Abstract
Abnormal expression of microRNA (miR)-21 has been reported in various types of cancers. However, the role and mechanism of miR-21 remain to be elucidated in acute myeloid leukemia (AML). In the present study, it was observed that miR-21 was upregulated and Krüppel-like factor 5 (KLF5) was downregulated in AML cells compared with normal bone marrow cells. Dual luciferase reporter assays revealed that KLF5 was a direct target of miR-21. Indeed, miR-21 overexpression resulted in a downregulation of KLF5 expression, while miR-21 inhibition had the opposite effect in AML cells. In addition, miR-21 overexpression promoted the proliferation of AML cells in vitro. Notably, using a mouse xenograft model, miR-21 overexpression was demonstrated to result in enhanced tumor growth and suppressed KLF5 expression in the xenograft tumors in vivo. In conclusion, the present results indicated that miR-21 promoted proliferation through directly regulating KLF5 expression in AML cells. miR-21 may thus serve as an oncogene in AML, providing a potential target for AML therapy.
Keywords: microRNA-21, Krüppel-like factor 5, acute myeloid leukemia, proliferation
Introduction
Acute myeloid leukemia (AML) is the most prevalent malignant myeloid tumor, primarily occurring in elderly patients and characterized by the accumulation of blast cells and the blockage of myeloid differentiation in bone marrow (1). Molecular, genetic and cytogenetic abnormalities cause clonal expansion of early hematopoietic progenitor cells and obstruct hematopoiesis of normal bone marrow (2). Although several patients with AML respond to the induction chemotherapy, the perseverance of residuary blast cells causes an unusual relapse rate in the bone marrow (3,4). Consequently, it is required to elucidate the pathogenesis and discover novel therapeutic targets for AML.
MicroRNAs (miRNAs or miR) are small endogenous non-coding RNA molecules consisting of 21–25 nucleotides (5). miRNAs bind to the 3′-untranslated region (UTR) of their target genes, resulting in decreased mRNA expression or transcriptional inhibition of protein synthesis (6). miRNAs have been used as diagnostic and prognosis biomarkers due to their role as oncogenes (7) or tumor suppressors (8) in cancer. Evidence has demonstrated that various miRNAs are closely associated with AML, including miR-143, miR-34a and miR-126 (9–11). miR-21 has been demonstrated to serve an essential role in various types of cancer. For instance, miR-21 acts as a predictor and prognostic factor for trastuzumab therapy in human epidermal growth factor receptor 2-positive metastatic breast cancer (12). In addition, a previous study demonstrated that miR-21 is overexpressed in nucleophosmin 1-mutant acute myeloid leukemias (13). However, the regulatory mechanism of miR-21 in AML progression remains unknown.
Krüppel-like factor 5 (KLF5), a zinc-finger transcription factor, functions as a tumor suppressor or an oncogene (14,15). It serves an important role in cell proliferation and metastasis by regulating expression of several downstream target genes, such as tumor necrosis factor α-induced protein 2, hypoxia inducible factor-1α, FYN proto-oncogene (16–18). A previous study suggested that KLF5 functions as a tumor suppressor in AML (19). However, the role of KLF5 in AML progression is not fully understood.
In the present study, it was suggested that KLF5 may be a potential direct target of miR-21 in AML cells, with a binding site at the 3′-UTR. The study aimed to investigate the role of miR-21 on AML cells and its underlying molecular mechanisms.
Materials and methods
Cell culture
The human AML cell lines HL-60 and SKM-1 and the bone marrow stromal cell line HS-5 were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. Each cell line was cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences) and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.). Cells were maintained in a humidified incubator at 37°C with 5% CO2.
Reverse transcription-quantitative PCR (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to isolate the total RNA from the harvested HL-60, SKM-1 and HS-5 cells. cDNA was generated using a PrimeScript™ RT-qPCR (Takara Biotechnology Co, Ltd.). The reverse transcription reaction conditions are as follows: 37°C for 15 min, 85°C for 5 sec and finally at 4°C. An Applied Biosystems 7500 real-time PCR system (Thermo Fisher Scientific, Inc.) was used to perform the qPCR using a SYBR premix ex Taq™ kit (Takara Bio, Inc.) according to the manufacturer's protocol. The PCR thermocycling conditions were as follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec. The relative expression levels of miR-21 and KLF5 mRNA were analyzed with GAPDH and U6 as internal references, respectively, using a TaqMan microRNA assay (Ambion; Thermo Fisher Scientific, Inc). Analysis of relative gene expression data was performed using the real-time quantitative PCR and the 2−ΔΔCq method. A total of three repeats were performed. The primer sequences were: U6 forward, 5′-CTCGCTTCGGCAGCACATATACT-3′; U6 reverse, 5′-ACGCTTCACGAATTTGCGTGTC-3′; GAPDH forward, 5′-TCAACGACCACTTTGTCAAGCTCA-3′; GAPDH reverse, 5′-GCTGGTGGTCCAGGGGTCTTACT-3′; miR-21 forward, 5′-TGCGCTAGCTTATCAGACTGAT-3′; miR-21 reverse, 5′-CCAGTGCAGGGTCCGAGGTATT-3′; KLF5 forward, 5′-AGCTACAATACGCTTGGCCT-3′; and KLF5 reverse, 5′-ATGTGTGTTACGCACGGTCT-3′.
Western blot analysis
Protein was extracted from the cells and tissues using RIPA lysis buffer [1% NP40, 0.1% sodium dodecyl sulfate (SDS), 100 µg/ml phenylmethylsulfonyl fluoride and 0.5% sodium deoxycholate in PBS] on ice. The supernatants were collected following centrifugation at 12,000 x g at 4°C for 20 min.
The bicinchoninic acid method (Thermo Fisher scientific, Inc.) was used to determine the protein concentrations in extracts from cultured cells or resected tumors. Subsequently, 10 µg of protein samples were separated by 10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore). Following blocking with 5% skimmed milk at room temperature for 2 h, the membranes were incubated with primary antibodies against KLF5 (1:1,000; cat. no. ab137676; Abcam), the proliferation marker protein Ki67 (1:1,000; cat. no. ab15580; Abcam) and GAPDH (1:3,000; cat. no. ab9485; Abcam) overnight at 4°C. The membranes were then incubated with secondary antibody, goat anti-rabbit IgG H&L (horseradish peroxidase-conjugated; 1:5,000; cat. no. ab205718; Abcam). Antibodies bound to the target proteins were visualized using the Enhanced Chemiluminescence Western Blotting Detection reagent (Santa Cruz Biotechnology, Inc.). The protein bands were quantified using the LAS3000 imaging system (Fujifilm Corporation).
Cell transfection
miR-21 mimics (mimic), miR-21 inhibitor (inhibitor) and scramble negative control (s-MiR) were synthesized by GE Healthcare Dharmacon, Inc. To overexpress or knockdown miR-21, SKM-1 and HL-60 cells were transfected with miR-21 mimics, inhibitor or negative control (all 50 nM) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Their sequences were as follows: miR-21 mimic sense, 5′-UAGCUUAUCAGACUGAUGUUGA-3′; miR-21 mimic antisense, 5′-AACAUCAGUCUGAUAAGCUAUU-3′; s-MiR sense, 5′-UUCUCCGAACGUGUCACGUTT-3′; s-MiR antisense, 5′-ACGUGACACGUUCGGAGAATT-3′; and miR-21 inhibitor, 5′-UCAACAUCAGUCUGAUAAGCUA-3′. Subsequent experiments were conducted following 24 h post-transfection.
Cell proliferation assays
HL-60 and SKM-1 cells transfected with miR-21 mimic, inhibitor and negative control were added to 96-well plates (5×103 cells/ml) at 0–5 days post-transfection and incubated with the CCK-8 reagent for 4 h at 37°C. Cell proliferation was determined using the Cell Counting Kit-8 assay (Beyotime Institute of Biotechnology) according to the manufacturer's instructions. The absorbance was recorded at 450 nm using an ELISA plate reader (BioTek Instruments, Inc.).
Luciferase reporter assay
The potential targets of miR-21 were predicted using TargetScan 7.2 (http://www.targetscan.org/vert_72/) and miRBase (http://www.Microrna.org/microrna/home.do). Using the QuikChange lightning site-directed mutagenesis kit (Stratagene; Agilent Technologies, Inc.), the present study constructed a version of the 3′-UTR sequence of the KLF5 gene that had the potential miR-21 binding sites mutated (MT). The wild-type (WT) or MT KLF5 3′-UTR fragments were then inserted downstream of the firefly luciferase gene in the pGL3 vector (Promega Corporation). The reporter plasmids were co-transfected with miR-21 mimic into AML cells for 36 h in 96-well plates using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Dual luciferase assays (Promega Corporation) were performed to analyze luciferase activity, normalized to Renilla luciferase activity, using a GloMax fluorescence reader (Promega Corporation).
Tumour formation in nude mice
Male athymic nude mice (n=20; weights, 12–15 g; age, 4 weeks) were purchased from the Animal Center of the Chinese Academy of Science and housed in sterile laminar flow cabinets, maintained with temperatures between 18 and 28°C, and ventilated air flow. The mice were exposed to a light/dark cycle of 12/12 h and were given continuous supplies of food, as well as disinfected and filtered drinking water. Mice (at the age of 6 weeks old) were randomly divided into two groups and inoculated subcutaneously with 1×106 HL-60 cells (in 100 µl PBS) transfected with s-MiR or miR-21 mimic. A slide calliper was used to measure the tumour width and length every 5 days, in order to calculate the tumour volume. After 35 days, the animals were sacrificed, the tumours were removed and the tumour volume was calculated using the following formula: Volume=1/2 x length × width. Western blot and RT-qPCR analyses were performed to evaluate the KLF5 expression levels in the resected tumours. All animal care and procedures were approved by the Guizhou Provincial People's Hospital for Animal Experiments committee.
Statistical analysis
Statistical analysis was performed using SPSS v18.0 (SPSS Inc.). Student's t-test or one-way analysis of variance, followed by Tukey's post hoc test, was used to compare the difference between two or more groups. Results were presented as mean ± SEM. Experiments were performed in triplicate. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of miR-21 and KLF5 in AML cells
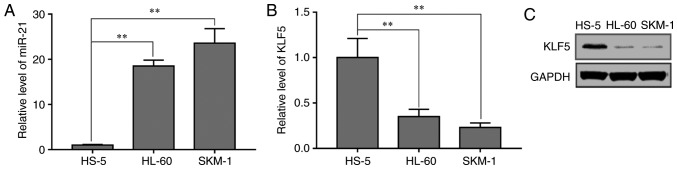
To explore the relative expression levels of miR-21 and KLF5 in SKM-1, HL-60 and HS-5 cells, RT-qPCR analysis was performed. The results demonstrated that miR-21 expression levels were increased in the AML SKM-1 and HL-60 cells compared with that in the normal bone marrow stromal HS-5 cells (Fig. 1A). KLF5 mRNA (Fig. 1B) and protein (Fig. 1C) expression levels were decreased in HL-60 and SKM-1 cells compared with that in HS-5 cells. These data suggested that KLF5 and miR-21 may be associated with the development of AML.
Figure 1.
Expression of miR-21 and KLF5 in AML cell lines. (A) Reverse transcription-quantitative PCR analysis was conducted to determine the expression levels of miR-21 and (B) KLF5 in the AML cell lines HL-60 and SKM-1 and the normal bone marrow stromal cell line HS-5. (C) Western blot analysis was performed to detect the protein expression levels of KLF5 in HL-60, SKM-1 and HS-5 cells. **P<0.01, with comparisons indicated by lines. AML, acute myeloid leukemia.
KLF5 is a direct target of miR-21 in AML cells
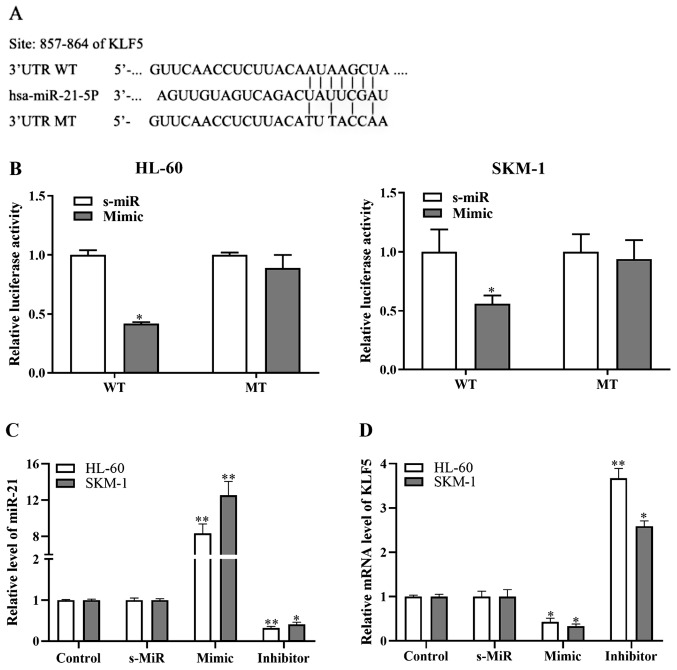
To further elucidate the function of miR-21 in AML, TargetScan 7.2 and miRBase database analysis was performed. The results demonstrated that the 3′-UTR of KLF5 contained a putative binding sequence for miR-21 (Fig. 2A). Using a dual luciferase activity assay, it was demonstrated that miR-21 overexpression by mimic transfection significantly suppressed luciferase activity in SKM-1 and HL-60 cells transfected with the KLF5-3′-UTR-WT construct (Fig. 2B); by contrast, the KLF5-3′-UTR-MT construct had no effect (Fig. 2B). The efficiency of the miR-21 mimic and a miR-21 inhibitor was confirmed by RT-qPCR (Fig. 2C). Notably, the KLF5 mRNA expression levels in SKM-1 and HL-60 cells were significantly decreased following miR-21 overexpression, while they were significantly enhanced following miR-21 inhibition (Fig. 2D). These results suggested that miR-21 could downregulate KLF5 expression by directly targeting its 3′-UTR.
Figure 2.
KLF5 is a direct target of miR-21 in acute myeloid leukemia cells. (A) The putative WT and MT miR-21 binding sequences in the 3′-UTR of KLF5. (B) Luciferase reporter assay in HL-60 and SKM-1 cells co-transfected with WT or MT KLF5 3′-UTR vector and miR-21 mimic or s-MiR. (C) RT-qPCR analysis of miR-21 levels in HL-60 and SKM-1 cells transfected with miR-21 mimic, inhibitor or s-MiR. (D) RT-qPCR analysis of KLF5 mRNA expression levels in HL-60 and SKM-1 cells transfected with miR-21 mimic, inhibitor or s-MiR. Untransfected cells were used as control. *P<0.05 and **P<0.01 vs. s-MiR. KLF5, Krüppel-like factor 5; miR, microRNA; WT, wild-type; MT, mutant; UTR, untranslated region; s-MiR, scramble/negative control microRNA; RT-qPCR, reverse transcription-quantitative PCR.
miR-21 promotes proliferation in AML cells
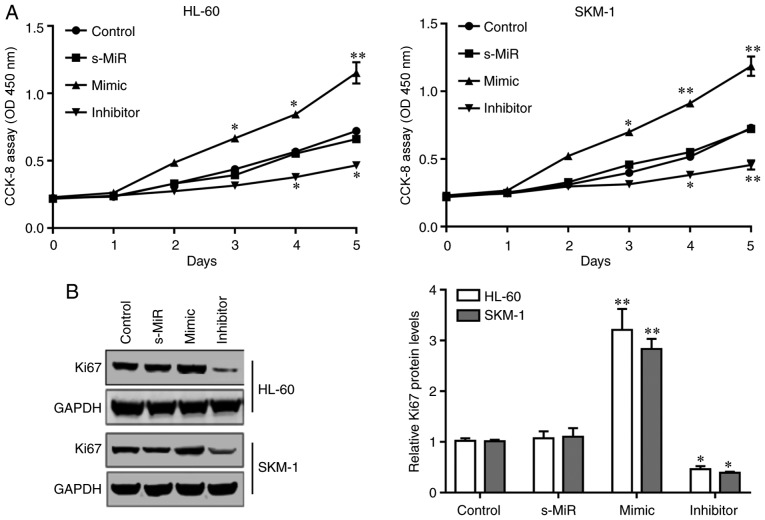
CCK-8 assays were performed to examine the effect of miR-21 on the proliferation of SKM-1 and HL-60 cells. As presented in Fig. 3A, transfection with miR-21 mimic significantly increased the total cell numbers of HL-60 and SKM-1 cells compared with that in the s-MiR group. By contrast, the total cell numbers were significantly reduced following transfection with the miR-21 inhibitor. In addition, western blot analysis demonstrated that the protein expression levels of the proliferation marker Ki67 were increased following transfection of the HL-60 and SKM-1 cells with miR-21 mimic (Fig. 3B). By contract, Ki67 protein expression levels were significantly reduced following transfection with the miR-21 inhibitor (Fig. 3B). These results indicated that high levels of endogenous miR-21 may promote AML cell proliferation by inhibiting KLF5.
Figure 3.
miR-21 promotes proliferation in acute myeloid leukemia cells. HL-60 and SKM-1 cells were transfected with either miR-21 mimic, inhibitor or s-MiR. (A) Cell proliferation was detected at 0–5 days via Cell Counting Kit-8 assays. (B) Western blot analysis of the protein expression levels of the proliferation marker Ki67 at 48 h post-transfection. Untransfected cells were used as control. *P<0.05 and **P<0.01 vs. s-MiR. miR, microRNA; s-MiR, scramble/negative control microRNA.
miR-21 promotes AML tumor growth in vivo
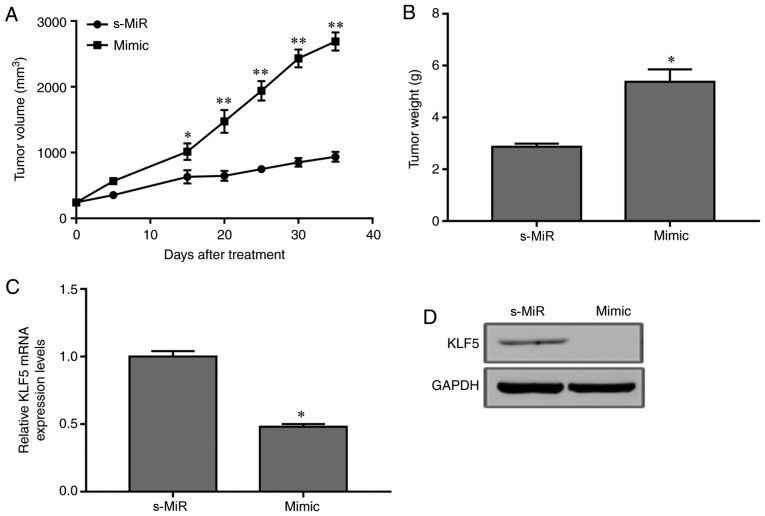
To validate the biological role of miR-21, an AML mouse model was established by subcutaneously injecting HL-60 cells transfected with miR-21 mimic or scramble s-MiR control into nude mice. The tumor volume was determined every 5 days. At 35 days, mice were sacrificed and the final tumor weights were evaluated. A significant increase was detected in the xenograft tumors overexpressing miR-21 compared with those in the s-MiR group (Fig. 4A). In addition, the weights of the tumors from animals injected with miR-21-overexpressing cells were significantly increased compared with those in the s-MiR group (Fig. 4B). Furthermore, miR-21 overexpression resulted in a decrease in KLF5 protein and mRNA expression levels in the xenograft tumors (Fig. 4C and D). Consequently, overexpression of miR-21 downregulated KLF4 expression and promoted AML tumorigenesis in vivo.
Figure 4.
miR-21 overexpression enhances acute myeloid leukemia tumor growth in vivo. A xenograft model was constructed by subcutaneous injection of HL-60 cells transfected with miR-21 mimic or s-MiR scramble control. (A) Tumor size was measured with a caliper every 5 days. (B) Tumors were isolated and weights were assessed at day 35. (C) The mRNA and (D) protein expression levels of KLF5 in the xenograft tumors. *P<0.05 and **P<0.01 vs. s-MiR. miR, microRNA; s-MiR, scramble/negative control microRNA; KLF5, Krüppel-like factor 5.
Discussion
Differential expression of miRNAs has been reported in various studies on patients with AML, including of miR-143, miR-34a, miR-126, miR-21 and miR-425 (9–11,13,20). The potential mechanisms underlying the effects of miRNAs in AML progression require further elucidation. Previous reports demonstrated that miR-21 is significantly upregulated in AML (13), which may identify this miRNA as a potential novel biomarker in the early diagnosis of AML.
It is well established that miRNAs are involved in tumorigenesis by modulating the expression of their target genes (7). Preceding studies revealed that miR-21 functions as a tumor suppressor or an oncogene in gastric carcinoma (21), colorectal carcinoma (22), hepatocellular carcinoma (23) and acute myeloid leukemias (13). In the present study, it was demonstrated that miR-21 was significantly upregulated and KLF5 was significantly downregulated in AML cells, compared with normal bone marrow cells. TargetScan 7.2 and miRBase databases predicted that KLF5 may be a target of miR-21. Subsequent dual luciferase reporter analysis confirmed that KLF5 was a direct target of miR-21. Furthermore, miR-21 overexpression resulted in KLF5 downregulation, while miR-21 inhibition resulted in KLF5 upregulation in AML cells in vitro. Finally, miR-21 overexpression promoted AML cell proliferation in vitro and enhanced AML tumor growth in vivo.
In conclusion, the present results provided experimental evidence that miR-21 promoted AML tumorigenesis by promoting cell proliferation and by directly regulating KLF5 expression in vitro and in vivo. Thus, miR-21 may serve as a potential diagnostic marker and therapeutic target in AML.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in the published article.
Authors' contributions
ChaL and LW were involved in the study concept and design, and analysis and interpretation of data. HY, TC and GL were involved in acquisition of data, analysis and interpretation of data, statistical analysis, and drafting of the manuscript. ChoL, JY and ZD performed bioinformatics analysis. SY, LJW and YH performed the luciferase reporter assay. AL and BY performed the cell proliferation assay and western blotting. JM and LJW revised the manuscript, and performed analysis and interpretation of data.
Ethics approval and consent to participate
All animal care and procedures were approved by Guizhou Provincial People's Hospital for Animal Experiments committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Graubert TA, Mardis ER. Genomics of acute myeloid leukemia. Cancer J. 2011;17:487–491. doi: 10.1097/PPO.0b013e31823c5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosshard R, O'Reilly K, Ralston S, Chadda S, Cork D. Systematic reviews of economic burden and health-related quality of life in patients with acute myeloid leukemia. Cancer Treat. Rev. 2018;69:224–232. doi: 10.1016/j.ctrv.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Wang J. Precision therapy for acute myeloid leukemia. J Hematol Oncol. 2018;11:3. doi: 10.1186/s13045-017-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao J, Li S, Zhao H, Zhu Y, Hong M, Zhu H, Qian S, Li J. Effects of chidamide and its combination with decitabine on proliferation and apoptosis of leukemia cell lines. Am J Transl Res. 2018;10:2567–2578. [PMC free article] [PubMed] [Google Scholar]
- 5.McGuire A, Brown JA, Kerin MJ. Metastatic breast cancer: The potential of miRNA for diagnosis and treatment monitoring. Cancer Metastasis Rev. 2015;34:145–155. doi: 10.1007/s10555-015-9551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Bai G, Zhu W, Bai D, Bi G. Identification of miRNA-mRNA network associated with acute myeloid leukemia survival. Med Sci Monit. 2017;23:4705–4714. doi: 10.12659/MSM.903989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Cai X, Liu E, Tian X, Tian C. MicroRNA-18a promotes proliferation and metastasis in hepatocellular carcinoma via targeting KLF4. Oncotarget. 2017;8:68263–68269. doi: 10.18632/oncotarget.19293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian C, Wu H, Li C, Tian X, Sun Y, Liu E, Liao X, Song W. Downreguation of FoxM1 by miR-214 inhibits proliferation and migration in hepatocellular carcinoma. Gene Ther. 2018;25:312–319. doi: 10.1038/s41434-018-0029-4. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann JU, Bräuer-Hartmann D, Kardosova M, Wilke F, Schödel C, Gerloff D, Katzerke C, Krakowsky R, Namasu CY, Bill M, et al. MicroRNA-143 targets ERK5 in granulopoiesis and predicts outcome of patients with acute myeloid leukemia. Cell Death Dis. 2018;9:814. doi: 10.1038/s41419-018-0837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Zou Y, Lin L, Ma X, Chen H. Identification of serum miR-34a as a potential biomarker in acute myeloid leukemia. Cancer Biomark. 2018;22:799–805. doi: 10.3233/CBM-181381. [DOI] [PubMed] [Google Scholar]
- 11.Ding Q, Wang Q, Ren Y, Zhu HQ, Huang Z. MicroRNA-126 attenuates cell apoptosis by targeting TRAF7 in acute myeloid leukemia cells. Biochem Cell Biol. 2018;25 doi: 10.1139/bcb-2018-0017. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 12.Badr M, Said H, Louka ML, Elghazaly HA, Gaballah A, Atef Abd El Mageed M. MicroRNA-21 as a predictor and prognostic factor for trastuzumab therapy in human epidermal growth factor receptor 2-positive metastatic breast cancer. J Cell Biochem. 2018;120:3459–3466. doi: 10.1002/jcb.27620. [DOI] [PubMed] [Google Scholar]
- 13.Riccioni R, Lulli V, Castelli G, Biffoni M, Tiberio R, Pelosi E, Lo-Coco F, Testa U. miR-21 is overexpressed in NPM1-mutant acute myeloid leukemias. Leuk Res. 2015;39:221–228. doi: 10.1016/j.leukres.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Sui X, Hu X, Hu Z. Overexpression of KLF5 inhibits puromycin-induced apoptosis of podocytes. Mol Med Rep. 2018;18:3843–3849. doi: 10.3892/mmr.2018.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Wang Q, Liu F, Ma X, Wu L, Guo F, Zhao S, Huang F, Qin G. KLF5 promotes the tumorigenesis and metastatic potential of thyroid cancer cells through the NF-κB signaling pathway. Oncol Rep. 2018;40:2608–2618. doi: 10.3892/or.2018.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia L, Zhou Z, Liang H, Wu J, Shi P, Li F, Wang Z, Wang C, Chen W, Zhang H, et al. KLF5 promotes breast cancer proliferation, migration and invasion in part by upregulating the transcription of TNFAIP2. Oncogene. 2016;35:2040–2051. doi: 10.1038/onc.2015.263. [DOI] [PubMed] [Google Scholar]
- 17.Gong T, Cui L, Wang H, Wang H, Han N. Knockdown of KLF5 suppresses hypoxia-induced resistance to cisplatin in NSCLC cells by regulating HIF-1α-dependent glycolysis through inactivation of the PI3K/Akt/mTOR pathway. J Transl Med. 2018;16:164. doi: 10.1186/s12967-018-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du C, Gao Y, Xu S, Jia J, Huang Z, Fan J, Wang X, He D, Guo P. KLF5 promotes cell migration by up-regulating FYN in bladder cancer cells. FEBS Lett. 2016;590:408–418. doi: 10.1002/1873-3468.12069. [DOI] [PubMed] [Google Scholar]
- 19.Diakiw SM, Kok CH, To LB, Lewis ID, Brown AL, D'Andrea RJ. The granulocyte-associated transcription factor Krüppel-like factor 5 is silenced by hypermethylation in acute myeloid leukemia. Leuk Res. 2012;36:110–116. doi: 10.1016/j.leukres.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Shao T, Zhang H, Zhang N, Shi X, Liu X, Yao Y, Xu L, Zhu S, Cao J, et al. MiR-425 expression profiling in acute myeloid leukemia might guide the treatment choice between allogeneic transplantation and chemotherapy. J Transl Med. 2018;16:267. doi: 10.1186/s12967-018-1647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Abraham JM, Cheng Y, Wang Z, Wang Z, Zhang G, Ashktorab H, Smoot DT, Cole RN, Boronina TN, et al. Synthetic circular RNA functions as a miR-21 sponge to suppress gastric carcinoma cell proliferation. Mol Ther Nucleic Acids. 2018;13:312–321. doi: 10.1016/j.omtn.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding T, Cui P, Zhou Y, Chen C, Zhao J, Wang H, Guo M, He Z, Xu L. Antisense oligonucleotides against miR-21 inhibit the growth and metastasis of colorectal carcinoma via the DUSP8 pathway. Mol Ther Nucleic Acids. 2018;13:244–255. doi: 10.1016/j.omtn.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Yang H, Ren L. MiR-21 promoted proliferation and migration in hepatocellular carcinoma through negative regulation of Navigator-3. Biochem Biophys Res Commun. 2015;464:1228–1234. doi: 10.1016/j.bbrc.2015.07.110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.