We have carefully read the manuscript entitled “The Relationships between Thyroid Hormone Levels and Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia” [1] published in The World Journal of Men's Health. We thank the authors for citing one of our references in the selected bibliography [2] and we take the opportunity to comment this article with its possible effects on clinical practice and suggest an original point of view for the interpretation of these data.
The authors demonstrated the existence of a relationship between some functional prostatic parameters and serum concentrations of free thyroxine (FT4). Measurement of thyroid stimulating hormone (TSH) levels, does not correlate with the parameters examined. The authors showed an increase in the percentage of patients with international prostatic symptoms score (IPSS) >7, maximal flow rate (peak urinary flow) <10 mL/s and total prostate volume (TVP) >30 mL among patients with FT4 levels in the highest quartile. The relationship between FT4 and TVP levels is maintained only in patients with high testosterone levels (>5.06 ng/mL).
The results of this study justify the hypothesis of a functional involvement of thyroid hormones in the pathogenesis of prostatic hyperplasia, as previously reported in other studies [3,4].
In the discussion, a series of potential mechanisms concerning the connections between thyroid hormones and cancer have been analyzed, underlining the stimulating action of thyroid hormones on some functional systems involved in glandular proliferation (phosphatidylinositol-3-kinase; hypoxia-inducible factor 1; αVß3; ERK1/2; fibroblast growth factor 2; mitogen-activated protein kinase), in addition to the slight hyperestrogenism favored by the increase in levels of thyroid hormones, which represents a further stimulus of prostatic glandular proliferation.
We share the hypotheses of mechanism advanced by the authors and we believe that this evidence could contribute to suggest pharmacological improvements for the treatment of prostatic hyperplasia, however we report briefly our clinical experience (unpublished data) which serves as a possible further explanation of these results.
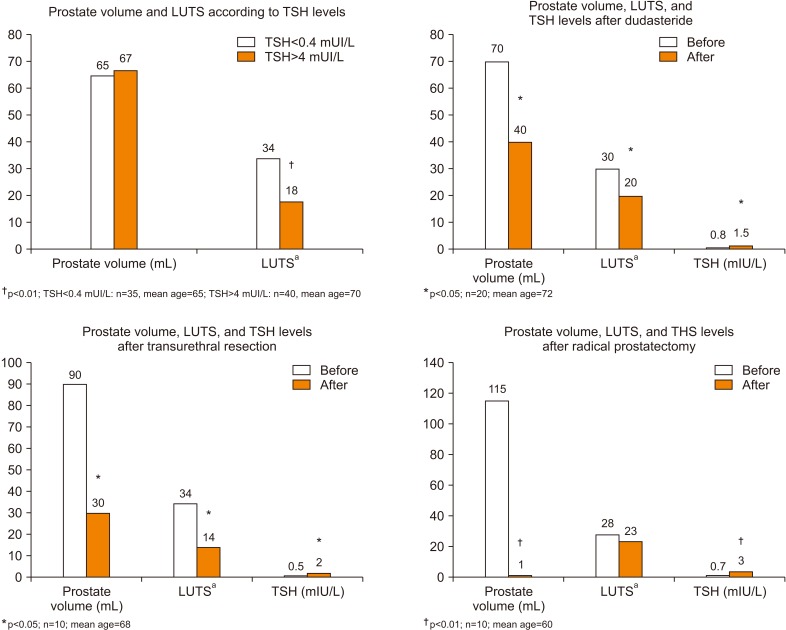
In our experience (Fig. 1) we evaluated the differences on prostate volume and severity of lower urinary tract symptoms (LUTS) between patients with subclinical hypertirodism (n=35; TSH levels <0.4 mU/L and normal serum concentrations of FT4 and FT3) and patients with subclinical hypothyroidism (n=40; TSH levels >4 mU/L and normal serum concentrations of FT4 and FT3). There are no statistically significant differences regarding TVP but patients with TSH levels <0.4 mIU/L showed a significant worsening of the LUTS assessed by IPSS questionnaire (upper left panel).
Fig. 1. Evaluation of thyroid function in patients with prostatic hyperplasia subjected to medical and surgical treatment. LUTS: lower urinary tract symptoms, TSH: thyroid stimulating hormone. aEvaluated by questionnaire's score.
Among patients with subclinical hyperthyroidism, TSH serum concentrations increase significantly after treatment with dutasteride (upper right panel). The increase in TSH levels is confirmed with the same significance values after transurethral resection of the prostate (lower left panel) and with a greater level of statistical significance after radical prostatectomy (lower right panel). Thyroid hormone evaluation was performed six months after therapy. Dutasteride was taken as tablets 0.5 g daily.
Evaluation of thyroid function was performed by chemiluminescence – Roche COBAS 6000 (TSH range: 0.3–4.2 mIU/L; FT4 range: 9.3–17 ng/L; FT3 range: 2–4.4 pg/mL). The evaluation of the severity of the LUTS was carried out using an IPSS questionnaire. Prostate volume assessment was performed by transrectal prostatic ultrasound (Esaote MyLab 25 endocavitary probe) applying the following formula: anteroposterior×transv erse×longitudinal diameter×0.52).
The reduction of TVP in these patients is associated with increased levels of TSH and resolution of the subclinical hyperthyroidism, in turn this endocrinological condition is associated with worsening of these patients' symptoms even in the absence of significant changes in prostate volume, suggesting a dysfunctional mechanism (a consequence of hyperactivation of the beta adrenergic system?) [5]. Previous evidence showed the production of TRH and similar TSH molecules from prostatic cells with endocrine function, as demonstrated from the reduction of FT4 and T3 levels in rats subjected to a ventral prostatectomy [3,4].
Footnotes
Conflicts of Interest: The authors have nothing to disclose.
- Conceptualization: SLV.
- Data curation: RAC.
- Formal analysis: RC.
- Project administration: AEC.
- Writing–original draft: SLV.
- Writing–review & editing: AEC.
References
- 1.Lee JH, Park YW, Lee SW. The relationships between thyroid hormone levels and lower urinary tract symptoms/benign prostatic hyperplasia. World J Mens Health. 2019;37:364–371. doi: 10.5534/wjmh.180084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Vignera S, Condorelli RA, Russo GI, Morgia G, Calogero AE. Endocrine control of benign prostatic hyperplasia. Andrology. 2016;4:404–411. doi: 10.1111/andr.12186. [DOI] [PubMed] [Google Scholar]
- 3.Mani Maran RR, Subramanian S, Rajendiran G, Sunil N, Archunan G, Arunakaran J, et al. Prostate-thyroid axis: stimulatory effects of ventral prostate secretions on thyroid function. Prostate. 1998;36:8–13. doi: 10.1002/(sici)1097-0045(19980615)36:1<8::aid-pros2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Maran RR, Ravichandran K, Arunakaran J. Prostate-thyroid axis: prostatic TRH is one of the stimulators of thyroid hormone. Endocr Res. 2001;27:143–152. doi: 10.1081/erc-100107176. [DOI] [PubMed] [Google Scholar]
- 5.Goswami R, Seth A, Goswami AK, Kochupillai N. Prevalence of enuresis and other bladder symptoms in patients with active Graves' disease. Br J Urol. 1997;80:563–566. doi: 10.1046/j.1464-410x.1997.00426.x. [DOI] [PubMed] [Google Scholar]