Abstract
The objective of this paper was to collect normative data essential for analyzing the subplate (SP) role in pathogenesis of developmental disorders, characterized by abnormal circuitry, such as hypoxic‐ischemic lesions, autism and schizophrenia. The main cytological features of the SP, such as low cell density, early differentiation of neurons and glia, plexiform arrangement of axons and dendrites, presence of synapses and a large amount of extracellular matrix (ECM) distinguish this compartment from the cell‐dense cortical plate (CP; towards pia) and large fiber bundles of external axonal strata of fetal white matter (towards ventricle). For SP delineation from these adjacent layers based on combined cytological criteria, we analyzed the sublaminar distribution of different microstructural elements and the associated maturational gradients throughout development, using immunocytochemical and histological techniques on postmortem brain material (Zagreb Neuroembryological Collection). The analysis revealed that the SP compartment of the lateral neocortex shows changes in laminar organization throughout fetal development: the monolayer in the early fetal period (presubplate) undergoes dramatic bilaminar transformation between 13 and 15 postconceptional weeks (PCW), followed by subtle sublamination in three ‘floors’ (deep, intermediate, superficial) of midgestation (15–21 PCW). During the stationary phase (22–28 PCW), SP persists as a trilaminar compartment, gradually losing its sublaminar organization towards the end of gestation and remains as a single layer of SP remnant in the newborn brain. Based on these sublaminar transformations, we have documented developmental changes in the distribution, maturational gradients and expression of molecular markers in SP synapses, transitional forms of astroglia, neurons and ECM, which occur concomitantly with the ingrowth of thalamo‐cortical, basal forebrain and cortico‐cortical axons in a deep to superficial fashion. The deep SP is the zone of ingrowing axons – ‘entrance (ingrowth) zone’. The process of axonal ingrowth begins with thalamo‐cortical fibers and basal forebrain afferents, indicating an oblique geometry. During the later fetal period, deep SP receives long cortico‐cortical axons exhibiting a tangential geometry. Intermediate SP (‘proper’) is the navigation and ‘nexus’ sublamina consisting of a plexiform arrangement of cellular elements providing guidance and substrate for axonal growth, and also containing transient connectivity of dendrites and axons in a tangential plane without radial boundaries immersed in an ECM‐rich continuum. Superficial SP is the axonal accumulation (‘waiting compartment’) and target selection zone, indicating a dense distribution of synaptic markers, accumulation of thalamo‐cortical axons (around 20 PCW), overlapping with dendrites from layer VI neurons. In the late preterm brain period, superficial SP contains a chondroitin sulfate non‐immunoreactive band. The developmental dynamics for the distribution of neuronal, glial and ECM markers comply with sequential ingrowth of afferents in three levels of SP: ECM and synaptic markers shift from deep to superficial SP, with transient forms of glia following this arrangement, and calretinin neurons are concentrated in the SP during the formation phase. These results indicate developmental and morphogenetic roles in the SP cellular (transient glia, neurons and synapses) and ECM framework, enabling the spatial accommodation, navigation and establishment of numerous connections of cortical pathways in the expanded human brain. The original findings of early developmental dynamics of transitional subtypes of astroglia, calretinin neurons, ECM and synaptic markers presented in the SP are interesting in the light of recent concepts concerning its functional and morphogenetic role and an increasing interest in SP as a prospective substrate of abnormalities in cortical circuitry, leading to a cognitive deficit in different neurodevelopmental disorders.
Keywords: extracellular matrix, glia, human fetal brain development, interneurons, laminar distribution, neurons, subplate, synapses
Introduction
The laminar organization of cell bodies, dendrites and axons is the most remarkable feature of both developing and adult cortex (Ramón y Cajal, 1909; DeFelipe et al. 2002; Kostović & Judaš, 2015). In the fetal cortex, synapses also exhibit a characteristic laminar distribution (Molliver et al. 1973; Kostović & Rakić, 1990; Bourgeois & Rakic, 1993; Huttenlocher & Dabholkar, 1997), a phenomenon described as synaptic strata (Molliver & Van Der Loos, 1970).
Synaptic strata, bands of high and low synaptic density, were first described in the human neocortex by Molliver et al. (1973) and Kostović & Molliver (1974). In the early fetal human neocortex, synaptic strata can be detected after cortical plate (CP) formation, when synaptic contacts are present in the marginal zone (MZ; Molliver et al. 1973) and below the CP, in the subplate (SP) zone (Molliver et al. 1973; Kostović & Molliver, 1974; Kristt & Molliver, 1976; Kostović & Rakić, 1990). The bilaminar distribution of synapses continues to be the main feature of spatial cortical synaptic organization throughout midgestation in humans (Molliver et al. 1973; Kostović & Molliver, 1974). It has been proposed (Molliver & Van Der Loos, 1970; Molliver et al. 1973) that synaptic strata exhibit spatial and temporal factors in the establishment of pioneering interaction between presynaptic axons and postsynaptic neurons of a developing cortex. Deep synapses are situated below CP and distributed throughout the prominent SP zone, which has been described as deep synaptic compartment (Kostović & Rakić, 1990) and which becomes more complex during midgestation. Sub‐strata of higher and lower synaptic density, located parallel to the pial surface, have been identified using systematic analysis of electron‐microscopic sections throughout the entire thickness of the SP (Kostović & Molliver, 1974; Kostović & Rakić, 1990). In fact, the presence of synapses in cyto‐architectonically defined compartments is a crucial, basic criterion for defining the SP and gaining a new view of cortical laminar development (Kostović & Molliver, 1974). Based on early development of synapses (Molliver et al. 1973; Kostović & Rakić, 1990) and associated stratified distribution throughout the voluminous SP compartment (Vasung et al. 2016), it has been proposed that this prominent compartment plays a crucial role in development of sensory, executive and associative circuitries in the human cortex (Kostović & Molliver, 1974; Kostović & Rakić, 1990; Volpe, 1996; Kostović & Judaš, 1998, 2002, 2006; Moore et al. 2009, 2011; Judaš et al. 2010a; Kostović et al. 2014a,b; Dubois et al. 2015; Smyser et al. 2010). This proposal is in agreement with experimental evidence on the role of SP in development of afferent/thalamo‐cortical pathways (Luskin & Shatz, 1985; Blakemore & Molnar, 1990; Ghosh et al. 1990; Allendoerfer & Shatz, 1994; Molnár & Blakemore, 1995; Molnár et al. 1998; Hanganu et al. 2002, 2009; Kanold & Luhmann, 2010; Hoerder‐Suabedissen & Molnár, 2015; Hoerder‐Suabedissen et al. 2018). Evidence which supports the important role of the SP compartment in cortical connectivity development, both in human and experimental mammals, has been drawn from several areas of research. First, the SP compartment provides extracellular matrix (ECM) substrate and axonal guidance for ingrowth of afferent axons, their waiting period and target finding (Rakić, 1977; Kostović & Rakić, 1990; Molnár & Blakemore, 1995; Judaš et al. 2005; López‐Bendito et al. 2006; Kanold & Luhmann, 2010; Kostović & Judaš, 2010; Hoerder‐Suabedissen & Molnár, 2015). Secondly, SP was recognized as the crucial substrate for early spontaneous (endogenous) transient activity (Friauf & Shatz, 1991; Ghosh & Shatz, 1993; Hanganu et al. 2002; Feldman & Brecht, 2005; Hanganu et al. 2006; Khazipov & Luhmann, 2006; Luhmann et al. 2009; Moore et al. 2009; Kanold & Luhmann, 2010; Tolner et al. 2012). Thirdly, SP neurons serve as a crucial, transient link of afferent axons with developing neurons of the CP, forming a framework for cortical circuitry establishment (Ghosh & Shatz, 1992; Lein et al. 1999; Kanold et al. 2003; Kostović & Judaš, 2006, 2007; Hanganu et al. 2009). Fourthly, SP neurons pioneer the first efferent axon pathway from the cerebral cortex and formation of descending connections (McConnell et al. 1989, 1994). The pathfinding role was proposed for axons of SP neurons in organizing thalamo‐cortical afferents (Molnár & Blakemore, 1995).
The most interesting role of the SP in the human brain development is the sequential growth of afferent pathways (Nobin & Björklund, 1973; Kostović & Goldman Rakić, 1983; Kostović & Rakić, 1990; Verney et al. 1993; Verney, 1999; Kostović & Judaš, 2010, 2015; Duque et al. 2016; Krsnik et al. 2017) and their complex connections during protracted intrauterine development. During growth, afferent fibers may change position within the SP in radial (‘subpial depth’) and tangential direction, and contact postsynaptic elements of SP neurons at different positions (considering layer depth), forming transient or permanent synapses (Molliver et al. 1973; Chun & Shatz, 1988; Kostović & Rakić, 1990; Kanold & Luhmann, 2010). It is not completely understood whether and how the heterogeneous population of SP neurons of the primate cortex changes its laminar position within the SP and how this process is related to the sublaminar shifts during sequential ingrowth of afferent fibers (Kostović & Judaš, 2007; Kostović et al. 2015). It has been well documented that prominent laminar changes occur in the human brain at around 13 PCW, during SP expansion (Duque et al. 2016). Interestingly, this SP expansion (second CP; Kostović & Rakić, 1990) can be visualized on MRI in vivo in utero images (Judaš et al. 2005). Analysis of the laminar distribution of SP neurons in humans depends on the selection of reliable markers. In this respect, enormous progress has been made in the identification of molecular diversity of SP neurons in rodent brains (Wang et al. 2010; Hoerder‐Suabedissen & Molnár, 2013, 2015), expanding on findings in developing cats (Chun & Shatz, 1989), monkeys (Meinecke & Rakic, 1992) and the human brain (Kostović et al. 1991b; Delalle et al. 1997; Bayatti et al. 2008; Molnár & Clowry, 2012).
The aim of the present paper was to reveal developmental changes in the laminar distribution and maturational gradient of immunolabeled neurons and glia, ECM components and synaptic markers in the human SP compartment, and to link these findings to previously reported developmental changes in afferent pathways (Kostović & Goldman Rakić, 1983; Krmpotic‐Nemanic et al. 1983; Kostović & Rakić, 1984; Kostović & Judaš, 2010), as well as synapse distribution (Molliver et al. 1973; Kostović & Rakić, 1990). We hope that new data on the development of sublaminar complexity and structural diversity of neuronal, glial and ECM constituents of the SP will help to explain human characteristic features and contribute to the elucidation of transient functional phenomenon and the role of SP in the development of the subcortico‐cortical (Kostović & Judaš, 2002) and cortico‐cortical (Kostović & Rakić, 1990) pathways. In addition, these new data provide a solid basis for identifying the vulnerability of the SP connectivity compartment after hypoxic‐ischemic episodes and other pathogenetic events, which may lead to major developmental disorders of an unknown etiology.
Materials and methods
We processed and analyzed 35 brains, from approximately 8.5 PCW to newborn age, using different cellular, fibrillar, synaptic and ECM markers to study laminar distribution and the maturational gradient of neurons, fibers, glia, synapses and ECM in the SP compartment. These brain specimens, without macroscopic or microscopic pathological changes, are part of the Zagreb Neuroembryological Collection (Kostović et al. 1991a; Judaš et al. 2011), and were obtained during regular autopsies after medically indicated or spontaneous abortions, or following the death of infants who were born prematurely or at term age, at a number of clinical hospitals affiliated with the University of Zagreb, School of Medicine. Sampling of brain tissue was performed in accordance with the Declaration of Helsinki (2000) and was approved by the Internal Review Board of the Ethical Committee of the School of Medicine, University of Zagreb. Fetal age was estimated on the basis of crown–rump length (CRL, in millimeters) and pregnancy records, and was expressed as PCW.
Postmortem brains tissue was sliced into blocks and fixed in 4%, or 1.25% (when processing tissue for plastic Nissl sections), paraformaldehyde in 0.1 m phosphate‐buffered saline (PBS) with pH 7.4. Tissue blocks were then embedded in paraffin wax and sections prepared using a microtome with a 20‐μm thickness, 1‐μm thickness for plastic sections, and 100‐μm thickness for Golgi preparations. Classical histological Cresyl violet (Nissl) or histochemical (acetylcholinesterase, AChE) staining was then performed as described previously in our papers (Kostović & Goldman Rakić, 1983). Periodic Acid Schiff–Alcian Blue (PAS‐Alcian) histochemistry was used to analyze the laminar location and regional abundance of the ECM. To analyze the diversity of structural/morphological phenotypes of neurons and glia, we used a Stensaas modification of the Del‐Rio Hortega Golgi method (referred to below as ‘Stensaas Golgi’), as previously described in Mrzljak et al. (1988). Immunohistochemistry was performed following deparaffinization of the paraffin‐embedded blocks. In brief, sections were treated with 0.3% hydrogen peroxide, incubated in blocking solution (3% bovine serum albumin (BSA) and 0.5% Triton X‐100, Sigma, St. Louis, MO, USA) in 0.1 m PBS, and incubated overnight with primary antibodies at room temperature. We used the following primary antibodies to identify microstructural elements such as neurons (anti‐neuroserpin, Abcam, ab55587, 3 μg mL–1), glia fibers (anti‐vimentin, DAKO, m7020, 1 : 100); glia cells (anti‐GFAP, DAKO, z‐0334, 1 : 1000; anti‐Pax6, Abcam, ab5790, 1 : 75), interneurons (calretinin, 63B, Swant, 010399, 1 : 2000), indicators of histogenetic events such as synaptogenesis markers (anti‐SNAP‐25, Biolegend, 836301, 1 : 1000; anti‐synaptophysin, DAKO, m‐7315, 1 : 100). Also, different ECM markers were shown (anti‐NCAN, Sigma, HPA036814, 1 : 600; anti‐fibronectin, Sigma, F3648, 1 : 400; anti‐chondroitin‐sulfate, CS‐56, Sigma, C8035, 1 : 200). Corresponding secondary biotinylated antibodies (Vectastain ABC kit, Vector Laboratories, Burlingame, CA, USA) were subsequently applied in accordance with the manufacturer's protocol. Finally, positive staining was visualized using 3,3‐diaminobenzidine with a metal enhancer in accordance with the manufacturer's protocol (Sigma), and sections were mounted and cover‐slipped (Histamount, National Diagnostics, Charlotte, NC, USA). Images were acquired using a high‐resolution digital slide scanner NanoZoomer 2.0RS (Hamamatsu, Japan) and images prepared using Microsoft publisher (Microsoft Office 2016).
For every section used in the semiquantitative analysis part of the study, vertical ‘probes’ were designed (average width of probes approximately 1.5 mm) and the depth of cells from CP to intermediate zone (IZ) was recorded. The depth of the neuron position was measured as the distance from the pial surface and analyzed within a square area covering interrupted superficial, intermediate and deep SP defined on adjacent Nissl‐stained section. When necessary, correction for the difference in tissue shrinkage of the adjacent section was applied. For older specimens, cells were attributed to CP, SP remnants and gyral white matter. The percentage of a given type of glia and neurons was calculated and expressed as a percentage of the total number of recorded neurons within one probe (see Figs 8 and 9). The purpose of semiquantitative analysis of GFAP‐immunoreacted preparations was to reveal the maturational gradient of glia, thus the astroglia were analyzed in superficial, intermediate and deep SP and CP. The percentage of a given cell type was calculated from the total number of recorded cells and also expressed as a percentage of a given type in the superficial SP, intermediate SP and deep SP, with respect to depth in given squares. The Golgi method provides reliable results, although only staining approximately 5% of all cells. All cells in a given square of a scanned section were classified if the staining showed cell bodies and processes, and the relationship between the cells encountered in sublaminas identified on matching Nissl staining was expressed as a relative proportion of different cells in a given SP depth (deep, intermediate, superficial).
To gain a quantitative representation of the relative distribution of cells immunoreactive to calretinin in the transient zones of the fetal brain wall at 13, 15 and 17 PCW, the Fiji program (imagej) was used (Schindelin et al. 2012), after which the obtained data were processed using the python programming language (https://www.python.org/). The processed surface of the coronal sections on the cerebral wall used for obtaining the respective data was 1.3 mm2 in 13 PCW, 2.85 mm2 in 1h PCW and 5.45 mm2 in 17 PCW. The distribution of the number of calretinin‐immunoreactive cells was presented as a percentage of all encountered cells in a given area and plotted as a histogram, where one bar represents 50 μm of wall, measured from the ventricular to the pial surface.
Results
Developmental phases of the human SP were first described by Kostović & Rakić (1990) and later by Bayatti et al. (2008), Hoerder‐Suabedissen & Molnár (2015), Wang et al. (2015) and Duque et al. (2016). In this paper, we have confirmed these broad developmental phases and added additional descriptive classification of sublaminar development, taking into consideration the laminar distribution of the markers. Three criteria were used for the delineation of developmental phases: (1) the classification used by Kostović & Rakić (1990) and Duque et al. (2016); (2) developmental periods corresponding to the postconceptional age; (3) descriptive terms relating to the grade of SP sublamination. The later stationary phase (Kostović & Rakić, 1990) of SP development (after 28 PCW) was not analyzed in this paper.
Presubplate phase (8–12 PCW): SP monolayer
During the early fetal phase (8–12 PCW), after formation of the CP (around 8 PCW), presubplate can be defined as a thin disrupted layer situated between cell dense CP and fiber bundle rich IZ (Fig. 1A,B). A systemic analysis using vertical probes of section immunoreacted for calretinin showed that some of the calretinin‐immunoreactive cells are present in the presubplate zone (Fig. 1C; Meyer et al. 2000). Their presence is not specifically related to the presubplate zone because the calretinin cells are also present in IZ and the subventricular zone (SVZ), and in a dense row within the MZ. Analysis of sections impregnated with a Stensaas modification of the Del Rio‐Hortega Golgi method revealed three types of cellular elements in presubplate: sparse populations of non‐radially oriented immature neurons, transversing axons and radial glia fibers. These glia fibers (Fig. 1B) exhibited a broken appearance, which corresponds to the broken appearance of vimentin and GFAP‐positive fibers. Vimentin‐reactive fibers, presumably from radial glia, extend throughout the entire thickness of the cerebral wall, and similar reactivity is also shown on GFAP‐reacted sections (Fig. 2B). This characteristic ‘broken’ appearance distinguishes the presubplate layer from the CP and IZ. Immunostained GFAP cell bodies were not found in the presubplate or other layers, except for MZ. AChE preparations at this early stage indicated the presence of AChE‐reactive fibers in the internal and external capsule, and only in basal portions of telencephalon, with no indication of fibers entering the presubplate zone (see Kostović & Goldman Rakić, 1983; Kostović, 1986). During this early fetal phase, synaptophysin and SNAP‐25 immunoreactivity was present in the fibers of IZ and SVZ (Fig. 2D,E; see Žunić Išasegi et al. 2018). Synaptophysin reactivity just below the CP in the presubplate layer (see Kostović & Rakić, 1990) appears as a darker band at low magnification (Fig. 2E). Synaptophysin immunoreactivity and immunoreactivity for another synaptic marker SNAP‐25 both decrease towards the ventricle, in IZ and SVZ (Fig. 2D,E). Immunostaining with ECM markers (NCAN) and histochemical staining (PAS‐Alcian) were the best methods for delineating presubplate for light microscopy (Fig. 2F,G). On sections stained for these ECM markers (Fig. 2F,G), presubplate appeared as a narrow conspicuous band extending from the basal to the medial portion of the neocortical telencephalic wall. This band is somewhat wider in the basal and midlateral portions of the telencephalic wall and becomes very thin when approaching the dorsal part of cerebral hemispheres. The conclusion is that presubplate is a single layer characterized by the presence of ECM‐rich neuropil, early calretinin immunoreactive neurons, an atypical arrangement of glial fibers and synaptic markers.
Figure 1.
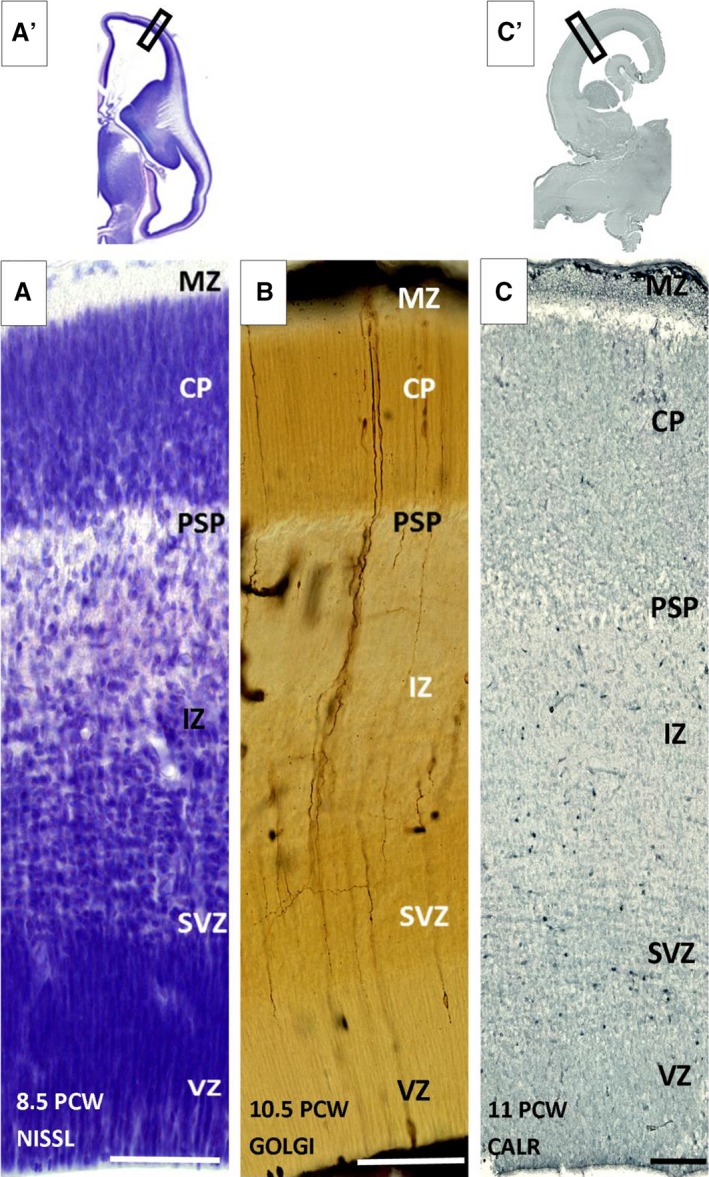
Laminar organization of the human fetal neocortex during the presubplate phase at 8.5 (A,A′), 10.5 (B) and 11 postconceptional weeks (PCW; C,C′). SP monolayer (PSP) shown on Nissl (A,A′), Golgi (B) and calretinin (C,C′) immunostained sections as a thin, disrupted cell poor monolayer between the cell‐dense cortical plate (CP) and fiber‐rich intermediate zone (IZ). CP, cortical plate; MZ, marginal zone; SVZ, subventricular zone; VZ, ventricular zone. Rectangles on low magnification images (A′,C′) correspond to the positions of enlarged images (A) and (C). Scale bar: 100 μm.
Figure 2.
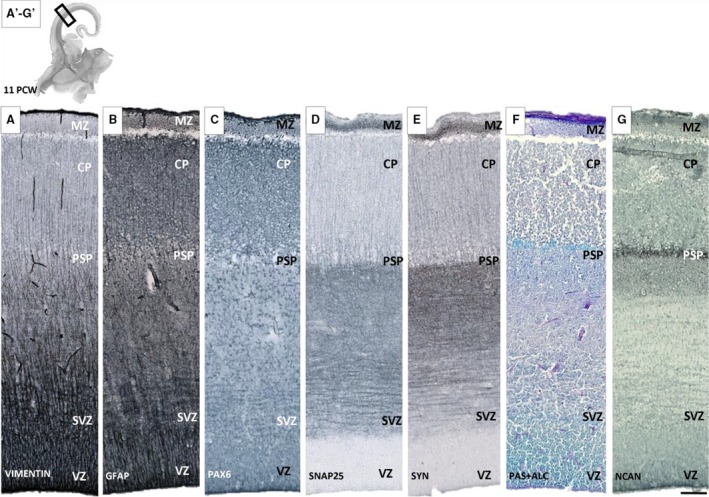
Presubplate (PSP) monolayer shown with different glial (A,B,C), synaptic (D,E) and extracellular matrix (F,G) markers on sequent coronal sections of brain aged 11 postconceptional weeks (PCW). The most obvious delineation of PSP is visible on PAS+Alcian (F) and NCAN section (G), while on sections reacted for synaptic markers such as SNAP‐25 (D) and synaptophysin (E), immunoreactivity for synaptic markers is somewhat stronger in the presubplate layer and gradually merges with moderate (‘fibrillar‐like’) reactivity of deeper layers [intermediate (IZ) and subventricular zone (SVZ)]. Glial markers vimentin (A) and GFAP (B) show a modest change in geometry at the PSP level. Rectangles on the low magnification image (A′‐G′) correspond to the position of enlarged sequential sections below. CP, cortical plate; MZ, marginal zone; SVZ, subventricular zone; VZ, ventricular zone. Scale bar: 100 μm.
SP formation (13–14 PCW; second cortical plate): bilaminar expanded SP
The overall developmental status and cyto‐architectonics are characterized by a ‘loss’ of the deep portion of the (‘second’) CP and its transformation into a new cyto‐architectonic entity, which is one of the most prominent histogenetic events in laminar development of the human neocortex (Fig. 3A). This event was described in our previous paper (Kostović & Rakić, 1990; Duque et al. 2016) and by Wang et al. 2010, with the event documented and based on immunostaining by Bayatti et al. (2008). After this event, SP can be divided into superficial (upper) and deep (lower) lamina, which gradually merge with the former presubplate (Kostović & Rakić, 1990; Duque et al. 2016). Stensaas Golgi‐impregnated sections have shown that cells in transforming the SP (‘second’ plate, SP information), display a non‐radial orientation, which is substantially different from strictly radially oriented neurons of cell dense CP (Fig. 3B). GFAP and vimentin‐immunoreactive sections displayed a well‐developed system of radial glia spanning the entire thickness of the transforming SP. However, at the lower edge of the deep transforming SP, radial glia fibers are not straight but ‘banded’, forming an angle. This corresponds to the morphology of radial fibers seen on Stensaas Golgi‐impregnated sections (Fig. 3B). In this phase, the distribution of calretinin neurons indicated a laminar preference for the deep (lower) SP (Fig. 4A,A′). Neurons are most numerous at that subpial depth, where the transforming deep (lower) SP merges with former presubplate. Semiquantitative systematic analysis of vertically oriented probes across all compartments of the telencephalic wall confirmed the qualitative observations: two ‘bins’ in the deep SP contain approximately 15% of all neurons encountered below the CP. A high density of calretinin neurons is also characteristic for the MZ (Fig. 4A,A′). A higher concentration of calretinin in two evident bins (see Fig. 4A′), namely, the deep (lower) SP and MZ, exhibit a bilaminar distribution of calretinin neurons (Fig. 4A,A′). This bilaminar distribution of calretinin in MZ and SP undergoing formation corresponds to two synaptic strata, above and below the CP, as described by Molliver et al. (1973) and Kostović & Rakić (1990). Synaptic markers are expressed in SVZ, IZ, the multilaminar axonal cellular compartment (MACC; Žunić Išasegi et al. 2018) and the deep transforming SP. ECM markers of NCAN showed increased immunoreactivity in the deep (lower) SP (Fig. 3D, dark band), indicating transforming SP as an area of increased activity and an increased amount of ECM (Kostović et al. 2002).
Figure 3.
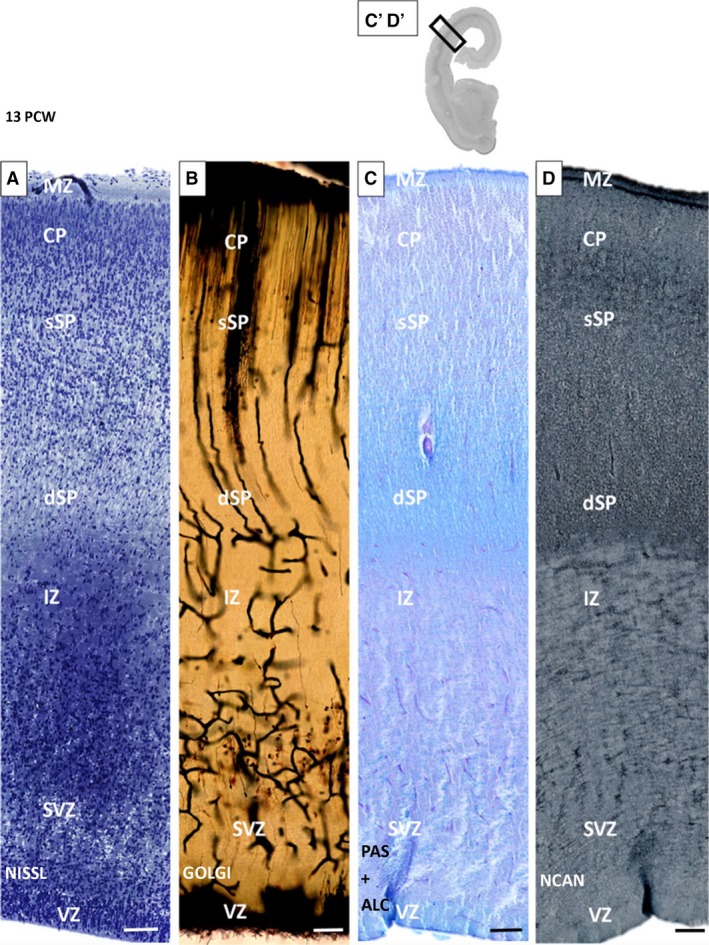
Subplate (SP) formation phase. Bilaminar expanded SP shown on histological and immunocytochemical preparations on an old human neocortex at 13 postconceptional weeks (PCW). Loss of the deep portion of the cortical plate (CP; superficial ‘upper’ SP‐sSP) and gradual merging with the presubplate (deep ‘lower’ SP; dSP) are characteristic of the bilaminar SP. Below the SP is an intermediate zone (IZ) with darker staining due to the osmification of fibers on a 1‐μm plastic section prepared for electron microscopy (A). Prominent changes in orientation of radial glia fibers and vessels within the dSP are visible on Stensaas Golgi‐impregnated sections (B). Distribution of extracellular matrix markers PAS (C) and NCAN (D) shows a gradual increasing tendency towards the deep (lower) SP (D). MZ, marginal zone; SVZ, subventricular zone; VZ, ventricular zone. Rectangles on (C′‐D′) correspond to the position of enlarged sequential sections (C) and (D). Scale bar: 100 μm.
Figure 4.
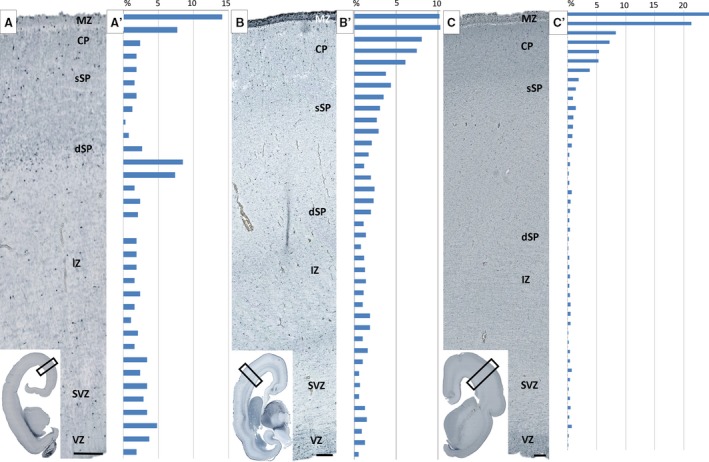
Histograms show distribution of calretinin‐reactive neurons in 13 (A,A′), 15 (B,B′) and 17 (C,C′) postconceptional weeks (PCW) old specimens. The height of each bin in the histograms represents 50 μm of ventricle–pia distance. Histograms are juxtaposed to histological sections on the left side with indicated laminar compartments. Note higher concentration of calretinin cells in the deep lamina of SP (dSP) during SP formation (A′) and increased number of calretinin cells in the cortical plate (CP) and marginal zone (MZ) during midgestation. Cells are counted in observed surface areas of 1.3 mm2 in 13 PCW, 2.85 mm2 in 15 PCW, and 5.45 mm2 in 17 PCW specimens, and number of cells is expressed as a percentage noted above (see Materials and methods). IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone. Scale bar: 100 μm.
Midgestation (15–21 PCW): trilaminar organization of the SP
Overall developmental status and architectonics
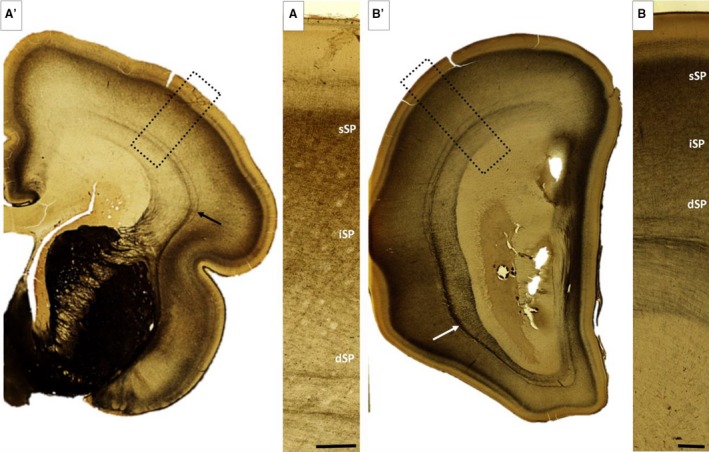
During midgestation, SP becomes the thickest compartment of the lateral cerebral wall, approximately three to four times thicker than CP (Fig. 5A). Due to lower cell packing density, a high content of ECM and absence of large fiber bundles, SP is prominent on both Nissl‐stained sections and MR images (not shown; Kostović et al. 2002). Three different floors (sublaminas) were distinguished in the SP compartment, in fact, shortly prior to this period: deep, intermediate (proper) and superficial (Fig. 5A). These three floors of the SP do not have distinct borders but gradually transition from one to the other. The superficial SP is characterized by higher cellular density compared with the intermediate SP. Cell density increases again in the deep SP. In older specimens of this developmental group, the trilaminar pattern is still present, although not so noticeable. In the occipital cortex, a band of proliferative cells was inserted between the deep SP and external sagittal stratum. The delineation of the deep SP and this cellular band became more noticeable in the older specimen (Fig. 6A) of this group, given that the fibrillar zone develops between the deep SP and this proliferative band (Fig. 6A, asterisk). We viewed this fibrillar band as a marker of developing cortico‐cortical fibers (Žunić Išasegi et al. 2018). Stensaas Golgi‐impregnated sections revealed a presence of postmigratory multipolar neurons with a relatively mature dendritic arborization and predominantly random orientation of the associated main soma‐dendritic three axes. In addition, migratory neurons displayed a typical bipolar shape, with leading and trailing processes visible throughout the entire thickness of SP (Fig. 7B: N5). Four types of SP postmigratory neurons can be distinguished on Stensaas Golgi‐impregnated sections: (1) multipolar neurons with big bodies and long dendrites (up to 0.5 mm; N1 type; dendrites rather smooth; Fig. 7B: N1); (2) neurons with a polymorphous shape, usually with a predominantly polarized arborization of dendrites (Fig. 7B: N2). Some of these neurons have bizarre shapes and look like glia (glia‐like neurons) but, in contrast to glia, processes associated with these cells emanate from the smooth body and are thick and tapered (N2 type shown on Figs 7B and 8C). The third type of neuron contains small fusiform bodies and a small number of dendrites (Fig. 7, N3). The fourth type of cell is the rarely encountered inverted pyramid (Fig. 7, N4, and Fig. 8D). A systematic semiquantitative analysis of different SP neuron types has shown that the largest population of neurons is polymorphic (38% of all encountered impregnated neurons) and fusiform (25% of all encountered impregnated cells). Both classes are evenly distributed throughout the entire thickness of the SP. Inverted pyramids are situated predominantly in the superficial portion of the SP, while multipolar neurons represent 13% of impregnated cells and are cells typical of SP proper. A semiquantitative analysis of calretinin‐immunoreacted sections indicated an increased number of reactive neurons within the superficial portion of CP (Fig. 4B,B′). Within the SP, calretinin distribution is relatively even. It is interesting to note that the moderately expressed ‘bin’ is present in the MACC, which is composed of fibers in the sagittal strata (former IZ), and disperses the outer SVZ (Žunić Išasegi et al. 2018).
Figure 5.
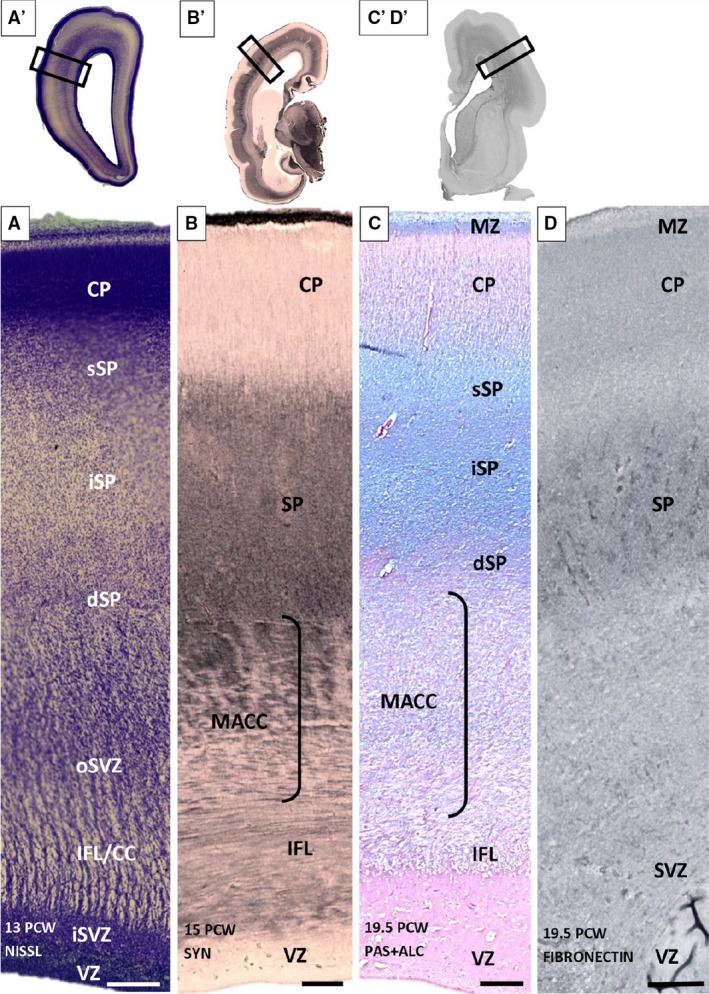
Trilaminar subplate (SP) organization shown on Nissl (A) synaptophysin (B), PAS‐Alcian (C) and fibronectin (D) immunoreacted coronal sections of 13 (A′), 15 (B,B′) and 19.5 (C,C′,D,D′) postconceptional (PCW) brains. Three SP laminas [superficial (sSP), intermediate (iSP) and deep (dSP)] have no sharp borders but are recognizable due to different cell packing density (A) on the Nissl‐stained section. The most obvious sublamination is evident on PAS‐Alcian stained sections (C). (C′D′) Section used as an example for the approximate position (rectangle) of sequent (C,D) sections. (A′,B′,C′,D′) Rectangles mark the positions of the enlarged images (A,B,C,D). CC, corpus callosum; CP, cortical plate; dSP, deep SP; IFL, inner fibrillar layer; ; iSP, intermediate SP; MACC, multilaminar axonal‐cell layer; MZ, marginal zone; oSVZ, outer SVZ; SP, superficial SP; SVZ, subventricular zone; VZ, ventricular zones. Scale bar: 200 μm.
Figure 6.
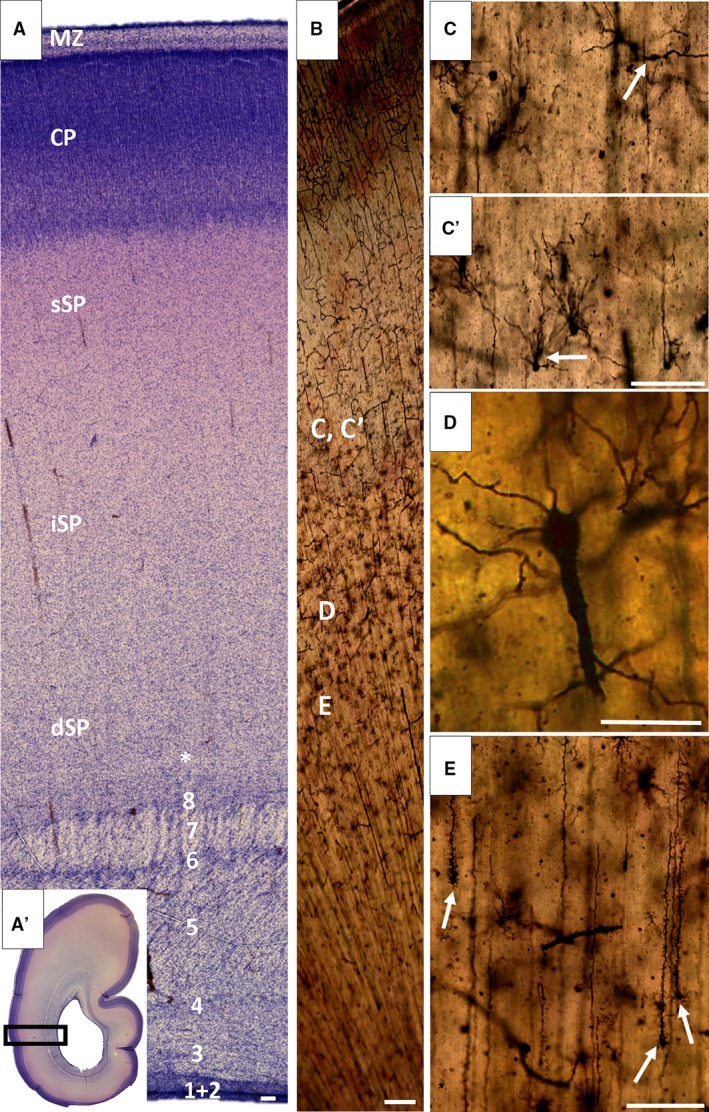
Stationary phase is characterized by the subplate (SP) as a prominent compartment, several times thicker than the cortical plate (CP). The Nissl‐stained section through to the occipital lobe of a developing cortex (25/26 postcconceptional weeks, PCW) shows a trilaminar organization of the SP, cellular superficial SP (sSP) characterized by metachromatic staining, thick intermediate (iSP) characterized by low cell density, and cellular deep SP (dSP) with cell‐poor band (A, asterisk) at its deep border, which provides evidence of initial cortico‐cortical fiber growth. The external sagittal stratum (A, 7) is well developed and marks the outermost fibrillar zone of fetal white matter. The Golgi section of the fetal brain (B) indicates the approximate position of cells in high magnified images (C,C′,D,E). During this developmental phase (26 PCW), neurons and glia of the SP show advanced maturation, characterized by different cell types (C,C′, arrow). Two special prominent types of cells are transforming radial glia (E, arrow) and rarely seen inverted pyramids (D). Numbers (A) indicate sublaminas from the ventricle towards the pia (B,D): (1) ventricular zone; (2) inner subventricular zone; (3) periventricular (callosal) fiber‐rich zone; (4–6) complex fibrillar/cellular stratum composed of the outer subventricular zone mixed with fibers of the internal sagittal stratum and composed of an inner (proliferative) cell layer (4), bulk of sagittal fibers (5) which disperse cells from the oSVZ and (6) outer (proliferative) cell layer; (7) external sagittal stratum with thalamo‐cortical projection fibers and an outermost fiber stratum (external capsule) containing fibers from the basal forebrain and (8) external transient (proliferative) cell band. CP, cortical plate; MZ, marginal zone. Scale bars = 100 μm (B‐C,E); 25 μm (D).
Figure 7.
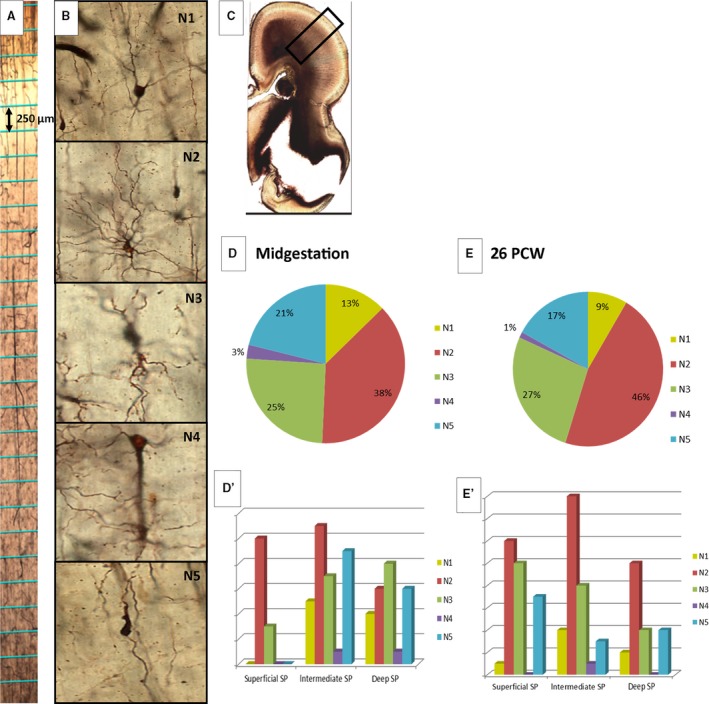
Classification of different neuronal types (B: N1–N5) on Stensaas modification of Del Rio‐Hortega Golgi method preparations. Squares marked on the real preparation (A; divided by height into smaller portions indicated in the figure), shown as an example of vertical probes for the semiquantitative method used for analyzing different neuron types. Graphical representation of different neurons found during analysis of vertical probes in midgestation (D) and at 26 postconceptional weeks (PCW; E) and their distribution according to subpial depths are shown on (D′) and (E′). Transient forms of neurons with predominantly polarized arborization processes (glia‐like neurons; B: N2) are the most numerous type of neuron. Rectangle on (C) corresponds to the position of the enlarged image (A). (SP‐subplate).
Figure 8.
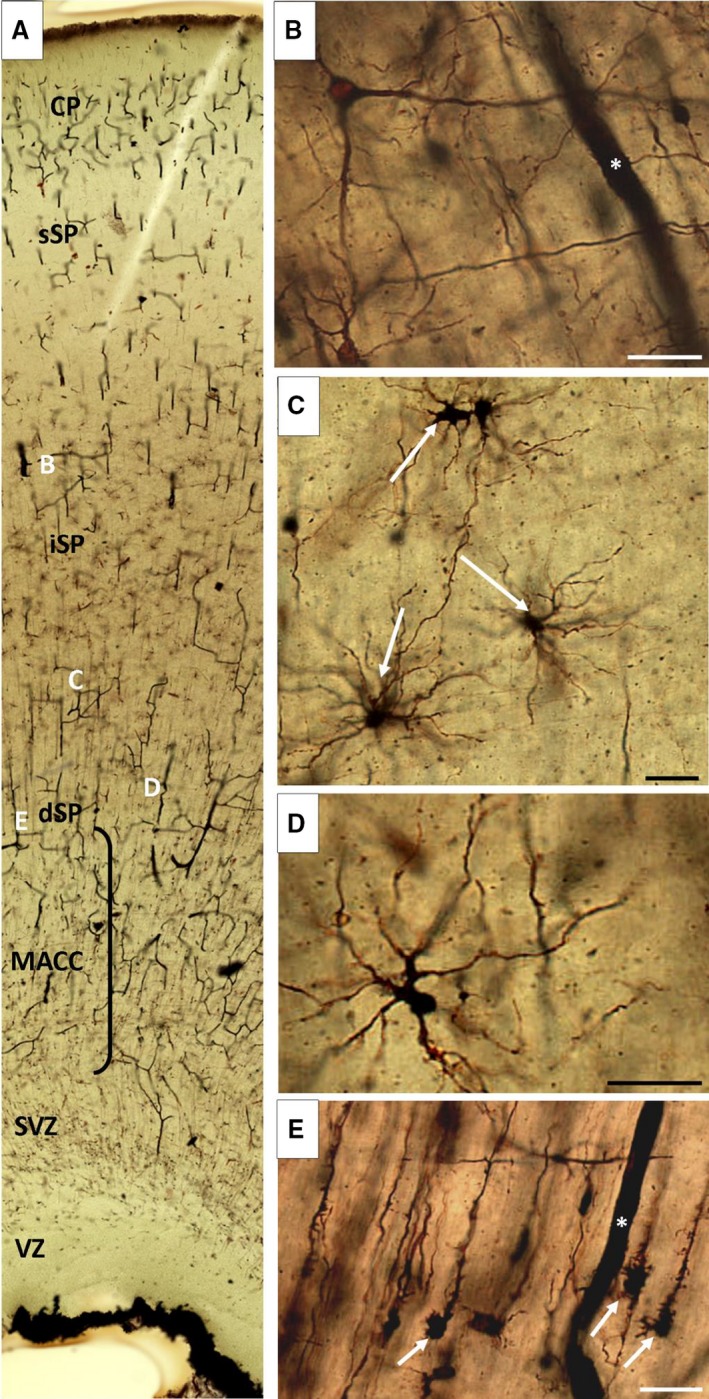
Diversity of neuronal and glial phenotypes. (A) Well impregnated Golgi section of a fetal brain at 21.5 postconceptional weeks (PCW), indicating the approximate position of cells shown at higher magnification (B–E). The most prominent type of glia are transforming radial glia (E, arrow), concentrated in the deep subplate (dSP). Large neurons with long smooth dendrites stretch their processes randomly in all directions (nexus‐neurons; A). Transient glia‐like neurons frequently have a bizarre shape (D) and predominantly polarized arborization. The group of glial and neuron‐like glial cells is shown on (C). Note that the Golgi method is great for staining capillary blood vessels, which are marked with an asterisk (B,E). CP, cortical plate; MACC, multilaminar axonal‐cell layer; iSP, intermediate subplate; sSP, superficial SP; SVZ, subventricular zone; VZ, ventricular zone. Scale bar: 25 μm.
Glia architectonics
Stensaas Golgi‐impregnated sections (Fig. 8) showed a diversity of cells displaying glia‐like morphology. At this stage, the transforming radial glia cells (Fig. 8E) were the most numerous in the deep SP and were missing from the superficial SP. The star‐like astrocytes have long processes and appear relatively mature, compared with astrocyte‐like cells. Many cells show neuron‐like morphology and asymmetric aborization of processes (Fig. 8C). Large polygonal bodies are possibly immature protoplasmic astrocytes (Fig. 9B: A5). GFAP‐immunoreactive preparations were analyzed to reveal possible maturational gradients of GFAP‐reactive astrocytes (Fig. 9). During this developmental phase, GFAP‐immunoreactive sections provided numerous GFAP‐reactive particles, but the typical star‐like cells seen in Golgi‐impregnated sections were not apparent.
Figure 9.
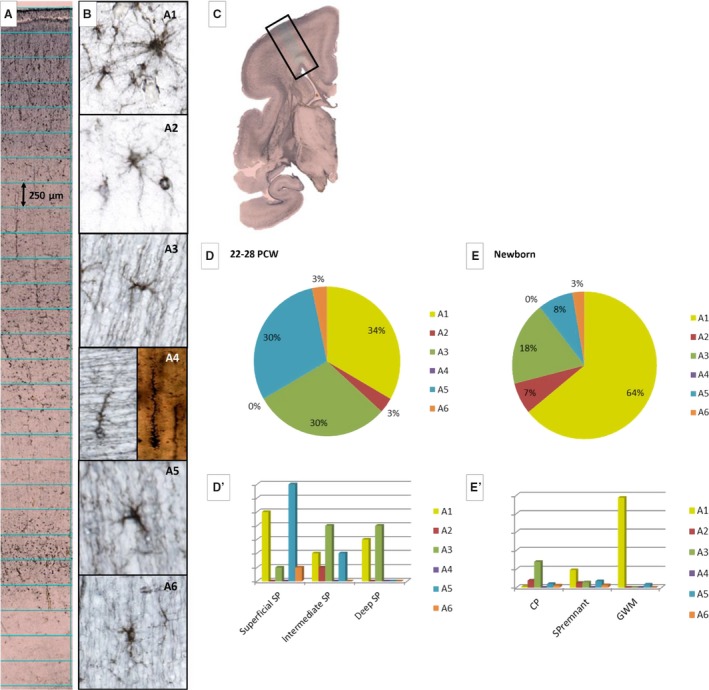
Glial type classification used for semiquantitative analysis (B: A1–A6). The most numerous cells are fibrous‐like astrocytes with long radiating processes (B: A1) and their number increases significantly during late fetal development (compare D and E). Different transient types of astrocytes are shown as A3 and A5 (B). The most ambiguous population is the one containing immature protoplasmic astrocytes (B: A2). Actual squares (height 250 μm) used for quantification of laminar distribution are marked on a vertical probe, as an example of the method used. In extremely premature brains (22–28 postconceptional weeks – PCW), transient types of glia are more numerous (B: A3) than in newborns (compare D and E) because they become more mature astrocytes later. The number of GFAP‐reacted transforming radial glia (B: A4) is significantly lower compared with the Stensaas modification using the Del Rio‐Hortega Golgi method, probably due to incomplete ‘staining’ of cell processes. The transient type of glia (B: A5) is more numerous at 22–28 PCW compared with the newborn period. Note the dramatic increase of astrocytes in gyral white matter (GWM) and subplate (SP) remnant (E′). Rectangle on (C) corresponds to the position of enlarged image (A).
During this period, there was a significant shift of synaptophysin immunoreactivity towards the SP (Fig. 5B) with a gradual increase of immunoreactivity in the superficial SP. The dispersion of immunoreacted synaptic terminals throughout the SP is a significant indicator of synaptogenesis and clearly corresponds to the data on synapse formation presented in our previous papers (Molliver et al. 1973; Kostović & Rakić, 1990).
AChE fibrillar staining showed predominant activity in the deep SP, which corresponds to the ingrowth of thalamo‐cortical and basal forebrain afferents (Kostović & Goldman Rakić, 1983; Kostović & Rakić, 1984; Krsnik et al. 2017; Žunić Išasegi et al. 2018). In this period, PAS‐Alcian staining showed a spread of activity throughout the SP across the hemisphere (Fig. 5C), where one can see three SP sublaminas stained with different intensity. A similar staining pattern was observed for other ECM markers such as fibronectin. Increased fibronectin immunoreactivity (Fig. 5D) and NCAN (not shown) was present in the zone of fiber ingrowth (deep SP; entrance zone) and the superficial SP, where fibers accumulate and find cues for the cortical target selection.
Stationary phase (22–28 PCW): trilaminar organization of the still present SP with different markers involved
Overall developmental status and cyto‐architectonics
During 22–28 PCW, the SP increased in size and provided the most prominent (thickest) and voluminous compartment of the human cortical anlage and the most voluminous compartment in the entire cerebral wall (Vasung et al. 2016). Knowing the deep landmark (external sagittal stratum) and superficial border (cell densely packed CP), it becomes easy to delineate the SP, even on Nissl‐stained sections (Fig. 6A,A′). SP delineation occurs relatively easily in the occipital cortex (Fig. 6A′), but in the frontal cortex, deep delineation depends on recognition of the external capsule radiation, which is well defined on AChE preparations (Fig. 11, arrow). Three broadly defined floors – sublaminas – are discernible: the most superficial part is metachromatic (pinkish red), the middle portion is lighter and less cellular, and the deep portion shows a less cell‐poor band, presumably presenting a zone of trajectory and ingress of associative fibers (Fig. 6A, asterisk). These modest differences in staining properties of the SP compartment led us to define this phase as having a trilaminar organization. Using other markers, especially AChE staining, trilaminar organization was confirmed (Fig. 11). In this phase of SP development, the distribution of neurons was qualitatively analyzed on Stensaas Golgi preparations and GFAP‐immunoreacted histological sections. Analysis of the Stensaas Golgi sections revealed a great diversity of neuronal phenotypes, which were classified as shown in Fig. 7B (N1–N5): the most prominent neuronal type is N1 type exhibiting a polymorphic cell body and very few long dendrites up to 2 mm in length. These peculiar neurons were not numerous (approximately 10%) but showed a slight laminar preference for intermediate (nexus) sublamina of the SP (Fig. 7E). The second type is N2 neurons with a small cell body and numerous processes – multipolar neurons – which branch predominantly on one side of the cell and are the most numerous, with a tendency to become concentrated in the intermediate SP. Some of these neurons resemble glial cells in terms of morphology and the best term for these neurons is ‘glia‐like neurons’ (Fig. 7B: N2). The third type of neuron (Fig. 7B: N3) has a small fusiform cell body displaying few processes, usually elongated in the direction of the main axis of the soma. They form the second largest population on Stensaas Golgi preparations (Fig. 7E). The fourth type of neuron (inverted pyramids; Fig. 7B: N4) is not very frequent. Interestingly, during this phase, migratory neurons, characterized by a bipolar shape and a thick leading process as well as thin trailing process (Fig. 7B, N5), are still numerous in the SP. Sections immunoreacted for calretinin showed an even distribution across all three SP floors (Fig. 4C, C′). Neuroserpin immunoreactive neurons show a clearly laminar preference: the majority are located in the superficial SP and CP (Fig. 10A; Kondo et al. 2015). Stensaas Golgi preparations showed well impregnated and relatively mature glia cells (classification of the following types are presented in Fig. 9B). The first type are fibrous‐like astrocytes with long, radiating processes (Fig. 9B: A1). The second type are prospective protoplasmic astroglia with a larger cell body and densely arranged cell processes (Fig. 9B: A2). The third type are characterized by small cells with few processes (Fig. 9B: A3). Transforming radial glia (Fig. 9B: A4) is the most prominent glia cell type, characterized by numerous laminated expansions along the main, usually radially oriented, axis and very spiny cell body (Fig. 9B: A5). We considered glia cells with polarized arborization of processes as a transient type of glia; they formed the second largest population of glia during this period (Fig. 9D). These glia cells show an arborization of processes similar to neurons (Fig. 9B: A5), and we described them as neuron‐like glia. Structural differences between this type of glia and the glia‐like neurons are not clear on Stensaas Golgi preparations. However, these cells appear regularly on GFAP‐immunoreacted sections. There are two possible structural criteria to distinguish neuron‐like glia from glia‐like neurons: tapered thickness of the main processes and the presence of small rough cell body (spiny) in glia. With respect to the 100 visualized scanned cells, fibrous astrocytes represented 34%, protoplasmic 3%, neuron‐like glia 30% and immature glia with few processes 30% of all cells. Transient neuron‐like glia (Fig. 9B:A6) showed a preference for the superficial SP (Fig. 9D′).
In this phase, GFAP cell bodies were seen throughout the SP for the first time. Poorly differentiated GFAP cells were seen in the CP (Fig. 10C). In this developmental phase, GFAP‐reactive fibers were seen across SP and CP. The appearance of immature astrocytes differs from the morphological phenotype observed on Golgi preparations. The basic difference is that GFAP‐reactive astrocytes show a less rich arborization of processes (number and length of processes) and therefore appear less differentiated.
Figure 10.

Laminar distribution of neuronal (A,A′), glial (B,B′,C,C′), synaptic (D,D′) and extracellular matrix (ECM) markers (E,E′) in the neocortical subplate (SP) during the trilaminar organization phase, shown on sections of 23‐ (E′), 24.5‐ (C′) and 25‐ (A′,B′,D′) postconceptional weeks (PCW) old brains. Neuroserpin neurons (A) are concentrated in the cortical plate (CP) and superficial SP (sSP). GFAP architecture (B,C) shows gradual changes in GFAP‐reactive cells from sagittal strata to CP. Note the presence of regularly aligned radial glia fibers in the intermediate portion of the subplate (marked with 2 on C). (C1,C2,C3) Differences in glial architecture of sSP (marked with 1 on C), iSP (marked with 2 on C) and dSP (marked with 3 on C). Synaptic markers show characteristic reactivity in sSP and CP (D) in concordance with distribution of the synapses. ECM marker fibronectin (E,E′) is expressed throughout the SP, with the highest concentration in the sSP of frontal and medial occipital cortex. Rectangles on (A′,B′,C′,D′) correspond to the positions of enlarged images below them. Numbers 1, 2, 3 on (C) correspond approximately to the positions of enlarged (C1,C2,C3). CC, corpus callosum; dSP, deep SP; iSP, intermediate SP; MZ, marginal zone. Scale bars: 500 μm (A–E); 50 μm (C1–C3).
Laminar distribution of AChE reactivity and synaptophysin reactivity
The most prominent finding in sublaminar SP distribution in this developmental period is sublaminar AChE histochemical reactivity and synaptophysin immunoreactivity, where AChE reactivity of deep and intermediate SP is matched by a similar distribution of synaptophysin immunoreactivity (Figs 10D and 11). ‘Accumulation’ of AChE reactivity in the superficial SP conformed to stronger synaptophysin reactivity. Finally, the AChE‐reactive band in the CP aligned closely with the synaptophysin‐immunoreactive band in the middle of CP (Figs 10D and 11). ECM markers, the AChE fibrillar marker and synaptic marker remained accumulated in superficial SP, but were also present in the CP (Figs 10D,E and 11). Glia maturation follows the distribution of ECM (Fig. 10E) and synaptic markers (Fig. 10D), ascending towards the CP.
Figure 11.
AChE histochemical sections during 24–26 postconceptional weeks (PCW). Subplate (SP) shows clear trilaminar distribution with the highest intensity in the superficial SP (sSP), moderate staining in intermediate SP (iSP) and the weakest staining in the deep SP (dSP). (A,B) Arrow indicates the external sagittal stratum, which serves as a reliable marker of the deep border of the SP. Rectangles in (A′) and (B′) mark the approximate position of the enlarged images (A,B). Scale bar: 500 μm.
Resolution of the SP, near term (after 36 PCW) and newborn‐age: the SP layer as a monolayer
Overall cyto‐ and fiber‐architectonics of the neocortical portion of the cerebral wall is characterized by the gradual resolution of transient laminar organization and significant differences in the cyto‐architectonic organization of transient fetal compartments. First, the volume of proliferative zones, VZ and SVZ, is decreased (Vasung et al. 2016). Despite the six‐layer pattern of laminar organization (Brodmann, 1909), the neocortex shows immature features and significant differences when compared with the newborn cortex. First, MZ shows fetal features expressed in sublamination and the presence of a subpial granular layer. Secondly, in the CP, supragranular layers are densely packed, which is characteristic of immaturity. Thirdly, the SP is transformed into a single layer – band – situated between the CP and white matter of developing gyri and sulci, but is nonetheless well developed. It gradually diminishes in thickness and volume, and is described as a narrow, plexiform, ECM‐rich SP‐remnant in the newborn brain (Kostović et al. 2014a). After 36 PCW, the laminar distribution of synaptophysin immunoreactivity gradually diminished (Fig. 12D), and in the newborn brain, synaptophysin immunoreactivity of the SP remnant showed a pattern similar to the CP. In the CP, synaptophysin was evenly distributed across layers. Coinciding with this process was a decrease in overall CP and SP remnant AChE reactivity, but the AChE‐reactive band remained as an interface between the SP remnant and layer VI (Kostović et al. 2014a,b). The most interesting change is the loss of chondroitin sulfate (CS‐56) immunoreactivity in the SP remnant (Fig. 12A–C) of near term and term specimens. This phenomenon was described by Kostović et al. (2014a) as a very prominent feature of all neocortical regions (Fig. 12A–C).
Figure 12.
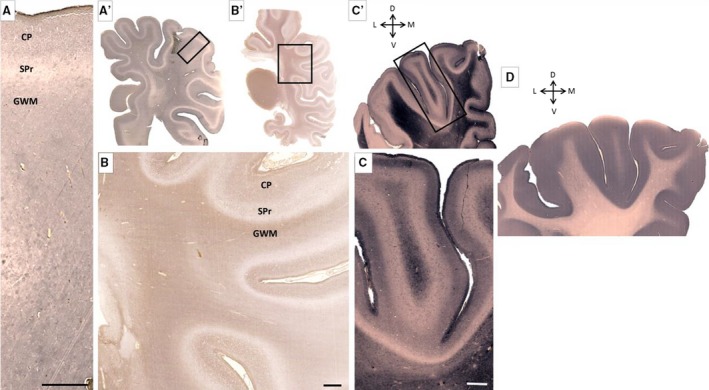
Characteristic distribution of extracellular matrix marker chondroitin sulfate (A–C) and synaptophysin (D) at the newborn age. Subplate remnant (SPr), seen as wavy unstained line, along the hemisphere (A,A′,B,B′,C,C′) is in contrast to moderately stained underlying gyral white matter (GWM) and cortical plate (CP). Changes in synaptophysin distribution are very prominent and immunoreactivity involves both SPr and CP (D). Rectangles in (A′, B′ and C′) mark approximate positions on (A, B and C), respectively. Scale bar: 1 mm.
Glia architectonics and distribution of GFAP‐positive cells
GFAP‐immunoreacted sections, at low power magnification, showed three broad compartments. The first was the superficial cortical compartment consisting of MZ and CP and indicating a somewhat darker staining than the underlying second, deeper cortical compartment, the SP remnant, which contains dispersed cellular elements. The third compartment of this glial cytoarchitecture is gyral white matter with a somewhat darker staining and GFAP‐reactive star‐like cells (astrocytes), which are aligned along the main axis of gyri. Thus, a lower cell packing density and random orientation of GFAP‐reactive astroglia are characteristics of the SP, and distinguishing this layer from the cortical layer above and gyral white matter below. Analysis of the distribution of GFAP‐reactive cells showed that glia cells in the CP are immature in terms of their length and number of processes. The SP remnant is characterized by its diverse glial reactive elements. Some cells resemble the phenotype of fibrous astrocytes with radiating unbranched processes (Fig. 13C1, C2), whereas others are similar to CP astrocytes (Fig. 13C4). Deep in the SP remnant is a change in glial architectonics, caused by a rather uniform type of GFAP‐reactive cell and resembling a phenotype of fibrous astrocytes with long processes of uniform thickness emanating from around the entire small cell body. In terms of maturity and a well‐defined fibrous astrocyte shape, gyral white matter GFAP cells are similar to astrocytes from the major fiber bundles, such as the internal capsule (Fig. 13D). In conclusion, gyral white matter has only fibrous astrocytes, CP has immature protoplasmic astrocytes and the SP remnant seems to contain a mixture of different glial phenotypes. Neuron‐like GFAP‐reactive cells nonetheless may be readily found in the SP remnant (Fig. 13C1). A semiquantitative analysis of the distribution of GFAP‐reactive glia cells revealed significant maturational shifts of astrocytes during the last trimester of gestation. The percentage of well differentiated astrocytes in the general population of GFAP‐reactive cells almost doubled (64% of all glia GFAP‐reactive cells; Fig. 9E). The number of mature‐looking astrocytes (Fig. 9B: A1) is greatest in gyral white matter, where these cells form the vast majority of GFAP‐reactive cells (Fig. 9E′). A high concentration of aligned fibrous astrocytes in gyral white matter helped to distinguish this compartment from the more randomly organized SP. The maturational trend was also seen as a decrease in the number of immature cells (Fig. 9B: A3). However, in terms of SP organization, we found that the most interesting developmental change was a decrease in the portion of transient forms of glial cells (Fig. 9B: A5), which were more likely involved in some transient developmental events (compare Figs 9E,D).
Figure 13.
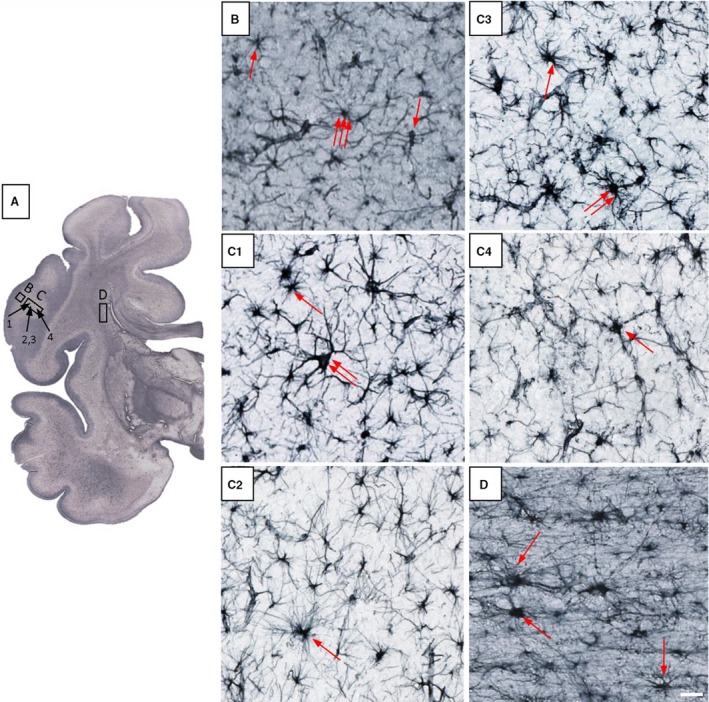
Glia architectonics in 38 postconceptional weeks (PCW) old brain shows clear differences between cortical plate (CP; B), subplate remnant (SPr; C1–C4) and fetal white matter – capsula interna (D). SPr shows typical astrocytes (C1,C2 – one arrow), some giant neuron‐like GFAP‐reactive cells (C1 – two arrows) and prospective protoplasmic astrocytes (C3 – two arrows; C3,C4 – single arrow). Identification of fibrous astrocytes is possible due to their radiating unbranched processes, whereas protoplasmic astrocytes are not easy to identify because they show immature, partially branched processes. Rectangles marked with (B, C and D) indicate approximate positions of cells shown on higher magnified images, juxtaposed to the right. Scale bar: 25 μm.
Discussion
In this study, we have been able to confirm that combined use of neuronal, glial, ECM, fibrillar and synaptic markers (Kostović & Molliver, 1974; Kostović & Rakić, 1990; Kostović et al. 2002, 2014a; Hoerder‐Suabedissen & Molnár, 2013, 2015) allows delineation of the SP zone. We subsequently extended these findings to previous unexplored subtle laminar changes and maturational gradients of glia and neurons.
In the following paragraphs of this discussion, we will compare this new data on laminar development of cellular and extracellular markers with (1) previous descriptions of the sublaminar distribution of synapses (Molliver et al. 1973; Kostović & Molliver, 1974; Kostović & Rakić, 1990), interneurons (Kostović et al. 1991b; Meinecke & Rakic, 1992; Delalle et al. 1997; Hoerder‐Suabedissen & Molnár, 2013; Pedraza et al. 2014) and fiber systems (Kostović & Goldman Rakić, 1983; Kostović & Rakić, 1984; Molnár et al. 1998; López‐Bendito & Molnár, 2003; Kostović & Judaš, 2010; Pedraza et al. 2014) and (2) distribution of glial elements (Kostović et al. 1975; Schmechel & Rakic, 1979; Zecevic, 2004; Jakovcevski & Zecevic, 2005; Jackocevski et al. 2009). We will elaborate on the significance of SP laminar distribution for studying the vulnerability of developing human cerebral wall (Volpe, 1996; McQuillen et al. 2003; Kanold & Luhmann, 2010; Kostović et al. 2014b).
Changes in the sublaminar organization of SP, spatiotemporal growth pattern of afferent pathways and development of synaptic strata
One of the most interesting observations in our study is the timing of changes in sublaminar distribution of SP neurons, synaptic markers, ECM and astroglia, which occur concomitantly with previously described sequential ingrowth of afferent fibers on human postmortem material (Kostović & Krmpotić Nemanić, 1975; Kostović & Goldman Rakić, 1983; Kostović & Rakić, 1984; Hevner, 2000; 2010Kostović & Judaš, 2002, 2010; Kostović & Jovanov‐Milošević, 2006; Vasung et al. 2010) and development of synapses (Molliver et al. 1973; Kostović & Molliver, 1974; Kostović & Rakić, 1990). In addition, we found the existence of sublaminar changes in the reactivity of ECM (GAGs and proteoglycans markers) which correspond to a general developmental pattern of ECM reactivity in the SP (Kostović et al. 2002; Jovanov Milošević et al. 2014). Thus, it seems that all elements that constitute the SP (axons, neurons, ECM, synapses, glia) participate in its morphogenetic shaping, during afferent fiber ingrowth concomitant with a characteristic time schedule and spatial distribution. The most dramatic change occurs between 13 and 15 PCW with the appearance of bilaminar SP during secondary expansion of the SP (Duque et al. 2016), when major classes of afferents participate in this process of SP proper formation, which is clearly detectable on human Nissl‐stained sections (Kostović & Rakić, 1990; Kostović et al. 2011) and with the use of MR in vivo in utero imaging (Judaš et al. 2005). The process of secondary expansion and development of bilaminar SP was noticed not only in primates (Duque et al. 2016) but also in the fetal cat (Luskin & Shatz, 1985). The contribution of this paper to a better understanding of cellular events related to bilaminar SP development, consists in showing the associated involvement of calretinin neurons and changes in radial glia orientation. The curved and wavy radial glia fibers, at the level of the expanded SP, are perhaps related to a loss of the radial orientation of neurons in the deep portion of CP, whereupon, ‘losing’ their radial orientation, they become SP constituents (Kostović et al. 2011; Duque et al. 2016).
Considering the early development of calretinin neurons in the human brain (Bayatti et al. 2008; González Gómez & Meyer, 2014; Hladnik et al. 2014; Radonjic et al. 2014; Alzubi et al. 2017) and monkey fetal cortex (Petanjek et al. 2009), our observation of the transient accumulation of calretinin neurons between CP and IZ in the presubplate during SP formation is of particular interest. This phenomenon can be explained by the increased interaction and synaptic engagement of calretinin neurons with new, dislocated populations of cells originating from the expanded SP (Bayatti et al. 2008; Duque et al. 2016), changing the orientation from radial to non‐radial (Kostović & Rakić, 1990). The other possibility is that dislocated neurons of the deep (‘second’) CP (Kostović & Rakić, 1990) indicate calretinin. This second possibility would imply that a certain percentage of neurons in the expanded ‘second plate’ (Kostović & Rakić, 1990) may express a calretinin‐GABAergic neurotransmitter profile (Chun & Shatz, 1989; Hanganu et al. 2002; Al‐Jaberi et al. 2015), while other dislocated neurons might show a glutamatergic neurotransmitter profile (Finney et al. 1998; Hanganu et al. 2002). Engagement of calretinin neurons in the formation of expanded SP conforms to the concept of an extraordinary increased number of calretinin neurons and their functional role in both the fetal (Bayatti et al. 2008; González Gómez & Meyer, 2014; Hladnik et al. 2014), and adult cortex (DeFelipe et al. 1999).
During midgestation, thalamic and basal forebrain afferents gradually invade the SP along the extension of the hemispheres (Kostović & Goldman Rakić, 1983; Kostović & Rakić, 1984; Hoerder‐Suabedissen & Molnár, 2015) and undergo a ‘waiting’ period (Rakić, 1977). A more illustrative combined term for this period might be ‘growing, navigating and waiting’, given that axons grow long in length in the sagittal strata before reaching the target point in the SP, where they enter the SP in small fascicles and grow to a significant length (in humans up to 5 mm), in order to reach the point below their target cortex. In trilaminar midgestational SP, the deepest SP portion is lamina of small fascicle afferent ingrowth (Žunić Išasegi et al. 2018), the middle portion is the associated substrate for individual navigation, and the most superficial stratum of SP is the layer for frequent synaptic contacts (Molliver et al. 1973; Kostović & Rakić, 1990) and accumulation of afferents prior to entering the cortex. Laminar correlations of the histogenetic role of the SP in pathway development are most obvious during the stationary phase, when major axonal classes (thalamo‐cortical, cortico‐cortical) are still growing. This process was illustrated in different publications on human postmortem material using AChE staining (Kostović & Goldman Rakić, 1983; Krmpotic‐Nemanic et al. 1983; Kostović & Rakić, 1984; Kostović & Judaš, 2010; Krsnik et al. 2017) and may be correlated with the presence of synaptic strata, where the most dense concentration of synapses is found in the superficial third of the SP and where axons actually make more frequent contacts during the ‘waiting period’ (Rakić, 1977; Kostović & Rakić, 1990). We refer to the concomitant development of AChE reactivity and synaptic markers in the SP and CP as presented in this study. It is highly likely that some of the synapses in the most superficial SP stratum are located on dendrites of CP neurons, which extend a considerable distance into the SP compartment (Mrzljak et al. 1988). Finally, the deep zone of SP is well developed during the ingrowth of long cortico‐cortical pathways (Huang et al. 2006, 2009; Kasprian et al. 2008; 2009Vasung et al. 2017; Žunić Išasegi et al. 2018) during a significant period after the thalamo‐cortical fibers have already been translocated from the SP to CP. However, the phenomenon of the ingrowth of long cortico‐cortical fibers requires further investigation. At the moment, it is important to emphasize the sublaminar and voluminous organization of the SP during growth of the long cortico‐cortical pathways. In the present study, we have documented developmental changes in the sublaminar distribution of ECM markers during the midfetal, late fetal and near term periods. These data confirm our previous studies of the distribution of ECM GAGs and proteoglycans (Kostović et al. 2002, 2014a; Milošević et al. 2014). It has been shown that ingrowth of fibers is accompanied during increased synthesis of GAGs and proteoglycans in ECM (Bicknese et al. 1994; Miller et al. 1995; Pearlman & Sheppard, 1996). In the context of observed changes in deep to superficial reactivity of ECM markers, it would be interesting to find out which of the multiple roles of GAGs and proteoglycans can be attributed to the SP ECM. In addition to well known roles in secreting axonal guidance molecules (Tessier‐Lavigne & Goodman, 1996; Judaš et al. 2003; Molnár et al. 2012), and providing gradients for growing axons, which navigate through an enormous volume of the hydrophilic environment, we propose that the crucial role of SP is to accommodate large numbers of ingrowing axons, without a proportional increase of volume. This ‘mechanical’ role of SP is derived from general tissue mechanics by absorbing capacity due to spatial factors and high ECM content (Pogoda & Janmey, 2018). Indeed, the intensity of general staining in the earlier stages for GAGs is concentrated along the deep border of SP, whereas in the later stages it is concentrated in the most superficial part of the SP, where there is accumulation of waiting thalamo‐cortical axons. The absence of one particular GAG component, chondroitin sulfate‐staining, during resolution of the SP was interpreted as the existence of a new phase of cortico‐cortical pathway development (Kostović et al. 2014a). Namely, in the first phase of pathway development, chondroitin sulfate serves as a growth‐promoting molecule for the ingrowth of thalamo‐cortical fibers (Bicknese et al. 1994; Miller et al. 1995; Kostović et al. 2002) and is ‘removed’ from resolving SP during growth of cortico‐cortical fibers, given that in more mature tissue, chondroitin sulfate acts as an inhibitor of growing axons (Rhodes & Fawcett, 2004).
Development, distribution and maturational gradients of (astro)glia and the transient form of neurons in the SP
In our study, we arrived at several interesting results concerning astroglial development. The first set of findings concerns developmental timing of GFAP‐reactive fibers and astroglial cells. The second set of findings relates to the laminar distribution of GFAP‐reactive astroglia based on laminar shifts of growing cortical pathways and distribution of synaptic markers. The following data on the developmental schedule of GFAP‐reactive fibers and cells will be discussed from the aspect of the early development of GFAP and vimentin‐reactive fibers in the SP, the early development of fibrous and protoplasmic astrocyte forms and, finally, the fact that the appearance of the astroglial phenotype (as seen in the Stensaas Golgi method) precedes development of GFAP immunoreactivity of astrocytes.
First, vimentin‐positive radial glial fibers were found in the earliest phases, were present in midgestation and were readily found in the SP at around 24 PCW. GFAP‐positive radial fibers were found somewhat later, at around 15 PCW. This observation is consistent with previous data on the early development of glial fiber immunoreactivity (Choi & Lapham, 1978; Levitt & Rakic, 1980; Ulfig et al. 2000; Zecevic, 2004). There is a large amount of data on the early development of radial glia fibers, but less data are available on the development of astroglial cells. According to Ulfig et al. (2000), immature astroglia‐like GFAP‐reactive elements were seen in the human neocortex by 23 PCW (Rezaie et al. 2003). Using the Stensaas Golgi method, we identified astroglia as early as 14 PCW (Kostović et al. 1975). In the present paper, we have confirmed these findings on more recently acquired specimens. Using GFAP, we identified different cellular processes and particles within the SP, as early as 15 PCW, and when subjected to immunohistochemical procedures. The first immature non‐radially oriented fully stained glial cells were clearly seen at around 20 PCW. A maturational gradient ‘from deep to superficial’ was noticeable, corresponding to the gradient of ingrowth of afferent fibers in the SP. The close correlation between distribution and packing density of GFAP‐reactive cells and laminar organization of the SP and within the whole cerebral wall has led us to conclude that ‘glia architectonics’ may serve as a reliable marker in investigating normal cerebral cortex development on postmortem material. The maturational schedule of GFAP elements described in this study corresponds to an increase in the number of synapses in the SP zone during the second trimester of gestation. Moreover, the period of astrogenesis corresponds to a period of intensive ECM production, as demonstrated by previous histochemical, immunocytochemical and electron‐microscopic studies (Kostović & Rakić, 1990; Kostović et al. 2002, 2014a). These two events (production of synapses and ECM production as a substrate for axonal growth; Pearlman & Sheppard, 1996; Tessier‐Lavigne & Goodman, 1996) suggest the possibility that early astroglia are involved in both the formation of synapses (Dermietzel & Spray, 1993; Araque et al. 1999; Elmariah, 2005; Sutor & Hagerty, 2005; Bruzzone & Dermietzel, 2006; Eroglu & Barres, 2010; Faissner et al. 2010; Allen, 2014) and ECM production (Wiese et al. 2012; Oberheim et al. 2012). If the aim is to define the roles of astrocytes, the support of different functions such as iron and water homeostasis (Mayorquin et al. 2018), neurotransmitter production (Araque et al. 1999), modulation of activity in neighboring cells (Oberheim et al. 2006, 2009) and glial‐initiated excitotoxic cell death in ischemia (Pennypacker et al. 2000), our data become important for elucidating the glial developmental role in the human SP. At the moment, it is not completely clear what the astroglial function in axonal growth is. In this respect, what is most interesting is the presence of deep SP astroglia at the interface between growing fibers of sagittal strata, where glia provide corridors for growth within the sagittal strata and into the SP (Žunić Išasegi et al. 2018). In the current search for different classes of progenitor cells, the most interesting phenomenon is radial glia transformation and a retraction of their process from the apical (ventricular) and basal (pial) surfaces, and also later from the basal (pial) surface (Schmechel & Rakic, 1979; Malatesta et al. 2000; Rakić, 2003; DeAzevedo et al. 2003; Noctor et al. 2004; Götz & Huttner, 2005; Hansen et al. 2010; Hevner & Haydar, 2012; Reillo & Borrell, 2012; Dehay et al. 2015). The question remains as to how radial glia cells, after retracting their apical process and translocating soma and nucleus, remain in the SP and temporarily maintain radial orientation. Schmechel & Rakic (1979) described this process in the cerebral wall of the fetal monkey as a transformation of radial glia into astrocytes, and although they did not mention it specifically, it is obvious that some of the cells they described are located in the SP (Kostović et al. 1975). In the present study, this type of transforming cell was easily identified using the Stensaas Golgi method by 20 PCW, but it was rarely seen in GFAP‐stained sections. However, after 20 PCW, transforming radial glia was frequently observed, with a peak at around 26 PCW, indicating that this period may be a developmental maximum for this interesting process. Observations reported in this paper, together with our original results (Kostović et al. 1975) and results of Schmechel & Rakic (1979) and Choi & Lapham (1978), confirm that astrogenesis in the primate cortex starts during midgestation and coincides with neurogenesis.
The most interesting scientific observation in this paper is the presence of different transitional forms of astroglia, especially GFAP‐positive cells with an arborization of processes, which emanate from one side of the cell, and neuron‐like glia. These cells were described by DeAzevedo et al. (2003) in the SP from 24 to 35 PCW in the human brain, as transitional ‘monopolar’ cells with pyriform soma and by Rezaie et al. (2003) as GFAP‐positive cells with a polarized morphology (A5 type by our classification). In our opinion, these cells are special transient forms of astroglia and not simply maturational transitional forms of a future final astrocyte shape. It is possible that the polarized arborization domain is directed towards certain specific cellular spots such as small fascicles of bundles, groups of synaptic junctions or blood vessels, as was proposed for astroglial participation in neuroglial units (Oberheim et al. 2006). On the other hand, random orientation of the long processes of astrocytes in the SP and associated partial interdigitation may be an indicator of the integrative function of astrocytes in a developing cortex. These results support the notion that the developmental pattern of gliogenesis in the human brain is one of the main processes of histogenetic timing which distinguish the primate from the rodent cortex. Our results do not provide a definitive answer as to when SP astroglia complete maturation and achieve a full‐size phenotype as previously described (Oberheim et al. 2009, 2012; Colombo, 2018), or as to when human astrocytes reach their full maturational state. Namely, even in the oldest specimen examined (near term age), when fibrous astrocytes seem to be relatively mature, transient forms of astroglia are still present in the SP remnant. It remains unknown at the moment whether transient forms of astroglia disappear due to cell death after completing their morphogenetic role or transform into astroglia of gyral white matter. In this respect, their developmental fate may be similar to SP neurons, which indeed become a part of gyral white matter, known as interstitial neurons (Kostović & Rakić, 1980; Judaš et al. 2010a,b; Kostović et al. 2011). Some of the transient astrocytes may become incorporated into layer 6 at the gyral white matter interface and become protoplasmic astrocytes. It should be emphasized that evidence of the presence of the complex composition of immature protoplasmic, fibrous and diverse transient astrocytes supports the concept of the SP as an essential connectivity compartment of fetal cortex which is not only a major site of synaptogenesis but is also specialized for ingrowth of the fiber system, thus explaining the presence of fibrous astrocytes. If we consider the role of glial radial fibers, which transverse the SP and support migration even during the last third of gestation (Kostović et al. 2015), it becomes evident that SP glia play a crucial role in cortical histogenesis. Finally, transformation of the radial glia into astrocytes in the deep third of the SP proves that SP is an important source of newly generated astroglia. Morphogenetic roles and developmental changes of astrocytes support the idea that glia cells participate as an active factor in synaptogenesis. Regarding the functional integrative role of astrocytes in fetal SP, it is important to determine whether these cells form functional domains, as has been proposed for the adult cortex. Some of the characteristically human glia, such as interlaminar glia, develop postnatally (Colombo & Reisin, 2004; Colombo, 2018), which is also in line with the prolonged process of postnatal synaptogenesis (Bourgeois et al. 1994; Huttenlocher & Dabholkar, 1997; Petanjek et al. 2011), dendritic development (Petanjek et al. 2008) and intracortical connectivity (Burkhalter, 1993).
All of the data discussed above indicate that developmental timing, laminar distribution and diversity of the glia cell phenotype correlate with multiple morphogenetic and functional roles of the SP. At this point, we would like to point out several similarities between laminar development and glial cell diversity, as well as the diversity of transient neurons. The transient type of neurons, for example glia‐like neurons (N2 type), and large nexus neurons (N1 type) are found in the same sublaminas of the SP, where transient forms of glia (A5 type) are frequently encountered. The transient type of neurons show a preference for the superficial two‐thirds of the SP, where most of the synapses are formed (Molliver et al. 1973; Kostović & Rakić, 1990). In midgestational cortex, in this portion of the SP, glial transient forms are more frequently represented. The asymmetric arborization of processes involving glia‐like neurons and neuron‐like glia is oblique and seems to be directed to some prospective ‘hot points’, possibly towards ‘clusters’ of synaptic engagements and communication via intercellular junctions. The molecular identity of neurons described in the present study is not clear, and further studies are necessary to explain the molecular diversity of SP neurons, as shown in experimental material (Chun et al. 1987; Meinecke & Rakic, 1992; Hoerder‐Suabedissen et al. 2009; Kanold, 2009; Kanold & Luhmann, 2010; Hoerder‐Suabedissen & Molnár, 2013; Pedraza et al. 2014). The exceptions are calretinin and GAD‐positive neurons, which are smaller than typical SP neurons and most likely correspond to a phenotype of fusiform neurons. The molecular diversity of SP neurons was also described in the human cortex by Kostović et al. (1991b), Delalle et al. (1997), Bayatti et al. (2008), Wang et al. (2010) and Harkin et al. (2017). Experimental studies have also shown that the distribution of diverse SP neurons conforms to the multiple functional roles of SP (Kanold, 2004; Khazipov & Luhmann, 2006; Luhmann et al. 2009; Moore et al. 2009; Kanold & Luhmann, 2010). However, morphogenetic roles of SP in different histogenetic events seem to be equally important. Kostović et al. (2015) stressed the role of the SP in migration and Kondo et al. (2015) demonstrated a neurosecretory role, but the most enigmatic is the role of the SP in glial and neuronal proliferation. In this respect, it is interesting that proliferative markers, such as Ki67, are present in the SP (see Žunić Išasegi et al. 2018), which raises the question as to whether the SP serves as an additional source of newly formed neurons in the human cortex. We believe analysis should focus on deep SP, where transforming radial glia cells are found, even after 24 PCW. Although transforming glia of the SP were described as an important source of astrocytes in monkeys (Schmechel & Rakic, 1979), so far this type of glia was not previously specifically linked to the SP. We can for the moment state that well developed transforming glia in the deep SP are a major source of astrocytes and possibly other cell types.
All the above‐mentioned analysis leads to the conclusion that the diversity of neural phenotypes, and molecular and transmitter profiles in the human cortex is indeed extremely complex. The significant diversity of molecular and structural phenotypes of SP can partly be explained by different sources of origin. So far, two major sources of SP neurons have been identified: the early born preplate cells, which are predominantly GABA‐ergic (Meyer et al. 2000; Zecevic et al. 2005, 2011; Bystron et al. 2008; Al‐Jaberi et al. 2015), and cells which are born at the same time as layer VI neurons, which form the majority of SP cells (Duque et al. 2016). Some of these cells, which are displaced from the cortex during SP formation, may be glutamatergic (Finney et al. 1998). The late arrival of some migratory neurons may also contribute to the diverse population of SP neurons (Kostović et al. 2015). Finally, the extracortical origin of SP neurons was demonstrated by Pedraza et al. (2014).
(Selective) vulnerability of different sub‐compartments of SP
Volpe (1996) was the first to propose that SP neurons might be the ‘missing link’ in understanding cognitive deficit after encephalopathy of prematurity. The role of the SP in structural plasticity after the perinatal lesion was proposed by Kostovic et al. (1989). Increased interest in the SP layer and its pathology in encephalopathy of prematurity has led to numerous experimental studies on the effects of hypoxic‐ischemic events (McQuillen et al. 2003; Kanold, 2009; Millar et al. 2017; McClendon et al. 2017; Sheikh et al. 2018). However, these studies were focused on SP neurons, not on a significant content of afferent axons, and ECM organization. Therefore, we have proposed the concept of radial vulnerability (Kostović et al. 2014b) and pointed out different axonal classes, in particular thalamo‐cortical axons, which can become damaged in the SP during growth, navigation and ‘waiting’ in the SP. This concept is consistent with recent clinical studies showing that one of the most frequently damaged fiber systems is the thalamo‐cortical system (Ball et al. 2012, 2013). In reviewing literature which considers the pathological substrate in the SP, in addition to studies on matrix metalloproteinases (Pennypacker et al. 2000), Baud et al. (2004) was the first to provide evidence of changes to some ECM components in experimental hypoxia‐ischemia. Based on the results of this study, we predict that sublaminar ECM organization may also influence the outcome after the occurrence of hypoxic‐ischemic lesions due to impairment of axonal growth, as well as navigation and exchange of metabolites through ECM. The question remains as to which one of the three sublaminas/floors of the SP is most vulnerable. It is very likely that the most vulnerable part is the deep SP, located adjacent to the sagittal strata and centrum semiovale of white matter, which is likely a target zone of ‘diffuse’ leukomalacia (Volpe, 2009). The vulnerability of the deep SP may also be partially explained by the transient position within the cerebral wall, where terminal fields of medullary arteries of cerebrum (deep system) and perforating arterial branches (superficial system) form potential ‘watershed’ zones. On the other hand, transforming glia cells in the superficial SP may be vulnerable because of the intensive glutamatergic synaptogenesis which may mediate mechanisms of excitotoxicity (Oka et al. 1993). The finding of early gliogenesis in the human fetal cortex opens new areas in the search for cellular targets in encephalopathy of prematurity. Indeed, Pogledic et al. (2014) found reactive glia in the SP of the postmortem brains of children after death caused by hypoxic‐ischemia. The normative data presented in this study may serve as a solid basis for analyzing the effects of hypoxia on the SP compartment, its transient neurons and, in particular, its classes of glia, especially transforming radial glia and transient neuron‐like glia. So far, involvement of glia has only been described during late pathological changes in encephalopathy (glia‐scars; Khwaja & Volpe, 2008) or damage of oligodendroglia and consequent failure of myelination (Back et al. 2001). The impairment of astroglial function in encephalopathy of prematurity is not necessarily accompanied by an apparent pathology; namely, glia may have an important function in hemichannel and channel functions (Sutor & Hagerty, 2005; Dere & Zlomuzica, 2012; Moore et al. 2014; Talaverón et al. 2015; Galinsky et al. 2018). The lesion of glial junctions causes abnormal channel and hemichannel activity (Rodríguez et al. 2012; Galinsky et al. 2018). This fine ultrastructural and metabolic change without an obvious pathology of the SP neurons may lead to depression of spontaneous electrical activity (Hanganu, 2001; Khazipov et al. 2004; Luhmann et al. 2009; Kanold & Luhmann, 2010; Moore et al. 2011; Singh et al. 2018). In a developing cortex, spontaneous activity obviously involves two synaptic compartments – the SP and MZ – as they both participate in the oscillatory network before CP. It is generally accepted that spontaneous activity is necessary for proper cortical maturation (Friauf et al. 1990; Friauf & Shatz, 1991; Khazipov et al. 2004; Khazipov & Luhmann, 2006; Moore et al. 2009; Kanold, 2009; Moore et al. 2011).
Conclusion
The distribution and packing density of neurons and glia, arrangement of ingrowing axons, distribution of synaptic markers, and the amount of ECM immunoreactivity of neocortical SP compartment changes with respect to laminar organization throughout fetal development. The monolayer of the early fetal period undergoes dramatic bilaminar transformation between 13 and 14 PCW, followed by three cellular floors (sublaminas) which are not sharply delineated in midgestation. At the beginning of the last third of gestation, SP persists as a bilaminar compartment (deep and superficial) and remains a single layer, an SP remnant, in the newborn brain. We have found that changes in laminar distribution of cells involve transient types of neurons and glia, indicating their possible role in synaptogenesis, axonal growth, ECM production and establishment of transient connectivity, along the cerebral hemisphere. Early differentiation of glia and the sublaminar organization of the SP are characteristic of the primate cortex and are particularly prominent in the human brain.
Conflict of interest
Authors of this manuscript declare no conflict of interest.
Author contributions
I.K., I.Ž.I. and Ž.K. performed the research; I.K., I.Ž.I and Ž.K. analyzed data; I.Ž.I. prepared images for publications. All authors contributed equally in writing this paper.
Acknowledgements
This work was supported by Croatian Science Foundation projects CSF‐IP‐09 2014‐4517 and CSF‐DOK‐10‐2015; co‐financed by the European Union through the European Regional Development Fund, Operational Programme Competitiveness and Cohesion, grant agreement No. KK.01.1.1.01.0007, CoRE ‐ Neuro. Some of the human embryonic and fetal material was provided by the Joint MRC/Wellcome Trust grant #099175/Z/12/Z Human Developmental Biology Resource. We would also wish to acknowledge the excellent technical assistance of Ana Bosak. We would like to thank Andrija Štajduhar and Martina Rinčić for help with processing data obtained with semiquantitative analysis and technical support.
References
- Al‐Jaberi N, Lindsay S, Sarma S, et al. (2015) The early fetal development of human neocortical GABAergic interneurons. Cereb Cortex 25(3), 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ (2014) Synaptic plasticity: astrocytes wrap it up. Curr Biol 24(15), 697–699. [DOI] [PubMed] [Google Scholar]
- Allendoerfer K, Shatz C (1994) The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci 17, 185–218. [DOI] [PubMed] [Google Scholar]
- Alzubi A, Lindsay S, Kerwin J, et al. (2017) Distinct cortical and sub‐cortical neurogenic domains for GABAergic interneuron precursor transcription factors NKX2.1, OLIG2 and COUP‐TFII in early fetal human telencephalon. Brain Struct Funct 222, 2309–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, et al. (1999) Tripartite synapses: glia, the unacknowledged partner. Trend Neurosci 22(5), 208–215. [DOI] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, et al. (2001) Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21(4), 1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Boardman JP, Rueckert D, et al. (2012) The effect of preterm birth on thalamic and cortical development. Cereb Cortex 22(5), 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Boardman JP, Aljabar P, et al. (2013) The influence of preterm birth on the developing thalamo‐cortical connectome. Cortex 49(6), 1711–1721. [DOI] [PubMed] [Google Scholar]
- Baud O, Daire J‐L, Dalmaz Y, et al. (2004) Gestational hypoxia induces white matter damage in neonatal rats: a new model of periventricular leukomalacia. Brain Path 14(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, et al. (2008) A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex 18(7), 1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknese A, Sheppard A, O'Leary D, et al. (1994) Thalamo‐cortical axons extend along a chondroitin sulfate proteoglycan‐enriched pathway coincident with the neocortical subplate and distinct from the efferent path. J Neurosci 14(6), 3500–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Molnar Z (1990) Factors involved in the establishment of specific interconnections between thalamus and cerebral cortex. Cold Spring Harb Symp Quant Biol 55, 491–504. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P (1993) Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci 13(7), 2801–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman‐Rakic PS, Rakic P (1994) Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex 4(1), 78–96. [DOI] [PubMed] [Google Scholar]
- Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde im ihrem Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: J.A Barth. [Google Scholar]
- Bruzzone R, Dermietzel R (2006) Structure and function of gap junctions in the developing brain. Cell Tissue Res 326(2), 239–248. [DOI] [PubMed] [Google Scholar]
- Burkhalter A (1993) Development of forward and feedback connections between areas v1 and v2 of human visual cortex. Cereb Cortex 3(5), 476–487. [DOI] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P (2008) Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 9(2), 110–122. [DOI] [PubMed] [Google Scholar]
- Choi BH, Lapham LW (1978) Radial glia in the human fetal cerebrum: a combined golgi, immunofluorescent and electron microscopic study. Brain Res 148(2), 295–311. [DOI] [PubMed] [Google Scholar]
- Chun J, Shatz C (1988) Redistribution of synaptic vesicle antigens is correlated with the disappearance of a transient synaptic zone in the developing cerebral cortex. Neuron 1(4), 297–310. [DOI] [PubMed] [Google Scholar]
- Chun J, Shatz C (1989) The earliest‐generated neurons of the cat cerebral cortex: characterization by MAP2 and neurotransmitter immunohistochemistry during fetal life. J Neurosci 9(5), 1648–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JJM, Nakamura MJ, Shatz CJ (1987) Transient cells of the developing mammalian telencephalon are peptide‐immunoreactive neurons. Nature 325(6105), 617–620. [DOI] [PubMed] [Google Scholar]
- Colombo JA (2018) Interlaminar glia and other glial themes revisited: pending answers following three decades of glial research. Neuroglia 1, 3. [Google Scholar]
- Colombo JA, Reisin HD (2004) Interlaminar astroglia of the cerebral cortex: a marker of the primate brain. Brain Res 1006(1), 126–131. [DOI] [PubMed] [Google Scholar]
- DeAzevedo LC, Fallet C, Moura‐Neto V, et al. (2003) Cortical radial glial cells in human fetuses: depth‐correlated transformation into astrocytes. J Neurobiol 55(3), 288–298. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, González‐Albo MC, Del Río MR, et al. (1999) Distribution and patterns of connectivity of interneurons containing calbindin, calretinin, and parvalbumin in visual areas of the occipital and temporal lobes of the macaque monkey. J Comp Neurol 412(3), 515–526. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Alonso‐Nanclares L, Arellano JI (2002) Microstructure of the neocortex: comparative aspects. J Neurocytol 31(3–5 Spec. Iss.), 299–316. [DOI] [PubMed] [Google Scholar]
- Dehay C, Kennedy H, Kosik KS (2015) The outer subventricular zone and primate‐specific cortical complexification. Neuron 85(4), 683–694. [DOI] [PubMed] [Google Scholar]
- Delalle I, Evers P, Kostovic I, et al. (1997) Laminar distribution of neuropeptide Y‐immunoreactive neurons in human prefrontal cortex during development. J Comp Neurol 379(4), 515–522. [DOI] [PubMed] [Google Scholar]
- Dere E, Zlomuzica A (2012) The role of gap junctions in the brain in health and disease. Neurosci and Biobeh Rev 36(1), 206–217. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Spray DC (1993) Gap junctions in the brain: where, what type, how many and why? Trends Neurosci 16(5), 186–192. [DOI] [PubMed] [Google Scholar]
- Dubois J, Kostović I, Judaš M (2015) Development of structural and functional connectivity In: Brain Mapping: An Encyclopedic Reference. (ed. Toga AW.), pp. 423–437, Oxford, UK: Academic Press, Elsevier: . [Google Scholar]
- Duque A, Krsnik Z, Kostović I, et al. (2016) Secondary expansion of the transient subplate zone in the developing cerebrum of human and nonhuman primates. Proc Natl Acad Sci USA 113(35), 9892–9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmariah SB (2005) Astrocytes regulate inhibitory synapse formation via Trk‐mediated modulation of postsynaptic GABAA receptors. J Neurosci 25(14), 3638–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Barres BA (2010) Regulation of synaptic connectivity by glia. Nature 468(7321), 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner A, Pyka M, Geissler M, et al. (2010) Contributions of astrocytes to synapse formation and maturation ‐ potential functions of the perisynaptic extracellular matrix. Brain Res Revi 63(1–2), 26–38. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M (2005) Map plasticity in somatosensory cortex. Science 310(5749), 810–815. [DOI] [PubMed] [Google Scholar]
- Finney EM, Stone JR, Shatz CJ (1998) Major glutamatergic projection from subplate into visual cortex during development. J Comp Neurol 398(1), 105–118. [PubMed] [Google Scholar]
- Friauf E, Shatz CJ (1991) Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. J Neurophysiol 66(6), 2059–2071. [DOI] [PubMed] [Google Scholar]
- Friauf E, McConnell SK, Shatz CJ (1990) Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci 10(8), 2601–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinsky R, Davidson J, Dean J, et al. (2018) Glia and hemichannels: key mediators of perinatal encephalopathy. Neural Regen Res 13(2), 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ (1992) Involvement of subplate neurons in the formation of ocular dominance columns. Science 255(5050), 1441–1443. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ (1993) A role for subplate neurons in the patterning of connections from thalamus to neocortex. Development 117(3), 1031–1047. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, et al. (1990) Requirement for subplate neurons in the formation of thalamo‐cortical connections. Nature 347(6289), 179–181. [DOI] [PubMed] [Google Scholar]
- González Gómez M, Meyer G (2014) Dynamic expression of calretinin in embryonic and early fetal human cortex. Front Neuroanat 8, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6(10), 777–788. [DOI] [PubMed] [Google Scholar]
- Hanganu IL (2001) Spontaneous synaptic activity of subplate neurons in neonatal rat somatosensory cortex. Cereb Cortex 11(5), 400–410. [DOI] [PubMed] [Google Scholar]
- Hanganu I, Kilb W, Luhmann H (2002) Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci 22(16), 7165–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Ben‐Ari Y, Khazipov R (2006) Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci 26(25), 6728–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu I, Okabe A, Lessmann V, et al. (2009) Cellular mechanisms of subplate‐driven and cholinergic input‐dependent network activity in the neonatal rat somatosensory cortex. Cereb Cortex 19(1), 89–105. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PRL, et al. (2010) Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464(7288), 554–561. [DOI] [PubMed] [Google Scholar]
- Harkin LF, Lindsay SJ, Xu Y, et al. (2017) Neurexins 1–3 each have a distinct pattern of expression in the early developing human cerebral cortex. Cereb Cortex 27, 216–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF (2000) Development of connections in the human visual system during fetal mid‐gestation: a DiI‐tracing study. J Neuropathol Exp Neurol 59(5), 385–392. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Haydar TF (2012) The (not necessarily) convoluted role of basal radial glia in cortical neurogenesis. Cereb Cortex 22(2), 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladnik A, Dzaja D, Darmopil S, et al. (2014) Spatio‐temporal extension in site of origin for cortical calretinin neurons in primates. Front Neuroanat 8, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerder‐Suabedissen A, Molnár Z (2013) Molecular diversity of early‐born subplate neurons. Cereb Cortex 23(6), 1473–1483. [DOI] [PubMed] [Google Scholar]
- Hoerder‐Suabedissen A, Molnár Z (2015) Development, evolution and pathology of neocortical subplate neurons. Nat Rev Neurosci 16(3), 133–146. [DOI] [PubMed] [Google Scholar]
- Hoerder‐Suabedissen A, Wang WZ, Lee S, et al. (2009) Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cereb Cortex 19(8), 1738–1750. [DOI] [PubMed] [Google Scholar]
- Hoerder‐Suabedissen A, Hayashi S, Upton L, et al. (2018) Subset of cortical layer 6b neurons selectively innervates higher order thalamic nuclei in mice. Cereb Cortex 28(5), 1882–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, et al. (2006) White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage 33(1), 27–38. [DOI] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, et al. (2009) Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci 29(13), 4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387(2), 167–178. [DOI] [PubMed] [Google Scholar]
- Jackocevski I, Filipovic R, Mo Z, et al. (2009) Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N (2005) Sequence of oligodendrocyte development in the human fetal telencephalon. Glia 49(4), 480–491. [DOI] [PubMed] [Google Scholar]
- Jovanov Milošević N, Judaš M, Aronica E, et al. (2014) Neural ECM in laminar organization and connectivity development in healthy and diseased human brain. Prog Brain Res 214, 159–178. [DOI] [PubMed] [Google Scholar]
- Judaš M, Jovanov Milošević N, Rašin MR, et al. (2003) Complex patterns and simple architects: molecular guidance cues for developing axonal pathways in the telencephalon. Prog Mol Subcell Biol 32, 1–32. [DOI] [PubMed] [Google Scholar]
- Judaš M, Radoš M, Jovanov‐Miloševic N, et al. (2005) Structural, immunocytochemical and MR imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am J Neuroradiol 26(10), 2671–2684. [PMC free article] [PubMed] [Google Scholar]
- Judaš M, Sedmak G, Pletikos M (2010a) Early history of subplate and interstitial neurons: from Theodor Meynert (1867) to the discovery of the subplate zone (1974). J Anat 217(4), 344–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judaš M, Sedmak G, Pletikos M, et al. (2010b) Populations of subplate and interstitial neurons in fetal and adult human telencephalon. J Anat 217(4), 381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judaš M, Šimić G, Petanjek Z, et al. (2011) The Zagreb collection of human brains: a unique, versatile, but underexploited resource for the neuroscience community. Ann N Y Acad Sci 1225(SUPPL. 1), 105–130. [DOI] [PubMed] [Google Scholar]
- Kanold PO (2004) Transient microcircuits formed by subplate neurons and their role in functional development of thalamo‐cortical connections. NeuroReport 15(14), 2149–2153. [DOI] [PubMed] [Google Scholar]
- Kanold P (2009) Subplate neurons: crucial regulators of cortical development and plasticity. Front Neuroanat 3, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold P, Luhmann H (2010) The subplate and early cortical circuits. Ann Rev Neurosci 33, 23–48. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, et al. (2003) Role of subplate neurons in functional maturation of visual cortical columns. Science 301(5632), 521–525. [DOI] [PubMed] [Google Scholar]
- Kasprian G, Brugger PC, Weber M, et al. (2008) In utero tractography of fetal white matter development. NeuroImage 43(2), 213–224. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ (2006) Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci 29(7), 414–418. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Sirota A, Leinekugel X, et al. (2004) Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 432(7018), 758–761. [DOI] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ (2008) Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed 93(2), F153–F161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Al‐Hasani H, Hoerder‐Suabedissen A, et al. (2015) Secretory function in subplate neurons during cortical development. Front Neurosci 9, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I (1986) Prenatal development of nucleus basalis complex and related fiber systems in man: a histochemical study. Neuroscience 17(4), 1047–1077. [DOI] [PubMed] [Google Scholar]
- Kostović I, Goldman Rakić PS (1983) Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol 219(4), 431–447. [DOI] [PubMed] [Google Scholar]
- Kostović I, Jovanov‐Milošević N (2006) The development of cerebral connections during the first 20‐45 weeks’ gestation. Semin Fetal Neonatal Med 11(6), 415–422. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M (1998) Transient patterns of organization of the human fetal brain. CMJ 39, 107–114. [PubMed] [Google Scholar]
- Kostović I, Judaš M (2002) Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec 267(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M (2006) Prolonged coexistence of transient and permanent circuitry elements in the developing cerebral cortex of fetuses and preterm infants. Dev Med Child Neurol 48(5), 388–393. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M (2007) Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobeh Rev 31(8), 1157–1168. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M (2010) The development of the subplate and thalamo‐cortical connections in the human foetal brain. Acta Paediatr 99(8), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M (2015) Embryonic and fetal development of the human cerebral cortex In: Brain Mapping: An Encyclopedic Reference (ed. Toga AW.), pp. 167–175. Oxford, UK: Academic Press, Elsevier. [Google Scholar]
- Kostović I, Krmpotić Nemanić J (1975) The comparative analysis of the laminar pattern of the synaptogenesis in limbic and neocortical areas of human fetal cortex. Exp Brain Res 23(Suppl), 111. [Google Scholar]
- Kostović I, Molliver ME (1974) A new interpretation of the laminar development of cerebral cortex: synaptogenesis in different layers of neopallium in the human fetus. Anat Rec 178, 95. [Google Scholar]
- Kostović I, Rakić P (1980) Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol 9(2), 219–242. [DOI] [PubMed] [Google Scholar]
- Kostović I, Rakić P (1984) Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci 4(1), 25, LP‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Rakić P (1990) Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 297(3), 441–470. [DOI] [PubMed] [Google Scholar]
- Kostoví I, Lukinovic N, Judas M, et al. (1989) Structural basis of the developmental plasticity in the human cerebral cortex: the role of the transient subplate zone. Metab Brain Dis 4(1), 17–23. [DOI] [PubMed] [Google Scholar]
- Kostović I, Krmpotić Nemanić J, Kelović Z (1975) The early development of glia in human neocortex. Rad Jug Akad Znan Unijet Nat Sci Ser 17, 155–159. [Google Scholar]
- Kostović I, Judaš M, Kostović‐Knežević L, et al. (1991a) Zagreb research collection of human brains for developmental neurobiologist and clinical neuroscientist. Int J Dev Biol 35, 215–230. [PubMed] [Google Scholar]
- Kostović I, Štefulj‐Fučić A, Mrzljak L, et al. (1991b) Prenatal and perinatal development of the somatostatin‐immunoreactive neurons in the human prefrontal cortex. Neurosci Lett 124(2), 153–156. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M, Radoš M, et al. (2002) Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex 12, 536–544. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M, Sedmak G (2011) Developmental history of the subplate zone, subplate neurons and interstitial white matter neurons: relevance for schizophrenia. Int J Dev Neurosci 29(3), 193–205. [DOI] [PubMed] [Google Scholar]
- Kostović I, Jovanov‐Milošević N, Radoš M, et al. (2014a) Perinatal and early postnatal reorganization of the subplate and related cellular compartments in the human cerebral wall as revealed by histological and MRI approaches. Brain Struct Funct 219(1), 231–253. [DOI] [PubMed] [Google Scholar]
- Kostović I, Kostović‐Srzentić M, Benjak V, et al. (2014b) Developmental dynamics of radial vulnerability in the cerebral compartments in preterm infants and neonates. Front Neurol 5, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Sedmak G, Vukšić M, et al. (2015) The relevance of human fetal subplate zone for developmental neuropathology of neuronal migration disorders and cortical dysplasia. CNS Neurosci Ther 21(2), 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristt D, Molliver M (1976) Synapses in newborn rat cerebral cortex: a quantitative ultrastructural study. Brain Res 108(1), 180–186. [DOI] [PubMed] [Google Scholar]
- Krmpotic‐Nemanic J, Kostovic I, Kelovic Z, et al. (1983) Development of the human fetal auditory cortex: growth of afferent fibres. Acta Anat 116(1), 69–73. [PubMed] [Google Scholar]
- Krsnik Ž, Majić V, Vasung L, et al. (2017) Growth of thalamo‐cortical fibers to the somatosensory cortex in the human fetal brain. Front Neurosci 11, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Finney EM, McQuillen PS, et al. (1999) Subplate neuron ablation alters neurotrophin expression and ocular dominance column formation. Proc Natl Acad Sci USA 96(23), 13491–13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Rakic P (1980) Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol 193(3), 815–840. [DOI] [PubMed] [Google Scholar]
- López‐Bendito G, Molnár Z (2003) Thalamo‐cortical development: how are we going to get there? Nat Rev Neurosci 4(4), 276–289. [DOI] [PubMed] [Google Scholar]
- López‐Bendito G, Cautinat A, Sánchez JA, et al. (2006) Tangential neuronal migration controls axon guidance: a role for neuregulin‐1 in thalamo‐cortical axon navigation. Cell 125(1), 127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann H, Kilb W, Hanganu‐Opatz I (2009) Subplate cells: amplifiers of neuronal activity in the developing cerebral cortex. Front Neuroanat 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ (1985) Studies of the earliest generated cells of the cat's visual cortex: cogeneration of subplate and marginal zones. J Neurosci 5(4), 1062–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Götz M (2000) Isolation of radial glial cells by fluorescent‐activated cell sorting reveals a neuronal lineage. Development 127(24), 5253–5263. [DOI] [PubMed] [Google Scholar]
- Mayorquin LC, Rodriguez AV, Sutachan J‐J, et al. (2018) Connexin‐mediated functional and metabolic coupling between astrocytes and neurons. Front Mol Neurosci 11, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClendon E, Shaver DC, Degener‐O'Brien K, et al. (2017) Transient hypoxemia chronically disrupts maturation of preterm fetal ovine subplate neuron arborization and activity. J Neurosci 37(49), 11912–11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ (1989) Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science 245, 978–982. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ (1994) Subplate pioneer and the formation of descending connections from cerebral cortex. J Neurosci 14, 1892–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen PS, Sheldon RA, Shatz CJ, et al. (2003) Selective vulnerability of subplate neurons after early neonatal hypoxia‐ischemia. J Neurosci 23(8), 3308–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinecke DL, Rakic P (1992) Expression of GABA and GABAa receptors by neurons of the subplate zone in developing primate occipital cortex: evidence for transient local circuits. J Comp Neurol 317(1), 91–101. [DOI] [PubMed] [Google Scholar]
- Meyer G, Schaaps JP, Moreau L, et al. (2000) Embryonic and early fetal development of the human neocortex. J Neurosci 20(5), 1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar LJ, Shi L, Hoerder‐Suabedissen A, et al. (2017) Neonatal hypoxia ischaemia: mechanisms, models, and therapeutic challenges. Front Cell Neurosci 11, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Sheppard AM, Bicknese AR, et al. (1995) Chondroitin sulfate proteoglycans in the developing cerebral cortex: the distribution of neurocan distinguishes forming afferent and efferent axonal pathways. J Comp Neurol 355(4), 615–628. [DOI] [PubMed] [Google Scholar]
- Milošević NJ, Judaš M, Aronica E, et al. (2014) Neural ECM in laminar organization and connectivity development in healthy and diseased human brain. Prog Brain Res 214, 159–178. [DOI] [PubMed] [Google Scholar]
- Molliver ME, Van Der Loos H (1970) The ontogenesis of cortical circuitry: the spatial distribution of synapses in somesthetic cortex of newborn dog. Ergeb Anat Entwicklungsgesch 42(4), 5–53. [PubMed] [Google Scholar]
- Molliver ME, Kostović I, Van Der Loos H (1973) The development of synapses in cerebral cortex of the human fetus. Brain Res 50(2), 403–407. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Blakemore C (1995) How do thalamic axons find their way to the cortex? Trends Neurosci 18(9), 389–397. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Clowry G (2012) Cerebral cortical development in rodents and primates. Prog Brain Res 195, 45–70. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Adams R, Blakemore C (1998) Mechanisms underlying the early establishment of thalamo‐cortical connections in the rat. J Neurosci 18(15), 5723–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Garel S, López‐Bendito G, et al. (2012) Mechanisms controlling the guidance of thalamo‐cortical axons through the embryonic forebrain. Eur J Neurosci 35(10), 1573–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AR, Filipovic R, Mo Z, et al. (2009) Electrical excitability of early neurons in the human cerebral cortex during the second trimester of gestation. Cereb Cortex 19(8), 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AR, Zhou W‐L, Jakovcevski I, et al. (2011) Spontaneous electrical activity in the human fetal cortex in vitro. J Neurosci 31(7), 2391–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AR, Zhou W‐L, Sirois CL, et al. (2014) Connexin hemichannels contribute to spontaneous electrical activity in the human fetal cortex. Proc Natl Acad Sci USA 111(37), E3919–E3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostović I, et al. (1988) Prenatal development of neurons in the human prefrontal cortex: I. a qualitative Golgi study. J Comp Neurol 271(3), 355–386. [DOI] [PubMed] [Google Scholar]
- Nobin A, Björklund A (1973) Topography of the monoamine neuron systems in the human brain as revealed in fetuses. Acta Physiol Scand Suppl 388, 1–40. [PubMed] [Google Scholar]
- Noctor SC, Martinez‐Cerdeño V, Ivic L, et al. (2004) Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 7(2), 136–144. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Wang X, Goldman S, et al. (2006) Astrocytic complexity distinguishes the human brain. Trends Neurosci 29(10), 547–553. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, et al. (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29(10), 3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814, 23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A, Belliveau MJ, Rosenberg PA, et al. (1993) Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci 13(4), 1441–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman AL, Sheppard AM (1996) Extracellular matrix in early cortical development. Prog Brain Res 108, 117–134. [PubMed] [Google Scholar]
- Pedraza M, Hoerder‐Suabedissen A, Albert‐Maestro MA, et al. (2014) Extracortical origin of some murine subplate cell populations. Proc Natl Acad Sci USA 111(23), 8613–8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennypacker KR, Kassed CA, Eidizadeh S, et al. (2000) Brain injury: prolonged induction of transcription factors. Acta Neurobiol Exp (Wars) 60(4), 515–530. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Kostović I, et al. (2008) Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer‐specific pattern. Cereb Cortex 18(4), 915–929. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Berger B, Esclapez M (2009) Origins of cortical GABAergic neurons in the cynomolgus monkey. Cereb Cortex 19(2), 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, et al. (2011) Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA 108(32), 13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogledic I, Kostovic I, Fallet‐Bianco C, et al. (2014) Involvement of the subplate zone in preterm infants with periventricular white matter injury. Brain Pathol 24(2), 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoda K, Janmey PA (2018) Glial tissue mechanics and mechanosensing by glial cells. Frontiers in Cellular Neuroscience 12, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonjic NV, Ortega JA, Memi F, et al. (2014) The complexity of the calretinin expressing progenitors in the human cerebral cortex. Front Neuroanat 8, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakić P (1977) Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci 278(961), 245–260. [DOI] [PubMed] [Google Scholar]
- Rakić P (2003) Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex 13(6), 541–549. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S (1909) Histologie du système nerveux de l'homme & des vertébrés. Paris: Maloine. [Google Scholar]
- Reillo I, Borrell V (2012) Germinal zones in the developing cerebral cortex of ferret: ontogeny, cell cycle kinetics, and diversity of progenitors. Cereb Cortex 22(9), 2039–2054. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Ulfig N, Male D (2003) Distribution and morphology of GFAP‐positive astrocytes in the human fetal brain at second trimester. Neuroembryology 2(2), 50–63. [Google Scholar]
- Rhodes KE, Fawcett JW (2004) Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J Anat 204(1), 33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez EM, Guerra MM, Vío K, et al. (2012) A cell junction pathology of neural stem cells leads to abnormal neurogenesis and hydrocephalus. Biol Res 45(3), 231–241. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda‐Carreras I, Frise E, et al. (2012) Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9(7), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE, Rakic P (1979) A golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol 156(2), 115–152. [DOI] [PubMed] [Google Scholar]
- Sheikh A, Meng X, Liu J, et al. (2018) Neonatal hypoxia–ischemia causes functional circuit changes in subplate neurons. Cereb Cortex. 10.1093/cercor/bhx358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, White J, McKimm E, et al. (2018) Mechanisms of spontaneous electrical activity in the developing cerebral cortex–mouse subplate zone. Cereb Cortex. doi.org/10.1093/cercor/bhy205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, et al. (2010) Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 20(12), 2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor B, Hagerty T (2005) Involvement of gap junctions in the development of the neocortex. Biochim Biophys Acta 1719(1–2), 59–68. [DOI] [PubMed] [Google Scholar]
- Talaverón R, Fernández P, Escamilla R, et al. (2015) Neural progenitor cells isolated from the subventricular zone present hemichannel activity and form functional gap junctions with glial cells. Front Cell Neurosci 9, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier‐Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274(5290), 1123–1133. [DOI] [PubMed] [Google Scholar]
- Tolner EA, Sheikh A, Yukin AY, et al. (2012) Subplate neurons promote spindle bursts and thalamo‐cortical patterning in the neonatal rat somatosensory cortex. J Neurosci 32(2), 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N, Neudorfer F, Bohl J (2000) Transient structures of the human fetal brain: subplate, thalamic reticular complex, ganglionic eminence. Histol Histopathol 15(3), 771–790. [DOI] [PubMed] [Google Scholar]
- Vasung L, Huang H, Jovanov‐Milošević N, et al. (2010) Development of axonal pathways in the human fetal fronto‐limbic brain: histochemical characterization and diffusion tensor imaging. J Anat 217(4), 400–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L, Lepage C, Radoš M, et al. (2016) Quantitative and qualitative analysis of transient fetal compartments during prenatal human brain development. Front Neuroanat 10, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L, Raguz M, Kostovic I, et al. (2017) Spatiotemporal relationship of brain pathways during human fetal development using high‐angular resolution diffusion MR imaging and histology. Front Neurosci 11, 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney C (1999) Distribution of the catecholaminergic neurons in the central nervous system of human embryos and fetuses. Microsc Res Tech 46(1), 24–47. [DOI] [PubMed] [Google Scholar]
- Verney C, Milosevic A, Alvarez C, et al. (1993) Immunocytochemical evidence of well developed dopaminergic and noradrenergic innervations in the frontal cerebral cortex of human fetuses at midgestation. J Comp Neurol 336(3), 331–344. [DOI] [PubMed] [Google Scholar]
- Volpe JJ (1996) Subplate neurons – missing link in brain injury of the premature infant? Pediatrics 97(1), 112–113. [PubMed] [Google Scholar]
- Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8(1), 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WZ, Hoerder‐Suabedissen A, Oeschger FM, et al. (2010) Subplate in the developing cortex of mouse and human. J Anat 217(4), 368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pettersson DR, Studholme C, et al. (2015) Characterization of laminar zones in the midgestation primate brain with Magnetic Resonance Imaging and histological methods. Front Neuroanat 9, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese S, Karus M, Faissner A (2012) Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharm 3, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N (2004) Specific characteristic of radial glia in the human fetal telencephalon. Glia 48(1), 27–35. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R (2005) Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol 491(2), 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Hu F, Jakovcevski I (2011) Interneurons in the developing human neocortex. Dev Neurobiol 71(1), 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žunić Išasegi II, Radoš M, Krsnik Ž, et al. (2018) Interactive histogenesis of axonal strata and proliferative zones in the human fetal cerebral wall. Brain Struct Funct. 10.1007/s00429-018-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


