Abstract
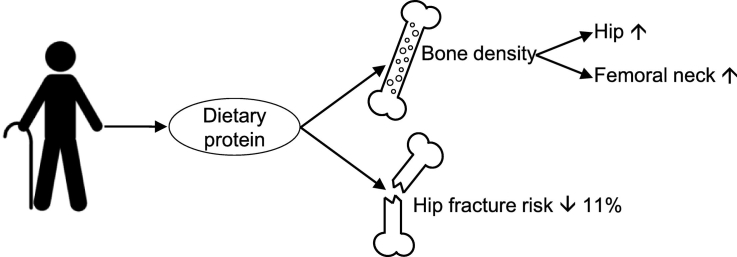
Protein may play a beneficial role in the prevention of bone loss and in slowing down osteoporosis. The effect of dietary protein may be different in older adults compared to younger adults, since this population has a greater need for protein. The aim of this systematic review and meta-analysis was to investigate the impact of a dietary protein intake above the Recommended Dietary Allowance (RDA) of 0.8 g/kg body weight/day from any source on Bone Mineral Density (BMD)/Bone Mineral Content (BMC), bone turnover markers, and fracture risk in older adults compared to a lower dietary protein intake. A systematic search was conducted through October 2018 in 3 databases: CENTRAL, MEDLINE, and EMBASE. We included all prospective cohort studies and Randomized Controlled Trials (RCTs) among adults aged ≥65 years that examined the relation between protein intake on bone health outcomes. Two investigators independently conducted abstract and full-text screenings, data extractions, and risk of bias assessments. Authors were contacted for missing data. After screening of 523 records, twelve cohort studies and one RCT were included. Qualitative evaluation showed a positive trend between higher protein intakes and higher femoral neck and total hip BMD. Meta-analysis of four cohort studies showed that higher protein intakes resulted in a significant decrease in hip fractures (pooled hazard ratio: 0.89; 95% confidence interval: 0.84, 0.94). This systematic review supports that a protein intake above the current RDA may reduce hip fracture risk and may play a beneficial role in BMD maintenance and loss in older adults.
Keywords: Protein, Bone, Bone density, Fractures, Older adults
Graphical abstract
1. Introduction
Osteoporosis is an increasing public health problem worldwide [1]. The prevalence in nine industrialized countries is estimated at 9–38% for women and 1–8% for men, affecting up to 49 million people [2]. The rising prevalence of osteoporosis leads to an increase in the number of falls and fractures, which in turn affects mortality and morbidity, and increases the economic burden [1]. Protein may play a role in the prevention of bone loss and in slowing down osteoporosis [3].
An adequate intake of dietary protein is important for bone acquisition and maintenance. Older adults may become protein malnourished due to an inadequate intake of protein and a reduced ability to use available protein, because of age-related changes in metabolism, immunity, and hormone levels and sensitivity [4,5]. At the same time there is a greater need for protein [5]. The current recommended dietary allowance (RDA) for protein is 0.8 g/kg body weight/day [6]. For the preservation of muscle function, evidence supports a protein intake of 1.0–1.2 for healthy older adults and 1.2–1.5 g/kg body weight/day for older adults suffering from acute or chronic illnesses [7].
Over the past years, the relation between dietary protein intake and bone health has received much attention. Safety concerns of a high dietary protein intake have been raised, but beneficial effects on bone health have also been found. As yet, several systematic reviews and meta-analyses [[8], [9], [10], [11]] have been published investigating the effect of dietary protein intake on bone health. All of these publications pooled cohort studies and trials over a wide age range with specific exclusion criteria to find beneficial effects of protein intake on Bone Mineral Density (BMD), Bone Mineral Content (BMC), bone turnover markers, and/or fracture risk. Some reviews include trials that have an intervention duration of <6 months [8,10]. However, it is questionable if dietary interventions can already lead to a measurable change in BMD within such a short time span. To get a reliable estimate of the impact on changes in BMD, a minimum intervention duration of 6 months seems appropriate [12].
An expert consensus paper from 2018 summarized the systematic reviews and meta-analyses looking at the effects of dietary protein on bone health in adults [3]. It states that protein intakes above the RDA, in combination with an adequate calcium intake, is associated with higher BMD, a lower rate of bone loss, and a modestly reduced fracture risk. Furthermore, it was not proven that the acid load caused by a high protein intake is harmful for bone health.
None of the above reviews focussed on older adults (age of 65 years and older) specifically. The effect of dietary protein may be different in older adults compared to adults, since this population has a greater need for protein. The aim of this systematic review and meta-analysis was to investigate the impact of a dietary protein intake above the RDA of 0.8 g/kg body weight/day from any source on BMD/BMC, bone turnover markers, and fracture risk in older adults compared to a lower dietary protein intake.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement is followed in reporting this systematic review [13].
2.1. Data Sources and Searches
A systematic search was conducted through October 29, 2018, in 3 databases: CENTRAL (http://www.thecochranelibrary.com), MEDLINE (https://www.ncbi.nlm.nih.gov/pubmed/), and EMBASE (https://www.ovid.com/). The searches were limited to the English language, and prospective cohort and human intervention studies that examined the relations of protein intake (food or supplemental sources) on bone health outcomes of interest. The search strategy per database is presented in Supplementary Table A1.
2.2. Study Eligibility Criteria
We included all prospective cohort studies and randomized controlled trials (RCTs) among older adults aged ≥65 years that examined the relation between protein intake from any source on several bone health outcomes (Table 1). Studies including both young and older individuals were still included if stratification was performed. Studies had to have an intervention duration of at least 6 months. Studies enrolling exclusively subjects with a diagnosed disease or where >20% of the baseline population was diagnosed with a disease were excluded. Furthermore, studies were excluded if they were designed to examine outcomes in response to protein type but not protein quantity and if protein was supplemented in the form of soy isoflavone. The reason for the latter is that these plant oestrogens present in soy can independently have an effect on bone loss [14]. Also studies designed for weight loss were excluded.
Table 1.
Bone health outcomes of interest.
| Outcome | Sites or markers |
|---|---|
| BMC | Total body |
| BMD | Total body, total hip, femoral neck, lumbar spine |
| Bone turnover markers | Alkaline phosphatase, bone alkaline phosphatase, bone-specific alkaline phosphatase, collagen type I cross-linked C-terminal telopeptide, collagen type I cross-linked N-terminal telopeptide, C-terminal type 1 procollagen, N-terminal type 1 procollegen, deoxypyridinoline, hydroxyproline, pyridinoline, osteocalcin |
| Fracture | All sites |
Note. BMC = Bone Mineral Content; BMD = Bone Mineral Density.
2.3. Study Selection Process
First, citation duplicates across the 3 literature searches were removed. Second, titles were screened by a single investigator to exclude cross-sectional, animal, in vitro studies, and review articles. All abstracts were then independently screened by 2 investigators. When an abstract was regarded as potentially relevant, full-text articles were retrieved and independently screened by 2 investigators based on study eligibility criteria. All abstract and full-text articles screening conflicts were resolved through discussion between the 2 investigators and a final decision was made by the consensus of the entire research team.
2.4. Data Extraction
To capture data of interest from the eligible studies, a data extraction sheet was created in Excel. One investigator extracted the data from all studies, which was reviewed and confirmed by another investigator. The following items were extracted: study characteristics, baseline population characteristics, intervention details, relevant outcomes and their assessment methods, data details (including dropouts), confounders and effect modifiers used in statistical analysis, and results.
2.5. Risk of Bias in Individual Studies
The Newcastle-Ottawa Scale (NOS) was used to assess risk of bias of included prospective cohort studies [15]. For intervention studies, the Cochrane Collaboration's tool for assessing risk of bias was used to assess internal validity [16]. This tool addresses risk of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases. Two investigators independently assessed the risk of bias in included studies. Disagreements were discussed among the research team and resolved via group consensus.
2.6. Data Synthesis
All included studies were summarized in narrative form and in tables. Items of the summary tables include study characteristics (first author, publication year, cohort name), participant characteristics, baseline mean age or age range, exposure assessment, follow-up period, and outcomes. Summary tables were organized by study type (cohort and RCT). Results were qualitatively and, if possible, quantitatively summarized by study type and outcome of interest. Meta-analysis was performed using R (v3.5.3; package meta) [17].
2.6.1. Qualitative Synthesis
Quality of the evidence was judged with the use of a grading system developed by the GRADE collaboration [18]. According to the GRADE approach, evidence was graded as ‘High’, ‘Moderate’, ‘Low’, or ‘Very Low’ depending on several criteria. Quality of evidence was downgraded based on five GRADE categories: risk of bias, imprecision, inconsistency, indirectness, and publication bias [19]. Quality was graded upwards when effects were sufficiently large, when all plausible biases would underestimate the effect, or when there was a dose-response gradient [20].
2.6.2. Quantitative Synthesis
If sufficient data were available and homogeneity in terms of participants, interventions and outcomes between studies were reasonable, a meta-analysis was conducted. Authors of relevant articles were contacted if required data were not reported. The methods described in the Cochrane Handbook for conducting meta-analyses was followed [16]. Results were pooled using a random-effects meta-analysis, with standardized mean differences for continuous outcomes and risk ratios (RR) or hazard ratios (HR) for binary outcomes. The extent of statistical heterogeneity was quantified using both the chi-squared test and the I-squared statistic. An I-squared value >50% was used as a threshold for indicating substantial statistical heterogeneity [16]. Random-effects model was used when studies were drawn from populations that differ from each other in such a way that it could influence the effect estimate. When heterogeneity was not present, a fixed-effect model was used. Sensitivity analyses were performed to explore the impact of excluding studies that were judged to be at high risk of bias and to detect the influence of a single study on the overall estimate.
3. Results
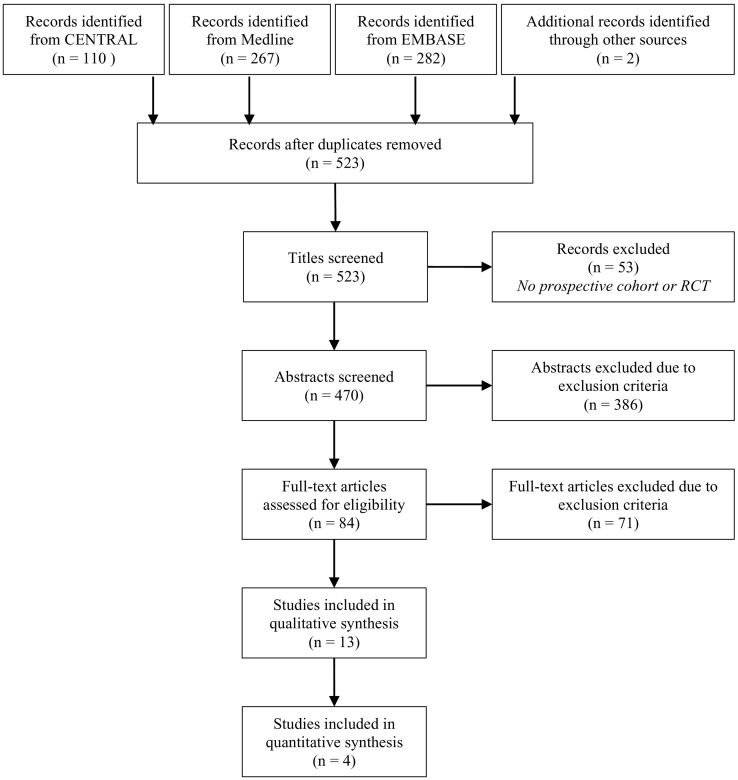
The search yielded 659 citations and two additional articles [21,22] were identified by searching in the reference lists of the other articles included in the analyses. After removal of 138 duplicates and exclusion of 53 articles because the title made clear that it was not a prospective cohort study or RCT, 470 articles were identified for dual abstract screening. A total number of 84 articles were identified for full-text screening, of which 13 were included for data extraction (12 prospective cohort studies and 1 RCT; Fig. 1). The characteristics of the included studies are shown in Table 2 and Table 3.
Fig. 1.
Flow diagram of study selection.
Table 2.
Summary table of cohort studies included in the analysis.
| First author, year [ref] | Cohort name (country) | Participants | N baseline/ analysed | Baseline mean age(SD) or age range (y) | Exposure assessment | Mean protein intakea | Follow-up (y) | Relevant outcomes | Effect sizesb |
|---|---|---|---|---|---|---|---|---|---|
| Beasley, 2014 [23] | Women's Health Initiative (US) | Post- menopausal women | 161,808/ 144,580 (whole sample) | 55–79; subgroups: 65 & 75 | FFQ + calibrated with biomarkers | 0.52, 0.75, 0.92, 1.11, 1.50 (quantiles, whole sample) | 6 | TB BMD | Per 20% increase in protein intake: 65 y: mean 0.003 (0.001, 0.005) ns 75 y: mean 0.003 (0.001, 0.007) ns |
| Hip BMD | 65 y: mean 0.003 (0.001, 0.005) ns 75 y: mean 0.004 (0.001, 0.007) ns | ||||||||
| Any fracture | 65 y: HR 0.99 (0.96, 1.01) ns 75 y: HR 0.96 (0.91, 1.02) ns | ||||||||
| Hip fracture | 65 y: HR 0.91 (0.82, 0.99) ns 75 y: HR 0.92 (0.83, 1.02) ns |
||||||||
| Cauley, 2016 [24] | Osteoporotic Fractures in Men Study (US) | Men >65 y | 5994/5876 | No fracture: 73.5(5.8); fracture: 77.8(6.1) | Block semi-quantitative FFQ | No fracture: 16.1%; fracture: 15.3% of EI | 8.6 | Hip fracture | Per SD increase in protein intake (2.9% of EI): HR 0.82 (0.69, 0.97) p<0.05 |
| Chan, 2011 [25] | - (China) | Men and women ≥65 y | 2944/2217 (1225 men, 992 women) | Men: 71.6(4.6); women: 72.0(5.1) | FFQ | Men 88.8, women 65.7 g/d. Men 1.42; women 1.19 g/kg bw/d (estimated values) |
4 | Per unit increase in energy-adjusted protein intake: | |
| Hip BMD | Men: B -0.007 SE 0.005 p 0.147 Women: B 0.003 SE 0.009 p 0.744 |
||||||||
| FN BMD | Men: B -0.013 SE 0.008 p 0.088 Women: B 0.010 SE 0.013 p 0.416 |
||||||||
| Dawson-Hughes, 2002 [26] | - (US) | Men and women ≥65 y | 389/342 | Supplemented group: 70(5), 71(4), 70(4); placebo group: 71(5), 71(5), 71(5) (tertiles) | Willett semi-quantitative FFQ | 9.6–15.5, 15.5–18.2, 18.2–29.1% of EI (tertiles). Supplemented: 0.96, 1.07, 1.17; placebo: 0.90, 1.08, 1.20 g/kg bw/d (estimated values) | 3 | Supplemented group protein T3 vs T1: | |
| TB BMD | NR, less loss/gain p 0.042 | ||||||||
| FN BMD | NR, less loss/gain p 0.011 | ||||||||
| Spine BMD | NR, no difference ns | ||||||||
| Osteocalcin | NR, no difference ns | ||||||||
| N-telopeptide | NR, no difference ns | ||||||||
| Devine, 2005 [27] | - (Australia) | Women >70 y | 1077 | 75(3) | ACCV semi-quantitative FFQ | <0.84, 0.84–1.6, >1.6 (tertiles) | 1 | Hip BMD | Protein T3 vs T1: NR, higher p<0.05 |
| FN BMD | NR, higher p<0.05 | ||||||||
| Fung, 2017 [21] | Nurses' Health Study & Health Professionals Follow-Up Study (US) | Men ≥50 y and post-menopausal women | 74,443 women; 35,439 men | Whole sample ≥50 y; stratification: <65, 65–75, ≥75 |
Semi-quantitative FFQ | Women 14.3, 18.6, 24.4% of EI; men 14.2, 18.3, 23.4% of EI (whole sample). Women 0.88, 1.12, 1.41; men 0.87, 1.12, 1.40 g/kg bw/d (estimated values) |
32 | Hip fracture | Protein Q5 vs Q1: Women 65–75 y: RR 0.92 (0.71, 1.18) Women 75+ y: RR 0.91 (0.69, 1.20) Men 65–75 y: RR 0.59 (0.33, 1.07) Men 75+ y: RR 0.77 (0.51, 1.15) |
| Hannan, 2000 [28] | Framingham Osteoporosis Study (US) | Men and women | 855/615 | 75(4.4), 68–91 | Willett semi-quantitative FFQ | 0.21–0.71; 0.72–0.96; 0.97–1.23; 1.24–2.78 (quartiles) | 4 | FN BMD | Protein Q4 vs Q1: Mean − 2.32(0.74)% vs −4.61(0.70)% p<0.001 |
| LS BMD | Mean − 1.11(1.10)% vs −3.72(0.97)% p<0.05 | ||||||||
| Isanejad, 2017 [29] | Osteoporosis Risk Factor and Fracture Prevention Study (Finland) | Women ≥65 y | 750/544 | 68.1(1.9), 65–72 | 3 d food records | 0.79, 0.90, 0.96, 1.18 (quartiles) | 3 | TB BMC | Per unit increase in energy-adjusted protein intake: B -0.16 SE 30.04 p 0.159 |
| TB BMD | B 0.04 SE 0.01 p 0.507 | ||||||||
| FN BMD | B -0.01 SE 0.01 p 0.918 | ||||||||
| LS BMD | B -0.31 SE 0.01 p 0.001 | ||||||||
| Langsetmo, 2017 [30] | Osteoporotic Fractures in Men Study (US) | Men ≥65 y | 5994/5875 | 73.6(5.9) | Modified Block FFQ | 0.67, 0.75, 0.83, 0.93 (quartiles) | 10.5–11.2 | Hip BMD | Per SD increase in protein intake (2.9% of EI): B 0.06 SE 0.01 p<0.001 |
| Spine fracture | HR 1.06 (0.92, 1.22) p 0.45 | ||||||||
| Hip fracture | HR 0.84 (0.73, 0.95) p 0.01 | ||||||||
| Meng, 2009 [31] | - (Australia) | Women 70–85 y | 1500/862 | 74.9(2.6) | ACCV quantitative FFQ | <0.84, 0.84–1.6, >1.6 (tertiles) | 5 | TB BMC | Protein T3 vs T1: 5.3% higher p<0.05 |
| Misra, 2011 [22] | Framingham Osteoporosis Study (US) | Men and women ≥68 y | 976/946 | No fracture: 75(5.0); fracture: 76(5.2) | Willett semi-quantitative FFQ | 46.5, 59.6, 67.7, 82.7 g/d (quartiles); 0.69, 0.88, 1.00, 1.22 g/kg bw/d (estimated values) |
11.6 (median) | Hip fracture | Protein Q2–4 vs Q1: HR 0.63 (0.37, 1.09) p 0.04 |
| Rapuri, 2003 [32] | Sites Testing Osteoporosis Prevention/ Intervention (US) | Women 65–77 y | 489/92 | 71.3(0.8), 72.2(0.8), 70.1(0.8), 69.9(0.8) (quartiles) | 7 d food diaries | 0.95, 0.94, 0.98, 0.99 g/kg bw/d; 13.3, 15.2, 16.7, 19.5% of EI (quartiles) |
3 | TB BMD | Protein Q4 vs Q1: NR, no difference ns |
| Hip BMD | NR, no difference ns | ||||||||
| FN BMD | NR, no difference ns | ||||||||
| Spine BMD | NR, no difference ns | ||||||||
| Osteocalcin | Mean − 6.5(7.6)% vs −5.8(7.6)% p 0.042 | ||||||||
| N-telopeptide | Mean 12.1(11.2)% vs 10.4(11.0)% p 0.226 |
Note. BMC = bone mineral content; BMD = bone mineral density; EI = energy intake; FFQ = Food Frequency Questionnaire; FN = femoral neck; HR = hazard ratio; TB = total body; LS = lumbar spine; NR = not reported; ns = not significant; RR = risk ratio; SD = standard deviation; SE = standard error; US = United States.
Unit is g/kg bw/d, unless stated otherwise. Values presented as mean or range.
Values presented as mean(SE), mean (95% CI) or RR/HR (95% CI). BMD in g/cm2.
Table 3.
Summary table of the intervention study included in the analysis.
| First author, year [ref] | RCT name (country) | Participants | N baseline/ analysed | Baseline mean age(SD) | Protein source | Control diet intake | High protein intake | Study length (y) | Relevant outcomes | Effect sizes |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhu, 2011 [33] | - (Australia) | Healthy ambulant post-menopausal women 70–80 y | 219/196 (after 1y) /179 (after 2y) | Protein: 74.2(2.8); placebo: 74.3(2.6) | Skim milk + whey protein isolate | 2.1 g (skim milk); after 2y: 1.1 g/kg bw/d | 30 g (skim milk + whey protein isolate); after 2y: 1.4 g/kg bw/d | 2 | Hip BMD | Mean change(SD) protein vs placebo (mg/cm2): After 1y: −8.3(21) vs −5.1(22) p 0.31 After 2y: −10.8(25) vs −8.2(22) p 0.46 |
| FN BMD | After 1y: −5.7(22) vs −2.6(24) p 0.34 After 2y: −8.7(26) vs −7.9(27) p 0.84 |
Note. BMD = Bone Mineral Density; NR = Not Reported; ns = not significant; RCT = Randomized Controlled Trial; SD = Standard Deviation.
3.1. Quality of Evidence
Risk of bias assessment using NOS in selected prospective cohort studies is presented in Table 4. Risk of bias was classified as high (score 1–3), potential limitations (score 4–6), or low (score 7–9). However, one study with a score of 4 was classified as having a high risk of bias due to a substantial large loss of follow-up (42.5%) [31]. Six cohort studies were classified as having a low risk of bias, five had potential limitations, and one study had a high risk of bias. With respect to adjustment of important confounders, 1 point was given if they controlled for age, gender, weight or BMI, energy intake, physical activity, smoking, alcohol, vitamin D, and calcium. Another point was given if family history of osteoporosis, fractures, (certain) illnesses, and (certain) drugs were included. Only one study took all those confounders into account [24]. Nine studies adjusted for a part of the relevant confounders [[21], [22], [23],25,26,29,30,32,33] and in three studies adjustment was inadequate [27,28,31]. The dropout rate was not clear in one cohort study [27] and another study stated that the rate was around 10% per 4 years with a total follow-up of 32 years [21]. Dropout rates were high (≥20%) in five studies varying from 24.7% to 81.2%, which (potentially) leads to attrition bias [25,28,29,31,32]. In the other five cohort studies, rates varied from 2.0% to 12.1%. Publication bias could not be assessed, because the numbers of studies in the meta-analysis were too small.
Table 4.
Newcastle - Ottawa quality assessment scale for selected cohort studies.
| First author, year [ref] | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of the exposure | Outcome of interest absent at baseline | Control for important confounders | Outcome assessment | Adequate follow-up duration | Completeness of cohort follow-up | Total points out of 9 | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Beasley, 2014 [23] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 | Low |
| Cauley, 2016 [24] | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 8 | Low |
| Chan, 2011 [25] | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 4 | Some concerns |
| Dawson-Hughes, 2002 [26] | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 6 | Low |
| Devine, 2005 [27] | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 4 | Some concerns |
| Fung, 2017 [21] | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 6 | Some concerns |
| Hannan, 2000 [28] | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 5 | Some concerns |
| Isanejad, 2017 [29] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 | Low |
| Langsetmo, 2017 [30] | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 | Low |
| Meng, 2009 [31] | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 4 | High |
| Misra, 2011 [22] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | Low |
| Rapuri, 2003 [32] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 | Some concerns |
Note. A study can be awarded a maximum of one point for each numbered item within the selection and outcome categories. A maximum of two points can be given for comparability.
Using the Cochrane Collaboration's tool, overall risk of bias in the selected intervention study [33] was classified as ‘low to some concerns’ (Table 5). Those concerns were raised with respect to attrition and reporting bias. The dropout rate was 10.5% after 1 year and 18.3% after 2 years. An expected dropout rate of 30% was taken into account in the sample size calculation. Furthermore, there were no significant differences between the protein and control group in the number of people lost to follow-up.
Table 5.
Risk of bias in the selected intervention study using Cochrane Collaboration's tool.
| Author | Risk of selection bias |
Risk of performance bias |
Risk of attrition bias |
Risk of detection bias |
Risk of reporting bias |
Overall risk of bias | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Random sequence allocation | Allocation concealment | Baseline differences | Total | Blinding of participants | Blinding of personnel | Appropriate analysis | Total | Complete outcome data | Missing outcome data | Total | Inappropriate outcome assessment | Outcome assessment group differences | Blinding | Total | Analysis as pre-specified | Selection on multiple outcome measurements | Selection on multiple analyses | Total | ||
| Zhu | L | L | L | Low | L | L | L | Low | PH | PL | Some concerns | L | L | L | Low | NI | L | NI | Some concerns | Low to some concerns |
Note. H = high; L = low; NI = no information; PH = probably high; PL = probably low.
According to the GRADE approach, evidence from observational studies should be rated as low quality (Table 6). Five cohort studies reported the protein intake per a defined unit increase in energy-adjusted protein intake (dose-response gradient). In absence of serious limitations in other categories, three of those cohort studies were rated up one level to moderate quality with respect to their BMD and fracture outcomes [24,29,30]. Quality of evidence for the outcomes total body BMC and bone turnover markers was rated down to ‘very low’ due to risk of bias, imprecision, and limited number of studies. In addition, quality of evidence for total fractures and spine fractures was rated ‘very low’, because both were assessed in one study only.
Table 6.
Quality of evidence per outcome of interest from selected studies.
| Outcome | Number of cohort studies | Number of RCTs | Risk of bias | Imprecision | Consistency | Number of moderate quality studies | Overall quality rating |
|---|---|---|---|---|---|---|---|
| BMC - total body | 2 | 0 | Some concerns | Some concerns | 1 p, 1 ns | 0 | Very low |
| BMD | |||||||
| Total body | 4 | 0 | Low | Low | 1 p, 3 ns | 1 | Low |
| Total hip | 5 | 1 | Some concerns | Low | 2 p, 3 ns | 1 | Low |
| Femoral neck | 6 | 1 | Some concerns | Low | 3 p, 3 ns | 1 | Low |
| Lumbar spine | 4 | 0 | Some concerns | Low | 1 p, 1 n, 2 ns | 1 | Low |
| Bone turnover markers | |||||||
| Osteocalcin | 2 | 0 | Some concerns | Some concerns | 2 ns | 0 | Very low |
| N-telopeptide | 2 | 0 | Some concerns | Some concerns | 2 ns | 0 | Very low |
| Fracture | |||||||
| Total | 1 | 0 | Low | Low | 1 ns | 0 | Very low |
| Spine | 1 | 0 | Low | Low | 1 ns | 1 | Very low |
| Hip | 5 | 0 | Low | Low | 4 p, 1 ns | 2 | Low |
Note. According to the GRADE approach, evidence was graded as ‘High’, ‘Moderate’, ‘Low’, or ‘Very Low’ depending on several criteria. Risk of bias is a combined judgement from risk of bias in the individual studies. Indirectness was rated low for all outcomes. BMC = Bone Mineral Content; BMD = Bone Mineral Density; n = negative; ns = not significant; p = positive; RCT = Randomized Controlled Trial.
3.2. Prospective Cohort Studies
3.2.1. BMC–Total Body
Two studies assessed the effect of protein intake on total body BMC and showed different results [29,31]. Meng et al. [31] found that postmenopausal women with a protein intake above the RDA (>1.6 g/kg bw/d) had a significant 5.3% higher whole body BMC compared to women with a low protein intake (<0.8 g/kg bw/d) after 5 years of follow-up. Isanejad et al. [29] found that protein intake was not significantly associated with total body BMC over 3 years of follow-up.
3.2.2. BMD–Total Body
Four studies assessed the effect of protein intake on total body BMD, of which one saw a beneficial effect [26] and three found no significant effects [23,29,32]. Dawson-Hughes et al. [26] found that the highest tertile of protein intake (18.2–29.1% of total energy; estimated mean 1.17 g/kg bw/d) was associated with significantly higher total body BMD after 3 years of follow-up compared to the lowest tertile of protein intake (9.6–15.5% of total energy; estimated mean 0.96 g/kg bw/d; figure-derived: mean percent change 0.6% vs -0.2%, respectively). This effect was seen in a group who took calcium and vitamin D supplements for 3 years, but no association was found in the placebo group. Beasley et al. [23] found that each 20% increase in calibrated protein intake was associated with a non-significant increase in total body BMD in women aged 65 and 75 years at baseline after 3 years of follow-up. Isanejad et al. [29] found that protein intake was not significantly associated with total body BMD over 3 years of follow-up and Rapuri et al. [32] found no differences in total body BMD between four quartiles of protein intake above the RDA (0.95, 0.94, 0.98, 0.99 g/kg bw/d; 13.3, 15.2, 16.7, 19.5% of energy intake) after 3 years of follow-up.
3.2.3. BMD–Total Hip
Five studies assessed the effect of protein intake on total hip BMD, of which two found beneficial effects [27,30] and three found no significant effects [23,25,32]. Devine et al. [27] found that women with a protein intake above the RDA (>1.6 g/kg bw/d) had a significantly higher hip BMD compared to women with a low protein intake (<0.8 g/kg bw/d) after 1 year of follow-up (figure-derived: mean 824 vs 798 mg/cm2, respectively). Langsetmo et al. [30] showed that a higher protein intake (each standard deviation (SD) increase in total energy from protein intake) was associated with higher hip BMD in men after 10.5–11.2 years of follow-up (beta = 0.06, standard error = 0.01). On the other hand, Beasley et al. [23] found that each 20% increase in calibrated protein intake was associated with a non-significant increase in hip BMD in women aged 65 and 75 years at baseline after 3 years of follow-up. Chan et al. [25] found that protein intake was not associated with % change in hip BMD in men and women after 4 years of follow-up. Rapuri et al. [32] found no differences in hip BMD between four quartiles of protein intake above the RDA (0.95, 0.94, 0.98, 0.99 g/kg bw/d; 13.3, 15.2, 16.7, 19.5% of energy intake) after 3 years of follow-up.
3.2.4. BMD–Femoral Neck
Six studies assessed the effect of protein intake on femoral neck BMD, half of them showed beneficial effects [[26], [27], [28]] and the other half showed no significant effects [25,29,32]. Dawson-Hughes et al. [26] found that highest tertile of protein intake (18.2–29.1% of total energy; estimated mean 1.17 g/kg bw/d) was associated with significantly higher femoral neck BMD after 3 years of follow-up compared to the lowest tertile of protein intake (9.6–15.5% of total energy; estimated mean 0.96 g/kg bw/d; figure-derived: mean percent change 2.5% vs -0.4%, respectively). This effect was seen in a group who took calcium and vitamin D supplements for 3 years, but no association was found in the placebo group. Devine et al. [27] found that women with a protein intake above the RDA (>1.6 g/kg bw/d) had a significantly higher femoral neck BMD compared to women with a low protein intake (<0.8 g/kg bw/d) after 1 year of follow-up (figure-derived: mean 702 vs 679 mg/cm2, respectively). Hannan et al. [28] showed that men and women with a protein intake above the RDA (1.24–2.78 g/kg bw/d) had significantly less femoral neck BMD loss than those with a protein intake below the RDA (0.21–0.71 g/kg bw/d) after 4 years of follow-up (mean percent change -2.32% vs -4.61%). With regard to the studies showing no effect, Chan et al. [25] found that protein intake was not associated with % change in femoral neck BMD in men and women after 4 years of follow-up. Isanejad et al. [29] found that protein intake was not significantly associated with femoral neck BMD over 3 years of follow-up and Rapuri et al. [32] found no differences in femoral neck BMD between four quartiles of protein intake above the RDA (0.95, 0.94, 0.98, 0.99 g/kg bw/d; 13.3, 15.2, 16.7, 19.5% of energy intake) after 3 years of follow-up.
3.2.5. BMD–(Lumbar) Spine
Four studies assessed the effect of protein intake on (lumbar) spine BMD, of which one found a beneficial effect [28], one found a negative effect [29], and two found no significant effects [26,32]. Hannan et al. [28] found a beneficial effect, they showed that men and women with a protein intake above the RDA (1.24–2.78 g/kg bw/d) had significantly less lumbar spine BMD loss than those with a protein intake below the RDA (0.21–0.71 g/kg bw/d) after 4 years of follow-up (mean percent change −1.11% vs −3.72%). On the contrary, Isanejad et al. [29] found that in women with protein intakes ranging from 0.79 to 1.18 g/kg bw/d, protein was significantly negatively associated with total body BMD over 3 years of follow-up (beta = −0.31, standard error = 0.01). Dawson-Hughes et al. [26] found no association between the protein tertile of 18.2–29.1% of total energy (estimated mean 1.17 g/kg bw/d) vs 9.6–15.5% of total energy (estimated mean 0.96 g/kg bw/d) for spine BMD after 3 years of follow-up. In addition, Rapuri et al. [32] found no differences in spine BMD between four quartiles of protein intake above the RDA (0.95, 0.94, 0.98, 0.99 g/kg bw/d; 13.3, 15.2, 16.7, 19.5% of energy intake) after 3 years of follow-up.
3.2.6. Bone Turnover Markers
Two studies assessed the effect of protein intake on the bone turnover markers osteocalcin and N-telopeptide [26,32]. Dawson-Hughes et al. [26] found no association between the highest and lowest protein tertile (18.2–29.1% vs 9.6–15.5% of total energy; estimated means 1.17 vs 0.96 g/kg bw/d) for both serum osteocalcin and urinary N-telopeptide cross-links after 3 years of follow-up. Rapuri et al. [32] also found no differences in serum osteocalcin and urinary N-telopeptides between four quartiles of protein intake above the RDA (0.95, 0.94, 0.98, 0.99 g/kg bw/d; 13.3, 15.2, 16.7, 19.5% of energy intake) after 3 years of follow-up.
3.2.7. Fractures
Only Beasley et al. [23] looked at total fractures and found that each 20% increase in calibrated protein intake was associated with a non-significant decrease in risk of total fractures in women aged 65 and 75 years at baseline after 3 years of follow-up.
With regard to risk of spine fracture specifically, only Langsetmo et al. [30] studied this outcome and found no association between a higher protein intake (each SD increase in total energy from protein intake) and risk of spine fracture in men after 10.5–11.2 years of follow-up.
More often investigated was the risk of getting a hip fracture. Five studies assessed the effect of protein intake on hip fracture risk, of which four showed a beneficial effect [21,22,24,30] and one found no effect [23]. Cauley et al. [24] showed that each SD increase in total energy from protein was associated with an 18% decrease in risk of hip fractures in men after 8.6 years of follow-up. In the same cohort population but after 10.5–11.2 years of follow-up, Langsetmo et al. [30] found a significant association between each SD increase in total energy from protein intake and a decreased hip fracture risk of 16%. Fung et al. [21] showed an 8% lower risk of hip fractures in women aged 65–75 years consuming 1.4 g protein/kg bw/d (estimated value, 24.4% of energy intake) compared to a protein intake of 0.9 g/kg bw/d (estimated value, 14.3% of energy intake) and a 9% lower risk in women aged ≥75 years after 32 years of follow-up. In men aged 65–75 years, a 41% lower hip fracture risk was found between the highest vs lowest protein tertile (23.4% vs 14.2% of energy intake, estimated mean 1.4 vs 0.9 g/kg bw/d) and this reduction in risk was 23% in men aged ≥75 years. Misra et al. [22] found that a mean protein intake above the RDA (three quartiles: 59.6, 67.7, 82.7 g/d; estimated 0.88, 1.00, 1.22 g/kg bw/d) was significantly positively associated with a decreased hip fracture risk of 37% after 11.6 years of follow-up compared to a mean protein intake below the RDA (46.5 g/d; estimated 0.69 g/kg bw/d). However, Beasley et al. [23] found that each 20% increase in calibrated protein intake was associated with a non-significant decrease in risk of hip fractures in women aged 65 and 75 years at baseline after 3 years of follow-up (9% and 8% respectively).
3.3. RCTs
No eligible RCTs were found investigating the effect of dietary protein intake on BMC, total body and lumbar spine BMD, and bone turnover markers. Only one RCT with total hip and femoral neck BMD as outcome was suitable for inclusion [33]. Zhu et al. [33] investigated the effect of a high-protein drink containing 30 g of protein compared to a placebo drink with 2.1 g of protein. Protein intake after 2 years was above the RDA for both groups (1.4 and 1.1 g/kg bw/d for protein and placebo group, respectively). No significant differences in hip and femoral neck BMD between women in the protein or placebo group after 1 and 2 years of protein supplementation were found. Hip and femoral neck BMD in both groups fell significantly from baseline.
3.4. Meta-analysis
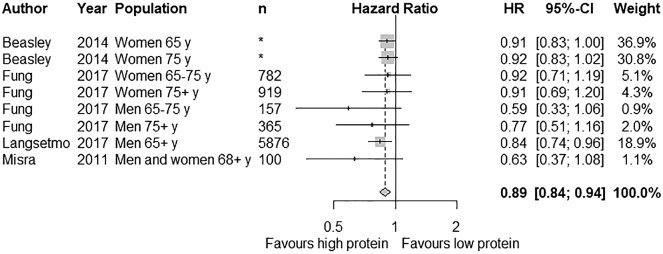
A meta-analysis could be conducted for the outcome hip fracture among four cohort studies including eight different groups (Fig. 2). The study of Cauley et al. [24] was excluded from the meta-analysis, since the same cohort population was used as in the study of Langsetmo et al. [30]. The latter was preferred because the follow-up duration was longer. The association between protein intake and risk of hip fracture was expressed as weighted HR (RR reported in one study was considered as HR). HR was transformed to its natural logarithm (ln) and the corresponding 95% CIs were used to calculate the standard errors. Inverse variance weighting was used to obtain the pooled HR for higher compared to lower protein intakes. Heterogeneity was not significantly present for hip fracture (I2 = 0.0%, heterogeneity chi-squared p = 0.614) and a fixed-effect model was used. The meta-analysis of the cohort studies showed that high vs low protein intake resulted in a statistically significant decrease in hip fractures (pooled HR: 0.89; 95% CI: 0.84, 0.94; p<0.001).
Fig. 2.
Effect of protein intake on hip fractures. Fixed-effect pooled hazard ratio (HR) analysis was used. Grey boxes represent the point estimates with the size of the box representing the weight of the study. Horizontal lines depict the length of the 95% CI. The diamond represents the pooled effect estimate.
* No exact sample size can be stated; the hazard ratio is the estimate of the effect at specific age levels (65 and 75 y) selected from a continuous distribution. Total sample size was 144,580 persons.
Sensitivity analyses showed that there was no single study affecting the overall estimate considerably. A subgroup analysis was performed for gender. One study had to be excluded because no separate data was available for men and women [22]. A pooled HR of 0.82 (95% CI: 0.73, 0.93; p = 0.002) was found for men and 0.91 (95% CI: 0.86, 0.98; p = 0.007) for women. This difference was not statistically different (p = 0.126).
Meta-analyses with other outcomes of interest could not be performed, because missing quantitative data could not be provided for each relevant article after contact with authors.
4. Discussion
The aim of this systematic review and meta-analysis was to investigate the impact of a dietary protein intake above the RDA of 0.8 g/kg body weight/day from any source on BMD/BMC, bone turnover markers, and fracture risk in older adults compared to a lower dietary protein intake. Our findings showed a positive trend between higher protein intakes and higher femoral neck and total hip BMD. Conflicting results were reported for lumbar spine BMD and no association was found for total body BMD. No conclusions could be drawn regarding BMC and bone turnover markers. Meta-analysis of four prospective cohort studies showed a statistically significant decrease of 11% in hip fracture risk.
Total body BMC was measured in only two cohort studies, of which one had a high risk of bias [31] and the other one had imprecise results [29]. Therefore, quality of evidence for total body BMC was rated as very low. Quality of evidence for the bone turnover markers was also rated as very low due to eligibility of only two cohort studies, of which one was rated down due imprecision [32]. Risk of bias of the other cohort studies did not affect the quality of evidence.
The selected cohort studies compared varying levels of protein intakes, which makes comparisons between studies difficult and it can influence the magnitude of the measured effect. In addition, some studies looked at very high levels above the RDA, while others reported protein intakes closer to the RDA. For instance, two studies divided the protein intake in tertiles with the highest protein category being >1.6 g/kg bw/d [27,31] and the highest category in the study of Fung et al. was 1.4 g/kg bw/d. While in three other cohort studies, the highest protein category was around 1.2 g/kg bw/d [22,26,29]. Rapuri et al. divided protein intake in four quartiles, which were very close to each other when transferred from percentage of energy intake to g/kg bw/d as unit. This minimal difference in protein intake may be one of the reasons why in this study no significant differences in bone health outcomes were found.
No sufficient evidence was available from intervention studies, only one RCT was eligible for inclusion in this review. This RCT showed no significant difference in hip and femoral neck BMD between protein and placebo group after 2 years. However, these outcomes fell significantly from baseline in both groups. Since protein intake after 2 years was above the RDA for both groups, this may indicate that a protein intake above the RDA is beneficial for hip and femoral neck BMD. This is in line with some of the cohort studies investigating hip BMD (3 studies showed no differences and 2 showed beneficial differences) and femoral neck BMD (3 studies showed no differences and 3 showed beneficial differences). The overall risk of bias in this RCT was classified as low to some concerns. Since this study was only done in women, representativeness is limited.
Over the years, several systematic reviews and meta-analyses assessing the impact of protein intake on bone health have been published. Darling et al. (2009) investigated the effect of varying protein intakes in healthy adults of 18 years and older and included 31 observational studies and 19 RCTs [8]. They concluded that there might be a small beneficial effect of protein supplementation on lumbar spine BMD (weighted mean difference = 0.02 g/cm2; 95% CI: 0.00, 0.04), but that it is unknown if this also results in a reduced fracture risk. However, they also included studies with a duration of <6 months, leading to a less a reliable estimate of the impact on changes in BMD [12]. In comparison with the current review, we did not have enough data to assess lumbar spine BMD. We did find an effect on hip fracture risk, which can partly be explained by the differences between study populations (adults ≥18 years vs adults ≥65 years).
A more recent systematic review of Shams-white et al. [9] investigated the effect of varying protein intakes in healthy adults of 18 years and older and excluded studies with a duration of <6 months [9]. A total of 20 observational studies and 16 RCTs were included, which showed that there were positive trends of high compared to low protein intakes on BMD at different bone sites, especially the lumbar spine (net percentage change = 0.52%; 95% CI: 0.06, 0.97). Meta-analyses of RCTs assessing lumbar spine, total hip and femoral neck BMD were performed; no meta-analysis was done with hip fracture risk. Since insufficient evidence is available from RCTs in the current review, meta-analysis results cannot be compared.
The systematic review of Wallace & Frankenfeld [10] included healthy adults of 18 years and older and specifically focused on protein intakes above the current RDA [10]. They also excluded weight loss studies and studies in which women used hormone replacement therapy (HRT), resulting in the inclusion of 15 observational studies and 16 RCTs. They conclude that a protein intake above 0.8 g/kg body weight/day can potentially have a beneficial effect on hip fracture risk (pooled RR = 0.84; 95% CI: 0.73, 0.95) and BMD loss (qualitative evaluation). Although this meta-analysis included a younger population, the reduction in hip fracture risk is similar to what we have found. They also included studies with a duration of <6 months, leading to a less a reliable estimate of the impact on changes in BMD [12]. Therefore, results for BMD outcomes cannot be compared.
Lastly, results of the meta-analysis performed by Wu et al. [34] were the same as the current meta-analysis: a significant decrease in hip fracture risk of 11% (RR 0.89; 95% CI 0.82, 0.97) [34]. Two of the six included prospective cohort studies were also added to the current meta-analysis, the other four were performed with adults aged <65 years. Despite addition of recent publications investigating older adults in our analysis, no differences in hip fracture risk reduction could be observed.
The prevalence of osteoporosis and fracture risk are higher in women than in men [35,36]. Therefore, the effect of a high protein intake on hip fracture risk may be gender-specific. However, subgroup analysis for gender showed no statistically significant difference in hip fracture risk reduction and therefore it was assumed that pooling the effect of men and women together was fair. Currently, much research is focused on postmenopausal women, but since men tend to have a higher mortality risk after a fracture, men should not be overlooked [36,37].
Previously it was believed that a high dietary protein intake could have a negative effect on bone health by inducing chronic metabolic acidosis, which eventually leads to osteoporosis [38]. However, an increase in urinary calcium excretion observed after a high protein diet likely originates from an increased intestinal absorption instead of from bone calcium loss [39]. In addition, an expert consensus paper from 2018, assessing the risks and benefits of dietary protein for bone health, concluded that a protein intake above the RDA is beneficial for older adults [3]. A long-term protein intake of 2 g/kg body weight/day is reported safe for healthy adults, higher values may lead to digestive, renal, and vascular problems [40]. No conclusive evidence is available to set a new RDA for older adults in the light of bone health.
Two comments must be made with respect to the study eligibility criteria of the current review. In the pre-specified protocol, it was mentioned that studies including women using oral contraceptives or HRT would be excluded, except when stratification was done or when it was controlled for in the analyses. In principle, women aged ≥65 years do not take oral contraceptives anymore. Regarding HRT, a majority of the included studies did not report if women used this. Since we have older adults as study population, exclusion of this group of people might harm the generalizability. Therefore, we decided to remove this exclusion criterion. Secondly, it was stated that vegan individuals would be excluded, but this was not reported in the included studies. We assumed that the prevalence of vegan individuals in this generation is negligible [41] and therefore the expected impact on the results is minimal.
For the meta-analysis, standard errors were estimated from the HR or RR and its 95% CI, reported by the individual studies. When standard errors were back transformed to CIs, this indirect variance estimation resulted into a close but not similar value of the actually reported CIs. However, the differences were small (maximum interval difference of 0.02) and assumed not to influence the results.
A strength of this study was that only studies investigating older adults (aged ≥65 years) were included, making the recommendation specific for this more vulnerable group that has a greater protein need than younger adults. As a consequence, the review is limited by inclusion of only one intervention study. This makes clear that large and long-term RCTs in older adults are needed to judge if a protein intake above the current RDA can improve bone health and/or prevent osteoporosis. Another strength was the exclusion of studies with a duration of <6 months, studies that supplemented protein in the form of soy isoflavone, and studies designed for weight loss. These exclusion criteria eliminate a proportion of confounding issues.
5. Conclusions
This systematic review supports that there is an association between a dietary protein intake above the current RDA of 0.8 g/kg body weight/day and a reduced hip fracture risk in older adults. In addition, positive trends for total hip and femoral neck BMD were found. In comparison with younger adults, the body of evidence from the included studies is not strong enough to increase the protein recommendation for older adults with respect to bone health.
Declaration of Competing Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
LdG and LvL have received grants from Friesland Campina (FC) for other studies. FC was not involved in the current review.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). She is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from.
Acknowledgements
Funding for this research was provided through a grant from Jaap Schouten Foundation under grant agreement JSF_SU_17_8. The funder had no role in the design, analyses, interpretation, or writing of this article.
We thank Joseph Larson, Ruth Chan, Teresa Fung, Lisa Langsetmo, Marian T. Hannan, and Kun Zhu for providing original data from their research studies upon request.
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health Funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2019.07.005.
Appendix A. Supplementary data
Supplementary material
References
- 1.Cauley J.A. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243–1251. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wade S.W., Strader C., Fitzpatrick L.A., Anthony M.S., O'Malley C.D. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014;9:182. doi: 10.1007/s11657-014-0182-3. [DOI] [PubMed] [Google Scholar]
- 3.Rizzoli R., Biver E., Bonjour J.P., Coxam V., Goltzman D., Kanis J.A. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European society for clinical and economical aspects of osteopororosis, osteoarthritis, and musculoskeletal diseases and by the international osteoporosis foundation. Osteoporos Int. 2018 doi: 10.1007/s00198-018-4534-5. [DOI] [PubMed] [Google Scholar]
- 4.Boirie Y. Physiopathological mechanism of sarcopenia. J Nutr Health Aging. 2009;13(8):717–723. doi: 10.1007/s12603-009-0203-x. [DOI] [PubMed] [Google Scholar]
- 5.Bauer J., Biolo G., Cederholm T., Cesari M., Cruz-Jentoft A.J., Morley J.E. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc. 2013;14(8):542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine . The National Academies Press; Washington, DC: 2005. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. [Google Scholar]
- 7.Deutz N.E.P., Bauer J.M., Barazzoni R., Biolo G., Boirie Y., Bosy-Westphal A. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin nutr (Edinb , Scotl) 2014;33(6):929–936. doi: 10.1016/j.clnu.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darling A.L., Millward D.J., Torgerson D.J., Hewitt C.E., Lanham-New S.A. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr. 2009;90(6):1674–1692. doi: 10.3945/ajcn.2009.27799. [DOI] [PubMed] [Google Scholar]
- 9.Shams-White M.M., Chung M., Du M., Fu Z., Insogna K.L., Karlsen M.C. Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr. 2017;105(6):1528–1543. doi: 10.3945/ajcn.116.145110. [DOI] [PubMed] [Google Scholar]
- 10.Wallace T.C., Frankenfeld C.L. Dietary protein intake above the current RDA and bone health: a systematic review and meta-analysis. J Am Coll Nutr. 2017;36(6):481–496. doi: 10.1080/07315724.2017.1322924. [DOI] [PubMed] [Google Scholar]
- 11.van den Heuvel E., Steijns J. Dairy products and bone health: how strong is the scientific evidence? Nutr Res Rev. 2018:1–15. doi: 10.1017/S095442241800001X. [DOI] [PubMed] [Google Scholar]
- 12.Weaver C.M., Gordon C.M., Janz K.F., Kalkwarf H.J., Lappe J.M., Lewis R. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–1386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng X., Lee S.-K., Chun O.K. Soy Isoflavones and osteoporotic bone loss: a review with an emphasis on modulation of bone remodeling. J Med Food. 2016;19(1):1–14. doi: 10.1089/jmf.2015.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available from:
- 16.Higgins J.P.T., Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011. http://handbook-5-1.cochrane.org/ Retrieved from.
- 17.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. A language and environment for statistical computing. [Google Scholar]
- 18.Atkins D., Best D., Briss P.A., Eccles M., Falck-Ytter Y., Flottorp S. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balshem H., Helfand M., Schunemann H.J., Oxman A.D., Kunz R., Brozek J. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt G.H., Oxman A.D., Sultan S., Glasziou P., Akl E.A., Alonso-Coello P. GRADE guidelines: 9. rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Fung T.T., Meyer H.E., Willett W.C., Feskanich D. Protein intake and risk of hip fractures in postmenopausal women and men age 50 and older. Osteoporos Int. 2017;28(4):1401–1411. doi: 10.1007/s00198-016-3898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra D., Berry S.D., Broe K.E., McLean R.R., Cupples L.A., Tucker K.L. Does dietary protein reduce hip fracture risk in elders? the framingham osteoporosis study. Osteoporos Int. 2011;22(1):345–349. doi: 10.1007/s00198-010-1179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beasley J.M., LaCroix A.Z., Larson J.C., Huang Y., Neuhouser M.L., Tinker L.F. Biomarker-calibrated protein intake and bone health in the women's health initiative clinical trials and observational study. Am J Clin Nutr. 2014;99(4):934–940. doi: 10.3945/ajcn.113.076786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cauley J.A., Cawthon P.M., Peters K.E., Cummings S.R., Ensrud K.E., Bauer D.C. Risk factors for hip fracture in older men: the osteoporotic fractures in men study (MrOS) J Bone Miner Res. 2016;31(10):1810–1819. doi: 10.1002/jbmr.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan R., Woo J., Leung J. Effects of food groups and dietary nutrients on bone loss in elderly Chinese population. J Nutr Health Aging. 2011;15(4):287–294. doi: 10.1007/s12603-010-0279-3. [DOI] [PubMed] [Google Scholar]
- 26.Dawson-Hughes B., Harris S.S. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr. 2002;75(4):773–779. doi: 10.1093/ajcn/75.4.773. [DOI] [PubMed] [Google Scholar]
- 27.Devine A., Dick I.M., Islam A.F.M., Dhaliwal S.S., Prince R.L. Protein consumption is an important predictor of lower limb bone mass in elderly women. Am J Clin Nutr. 2005;81(6):1423–1428. doi: 10.1093/ajcn/81.6.1423. [DOI] [PubMed] [Google Scholar]
- 28.Hannan M.T., Tucker K.L., Dawson-Hughes B., Cupples L.A., Felson D.T., Kiel D.P. Effect of dietary protein on bone loss in elderly men and women: the framingham osteoporosis study. J Bone Miner Res. 2000;15(12):2504–2512. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- 29.Isanejad M., Sirola J., Mursu J., Kroger H., Tuppurainen M., Erkkila A.T. Association of protein intake with bone mineral density and bone mineral content among elderly women: the OSTPRE fracture prevention study. J Nutr Health Aging. 2017;21(6):622–630. doi: 10.1007/s12603-016-0800-4. [DOI] [PubMed] [Google Scholar]
- 30.Langsetmo L., Shikany J.M., Cawthon P.M., Cauley J.A., Taylor B.C., Vo T.N. The association between protein intake by source and osteoporotic fracture in older men: a prospective cohort study. J Bone Miner Res. 2017;32(3):592–600. doi: 10.1002/jbmr.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng X., Zhu K., Devine A., Kerr D.A., Binns C.W., Prince R.L. A 5-year cohort study of the effects of high protein intake on lean mass and BMC in elderly postmenopausal women. J Bone Miner Res. 2009;24(11):1827–1834. doi: 10.1359/jbmr.090513. [DOI] [PubMed] [Google Scholar]
- 32.Rapuri P.B., Gallagher J.C., Haynatzka V. Protein intake: effects on bone mineral density and the rate of bone loss in elderly women. Am J Clin Nutr. 2003;77(6):1517–1525. doi: 10.1093/ajcn/77.6.1517. [DOI] [PubMed] [Google Scholar]
- 33.Zhu K., Meng X., Kerr D.A., Devine A., Solah V., Binns C.W. The effects of a two-year randomized, controlled trial of whey protein supplementation on bone structure, IGF-1, and urinary calcium excretion in older postmenopausal women. J Bone Miner Res. 2011;26(9):2298–2306. doi: 10.1002/jbmr.429. [DOI] [PubMed] [Google Scholar]
- 34.Wu A.M., Sun X.L., Lv Q.B., Zhou Y., Xia D.D., Xu H.Z. The relationship between dietary protein consumption and risk of fracture: a subgroup and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015:5. doi: 10.1038/srep09151. doi:UNSP 9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nih Consensus Development Panel on Osteoporosis Prevention, D., & Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 36.Alswat K.A. Gender disparities in osteoporosis. J Clin Med Res. 2017;9(5):382–387. doi: 10.14740/jocmr2970w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haentjens P., Magaziner J., Colon-Emeric C.S., Vanderschueren D., Milisen K., Velkeniers B. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–390. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonjour J.P. The dietary protein, IGF-I, skeletal health axis. Horm Mol Biol Clin Invest. 2016;28(1):39–53. doi: 10.1515/hmbci-2016-0003. [DOI] [PubMed] [Google Scholar]
- 39.Calvez J., Poupin N., Chesneau C., Lassale C., Tome D. Protein intake, calcium balance and health consequences. Eur J Clin Nutr. 2012;66(3):281–295. doi: 10.1038/ejcn.2011.196. [DOI] [PubMed] [Google Scholar]
- 40.Wu G. Dietary protein intake and human health. Food Funct. 2016;7(3):1251–1265. doi: 10.1039/c5fo01530h. [DOI] [PubMed] [Google Scholar]
- 41.Leitzmann C. Vegetarian nutrition: past, present, future. Am J Clin Nutr. 2014;100(Suppl_1):496S–502S. doi: 10.3945/ajcn.113.071365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material