Abstract
The pulvinar is primarily referred to for its role in visual processing. However, the ‘visual pulvinar’ only encompasses the inferior and lateral regions of this complex thalamic nucleus. The remaining medial portion (medial pulvinar, PM) establishes distinct cortical connectivity and has been associated with directed attention, executive functions and working memory. These functions are particularly impaired in neurodevelopmental disorders, including schizophrenia and attention deficit and hyperactivity disorder (ADHD), both of which have been associated with abnormal PM architecture and connectivity. With these disorders becoming more prevalent in modern societies, we review the literature to better understand how the PM can participate in the pathophysiology of cognitive disorders and how a better understanding of the development and function of this thalamic nucleus, which is most likely exclusive to the primate brain, can advance clinical research and treatments.
Keywords: pulvinar, primate, cognitive disorders
Introduction
In the light of recent evidence, it is now undeniable that the pulvinar complex is not a single structure with a single primary function. Through a suite of experimental approaches, including traditional anatomical staining, in situ hybridization, electrophysiology and more advanced MRI‐guided tracer injections, it has been established that the structure known as the pulvinar is actually a collection of discernible nuclei. Early anatomical studies in Macaca mulatta had already suggested the presence of the anterior (PA), medial (PM), inferior (PI) and lateral pulvinar (PL) nuclei (Walker, 1938; Olszewski, 1952), although the position of the border between the PL and the PI remained contentious. Over time, the PL and PI have been further subdivided, with the most recent map of the PI including five subdivisions (posterior, medial, caudomedial, caudolateral and lateral inferior) and the PL encompassing a dorsomedial and ventrolateral subdivisions in macaques (Lyon et al. 2010). These subdivisions are collectively referred to as the ‘visual pulvinar’ and have dominated the focus of research (for review see Bridge et al. 2015; Baldwin et al. 2017).
In the current review, we focus on the PM, which is the largest but also the least studied nucleus of the pulvinar complex. The evolution of the PM in the mammalian brain is relatively recent and its emergence, causing the PI and PL to migrate ventrally, has been traced to the early primates (Baldwin et al. 2017). The PM has undergone continuous expansion within the Primate Order mirroring that of the neocortex, reaching a maximum in humans in terms of both size and connectivity. The PM is the largest nuclei of the pulvinar complex, contributing 40% of the thalamus in humans. From its connectivity pattern in a wide range of New and Old World monkeys, including cynomologus (Macaca fascicularis), squirrel (Saimiri sciureus), rhesus (M. mulatta) and pigtail monkeys (Macaca nemestrina), it was demonstrated that unlike other pulvinar nuclei, the PM is polymodal and forms connections with cortical areas involved in visuomotor and auditory processing, as well as associative and higher cognitive processing (Burton & Jones, 1976; Baleydier & Morel, 1992; Romanski et al. 1997). From these important mapping efforts, it was proposed that the PM performs an integrative function and participates in directed attention networks. However, the question ‘what does the PM do?’ still remains largely unanswered.
Providing an answer has become more pressing, as mounting evidence suggests that the structure and function of the PM are abnormal in patients with autism spectrum disorder (ASD) and schizophrenia. Moreover, the neurodevelopmental origin of these cognitive disorders suggests that the formation of the PM might be altered in patients.
In this context, we believe it is necessary to conduct a comprehensive review of the literature available on the PM, encompassing all aspects, from its connectivity, evolution and development to its putative functions and implications in neurodevelopmental cognitive disorders. We hope that this will help bring this neglected thalamic nucleus to the forefront of brain research and subsequently benefit patients living with associated disorders.
Organisation of the medial pulvinar
Cellular and neurochemical anatomy
Due to distinctive cytoarchitecture, the boundaries and structure of the nuclei forming the pulvinar complex are readily identified with histological stains, as illustrated in the adult marmoset (Callithrix jacchus, Fig. 1). Neurochemical approaches targeting specific neurotransmitter systems such as acetylcholinesterase (AChE) histochemistry, or a component of the cell physiology, including calbindin (Cb) and parvalbumin (Pv) immunohistochemistry, have proven effective in further defining functional subdivisions within the main nuclei, refining our understanding of the pulvinar organisation. This is particularly true for the inferior pulvinar (PI), enabling the demarcation of four subdivisions (Gutierrez et al. 1995; Fig. 1).
Figure 1.

Architecture of the adult marmoset monkey pulvinar complex. Adjacent coronal sections through the adult marmoset brain were stained for Nissl substance (A), to stain cell bodies; modified Gallyas stain (B), to reveal myelin; acetylcholinesterase (AChE) histochemistry (C), to label cholinergic neurones; and the calcium‐binding proteins calbindin (D) and parvalbumin (E) immunohistochemistry. This combination enables the demarcation of all the subnuclei forming the pulvinar complex, including potential subdvisions within the PM (see C, arrowhead); PIcl, caudolateral inferior pulvinars; PIcm, caudomedial inferior pulvinar; PIm, medial inferior pulvinar; PIp, posterior inferior pulvinar; PL, lateral pulvinar; PM, medial pulvinar; PM‐l, lateral medial pulvinar; PM‐m, medial medial pulvinar; PM‐mc, medial central medial pulvinar. Scale bar: 1000 μm.
Unfortunately, when applied to the PM, this method does not yield such accurate boundary demarcation, as the cytoarchitecture exhibits only subtle differences (Ma et al. 1998). In rhesus monkeys (M. mulatta), the lateral PM (PMl) is characterised by medium AChE/Pv and light Cb staining, whereas the medial PM (PMm) exhibits the opposite profile of light AChE/Pv and medium Cb staining (Gutierrez et al. 2000; Fig. 1C in the marmoset). Within the PMm, Gutierrez and colleagues identified an additional AChE/Pv‐dense Cb‐light patch, which they termed the medial central PM or PMmc. However, none of these anatomically defined compartments has been demonstrated to be correlated with a particular connectivity pattern or function. This might explain why they have not become mainstream, and why the PM is still depicted as a homogeneous nucleus (see Baldwin et al. 2011). However, the absence of any subdivisions within the PM cannot be ruled out based on the minimal anatomical investigations that have been undertaken to date. Alternatively, the patchy distribution of cortical terminals in the PM, as well as the patches of heightened cytochrome oxidase or AChE staining, has led to the proposition of a mosaic organisation of the PM, supported by the identification of intrinsic long‐range interneurones labelled with Pv and GABA within the macaque PM. These have been suggested to interconnect the micro‐units of PM (Imura & Rockland, 2006).
Connectivity of the medial pulvinar
Cortical connectivity
Bidirectional tracer injections in the marmoset (C. jacchus) medial pulvinar (PM) reveal efferent terminals concentrated in two bands, in deep layer 3/layer 4 and in layer 1 of the cortex. The reciprocal PM afferents emerge from layers 5/6 of the cortex (Fig. 2). Cortical interconnectivity with PM is extensive (Fig. 3). Early tracer studies in adult M. mulatta principally, but also M. nemestrina, revealed reciprocal connectivity between the PM and inferior temporal areas TE (superior temporal sulcus) and TEO (occipital temporal sulcus; Baleydier & Morel, 1992; Burton & Jones, 1976; Webster et al. 1993; Yeterian & Pandya, 1985). PM connectivity with the inferotemporal gyrus increased along the posterior‐anterior axis, with injections in TEO yielding little labelling in PM, and terminal labelling mostly in the rostral portion of the nucleus. In comparison, tracer injections in the more anterior TE, namely, TEa and TEp, produced intense labelling in the ventral region of the caudal PM (Baleydier & Morel, 1992). An opposing gradient of connectivity is observed in the PL, exchanging more connections with posterior areas V4 and TEO than with anterior TE (Baleydier & Morel, 1992). Both retrograde and anterograde tracing produced overlapping labelling, suggesting intermingled reciprocal connectivity (Baleydier & Morel, 1992). Tracer injections in the superior temporal cortex of the marmoset monkey (C. jacchus) identified major PM inputs with the caudomedial (CM) area of the medial belt, characterised by its auditory and somatosensory activity, and multisensory and somatosensory retroinsular area (Ri; de la Mothe et al. 2012, 2006).
Figure 2.

Reciprocal connectivity between the medial pulvinar and the neocortex. Injection of the retrograde fluorescent tracer cholera toxin subunit b 488 (CTb‐488) and the anterograde fluorescent tracer biotinylated dextran amine 10 kDa 488 (BDA‐10k‐488) in an adult marmoset PM using an MRI‐guided injection technique (Mundinano et al. 2016) demonstrates that PM efferents innervate cortical layers 3 and 1, and are recipients of cortical inputs primarily from neurones in layer 6, with additional weaker projections arising from neurones in layer 5. (A) CTb‐488 injected in the DLPFC area 9 reveals the complex morphology of PM neurones. Scale bars: (A) 200 μm; (B) 100 μm.
Figure 3.
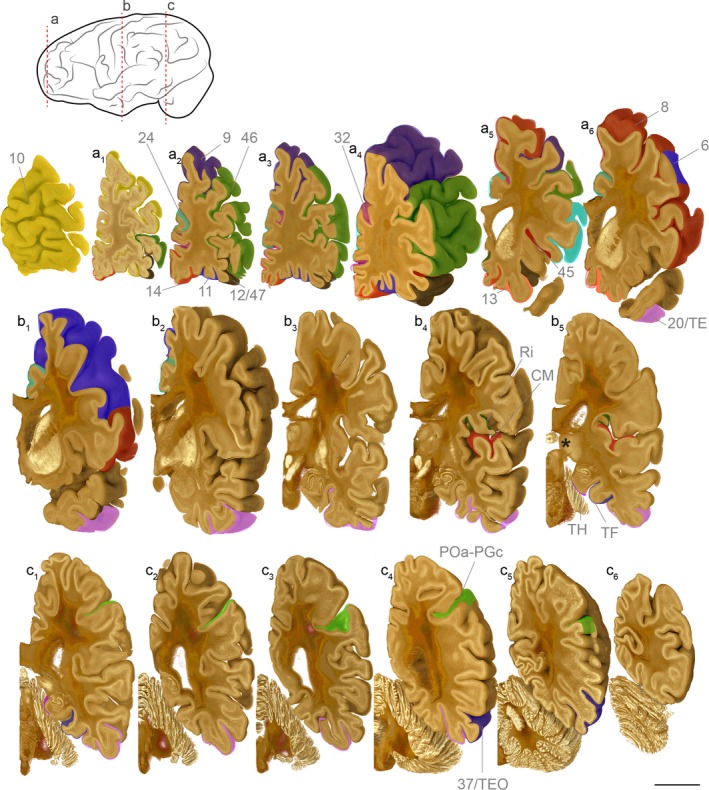
Schematic illustration of the cortical areas reciprocally connected with the medial pulvinar. The brain of an adult male human obtained from the Big Brain project (Amunts et al. 2013) was rendered in 3D using drishti (Limaye, 2012) and sliced in the coronal plane from the frontal lobe (a) to the occipital pole (c). Using the adult human brain reference atlas available on the Allen Brain portal ( www.brainspan.org/static/atlas), the position of cortical areas establishing reciprocal connections with the medial pulvinar (PM), based on primate connectivity studies, were delineated and highlighted. This figure illustrates the extensive network of areas that are connected through the PM. The position of the pulvinar is indicated by a white asterisk (slice b5) Scale bar: 15 mm.
The PM is also interconnected with the caudal inferior parietal lobe as demonstrated by tracer injections in M. mulatta, M. fascicularis and M. cynomologus, Erythrocebus patas and S. sciureus (Burton & Jones, 1976; Baleydier & Mauguière, 1977; Divac et al. 1977; Mesulam et al. 1977; Stanton et al. 1977; DeVito, 1978; Kasdon & Jacobson, 1978; Pearson et al. 1978; Asanuma et al. 1985). Following tracer injection in POa – in the ventral bank of the intraparietal sulcus, and PGc – part of the caudal inferior convexity, labelling in PM was observed (Baleydier & Morel, 1992). Labelled neurones were predominantly distributed in the central portion of PM, up to the rostral limit of the nucleus and extending laterally along the border with the PL, including a subset of neurones mainly projecting to POa and invading the adjacent dorsal fringe of the dorsal PL (PLd; Baleydier & Morel, 1992). PM projections to POa and PGc originate from the same region. However, the absence of double‐labelled cells suggests that they are distinct populations (Baleydier & Morel, 1992). Double‐tracer injections in TE and PGc/POa, to investigate the relative position of the projecting neurones in PM, revealed that PM neurones projecting to the inferoparietal cortex are more dorsal and rostral compared with the neurones projecting to the inferior temporal cortex (Baleydier & Morel, 1992).
Injections of tritiated amino acids and horseradish peroxidase (HRP) in the PM of baboons (Papio papio) and crab‐eating macaques (M. fascicularis) have also enabled the identification of reciprocal connectivity with the posterior cingulate cortex (area 23), the restrosplenial areas and the posterior parahippocampal gyrus (areas TH and TF; Baleydier & Mauguière, 1985).
The PM has also been demonstrated in rhesus and cynomologus monkeys (M. mulatta and M. fascicularis) to establish a dense network of reciprocal connections with the prefrontal cortex, in particular the frontal eye fields (area 8; Kuypers & Lawrence, 1967; Trojanowski & Jacobson, 1974; Bos & Benevento, 1975; Künzle et al. 1976; Kievit & Kuypers, 1977; Fallon & Benevento, 1978; Barbas & Mesulam, 1981; Leichnetz, 1982). A more recent study, where tracers were injected in the PM of rhesus monkeys (M. mulatta), contributed to providing a more exhaustive map of the PM. Connections with the anterior cortical areas of the brain, including the prefrontal cortex (areas 10–14, 24–25, 32 and 45), the dorsolateral prefrontal cortex (DLPFC; areas 9 and 46), the precentral opercular area (PrCo), the central sulcus area (CsS), the lateral sylvian fissure (Ls), and the ventral and dorsal branches of the arcuate sulcus (ASv and ASd, respectively, Romanski et al. 1997). Unfortunately, subcortical tracer injections often lack precision due to the difficulty in defining exact coordinates, leading to contamination of adjacent structures. This is particularly problematic in the case of the PM connectivity with the prefrontal cortex, as the adjacent medial dorsal nucleus (MD) is known to possess strong connections with the DLPFC. Therefore, the mapping of the PM connectivity would greatly benefit from newly developed protocols, including the use of MRI to calculate precisely the coordinates of the injection site (Mundinano et al. 2016).
Subcortical connectivity
In addition to reciprocal connectivity with the cortex, the PM possesses connectivity with several subcortical structures. As described for other pulvinar nuclei, the PM is the recipient of afferents from the superior colliculus in rhesus monkeys (M. mulatta; Benevento & Fallon, 1975; Benevento & Standage, 1983). However, in the particular case of the PM, these connections emerge from the deep, non‐retinorecipent layers of the superior colliculus, whereas the inferior and lateral pulvinar receive connections from the superficial, retinorecipient layers (Baldwin et al. 2011).
Injections of tritiated amino acids into the PM of rhesus (M. mulatta) and squirrel (S. sciureus) monkeys revealed projections to the amygdala (Jones & Burton, 1976). The afferent connections were concentrated in the anterior region of lateral nucleus of the amygdala, entering through the external capsule (Jones & Burton, 1976). The lateral nucleus of the amygdala projects to the perirhinal cortex, ultimately connecting with the hippocampal formation (Krettek & Price, 1974). Therefore, through its connection with the amygdala, the PM is brought into close relation to the limbic lobe, with a potential functional implication in associative learning and modulation of attention based on emotional states (for review on the roles of the amygdala complex in emotionally based learning, see Gallagher & Chiba, 1996).
PM connections with the brain stem, including the nucleus of the optic tract and the lateral terminal nucleus, have also been suggested in rhesus monkeys (M. mulatta; see Romanski et al. 1997) but have yet to be confirmed.
Mapping of PM connectivity with cortical and subcortical structures remains a work in progress. This is in part due to the difficulty of precisely targeting subcortical regions for tracer injections but also to the relative lack of interest for the PM in comparison with the visual pulvinar. However, this relative delay might work to the advantage of the PM, as it will benefit from advances in imaging techniques, including diffusion tensor imaging (DTI; Warner et al. 2015; Mundinano et al. 2018) and MRI‐guided tracer injection (Mundinano et al. 2016), recently applied to study the PIm. Importantly, MRI‐guided injections have been successfully employed specifically to deliver neurotoxic substances to the medial division of the inferior pulvinar in neonatal marmosets (Mundinano et al. 2018) in order to study its role in development of cortical areas and associated behaviours. Applied to the PM, this technique would enhance the current understanding of the PM complex functions, essentially inferred from the functions of the areas with which the PM is connected.
Function
The inferior and lateral pulvinar are interconnected with visual cortical areas, extending from the occipital lobe to the parietal and temporal lobes, hence the more common name ‘visual pulvinar’ (for review Bridge et al. 2015; Baldwin et al. 2017). However, the PM with its widespread network of cortical connections (Fig. 3) differs from the other pulvinar nuclei and is considered a multimodal integration nucleus. To grasp its functions, it is easier first to explain the roles of the cortical areas forming reciprocal connectivity with the PM.
The inferior parietal lobule is partially associated with the dorsal visual processing stream, chiefly referred to as the ‘where’ stream, responsible for locating objects in the visual field. Components of the posterior parietal lobe, including areas PGc and POa, contribute to forming an internal sensory map based on vision, including the ability to use spatial landmarks as cues in a choice task (Ungerleider & Mishkin, 1982). PG is important for attentional processes, in particular towards motivationally relevant objects like food. Neurones in PG will respond if a desirable object enters the visual field and is likely to become the target of subsequent actions. However, the amplitude of the response is modulated by the probability of these actions successful outcome. For example, response in rhesus and stump‐tail monkeys (M. mulatta and M. arctoides) PG is dampened if the object is beyond the animal's reach or its arm is restrained (Mountcastle et al. 1975). Lesions to the inferior parietal lobule result in an inability to register motivationally relevant events and disturbance in the distribution of attention (for review see Mesulam, 1981).
In comparison, the inferior temporal cortex, comprising TE and TEO, belongs to the ‘what’/ventral visual stream, which is involved in object recognition. Lesions to TE and TEO lead to difficulty in learning visual discrimination tasks, including impairment in visual pattern discrimination and recognition (Ungerleider & Mishkin, 1982).
PM is also strongly connected with the frontal eye fields (FEF), collectively known as area 8. The FEF are part of the attention network and are responsible for distributing and orienting exploratory eye movements. The function of the FEF is dependent on that of area PG, in the parietal lobe. PG is responsible for directing the visual attention to regions that are particularly relevant in the individual's current physiological state. For example, if one is hungry, PG becomes activated when one attends to the location of food, resulting in the subject spending disproportionally more time attending to these regions of the visual field. Once satiated, these regions lose their relevance, they no longer trigger a response in PG and they are not attended to more than average. Therefore, PG shapes the subjective attentional landscape and FEF plan a strategy for navigating it (Mesulam, 1981; Milner & Goodale, 1993).
Other cortical areas that PM establishes reciprocal connections with, are also part of the visual attention network, including the cingulate cortex, which assigns value to spatial coordinates based on sensory events of past experiences (Mesulam, 1981, 1990). Areas in the prefrontal cortex, including DLPFC area 46, are also involved in this large‐scale network, comprising widely separated but interconnected local networks, composed of immediately contiguous areas (for example V4, TEO and TE); for more information see Mesulam (1990).
In light of the reciprocal connectivity of PM with the posterior parietal, inferior temporal, cingulate, restrosplenial and prefrontal cortical areas, it has been concluded that PM is a multimodal association nucleus implicated in the modulation and selectivity of directed attention. This network appears a priori redundant, with all the areas connecting directly with each other and to each other through the PM (Baleydier & Mauguière, 1985). This organisation has been described as the replication principle where pulvinar connections replicate the cortico‐cortical connections but not their functions (Shipp, 2003, 2004).
In summary, the PM function is modulatory and it coordinates the simultaneous processing of many items of information in distinct modules of the network. PM plays a decisive role in determining the behavioural outcome of a situation when faced with ambiguous and competing constraints.
PM has also been demonstrated to participate in the network responsible for informing the visual system that stimulus motion results from self‐induced movement and not real stimulus motion. Following self‐generated image motion, the occulomotor system sends a corollary discharge or efference copy to the visual system to signal that the stimulus motion was self‐induced and prevents the triggering of eye movement (Robinson & Petersen, 1985, 1992; Sherman, 2016). Despite the lack of connections with low‐order visual processing areas, PM is nevertheless critically involved with important aspects of visual perception.
To date, all the functions associated with PM have been studied in adults and there is no information on the role PM fulfils in the developing and maturing brain. This is a critical aspect of PM that needs to be urgently addressed, as several studies over the past decade have demonstrated that other nuclei and subdivisions of the pulvinar, such as the PIm, play an essential role in shaping the networks they are connected with (Warner et al. 2012, 2015; Mundinano et al. 2018). Moreover, PIm connectivity undergoes significant remodelling during the course of postnatal development (Warner et al. 2010), suggesting that PM connectivity in young animals might differ significantly during development from what is known in the adult. This is particularly important, as abnormal development of the PM has been correlated with neurodevelopmental disorders.
Origin of the PM
Evolution
After a long‐standing debate in which it was notably argued that the pulvinar complex was a primate‐only structure (LeGros Clark, 1930) , a consensus has been reached and it is now accepted that the pulvinar complex is older than primates and was present in the common ancestor of Euarchontoglires (Baldwin et al. 2017). The lateral posterior (LP) nucleus in rodents, as originally defined by Jones (2007), is homologous to the primate pulvinar (Masterson et al. 2009) and distinct from the structure identified as the lateral posterior nucleus in a separate region of the primate thalamus, a source of much of the confusion surrounding the presence of a rodent pulvinar.
However, this is only true for the visual pulvinar, comprising the inferior and lateral pulvinar. The medial nucleus is a recent addition to the pulvinar complex and is likely exclusive to primates. The evolution of PM in the dorsal position has led to a rotation of the pre‐existing nuclei of the pulvinar complex. Whereas this rotation is incomplete in prosimian primates, including galago, in which PIp and PIcm exhibit a dorsal position (Baldwin et al. 2013), it is fully achieved in Haplorhines (tarsiers and simians) with the PI adopting the fully ventral position, which is conserved through to humans (Fig. 4; for review see Baldwin et al. 2017).
Figure 4.
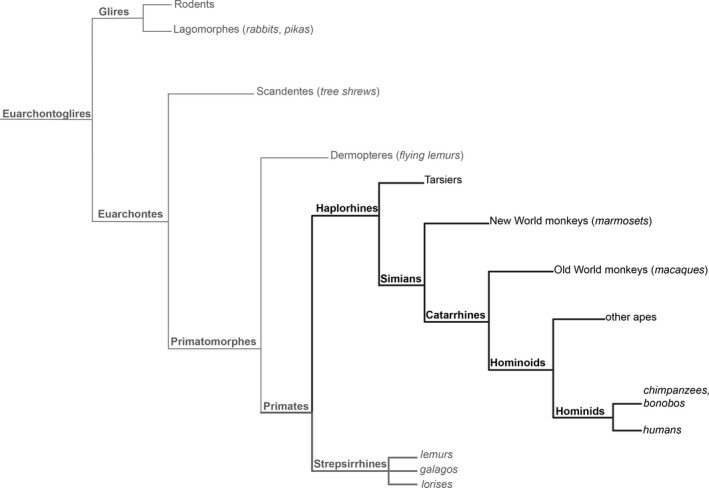
Phylogenetic tree of medial pulvinar evolution. The medial pulvinar (PM) emerged first in primates with strepsirrhines exhibiting a rudimentary PM, and an inferior pulvinar (PI) and lateral pulvinar (PL) that have not achieved their ventral rotation (thick grey line). The evolution of PM is considered complete in haplorhines (thick black lines) based on the full rotation of the PI into a ventral position. The evolution of the PM continues over the primate lineage as suggested by its progressive expansion, occupying 40% of the human adult thalamus.
It has been proposed that the evolution of the PM is tightly correlated with the evolutionary expansion of the association cortex in primates. However, the mechanisms underlying the evolution of this network remain unknown. Comparative analysis of brain development in human and nonhuman primates revealed that the pattern of evolutionary expansion of the cortex is remarkably similar to that of pre‐ and postnatal development (Hill et al. 2010). Therefore, elucidating the development of the PM could further our knowledge of how this nucleus evolved in primates. Fate‐mapping has proven very successful in establishing the evolutionary relationship between brain regions. The application of this method to the PM first requires establishing its genetic signature, in particular during development. In this context, Homman‐Ludiye and colleagues recently identified in the embryonic mouse dorsal thalamus, cell clusters expressing genes specifically enriched in the neonatal marmoset PM (Homman‐Ludiye et al. 2018), suggesting that the origin of the PM could be traced back beyond the primate lineage.
Embryonic development
The misconception that the pulvinar complex is not present in rodents has significantly hampered progress in elucidating its developmental profile. However, pulse‐chase experiments with a tritiated thymidine analogue in macaques (M. mulatta) demonstrated that the ontogenesis of the pulvinar nuclei recapitulates evolution, with PI and PL neurones being generated first and PM neurones forming last (Ogren & Rakic, 1981).
The current model of pulvinar development indicates a dichotomy between human and nonhuman primates (Ogren, 1982). It has been proposed that although the early onset of neurogenesis is common across all primates (first weeks of gestation), the production of pulvinar neurones rapidly terminates in macaques, lasting less than 10 days of the 165‐day‐long gestation (Ogren & Rakic, 1981). However, pulvinar neurogenesis appears to be significantly protracted in human, extending well into the final weeks of pregnancy (Rakic & Sidman, 1969). Moreover, the study by Rakic and Sidman suggests that the human pulvinar is populated in two successive waves of neuronal migration, the initial wave from the neurogenic zone lining the 3rd ventricle (Fig. 5), and the second wave, during the late stages of pregnancy, emerging from the telencephalon, with the stream of migrating cells forming the transient ‘gangliothalamic body’ (Rakic & Sidman, 1969; Letinic & Kostović, 1997; Letinic & Rakic, 2001). Analysis of macaque monkey pulvinar development failed to identify the second telencephalic wave of pulvinar neurones. It was thus concluded that the protracted period of neurogenesis and the additional neurogenic niche of pulvinar neurones in the telencephalon support an enlarged PM in humans compared with other primates.
Figure 5.
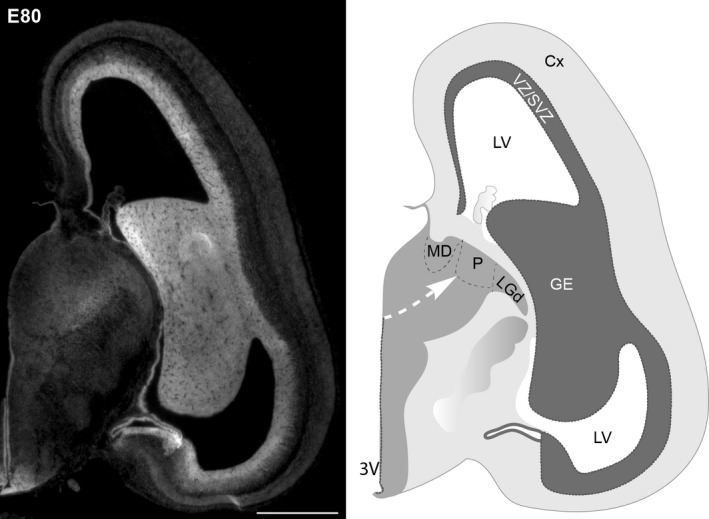
Development of the pulvinar in the embryonic brain. Coronal section through an embryonic marmoset brain at embryonic day (E) 80, ~ 65 days before birth, stained with the nuclear dye, Hoechst, to reveal the dense neurogenic zones lining the ventricles. The neurones populating the dorsal thalamus, including the pulvinar, originate from the layer lining the 3V along the path illustrated by the arrow on the schematic. 3V, third ventricle; Cx, cortex; GE, ganglionic eminences; LGd, dorsal lateral geniculate nucleus; LV, lateral ventricle; MD, medial dorsal nucleus; P, pulvinar; VZ/SVZ ventricular and subventricular zones. Scale bar: 1000 μm.
However, a recent study in the marmoset monkey (C. jacchus) demonstrated that pulvinar neurones are still being generated a few days before birth (Homman‐Ludiye et al. 2018), suggesting that ontogenesis of the pulvinar is more similar across primates than originally believed. Despite pulvinar neurogenesis spanning the entire period of brain development in the marmoset, there is no evidence of a second telencephalic wave of neurones. This signifies that this telencephalic population has exclusively evolved in humans, including the molecular regulatory pathways underpinning the proliferation of a separate pool of progenitors, the differentiation of a novel population of neurones and the establishment of an original migratory path for these telencephalic cells to follow. Furthermore, although there has been an expansion of the cortex, there is no evidence of a drastic difference in connectivity or function of the PM between human and nonhuman primates. Therefore, the question arises as to what is the purpose of this telencephalic ancillary population? Until this is fully addressed, the most realistic and parsimonious model suggests that the PM is populated with diencephalic neurones exclusively across all primates, including humans.
Postnatal development and maturation
At birth, the pulvinar complex does not exhibit the anatomical features characteristic of the adult nuclei. In particular, the calcium‐binding protein Cb, which is upregulated in functionally mature neurones, is almost absent from the neonatal pulvinar and becomes progressively upregulated according to a nucleus‐specific sequence. In the marmoset (C. jacchus), visual pulvinar nuclei PI and PL acquire their adult‐like profile around 2 weeks postnatally, whereas the Cb profile in PM is still immature at 3 months of age (Homman‐Ludiye et al. 2018). This sequence appears to parallel the sequential maturation of the respective cortical areas with which the pulvinar nuclei connect, with occipital areas maturing early, and frontal and parietal areas maturing last, as demonstrated in marmosets (C. jacchus) and humans (Gogtay et al. 2004; Bourne & Rosa, 2006; Burman, Lui, Rosa, & Bourne, 2007; Hill et al. 2010; Mundinano et al. 2015). The postnatal maturation of the pulvinar complex is not limited to the expression profile of maturation markers, as recent studies have demonstrated the profound remodelling of the PIm connectivity. Notably, the PIm in juvenile marmosets (C. jacchus) receives direct inputs from the retina, which are later pruned back (Warner et al. 2010, 2012). Therefore, the PIm switches from a primary relay nucleus in early life to a higher‐order nucleus, receiving greater input from the cerebral cortex in adulthood. Often called exuberant, these transient connections are essential for the appropriate development of the visual network, with disruption of this pathway in the neonatal marmoset (C. jacchus) leading to alteration of the cortical architecture and connectivity, and deficits in visually guided behaviours (Mundinano et al. 2018). Although these studies were focused on the PIm, it is expected that the maturation of PM connectivity follows similar principles, although a comparative analysis between 7‐day‐old and adult macaque (M. mulatta) failed to detect changes in the PM connectivity (Webster et al. 1995). The developmental remodelling of thalamocortical connectivity varies greatly across structures; therefore, more research is needed to determine the particular timing for each PM network.
Association with neurodevelopmental disorders
The demonstrated role of PM in the modulation of directed attention and higher cognitive function prompted the hypothesis that abnormal PM function could be an underpinning cause or contributing factor to neurodevelopmental cognitive disorders exhibiting altered attentional selectivity behaviour. Anatomical and MRI studies, including functional MRI, have in particular revealed altered PM structure and/or connectivity in three major neurodevelopmental cognitive disorders: attention deficit and hyperactivity disorder (ADHD), schizophrenia (SCZ) and autism spectrum disorders (ASD).
ADHD
ADHD is characterised by the inability to focus attention, associated with uncontrollable hyperactivity and impulsivity. This common neuropsychiatric disorder affects principally children, with 5% of school‐aged children affected worldwide (Polanczyk et al. 2007). Although the aetiology of the disorder is still unknown, studies have demonstrated the contribution of environmental and genetic factors (for review Dark et al. 2018). Observations in patients consistently reveal volume reduction in many brain regions, in particular those involved in attention, including the parietal and temporal lobes, the prefrontal and cingulate cortices (for a summary of the regions affected see Dark et al. 2018). Volume reduction has also been observed in the thalamus, specifically the pulvinar complex (Ivanov et al. 2010). Comparison of visual attention task‐based functional MRI data between children with ADHD and demographically matched controls revealed significantly reduced pulvinar activity in ADHD patients. Temporal correlation of activity patterns between pulvinar nuclei and the remainder of the brain also demonstrated decreased functional connectivity between the pulvinar and the prefrontal regions, confirming that these functional abnormalities in the pulvinar complex are associated with the inattentive symptoms of the disorder (Li et al. 2012). Unfortunately, the resolution of the studies does not allow for the discrimination of specific nuclei within the complex. Therefore, it is not possible to conclude if the PM is more affected than PL or PI.
Brain volume reduction in neurodevelopmental disorders is normally a consequence of loss of synapses or white matter, rather than an actual loss of neurones, which is more symptomatic of neurodegenerative disease. Whatever the cause, the structural changes correlated with ADHD are not permanent and can be reversed. First, the pulvinar volume reduction is attenuated in patients undergoing treatment (Ivanov et al. 2010). Secondly, the disorder persists into adulthood in only 30–50% of cases (Polanczyk et al. 2007), suggesting that the remodelling of specific networks during maturation can contribute to rebalancing the activity. Unfortunately, no data are available on the brain volumes of adults who have overcome ADHD as children to determine whether brain volume returns to normal. In any case, a better understanding of the development and maturation of the PM, and its connectivity, would be a great advance in our understanding and potentially the diagnosis and treatment of ADHD.
Autism spectrum disorders
The neurodevelopmental conditions grouped under autism spectrum disorders (ASD) are characterised by impairments in social interactions and communication as well as restricted, repetitive and stereotyped patterns of behaviour (American Psychiatric Association, 2013). The symptoms emerge in the first few years of life, typically around the age of 3 and persist lifelong (Volkmar et al. 2005). People with ASD often have co‐occurring conditions including seizures, ADHD and sleep disturbances (Levy et al. 2010). Long considered a failure of parenting, it is now accepted that ASD has a strong genetic component and complex environmental interactions; however, there is still limited information on what predisposes a child to develop an ASD. Prevalence estimates have detected a rise since the beginning of the 20th century, with current occurrence rate of around 1% of children affected, as well as a strong gender bias, with males being affected three to four times more than females (Rice et al. 2012).
The well‐documented differences in basic sensorimotor processing and attention skills associated with ASD have prompted intense research into the thalamocortical connectivity in patients. Imaging approaches, including functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI), have uncovered abnormalities in the thalamus (Nair et al. 2013), in particular a specific and persistent expansion in the surface area of the posterior medial thalamus encompassing the pulvinar complex (Schuetze et al. 2016). In the left medial thalamus, this increase was positively correlated with the severity of the disorder (Schuetze et al. 2016). The enlargement of the pulvinar in ASD is accompanied by increased connectivity with the cortex. A recent analysis of thalamocortical functional connectivity in 228 patients with ASD detected a hyperconnectivity between the PM and specifically the prefrontal and temporal cortices (Woodward et al. 2017). Abnormal PM structure and connectivity are consistent with the behavioural symptoms associated with ASD, which should encourage more research into the development of PM and its role in shaping the cortical areas with which it is reciprocally connected. In particular, it will be important to determine how and when PM connectivity is pruned back during development, as the PM hyperconnectivity and enlargement suggest that this phase of remodelling is abnormal in these individuals. Considering the important genetic component of ASD, the identification of the genes underpinning the formation of the PM and correlating them with genes associated with ASD can greatly advance our understanding on the origins of the disorders. A recent analysis of the genetic signature of the neonatal marmoset dorsal thalamus notably revealed expression of AUTS2 in the pulvinar complex (Homman‐Ludiye et al. 2018), suggesting that its development could be impaired in individuals carrying a faulty allele. Research into how the structure and functions of the PM are affected in patients has the potential to identify much needed therapeutic targets and enable differential diagnosis within the spectrum of disorders.
Schizophrenia
Schizophrenia (SCZ) occurs in 1% of the world population and the risk increases to 10% in people with a first‐degree relative affected by the disorder. It is a complex brain disorder characterised by, in part, psychosis, delusions and hallucinations, known as positive symptoms, which constitute the basis for diagnosis (American Psychiatric Association, 2013). These symptoms typically arise in young adults (20–30 years) and are preceded by a prodromal phase. This phase is characterised by negative symptoms, including apathy, social withdrawal, impaired attention and altered sensory processing (for review see Lewis & Sweet, 2009). These symptoms generally go undetected at this stage because of the idiosyncrasies associated with adolescence. Unfortunately, the delay between onset of negative symptoms and diagnosis, typically 10 years, negatively correlates with patient outcome. Current research strives to reduce the age of diagnosis, which requires a more robust framework. Despite onset during adolescence, SCZ is nevertheless a neurodevelopmental disorder and results from a combination of genetic and environmental factors disrupting normal brain development (Murray & Lewis, 1987; Weinberger, 1987; Rapoport et al. 2005). The transition into illness corresponds to a phase of intense brain changes, including remodelling of connectivity between the cortex and the thalamus, usually associated with a maturational phase which can be observed by longitudinal MRI (Alexander‐Bloch et al. 2014; Gogtay et al. 2011; for review Pantelis et al. 2005).
The cerebral structures of people with SCZ exhibit characteristic abnormalities, including enlargement of the ventricles and loss of grey matter, affecting particularly the temporal and prefrontal lobes (Haijma et al. 2013). Loss of cortical grey matter in SCZ is not a consequence of neuronal loss but results from a reduction of dendritic spines and synaptic density (Glantz & Lewis, 2000; Lewis & Gonzalez‐Burgos, 2000; Cannon et al. 2002). Recent research has linked the reduced synaptic density in patients to a particular allelic variation correlated to SCZ, which leads to the over‐activation of microglial cells responsible for the developmentally regulated elimination of redundant synapses (Sekar et al. 2016). Although research to date has predominantly focused on the defects affecting the DLPFC, considered a hallmark of the disease, evidence exists that thalamic volumes and connectivity are also affected. Initially, research focused on the medial dorsal nucleus (MD), densely interconnected with the DLPFC. However, further evidence suggests alteration of the PM. In particular, the volume of the PM is disproportionately reduced relative to the overall reduction in brain volume (Gilbert et al. 2001; Byne et al. 2002; Danos et al. 2003; Highley et al. 2003; Kemether et al. 2003; for review Dorph‐Petersen & Lewis, 2017). Postmortem quantification of neurones in PM reveals decreased density in SCZ patients, which in the absence of signs of neurodegeneration and scarring has been attributed to disrupted neurogenesis (Byne et al. 2007). Functional and resting state MRI demonstrate reduced PM–temporal lobe connectivity (Cobia et al. 2017) leading to abnormal thalamic activation and cognition. Associated symptoms, including impaired attentional performance, have also been attributed to disrupted PM function (Zhang et al. 2013).
With recent evidence that pulvinar connectivity drives the maturation of its cortical targets (Warner et al. 2012) and disruption of this connectivity leads to dramatic structural remodelling and abnormal vision‐guided behaviour (Mundinano et al. 2018), a new model for SCZ proposes that the structural and behavioural defects associated with the disorder are caused by disruption to PM development. It is therefore pressing to establish the mechanisms governing the formation of the PM and the remodelling of its connectivity. This includes the role of microglia in the pruning of PM–cortical synapses in order to determine whether PM can be used as a marker for diagnosis and/or prognosis, and potentially as a therapeutic target.
Collective efforts using animal models to elucidate the pathophysiology of SCZ have met with limited success. A potential explanation could be that the current animal models lack many of the networks affected in the disorder and can thus only paint an incomplete picture. Although endeavours such as the recently published collection of 132 human SCZ‐associated genes mutated in zebrafish to generate phenotypic atlases (Thyme et al. 2018) are certainly useful, it is not clear how effective they are at providing information about the function of these genes in primate‐only structures. The recent observation of dopamine D2 receptor (DRD2) selective expression exclusively in the neonatal marmoset PM, and in no other nuclei of the dorsal thalamus (Homman‐Ludiye et al. 2018), is another argument in favour of primate models in the context of SCZ. DRD2 is of utmost relevance in SCZ; not only have allelic variations been associated with the disorder (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014) but DRD2 antagonists have been used for decades to reduce and manage positive psychotic symptoms, including hallucinations (for review Li et al. 2012). The expression of DRD2 in the neonatal PM suggests that its development and function could be impaired in individuals carrying mutations in the gene and needs to be further examined in the development of the human PM and SCZ patients.
Conclusion
The medial pulvinar (PM) is a recently evolved heteromodal thalamic nucleus exclusive to the primate pulvinar complex that establishes extensive cortical and subcortical connectivity. This includes connections with the inferior temporal and posterior parietal lobes, frontal cortical areas, including the frontal eye fields and DLPFC, as well as subcortical connectivity with the amygdala and the non‐retinorecipient deep layers of the superior colliculus. Together with functional imaging and lesion studies, anatomical observations have contributed to positioning the PM within the directed attention modulation network. Despite its critical functions in normal cognitive processing, the PM remains largely understudied, in contrast to the visual pulvinar, which has been gaining interest recently. The fact that it is a primate‐only structure is often used to explain the lack of research, but this very fact should be an incentive to focus our interest on the PM and not a deterrent. Structures which have evolved exclusively in primates might hold the key to understanding the neural substrate of the human mind, including consciousness, and associated disorders.
Moreover, abnormal PM structure and connectivity have been associated with several neurodevelopmental cognitive disorders, including ADHD, ASD and SCZ. These disorders share many comorbidities and overlapping syndromes with gene wide association studies (GWAS) identifying copy number variants associated with all three. With the rapidly growing incidence rate of these disorders, new approaches are necessary to elucidate their aetiology. One such approach could be the comparative analysis of the genes regulating the development and the maturation of the PM with the genes associated with the disorders. Recent progress in imaging and molecular techniques provide the necessary tools to study the thalamus, and should be applied to the PM to advance our understanding of these disorders and the role the PM plays in shaping functioning brain networks. Correlative human and nonhuman primate studies are therefore essential in this future endeavour.
Acknowledgements
The authors wish to thank Robert Bryson‐Richardson for his help in the 3D rendering of the human brain. J.A.B. is supported by a Senior Research Fellowship support (APP 1077677) from the National Health and Medical Research Council (NHMRC). J.H.L. is supported by Stem Cells Australia. This work was funded through a Brain and Behavior Foundation (NARSAD) Independent Investigator Grant to J.A.B. The Australian Regenerative Medicine Institute is supported by grants from the State Government of Victoria and the Australian Government.
References
- Alexander‐Bloch AF, Reiss PT, Rapoport J, et al. (2014) Abnormal cortical growth in schizophrenia targets normative modules of synchronized development. Biol Psychiatry 76, 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders (DSM‐5®). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Amunts K, Lepage C, Borgeat L, et al. (2013) BigBrain: an ultrahigh‐resolution 3D human brain model. Science 340, 1472–1475. [DOI] [PubMed] [Google Scholar]
- Asanuma C, Andersen RA, Cowan WM (1985) The thalamic relations of the caudal inferior parietal lobule and the lateral prefrontal cortex in monkeys: divergent cortical projections from cell clusters in the medial pulvinar nucleus. J Comp Neurol 241, 357–381. [DOI] [PubMed] [Google Scholar]
- Baldwin MKL, Wong P, Reed JL, et al. (2011) Superior colliculus connections with visual thalamus in gray squirrels (Sciurus carolinensis): evidence for four subdivisions within the pulvinar complex. J Comp Neurol 519, 1071–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Balaram P, Kaas JH (2013) Projections of the superior colliculus to the pulvinar in prosimian galagos (Otolemur garnettii) and VGLUT2 staining of the visual pulvinar. J Comp Neurol 521, 1664–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Balaram P, Kaas JH (2017) The evolution and functions of nuclei of the visual pulvinar in primates. J Comp Neurol 525, 3207–3226. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguière F (1977) Pulvinar‐latero posterior afferents to cortical area 7 in monkeys demonstrated by horseradish peroxidase tracing technique. Exp Brain Res 27, 501–507. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguière F (1985) Anatomical evidence for medial pulvinar connections with the posterior cingulate cortex, the retrosplenial area, and the posterior parahippocampal gyrus in monkeys. J Comp Neurol 232, 219–228. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Morel A (1992) Segregated thalamocortical pathways to inferior parietal and inferotemporal cortex in macaque monkey. Vis Neurosci 8, 391–405. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam MM (1981) Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J Comp Neurol 200, 407–431. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Fallon JH (1975) The ascending projections of the superior colliculus in the rhesus monkey (Macaca mulatta). J Comp Neurol 160, 339–361. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Standage GP (1983) The organization of projections of the retinorecipient and nonretinorecipient nuclei of the pretectal complex and layers of the superior colliculus to the lateral pulvinar and medial pulvinar in the macaque monkey. J Comp Neurol 217, 307–336. [DOI] [PubMed] [Google Scholar]
- Bos J, Benevento LA (1975) Projections of the medial pulvinar to orbital cortex and frontal eye fields in the rhesus monkey (Macaca mulatta). Exp Neurol 49, 487–496. [DOI] [PubMed] [Google Scholar]
- Bourne JA, Rosa MGP (2006) Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: early maturation of the middle temporal area (MT). Cereb Cortex 16, 405–414. 10.1093/cercor/bhi119 [DOI] [PubMed] [Google Scholar]
- Bridge H, Leopold DA, Bourne JA (2015) Adaptive pulvinar circuitry supports visual cognition. Trends Cogn Sci 20, 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman KJ, Lui LL, Rosa MGP, Bourne JA (2007) Development of non‐phosphorylated neurofilament protein expression in neurones of the New World monkey dorsolateral frontal cortex. Eur J Neurosci 25, 1767–1779. 10.1111/j.1460-9568.2007.05442.x [DOI] [PubMed] [Google Scholar]
- Burton H, Jones EG (1976) The posterior thalamic region and its cortical projection in New World and Old World monkeys. J Comp Neurol 168, 249–301. [DOI] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Mattiace LA, et al. (2002) Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry 159, 59–65. [DOI] [PubMed] [Google Scholar]
- Byne W, Fernandes J, Haroutunian V, et al. (2007) Reduction of right medial pulvinar volume and neuron number in schizophrenia. Schizophr Res 90, 71–75. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Thompson PM, van Erp TGM, et al. (2002) Cortex mapping reveals regionally specific patterns of genetic and disease‐specific gray‐matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci U S A 99, 3228–3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobia DJ, Smith MJ, Salinas I, et al. (2017) Progressive deterioration of thalamic nuclei relates to cortical network decline in schizophrenia. Schizophr Res 180, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos P, Baumann B, Krämer A, et al. (2003) Volumes of association thalamic nuclei in schizophrenia: a postmortem study. Schizophr Res 60, 141–155. [DOI] [PubMed] [Google Scholar]
- Dark C, Homman‐Ludiye J, Bryson‐Richardson RJ (2018) The role of ADHD associated genes in neurodevelopment. Dev Biol 438, 69–83. [DOI] [PubMed] [Google Scholar]
- DeVito JL (1978) A horseradish peroxidase‐autoradiographic study of parietopulvinar connections in saimiri sciureus. Exp Brain Res 32, 581–590. [DOI] [PubMed] [Google Scholar]
- Divac I, Lavail JH, Rakic P, et al. (1977) Heterogeneous afferents to the inferior parietal lobule of the rhesus monkey revealed by the retrograde transport method. Brain Res 123, 197–207. [DOI] [PubMed] [Google Scholar]
- Dorph‐Petersen K‐A, Lewis DA (2017) Postmortem structural studies of the thalamus in schizophrenia. Schizophr Res 180, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Benevento LA (1978) Projections of lateral orbital cortex to sensory relay nuclei in the rhesus monkey. Brain Res 144, 149–154. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Chiba AA (1996) The amygdala and emotion. Curr Opin Neurobiol 6, 221–227. [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, et al. (2001) Thalamic volumes in patients with first‐episode schizophrenia. Am J Psychiatry 158, 618–624. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA (2000) Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57, 65–73. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101, 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Vyas NS, Testa R, et al. (2011) Age of onset of Schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull 37, 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C, Yaun A, Cusick CG (1995) Neurochemical subdivisions of the inferior pulvinar in macaque monkeys. J Comp Neurol 363, 545–562. [DOI] [PubMed] [Google Scholar]
- Gutierrez C, Cola MG, Seltzer B, et al. (2000) Neurochemical and connectional organization of the dorsal pulvinar complex in monkeys. J Comp Neurol 419, 61–86. [DOI] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, et al. (2013) Brain volumes in schizophrenia: a meta‐analysis in over 18 000 subjects. Schizophr Bull 39, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highley JR, Walker MA, Crow TJ, et al. (2003) Low medial and lateral right pulvinar volumes in schizophrenia: a postmortem study. Am J Psychiatry 160, 1177–1179. [DOI] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, et al. (2010) Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A 107, 13135–13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homman‐Ludiye J, Kwan WC, de Souza MJ, et al. (2018) Ontogenesis and development of the nonhuman primate pulvinar. J Comp Neurol 526, 2870–2883. [DOI] [PubMed] [Google Scholar]
- Imura K, Rockland KS (2006) Long‐range interneurons within the medial pulvinar nucleus of macaque monkeys. J Comp Neurol 498, 649–666. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Bansal R, Hao X, et al. (2010) Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry 167, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG (2007) The Thalamus. New York, NY: Cambridge University Press. [Google Scholar]
- Jones EG, Burton H (1976) A projection from the medial pulvinar to the amygdala in primates. Brain Res 104, 142–147. [DOI] [PubMed] [Google Scholar]
- Kasdon DL, Jacobson S (1978) The thalamic afferents to the inferior parietal lobule of the rhesus monkey. J Comp Neurol 177, 685–705. [DOI] [PubMed] [Google Scholar]
- Kemether EM, Buchsbaum MS, Byne W, et al. (2003) Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Arch Gen Psychiatry 60, 983–991. [DOI] [PubMed] [Google Scholar]
- Kievit J, Kuypers HG (1977) Organization of the thalamo‐cortical connexions to the frontal lobe in the rhesus monkey. Exp Brain Res 29, 299–322. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL (1974) A direct input from the amygdala to the thalamus and the cerebral cortex. Brain Res 67, 169–174. [DOI] [PubMed] [Google Scholar]
- Künzle H, Akert K, Wurtz RH (1976) Projection of area 8 (frontal eye field) to superior colliculus in the monkey. An autoradiographic study. Brain Res 117, 487–492. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Lawrence DG (1967) Cortical projections to the red nucleus and the brain stem in the Rhesus monkey. Brain Res 4, 151–188. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE (1930) The Thalamus of Tarsius. J Anat 64, 371–414. [PMC free article] [PubMed] [Google Scholar]
- Leichnetz GR (1982) Connections between the frontal eye field and pretectum in the monkey: an anterograde/retrograde study using HRP gel and TMB neurohistochemistry. J Comp Neurol 207, 394–404. [DOI] [PubMed] [Google Scholar]
- Letinic K, Kostović I (1997) Transient fetal structure, the gangliothalamic body, connects telencephalic germinal zone with all thalamic regions in the developing human brain. J Comp Neurol 384, 373–395. [DOI] [PubMed] [Google Scholar]
- Letinic K, Rakic P (2001) Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci 4, 931–936. [DOI] [PubMed] [Google Scholar]
- Levy SE, Giarelli E, Lee L‐C, et al. (2010) Autism spectrum disorder and co‐occurring developmental, psychiatric, and medical conditions among children in multiple populations of the United States. J Dev Behav Pediatr 31, 267–275. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez‐Burgos G (2000) Intrinsic excitatory connections in the prefrontal cortex and the pathophysiology of schizophrenia. Brain Res Bull 52, 309–317. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA (2009) Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest 119, 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sroubek A, Kelly MS, et al. (2012) Atypical pulvinar‐cortical pathways during sustained attention performance in children with attention‐deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 51, 1197–1207.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye A (2012) Drishti: a volume exploration and presentation tool, In: Developments in X‐Ray Tomography VIII, SPIE Proceedings. (eds Stock SR.), vol. 85060 International Society for Optics and Photonics: San Diego, CA: 10.1117/12.935640 [DOI] [Google Scholar]
- Lyon DC, Nassi JJ, Callaway EM (2010) A disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkey. Neuron 65, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TP, Lynch JC, Donahoe DK, et al. (1998) Organization of the medial pulvinar nucleus in the macaque. Anat Rec 250, 220–237. [DOI] [PubMed] [Google Scholar]
- Masterson SP, Li J, Bickford ME (2009) Synaptic organization of the tectorecipient zone of the rat lateral posterior nucleus. J Comp Neurol 515, 647–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (1981) A cortical network for directed attention and unilateral neglect. Ann Neurol 10, 309–325. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1990) Large‐scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28, 597–613. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Van Hoesen GW, Pandya DN, et al. (1977) Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res 136, 393–414. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA (1993) Visual pathways to perception and action. Prog Brain Res 95, 317–337. [DOI] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, et al. (2006) Thalamic connections of the auditory cortex in marmoset monkeys: core and medial belt regions. J Comp Neurol 496, 72–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mothe LA, Blumell S, Kajikawa Y, et al. (2012) Thalamic connections of auditory cortex in marmoset monkeys: lateral belt and parabelt regions. Anat Rec (Hoboken) 295, 822–836. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, et al. (1975) Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol 38, 871–908. [DOI] [PubMed] [Google Scholar]
- Mundinano I‐C, Kwan WC, Bourne JA (2015) Mapping the mosaic sequence of primate visual cortical development. Front Neuroanat 9, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundinano I‐C, Flecknell PA, Bourne JA (2016) MRI‐guided stereotaxic brain surgery in the infant and adult common marmoset. Nat Protoc 11, 1299–1308. [DOI] [PubMed] [Google Scholar]
- Mundinano I‐C, Fox DM, Kwan WC, et al. (2018) Transient visual pathway critical for normal development of primate grasping behavior. Proc Natl Acad Sci USA 115, 1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Lewis SW (1987) Is schizophrenia a neurodevelopmental disorder? BMJ (Clin Res Ed) 295, 681–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, et al. (2013) Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain 136, 1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren MP (1982) The development of the primate pulvinar, In: Primate Brain Evolution (ed. Armstrong E.), pp. 1–17. Boston, MA: Springer. [Google Scholar]
- Ogren MP, Rakic P (1981) The prenatal development of the pulvinar in the monkey: 3H‐thymidine autoradiographic and morphometric analyses. Anat Embryol 162, 1–20. [DOI] [PubMed] [Google Scholar]
- Olszewski J (1952) The Thalamus of the Macaca mulatta. An Atlas for Use with the Stereotaxic Instrument. Basel: S. Karger. [Google Scholar]
- Pantelis C, Yücel M, Wood SJ, et al. (2005) Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull 31, 672–696. [DOI] [PubMed] [Google Scholar]
- Pearson RC, Brodal P, Powell TP (1978) The projection of the thalamus upon the parietal lobe in the monkey. Brain Res 144, 143–148. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, et al. (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164, 942–948. [DOI] [PubMed] [Google Scholar]
- Rakic P, Sidman RL (1969) Telencephalic origin of pulvinar neurons in the fetal human brain. Z Anat Entwicklungsgesch 129, 53–82. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, et al. (2005) The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry 10, 434–449. [DOI] [PubMed] [Google Scholar]
- Rice CE, Rosanoff M, Dawson G, et al. (2012) Evaluating changes in the prevalence of the Autism Spectrum Disorders (ASDs). Public Health Rev 34, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Petersen SE (1985) Responses of pulvinar neurons to real and self‐induced stimulus movement. Brain Res 338, 392–394. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Petersen SE (1992) The pulvinar and visual salience. Trends Neurosci 15, 127–132. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Giguere M, Bates JF, et al. (1997) Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol 379, 313–332. [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014) Biological insights from 108 schizophrenia‐associated genetic loci. Nature 511, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze M, Park MTM, Cho IY, et al. (2016) Morphological alterations in the thalamus, striatum, and pallidum in autism spectrum disorder. Neuropsychopharmacology 41, 2627–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, et al., Schizophrenia Working Group of the Psychiatric Genomics Consortium , Daly MJ, Carroll MC, Stevens B, McCarroll SA (2016) Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM (2016) Thalamus plays a central role in ongoing cortical functioning. Nat Neurosci 19, 533–541. [DOI] [PubMed] [Google Scholar]
- Shipp S (2003) The functional logic of cortico‐pulvinar connections. Philos Trans R Soc Lond B Biol Sci 358, 1605–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S (2004) The brain circuitry of attention. Trends Cogn Sci 8, 223–230. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Cruce WLR, Goldberg ME, et al. (1977) Some ipsilateral projections to areas PF and PG of the inferior parietal lobule in monkeys. Neurosci Lett 6, 243–250. [DOI] [PubMed] [Google Scholar]
- Thyme S, Pieper LM, Li EH, et al. (2018) Phenotypic landscape of schizophrenia‐associated genes defines candidates and their shared functions. SSRN Journal. 10.2139/ssrn.3198823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Jacobson S (1974) Medial pulvinar afferents to frontal eye fields in rhesus monkey demonstrated by horseradish peroxidase. Brain Res 80, 395–411. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M (1982) Two cortical visual systems. In Analysis of Visual Behavior (eds Ingle DJ, Goodale MA, Mansfield RJW). Cambridge, MA: MIT Press. [Google Scholar]
- Volkmar FR, Paul R, Klin A, et al. (2005) Handbook of Autism and Pervasive Developmental Disorders, Diagnosis, Development, Neurobiology, and Behavior. 3rd ed., Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Walker AE (1938) The primate thalamus. Oxford, England: University of Chicago Press. [Google Scholar]
- Warner CE, Goldshmit Y, Bourne JA (2010) Retinal afferents synapse with relay cells targeting the middle temporal area in the pulvinar and lateral geniculate nuclei. Front Neuroanat 4, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner CE, Kwan WC, Bourne JA (2012) The early maturation of visual cortical area MT is dependent on input from the retinorecipient medial portion of the inferior pulvinar. J Neurosci 32, 17073–17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner CE, Kwan WC, Wright D, et al. (2015) Preservation of vision by the pulvinar following early‐life primary visual cortex lesions. Curr Biol 25, 424–434. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG (1993) Subcortical connections of inferior temporal areas TE and TEO in macaque monkeys. J Comp Neurol 335, 73–91. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG (1995) Transient subcortical connections of inferior temporal areas TE and TEO in infant macaque monkeys. J Comp Neurol 352, 213–226. [DOI] [PubMed] [Google Scholar]
- Weinberger DR (1987) Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44, 660–669. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Giraldo‐Chica M, Rogers B, et al. (2017) Thalamocortical dysconnectivity in autism spectrum disorder: an analysis of the Autism Brain Imaging Data Exchange. Biol Psychiatry Cogn Neurosci Neuroimaging 2, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN (1985) Corticothalamic connections of the posterior parietal cortex in the rhesus monkey. J Comp Neurol 237, 408–426. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chu K‐W, Teague EB, et al. (2013) fMRI assessment of thalamocortical connectivity during attentional performance. Magn Reson Imaging 31, 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]


