rapidly dissolving microneedle patches for transdermal delivery of exenatide. Pharm Res. 2014;31(12):3348–3360. doi: 10.1007/s11095-014-1424-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.


