Abstract
Long non-coding RNA (lncRNA) actin filament-associated protein 1 antisense RNA 1 (AFAP1-AS1) has been revealed to be associated with certain types of cancer. However, whether the lncRNA AFAP1-AS1 is involved in the development and progression of gastric cancer (GC) remains unknown. The present study investigated the clinical significance and biological functions of AFAP1-AS1 in GC. The expression levels of lncRNA AFAP1-AS1 in 52 patients with GC, and in 1 normal gastric mucosal cell line and 3 GC cell lines, were evaluated by reverse transcription quantitative polymerase chain reaction analysis. Small interfering RNAs were used to suppress AFAP1-AS1 expression in GC cell lines. The results indicated that AFAP1-AS1 expression levels were significantly increased in GC tissues and cell lines compared with the corresponding noncancerous tissues and normal gastric cells. In addition, the patients with GC with increased AFAP1-AS1 expression exhibited an advanced clinical stage and an association with the occurrence of lymph node metastasis compared with those with decreased AFAP1-AS1 expression. In vitro assays demonstrated that knockdown of AFAP1-AS1 decreased levels of cell proliferation and migration. In addition, the results of flow cytometry demonstrated that knockdown of AFAP1-AS1 caused cell cycle arrest. In conclusion, AFAP1-AS1 is a novel molecule involved in GC progression, which may be a potential prognostic biomarker and target for therapeutic intervention.
Keywords: gastric cancer, tumorigenesis, genes, proliferation, migration invasion
Introduction
Gastric cancer (GC), a genetic disease involving multiple changes in the genome, remains a primary cause of male mortality in developing countries, with its incidence continuously increasing in recent years (1,2). In spite of the improvements achieved in the techniques employed for diagnosis and treatment, the majority of patients are diagnosed during the advanced stages of disease, and thus suffer a poor prognosis (3–5). Therefore, improving the early diagnosis and treatment of GC is a major strategy for decreasing the rate of mortality.
Over the past few decades, a large number of studies have focused on the aberrant expression of protein-coding RNAs in different types of cancer, which have provided promising approaches for cancer diagnosis and treatment (6,7). However, along with the advances made in high-resolution microarrays and genome-wide sequencing technology, an increasing body of evidence from genomics and transcriptomics studies has suggested that long non-coding RNAs (lncRNAs) may be effective biomarkers for cancer detection and molecular targets for cancer therapy (8–11). The lncRNA actin filament-associated protein 1 antisense RNA 1 (AFAP1-AS1) has been demonstrated to be upregulated in ovarian cancer, gallbladder cancer, esophageal squamous cell carcinoma, pancreatic ductal adenocarcinoma and colorectal cancer (12–16). However, whether lncRNA AFAP1-AS1 is involved in the development and tumorigenesis of GC remains unknown. The aim of the present study was to determine the clinical significance and biological functions of AFAP1-AS1 in GC.
Materials and methods
Patients and tissue samples
A total of 52 GC samples and the corresponding adjacent tissues were obtained from patients who underwent surgery during October 2013 and August 2015 at Nanjing Hospital Affiliated with Nanjing Medical University (Nanjing, China). The inclusion criteria for patient recruitment were as follows: i) The first diagnosis of the patient is GC (age 20–85 years old); ii) the patients' cardiopulmonary function was confirmed to be normal by pathological examination prior to surgery; iii) the patients' blood, liver and kidney function were basically normal; iv) none of the patients received radiotherapy or chemotherapy prior to surgery; and v) all patients were diagnosed with clinical TNM stage (cTNM) I–IV GC (17) and had detailed clinical data and basic personal information available. The exclusion criteria for patient recruitment were: i) Patients who has undergone radiotherapy and chemotherapy prior to surgery; ii) patients who were in poor physical condition and would not be able to tolerate the required examination; iii) patients in which GC was not the primary tumor, but a result of secondary tumor metastasis; and iv) diagnosis of secondary diseases upon admission, in particular other types of cancer besides GC. The tumor tissues and adjacent non-tumor tissue (the distance from tumor surgical margin ≥5 cm) were collected during surgical resection. All specimens were immediately stored at −80°C in a freezer until subsequent use. The present study was approved by the Ethics Committee of Nanjing First Hospital, Nanjing Medical University (Jiangsu, China). Written informed consent was obtained from all of the patients prior to participation.
RNA isolation, reverse transcription (RT), and RT-quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from the cancerous and paracancerous tissues using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was obtained with the Prime-Script™ RT-PCR kit (Takara Biotechnology Co., Ltd.). AFAP1-AS1 expression was measured by RT-qPCR with the following primer sequences: AFAP1-AS1 forward, 5′-TCGCTCAATGGAGTGACGGCA-3′; and AFAP1-AS1 reverse, 5′-CGGCTGAGACCGCTGAGAACTT-3′. The results were normalized to GAPDH using the following primers: GAPDH forward, 5′-GTCAACGGATTTGGTCTGTATT-3′; and GAPDH reverse, 5′-AGTCTTCTGGGTGGCAGTGAT-3′. RT-qPCR reactions were performed with ABI7500 System and SYBR Green PCR Master Mix (Thermo Fisher Scientific, Inc.). The PCR was performed using the following conditions: Initial denaturation at 95°C for 30 sec; 40 cycles of 95°C for 5 sec and 60°C for 30 sec with final annealing and extension at 60°C for 60 sec and 95°C for 15 sec. The expression level of AFAP1-AS1 in GC tissues and corresponding paracancerous tissues was analyzed using the 2ΔΔCq method (18), and the fold change of target genes was analyzed.
Cell culture
The human GC MKN-45, MGC-803 and AGS cell lines were purchased from Shanghai Institute of Cell Biology. Cells were cultured in RPMI-1640 medium supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml), and streptomycin (100 U/ml) at 37°C in a humidified incubator with 5% CO2.
Small interfering RNA (siRNA) preparation and transfection
For gene knockdown, cells were seeded for 10–12 h and transfected with either 10 nM siRNA or scramble control siRNA (Invitrogen; Thermo Fisher Scientific, Inc.) using Lipofectamine™ Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc.). The sequences of the AFAP1-AS1 targeting siRNAs are presented in Table I.
Table I.
Sequences of the AFAP1-AS1-targeting siRNAs.
| siRNAs | Forward | Reverse |
|---|---|---|
| Negative control | 5′-GCGACGAUCUGCCUAAGA-3′ | 5′-AUCUUAGGCAGAUCGUCG-3′ |
| siRNA1 | 5′-GUCCCAGCUUACACUUGUATT-3′ | 5′-UACAAGUGUAAGCUGGGACTT-3′ |
| siRNA2 | 5′-GGGCUUCAAUUUACAAGCATT-3′ | 5′-UGCUUGUAAAUUGAAGCCCTT-3′ |
| siRNA3 | 5′-CCUAUCUGGUCAACACGUATT-3′ | 5′-UACGUGUUGACCAGAUAGGTT-3′ |
siRNA, small interfering RNA.
Transfection experiments were performed at 10–12 h after cell growth. Cell proliferation was in the logarithmic growth phase, and the cell area size occupied 50–60% of the culture dish area. Initial detection may be performed at multiple concentration gradients, to detect the interference efficiency of different concentrations of siRNA. Using a 10 nmol (1.2 µl) interference concentration as an example, 100 µl Opti-MEM, 1.2 µl negative control (NC)/interference reagents (siRNA), and 12 µl HiPerFect (Qiagen GmbH) were added to each tube. After 48 h, the cells were harvested and RT-qPCR was performed as aforementioned to detect the interference efficiency.
MTT assays
Cells were seeded onto 96-well culture plates at a density of 1×103 cells per well and incubated at 37°C overnight. Then, the cells were incubated with different concentration (1–10 µm) of curcumin for 72 h. After 24, 48, 72 or 96 h, 20 µl MTT dissolved in 100 µl RPMI-1640 was added to each well and incubated for an additional 4 h. Then, 150 µl dimethyl sulfoxide was added to dissolve formazan crystals. Optical density was detected at a wavelength of 490 nm using an enzyme-labeled analyzer.
Flow cytometry analysis
The GC MGC-803 cell line was harvested following treatment and washed with PBS. Then, cells were resuspended and stained with fluorescein isothiocyanate-Annexin V and propidium iodide Apoptosis Detection Kit (cat. no. 556547; BD Biosciences) for apoptosis analysis. A flow cytometer (CYTOMICS FC 500; Beckman Coulter, Inc.) was used to analyze the number of apoptotic MGC-803 cells, and the cell cycle distribution. The fluorescence signal was detected at an emission wavelength of 530 nm. The percentage of cells in the G0-G1, S, and G2-M phases was counted and compared. All data were analyzed using Flowjo v10 software (Tree Star, Inc.).
In vitro wound healing assay
Wounds were created in adherent cells using a 20 µl sterile pipette tip after 48 h of transfection with si-AFAP1-AS1 or si-NC. Cells were then washed 3 times with PBS to remove any free-floating cells and debris. Medium without serum was added, and the cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. Wound healing was observed after 0, 12, or 24 h, respectively, and images of the cells were captured using digital light microscope at ×200 magnification.
Statistical analysis
All experiments were performed at least 3 times, and the data are presented as the mean ± standard deviation. The differential expression levels of AFAP1-AS1 between the cancerous and adjacent tissues were analyzed with paired Student's t-tests. Categorical data in Table II were analyzed using the χ2 test. A one-way analysis of variance followed by the Bonferroni's post-hoc test was used to evaluate numerical differences for multiple comparisons. For GC tissues and the corresponding non-cancerous adjacent tissues, the fold change of the target gene was calculated using the 2-∆∆Cq method (15). All statistical analyses were performed with SPSS 23 software (IBM Corp.) and GraphPad Prism 7.0 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Table II.
Associations between AFAP1-AS1 and clinicopathological characteristic of 52 primary gastric cancer samples.
| Variables | Over expression of AFAP1-AS1, n (%) | Normal expression of AFAP1-AS1, n (%) | χ2 value | P-value |
|---|---|---|---|---|
| Age, y | 0.084 | 0.772 | ||
| ≥60 | 20 (52.6) | 6 (42.9) | ||
| <60 | 18 (47.4) | 8 (57.1) | ||
| Sex | 2.608 | 0.106 | ||
| Male | 28 (73.7) | 7 (50) | ||
| Female | 10 (26.3) | 7 (50) | ||
| Diameter, cm | 3.791 | 0.052 | ||
| ≥5 | 13 (34.2) | 9 (64.3) | ||
| <5 | 25 (65.8) | 5 (35.7) | ||
| Sites | 0.259 | 0.611 | ||
| Upper | 16 (47.1) | 7 (50) | ||
| Lower | 22 (52.9) | 7 (50) | ||
| Differentiation | 1.481 | 0.224 | ||
| Well/Mid | 18 (47.4) | 4 (28.6) | ||
| Poorly | 20 (52.6) | 10 (71.4) | ||
| Lymphatic metastasis | 6.656 | 0.010 | ||
| Yes | 12 (31.6) | 10 (71.4) | ||
| No | 26 (68.4) | 4 (28.6) | ||
| TNM stage | 7.137 | 0.008 | ||
| I+II | 14 (36.8) | 11 (78.6) | ||
| III+IV | 24 (63.2) | 3 (21.4) |
AFAP1-AS, actin filament-associated protein 1 antisense RNA 1; TNM, tumor node metastasis staging.
Results
Expression of LncRNA AFAP1-AS1 in GC tissues
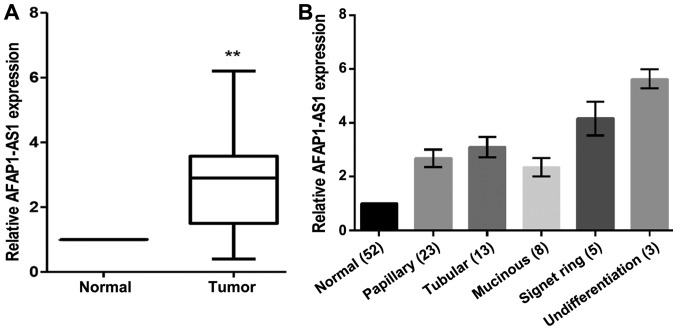
RT-qPCR was used to examine AFAP1-AS1 mRNA expression in 52 cases of GC. The results demonstrated that lncRNA AFAP1-AS1 expression levels were significantly upregulated in GC tissues when compared with the corresponding non-cancerous tissues (n=52; P<0.05; Fig. 1). Evaluation of the differences in the clinicopathological characteristics between the patients with primary GC with and without AFAP1-AS1 overexpression revealed that the overexpression of AFAP1-AS1 was positively associated with clinical stage and lymph node metastasis, although they were not significantly associated with sex, age, tumor size, tumor location or degree of differentiation in patients with GC (Table II).
Figure 1.
Expression of LncRNA AFAP1-AS1 is detected by reverse transcription-quantitative polymerase chain reaction in 52 GC tissues. (A) AFAP1-AS expression is upregulated in GC tissues when compared with adjacent para-cancer tissues (n=52). (B) AFAP1-AS1 expression in different pathological types of GC tissues when compared with adjacent para-cancer tissues. **P<0.01 vs. Normal. AFAP1-AS, actin filament-associated protein 1 antisense RNA 1; lncRNA, long non-coding RNA; GC, gastric cancer.
Expression of LncRNA AFAP1-AS1 in GC cells
The expression levels of AFAP1-AS1 in the GC MGC-803 and AGS cell lines were significantly increased compared with that observed in the normal gastric GES-1 cell line (P<0.05); the expression level in MGC-803 cells was the highest. However, the expression of AFAP1-AS1 in the GC cell line MKN45 was not significantly different when compared with the normal gastric cells (Fig. 2).
Figure 2.
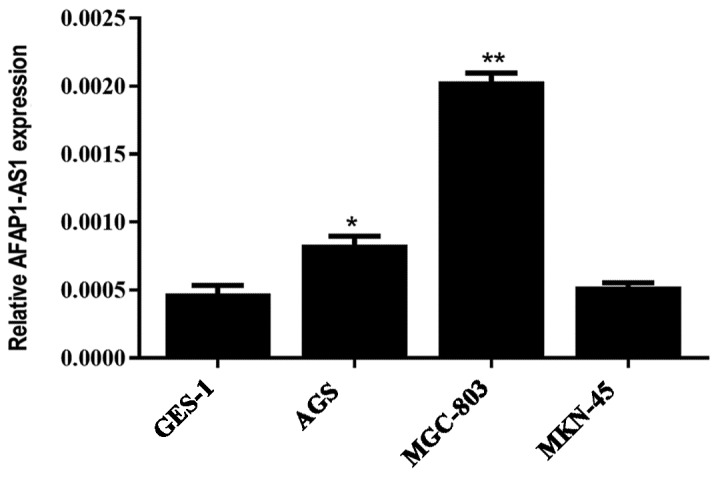
AFAP1-AS1 expression in GES-1 cells and 3 gastric cancer cell lines was evaluated using reverse transcription-quantitative polymerase chain reaction and presented as fold change relative to the expression in GES-1. *P<0.05 and **P<0.01 vs. GES-1. AFAP1-AS, actin filament-associated protein 1 antisense RNA 1.
Effects of AFAP1-AS1 knockdown on the proliferation of the GC cell line MGC-803
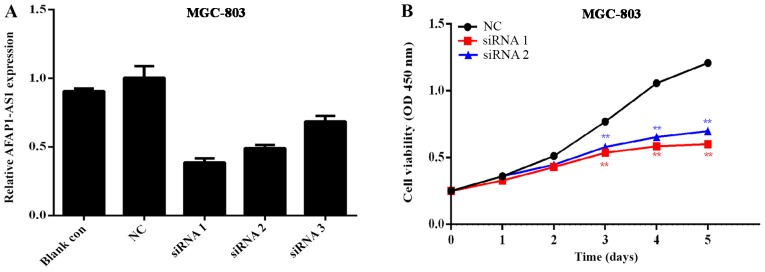
The GC cell line MGC-803, which exhibited the highest level of AFAP1-AS1 expression, was selected for subsequent experiments to determine the transfection efficiency. As indicated in Fig. 3A, the knockout effects of siRNA1 and siRNA2 were more efficient compared with siRNA3, with knockdown efficiencies of ~65 and ~50%, respectively. Therefore, these 2 interference sequences were selected for co-transfection in order to examine the effects on the functions of GC cells.
Figure 3.
The AFAP1 expression and cell viability following MGC803 knock down. (A) Detection of AFAP1-AS1 knockdown efficiency in the gastric cancer cell line MGC-803. Compared to the NC group, the knockdown efficiency of siRNA1 was ~65% and for siRNA2 was ~50%. (B) MTT assays in the MGC803 cell line following alteration of the expression of AFAP1-AS1. AFAP1-AS, actin filament-associated protein 1 antisense RNA 1; siRNA, small interfering RNA; NC, negative control; OD, optical density. **P<0.01 vs. control.
Using the GC cell line MGC-803, an MTT assay was performed to generate curves of cell growth over 5 days and the results determined the proliferation rate of GC MGC-803. Cell proliferation was significantly inhibited following AFAP1-AS1 downregulation (Fig. 3B). The results revealed that transfection of MGC-803 cells with siRNA1 and siRNA2 resulted in a significant decrease in cell proliferation when compared with the control siRNA-transfected MGC-803 cells (Fig. 3B).
Effects of AFAP1-AS1 on apoptosis and the cell cycle in MGC-803 cells
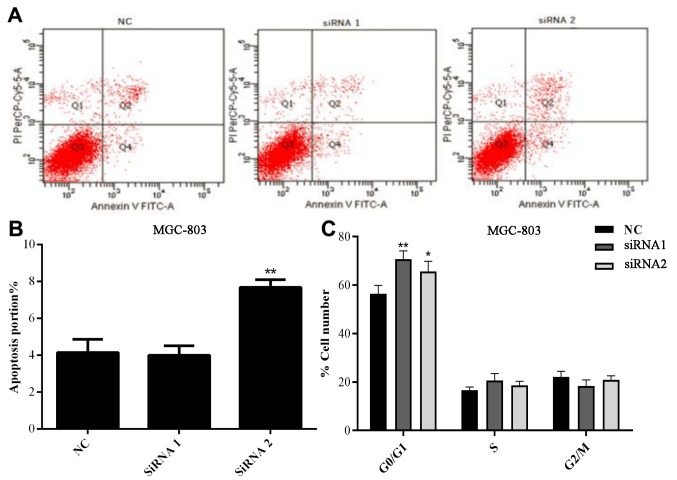
Flow cytometry was conducted to detect the changes in apoptosis and the cell cycle in MGC-803 cells following transfection with si-AFAP1-AS1. As demonstrated in Fig. 4A and B, there was no significant difference in the rate of apoptosis between the NC and siRNA1 groups, but the MGC cells apoptosis rate was significantly increased in the siRNA2 group (P<0.01).
Figure 4.
Effects of AFAP1-AS1 on apoptosis and the cell cycle in MGC-803 cells. (A and B) Flow cytometry of apoptosis in MGC-803 cells transfected with siRNA1 and siRNA2. Specific AFAP1-AS1 siRNAs increased the rate of apoptosis in MGC-803 cells. (A) Representative scatter plot demonstrating levels of apoptosis. (B) Quantification of flow cytometry data. (C) Cell cycle phase distributions were analyzed using flow cytometry. AFAP1-AS, actin filament-associated protein 1 antisense RNA 1; siRNA, small interfering RNA; NC, negative control; FITC, fluorescein isothiocyanate; PI, propidium iodide. *P<0.05 and **P<0.01 vs. negative control.
Following transfection of siRNAs in the MGC803 cell line, it was demonstrated that there was no marked difference in the rate of apoptosis between the NC and siRNA1 groups following APAP1-AS1 silencing (P=0.1064). However, the proportion of cells in the G0/G1 phase following APAP1-AS1 knockdown was significantly increased in the siRNA1 and siRNA2 groups compared with that observed in the NC group (P<0.05; P<0.01 respectively; Fig. 4C), suggesting that APAP1-AS1 may affect the regulation of the cell cycle in GC.
Effects of AFAP1-AS1 on migratory capabilities in the GC MGC-803 cell line
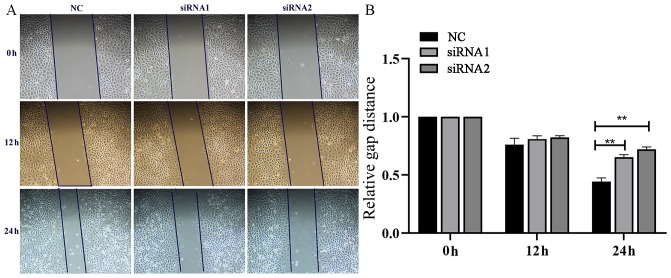
Using the GC cell line MGC-803, cell scratches were created at 0, 12, and 24 h after cell interference and images of the cells were captured. As indicated in Fig. 5, the migratory ability of the GC MGC-803 cell line was significantly inhibited following knockdown of AFAP1-AS1.
Figure 5.
Effects of AFAP1-AS1 on the migratory ability of the gastric cancer MGC-803 cell line. (A) Wound healing assays were conducted to assess the migratory capabilities of MGC-803 cells transfected with si-AFAP1-AS1. (B) The data are summarized as the relative gap distance. AFAP1-AS, actin filament-associated protein 1 antisense RNA 1; siRNA, small interfering RNA; NC, negative control. **P<0.01 vs. NC.
Discussion
An increasing body of evidence has demonstrated that the upregulation of lncRNAs is involved in the tumorigenesis and metastasis of GC, a type of malignant neoplasm frequently observed in East Asia. lncRNAs are a class of nucleic acid sequences measuring >200 bps in length (19). A number of studies have demonstrated that lncRNAs participate in a wide range of physiological processes; they may regulate gene expression at the transcriptional and post-transcriptional level, and may also regulate cellular functions through various mechanisms (20). lncRNAs are known to be closely associated with the development and progression of cancer, and recently have become a key area of medical research, with potential in future clinical practice.
GC is one of the most common types of malignant tumors in humans and is a serious threat to human health. Its incidence rate has continuously risen over previous years and China has one of the highest incidence rates of GC in the world. Chen et al (21) demonstrated that in China, the GC incidence was the second most common type of tumor diagnosed. The traditional treatments, including surgery, radiotherapy and chemotherapy, are not sufficiently effective for patients with advanced stage GC. Therefore, it is important to identify valuable biomarkers to improve the percentage of accurate early diagnoses in patients with GC. According to previous studies, a number of biomarkers have been identified for the diagnosis or prognosis of patients with cancer (22,23); among these, lncRNAs are a key area of interest.
Lately, AFAP1-AS1 has been revealed to function primarily as an oncogene, regulating gene expression by affecting mRNA and the corresponding transcriptional protein. A previous profiling study suggested that the upregulation of lncRNA AFAP1-AS1 affected the proliferation, invasion and survival rates of tongue squamous cell carcinoma via the Wnt/β-catenin signaling pathway (24). Yuan et al (25) revealed that the AFAP1-AS1/miR-320a/RBPJ axis regulates laryngeal carcinoma cell stemness and chemoresistance. An additional previous study indicated that the upregulation of lncRNA AFAP1-AS1 expression was associated with progression and poor prognosis in nasopharyngeal carcinoma (26). However, to the best of our knowledge, its function in carcinogenesis and tumor progression in GC remains unknown.
In the present study, the results of RT-qPCR demonstrated that lncRNA AFAP1-AS1 expression in tumor tissues was significantly increased compared with that of non-cancerous tissues in 52 matched pairs of patient samples, which is consistent with a previous study (27). In addition, the association between AFAP1-AS1 and clinicopathological characteristics revealed that the overexpression of AFAP1-AS1 was not aberrantly associated with ex, age, tumor size, tumor location or degree of differentiation in patients with GC, but was positively associated with clinical stage and lymph node metastasis. Higher AFAP1-AS1 mRNA expression levels were also associated with the occurrence of lymph node metastasis.
To further understand the biological function of AFAP1-AS1 in GC cells, in vitro experiments were conducted. The GC MGC-803 cell line exhibited the highest AFAP1-AS1 expression when compared with the gastric normal cell line GES-1, within a panel of GC cell lines. Therefore, the MGC-803 cell line was selected for further analysis. The results demonstrated that the proliferation rate of MGC-803 cells was significantly inhibited following knockdown of AFAP1-AS1 expression.
In addition, the proportion of cells in the G0/G1 phase following knockdown was significantly increased compared with that observed in the NC group, suggesting that AFAP1-AS1 may have an effect on the regulation of the GC cell cycle. However, the effect of APAP1-AS1 on the rate of apoptosis was not as marked as expected. In addition, the migratory ability of the GC MGC-803 cell line was significantly inhibited following knockdown of AFAP1-AS1 expression.
The present study confirmed that AFAP1-AS1 is highly expressed in human GC tissues and cell lines, and that it significantly affected tumor cell invasion and proliferation. However, its specific mechanism has not been fully elucidated and requires additional investigation.
In conclusion, the results of the present study demonstrated that lncRNA AFAP1-AS1 is highly expressed in GC, suggesting that AFAP1-AS1 may be a GC-specific lncRNA and serve play an important role in the development of GC. Knockdown of lncRNA AFAP1-AS1 inhibited cell proliferation and migration in MGC-803 cells. These results indicated that lncRNA AFAP1-AS1 may function as an oncogene in GC and may be a novel marker for the diagnosis and therapeutic targeting of GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jiangsu Natural Science Foundation (grant no. BK20151087), awarded to Professor Hongyong Cao.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZXL designed the project, collected patient data and performed the experiments. ZLD collected the patient data and performed the experiments. DWR analyzed the data. WWT contributed to the design of the project, wrote the original draft manuscript and provided funding. HYC supervised the project throughout, reviewed and edited the manuscript, provided funding and collected the patient data.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Nanjing First Hospital, Nanjing Medical University (Jiangsu, China). Written informed consent was obtained from all of the patients prior to participation.
Patient consent for publication
Written informed consent was obtained from all of the patients prior to participation.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Yagi K, Nozawa Y, Endou S, Nakamura A. Diagnosis of early gastric cancer by magnifying endoscopy with NBI from viewpoint of histological imaging: Mucosal patterning in terms of white zone visibility and its relationship to histology. Diagn Ther Endosc. 2012;2012:954809. doi: 10.1155/2012/954809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon SH, Kim YH, Lee YJ, Park J, Kim JW, Lee HS, Kim B. Tumor heterogeneity in human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer assessed by CT texture analysis: Association with survival after trastuzumab treatment. PLoS One. 2016;11:e0161278. doi: 10.1371/journal.pone.0161278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biffi R, Botteri E, Cenciarelli S, Luca F, Pozzi S, Valvo M, Sonzogni A, Chiappa A, Leal Ghezzi T, Rotmensz N, et al. Impact on survival of the number of lymph nodes removed in patients with node-negative gastric cancer submitted to extended lymphnode dissection. Eur J Surg Oncol. 2011;37:305–311. doi: 10.1016/j.ejso.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Yang G, Lu X, Yuan L. LncRNA: A link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: A new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.CAN-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 9.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Zheng J, Deng J, You Y, Wu H, Li N, Lu J, Zhou Y. Increased levels of the long intergenic non-protein coding RNA POU3F3 promote DNA methylation in esophageal squamous cell carcinoma cells. Gastroenterology. 2014;146:1714–1726.e5. doi: 10.1053/j.gastro.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Tsang WP, Ng EK, Ng SS, Jin H, Yu J, Sung JJ, Kwok TT. Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis. 2010;31:350–358. doi: 10.1093/carcin/bgp181. [DOI] [PubMed] [Google Scholar]
- 12.Yang SL, Lin RX, Si LH, Cui MH, Zhang XW, Fan LM. Expression and functional role of long non-coding RNA AFAP1-AS1 in ovarian cancer. Eur Rev Med Pharmacol Sci. 2016;20:5107–5112. [PubMed] [Google Scholar]
- 13.Ma F, Wang SH, Cai Q, Zhang MD, Yang Y, Ding J. Overexpression of LncRNA AFAP1-AS1 predicts poor prognosis and promotes cells proliferation and invasion in gallbladder cancer. Biomed Pharmacother. 2016;84:1249–1255. doi: 10.1016/j.biopha.2016.10.064. [DOI] [PubMed] [Google Scholar]
- 14.Luo HL, Huang MD, Guo JN, Fan RH, Xia XT, He JD, Chen XF. AFAP1-AS1 is upregulated and promotes esophageal squamous cell carcinoma cell proliferation and inhibits cell apoptosis. Cancer Med. 2016;5:2879–2885. doi: 10.1002/cam4.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han X, Wang L, Ning Y, Li S, Wang Z. Long non-coding RNA AFAP1-AS1 facilitates tumor growth and promotes metastasis in colorectal cancer. Biol Res. 2016;49:36. doi: 10.1186/s40659-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu XL, Liu DJ, Yan TT, Yang JY, Yang MW, Li J, Huo YM, Liu W, Zhang JF, Hong J, et al. Analysis of long non-coding RNA expression profiles in pancreatic ductal adenocarcinoma. Sci Rep. 2016;6:33535. doi: 10.1038/srep33535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahul BA, Stephen B, Edge, Frederick L, et al. AJCC Cancer Staging Manual. https://cancerstaging.org/references-tools/deskreferences/pages/default.aspx. [Sep 5;2018 ];
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann JH, Spector DL. Long non-coding RNAs: Modulators of nuclear structure and function. Curr Opin Cell Biol. 2014;26:10–18. doi: 10.1016/j.ceb.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Qu X. Cancer biomarker detection: Recent achievements and challenges. Chem Soc Rev. 2015;44:2963–2997. doi: 10.1039/C4CS00370E. [DOI] [PubMed] [Google Scholar]
- 23.Armitage EG, Barbas C. Metabolomics in cancer biomarker discovery: Current trends and future perspectives. J Pharm Biomed Anal. 2014;87:1–11. doi: 10.1016/j.jpba.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Wang ZY, Hu M, Dai MH, Xiong J, Zhang S, Wu HJ, Zhang SS, Gong ZJ. Upregulation of the long non-coding RNA AFAP1-AS1 affects the proliferation, invasion and survival of tongue squamous cell carcinoma via the Wnt/β-catenin signaling pathway. Mol Cancer. 2018;17:3. doi: 10.1186/s12943-017-0752-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Yuan Z, Xiu C, Song K, Pei R, Miao S, Mao X, Sun J, Jia S. Long non-coding RNA AFAP1-AS1/miR-320a/RBPJ axis regulates laryngeal carcinoma cell stemness and chemoresistance. J Cell Mol Med. 2018;22:4253–4262. doi: 10.1111/jcmm.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bo H, Gong Z, Zhang W, Li X, Zeng Y, Liao Q, Chen P, Shi L, Lian Y, Jing Y, et al. Upregulated long non-coding RNA AFAP1-AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget. 2015;6:20404–1829. doi: 10.18632/oncotarget.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu X, Zhou C, Li R, Liang Z, Zhai W, Zhao L, Zhang S. Critical role for the long non-coding RNA AFAP1-AS1 in the proliferation and metastasis of hepatocellular carcinoma. Tumor Biol. 2016;37:9699–9707. doi: 10.1007/s13277-016-4858-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.