Abstract
Despite advances in the field of male reproductive health, idiopathic male infertility, in which a man has altered semen characteristics without an identifiable cause and there is no female factor infertility, remains a challenging condition to diagnose and manage. Increasing evidence suggests that oxidative stress (OS) plays an independent role in the etiology of male infertility, with 30% to 80% of infertile men having elevated seminal reactive oxygen species levels. OS can negatively affect fertility via a number of pathways, including interference with capacitation and possible damage to sperm membrane and DNA, which may impair the sperm's potential to fertilize an egg and develop into a healthy embryo. Adequate evaluation of male reproductive potential should therefore include an assessment of sperm OS. We propose the term Male Oxidative Stress Infertility, or MOSI, as a novel descriptor for infertile men with abnormal semen characteristics and OS, including many patients who were previously classified as having idiopathic male infertility. Oxidation-reduction potential (ORP) can be a useful clinical biomarker for the classification of MOSI, as it takes into account the levels of both oxidants and reductants (antioxidants). Current treatment protocols for OS, including the use of antioxidants, are not evidence-based and have the potential for complications and increased healthcare-related expenditures. Utilizing an easy, reproducible, and cost-effective test to measure ORP may provide a more targeted, reliable approach for administering antioxidant therapy while minimizing the risk of antioxidant overdose. With the increasing awareness and understanding of MOSI as a distinct male infertility diagnosis, future research endeavors can facilitate the development of evidence-based treatments that target its underlying cause.
Keywords: Infertility, male; MOSI; Oxidation reduction potential; Oxidative stress; Semen
INTRODUCTION
Natural conception is a complex process that is achieved in only 76% to 85% of couples within 12 months of regular unprotected intercourse [1,2,3,4,5]. The International Committee for Monitoring Assisted Reproductive Technologies (ICMART) defines infertility as the inability to conceive after 1 year of regular, unprotected intercourse [6,7]. The World Health Organization estimates that nearly 190 million people struggle with infertility worldwide and the number of couples seeking medical assistance is steadily rising [8,9]. Among couples unable to conceive, infertility is partially or wholly attributable to a male factor in approximately 50% of cases (Fig. 1) [10,11,12]. A variety of conditions can affect male reproductive potential to different extent and they often coexist (Fig. 2) [13,14,15,16,17,18,19]. Paradoxically, on routine assessment, the precise etiology of male factor infertility remains undefined in 30% to 50% of patients, who are subsequently classified as having idiopathic male infertility [20,21,22]. Unlike unexplained male infertility with its normal semen parameters, idiopathic male infertility is diagnosed in the presence of altered semen characteristics without an identifiable cause and the absence of female factor infertility [23].
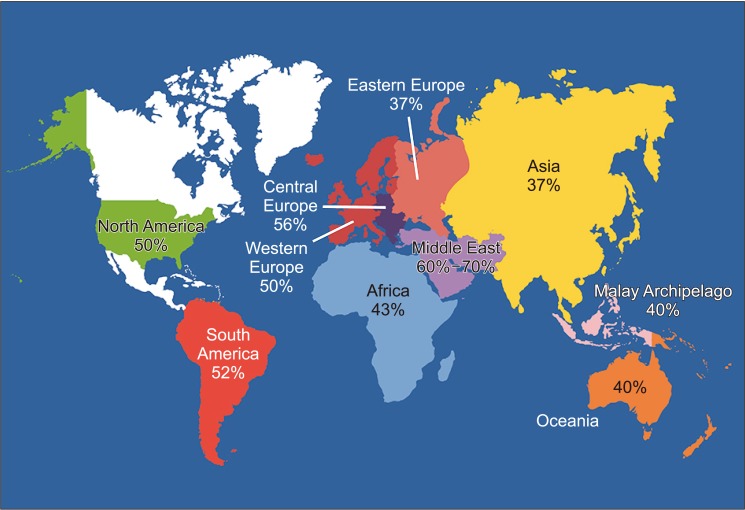
Fig. 1. World map containing percentages of infertility cases per region that are due to male factor involvement among regions studied. Asia includes all of Russia. Data from Agarwal et al (Reprod Biol Endocrinol 2015;13:37) [10].
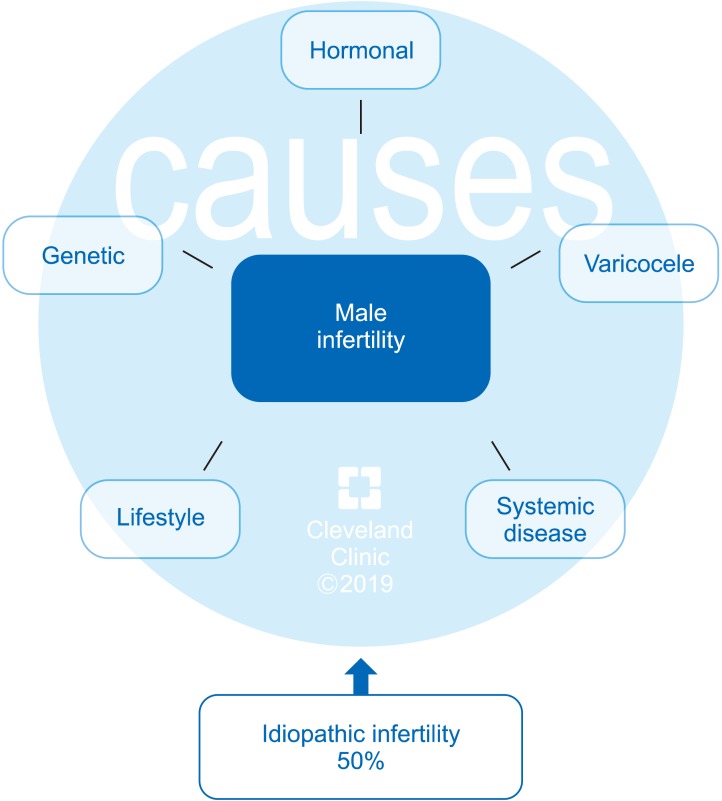
Fig. 2. Conditions affecting male reproductive potential.
THE CONCEPT OF MALE OXIDATIVE STRESS INFERTILITY (MOSI)
There is overwhelming evidence that oxidative stress (OS) plays a significant role in the etiology of male infertility [24,25,26,27,28,29,30]. Seminal reactive oxygen species (ROS) are produced mainly by leukocytes or abnormal and immature spermatozoa, and are a natural byproduct of oxidative metabolic pathways as well as cytosolic and plasma membrane oxidases [31,32,33,34]. ROS are also a natural byproduct of adenosine triphosphate production within sperm cell mitochondria [35]. Small quantities of ROS are required to ensure normal cellular physiological functions, including spermatogenesis and various sperm functions preceding fertilization, such as capacitation and acrosome reaction [32,36,37,38]. When ROS levels increase to a pathological level, the body uses dietary and endogenously produced antioxidants to bring the system back to homeostasis [39]. An imbalance between these two opposing forces, in which ROS outweigh antioxidants, can result in OS, which can negatively affect fertility via a number of pathways. OS interferes with capacitation and may cause sperm membrane and DNA damage, thereby affecting the sperm's potential to fertilize an egg and generate a healthy embryo [32,40,41,42,43,44,45]. Also, OS can trigger formation of genotoxic and mutagenic byproducts in the sperm that may increase the risk of disease in the offspring [46]. Depending on the assay methodology used, recent literature suggests that 30% to 80% of infertile men have elevated seminal ROS levels, a potentially treatable condition [28,30,44,47,48,49,50,51,52,53,54,55,56]. A similarly high incidence of OS was reported in a recent clinical trial, with 83.8% (124 of 148 cases) of idiopathic infertile men having positive seminal oxidation-reduction potential (ORP), a measure of ROS-antioxidant discrepancy [unpublished data].
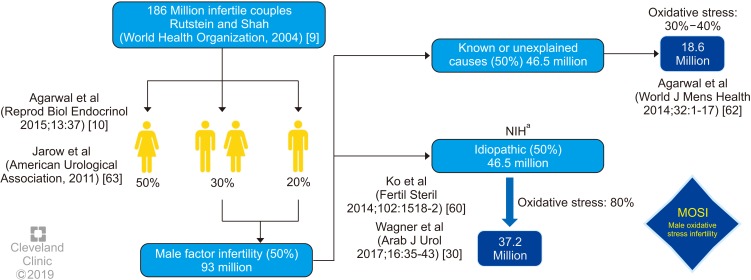
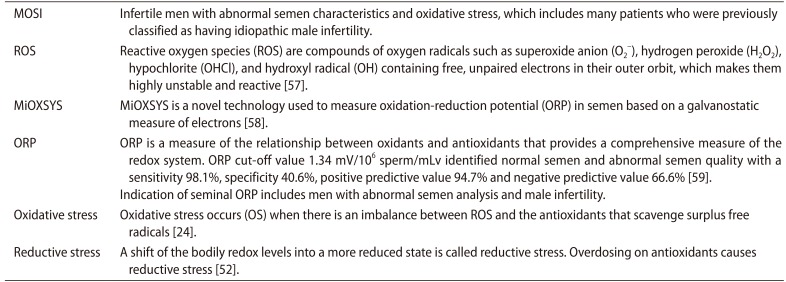
Male reproductive potential cannot be adequately assessed if seminal OS is overlooked. However, there is currently no consensus concerning either the preferred method to measure OS in the clinical setting nor the diagnostic terminology to define this condition. Therefore, we propose the term Male Oxidative Stress Infertility, or MOSI, as a novel descriptor for infertile men with abnormal semen characteristics and OS, which includes many patients who were previously classified as having idiopathic male infertility (Appendix) [24,52,57,58,59]. Based on several epidemiologic studies, OS may be present in about 56 million males complaining of infertility, two-thirds of whom are considered to have MOSI (https://www.nichd.nih.gov/health/topics/menshealth/conditioninfo/infertility) (Fig. 3) [30,60,61,62,63]. In men with normal semen characteristics who are part of couples experiencing unexplained infertility, the role of OS is not well defined. In our experience, 29.4% (10 of 34) of men in this group have leukocytospermia as opposed to 12.2% (77 of 629) in the general population of men with infertility [unpublished data].
Fig. 3. Worldwide incidence of MOSI in infertile men. aNational Institutes of Health (NIH) (https://www.nichd.nih.gov/health/topics/menshealth/conditioninfo/infertility) [61], Agarwal et al (2014) [60], Jarow et al (2011) [63].
DIAGNOSIS OF MALE OXIDATIVE STRESS INFERTILITY (MOSI)
Conventional semen analysis was introduced about a century ago and remains the most widely used test for measuring sperm production and quality. In recent years, it has become clear that conventional semen analysis alone is not an adequate surrogate measure of male fecundity [64], as it is plagued with critical shortcomings such as poor reproducibility, subjectivity, and poor prediction of fertility [65,66,67,68]. Given the limited clinical utility of conventional semen analysis and the pathological consequences and ubiquity of OS among the subfertile male population, we propose the incorporation of ORP as a useful clinical biomarker for MOSI in men with abnormal semen analysis and male infertility [58,69,70,71,72]. ORP may be used to measure the levels of reductants (antioxidants) and oxidants in a variety of biological fluids [73] and could become an adjunct component of semen analysis due to its robust association with impaired sperm function. A number of assays are available to measure OS including chemiluminescence for ROS, total antioxidant capacity for antioxidants, and the malondialdehyde assay for post-hoc damage from lipid peroxidation [74,75,76]. Though useful, these tests are difficult to incorporate into routine use because they are expensive, complex, and time-sensitive, and may also require complex instrumentation, large and neat sample volumes, and extensive technical training (Table 1) [76]. Additionally, assay results do not correlate with one another and provide only a single marker of OS-either oxidant levels, antioxidant levels, or post-hoc damage [77].
Table 1. Advantages and disadvantages of commonly used techniques to measure seminal oxidative stress.
| Assay | Advantages | Disadvantages |
|---|---|---|
| ROS by chemiluminescence | • Chemiluminescence is robust | • Time-consuming method |
| • High sensitivity and specificity | • Requires large and expensive equipment | |
| • Luminol measures global ROS levels – both extracellular and intracellular (superoxide anion, hydrogen peroxide, hydroxyl radical) | • Variables such as semen age, volume, repeated centrifugation, temperature control and background luminescence may interfere with measurement | |
| TAC | • Rapid colorimetric method | • Does not measure enzymatic antioxidants |
| • Measures total antioxidants in seminal plasma | • Length of inhibition time is a critical aspect of the test | |
| • Requires expensive microplate readers | ||
| ROS-TAC score | • Better predictor compared with ROS or TAC alone | • Requires statistical modeling |
| • Not a direct measure of ROS or TAC, rather a prediction of oxidative stress | ||
| MDA-TBA adduct detection by colorimetry or fluoroscopy | • Measures lipid peroxidation | • Rigorous controls required |
| • Detects MDA-TBA adduct by colorimetry or fluoroscopy | • Non-specific test providing post hoc measure only | |
| ORP | • Provides redox balance in real time | • Affected by viscosity of the sample |
| • Measures all known and unknown oxidants and antioxidants | ||
| • Less time-consuming and requires less expertise | ||
| • Can be measured in semen and seminal plasma, including frozen specimens |
ROS: reactive oxygen species, TAC: total antioxidant capacity, MDA: malondialdehyde, TBA: thiobarbituric acid, ORP: oxidation-reduction potential. Data from Agarwal et al (Ther Adv Urol 2016;8:302-18) [76].
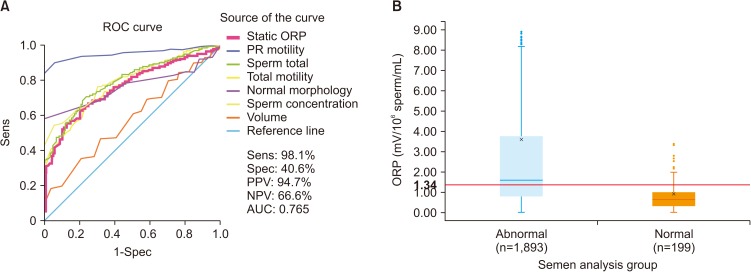
To date, measurement of ROS in semen is not often utilized as, depending on the method for ROS assessment, it may be prone to intra- and inter-laboratory variability, high turnaround time and high costs [58,69,78]. The advent of new technologies that rapidly detect seminal OS through the assessment of ORP in a reproducible manner using a bench-top analyzer can allow for an accurate and cost-effective diagnosis of MOSI [76,78,79]. The Male Infertility Oxidative System (MiOXSYS) is a recently developed assay for the assessment of ORP [69]. The ORP test is novel in the area of infertility and is based on a galvanostatic measure of electrons. MiOXSYS has been developed for easy and quick measurement of ORP in semen [80]. Several studies have validated the reproducibility and reliability of the MiOXSYS in measuring ORP levels in semen samples from patients being evaluated for male infertility [58,69,71,81]. More importantly, ORP levels have been shown to be significantly negatively correlated with sperm concentration, sperm motility, normal morphology and total motile count [72]. ORP levels are also significantly positively correlated with sperm DNA fragmentation (SDF) [72,79,81], although normal levels of SDF do not exclude the presence of OS. At a cutoff value of 1.34 (mV/106 sperm/mL), ORP may be used to differentiate between normal and abnormal semen quality in infertile men with 98.1% sensitivity, 40.6% specificity, 94.7% positive predictive value, and 66.6% negative predictive value [58,59,69] (Fig. 4).
Fig. 4. (A) A receiver operating characteristic (ROC) curve was used to identify the oxidation-reduction potential (ORP) (mV/106 sperm/mL) cutoff that best predicted normal and abnormal semen parameters based on sensitivity (Sens), specificity (Spec), positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC). (B) Distribution of ORP in patients with at least one abnormal semen parameter versus patients with normal semen parameters, showing the established cutoff value of 1.34 mV/106 sperm/mL. Data from Agarwal et al (Asian J Androl 2019 [in press]) [59].
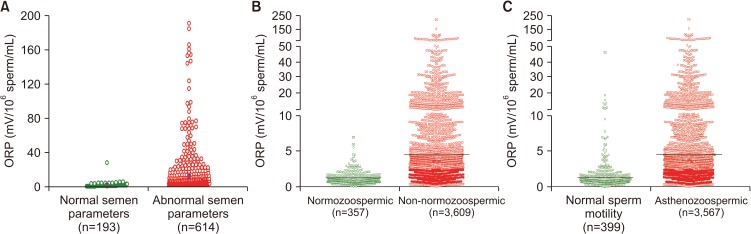
Among infertile men, higher ORP levels are observed in cases with abnormal semen parameters versus normal parameters (Fig. 5A, 5B). Analysis of data of 3,966 patients at Hamad Medical Corporation, Doha, Qatar, revealed statistically significant negative correlations between ORP and normal sperm morphology (r=−0.529, p<0.0001), progressive motility (r=−0.463, p<0.0001), and sperm concentration (r=−0.844, p<0.0001). The difference in ORP between normozoospermic (mean: 1.14±0.97 mV/106 sperm/mL; median: 0.86 mV/106 sperm/mL) and non-normozoospermic (mean: 5.65±11.34 mV/106 sperm/mL; median: 2.04 mV/106 sperm/mL) patients was also significant (p<0.0001) (Fig. 5B). Fig. 5C depicts ORP values of asthenozoospermic (mean: 5.63±11.36 mV/106 sperm/mL; median: 2.03 mV/106 sperm/mL) versus non-asthenozoospermic patients (mean: 1.79±3.80 mV/106 sperm/mL; median: 0.92 mV/106 sperm/mL) [unpublished data].
Fig. 5. Distribution of oxidation-reduction potential (ORP) values in the infertile men with normal and abnormal semen parameters. (A) Data from Cleveland Clinic, Cleveland OH, USA (n=807); (B) Data from Hamad Medical Corporation, Doha, Qatar (n=3,966); (C) Data of asthenozoospermic patients from Hamad Medical Corporation, Doha, Qatar (n=3,966).
MANAGEMENT AND TREATMENT OF MALE OXIDATIVE STRESS INFERTILITY (MOSI)
Despite significant advances in the diagnosis and management of male infertility, there are no evidencebased treatment guidelines available for idiopathic male infertility. Understandably, it is difficult to develop an evidence-based approach for a condition with an unclear etiology. A survey among members of the American Urological Association (AUA) indicated that two-thirds of clinicians use empirical medical therapy (EMT) such as selective estrogen receptor modulators, aromatase inhibitors, and gonadotropins to treat idiopathic male factor infertility [82]. While the role of hormonal therapy in men with an identified abnormality such as hypogonadotropic hypogonadism is well-defined [83], endocrine imbalance is responsible for approximately 10% of all known causes of infertility [21]. The literature remains inconclusive and controversial regarding off-label EMTs for men with idiopathic infertility [20,82,84,85,86,87], especially in light of their cost and side effects (Table 2). Although there are several small studies that provide support for pharmacological EMT to treat idiopathic male infertility, there is a lack of robust placebo-controlled trials demonstrating improved live birth outcomes [87,88,89,90].
Table 2. Empiric medical treatment for idiopathic male infertility (ICD10 Code: Z31.41).
| Medication | Administration | Common dosages | Adverse effects | Estimated cost per 3 months |
|---|---|---|---|---|
| Selective estrogen receptor modulators | ||||
| Clomiphene citratea | Oral | 50 mg daily | Hot flashes, weight gain, gynecomastia, hair loss, dizziness, gastrointestinal distress | $185.40c |
| Tamoxifen citratea | Oral | 20 mg daily | See above | $99.60c |
| Aromatase inhibitors | ||||
| Anastrozolea | Oral | 1 mg, 3 times/wk | Decreased libido, headache, elevated liver function tests | $35.40c |
| Human chorionicgonadotropinb | Subcutaneous | 1,500–3,000 IU, 3 times/wk | Injection site pain, headache, depression, gynecomastia, hyperglycemia | $337.50–$675.00d |
| Recombinant folliclestimulating hormoneb | Subcutaneous | 75 IU, 3 times/wk | Injection site pain | $2,160.00d |
aOff label use; bFood and Drug Administration approved for treatment of infertility secondary to gonadotropin deficiency; cAverage cost at Walmart, CVS and Walgreens; dCompound pharmacy cost.
For the vast majority of infertile men with no underlying endocrine, bacterial, genetic or anatomical causes of infertility, an alternative approach may be to shift from administering EMTs to identifying potential sources of MOSI and mitigating the sequela. The human body produces endogenous antioxidants in an effort to prevent the damage caused by ROS [91,92], but this response is not always adequate, resulting in OS. Several studies have shown that exogenous antioxidants have the capacity to counteract oxidative damage or OS, improving both sperm motility and DNA integrity for infertile men with OS (Table 3) [87,88,89,90,91,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111]. Indeed, many oral formulations of antioxidants are readily available in the market and are commonly used to treat men with infertility. However, there is growing awareness that the indiscriminate use of antioxidants may paradoxically exacerbate sperm cell damage in men without elevated MOSI by inducing a state of reductive stress [52,112]. In order to prevent the inappropriate use of antioxidants, clinical guidelines outlining the effective diagnosis and treatment of MOSI are critical. Several clinical trials and systemic reviews involving the use of various combinations of antioxidants (L-carnitine, selenium, N-acetyl-cysteine, Coenzyme Q10, ubiquinol, vitamin E, vitamin C, and lycopene) in infertile men have reported beneficial effects of antioxidants on sperm concentration, motility, and DNA integrity (Table 4) [113,114,115,116,117,118,119,120]. Preliminary results from a 2018 clinical trial involving 148 idiopathic infertile men indicated that intake of oral antioxidants for a period of three months significantly increased sperm concentration (36%, p<0.0001), progressive motility (100%, p<0.0001), and motility (12%, p=0.0033). Moreover, a significant decrease in ORP (39%, p<0.0001) and SDF (20%, p=0.0002) was observed post-treatment [unpublished data]. These beneficial changes in semen quality have been reported to improve the chance of natural conception in several but not all studies [53,121]. This benefit could be augmented, and harm prevented, by directing therapy through measuring and monitoring seminal ORP [113,114,115,116,122,123].
Table 3. Antioxidant classification in relation to its action on sperm characteristics.
| Type | Function | References |
|---|---|---|
| Enzymatic: | ||
| Superoxide dismutase | First line defense antioxidants | [94,95] |
| Catalase | First line defense antioxidants | [54,96] |
| Glutathione peroxidase | Scavenges lipid peroxides and hydrogen peroxide | [97,98] |
| Glutathione reductase | Scavenges lipid peroxides and hydrogen peroxide | [99] |
| Non enzymatic: | ||
| Vitamin C | Neutralizes free radicals | [100,101] |
| Vitamin E | Neutralizes free radicals | [102,103] |
| Ferritin and carnitines | Neutralizes free radicals and acts as an energy source | [126] |
| Coenzyme Q10 | In its reduced form, scavenges free radicals intermediate in mitochondrial electron transport system | [104] |
| Transferrin | Sperm vitality, DNA integrity and OS homeostasis | [105] |
| Zinc | Formation of free oxygen radicals and sperm chromatin stability | [106] |
| Selenium | Sperm motility and OS homeostasis | [107] |
| N-acetyl L-cysteine | Free radical scavenging activity | [108,109] |
| L-arginine | Formation of free oxygen radicals | [110] |
| Folic acid | Sperm DNA integrity | [111] |
OS: oxidative stress.
Table 4. Effect of antioxidants on male infertility: Double blind placebo controlled studiesa.
| Study reference | Infertility type | Cases | Antioxidants | Duration | Outcome |
|---|---|---|---|---|---|
| Micic et al (2019) [119] | Idiopathic oligoasthenozoospermia | Placebo group (n=50) | Proxeed plus=2 times/d | 3 months | Increase in semen volume, progressive motility and vitality |
| Treatment group (n=125) | • LC=1,000 g, LAC=0.5 g, fumarate=0.725 g, fructose=1 g, citric acid=50 mg, zinc=10 mg, coenzyme Q10=20 mg, selenium=50 µg, Vit C=90 mg, folic acid=200 µg, Vit B12=1.5 µg | Decrease in sperm DNA fragmentation index | |||
| Busetto et al (2018) [113] | Idiopathic OAT, with and without varicocele | Varicocele (n=45) | LC=1,000 mg, LAC=500 mg, fumarate=725 mg, fructose= 1,000 mg, Coenzyme Q10=20 mg, Vit C=90 mg, Zinc=10 mg, folic acid=200 μg, Vit B12=1.5 μg | 6 months | Increase in sperm concentration, total sperm count, motility, and progressive motility |
| Without varicocele (n=49) | |||||
| Safarinejad et al (2012) [116] | Idiopathic infertility | Placebo group (n=114) | Coenzyme Q10=200 mg/d | 26 weeks | Increase in sperm concentration, motility and normal sperm morphology |
| Treatment group (n=114) | |||||
| Safarinejad (2009) [114] | Idiopathic OAT | Placebo group (n=106) | Coenzyme Q10=300 mg/d | 26 weeks | Increase in sperm concentration and motility |
| Treatment group (n=106) | |||||
| Balercia et al (2009) [120] | Idiopathic asthenozoospermia | Placebo group (n=30) Treatment group (n=30) | Coenzyme Q10=200 mg/d | 3 months | Increase in sperm concentration and motility |
| Tremellen et al (2008) [28] | Male factor infertility | Placebo group (n=20) | Menevit=1 capsule/d | 3 months | Improved pregnancy rates in couples undergoing IVF-ICSI treatment for severe male factor infertility |
| Infertile men (n=40) | • Lycopene=6 mg, Vit E=400 IU, Vit C=100 mg, Zinc=25 mg, selenium=26 μg, folate=0.5 mg, garlic-1,000 mg, palm oil (vehicle) | ||||
| Balercia et al (2005) [115] | Idiopathic asthenozoospermia | Placebo group (n=15) | LC=3 g/d | 6 months | Increase in sperm motility and normal sperm morphology |
| Treatment group (n=45): LC group: n=15; LAC group: n=15; LC+LAC group: n=15 | LAC=3 g/d | ||||
| LC+LAC=2 g+1 g/d |
OAT: oligoasthenoteratozoospermia, LC: L-carnitine, LAC: L-acetylcarnitine, Vit: vitamin, IVF-ICSI: in vitro fertilization/intracytoplasmic sperm injection. aOnly double blind placebo control studies on idiopathic male infertility patients were included. Except for three studies (94, 96, and 142), others used a combination of antioxidant supplements for a period of 3 to 6 months.
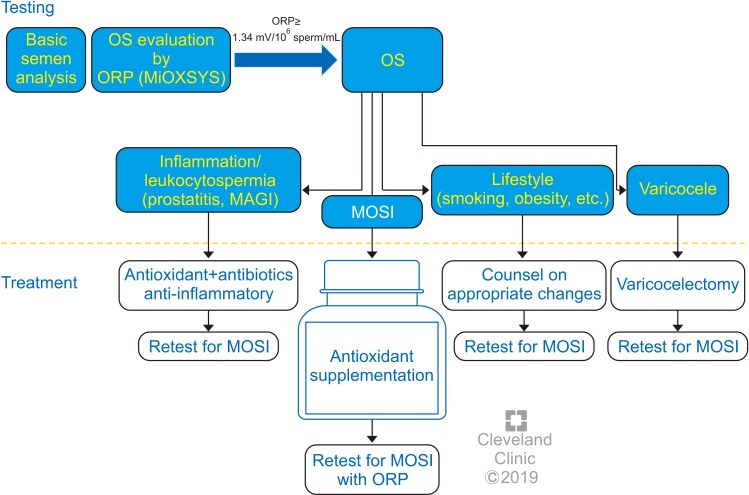
Identifying and treating MOSI in cases where the use of assisted reproductive technology (ART) is indicated is especially important, as many of the sperm preparation and handling methods used during ART may induce OS, further aggravating the negative impact of MOSI [124,125]. In couples undergoing ART, diagnosis of MOSI and subsequent antioxidant therapy may improve ART success [122,126,127]. Additionally, there is emerging evidence that antioxidant therapy may improve pregnancy outcomes in couples with recurrent pregnancy loss [128]. Evidence-based guidelines should provide recommendations on ways to best manage other causes of OS, including lifestyle modifications (improved diet, smoking cessation, exercise, and weight loss), treatment of clinically relevant varicoceles, and treatment of male accessory gland infection (MAGI) as well as other inflammatory pathologies linked with MOSI (Fig. 3). The treatment of MAGI with antibiotics, and the decrease in the numbers of ROS-producing seminal leukocytes using anti-inflammatories are likely to add benefit in combination with neutralization of ROS by antioxidant therapy [129,130,131,132]. Treatment success and adherence for the above conditions can be monitored by measuring seminal ORP, as well.
The diagnosis and management of idiopathic male infertility is an integral component of comprehensive sexual and reproductive health services. Idiopathic male infertility can be an emotional burden and financial strain for couples. Current treatment protocols for male infertility are not evidence-based and have the potential risk of complications and increased healthcare-related expenditures [20,84]. MOSI provides clinicians and patients with a diagnostic classification to guide future research and treatment, while simultaneously reducing apprehension and uncertainty for many couples. A recent consensus guideline by the European Society for Human Reproduction & Embryology (ESHRE) concluded that there is currently insufficient evidence to support the use of antioxidants for male infertility due to lack of a standardized measure of OS and inconsistent selection of eligible patients across studies [133]. MOSI diagnosis combined with ORP monitoring may provide a more targeted, reliable approach for using antioxidant therapy in both research and practice.
Compared with hormonal EMT and ART, antioxidants are relatively safe, inexpensive and widely available, with a growing body of data supporting their effectiveness at improving semen parameters and live birth rates [53]. Further clinical studies are indicated to directly compare live birth rates among men with MOSI assigned to receive antioxidants versus EMT and ART. Treatment guidelines providing individualized antioxidant therapy protocols based on ORP status for men with MOSI could provide a significant advancement in the management of male factor infertility and facilitate future investigations (Fig. 6) [134]. Guidelines are also necessary to avoid possible overuse of antioxidants leading to reductive stress, which can be as detrimental to sperm health as OS [52,135,136,137] and has been associated with defects in embryogenesis [138]. Supra-physiologic levels of antioxidants may also scavenge the ROS necessary to induce sperm capacitation [32,38], leading to infertility. Because antioxidants are readily available online or over-the-counter, they may appear to be a benign first-line treatment. Without clear guidelines for appropriate use, however, there is a risk of overuse in men without evidence of MOSI who may then experience delay accessing more effective therapies (e.g., ART or varicocele repair). Therefore, the oxidative status of male infertility patients should be evaluated before antioxidants are recommended and used only in those cases where MOSI is present.
Fig. 6. options for male oxidative stress infertility. OS: oxidative stress, ORP: oxidation-reduction potential, MiOXSYS: Male Infertility Oxidative System, MAGI: male accessory gland infection, MOSI: Male Oxidative Stress Infertility.
RECOMMENDATIONS AND FUTURE DIRECTIONS
Therefore, the authors recommend that men with idiopathic infertility should be screened for MOSI using an efficient, inexpensive, high sensitivity/specificity test for ORP such as MiOXSYS, which has practical advantages over alternative techniques (Table 1). Those men screening positive for MOSI should then undergo more extensive examination to identify treatable triggers and be counseled on appropriate steps to mitigate known causes of OS (e.g., smoking, alcohol consumption, lifestyle risk factors, radiation, toxins, etc.) [139,140]. ORP testing should be repeated no less than 3 months following the appropriate management plan in infertile men with no explanation for MOSI. Ultimately, infertile men with MOSI should be advised to take antioxidants for a minimum of three months after other known causes of OS have been eliminated. Infertile men without MOSI should be advised against antioxidant therapy. Follow-up testing of ORP levels is recommended to confirm compliance and monitor the efficacy of antioxidant supplementation and continued lifestyle changes 6 to 8 weeks post treatment. We recommend that these approaches be tested in double blind randomized controlled trials to establish whether time to pregnancy and live birth rate is improved in couples where the man is undergoing antioxidant treatment.
With the increasing awareness and understanding of MOSI as a distinct male infertility diagnosis, the development of evidence-based guidelines that target the underlying causes, while balancing the risks and benefits of individual therapies, is imperative. The authors feel that measurement of ORP and stratification of male fertility/infertility on the basis of ORP will be an important tool in the management of infertile couples. The exact role will be defined in future trials and could validate a reclassification of male infertility that incorporates MOSI as a diagnostic category. A better understanding of the etiology of this diagnosis will help identify those men likely to benefit from antioxidant therapy while minimizing the harmful effects of antioxidant overdosing.
ACKNOWLEDGEMENTS
The authors thank Ken Kula, Mary Reagan, and Bernastine Buchanan from the Center for Medical Art and Photography for assistance with the figures.
Financial support for this study was provided by the American Center for Reproductive Medicine.
Appendix
Key terminologies
Footnotes
Disclosure: None of the authors declares competing financial interests. The authors do not have any potential interest in promoting MiOXSYS.
- Conceptualization: AA.
- Writing–original draft: NP, AA, MKPS.
- Writing–review & editing: all the authors.
References
- 1.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–1331.e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassa EM, Kebede E. Time-to-pregnancy and associated factors among couples with natural planned conception in Addis Ababa, Ethiopia. Afr J Reprod Health. 2018;22:33–42. doi: 10.29063/ajrh2018/v22i3.4. [DOI] [PubMed] [Google Scholar]
- 3.Slama R, Hansen OK, Ducot B, Bohet A, Sorensen D, Giorgis Allemand L, et al. Estimation of the frequency of involuntary infertility on a nation-wide basis. Hum Reprod. 2012;27:1489–1498. doi: 10.1093/humrep/des070. [DOI] [PubMed] [Google Scholar]
- 4.Juul S, Karmaus W, Olsen J. Regional differences in waiting time to pregnancy: pregnancy-based surveys from Denmark, France, Germany, Italy and Sweden. Hum Reprod. 1999;14:1250–1254. doi: 10.1093/humrep/14.5.1250. [DOI] [PubMed] [Google Scholar]
- 5.Zinaman MJ, Clegg ED, Brown CC, O'Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65:503–509. [PubMed] [Google Scholar]
- 6.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013;99:63. doi: 10.1016/j.fertnstert.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21:411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 9.Rutstein SO, Shah IH ORC Macro; World Health Organization. Infecundity, infertility, and childlessness in developing countries. Calverton: World Health Organization; 2004. [Google Scholar]
- 10.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO laboratory manual for the examination and processing of human semen. Geneva: World Health Organization; 2010. [Google Scholar]
- 12.Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod. 1998;13(Suppl 1):33–44. doi: 10.1093/humrep/13.suppl_1.33. [DOI] [PubMed] [Google Scholar]
- 13.O'Flynn O'Brien KL, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril. 2010;93:1–12. doi: 10.1016/j.fertnstert.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 14.Sharma R, Biedenharn KR, Fedor JM, Agarwal A. Lifestyle factors and reproductive health: taking control of your fertility. Reprod Biol Endocrinol. 2013;11:66. doi: 10.1186/1477-7827-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurewicz J, Dziewirska E, Radwan M, Hanke W. Air pollution from natural and anthropic sources and male fertility. Reprod Biol Endocrinol. 2018;16:109. doi: 10.1186/s12958-018-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, Ladva R, et al. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod. 2017;32:18–31. doi: 10.1093/humrep/dew284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tournaye H, Krausz C, Oates RD. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol. 2017;5:544–553. doi: 10.1016/S2213-8587(16)30040-7. [DOI] [PubMed] [Google Scholar]
- 18.Gabrielsen JS, Tanrikut C. Chronic exposures and male fertility: the impacts of environment, diet, and drug use on spermatogenesis. Andrology. 2016;4:648–661. doi: 10.1111/andr.12198. [DOI] [PubMed] [Google Scholar]
- 19.Pasqualotto FF, Pasqualotto EB, Sobreiro BP, Hallak J, Medeiros F, Lucon AM. Clinical diagnosis in men undergoing infertility investigation in a university hospital. Urol Int. 2006;76:122–125. doi: 10.1159/000090873. [DOI] [PubMed] [Google Scholar]
- 20.Chehab M, Madala A, Trussell JC. On-label and off-label drugs used in the treatment of male infertility. Fertil Steril. 2015;103:595–604. doi: 10.1016/j.fertnstert.2014.12.122. [DOI] [PubMed] [Google Scholar]
- 21.Jungwirth A, Diemer T, Kopa Z, Krausz C, Minhas S, Tournaye H. EAU guidelines on male infertility. Arnhem: European Association of Urology; 2018. [DOI] [PubMed] [Google Scholar]
- 22.de Kretser DM. Male infertility. Lancet. 1997;349:787–790. doi: 10.1016/s0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- 23.Hamada A, Esteves SC, Agarwal A. Unexplained male infertility: potential causes and management. Hum Androl. 2011;1:2–16. [Google Scholar]
- 24.Agarwal A, Durairajanayagam D, Halabi J, Peng J, Vazquez-Levin M. Proteomics, oxidative stress and male infertility. Reprod Biomed Online. 2014;29:32–58. doi: 10.1016/j.rbmo.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Aitken RJ. Oxidative stress and the etiology of male infertility. J Assist Reprod Genet. 2016;33:1691–1692. doi: 10.1007/s10815-016-0791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bui AD, Sharma R, Henkel R, Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. 2018;50:e13012. doi: 10.1111/and.13012. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal A, Sharma RK, Nallella KP, Thomas AJ, Jr, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–885. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 28.Tremellen K. Oxidative stress and male infertility: a clinical perspective. Hum Reprod Update. 2008;14:243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 29.Aitken RJ, De Iuliis GN, Drevet JR. Role of oxidative stress in the etiology of male infertility and the potential therapeutic value of antioxidants. In: Henkel R, Samanta L, Agarwal A, editors. Oxidants, antioxidants, and impact of the oxidative status in male reproduction. London: Elsevier/Academic Press; 2018. pp. 91–100. [Google Scholar]
- 30.Wagner H, Cheng JW, Ko EY. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J Urol. 2017;16:35–43. doi: 10.1016/j.aju.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–850. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 32.Aitken RJ. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017;84:1039–1052. doi: 10.1002/mrd.22871. [DOI] [PubMed] [Google Scholar]
- 33.Robinson JM. Phagocytic leukocytes and reactive oxygen species. Histochem Cell Biol. 2009;131:465–469. doi: 10.1007/s00418-009-0565-5. [DOI] [PubMed] [Google Scholar]
- 34.Aitken RJ, West K, Buckingham D. Leukocytic infiltration into the human ejaculate and its association with semen quality, oxidative stress, and sperm function. J Androl. 1994;15:343–352. [PubMed] [Google Scholar]
- 35.Cassina A, Silveira P, Cantu L, Montes JM, Radi R, Sapiro R. Defective human sperm cells are associated with mitochondrial dysfunction and oxidant production. Biol Reprod. 2015;93:119. doi: 10.1095/biolreprod.115.130989. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 37.Du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. 2015;32:509–520. doi: 10.1007/s10815-014-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Flaherty C. Redox regulation of mammalian sperm capacitation. Asian J Androl. 2015;17:583–590. doi: 10.4103/1008-682X.153303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walczak-Jedrzejowska R, Wolski JK, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66:60–67. doi: 10.5173/ceju.2013.01.art19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal A, Cho CL, Esteves SC, Majzoub A. Reactive oxygen species and sperm DNA fragmentation. Transl Androl Urol. 2017;6:S695–S696. doi: 10.21037/tau.2017.05.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal A, Ikemoto I, Loughlin KR. Relationship of sperm parameters with levels of reactive oxygen species in semen specimens. J Urol. 1994;152:107–110. doi: 10.1016/s0022-5347(17)32829-x. [DOI] [PubMed] [Google Scholar]
- 42.Menezo Y, Evenson DP, Cohen M, Dale B. Effect of antioxidants on sperm genetic damage. In: Baldi E, Muratori M, editors. Genetic damage in human spermatozoa. New York: Springer; 2014. pp. 173–189. [DOI] [PubMed] [Google Scholar]
- 43.Truong T, Gardner DK. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum Reprod. 2017;32:2404–2413. doi: 10.1093/humrep/dex330. [DOI] [PubMed] [Google Scholar]
- 44.Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14:470–485. doi: 10.1038/nrurol.2017.69. [DOI] [PubMed] [Google Scholar]
- 45.Muratori M, Tamburrino L, Marchiani S, Cambi M, Olivito B, Azzari C, et al. Investigation on the origin of sperm DNA fragmentation: role of apoptosis, immaturity and oxidative stress. Mol Med. 2015;21:109–122. doi: 10.2119/molmed.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aitken RJ. DNA damage in human spermatozoa; important contributor to mutagenesis in the offspring. Transl Androl Urol. 2017;6:S761–S764. doi: 10.21037/tau.2017.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agarwal A, Allamaneni SS. Free radicals and male reproduction. J Indian Med Assoc. 2011;109:184–187. [PubMed] [Google Scholar]
- 48.Tremellen K. Treatment of sperm oxidative stress: a collaborative approach between clinician and embryologist. In: Henkel R, Samanta L, Agarwal A, editors. Oxidants, antioxidants, and impact of the oxidative status in male reproduction. London: Elsevier/Academic Press; 2018. pp. 225–235. [Google Scholar]
- 49.Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018;50:e13126. doi: 10.1111/and.13126. [DOI] [PubMed] [Google Scholar]
- 50.Shekarriz M, Thomas AJ, Jr, Agarwal A. Incidence and level of seminal reactive oxygen species in normal men. Urology. 1995;45:103–107. doi: 10.1016/s0090-4295(95)97088-6. [DOI] [PubMed] [Google Scholar]
- 51.Ochsendorf FR, Thiele J, Fuchs J, Schüttau H, Freisleben HJ, Buslau M, et al. Chemiluminescence in semen of infertile men. Andrologia. 1994;26:289–293. doi: 10.1111/j.1439-0272.1994.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 52.Henkel R, Sandhu IS, Agarwal A. The excessive use of antioxidant therapy: a possible cause of male infertility? Andrologia. 2019;51:e13162. doi: 10.1111/and.13162. [DOI] [PubMed] [Google Scholar]
- 53.Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 2010;20:711–723. doi: 10.1016/j.rbmo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Adewoyin M, Ibrahim M, Roszaman R, Isa MLM, Alewi NAM, Rafa AAA, et al. Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases. 2017;5:E9. doi: 10.3390/diseases5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dada R, Shamsi MB, Venkatesh S, Gupta NP, Kumar R. Attenuation of oxidative stress & DNA damage in varicocelectomy: implications in infertility management. Indian J Med Res. 2010;132:728–730. [PMC free article] [PubMed] [Google Scholar]
- 56.Chen SS, Huang WJ, Chang LS, Wei YH. Attenuation of oxidative stress after varicocelectomy in subfertile patients with varicocele. J Urol. 2008;179:639–642. doi: 10.1016/j.juro.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal A, Gupta S, Sharma R. Reactive oxygen species (ROS) measurement. In: Agarwal A, Gupta S, Sharma R, editors. Andrological evaluation of male infertility: a laboratory guide. Cham: Springer International Publishing; 2016. pp. 155–163. [Google Scholar]
- 58.Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, Sabanegh E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online. 2017;34:48–57. doi: 10.1016/j.rbmo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Agarwal A, Panner Selvam MK, Arafa M, Okada H, Homa S, Killeen A, et al. Multi-center evaluation of oxidation-reduction potential by the MiOXSYS System in males with abnormal semen. Asian J Androl. 2019 doi: 10.4103/aja.aja_5_19. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ko EY, Sabanegh ES, Jr, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. 2014;102:1518–1527. doi: 10.1016/j.fertnstert.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 61.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989) Hum Reprod. 1991;6:811–816. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jarow J, Sigman M, Kolettis PN, Lipshultz LR, McClure RD, Nangia AK, et al. The optimal evaluation of the infertile male: AUA best practice statement reviewed and validity confirmed 2011. Linthicum: American Urological Association; 2011. [Google Scholar]
- 64.Esteves SC, Sharma RK, Gosálvez J, Agarwal A. A translational medicine appraisal of specialized andrology testing in unexplained male infertility. Int Urol Nephrol. 2014;46:1037–1052. doi: 10.1007/s11255-014-0715-0. [DOI] [PubMed] [Google Scholar]
- 65.Patel AS, Leong JY, Ramasamy R. Prediction of male infertility by the World Health Organization laboratory manual for assessment of semen analysis: a systematic review. Arab J Urol. 2017;16:96–102. doi: 10.1016/j.aju.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502–1507. doi: 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES, Jr, Agarwal A. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79:16–22. doi: 10.1016/j.urology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, Henriksen TB, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–1177. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 69.Agarwal A, Sharma R, Roychoudhury S, Du Plessis S, Sabanegh E. MiOXSYS: a novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil Steril. 2016;106:566–573.e10. doi: 10.1016/j.fertnstert.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 70.Samanta L, Agarwal A, Swain N, Sharma R, Gopalan B, Esteves SC, et al. Proteomic signatures of sperm mitochondria in varicocele: clinical use as biomarkers of varicocele associated infertility. J Urol. 2018;200:414–422. doi: 10.1016/j.juro.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Arafa M, Agarwal A, Al Said S, Majzoub A, Sharma R, Bjugstad KB, et al. Semen quality and infertility status can be identified through measures of oxidation-reduction potential. Andrologia. 2018;50:e12881. doi: 10.1111/and.12881. [DOI] [PubMed] [Google Scholar]
- 72.Homa ST, Vassiliou AM, Stone J, Killeen AP, Dawkins A, Xie J, et al. A comparison between two assays for measuring seminal oxidative stress and their relationship with sperm DNA fragmentation and semen parameters. Genes (Basel) 2019;10:E236. doi: 10.3390/genes10030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okouchi S, Suzuki M, Sugano K, Kagamimori S, Ikeda S. Water desirable for the human body in terms of oxidation-reduction potential (ORP) to pH relationship. J Food Sci. 2002;67:1594–1598. [Google Scholar]
- 74.Grotto D, Santa Maria LD, Boeira S, Valentini J, Charão MF, Moro AM, et al. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharm Biomed Anal. 2007;43:619–624. doi: 10.1016/j.jpba.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 75.Vessey W, Perez-Miranda A, Macfarquhar R, Agarwal A, Homa S. Reactive oxygen species in human semen: validation and qualification of a chemiluminescence assay. Fertil Steril. 2014;102:1576–1583.e4. doi: 10.1016/j.fertnstert.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 76.Agarwal A, Roychoudhury S, Bjugstad KB, Cho CL. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol. 2016;8:302–318. doi: 10.1177/1756287216652779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agarwal A, Qiu E, Sharma R. Laboratory assessment of oxidative stress in semen. Arab J Urol. 2017;16:77–86. doi: 10.1016/j.aju.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agarwal A, Wang SM. Clinical relevance of oxidation-reduction potential in the evaluation of male infertility. Urology. 2017;104:84–89. doi: 10.1016/j.urology.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 79.Agarwal A, Arafa MM, Elbardisi H, Majzoub A, Alsaid SS. Relationship between seminal oxidation reduction potential and sperm DNA fragmentation in infertile men. Fertil Steril. 2017;108:e316 [Google Scholar]
- 80.Agarwal A, Gupta S, Sharma R. Oxidation-reduction potential measurement in ejaculated semen samples. In: Agarwal A, Gupta S, Sharma R, editors. Andrological evaluation of male infertility: a laboratory guide. Cham: Springer International Publishing; 2016. pp. 165–170. [Google Scholar]
- 81.Majzoub A, Arafa M, Mahdi M, Agarwal A, Al Said S, Al-Emadi I, et al. Oxidation-reduction potential and sperm DNA fragmentation, and their associations with sperm morphological anomalies amongst fertile and infertile men. Arab J Urol. 2018;16:87–95. doi: 10.1016/j.aju.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES., Jr Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187:973–978. doi: 10.1016/j.juro.2011.10.137. [DOI] [PubMed] [Google Scholar]
- 83.Kim HH, Schlegel PN. Endocrine manipulation in male infertility. Urol Clin North Am. 2008;35:303–318. doi: 10.1016/j.ucl.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Jung JH, Seo JT. Empirical medical therapy in idiopathic male infertility: promise or panacea? Clin Exp Reprod Med. 2014;41:108–114. doi: 10.5653/cerm.2014.41.3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tadros NN, Sabanegh ES. Empiric medical therapy with hormonal agents for idiopathic male infertility. Indian J Urol. 2017;33:194–198. doi: 10.4103/iju.IJU_368_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nieschlag E, Kamischke A. Empirical therapies for idiopathic male infertility. In: Nieschlag E, Behre HM, Nieschlag S, editors. Andrology: male reproductive health and dysfunction. Berlin, Heidelberg: Springer Berlin Heidelberg; 2010. pp. 457–467. [Google Scholar]
- 87.Attia AM, Abou-Setta AM, Al-Inany HG. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst Rev. 2013:CD005071. doi: 10.1002/14651858.CD005071.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar R, Gautam G, Gupta NP. Drug therapy for idiopathic male infertility: rationale versus evidence. J Urol. 2006;176:1307–1312. doi: 10.1016/j.juro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 89.Clark RV, Sherins RJ. Treatment of men with idiopathic oligozoospermic infertility using the aromatase inhibitor, testolactone. Results of a double-blinded, randomized, placebocontrolled trial with crossover. J Androl. 1989;10:240–247. doi: 10.1002/j.1939-4640.1989.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 90.Siddiq FM, Sigman M. A new look at the medical management of infertility. Urol Clin North Am. 2002;29:949–963. doi: 10.1016/s0094-0143(02)00085-x. [DOI] [PubMed] [Google Scholar]
- 91.Mirończuk-Chodakowska I, Witkowska AM, Zujko ME. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. 2018;63:68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Halliwell B, Gutteridge JMC. Antioxidant defences synthesized in vivo. In: Halliwell B, Gutteridge JMC, editors. Free radicals in biology and medicine. 5th ed. Oxford: Oxford University Press; 2015. [Google Scholar]
- 93.Majzoub A, Agarwal A. Antioxidant therapy in idiopathic oligoasthenoteratozoospermia. Indian J Urol. 2017;33:207–214. doi: 10.4103/iju.IJU_15_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsieh YY, Sun YL, Chang CC, Lee YS, Tsai HD, Lin CS. Superoxide dismutase activities of spermatozoa and seminal plasma are not correlated with male infertility. J Clin Lab Anal. 2002;16:127–131. doi: 10.1002/jcla.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kobayashi T, Miyazaki T, Natori M, Nozawa S. Protective role of superoxide dismutase in human sperm motility: superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum Reprod. 1991;6:987–991. doi: 10.1093/oxfordjournals.humrep.a137474. [DOI] [PubMed] [Google Scholar]
- 96.Ben Abdallah F, Dammak I, Attia H, Hentati B, Ammar-Keskes L. Lipid peroxidation and antioxidant enzyme activities in infertile men: correlation with semen parameter. J Clin Lab Anal. 2009;23:99–104. doi: 10.1002/jcla.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lenzi A, Lombardo F, Gandini L, Culasso F, Dondero F. Glutathione therapy for male infertility. Arch Androl. 1992;29:65–68. doi: 10.3109/01485019208987710. [DOI] [PubMed] [Google Scholar]
- 98.Lenzi A, Culasso F, Gandini L, Lombardo F, Dondero F. Placebo-controlled, double-blind, cross-over trial of glutathione therapy in male infertility. Hum Reprod. 1993;8:1657–1662. doi: 10.1093/oxfordjournals.humrep.a137909. [DOI] [PubMed] [Google Scholar]
- 99.Atig F, Raffa M, Habib BA, Kerkeni A, Saad A, Ajina M. Impact of seminal trace element and glutathione levels on semen quality of Tunisian infertile men. BMC Urol. 2012;12:6. doi: 10.1186/1471-2490-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dawson EB, Harris WA, Rankin WE, Charpentier LA, Mc-Ganity WJ. Effect of ascorbic acid on male fertility. Ann N Y Acad Sci. 1987;498:312–323. doi: 10.1111/j.1749-6632.1987.tb23770.x. [DOI] [PubMed] [Google Scholar]
- 101.Akmal M, Qadri JQ, Al-Waili NS, Thangal S, Haq A, Saloom KY. Improvement in human semen quality after oral supplementation of vitamin C. J Med Food. 2006;9:440–442. doi: 10.1089/jmf.2006.9.440. [DOI] [PubMed] [Google Scholar]
- 102.Suleiman AA, Alboqai OK, Yasein N, Al-Essa MK, El Masri K. Prevalence of vitamin-mineral supplement use among Jordan University students. Saudi Med J. 2008;29:1326–1331. [PubMed] [Google Scholar]
- 103.Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM, Cooke ID, et al. A double-blind randomized placebo cross-over controlled trial using the antioxidant vitamin E to treat reactive oxygen species associated male infertility. Fertil Steril. 1995;64:825–831. doi: 10.1016/s0015-0282(16)57861-3. [DOI] [PubMed] [Google Scholar]
- 104.Nadjarzadeh A, Shidfar F, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Effect of coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: a double-blind randomised clinical trial. Andrologia. 2014;46:177–183. doi: 10.1111/and.12062. [DOI] [PubMed] [Google Scholar]
- 105.Tvrda E, Peer R, Sikka SC, Agarwal A. Iron and copper in male reproduction: a double-edged sword. J Assist Reprod Genet. 2015;32:3–16. doi: 10.1007/s10815-014-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao J, Dong X, Hu X, Long Z, Wang L, Liu Q, et al. Zinc levels in seminal plasma and their correlation with male infertility: a systematic review and meta-analysis. Sci Rep. 2016;6:22386. doi: 10.1038/srep22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahsan U, Kamran Z, Raza I, Ahmad S, Babar W, Riaz MH, et al. Role of selenium in male reproduction: a review. Anim Reprod Sci. 2014;146:55–62. doi: 10.1016/j.anireprosci.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 108.Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology. 2009;74:73–76. doi: 10.1016/j.urology.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 109.Oeda T, Henkel R, Ohmori H, Schill WB. Scavenging effect of N-acetyl-L-cysteine against reactive oxygen species in human semen: a possible therapeutic modality for male factor infertility? Andrologia. 1997;29:125–131. doi: 10.1111/j.1439-0272.1997.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 110.Stanislavov R, Rohdewald P. Sperm quality in men is improved by supplementation with a combination of L-arginine, L-citrullin, roburins and Pycnogenol®. Minerva Urol Nefrol. 2014;66:217–223. [PubMed] [Google Scholar]
- 111.Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers-Theunissen RP. Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertil Steril. 2002;77:491–498. doi: 10.1016/s0015-0282(01)03229-0. [DOI] [PubMed] [Google Scholar]
- 112.Castagné V, Lefèvre K, Natero R, Clarke PG, Bedker DA. An optimal redox status for the survival of axotomized ganglion cells in the developing retina. Neuroscience. 1999;93:313–320. doi: 10.1016/s0306-4522(99)00138-4. [DOI] [PubMed] [Google Scholar]
- 113.Busetto GM, Agarwal A, Virmani A, Antonini G, Ragonesi G, Del Giudice F, et al. Effect of metabolic and antioxidant supplementation on sperm parameters in oligo-asthenoteratozoospermia, with and without varicocele: a double-blind placebo-controlled study. Andrologia. 2018:150. doi: 10.1111/and.12927. [DOI] [PubMed] [Google Scholar]
- 114.Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009;182:237–248. doi: 10.1016/j.juro.2009.02.121. [DOI] [PubMed] [Google Scholar]
- 115.Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril. 2005;84:662–671. doi: 10.1016/j.fertnstert.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 116.Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol. 2012;188:526–531. doi: 10.1016/j.juro.2012.03.131. [DOI] [PubMed] [Google Scholar]
- 117.ElSheikh MG, Hosny MB, Elshenoufy A, Elghamrawi H, Fayad A, Abdelrahman S. Combination of vitamin E and clomiphene citrate in treating patients with idiopathic oligoasthenozoospermia: a prospective, randomized trial. Andrology. 2015;3:864–867. doi: 10.1111/andr.12086. [DOI] [PubMed] [Google Scholar]
- 118.Majzoub A, Agarwal A, Esteves SC. Antioxidants for elevated sperm DNA fragmentation: a mini review. Transl Androl Urol. 2017;6:S649–S653. doi: 10.21037/tau.2017.07.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Micic S, Lalic N, Djordjevic D, Bojanic N, Bogavac-Stanojevic N, Busetto GM, et al. Double-blind, randomised, placebo-controlled trial on the effect of L-carnitine and L-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia. 2019:e13267. doi: 10.1111/and.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, Amoroso S, et al. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril. 2009;91:1785–1792. doi: 10.1016/j.fertnstert.2008.02.119. [DOI] [PubMed] [Google Scholar]
- 121.Tunc O, Thompson J, Tremellen K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online. 2009;18:761–768. doi: 10.1016/s1472-6483(10)60024-7. [DOI] [PubMed] [Google Scholar]
- 122.Tremellen K, Miari G, Froiland D, Thompson J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust N Z J Obstet Gynaecol. 2007;47:216–221. doi: 10.1111/j.1479-828X.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 123.Imamovic Kumalic S, Pinter B. Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia. Biomed Res Int. 2014;2014:426951. doi: 10.1155/2014/426951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lampiao F. Free radicals generation in an in vitro fertilization setting and how to minimize them. World J Obstet Gynecol. 2012;1:29–34. [Google Scholar]
- 125.Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, et al. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril. 2004;82:593–600. doi: 10.1016/j.fertnstert.2004.02.121. [DOI] [PubMed] [Google Scholar]
- 126.Showell MG, Mackenzie-Proctor R, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2014:CD007411. doi: 10.1002/14651858.CD007411.pub3. [DOI] [PubMed] [Google Scholar]
- 127.Mora-Esteves C, Shin D. Nutrient supplementation: improving male fertility fourfold. Semin Reprod Med. 2013;31:293–300. doi: 10.1055/s-0033-1345277. [DOI] [PubMed] [Google Scholar]
- 128.Gil-Villa AM, Cardona-Maya W, Agarwal A, Sharma R, Cadavid A. Role of male factor in early recurrent embryo loss: do antioxidants have any effect? Fertil Steril. 2009;92:565–571. doi: 10.1016/j.fertnstert.2008.07.1715. [DOI] [PubMed] [Google Scholar]
- 129.Calogero AE, Duca Y, Condorelli RA, La Vignera S. Male accessory gland inflammation, infertility, and sexual dysfunctions: a practical approach to diagnosis and therapy. Andrology. 2017;5:1064–1072. doi: 10.1111/andr.12427. [DOI] [PubMed] [Google Scholar]
- 130.La Vignera S, Vicari E, Condorelli RA, D'Agata R, Calogero AE. Male accessory gland infection and sperm parameters (review) Int J Androl. 2011;34:e330–e347. doi: 10.1111/j.1365-2605.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 131.Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, et al. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology. 2018;6:513–524. doi: 10.1111/andr.12502. [DOI] [PubMed] [Google Scholar]
- 132.Haidl G, Haidl F, Allam JP, Schuppe HC. Therapeutic options in male genital tract inflammation. Andrologia. 2019;51:e13207. doi: 10.1111/and.13207. [DOI] [PubMed] [Google Scholar]
- 133.Barratt CLR, Björndahl L, De Jonge CJ, Lamb DJ, Osorio Martini F, McLachlan R, et al. The diagnosis of male infertility: an analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum Reprod Update. 2017;23:660–680. doi: 10.1093/humupd/dmx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Atik RB, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018 doi: 10.1093/hropen/hoy002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Henkel RR. Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian J Androl. 2011;13:43–52. doi: 10.1038/aja.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gutteridge JM, Halliwell B. Antioxidants: molecules, medicines, and myths. Biochem Biophys Res Commun. 2010;393:561–564. doi: 10.1016/j.bbrc.2010.02.071. [DOI] [PubMed] [Google Scholar]
- 137.Pérez-Torres I, Guarner-Lans V, Rubio-Ruiz ME. Reductive stress in inflammation-associated diseases and the prooxidant effect of antioxidant agents. Int J Mol Sci. 2017;18:E2098. doi: 10.3390/ijms18102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ufer C, Wang CC, Borchert A, Heydeck D, Kuhn H. Redox control in mammalian embryo development. Antioxid Redox Signal. 2010;13:833–875. doi: 10.1089/ars.2009.3044. [DOI] [PubMed] [Google Scholar]
- 139.Hendin BN, Kolettis PN, Sharma RK, Thomas AJ, Jr, Agarwal A. Varicocele is associated with elevated spermatozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol. 1999;161:1831–1834. [PubMed] [Google Scholar]
- 140.Harlev A, Agarwal A, Gunes SO, Shetty A, du Plessis SS. Smoking and male infertility: an evidence-based review. World J Mens Health. 2015;33:143–160. doi: 10.5534/wjmh.2015.33.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]