Abstract
In randomized clinical trials (RCTs) proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors showed a favorable safety profile, however, “real‐world” data on adverse events (AEs) is scarce. Three datasets, a hospital registry (n = 164), and two Pharmacovigilance databases, Lareb (n = 149) and VigiLyze (n = 15,554), reporting AEs attributed to PCSK9 inhibitors (alirocumab or evolocumab) prescribed in clinical practice were analyzed. In the hospital registry, 41.5% of the patients reported any AE, most often injection‐site reactions (33.8%) and influenza‐like illness (27.9%). Twelve patients (7%) discontinued PCSK9 inhibitor treatment. Most common AE reported in the Lareb and VigiLyze database was myalgia (12.8% and 8.3%, respectively). No clinically relevant differences in gender or between drugs were observed. No specific subgroup of patients could be identified at risk of developing AEs. During follow‐up, AEs resolved in most patients (71.1%). In a real‐world setting, PCSK9 inhibitors are well tolerated with an overall safety profile comparable to RCTs.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ RCTs assessing clinical effects of PCSK9 inhibitors alirocumab and evolocumab showed a favorable safety profile with a low rate of AEs. Most common reported AEs in RCTs are nasopharyngitis, upper respiratory tract infection, influenza‐like illness, myalgia, back pain, arthralgia, headache, and ISRs.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ What are (the most common) AEs associated with the use of the PCSK9 inhibitors alirocumab and evolocumab prescribed in a real‐world setting?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ In a real‐world setting, PCSK9 inhibitors are well tolerated with an overall safety profile comparable to RCTs. Most common AEs are influenza‐like illness, nasopharyngitis, myalgia, and ISRs, which often resolve over time. No clinical relevant differences in gender or between drugs were observed. No specific subgroup of patients could be identified at risk of developing AEs.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Safety monitoring of PCSK9 inhibitors is indispensable to assess long‐term effects and reactions occurring in specific subgroups of patients. Therefore, healthcare providers should contribute to report to pharmacovigilance centers.
High low‐density lipoprotein‐cholesterol (LDL‐C) levels are associated with risk of cardiovascular disease (CVD).1 Lipid‐lowering therapy (LLT), including statins and ezetimibe, proved to be very effective in lowering CVD events.2 Cardiovascular prevention guidelines provide recommendations for optimal LDL‐C levels for patients at high risk of CVD.3 However, despite maximum tolerated LLT, only a minority of these patients reach the desired LDL‐C targets.4
Proprotein convertase subtilisin/kexin 9 (PCSK9) has been identified as a new player within the lipid metabolism.5 PCSK9 binds to the LDL‐receptor and promotes its lysosomal degradation, hence increasing LDL‐C plasma levels.6 People with hereditary high PCSK9 levels have a higher risk of cardiovascular events, whereas the opposite is true for people with hereditary low PCSK9 compared to the general population.7
Monoclonal antibodies binding PCSK9 have been successfully developed as a new class of LLT.6 In 2015, two PCSK9 inhibitors (alirocumab and evolocumab) have been approved by the US Food and Drug Administration and European Medicines Agency. In multiple large randomized placebo‐controlled trials, PCSK9 inhibitors showed reductions of LDL‐C levels up to 60% and recently also a decrease in cardiovascular outcomes.8, 9
According to current literature, alirocumab and evolocumab have a favorable safety profile and are generally well tolerated.10 However, real‐word data on adverse events (AEs) of PCSK9 inhibitors prescribed outside clinical trials is scarce. At present, real‐world observational studies are considered indispensable to provide complementary information to randomized clinical trials (RCTs), especially about AEs, as these studies involve a large and diverse population of patients in a real‐world setting beyond the rather homogenous group of patients who participated in RCTs. This allows for detection of rare complications, long‐term effects, and reactions occurring in specific subgroups of patients.11
The aim of this study is to assess the main AEs reported by patients using PCSK9 inhibitors alirocumab and evolocumab in clinical practice.
Results
Baseline characteristics per database are shown in Table 1 and Tables S1–S3 for data on alirocumab and evolocumab separately.
Table 1.
Patient characteristics
| Characteristics | EMC, n = 164a | Lareb, n = 149 | VigiLyze, n = 15,554 |
|---|---|---|---|
| Age (year), median (IQR) | 58 (48–65) | 63 (56–69) | |
| Age groups, n (%) | |||
| 0–17 years | 0 (0) | 0 (0.0) | 8 (0.1) d |
| 18–44 years | 24 (15) | 5 (3.4) | 289 (1.9) |
| 45–64 years | 91 (56) | 71 (47.7) | 4,210 (27.1) |
| 65–74 years | 46 (28) | 56 (37.6) | 4,835 (31.1) |
| ≥ 75 years | 3 (2) | 8 (5.4) | 2,670 (17.2) |
| Age unknown, n (%) | 0 (0) | 9 (6) c | 3,542 (22.8) |
| Gender, n (%) | |||
| Male | 90 (55) | 70 (47) | 5,975 (38.4) |
| Female | 74 (45) | 78 (52) | 8,772 (56.4) |
| Unknown | 0 (0) | 1 (1) | 807 (5.2) |
| BMI (kg/m2), median (IQR) | 27.4 (24.4–30.2) | ||
| Diabetes, n (%) | 29 (18) | ||
| Hypertension, n (%) | 75 (46) | ||
| Ever smoker, n (%) | 78 (48) | ||
| Current smoker, n (%) | 24 (15) | ||
| History of CVD, n (%) | 108 (66) | ||
| Familial hypercholesterolemia, n (%) | 148 (90) | ||
| Heterozygous | 98 (60) | ||
| Homozygousb | 7 (4) | ||
| Clinical | 43 (26) | ||
| Lipid‐lowering therapy, n (%) | |||
| Statin use | 100 (61) | ||
| High intensity | 63 (38) | ||
| Moderate intensity | 30 (18) | ||
| Low intensity | 7 (4) | ||
| Ezetimibe | 164 (100) | ||
| Ezetimibe monotherapy | 64 (39) | ||
| LDL‐C (mmol/L), median (IQR) | 4.28 (3.34–5.14) | ||
BMI, body mass index; CVD, cardiovascular disease; EMC, Erasmus Medical Centre; IQR, interquartile range; LDL‐C, low‐density lipoprotein‐cholesterol.
aBaseline characteristics before starting proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitor. bDouble heterozygous LDLR/APOB gene mutation (n = 1), compound heterozygous LDLR gene mutation (n = 6). cOne patient was reported to be 114 years old, which was put as missing value. dThese reports are considered either incorrect or due to off‐label use.
Erasmus Medical Centre hospital registry
Of the 183 patients registered in the database, 19 were excluded; 10 lacked follow‐up data, 8 participated in PCSK9 trials, and 1 patient had missing baseline data. Therefore, 164 patients were included in the current analysis (Table 1 ). The median age was 58 years (interquartile range (IQR) = 48–65 years), 55% were men. The majority of patients (90%) was diagnosed with familial hypercholesterolemia (FH). Sixty‐six percent of the patients had a history of CVD. All patients used LLT, however, more than a third of the patients (39%) used ezetimibe monotherapy because of statin intolerance. Baseline median LDL‐C was 4.28 mmol/l (166 mg/dL). Alirocumab and evolocumab were used by 49.4% and 50.6% of the patients, respectively. There was no difference between baseline characteristics of patients using alirocumab and evolocumab (Table S1 ).
Lareb database
Of the 152 patients in the database, 149 patients were included in the analysis (Table 1 ). Main reasons for exclusion were AEs not related to PCSK9 inhibitors (Figure S1 ). The median age was 63 years (IQR = 56–69 years) and 52% were women. Forty‐three patients used alirocumab and 110 evolocumab (Table S2 ). Age and gender distribution was similar between both groups.
VigiLyze database
The VigiLyze database contained 15,554 patients of AEs associated with PCSK9 inhibitors. Most patients (31.1%) were 65–74 years, 56.4% were women. A total of 4,650 patients used alirocumab and 10,931 evolocumab (Table S3 ). Age and gender distribution was similar between both groups.
Adverse events
Most common AEs are presented in Table 2 and Tables S4–S6 for data on alirocumab and evolocumab separately.
Table 2.
Overall frequencies of adverse events for both PCSK9 inhibitors with gender differences
| ERASMUS MC | Total (n = 68a) | OR (95% CI) male vs. female | Lareb | Total (n = 149) | OR (95% CI) male vs. female | VigiLyze | Total (n = 15,554) | OR (95% CI) male vs. female |
|---|---|---|---|---|---|---|---|---|
| Any TEAE, n (%) | 68 (100.0) | 0.58 (0.31–1.09)b | Any TEAE, n (%) | 149 (100.0) | Any TEAE, n (%) | 15,554 (100.0) | ||
| 1 event | 37 (54.4) | 1 event | 51 (34.2) | |||||
| 2 events | 21 (30.9) | 2 events | 41 (27.5) | |||||
| ≥3 events | 10 (14.7) | ≥ 3 events | 61 (38.3) | |||||
| Events, median (IQR) | 1.0 (1.0–2.0) | Events, median (IQR) | 2.0 (1.0–3.0) | |||||
| Total no. of TEAEs reported | 116 | Total no. of TEAEs reported | 375 | Total no. of TEAEs reported | 29,956 | |||
| TEAEs leading to discontinuation | 11 (16.2) | TEAEs leading to discontinuation | 60 (40.3) | TEAEs leading to discontinuation | N/A | |||
| TEAEs leading to death | 0 (0.0) | TEAEs leading to death | 1 (0.7) | TEAEs leading to death | N/A | |||
| Most common (≥4%) TEAEs, n (%) | Most common (≥4%) TEAEs, n (%) | Most common (≥4%) TEAEs, n (%) | ||||||
| Influenza‐like illness | 19 (27.9) | 0.56 (0.19–1.66) | Myalgia | 19 (12.8) | 1.63 (0.62–4.32) | Myalgia | 1,287 (8.3) | 1.11 (0.99–1.25) |
| Injection‐site hematoma | 13 (19.1) | 0.43 (0.12–1.56) | Influenza like illness | 14 (9.4) | 2.15 (0.69–6.77) | Drug dose omission | 1,151 (7.4) | 0.87 (0.77–0.99) |
| Nasopharyngitis | 11 (16.2) | 0.52 (0.16–2.25) | Fatigue | 12 (8.1) | 1.13 (0.35–3.67) | Injection‐site pain | 959 (6.2) | 0.55 (0.48–0.65) |
| Abdominal discomfort | 8 (11.8) | 2.04 (0.45–9.31) | Headache | 12 (8.1) | 0.20 (0.04–0.95) | Influenza like illness | 818 (5.3) | 1.06 (0.91–1.23) |
| Myalgia | 7 (10.3) | 0.41 (0.07–2.30) | Arthralgia | 10 (6.7) | 1.73 (0.47–6.42) | Back pain | 816 (5.2) | 0.95 (0.82–1.09) |
| Cognitive disorder | 6 (8.8) | 2.43 (0.41–14.25) | Dyspnea | 9 (6.0) | 0.13 (0.02–1.04) | Arthralgia | 789 (5.1) | 1.01 (0.87–1.17) |
| Fatigue | 6 (8.8) | 2.43 (0.41–14.25) | Nausea | 9 (6.0) | 0.54 (0.13–2.24) | Fatigue | 764 (4.9) | 0.92 (0.79–1.06) |
| Headache | 6 (8.8) | 0.53 (0.09–3.13) | Malaise | 8 (5.4) | 0.35 (0.07–1.81) | Pain in extremity | 755 (4.9) | 0.77 (0.66–0.90) |
| Injection‐site pain | 6 (8.8) | 1.14 (0.21–6.08) | Muscle spasms | 8 (5.4) | 0.65 (0.15–2.84) | Muscle spasms | 719 (4.6) | 0.81 (0.69–0.95) |
| Injection‐site swelling | 6 (8.8) | 2.43 (0.41–14.25) | Pain in extremity | 8 (5.4) | 0.35 (0.07–1.81) | Pain | 703 (4.5) | 0.66 (0.56–0.78) |
| Rash | 4 (5.9) | 0.36 (0.04–3.60) | Diarrhea | 6 (4.0) | 0.54 (0.10–3.07) | Headache | 651 (4.2) | 0.72 (0.61–0.86) |
| Dizziness | 6 (4.0) | 0.54 (0.10–3.07) | ||||||
| Injection‐site reactions, n (%) | 23(33.8) | 0.62 (0.22–1.71) | Injection‐site reactions, n (%) | 3 (2.0) | 2.27 (0.20–25.53) | Injection‐site reactions (≥ 1.0%), n (%) | 3,291 (21.2) | 0.55 (0.50–0.60) |
| Injection‐site hematoma | 13 (19.1) | 0.43 (0.12–1.56) | Injection‐site hematoma | 1 (0.7) | Injection‐site pain | 959 (6.2) | 0.55 (0.48–0.65) | |
| Injection‐site pain | 6 (8.8) | 1.14 (0.21–6.08) | Injection‐site hemorrhage | 1 (0.7) | Injection‐site bruising | 526 (3.4) | 0.56 (0.46–0.67) | |
| Injection‐site swelling | 6 (8.8) | 2.43 (0.41–14.25) | Injection‐site swelling | 1 (0.7) | Injection‐site hemorrhage | 373 (2.4) | 0.72 (0.58–0.89) | |
| Injection‐site erythema | 2 (2.9) | 1.13 (0.07–18.8) | Injection‐site erythema | 268 (1.7) | 0.49 (0.37–0.65) | |||
| Injection‐site infection | 1 (1.5) | Injection‐site swelling | 229 (1.5) | 0.61 (0.45–0.81) | ||||
| Injection‐site pruritus | 152 (1.0) | 0.42 (0.29–0.62) |
95% CI, confidence interval; IQR, interquartile range; N/A, not applicable; OR, odds ratio; PCSK9, proprotein convertase subtilisin/kexin 9; TEAE, treatment‐emergent adverse event.
Significant results are set in bold.
aOnly patients with adverse events at follow‐up 1. Total patients n = 164. bORs calculated on total population of men and women with and without adverse events.
Erasmus Medical Centre hospital registry
At first follow‐up, 68 patients (41.5%) reported ≥1 AE. A total of 116 events were reported with most patients reporting one event (54.4%; Table 2 ). Most common AEs were influenza‐like illness (27.9%), nasopharyngitis (16.2%), abdominal discomfort (11.8%), and myalgia (10.3%). Twenty‐three patients (33.8%) reported ≥1 injection‐site reactions (ISRs), most commonly injection‐site hematoma. No significant gender differences in AEs were observed.
The percentage of patients reporting AEs were similar for the alirocumab group and the evolocumab group (43.2% and 39.8%, respectively; Table S4 ). Influenza‐like illness was the most reported AE for both alirocumab and evolocumab (28.6% and 27.3%, respectively). The AE profile did not significantly differ between alirocumab and evolocumab.
Lareb database
A total of 375 events were reported by 149 patients of whom 38% reported ≥3 AEs (Table 2 ). The most common reported AEs were myalgia (12.8%), influenza‐like illness (9.4%), fatigue (8.1%), and headache (8.1%). ISRs were infrequently reported by 2.0% of the patients.
No significant differences in gender were observed, except for men reporting headache less frequently than women (odds ratio (OR) = 0.20, 95% confidence interval (CI) = 0.04–0.95; P = 0.042). The overall AE profile for alirocumab and evolocumab was similar (Table S5 ).
VigiLyze database
A total of 29,956 AEs were collected, reported by 15,554 patients (Table 2 ). Most common documented AEs were myalgia (8.3%), influenza‐like illness (5.3%), back pain (5.2%), and arthralgia (5.1%). ISRs were frequently reported (21.2%), and were most often injection‐site pain (6.2%). Most AEs, including pain in extremity (P < 0.001), muscle spasms (P = 0.010), pain (P < 0.001), headache (P < 0.001), diarrhea (P = 0.002), and nausea (P < 0.001), and the overall total of ISRs (P < 0.001) were significantly more often reported by women than men. Drug dose omission (7.4%) was not considered a drug‐related AE that was relevant for this study.
Myalgia was the most reported AE for both alirocumab and evolocumab (9.4% and 7.8%, respectively; Table S6 ). Back pain was reported nearly three times as frequent for evolocumab (6.4%) compared to alirocumab (2.4%). ISRs were reported at a similar rate (21.6% vs. 21.3%).
Predictors and time course of AEs
In the Erasmus Medical Centre (EMC) hospital registry, we analyzed for possible predictors of AEs, such as gender, statin use, or a very low LDL‐C. Univariate logistic regression analyses did not show any significant predictors for AEs, in particular very low LDL‐C and statin intolerance (Table 3 ).
Table 3.
Univariate logistic regression of possible predictors of adverse events at follow‐up 1
| OR (95% CI) | P value | |
|---|---|---|
| Age | 0.98 (0.96–1.01) | 0.202 |
| Gender (male) | 0.58 (0.31–1.09) | 0.091 |
| BMI | 0.99 (0.92–1.07) | 0.740 |
| Hypertension (yes) | 1.34 (0.72–2.50) | 0.356 |
| Current smoker (yes) | 0.63 (0.25–1.57) | 0.320 |
| Diabetes (yes) | 1.00 (0.44–2.25) | 0.992 |
| History of CVD (yes) | 1.44 (0.74–2.80) | 0.283 |
| FH (yes) | 0.90 (0.32–2.56) | 0.845 |
| FH – Genetic mutation (yes) | 1.46 (0.70–3.04) | 0.318 |
| Statin use (yes) | 1.18 (0.62–2.23) | 0.618 |
| Statin intensity (high vs. low + mod) | 1.68 (0.73–3.88) | 0.225 |
| Statin intolerance | 0.85 (0.45–1.61) | 0.618 |
| LDL‐C at baseline | 1.05 (0.88–1.24) | 0.590 |
| LDL‐C at follow‐up 1 | 1.14 (0.94–1.38) | 0.187 |
| LDL‐C <0.5 mmol/La at follow‐up 1 | 1.83 (0.47–7.07) | 0.383 |
| PCSK9 inhibitor (EVO vs. ALI) | 0.87 (0.47–1.62) | 0.654 |
ALI, alirocumab; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; EVO, evolocumab; FH, familial hypercholesterolemia; LDL‐C, low‐density lipoprotein‐cholesterol; OR, odds ratio; PCSK9, proprotein convertase subtilisin/kexin 9.
0.5 mmol/L = 19.3 mg/dL.
To study the time course of reported AEs we compared the reported AEs at the first, second, and third follow‐up visits (Table 4 ). Of the 164 patients with a first follow‐up visit, 131 had a second follow‐up visit and 94 had a third follow‐up visit. Nearly 60% of patients who reported AEs at the first follow‐up visit also had AEs at the second follow‐up visit. Notably, the majority (74%) of these patients reported different AEs at the first and second follow‐up visits. For 40.4% of the patients with AEs at the first follow‐up visit, AEs resolved at the second follow‐up. However, 22.8% of the patients without AEs at the first follow‐up developed new AEs at the second follow‐up. Compared to the first follow‐up visit, AEs resolved in 71.1% at the third follow‐up.
Table 4.
Comparison of adverse events occurrence at follow‐up
| Follow‐up 2 (n = 131) | Follow‐up 3 (n = 94) | |||
|---|---|---|---|---|
| AEs | No AEs | AEs | No AEs | |
| Follow‐up 1 | ||||
| AEs |
31 59.6% |
21 40.4% |
11 28.9% |
27 71.1% |
| No AEs |
18 22.8% |
61 77.2% |
7 12.5% |
49 87.5% |
| Follow‐up 2 | ||||
| AEs | 1133.3% |
22 66.7% |
||
| No AEs | 711.5% |
54 88.5% |
||
AEs, adverse events.
Follow‐up (FU)1 vs. FU2: McNemar's P = 0.749; FU2 vs. FU3: McNemar's P = 0.009; FU1 vs. FU3: McNemar's P = 0.001.
Drug discontinuation
In the EMC hospital registry, 12 patients (7%), 5 patients using alirocumab and 7 evolocumab, discontinued PCSK9 inhibitor treatment; 11 because of AEs and 1 due to nonresponse. The majority of patients (67%) who stopped treatment were women and 42% reported ≥3 events. Twenty‐four AEs were reported by 11 patients, which were most often influenza‐like illness (50%), cognitive disorders (25%), abdominal discomfort (17%), fatigue (17%), and malaise (17%; Table S7 ). ISRs did not lead to discontinuation of drug therapy. Notably, most patients with these AEs continued treatment.
In the Lareb database, 60 patients (40%) discontinued treatment due to AEs; 12 patients (20%) were using alirocumab and 48 patients (80%) evolocumab. In line with the hospital registry, most patients discontinued treatment due to ≥3 AEs (57%) and ISRs were not associated with discontinuation (Table S7 ). For alirocumab, discontinuation was higher in women compared to men (67% vs. 33%), whereas the women to men ratio was similar for those who stopped evolocumab treatment. The main reason for drug discontinuation was myalgia; in patients with myalgia 100% were alirocumab users and 70% of evolocumab users discontinued therapy (Figure 1 ).
Figure 1.
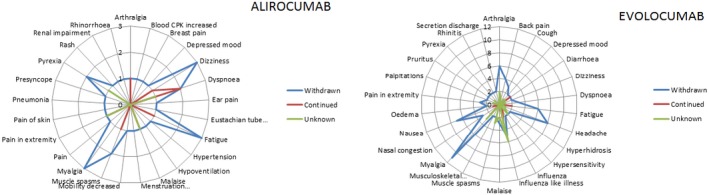
Drug action taken for alirocumab and evolocumab in Lareb database. Drug action taken per reported adverse event (AE) by patients who discontinued alirocumab (left) and evolocumab (right) in the Lareb database compared to patients who continued or for whom drug action was unknown. Axis shows number of patients who reported a specific AE. AEs with a frequency of n = 1 have been excluded for evolocumab to allow for better overview and visualization of the most common AEs.
Discussion
In this analysis, based on a hospital registry and two pharmacovigilance databases, the most reported AEs associated with PCSK9 inhibitors prescribed in a clinical setting were influenza‐like illness, nasopharyngitis, myalgia, and ISRs. There were no significant differences between alirocumab and evolocumab or between both genders. The AEs were usually mild and most AEs resolved during follow‐up. The rate of drug discontinuation was infrequent. No specific category of patients could be identified at increased risk of developing AEs.
Pharmacovigilance databases in general contain fewer reports of nonserious AEs or AEs not associated with drug discontinuation because of the threshold to report. Furthermore, it is known that women are at higher risk of developing AEs12 and more often report to pharmacovigilance centers compared to men.13 In our study, we found that women represented 52% of Lareb and 56% of VigiLyze reports. In the Netherlands, 42% of all patients using PCSK9 inhibitors were women14 leading to an AE report ratio of 1.23 supporting that women report AEs more often than men. This is in line with the women to men ratio of AE reports of 1.18 observed in the hospital registry.
RCTs assessing clinical effects of PCSK9 inhibitors showed a favorable safety profile with a low rate of AEs. The most common reported AEs are nasopharyngitis, upper respiratory tract infection, influenza‐like illness, myalgia, back pain, arthralgia, headache, and ISRs.10, 15, 16 Despite these findings, the application of PCSK9 inhibitors might be limited by their high costs, even in patients with FH. Hence, in daily clinical practice, cost and benefit need to be weighed against potential AEs.10, 15, 16Although meta‐analyses of published RCTs showed no significant difference in (serious) AE occurrence between patients receiving PCSK9 inhibitors and control,10, 17, 18 real‐world data are limited. Five studies published data of AEs associated with PCSK9 inhibitors in a limited number of patients.19, 20, 21, 22, 23 These previous studies described a similar set of AEs associated with PCSK9 inhibitors in 15–39% of the patients. The method of acquisition of AEs was often not described in detail. Only Saborowski et al.23 indicated that AEs were acquired by self‐reported questionnaires available in 31 of 38 patients (82%). We observed a higher (41.5%) incidence of AEs, which might be explained by the fact that patients were systematically questioned about AEs specifically attributed to PCSK9 inhibitors at every visit.
AEs related to monoclonal antibodies (mAbs) can be distinguished in nonspecific AEs and AEs specifically related to the mAb target, for example, infections with infliximab due to reduced activity of immune cells.24 The use of mAbs can lead to an immune response and even immunogenicity such as the development of neutralizing antibodies, depending on the type of mAb.24 The effect of a mAb on the immune system can range from immune suppression to immune stimulation leading to a wide variety of AEs.25 The cytokine‐mediated type alpha immune response is likely to be the main mechanism for common AEs associated with mAbs, such as flu‐like symptoms and ISRs.26
Alirocumab and evolocumab are fully humanized, which substantially reduces the risk of immunogenicity.24 The use of PCSK9 inhibitors is not related to target‐specific AEs. Very low LDL‐C levels achieved with PCSK9 inhibitors were not significantly associated with an increase in overall AE rates27, 28 or neurocognitive AEs.29, 30 Moreover, levels of vitamin E, steroid, or gonadal hormones were not affected in patients using PCSK9 inhibitors even at very low LDL‐C levels of <0.4 mmol/L (15 mg/dL).31
In RCTs, among the most reported AEs were nasopharyngitis (5.9–12.2%) and influenza (2.1–7.3%) in patients randomized to PCSK9 inhibitors.10, 15, 16 In the EMC registry, influenza‐like illness and nasopharyngitis were reported by 27.9% and 16.2%, respectively, and often resolved during follow‐up. Influenza‐like illness was also among the most frequent reported reactions in both the Lareb and VigiLyze databases.
ISRs were reported by 3.1–7.4% of patients randomized to PCSK9 inhibitors and in general occurred more often in patients randomized to a PCSK9 inhibitor compared to placebo.10, 15, 16 Overall, ISRs in RCTs were mild and transient and did not lead to drug discontinuation. In the EMC registry, ISRs were reported more frequently (33.8%) compared to RCTs, and were also mild and did not lead to drug discontinuation. Remarkably, ISRs were reported much more frequently in the VigiLyze database than in the Lareb database, which might be explained by differences in the types of information source between different countries.
In RCTs, myalgia was reported by 3.5–7.2% of patients randomized to a PCSK9 inhibitor.10, 15, 16 No significant differences were observed in myalgia between PCSK9 inhibitor and comparator arm.10, 17, 18 In the EMC hospital registry, myalgia was reported more frequently, whereas in the Lareb and the VigiLyze databases it was the most commonly reported AE. In the Lareb database, myalgia was a major reason for drug discontinuation. The number of reports in these databases reflects the reporting behavior and not incidence on a suspected AE. We do not have a conclusive explanation for the differences concerning myalgia between the RCTs and the hospital registry. A possible pathogenic mechanism of the development of myalgia as a result of the use of a PCSK9 inhibitor is unclear.
Two meta‐analyses showed that the use of PCSK9 inhibitors was associated with a significantly increased incidence of neurocognitive AEs compared to controls.32, 33 These results raised initial concern about the effect of PCSK9 inhibitors and led to a recommendation of the US Food and Drug Administration in 2014 to perform a long‐term trial prospectively evaluating neurocognitive function.34 The evaluating PCSK9 binding antibody influence on cognitive health in high cardiovascular risk subjects (EBBINGHAUS) study prospectively assessed cognitive function using formal tests and showed no effect on cognitive function in patients randomized to evolocumab compared to placebo.29 Moreover, no significant differences in neurocognitive AE rates were found between alirocumab vs. controls.30 Finally, a recent meta‐analysis, including a larger number of trials, showed no significant differences between the PCSK9 inhibitor and the control arm on neurocognitive outcomes.
In our study, cognitive disorders were reported more frequently (3.7%) than in RCTs and were one of the main reasons for drug discontinuation. An explanation could be that, in contrast to the placebo‐controlled trials, patients were unblinded to treatment and were aware of possible negative cognitive effects attributed to PCSK9 inhibitors via the media. Cognitive AEs reported were mainly mental dullness and forgetfulness and were nonspecific. No formal screening tools have been used in these patients.
Until now, it is not known whether the reported AEs are caused by the PCSK9 inhibition or are specific for the monoclonal antibody that is administered. A novel PCSK9‐based therapy is inclisiran, a small interfering RNA that inhibits translation of the PCSK9 protein. A phase II trial showed a maximum LDL‐C decrease of 41.9% after a single‐dose of 500 mg and 52.6% after two doses of 500 mg.35 The most common AEs of inclisiran were comparable to PCSK9 mAbs, namely myalgia, headache, fatigue, nasopharyngitis, back pain, hypertension, diarrhea, and dizziness. The overall AE rate in the patients who received inclisiran was similar to the patients who received placebo.10, 15, 35
To our knowledge, this is the largest in‐depth study of AEs of PCSK9 inhibitors prescribed in a clinical setting to date. Our study has several strengths. First, in the EMC hospital registry, the treating healthcare professionals enquired about AEs at every visit and only AEs considered to be directly related to the use of the PCSK9 inhibitor were included. Therefore, we consider that reporting bias is prevented as much as possible. We provide data up to a follow‐up duration of 42 weeks, which provides insight on how AEs develop over time. As we combined different data sources utilizing different methods of AE monitoring, a complete overview of possible AEs is provided. General limitations of using real‐world data, such as selection bias, confounding, the lack of a control group, and variable physician‐scheduled appointments, also apply for our study.11 Specific limitations are that the power of the EMC hospital registry was too low to detect significant differences in AEs between genders. A limitation of the Lareb and VigiLyze data was that the amount of available information varied between cases. Moreover, except for the VigiLyze data, this is a single‐country study and experience with these agents is limited. In conclusion, in a real‐world setting, PCSK9 inhibitors are well tolerated. Most common AEs are influenza‐like illness, nasopharyngitis, myalgia, and ISRs, which often resolve over time. ISRs are mild and do not lead to drug discontinuation. However, despite these findings, cost‐effectiveness has still to be taken in account regarding both patients with FH and those without.36 Long‐term safety monitoring of PCSK9 inhibitors prescribed in clinical practice to a diverse population is indispensable to discover new or rare AEs and to assess AE risk in specific subgroups. All healthcare professionals prescribing these medications should contribute to monitor AEs by reporting these to pharmacovigilance agencies and if possible by collecting long‐term data in a local, national, and ultimately an international database.
Methods
Patients and study design
Three different data sources were analyzed: (i) the EMC hospital registry; (ii) the Netherlands Pharmacovigilance Centre Lareb database37; and (iii) VigiLyze38 pharmacovigilance database maintained by the World Health Organization collaborating center for international drug monitoring Uppsala Monitoring Centre (UMC) in Sweden.
EMC hospital registry
Full details of this registry have been published before.20 Briefly, patients with hypercholesterolemia, mostly patients with FH not reaching target LDL‐C levels despite maximally tolerated statin and ezetimibe therapy who were eligible for treatment with PCSK9 inhibitors were recruited from the outpatient clinic in a tertiary university hospital setting. All patients fulfilled the Dutch criteria for reimbursement of PCSK9 inhibitors.39
Patients started with a PCSK9 inhibitor (evolocumab 140 mg subcutaneously every 2 weeks (heterozygous familial hypercholesterolemia) or 420 mg subcutaneously every 2 weeks (homozygous familial hypercholesterolemia) or alirocumab 75, or 150 mg subcutaneously every 2 weeks (heterozygous familial hypercholesterolemia)) between June 2015 and November 2017 as part of clinical care. There was no preference for alirocumab or evolocumab. All patients had at least two PCSK9 inhibitor subcutaneous injections between baseline and on‐treatment measurements. Only patients of whom at least one follow‐up visit was available were included in the analysis. Patients who participated in a PCSK9 trial were excluded from analysis.
Baseline date was defined as the date when the first injection of the PCSK9 inhibitor was administered. Routine laboratory investigations were performed before and after start of PCSK9 inhibitor to monitor treatment effects. Patients had regular appointments for follow‐up at 6 weeks, 18 weeks, and 42 weeks at the outpatient clinic. AEs, including ISRs and adherence to LLT, were systematically discussed during each consultation. Only PCSK9 inhibitor‐related AEs were included, which were defined as AEs not present prior to the start of PCSK9 inhibitor therapy, or an already present symptom that changed or worsened following treatment. AEs were classified using Medical Dictionary for Regulatory Activities terms. One patient may report multiple events. Clinical data, such as age, gender, body mass index (BMI), diabetes mellitus, hypertension, history of CVD, familial hypercholesterolemia, LLT, laboratory values, and AEs were collected from patients’ files and entered into a database.
According to the Medical Ethical Research Committee, this study (MEC‐2016‐698) was not subject to the Medical Research involving Human Subjects Act. We only used data of patients who provided written consent for research and anonymous publication of their clinical information.
Lareb database
The Netherlands Pharmacovigilance Centre Lareb37 identifies risks associated with the use of drugs in daily practice in the Netherlands. This database contains individual case safety reports of suspected AEs reported by healthcare professionals, manufacturers, patients, or others. The submitted reports are reviewed case‐by‐case by Lareb and AEs are defined with the use of Medical Dictionary for Regulatory Activities terms. One single report may refer to multiple AEs. It must be emphasized that the number of reports reflects reporting behavior and not the incidence of a reaction. Furthermore, it must be noted that the likelihood of a causal relationship can differ between cases, because the aim is to collect all reports of suspicions of AEs and reports may be incomplete concerning the provided information. The database was accessed on September 26, 2017, and contained all AE reports for alirocumab and evolocumab since its inception.
VigiLyze database
VigiLyze38 contains individual case safety reports of suspected AEs collected by national drug authorities in over 110 countries, including Lareb data. Similar to the Lareb database, one single report may refer to multiple AEs, the number of reports reflects reporting behavior and not the incidence of a reaction, and causality is not ensured. Collection of data is heterogeneous between countries, due to, for example, differing national legislation and policies. The database was accessed on November 21, 2017, and contained the dataset from inception to November 19, 2017, of all AE reports on alirocumab and evolocumab.
Statistical analysis
Dichotomous variables are reported as numbers and percentages. Continuous variables are presented as mean ± SD or median and IQR. Normality of data was assessed by visually exploring the distribution in normal plots, checking for skewness, and using The Shapiro‐Wilk test. Differences between categorical variables were evaluated using the χ2 or Fisher's exact test as appropriate. Differences between numeric variables were evaluated using The Student's t test or Mann‐Whitney U test as appropriate. Gender differences were assessed using ORs, which were obtained using binary logistic regression. Covariates were analyzed using univariate logistic regression to determine possible predictors. McNemar's test was performed to assess asymmetry in the distribution of AE occurrence during follow‐up. For all tests, a P value < 0.05 was considered statistically significant. Data were analyzed using IBM SPSS Statistics for Windows, version 21. When individual cases were not available for analysis, SAS Statistics version 9.4 was used to obtain ORs from counts.
Disclaimer
The authors are indebted to the national pharmacovigilance centers that contributed data to the worldwide database, maintained by the World Health Organization collaborating center for international drug monitoring UMC in Sweden. The opinions and conclusions, however, are not those of the various centers, or of the UMC in Sweden. The information originates from a variety of sources, and the likelihood that the suspected AEs are drug‐related can vary between cases.
Funding
No funding was received for this work.
Conflict of interest
J.E. Roeters van Lennep reports personal fees from AKCEA, grants from AMRYT, paid to the institution, outside the submitted work. A.M.H. Galema‐Boers reports personal fees from Sanofi‐Aventis Netherlands B.V. for publication of her thesis and Amgen for presentation at congress, outside the submitted work. All other authors declared that there is no conflict of interest regarding the publication of this article.
Author contributions
M.T.G., and J.E.R. wrote the manuscript; M.T.G., A.H.G.M., M.M.S., J.M.H.G., H.B., and J.E.R. made critical revisions to the manuscript; M.T.G., A.H.G.M., J.M.H.G., and J.E.R. designed the research; M.T.G., A.H.G.M., J.M.H.G., and J.E.R. performed the research; M.T.G., A.H.G.M., H.B., and J.E.R. analyzed the data.
Supporting information
Figure S1. Flowchart of patient inclusion and exclusion for the Lareb database.
Table S1. Baseline patient characteristics for EMC database split by drug.
Table S2. Patient characteristics for Lareb database split by drug.
Table S3. Patient characteristics for VigiLyze database split by drug.
Table S4. AEs split by PCSK9 inhibitor for EMC database.
Table S5. Adverse events split by PCSK9 inhibitor for Lareb database.
Table S6. Adverse events split by PCSK9 inhibitor for VigiLyze database.
Table S7. Drug discontinuation.
Acknowledgments
The authors would like to thank K.A. Steward for carefully scanning through patients’ files and data entry in the EMC hospital registry. All authors approved the final version of the submitted manuscript.
[The copyright line for this article was changed on August 16, 2019 after original online publication.]
References
- 1. Baigent, C. et al Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–1278 (2005). [DOI] [PubMed] [Google Scholar]
- 2. Cannon, C.P. et al Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397 (2015). [DOI] [PubMed] [Google Scholar]
- 3. Landmesser, U. et al 2017 Update of ESC/EAS Task Force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur. Heart J. 39, 1131–1143 (2018). [DOI] [PubMed] [Google Scholar]
- 4. Rallidis, L.S. & Lekakis, J. PCSK9 inhibition as an emerging lipid lowering therapy: unanswered questions. Hellenic J. Cardiol. 57, 86–91 (2016). [DOI] [PubMed] [Google Scholar]
- 5. Abifadel, M. et al Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 (2003). [DOI] [PubMed] [Google Scholar]
- 6. Gencer, B. , Lambert, G. & Mach, F. PCSK9 inhibitors. Swiss Med. Wkly. 145, w14094 (2015). [DOI] [PubMed] [Google Scholar]
- 7. Vlachopoulos, C. et al Prediction of cardiovascular events with levels of proprotein convertase subtilisin/kexin type 9: a systematic review and meta‐analysis. Atherosclerosis 252, 50–60 (2016). [DOI] [PubMed] [Google Scholar]
- 8. Robinson, J.G. et al Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372, 1489–1499 (2015). [DOI] [PubMed] [Google Scholar]
- 9. Sabatine, M.S. et al Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017). [DOI] [PubMed] [Google Scholar]
- 10. Zhang, X.L. et al Safety and efficacy of anti‐PCSK9 antibodies: a meta‐analysis of 25 randomized, controlled trials. BMC Med. 13, 123 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nallamothu, B.K. , Hayward, R.A. & Bates, E.R. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation 118, 1294–1303 (2008). [DOI] [PubMed] [Google Scholar]
- 12. Martin, R.M. , Biswas, P.N. , Freemantle, S.N. , Pearce, G.L. & Mann, R.D. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br. J. Clin. Pharmacol. 46, 505–511 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Montastruc, J.L. , Lapeyre‐Mestre, M. , Bagheri, H. & Fooladi, A. Gender differences in adverse drug reactions: analysis of spontaneous reports to a Regional Pharmacovigilance Centre in France. Fundam. Clin. Pharmacol. 16, 343–346 (2002). [DOI] [PubMed] [Google Scholar]
- 14. Zorginstituut Nederland . GIP databank. (version date: 2017). <https://www.gipdatabank.nl> (2017). Accessed 29 November 2017.
- 15. Jones, P.H. et al Safety of alirocumab (a PCSK9 monoclonal antibody) from 14 randomized trials. Am. J. Cardiol. 118, 1805–1811 (2016). [DOI] [PubMed] [Google Scholar]
- 16. Toth, P.P. et al Pooled safety analysis of evolocumab in over 6000 patients from double‐blind and open‐label extension studies. Circulation 135, 1819–1831 (2017). [DOI] [PubMed] [Google Scholar]
- 17. Karatasakis, A. et al Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J. Am. Heart Assoc. 6, pii: e006910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schmidt, A.F. , Pearce, L.S. , Wilkins, J.T. , Overington, J.P. , Hingorani, A.D. & Casas, J.P. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. (4), CD011748 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi, J. , Khan, A.M. , Jarmin, M. , Goldenberg, N. , Glueck, C.J. & Wang, P. Efficacy and safety of proprotein convertase subtilisin‐kexin type 9 (PCSK9) inhibitors, alirocumab and evolocumab, a post‐commercialization study. Lipids Health Dis. 16, 141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galema‐Boers, A.M.H. , Lenzen, M.J. , Sijbrands, E.J. & Roeters van Lennep, J.E. Proprotein convertase subtilisin/kexin 9 inhibition in patients with familial hypercholesterolemia: initial clinical experience. J. Clin. Lipidol. 11, 674–681 (2017). [DOI] [PubMed] [Google Scholar]
- 21. Kohli, M. et al Pro‐protein subtilisin kexin‐9 (PCSK9) inhibition in practice: lipid clinic experience in 2 contrasting UK centres. Int. J. Clin. Pract. 71, e13032 (2017). [DOI] [PubMed] [Google Scholar]
- 22. Pandey, A.S. , Bajaj, H.S. , Garg, V. , Pandey, A. & Verma, S. The emerging role of proprotein convertase subtilisin/kexin type‐9 inhibition in secondary prevention: from clinical trials to real‐world experience. Curr. Opin. Cardiol. 32, 633–641 (2017). [DOI] [PubMed] [Google Scholar]
- 23. Saborowski, M. , Dolle, M. , Manns, M.P. , Leitolf, H. & Zender, S. Lipid‐lowering therapy with PCSK9‐inhibitors in the management of cardiovascular high‐risk patients: effectiveness, therapy adherence and safety in a real world cohort. Cardiol. J., 25, 32–41 (2017). [DOI] [PubMed] [Google Scholar]
- 24. Catapano, A.L. & Papadopoulos, N. The safety of therapeutic monoclonal antibodies: implications for cardiovascular disease and targeting the PCSK9 pathway. Atherosclerosis 228, 18–28 (2013). [DOI] [PubMed] [Google Scholar]
- 25. Descotes, J. Immunotoxicity of monoclonal antibodies. MAbs 1, 104–111 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scherer, K. , Spoerl, D. & Bircher, A.J. Adverse drug reactions to biologics. J. Dtsch. Dermatol. Ges. 8, 411–426 (2010). [DOI] [PubMed] [Google Scholar]
- 27. Giugliano, R.P. et al Clinical efficacy and safety of achieving very low LDL‐cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet, 390, 1962–1971 (2017). [DOI] [PubMed] [Google Scholar]
- 28. Robinson, J.G. et al Safety of very low low‐density lipoprotein cholesterol levels with alirocumab: pooled data from randomized trials. J. Am. Coll. Cardiol. 69, 471–482 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Giugliano, R.P. et al Cognitive function in a randomized trial of evolocumab. N. Engl. J. Med. 377, 633–643 (2017). [DOI] [PubMed] [Google Scholar]
- 30. Harvey, P.D. et al No evidence of neurocognitive adverse events associated with alirocumab treatment in 3340 patients from 14 randomized Phase 2 and 3 controlled trials: a meta‐analysis of individual patient data. Eur. Heart J., 39, 374–381 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blom, D.J. et al Effects of evolocumab on vitamin E and steroid hormone levels: results from the 52‐week, phase 3, double‐blind, randomized, placebo‐controlled DESCARTES study. Circ. Res. 117, 731–741 (2015). [DOI] [PubMed] [Google Scholar]
- 32. Khan, A.R. et al Increased risk of adverse neurocognitive outcomes with proprotein convertase subtilisin‐kexin type 9 inhibitors. Circ. Cardiovasc. Qual. Outcomes 10, e003153 (2017). [DOI] [PubMed] [Google Scholar]
- 33. Lipinski, M.J. et al The impact of proprotein convertase subtilisin‐kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta‐analysis. Eur. Heart J. 37, 536–545 (2016). [DOI] [PubMed] [Google Scholar]
- 34. U. S. Food and Drug Administration . Summary review: BLA# 125559. <https://www.fda.gov/> (2014).
- 35. Ray, K.K. et al Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 376, 1430–1440 (2017). [DOI] [PubMed] [Google Scholar]
- 36. Arrieta, A. , Hong, J.C. , Khera, R. , Virani, S.S. , Krumholz, H.M. & Nasir, K. Updated cost‐effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the FOURIER trial. JAMA Cardiol. 2, 1369–1374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. The Netherlands pharmacovigilance centre Lareb database (version date: 2017). <www.lareb.nl> (2017). Accessed 26 September 2017.
- 38. WHO Global Individual Case Safety Reports database (VigiLyze). (version date: 2017). <https://tools.who-umc.org/webroot/> (access restricted). (2017). Accessed 21 November 2017.
- 39. Zorginstituut Nederland . GVS rapport. Farmaco‐Economisch rapport voor evolocumab (Repatha®) bij de behandeling van hypercholesterolemie 1‐111. <https://www.zorginstituutnederland.nl/publicaties/rapport/2015/12/14/evolocumabrepatha-bij-hypercholesterolemie-en-gemengde-dyslipidemiehomozygotefamiliaire-hypercholesterolemie> (2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flowchart of patient inclusion and exclusion for the Lareb database.
Table S1. Baseline patient characteristics for EMC database split by drug.
Table S2. Patient characteristics for Lareb database split by drug.
Table S3. Patient characteristics for VigiLyze database split by drug.
Table S4. AEs split by PCSK9 inhibitor for EMC database.
Table S5. Adverse events split by PCSK9 inhibitor for Lareb database.
Table S6. Adverse events split by PCSK9 inhibitor for VigiLyze database.
Table S7. Drug discontinuation.


