Abstract
Purpose
Hepatocellular carcinoma (HCC) is the most common form of liver cancer. In advanced cancer stages (metastatic disease and/or vascular invasion), the generally accepted standard of care is systemic therapy using sorafenib as first-line treatment and, recently, regorafenib and nivolumab as second-line treatment, but the quality of life and the prognosis of patients remain very poor. Our paper reports a case of US-guided radiofrequency ablation (RFA) of both intraparenchymal HCC and inferior vena cava tumor thrombus.
Methods
We treated a patient with HCC associated with tumor thrombus extending into vena cava after failure of sorafenib therapy using US-guided radiofrequency ablation (RFA).
Results
A good radiological and clinical response was observed in association with excellent tolerability. The patient has been followed up for 15 months from the ablation, is alive, and is in a good clinical condition without evidence of tumor recurrence.
Conclusion
This is the first case in which this minimally invasive percutaneous procedure has been successfully used to treat an HCC thrombus entering the vena cava.
Keywords: Hepatocellular carcinoma, Sorafenib, Locoregional therapy, Tumor thrombus, US-guided radiofrequency ablation
Introduction
Hepatocellular carcinoma (HCC) is the most prevalent form of primary liver cancer. HCC arises mainly in the cirrhotic liver and is one of the most common leading causes of cancer-related deaths [1]. The Barcelona clinic liver cancer (BCLC) staging system is the most widely accepted staging system for the management of HCC in cirrhosis. The system considers not only tumor spread but also hepatic function (Child–Pugh score, CPS), performance status (PST), and the presence of comorbidity [2]. Importantly, even in the same staging group patients are often quite heterogeneous in terms of patient-related characteristics, tumor burden, biology, and prognosis, which makes standardization of treatments difficult. In fact, the most representative treatment guidelines for the management of HCC around the world show differences between Europe [3], United States [4], Japan [5], and Asia [6]. In an advanced cancer stage—i.e., BCLC stage C, characterized by the presence of vascular invasion and/or metastatic disease—sorafenib is considered the standard of care therapy [3–7]. Even though sorafenib treatment allows disease control in about half of patients, it has a weak overall survival (OS) rate and is burdened by a poor safety profile [7]. Nevertheless, surgical or minimally invasive treatment modalities have been reported in the literature for the treatment of advanced HCC, such as transarterial chemoembolization (TACE), hepatic arterial infusion chemotherapy (HAIC), radiotherapy (RT), high-intensity focused ultrasound (HIFU), selective internal radiation therapy (SIRT), radiofrequency ablation (RFA), and microwave ablation (MWA). All these techniques have been used separately or in combination according to a multidisciplinary approach [8]. Especially in patients with vascular invasion the deficient blood supply contributes to hepatic failure, so eliminating thrombosis can be considered an important target to improve hepatic function and, probably, survival chances, as has been shown using locoregional therapy (LRT) [9, 10]. In case of tumor thrombus (TT) invading the inferior vena cava (IVC), the prognosis is particularly poor since the pulmonary embolization and the massive involvement of the right atrial cavity can rapidly lead to death [11–16]. In these cases, the treatment choice is a difficult challenge. Here, we report a case of a patient with HCC in cirrhosis associated with tumor thrombus extending into the inferior vena cava treated with percutaneous ultrasound (US)-guided radiofrequency ablation. To our knowledge, this is the first case in which this minimally invasive and liver-sparing procedure has been used in patients.
Case report
In August 2016, a 54-year-old HCV-positive man (genotype 1a, HCV RNA 76,000 UI/mL; a previous non-responder to interferon and ribavirin therapy and a history of previous alcohol abuse) was referred to our institution for RFA of three HCC nodules (segment I: 2.5 cm; VI: 1.7 cm; and VIII: 1.5 cm). He had Child B 9 cirrhosis (no ascites, INR 1.8, total bilirubin 1.6 mg/dL, albumin 2.9 g/dL, and AFP 195 ng/mL). RFA was requested by our regional liver transplantation center as a bridge to transplantation because the waiting time in this center was more than 6 months and all the three nodules were treated with percutaneous US-guided RFA, obtaining complete necrosis of HCC. While waiting for the liver transplantation, 6 months later, the patient developed a new HCC nodule in segment I (3 cm), accompanied by a 3.5 cm thrombus extending into the inferior vena cava (Figs. 1, 2). On contrast-enhanced computerized tomography (e-CT), the thrombus showed a rapid enhancement in the arterial phase and wash-out in the delayed and equilibrium phases, suggesting the diagnosis of HCC tumor thrombus; a fine needle biopsy confirmed the diagnosis of tumor thrombus. AFP was 716 ng/mL, total bilirubin 3.8 mg/dL, INR 1.5, albumin 3.9 g/dL, hemoglobin 10 g/dL, and platelet count 38,000/mcL. No ascites was present.
Fig. 1.
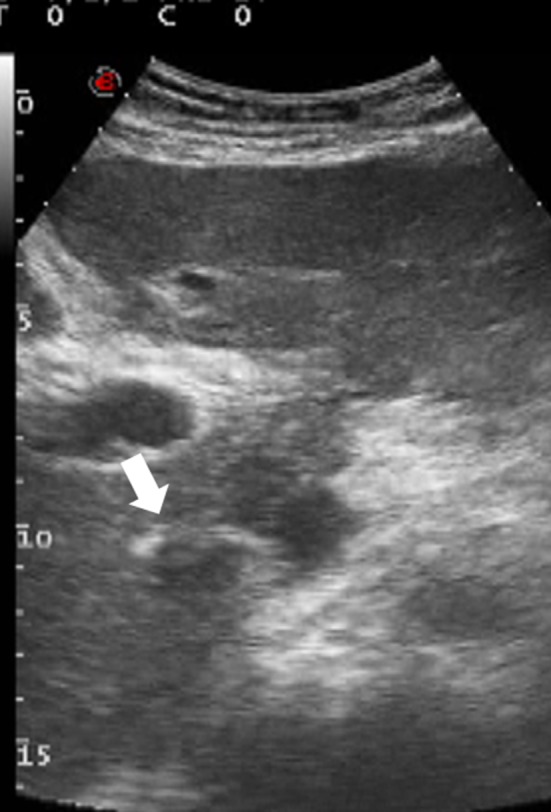
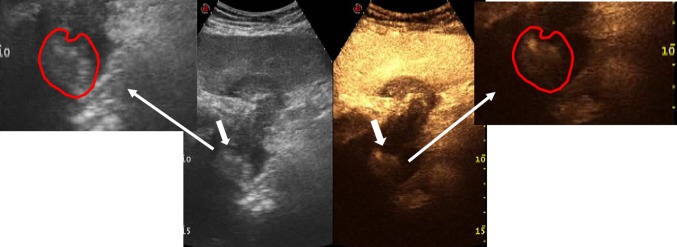
Conventional US shows thrombus (white arrow) in the IVC
Fig. 2.
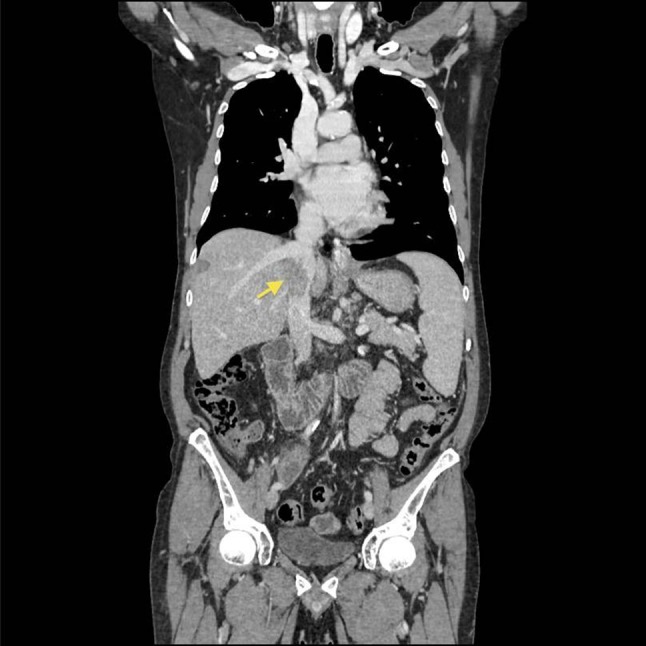
CT examination confirms the presence of thrombus in the IVC
Sorafenib was given at a dose of 400 mg daily for two months. In April 2017, a severe episode of anemia (hemoglobin 3.4 g/dL) occurred, and the patient needed blood transfusions. Endoscopy revealed severe erosive gastritis. Sorafenib was stopped. At this time, the patient was in C 10 Child’s Class. The Eastern Cooperative Oncology Group score was 2; AFP was 786 ng/mL, bilirubin 3.4 mg/dL, INR 2.3, albumin 2.5 g/dL, and PLT count 40,000/mcL. In May 2017, the nodule and part of the thrombus were treated with percutaneous ethanol injection (PEI), using 5 mL of sterile 95% ethyl alcohol: the procedure was safe, but no results were observed at contrast-enhanced US (CEUS). In the meantime, the patient developed deep weakness, grade II ascites, right pleural effusion, and severe edema of the legs that interfered significantly with deambulation: CPS was B9, and Eastern Cooperative Oncology Group score 2, while laboratory tests were mostly stable (bilirubin 2.4 mg/dL, INR 1.5, albumin 2.5 g/dL, and hemoglobin 10 g/dL). Therefore, we decided to treat the patient with two rounds of drug-eluting beads (DEB)-TACE (50 mg epirubicin), with an interval of 1 month between the two rounds: however, e-CT evaluation failed to show a reduction of tumor thrombus even though the patient showed an amelioration of ascites and edema (he was at the same time treated with diuretics). Considering the relatively young age of the patient, the clinical amelioration, the stability of the laboratory tests, but the absence of a radiological response and the increase in AFP (1401 ng/mL), in July we decided to perform a US-guided RFA, according to the results reported by Giorgio et al. on treating both parenchymal HCC nodules and portal vein tumor thrombus [9, 16]. The same operator, with more than 20 years of experience in interventional US, performed RFA of both the HCC nodule and IVC TT under US guidance and under general anesthesia. A perfused 17-gauge 20-cm-long needle with 3-cm-long active needle tip (Coll-tip Covidien) was deeply inserted percutaneously into the thrombus using the epigastric access (Fig. 3). The electrode was connected to a 472 kHz RF Cool-tip E series generator, which used a power output of 1.6 A 150 W, on standard modality. TT was treated for 7 min. The procedure went on for another 7 min after the needle was withdrawn for 1 cm, and for another 12 min after the needle was further withdrawn for 2 cm. Then, the needle was extracted, and using the same needle two other insertions made (using the technique of withdrawing to achieve proximal and distal ablation) in the lateral (8 min) and the medial portion (8 min) of the nodule. The procedure was considered concluded when the nodule and the TT appeared completely hyperechoic and did not show vascularization on CEUS (Fig. 4). Altogether, the treatment lasted 42 min. No complications occurred in the post-operative period and during the 15 months of follow-up. E-CT performed the day after the procedure was completed revealed complete necrosis of the HCC and a sensible initial shrinkage of the TT. The patient was discharged from the hospital days after completion of the procedure. One month later, a new e-CT revealed further shrinkage of the TT, as reported in Fig. 5. Concurrently, the patient’s general condition improved: complete disappearance of leg edema, ascites, and pleural effusion, and amelioration of the performance status (Eastern Cooperative Oncology Group score 1), of CPS (B7) and in part of the laboratory tests (AFP 10 ng/mL, bilirubin 2.3 mg/dL, INR 1.1, albumin 2.3g/dL, and PLT count 80,000/mcL). The patient was followed up for 15 months after the TT ablation and is still alive without evidence of recurrence. Moreover, US color-Doppler examination shows a completely patent IVC (Fig. 6a), as confirmed by CEUS (Fig. 6b). Clinically he is in a good condition: CPS is stable at B7, without ascites and pleural effusion recurrence; Eastern Cooperative Oncology Group score is 1; laboratory tests are stable (AFP 10 ng/mL, bilirubin 2.8 mg/dL, INR 1.5, albumin 2.4 g/dL, and PLT count 85,000/mcL).
Fig. 3.
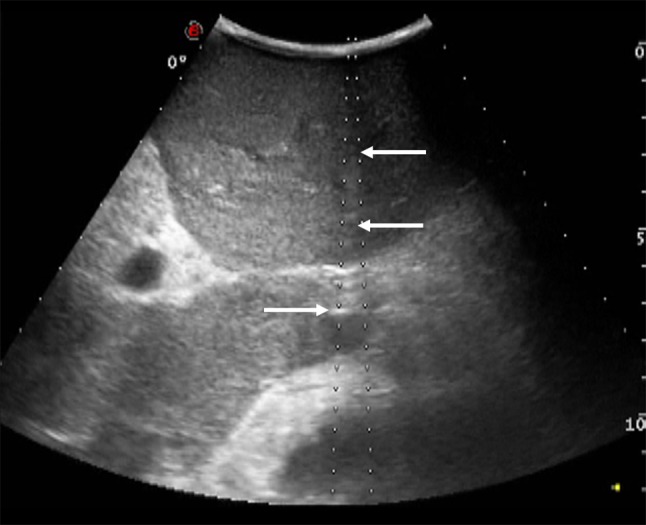
Needle (white arrows) entering tumor thrombus during US-guided RFA
Fig. 4.
Soon after RFA, CEUS examination shows that the IVC tumor thrombus (white arrows) is not enhanced in the arterial phase (white arrows on the left), demonstrating complete ablation
Fig. 5.
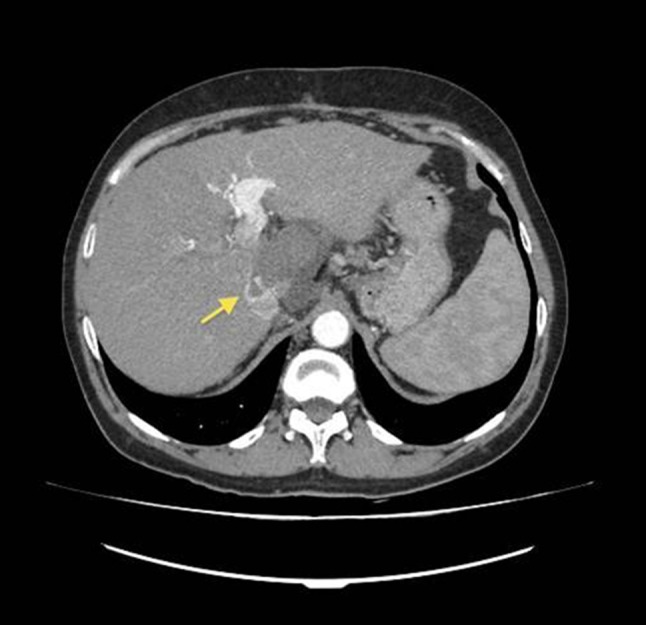
CT scan 1 month after RFA definitively confirms necrosis of HCC nodule in segment I and further regression of the IVC thrombus
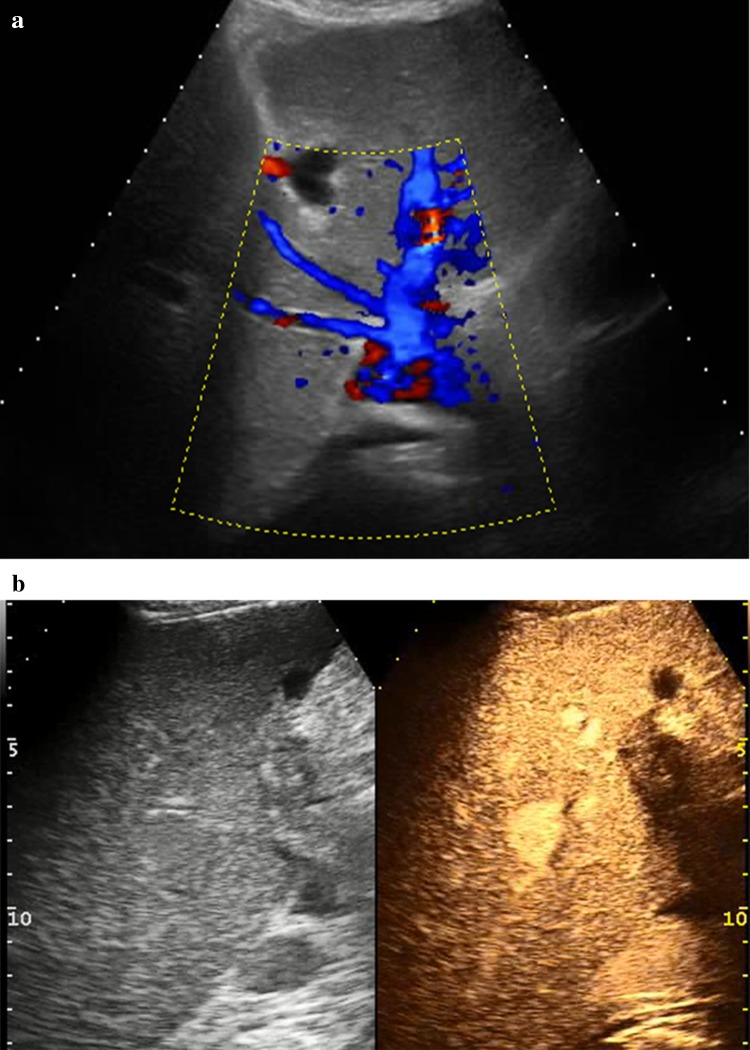
Fig. 6.
a US color-Doppler image 15 months after RFA of IVC tumor thrombus shows a still patent IVC. b CEUS 15 months after RFA confirms IVC patency: the vessel is completely visible in the portal phase without local defects
Discussion
Patients with advanced HCC (macrovascular invasion and/or metastatic disease; BCLC stage C) represent a unique clinical challenge. Macrovascular invasion generally involves the portal vein and, less commonly, the vena cava and often rapidly leads to tumor-related symptoms and death, particularly in cases where the vena cava is involved [11–16]. Even though the major international guidelines classify patients with macrovascular tumor invasion into the advanced HCC group (stage C), they specifically refer to portal vein TT and not to cava involvement [3–6]. For patients with TT, the American Association for the Study of Liver Disease (AASLD 2008) [4] and the European Association for the Study of the Liver [3] recommend the use of systemic therapy with sorafenib (an anti-angiogenetic drug) as first-line therapy because of a better OS (8.1 months in the sorafenib vs 4.9 months in the placebo group) in the SHARP study [17]. This study, as confirmed by other studies [18, 19], does not consider the type and extent of macrovascular invasion and, above all, does not compare systemic therapy with LRT. Anyway, the recent AASLD guidelines for the treatment of HCC [4], in the setting of patients with advanced HCC in Child A and in selected Child B patients, underline that it is not possible to recommend a systemic therapy over LRT because there is no evidence of superiority of one therapy over another. Moreover, in the choice of a therapeutic approach, some variables, such as the extent and localization of TT, the burden of the extrahepatic tumor, the underlying cirrhosis, and the performance status of the patient, must be taken in consideration: therapy must be tailored to the patient’s needs. Similarly, the APASL 2017 guidelines [6] indicate systemic therapy as the first-line approach, even though they consider TACE as an alternative approach. In contrast, Japanese 2014 guidelines [5] suggest the possibility of using multiple options (resection, sorafenib, TACE, HAIC) with preference according to the TT extent, but also in these guidelines there is no reference to cava TT, while locoregional therapy, particularly RFA and MWA, is not mentioned. Apart from incomplete consensus on the first-line therapy, there is also uncertainty about the second-line approach in case of sorafenib failure (loss of efficacy or the occurrence of side effects). Recently, a new anti-angiogenetic drug, regorafenib, has been approved as second-line treatment by the Food and Drug Administration (FDA) and European Medicines Agency (EMA), but not yet in Italy: unfortunately, the two drugs share the most common side effects, precluding in many cases the use of the new drug [7, 20]. Another new drug, nivolumab, which is a programmed-death 1 pathway (PD-1) blocking antibody, has been approved by FDA but not by EMA until now [21]. There are already several published and ongoing studies exploring the efficacy of LRT added to sorafenib, i.e., TACE and RT [22], HAIC [23]. A recent systematic review on the use of TACE found an overall median survival of 8 months in advanced HCC patients [24]. The only comparative trials are two recent phase III trials (SARAH and SIRveNIB) [25–27] in patients with macrovascular invasion. These trials compared sorafenib and SIRT and showed a trend to a better OS in the sorafenib group (9.9 months vs 8 months) but with a more favorable radiologic response, quality of life (QOL), and safety profile in the SIRT group. Other ongoing trials are comparing the added benefit of LRTs (radiation, TACE, and HAIC) when combined with sorafenib (NCT01730937, NCT01829035, NCT02774187, NCT01214343); interestingly, they include also patients with cava TT. Among LRTs, MWA and RFA can certainly have a role: already in 2004, a small Italian study [28] showed the possibility of ablating portal vein TT using RF and PEI, and a follow-up study by Hirooka [29] confirmed the efficacy of RF ablation of portal TT. In 2009 and 2014, Giorgio et al. [9, 10] confirmed the feasibility, safety, and efficacy (survival of 77% in treated vs 0% in untreated patients over a follow-up period of 3 years) of percutaneous RF treatment of both intraparenchymal HCC nodules and portal vein TT (“percutaneous RF thrombectomy”). In our opinion, the probable explanation for our result is that heat spreads into the vascular trunk because of a tunnel effect so that the solid neoplastic thrombus crumbles under the flux pressure in the vein and therefore is removed [9].
In patients with thrombus in the vena cava, the literature suggests that surgical therapy (extraction of the thrombus and resection of the tumor) is probably the most effective therapy because it obtains the best survival scores (mean survival varying from 11 to 19 months) [30, 31]. Unfortunately, the therapy also shows the surgery-related limit: the need of cardiopulmonary bypass (particularly if the atrial thrombus is present), risks of anesthesia and hepatic failure related to the loss of functioning hepatic tissue in such weak and sometimes decompensated patients. Recently, a case has been published of an asymptomatic and CPS grade A patient with HCC and cava TT extending in the right atria, who refused surgery and was treated with percutaneous CT-guided MWA, yielding safe and effective results [32]. Furthermore, a large clinical trial on the treatment of HCC patients with RFA of IVC TT is ongoing in China. It is hoped that such new research studies will also be carried out in Western Countries, particularly in Italy, considering the high prevalence of these kinds of tumors and patients in this country. In our case, we excluded the possibility of surgery because of the very high risk (CPS grade C) and the failure of TACE. We preferred RFA to MWA only because the needle has a smaller caliber and therefore a better safety profile. Though RF is generally considered to be burdened by limited efficacy (smaller ablation volumes, longer duration, and the heat-sink effect), we obtained a good ablation result only with a single procedure lasting 42 min thanks to the multiple insertions and the withdrawal of the needle during the ablation of the different parts of the nodule and of the TT. Apart from the good radiological response, we observed a rapid and very significant clinical improvement of the patient (probably related to amelioration of the blood flow) and excellent tolerability. It is worth mentioning that in case of neoplastic recurrence the treatment can be repeated; if necessary, and if the clinical condition of the patient allows it, also surgery can be considered, and in case of persistent HCC macrovascular invasion response, in time even transplantation (even though this is extremely more difficult). To our knowledge, this is the first report of the use of percutaneous RFA of cava TT; it demonstrates the feasibility, safety, and efficacy of this approach.
Conclusions
Percutaneous RFablation therapy might represent a promising approach in patients with HCC and tumor extension in the vena cava, even in difficult cases (decompensated patients or patients with a high risk of hepatic decompensation, contraindications, or refusal of surgery). Large-scale clinical trials are necessary to confirm this preliminary report and to clarify the benefits of a multidisciplinary approach.
Abbreviations
- HCC
Hepatocellular carcinoma
- BCLC
Barcelona clinic liver cancer
- OS
Overall survival
- TACE
Transarterial chemoembolization
- HAIC
Hepatic arterial infusion chemotherapy
- HIFU
High-intensity focused ultrasound
- US
Ultrasound
- MWA
Microwave ablation
- RFA
Radiofrequency ablation
- CPS
Child–Pugh score
- PST
Performance status
- RT
Radiotherapy
- SIRT
Selective internal radiation therapy
- LRT
Locoregional therapy
- TT
Tumor thrombus
- e-CT
Contrast-enhanced computerized tomography
- IVC
Inferior vena cava
- AFP
Alfa-fetoprotein
- PEI
Percutaneous ethanol injection
- DEB
Drug-eluting bead
- CEUS
Contrast-enhanced ultrasound
- INR
International normalised ratio
- PLT
Platelet
- FDA
Food and Drug Administration
- EMA
European Medicines Agency
- QOL
Quality of life
Funding
This study was not funded by anyone.
Compliance with ethical standards
Conflict of interest
All the authors (Pietro Gatti, Antonio Giorgio, Emanuela Ciracì, Italia Roberto, Alessandro Anglani, Sergio Spano, Fernando Rizzello, Valentina Giorgio, Stefano Semeraro) declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the participant included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Adhoute X, Penaranda G, Raoul JL, Le Treut P, Bollon E, Hardwigsen J, et al. Usefulness of staging systems and prognostic scores for hepatocellular carcinoma treatments. World J Hepatol. 2016;8:703–715. doi: 10.4254/wjh.v8.i17.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236 10.1016/j.jhep.2018.03.019 [DOI] [PubMed]
- 4.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y et al; Liver Cancer Study Group of Japan (2014) JSH consensus-based clinical practice guideline for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer 3:458–468. https://doi.org/ 10.1159/000343875 [DOI] [PMC free article] [PubMed]
- 6.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A. Chemotherapy for hepatocellular carcinoma: the present and the future. World J Hepatol. 2017;9:907–920. doi: 10.4254/wjh.v9.i21.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gbolahan OB, Schacht MA, Beckley EW, LaRoche TP, O'Neil BH, Pyko M. Locoregional and systemic therapy for hepatocellular carcinoma. J Gastrointest Oncol. 2017;8:215–228. doi: 10.21037/jgo.2017.03.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgio A, Di Sarno A, de Stefano G, Farella N, Scognamiglio U, de Stefano M, et al. Hepatocellular carcinoma with cirrhosis: are patients with neoplastic main portal vein invasion eligible for percutaneous radiofrequency ablation of both the nodule and the portal venous tumor thrombus? Am J Roentgenol. 2009;193(4):948–954. doi: 10.2214/AJR.08.2087. [DOI] [PubMed] [Google Scholar]
- 10.Giorgio A, Calisti G, Montesarchio L, Scognamiglio U, Matteucci P, Coppola C, et al. Hepatocellular carcinoma invading portal venous system in cirrhosis: long-term results of percutaneous radiofrequency ablation of both the nodule and portal vein tumor thrombus. A case control study. Anticancer Res. 2014;34:6785–6790. [PubMed] [Google Scholar]
- 11.Florman S, Weaver M, Primeaux P, Killackey M, Sierra R, Gomez S, et al. Aggressive resection of hepatocellular carcinoma with right atrial involvement. Am Surg. 2009;75:1104–1108. [PubMed] [Google Scholar]
- 12.Luo X, Zhang B, Dong S, Zhang B, Chen X. Hepatocellular carcinoma with tumor thrombus occupying the right atrium and portal vein: a case report and literature review. Medicine (Baltimore) 2015;94:e1049. doi: 10.1097/MD.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Treut YP, Hardwigsen J, Ananian P, Saïsse J, Grégoire E, Richa H et al (2006) Resection of hepatocellular carcinoma with tumor thrombus in the major vasculature. A European case–control series. J Gastrointest Surg 10:855–862 [DOI] [PubMed]
- 14.Lin HH, Hsieh CB, Chu HC, Chang WK, Chao YC, Hsieh TY. Acute pulmonary embolism as the first manifestation of hepatocellular carcinoma complicated with tumor thrombi in the inferior vena cava: surgery or not? Dig Dis Sci. 2007;52:1554–1557. doi: 10.1007/s10620-006-9129-x. [DOI] [PubMed] [Google Scholar]
- 15.Papp E, Keszthelyi Z, Kalmar NK, Papp L, Weninger C, Tornoczky T, et al. Pulmonary embolization as primary manifestation of hepatocellular carcinoma with intracardiac penetration: a case report. World J Gastroenterol. 2005;11:2357–2359. doi: 10.3748/wjg.v11.i15.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saynak M, Ozen A, Kocak Z, Cosar-Alas R, Uzal C. Sudden death: a case report of hepatocellular carcinoma with tumor thrombus extending into the right atrium. J BUON. 2007;12:556. [PubMed] [Google Scholar]
- 17.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 18.Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia–Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 19.Cheng A-L, Guan Z, Chen Z, Tsao C-J, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: Subset analyses of the phase III Sorafenib Asia–Pacific trial. Eur J Cancer. 2012;48:1452–1465. doi: 10.1016/j.ejca.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 21.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim G-A, Shim JH, Yoon SM, Jung J, Kim JH, Ryu M-H, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320–329. doi: 10.1016/j.jvir.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Song DS, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portalvein tumor thrombosis. J Gastroenterol. 2015;50:445–454. doi: 10.1007/s00535-014-0978-3. [DOI] [PubMed] [Google Scholar]
- 24.Silva JP, Berger NG, Tsai S, Christians KK, Clarke CN, MoGal H, et al. Transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis: a systematic review and meta-analysis. HPB (Oxford) 2017;19:659–666. doi: 10.1016/j.hpb.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Vilgrain V, Abdel-Rehim M, Sibert A, Ronot M, Lebtahi R, Castéra Let al; SARAH Trial Group (2014) Radioembolisation with yttrium-90 microspheres versus sorafenib for treatment of advanced hepatocellular carcinoma (SARAH): study protocol for a randomised controlled trial. Trials 15:474. 10.1186/1745-6215-15-474 [DOI] [PMC free article] [PubMed]
- 26.(2017) SARAH: a randomised controlled trial comparing efficacy and safety of selective internal radiation therapy (with yttrium-90 microspheres) and sorafenib in patients with locally advanced hepatocellular carcinoma (GS012). In: The international liver congress 2017
- 27.Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J et al; Asia–Pacific Hepatocellular Carcinoma Trials Group (2018) SIRveNIB: selective internal radiation therapy versus sorafenib in Asia–Pacific patients with hepatocellular carcinoma. J Clin Oncol 36:1913–1921. 10.1200/JCO.2017.76.0892 [DOI] [PubMed]
- 28.Poggi G, Gatti C, Teragni C, Delmonte A, Bernardo G. Radiofrequency ablation combined with percutaneous ethanol injection in the treatment of hepatocellular carcinoma and portal vein neoplastic thrombosis. Anticancer Res. 2004;24:2419–2421. [PubMed] [Google Scholar]
- 29.Hirooka M, Koizumi Y, Kisaka Y, Abe M, Murakami H, Matsuura B, et al. Mass reduction by radiofrequency ablation before hepatic arterial infusion chemotherapy improved prognosis for patients with huge hepatocellular carcinoma and portal vein thrombus. Am J Roentgenol. 2010;194:W221–W226. doi: 10.2214/AJR.09.2852. [DOI] [PubMed] [Google Scholar]
- 30.Inoue Y, Hayashi M, Katsumata T, Shibayama Y, Tanigawa N. Hepatocellular carcinoma with right atrial tumor thrombus: report of a case. Surg Today. 2011;41:1122–1129. doi: 10.1007/s00595-010-4443-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Yuan L, Ge RL, Sun Y, Wei G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol. 2013;20(3):914–922. doi: 10.1245/s10434-012-2646-2. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Wang Y, Gao W, Zheng J. HCC with tumor thrombus entering the right atrium and inferior vena cava treated by percutaneous ablation. BMC Surg. 2017;17:21. doi: 10.1186/s12893-017-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]