Abstract
Aim
To review the knowledge on the mechanisms controlling membrane–host interactions in guided bone regeneration (GBR) and investigate the possible role of GBR membranes as bioactive compartments in addition to their established role as barriers.
Materials and Methods
A narrative review was utilized based on in vitro, in vivo and available clinical studies on the cellular and molecular mechanisms underlying GBR and the possible bioactive role of membranes.
Results
Emerging data demonstrate that the membrane contributes bioactively to the regeneration of underlying defects. The cellular and molecular activities in the membrane are intimately linked to the promoted bone regeneration in the underlying defect. Along with the native bioactivity of GBR membranes, incorporating growth factors and cells in membranes or with graft materials may augment the regenerative processes in underlying defects.
Conclusion
In parallel with its barrier function, the membrane plays an active role in hosting and modulating the molecular activities of the membrane‐associated cells during GBR. The biological events in the membrane are linked to the bone regenerative and remodelling processes in the underlying defect. Furthermore, the bone‐promoting environments in the two compartments can likely be boosted by strategies targeting both material aspects of the membrane and host tissue responses.
1.
Clinical Relevance.
Scientific rationale for the study: The GBR membrane has been mainly considered as a passive barrier, whereas the potential bioactive role has scarcely been documented.
Principal findings: The biological events in both the membrane and the underlying defect are important for bone regeneration. The membrane has direct bone promotive effects, by virtue of hosting cells that express and secrete pro‐osteogenic and bone‐promoting factors, which are linked to the bone regeneration and restitution of the underlying defect. This bioactive effect has also been shown with cell and molecules intentionally incorporated in the membrane and/or in the underlying defect, with or without the bone grafting materials.
Practical implications: The evolving knowledge of the bioactive role of GBR membranes in modulating cellular and molecular events in membrane‐associated cells and adjacent regenerating tissues underpins the development of a new generation of GBR membranes. Further optimization of membrane properties is proposed, focusing on improving membrane bioactivity via well‐selected and scientifically documented biological cues, thereby developing novel GBR membranes suited for large oral bone defects and compromised cases.
2. INTRODUCTION
The original hypothesis of guided bone regeneration (GBR) was introduced almost three decades ago (Dahlin, Linde, Gottlow, & Nyman, 1988), implying that a non‐resorbable or biodegradable barrier could be placed to exclude certain cell types, such as rapidly proliferating epithelium and connective tissue, thus promoting the growth of slower‐growing cells capable of forming bone (Figure 1a). Hence, osteoprogenitors would be exclusively allowed to repopulate the bone defect site by preventing the entry of non‐osteogenic tissues (Dahlin et al., 1988; Dimitriou, Mataliotakis, Calori, & Giannoudis, 2012; Retzepi & Donos, 2010).
Figure 1.
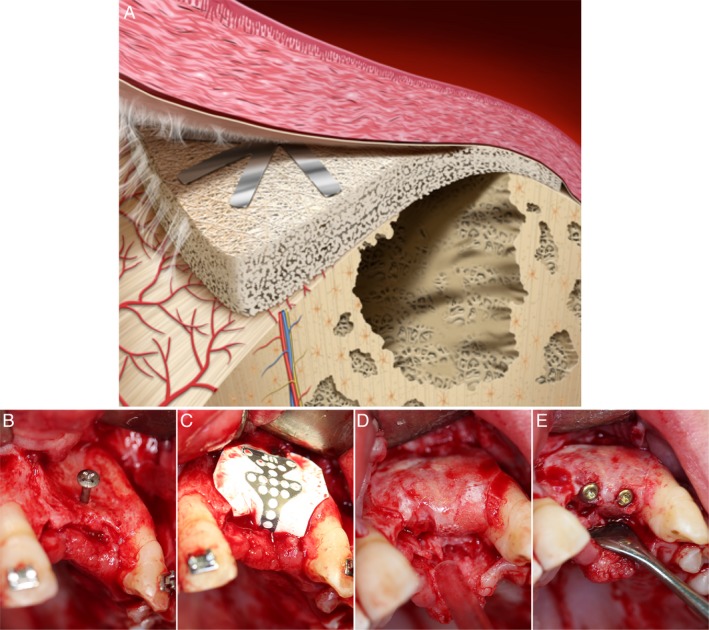
Schematic diagram and clinical photographs of guided bone regeneration. (a) Simple schematic diagram showing the membrane and defect compartments in the guided bone regeneration procedure. (b–e) Serial clinical photographs of a horizontal bone defect treated by guided bone regeneration using a barrier membrane (titanium‐reinforced polytetrafluoroethylene (PTFE)): (b) the anterior maxilla with predominant bone loss is exposed, and a titanium tenting screw is inserted. (c) The titanium‐reinforced PTFE membrane is positioned and stabilized above the defect area. (d) After 7 months, the membrane is removed, and the previous defect has now been filled with bone. (e) Two titanium implants are inserted in the regenerated region and are subsequently connected with abutments and restored with final crowns (Courtesy of Drs Miranda‐Burgos & Dahlin)
The early experimental studies on GBR were conducted using barrier membranes without the administration of bone substitute or grafting materials underneath the membrane (Dahlin, Sennerby, Lekholm, Linde, & Nyman, 1989; Dahlin et al., 1988; Schenk, Buser, Hardwick, & Dahlin, 1994). The advantage of the barrier membrane alone was then verified clinically, where bone fenestrations at titanium implants treated with PTFE membranes demonstrated higher amount of new bone formation compared to the control fenestration sites, left underneath the periosteum without membrane (Dahlin, Andersson, & Linde, 1991). Following the clinical introduction of the GBR concept, it has been realized that the addition of membrane‐supporting materials or grafts in combination with membranes may provide synergistic effects for the regenerative outcome (Donos, Mardas, & Chadha, 2008; Hermann & Buser, 1996). This has also been confirmed in multiple experimental studies (Dahlin, Alberius, & Linde, 1991; Stavropoulos, Dahlin, Ruskin, & Johansson, 2004). Nevertheless, some variable results have been obtained regarding the combined use of bone replacement grafts with the membrane when compared to the use of the membrane alone. The potential role of graft materials in conjunction with membrane placement will be discussed in this review.
Guided bone regeneration is considered the most clinically used (Khojasteh, Kheiri, Motamedian, & Khoshkam, 2017) and documented (Aghaloo & Moy, 2007) technique for local augmentation and defect restitution in the jaw bone in conjunction with oral implant treatment. Further, several reports have demonstrated that the survival rates of implants placed in the augmented sites by GBR are similar to those of implants placed into pristine bone (Clementini, Morlupi, Canullo, Agrestini, & Barlattani, 2012; Donos et al., 2008; Jensen & Terheyden, 2009). Moreover, data from a large cohort clinical report revealed that up to 40% of implant patients required the GBR procedure as part of the implant therapy (Bornstein, Halbritter, Harnisch, Weber, & Buser, 2008). In line with the clinical data, a considerable number of histological studies in different animal models show the promotion of bone formation in experimental defects treated with different types of GBR barrier membranes (Ahn, Kim, Kim, Oh, & Lim, 2012; Al‐Hezaimi et al., 2013; Benic et al., 2015; Bernabe et al., 2012; Busenlechner et al., 2005; Cho et al., 1998; De Marco, Jardini, Modolo, Nunes, & de Lima, 2012; Donos, Graziani, Mardas, & Kostopoulos, 2011; Donos, Kostopoulos, & Karring, 2002; Ge, Feng, & Wang, 2011; Guda et al., 2013; Jardini, De Marco, & Lima, 2005; Jung et al., 2013; Kim et al., 2008; Koerdt, Ristow, Wannhoff, Kubler, & Reuther, 2014; Kostopoulos & Karring, 1994; Lundgren, Lundgren, Sennerby, & Nyman, 1995; Melo, Nagata, Bosco, Ribeiro, & Leite, 2005; Queiroz, Hochuli‐Vieira, Gabrielli, & Cancian, 2006; Ramalingam et al., 2016; Schlegel, Donath, & Weida, 1998; Schwarz, Mihatovic, Golubovic, Hegewald, & Becker, 2012; Simion et al., 2007; Stetzer et al., 2002; Sverzut et al., 2008; Taga, Granjeiro, Cestari, & Taga, 2008; Thomaidis et al., 2008; Ueyama et al., 2002; Weng et al., 2009; Zubery, Goldlust, Alves, & Nir, 2007).
A variety of synthetic and naturally derived materials has been used clinically as GBR membranes (Table 1a). Further, several materials and material modifications, including metals and composites, have been experimentally investigated as potential GBR membranes (Elgali, Omar, Dahlin, & Thomsen, 2017). In addition, strategies to incorporate biological substances, cells and antibacterial agents, into the membrane or into the defect under the membrane, have been explored. During the course of GBR evolvement, a set of requirements for the membrane has been defined (Dahlin, 2010): (a) Biocompatibility: the material shall perform with an appropriate tissue response (Williams, 2017). Thus, the interaction between the material and the tissues should not adversely affect the surrounding tissues, the intended healing result or patient safety; (b) Occlusive properties: the material should prevent soft tissue invasion and provide some degree of protection from bacterial invasion if the membrane becomes exposed to the oral environment; (c) Space‐making capacity: the membrane should provide a suitable space in which the regeneration of bone can take place; (d) Attachment to or integration with the surrounding tissues: the integration of the membrane with the tissues stabilizes the wound healing environment and further contributes to the creation of a barrier between the soft tissue and the bone defect; (e) Manageability: the membrane must be clinically manageable. Over time, the search for the optimal set of properties has led to the development of GBR membranes that possess several of the characteristics listed above. A summary of the membrane classifications and material compositions is found in Table 1b. It is beyond the scope of this article to review the classes of membrane materials, the modifications of membrane physicochemical properties and the documented biological effects. Instead, for these topics, the reader is referred to a recent review (Elgali et al., 2017). To date, the choice of membrane has been dictated by the defect anatomy, the need for augmentation in horizontal or vertical directions, and the use of a predictable clinical protocol (Donos et al., 2008) (Figure 1b–e).
Table 1.
Membranes for guided bone regeneration
| a) Examples of clinically used membranes, presented according to resorbability, material and commercial name | |||
|---|---|---|---|
| Class | Material | Descriptiona | Examples of clinically used membranes |
| Non‐resorbable, synthetic | Polytetrafluorethylene (PTFE) | Expanded PTFE | Gore‐Tex® |
| Dense PTFE | Cytoplast™ TXT‐200 | ||
| Dual textured expanded PTFE | NeoGen® | ||
| Titanium‐reinforced PTFE | Gore‐Tex‐Ti; Cytoplast™ Ti‐250; NeoGen® Ti‐Reinforced | ||
| Titanium | Titanium mesh | Frios® BoneShields; Ridge‐Form Mesh™ | |
| Resorbable, naturally derived | Non‐cross‐linked collagen | Type I collagen | CollaTape®; Tutodent® |
| Type I and III collagen | BioGide®; botiss Jason® | ||
| Type I, III, IV, VI and other proteins | DynaMatrix® | ||
| Collagen with intermingled elastin | Creos xenoprotect™ | ||
| Cross‐linked collagen | Cross‐linked type I collagen | BioMend®; OSSIX® PLUS; OsseoGuard® | |
| Cross‐linked type I and type III | OsseoGuard Flex®; EZ Cure™; MatrixDerm™ EXT | ||
| Resorbable, synthetic | Aliphatic polyesters | Poly‐D, L‐lactide‐co‐glycolide | Resolut adapt® |
| D, D‐L, L polylactic acid | Epi‐Guide® | ||
| Poly‐D, L‐lactide and poly‐L‐lactide, blended with acetyl tri‐n‐butyl citrate | Guidor® | ||
| Polyglycolide, poly‐D, L‐lactide‐co‐glycosides, poly‐L‐lactide | BioMesh®‐S | ||
| b) Classes of materials used as GBR membranes in experimental studies | |||
|---|---|---|---|
| Synthetic polymers | Natural polymers | Metals | Inorganic compounds |
|
Collagen and extracellular matrices Chitosan Alginate | Titanium and titanium alloys Cobalt–chromium alloys | Calcium sulphate Calcium phosphate |
The information on the composition of the commercially available membranes is derived from the web pages of the producers. The degree of details and supporting information vary. Hence, the information should be treated carefully.
Despite the significant clinical use and documentation of GBR, which is supported by experimental histological evidence, the cellular and molecular mechanisms that govern the sequence of biological events during GBR and the possible bioactive role of the GBR membrane in modulating these events have been scarcely investigated. With the advent of cellular and molecular techniques, a correlative approach to deciphering the molecular and morphological basis of GBR has emerged. In a previous review focused on the membrane properties and their modifications, the cellular and molecular events of GBR have been highlighted (Elgali et al., 2017). In the present review, via a narrative approach, a comprehensive and up‐to‐date survey of the available scientific literature is mainly focused on the in vivo biological mechanisms related to GBR and the potential active role of the membrane. The aims of this review were (a) to summarize the current knowledge on the cellular and molecular processes of bone healing and regeneration in association with GBR, (b) to determine available evidence on the biological events in the membrane and their possible relationships with the biological processes in the underlying defect compartment, (c) to evaluate the role of the exogenous administration of biological cues (including growth factors and cells) to the membrane or bone grafts (in combination with membranes) to promote bone formation in the defect, and finally (d) to highlight potential strategies for future GBR optimization.
3. LITERATURE SEARCH AND INCLUSION CRITERIA
For this narrative review, the literature survey was conducted using the MEDLINE/PubMed electronic database, without limiting the years of publication. Only papers written in English were included. The search was restricted to in vitro, in vivo and human studies that reported data on GBR (and selected papers on guided tissue regeneration (GTR)). Papers published before October 2018 and relevant to the topic were included. Keywords based on MeSH terms as well as free text were used with the aim to determine published in vivo, in vitro and clinical studies that investigated cellular and molecular events during GBR and the possible bioactive role of the membrane. The following keywords were used in different combinations: “Guided bone regeneration,” “GBR,” “Guided tissue regeneration,” “GTR,” “Barrier,” “Active/Bioactive,” “Membrane,” “Materials,” “Polytetrafluoroethylene,” “PTFE,” “Collagen,” “Non‐resorbable,” “Resorbable,” “Synthetic,” “Natural,” “Naturally derived,” “Mechanisms,” “Cellular/molecular events,” “Cellular/molecular activities,” “Adherent cells,” “Associated cells,” “Membrane cells,” “Bone defect,” “In vivo,” In vitro,” “Gene expression,” “Immunohistochemistry,” “Histology,” “Histomorphometry,” “Cell recruitment,” “Inflammation,” “Bone formation,” “Bone remodeling/remodelling,” Cytokines,” “Growth factors,” “Osteoinductive” and “Drug delivery.” Since the focus of this narrative review is on the role of membranes during GBR, only selected studies on GTR were included (i.e. GTR studies that addressed cellular and molecular events in relation to the membrane).
4. RESULTS OF THE LITERATURE SURVEY
4.1. Cellular and molecular mechanisms during GBR
Most of the available information on bone healing and regeneration beneath a GBR membrane is based on histological studies. While the histological data are a fundamental proof of concept for this regenerative procedure, they do not fully reveal the sequential cellular and molecular cascades of cell recruitment, inflammation, bone formation and remodelling in membrane‐treated defects. Moreover, these data do not explain the exact role of the membrane in promoting the healing events within defect leading to the considerable filling of the membrane‐covered defect. Therefore, it is important to relate the membrane‐induced molecular patterns in the defect both to the recruitment and differentiation of different cell types involved in bone healing and regeneration and to the structural restitution of the membrane‐covered defect.
A pertinent question regards the biological role of the membrane in addition to its barrier function. Originally, the role of the membrane was hypothesized to be a physical barrier (Figure 2) for preventing the invasion of undesired soft tissue and providing and maintaining space for osteogenic cells to migrate and form bone in the defect (Dahlin et al., 1988; Retzepi & Donos, 2010). Further, histological observations suggested a biomechanical role for the non‐resorbable, relatively stiff, PTFE membrane in stabilizing and protecting the nascent clot and promoting vascularity and osteogenesis, especially in the central region of the membrane‐covered bone defect (Hammerle, Schmid, Lang, & Olah, 1995; Figure 2). An organized blood clot provides an intermediate tissue with appropriate mechanical properties necessary for osteogenesis (Hammerle et al., 1995). Further, notwithstanding that bone healing and regeneration is a complex process, it is plausible that the regenerative processes are influenced by the local microenvironment in the defect, of which the stability of the initially formed haematoma and blood clot may influence the migration of cells, vascularization and osteogenesis (Liu & Kerns, 2014). Nonetheless, few studies that addressed the biological processes within the membrane provided evidence that the membrane per se could play a biologically active role in bone regeneration in the defect beneath the membrane.
Figure 2.
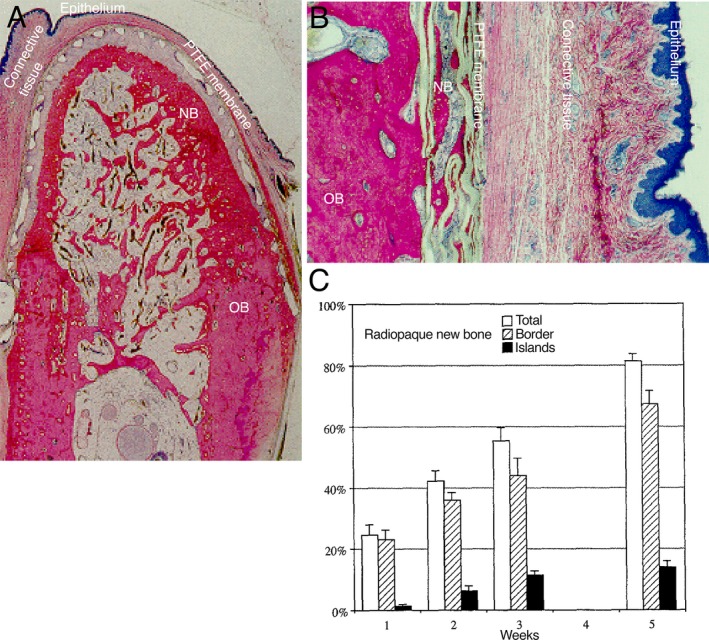
Guided bone regeneration (GBR) using a synthetic, polytetrafluorethylene (PTFE) barrier membrane. The histological images (a and b) represent undecalcified, resin‐embedded and toluidine blue‐stained sections showing GBR using a titanium‐reinforced PTFE barrier membrane on a surgically created mandible defect in the dog model. (a) An orofacial section showing the pattern of bone formation under the membrane after 4 months of healing. The newly regenerated bone (NB) is formed in direct continuity with the host old bone (OB) under the barrier membrane, which separated the bone from the overlying oral mucosa (epithelium and connective tissue). (b) Under the periphery of the PTFE membrane, NB is formed on the porous surface of the PTFE. (c) The bar chart shows the amounts of radiopaque new bone within a rabbit 15‐mm cranial defect treated with a PTFE membrane and evaluated on radiographs at 1, 2, 3 and 5 weeks after surgery. The spatial analysis reveals the progressive increase in the amount of regenerated bone with respect to the amounts of total new bone (white bars), bone originating at the defect borders (hatched bars) and new bone formed as islands in the central region of the defect (black bars). The images (a and b) are adapted and republished with the permission of Quintessence Publishing Company Inc. from the Int J Oral Maxillofac Implants: Healing pattern of bone regeneration in membrane‐protected defects: a histological study in the canine mandible., Schenk RK, Buser D, Hardwick WR, Dahlin C., 9 (1), 1994; permission conveyed through the Copyright Clearance Center, Inc. The image in (c) is adapted and reprinted from the J Oral Maxillofac Surg, 53 (2), Hämmerle CH, Schmid J, Lang NP, Olah AJ., Temporal dynamics of healing in rabbit cranial defects using guided bone regeneration, 167‐74, copyright (1995), with permission from Elsevier [via the Copyright Clearance Center]
4.1.1. The role of GBR membrane
Non‐resorbable membranes
An experimental study revealed that compared with no treatment, the application of a non‐resorbable PTFE membrane promoted earlier generation and a higher number of core‐binding factor alpha 1/runt‐related transcription factor 2 (Cbfa1/Runx2)‐positive osteoprogenitor cells in rat tibia defects, particularly in the upper region of the defects, after 6 days of healing (Tanaka, Matsuzaka, Sato, & Inoue, 2007). At 8 and 10 days of healing, the presence of the PTFE membrane enhanced the expression of the bone‐related gene osteocalcin (OC) in the underlying defect compared to the expression in the untreated sham defect (Tanaka et al., 2007). These data corroborated the observation that the presence of a PTFE membrane progressively promoted a higher percentage of newly formed bone at 6, 8 and 10 days, especially in the upper part of the defect, than in the sham defect (Matsuzaka, Shimono, & Inoue, 2001). Further, the immunoreactivity of the cell proliferation marker proliferating cell nuclear antigen (PCNA) did not differ between the membrane and sham groups, suggesting that the promotion of early bone formation under the PTFE membrane was due to enhanced osteogenic differentiation rather than the proliferation of osteoprogenitor cells. Another study also documented enhanced osteogenic activity in the regenerating tissue directly underneath the PTFE membrane in an experimental periodontal bone defect model in Rhesus monkeys (Amar et al., 1997). The latter study demonstrated high immunoreactivity of bone morphogenetic proteins (BMPs) (BMP‐2, BMP‐4, and BMP‐7), osteonectin (ON) and bone sialoprotein (BSP) as well as higher mRNA transcript levels of BMP‐2 and BMP‐4 in the tissue formed directly underneath the PTFE membrane compared to the undetectable or very low reactivity in untreated defects after 6 weeks of healing. Further, in rat calvarial model, although no control defect without membrane was included, the application of PTFE membrane was associated with positive upregulation of Wnt signalling pathway at day 7 compared to day 15, whereas the day 15 was characterized by overexpression of genes encoding inhibitors of the Wnt pathway, but overexpression of multiple growth factors, including insulin‐like growth factor‐1 (IGF‐1) and BMPs, in the underlying defect (Al‐Kattan, Retzepi, Calciolari, & Donos, 2017). The latter findings indicate that different pathways involved in bone regeneration are temporally regulated in the defect under the PTFE membrane.
The aforementioned experimental findings were corroborated in a human study comparing the GTR procedure using a PTFE membrane with flap surgery alone, without a membrane, for periodontal bone defects (Lima, Goncalves, Sallum, Casati, & Nociti, 2008). Gene expression analysis showed that the PTFE membrane led to a significant upregulation of bone formation genes, including osteopontin (OP), BSP and alkaline phosphatase (ALP), in the underlying defect after 3 weeks of healing, compared to the expression of these genes in the control defect (flap surgery without a membrane) (Lima et al., 2008). Moreover, the PTFE membrane‐induced effects in the defects not only promoted osteogenic activity but also enhanced the expression of genes encoding growth factors (fibroblast growth factor‐2 (FGF‐2)), inflammatory cytokines (interleukin (IL)‐6 and IL‐1) and proteins involved in bone and tissue remodelling (receptor activator of nuclear factor kappa B ligand (RANKL), osteoprotegerin (OPG) and matrix metallopeptidases (MMP‐2 and MMP‐9)), in the defect underneath the barrier membrane (Lima et al., 2008).
Resorbable membranes
As observed with non‐resorbable PTFE membranes, naturally derived, resorbable, collagen membranes promote an early coupled upregulation of genes related to bone formation (OC) and bone remodelling (the calcitonin receptor (CTR), cathepsin K (CatK) and RANKL)) in the underlying defect compared to the expression of these genes in the untreated sham defect in the rat femur (Turri et al., 2016; Figure 3). Further, the presence of a collagen membrane above the defect appeared to fine‐tune the expression of the pro‐inflammatory cytokine tumour necrosis factor alpha (TNF‐α) during the different phases of GBR, as indicated by the early peak of TNF‐α at day 3 followed by significant downregulation at day 6 and a second peak at day 28 in the defect treated with the collagen membrane (Turri et al., 2016). Despite the controversy regarding the role of inflammation and pro‐inflammatory cytokines during bone healing, TNF‐α is crucial for intra‐membranous bone formation (Gerstenfeld et al., 2001), and the observed early increase in TNF‐α expression in the membrane‐treated defect may positively impact the recruitment of cells, including mesenchymal stem cells (MSCs) (Bocker et al., 2008; Fu et al., 2009), and the osteogenic differentiation of these cells (Hess, Ushmorov, Fiedler, Brenner, & Wirth, 2009). However, the second peak in TNF‐α expression may be related to osteoclastic bone remodelling (Azuma, Kaji, Katogi, Takeshita, & Kudo, 2000; Katagiri & Takahashi, 2002). The multiple effects of the collagen membrane on different cellular activities in the underlying defect may explain the higher percentage of well‐remodelled mature bone in the defect under the membrane and the eventual higher degree of restitution of the membrane‐treated defect than in the untreated sham defect (Turri et al., 2016; Figure 3).
Figure 3.
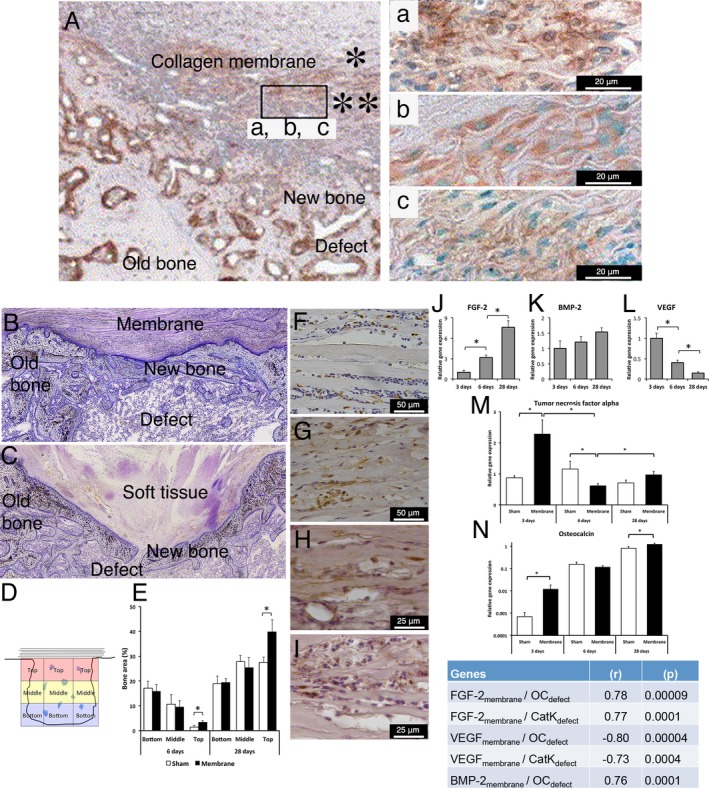
Cellular, molecular and structural events during guided bone regeneration (GBR) by collagen‐based membranes on surgically created bone defects in a rat model. (A) Immunohistochemical findings in decalcified and paraffin‐embedded sections showing GBR using a porcine type I/III collagen membrane consisting of a “compact” top part (*) and a “porous” bottom part (**) placed on a surgically created maxillary defect in the rat model after 2 weeks of healing. In (A), abundant newly formed bone (NB) is observed filling the defect, and an abundant cell infiltrate is observed in the porous part of the membrane facing the defect. The insert shows that the cells infiltrating the porous part of the porcine type I/III collagen membrane are positively stained for the bone proteins (a) alkaline phosphatase (ALP), (b) osteopontin (OPN) and (c) osteocalcin (OC), suggesting the active participation of the membrane‐associated cells in the bone regeneration process. (B and C) Histological images of undecalcified, resin‐embedded and toluidine blue‐stained sections showing that the application of a collagen membrane (derived from porcine small intestine extracellular matrix (ECM)) to a rat femur bone defect (B) results in structural restitution of the underlying defect with newly formed bone compared to the lesser restitution in the untreated defect (C). In the untreated sham defect (C), soft tissue invasion and poor restitution of the defect are evident. The histomorphometric analysis of the different regions of the defect (D and E) demonstrates a higher proportion of newly regenerated bone in the defect treated with the ECM collagen membrane than in the untreated sham defect, specifically in the top region of the defect directly underneath the membrane. The immunohistochemical analyses show that during GBR (exemplified here at 3 days), different cell types, including CD68‐positive macrophages (F) and periostin‐positive osteoprogenitor cells (G), are recruited and hosted within the ECM collagen membrane above the defect. Moreover, the immunohistochemical analysis reveals positive protein reactivity for the pro‐osteogenic, bone‐promoting growth factors FGF‐2 (H) and BMP‐2 (I) inside the membrane. The molecular analysis (qPCR) confirms the progressively increasing expression of the bone‐promoting growth factors FGF‐2 (J) and BMP‐2 (K) in conjunction with a temporal downregulation of the vascularization growth factor VEGF (L) in the membrane‐associated cells. The corresponding molecular qPCR analysis of the underlying defect reveals that the application of the ECM collagen membrane modulates the molecular activities of different healing processes, exemplified here by the pro‐inflammatory cytokine TNF‐α (M) and the bone formation gene OC (N), providing molecular evidence for membrane‐promoted bone healing and regeneration in the underlying defect. The significant correlations between the gene expression in the membrane and the gene expression in the underlying defect (insert table) demonstrate that the molecular activities in the two compartments are linked during the course of GBR. The upper panel of the figure (A and a, b c) is adapted and reprinted from Biomaterials, 26 (31), Taguchi Y, Amizuka N, Nakadate M, Ohnishi H, Fujii N, Oda K, Nomura S, Maeda T., A histological evaluation for guided bone regeneration induced by a collagenous membrane., 6158‐66, copyright (2005), with permission from Elsevier [via the Copyright Clearance Center]. The lower panel of the figure is adapted and reprinted from the Eur J Oral Sci, 125 (5), Elgali I, Omar O, Dahlin C, Thomsen P, Guided bone regeneration: materials and biological mechanisms revisited., 315–337, copyright (2017), published by John Wiley & Sons Ltd. under a Creative Commons license (CC‐BY‐NC‐ND): https://creativecommons.org/licenses/by-nc-nd/4.0/
The original hypothesis of the barrier function of the GBR membrane suggested that the secluded space created by the membrane allows the migration of osteogenic cells and their subsequent differentiation to bone‐forming cells (Dahlin et al., 1988; Retzepi & Donos, 2010), but the molecular mechanisms underlying such an effect long remained undetermined. Recent studies using a collagen membrane provided, at least in part, a molecular explanation; the presence of the collagen membrane prompted an early (3 days) and late (28 days) promotion of the expression of cell recruitment mediators (CXC chemokine receptor 4 (CXCR4) and monocyte chemoattractant protein‐1 (MCP‐1)) in the underlying defect (Turri et al., 2016). The chemokine receptor CXCR4 plays a major role in the recruitment of MSCs and osteoprogenitors (Ceradini et al., 2004; Karp & Leng Teo, 2009; Kitaori et al., 2009), while MCP‐1 has multiple chemotactic activities, including major roles in the recruitment of osteoclast progenitors (Binder et al., 2009; Xing et al., 2010). Further, experimental data revealed an important role for MCP‐1 in osteoclastic bone remodelling during fracture healing (Binder et al., 2009; Xing et al., 2010). For instance, the depletion of the CCR2 (the receptor for MCP‐1) gene in mice (CCR2‐null mice) resulted in a reduced macrophage number (at day 3), impaired vascularization and bone formation (at days 7 and 14, respectively), and delayed overall remodelling and fracture healing (at day 21) compared to these processes in normal mice (Binder et al., 2009; Xing et al., 2010). Taken together, these cellular and molecular findings, supported by histological observations, show that the GBR membrane evokes a local environment in the defect that favours the recruitment and differentiation of multiple cell types, including osteoblasts and osteoclasts. Moreover, the environment created by the membrane is conducive to the molecular mechanisms underlying coupled bone formation and remodelling in the defect underneath the membrane.
4.1.2. The role of scaffolds/grafts in combination with the GBR membrane
GBR membrane and bone graft/substitute materials are commonly applied together. The membrane isolates the bone defect site from non‐osteogenic soft tissue, whereas the bone substitute constitutes a three‐dimensional scaffold that supports osteogenic cells and the promotion of bone formation during healing as well as prevents the membrane from collapsing. Therefore, one of the major strategies to improve the results of GBR is to improve the properties of the bone grafts and the synthetic bone substitute materials.
Different types of bone filling materials, including autografts, allografts, xenografts, and alloplastic or synthetic bone substitutes, have been used. There is agreement that autogenous bone, the gold standard for bone augmentation, improves the outcome of GBR in both experimental (Sverzut et al., 2008) and clinical (Mattout & Mattout, 2000; Meijndert, Raghoebar, Schupbach, Meijer, & Vissink, 2005; Schlegel et al., 1998) studies. However, controversial data have been reported regarding other types of bone substitutes. On the one hand, synergistic effects have been suggested in several experimental (Bernabe et al., 2012; Hammerle, Chiantella, Karring, & Lang, 1998; Jung et al., 2011; Kohal et al., 1998; Martinez, Balboa, Gasamans, Otero‐Cepeda, & Guitian, 2015; Park et al., 2015; Sverzut et al., 2008) and clinical (De Angelis et al., 2011; Luczyszyn et al., 2005) studies. On the other hand, other reports have not demonstrated significant beneficial effect of combining bone substitutes with membranes in either experimental (Becker et al., 1992; Buser et al., 1998; Cho et al., 1998; Dupoirieux, Neves, & Pourquier, 2000; Stavropoulos, Kostopoulos, Mardas, Nyengaard, & Karring, 2001) or clinical (Dies, Etienne, Abboud, & Ouhayoun, 1996; Guarnieri, Stefanelli et al., 2017; Guarnieri, Testarelli et al., 2017; Mattout, Nowzari, & Mattout, 1995) contexts. The differences between the results of different studies might be attributed to differences in the experimental animal species and model; study design; defect site, size and configuration; and evaluation time. Furthermore, the manufacturing and preparation of the same type of bone substitute could be different or not standardized, which may affect the material properties and consequently the final results of each study.
Non‐resorbable membranes
Variable results have been reported when combining non‐resorbable membranes with different types of bone substitutes. For example, the combination of two different commercial types of allogeneic demineralized freeze‐dried bone matrix (DFDB) with e‐PTFE led to diverse results when used in conjunction with implants. Whereas one type reduced the bone gain in dehiscence defects related to titanium implants (Becker et al., 1992), the other type increased the bone‐implant contact (Kohal et al., 1998). These observations suggest that the osteoinductivity of DFDB is largely dependent on the extent of decalcification, the donor age and the size of the allograft particles. Xenografts (e.g. deproteinized or demineralized bovine bone) and synthetic bone substitutes (e.g. hydroxyapatite (HA)) are the most studied bone substitutes in combination with GBR. Interestingly, in studies on bone formation outside the rat skeleton, deproteinized bovine bone inhibited osseous healing, and applying only a dome‐shaped PTFE membrane led to better bone formation (Stavropoulos, Kostopoulos, Nyengaard, & Karring, 2003; Stavropoulos et al., 2001). Similarly, Buser et al. (1998) showed in minipigs that another type of natural HA (coral‐derived HA) did not enhance bone regeneration guided by an e‐PTFE membrane (Buser et al., 1998). In contrast, an experimental study showed that the combination of deproteinized bovine bone with dome‐shaped PTFE membrane maintained higher proportion of newly formed bone, for a prolonged period of time, compared to dome‐shaped PTFE membrane filled with blood in rabbit calvarial defect model (Okazaki, Shimizu, Xu, & Ooya, 2005). Further, a study in dog revealed that the combination of anorganic bovine bone matrix (ABM) with an e‐PTFE membrane enhanced the osseointegration of implants placed into extraction sockets (Tehemar, Hanes, & Sharawy, 2003).
Resorbable membranes
As observed with non‐resorbable membranes, controversial results have been reported on the combination of resorbable membranes with bone substitutes. Experimental study used resorbable polymeric dome membrane, with and without deproteinized bovine mineral on calvarial defects, concluded that whereas the deproteinized bovine mineral contributed to accelerate initial bone neogenesis, it did not contribute to increasing bone volume or bone height at later observation stages (Schmid et al., 1997). Moreover, experimental studies on the early events in bone healing and the cellular activities in response to the combination of deproteinized bovine bone or HA and a collagen membrane demonstrated no added bone formation in the defect in association with either substitute material (Elgali et al., 2016). However, when the HA substitute was doped with strontium ions and used with the same collagen membrane, the formation of new bone was significantly increased in the defect. This increase was attributed to the inhibition of osteoclast numbers and activity as well as a reduction in coupling between osteoblasts and osteoclasts, which was not shown in the presence of deproteinized bovine bone and stoichiometric HA (Elgali et al., 2016). Furthermore, the experimental finding that the collagen membrane alone was sufficient to promote bone formation without the addition of deproteinized bovine bone agrees with clinical data showing similar clinical and histological findings using collagen membrane alone or collagen membrane in combination with anorganic porcine‐derived bone mineral matrix (Guarnieri, Stefanelli et al., 2017; Guarnieri, Testarelli et al., 2017).
In contrast to several of these studies, a beneficial effect of deproteinized bovine bone was observed when it was combined with different types of resorbable membranes (Jung et al., 2011). Interestingly, in rat calvarial defect model, whereas the application of collagen membrane alone gained higher proportion of new bone compared to a combination of the collagen membrane and anorganic bovine minerals/cell‐binding peptide (P‐15) graft, the combined group (membrane + graft) showed greater vertical augmentation height in the central area (Artzi et al., 2008) compared to the membrane alone. The latter experimental findings have been corroborated by clinical data showing that the combination of resorbable acellular dermal matrix membrane with either anorganic bovine minerals/cell‐binding peptide (P‐15) graft (Fernandes et al., 2011) or HA (Luczyszyn et al., 2005) improved the preservation of the alveolar ridge compared to the use of the membrane alone. Moreover, in humans, the addition of deproteinized bovine bone granules with a collagen membrane results in increased bone regeneration in defects around post‐extraction implants and improves the aesthetic outcome of GBR (De Angelis et al., 2011).
4.2. The cellular and molecular events in the membrane and their relationships with the biological processes in the underlying defect compartment
4.2.1. The active involvement of the membrane in the regenerative process
Non‐resorbable membranes
Several indications in the literature support the assumption that PTFE membranes actively contribute to the healing and regenerative processes in the underlying defect. To this end, cells adherent to clinically retrieved PTFE membranes (in GTR and/or GBR procedures) were analysed. In one study, ex vivo culturing of the retrieved membranes in osteogenic media showed that PTFE membrane‐associated cells produce higher ALP activity than ex vivo‐cultured gingival cells harvested from the same patient cohort (Kuru, Griffiths, Petrie, & Olsen, 1999). In another study using the same approach, the host cells adherent to the retrieved PTFE membranes produced mineralized nodules when cultured ex vivo in osteogenic media for a relatively long time (Wakabayashi, Iha, Niu, & Johnson, 1997). Further, the latter study revealed that cells adherent to the PTFE membranes in either the GTR or GBR procedures express IL‐1α and IL‐4 cytokines, whereas the expression of IL‐1β was detected only in cells adherent to the PTFE membrane used in GTR (Wakabayashi et al., 1997). Taken together, the data from the few available studies investigating PTFE membrane‐associated cells indicate that the non‐resorbable synthetic PTFE membrane surface becomes populated with cells that not only possess regenerative capacity but also convey inflammatory signals.
Resorbable membranes
Previous immunohistochemical observations suggested that collagen membranes participate in the bone regenerative process, as indicated by the positive immunoreactivity for bone proteins (ALP, OP and OC) within the lower side of the membrane that interfaces with the underlying tibia bone defect (Figure 3; Taguchi et al., 2005). The immunohistochemical observations are corroborated by recent histological and electron microscopy findings showing bone formation and mineralization within the lower porous part of a similar collagen membrane, covering calvarial bone defect, after 4 weeks of healing (Kuchler et al., 2018). Recently, the emerging hypothesis of a bioactive role of the GBR membrane has been addressed in vivo with a collagen membrane derived from the porcine small intestinal submucosa (Turri et al., 2016). Using a combination of gene expression analysis, Western blotting, histology and immunohistochemistry, this study demonstrated that during GBR, the collagen membrane hosts different cell phenotypes, and these cells increasingly express and release important pro‐osteogenic factors, including BMP‐2 (Turri et al., 2016). Importantly, the correlation analysis revealed a strong relationship between the expression of growth factors (BMP‐2, FGF‐2 and transforming growth factor‐beta (TGF‐β)) in the membrane and the molecular activities of bone formation and remodelling in the defect underneath the membrane (Turri et al., 2016) (Figure 3). Thus, scientific evidence indicates that the GBR membrane is actively involved in promoting the healing and regenerative processes in the defect by stimulating the recruited cells that migrate into and/or become associated with the membrane. Concomitantly, the membrane enables the signals from the membrane‐associated cells to be communicated to cells in the underlying defect, thus creating a local environment conducive to bone formation and remodelling (Figure 3).
4.2.2. Cells associated with the membrane during GBR may exhibit features distinct from the cellular component in the underlying defect
The assumption that the cells in the membrane and in the underlying defect may differ with respect to their phenotype and secretory behaviour derives from two independent studies that explored and compared the two cellular compartments (i.e. cells associated with the membrane vs. cells in the defect compartment underneath the membrane).
Non‐resorbable membranes
In one study, cells associated with PTFE membranes from GTR procedures, clinically retrieved after 6 weeks, had a lower proliferation rate and larger size and expressed higher levels of collagen 1 and fibronectin than cells in the regenerated tissue underneath the membrane, the periodontal ligament tissue or the gingival tissue retrieved from the same cohort of patients (Kuru, Parkar, Griffiths, & Olsen, 2001).
Resorbable membranes
In another study, site‐specific immunohistochemical evaluation revealed comparable immunoreactivity for bone proteins (OC, OP and BSP) in the collagen membrane and the underlying defect; however, the cells in the collagen membrane exhibited higher collagenase activity (for MMP‐1 and MMP‐8) than the cells in the underlying defect (Calciolari, Ravanetti et al., 2018).
4.2.3. Different membranes may have different potentials to harbour and activate the membrane‐associated cells
Recent studies provide scientific evidence that the membrane is biologically active in the promotion of regenerative processes during GBR instead of being mainly a passive barrier. Moreover, a few reports show that different membranes may have different potentials to harbour and activate membrane‐associated cells, which then promote different degrees of defect restitution.
Non‐resorbable membranes
Proteomic data on non‐resorbable synthetic GBR materials with different properties revealed that a titanium dome with a hydrophilic surface promotes the activity of pathways involved in an enhanced osteogenic response and reduced inflammatory response during GBR in rabbit parietal bone (Calciolari, Mardas et al., 2018). Recently, dual‐sided, expanded PTFE with different texture configurations on each side of the membrane was compared with solid, dense PTFE in a rat femoral defect (Omar, Trobos et al., 2018). Preliminary findings revealed several molecular differences between the two types of PTFE membrane, but the differences were mainly observed in the overlying soft tissue and not in the underlying bone defects. For example, whereas a higher expression of the pro‐inflammatory cytokine TNF‐α was detected in the soft tissue covering the expanded PTFE membrane than in the tissue covering the dense PTFE membrane or the sham after 6 days, both the expanded and dense PTFE membranes lowered TNF‐α expression in the overlying soft tissue compared to that in the sham site after 28 days of healing. Moreover, after 28 days, the expression of growth factor (FGF‐2) and vascularity (vascular endothelial growth factor (VEGF)) genes in the overlying soft tissue was upregulated only with the expanded PTFE membrane (Omar, Trobos et al., 2018).
Resorbable membranes
In a recent study, two different naturally derived collagen membranes appeared to contribute differently to the pattern of bone formation during GBR when both membranes were combined with a deproteinized bovine bone (DBB) substitute in a rat femoral defect (Omar, Dahlin, Gasser, & Dahlin, 2018): compared to the membrane derived from type I/III porcine collagen (BG), the membrane derived from porcine collagen intermingled with porcine elastin (CXP) triggered higher expression of BMP‐2 and higher temporal increase in the expression of bone remodelling genes (CTR and CatK) in the underlying defects (Omar, Dahlin et al., 2018), along with a higher proportion of bone in the central region of the defect. Furthermore, the analysis of cells recruited into the membrane after 3 days of healing demonstrated the expression of several growth factors and cytokines in both collagen membranes; however, the expression of BMP‐2 and FGF‐2 in the membranes was primarily correlated with the expression of BMP‐2 and inflammatory cytokines, respectively, in the underlying defects. However, one discrepancy was that the BG membrane, which stimulated higher expression of BMP‐2 in the membrane‐associated cells at 3 days, exhibited a lower proportion of bone in the central region of the defect than did the CXP membrane. Similarly, a novel resorbable polymeric (Diplen‐Gam) membrane revealed enhanced BMP‐2 immunoreactivity during GBR in a rat calvarial defect (Ge et al., 2011). However, the defect treated with this novel membrane revealed lower bone formation after 12 weeks than a defect treated with a porcine‐derived collagen membrane.
4.3. Incorporation of biological cues, natural elements and synthetic bioactive materials during GBR
4.3.1. Incorporation of biological cues, natural elements and synthetic bioactive materials in the membrane
The incorporation of biological cues (growth factors or cells) and antibacterial agents in the membrane has been suggested as a promising approach in order to increase the bone promotive effects and/or providing the membrane with antibacterial properties.
Incorporation of biological cues to increase the bone promotive effects
Different growth factors have received the most attention because of their multiple functions during bone healing, including cell recruitment, proliferation and differentiation (Fei, Gronowicz, & Hurley, 2013; Poniatowski, Wojdasiewicz, Gasik, & Szukiewicz, 2015; Shah, Keppler, & Rutkowski, 2014). The majority of in vitro studies have used MSCs, osteoblast cell lines and endothelial cells to determine the effects of bone‐forming peptide‐1 (Lee et al., 2013), basic fibroblast growth factor (bFGF)/FGF‐2 (Lee, Lee, Cho, Kim, & Shin, 2015) and stromal cell‐derived factor‐1α (SDF‐1α) (Ji et al., 2013) immobilized on the membranes (Table 2). In general, these in vitro studies demonstrated increased spreading, proliferation, migration and osteogenic differentiation depending on the immobilized molecule.
Table 2.
In vitro studies evaluating cellular activities in response to membranes with incorporated biological/antimicrobial factors
| Modification | Cell type | Experimental groups (membrane materials) | Main findings | References |
|---|---|---|---|---|
| Incorporation of biological molecules | Human MSCs | PLGABFP1‐immobilized PLGA | Immobilization of BFP1 in PLGA increased hMSC spreading, ALP production and calcium deposition | Lee et al. (2013) |
| Human MSCsHuman HUVECs | PCL/gelatin PCL/gelatin immobilized with 50 or 100 ng/ml bFGF | Incorporation of bFGF in the PCL/gelatin composite fibre mesh enhanced the proliferation and migration of human MSCs as well as the tubule formation of HUVECs | Lee et al. (2015) | |
| Rat BMSCs | PCL/gelatin SDF‐1α loaded PCL/gelatin | The presence of SDF‐1α in PCL/gelatin membranes induced chemotactic migration of BMSCs | Ji et al. (2013) | |
| Incorporation of antibiotics or antimicrobial agents | Rat foetal calvarial osteoblasts | PLLATetracycline‐loaded PLLA | The level of cell attachment was higher on the tetracycline‐loaded membranes than on the unloaded membranes | Park et al. (2000) |
| L929 fibroblast cellsHuman PDLFsROS cellsAnaerobic bacterium Fusobacterium nucleatum | TCPPCL nanofibres incorporated with different concentrations of MNA (0, 1, 5, 10, 20, 30 and 40) (wt %) | In L929 cell culture, a membrane loaded with 30% MNA showed the best cell proliferation rate among the membrane groups, but cell proliferation on this membrane was still lower than that on a polystyrene surfaceA 30% MNA membrane and a TCP surface showed a comparable level of cell proliferation when tested in human PDLFs and ROS cell culturesA dose‐dependent inhibition of bacterial growth was found for MNA concentrations of between 5 and 40 wt% | Xue et al. (2014) | |
| Osteoblast‐like cells (MG63) | Polystyrene surfacenHA‐PA66Silver‐nHA/TiO(2)/PA66 | All groups showed comparable cell viability, proliferation and osteogenic differentiation | Li et al. (2012) | |
| Staphylococcus aureusOsteoblastic cells (MC3T3‐E1) | PLA/siloxane/calcium carbonate composite containing mercapto groups (PSC‐SH) Silver‐PSC‐SH | The presence of silver in the membrane reduced the number of bacteria after 24 hr of cultureSilver did not affect the proliferation of osteoblast‐like cells | Tokuda et al. (2009) | |
| Osteoblast‐like cells (MG63) | e‐PTFEnHA‐PA66Silver‐nHA‐nTiO(2)/(PA66) nanocomposite | Cell viability on the silver‐nHA‐nTiO(2)/PA66 membrane was significantly lower than that on the other membranesThe ALP activity and Ca concentration did not differ among the different types of membranes | Ye et al. (2011) |
ALP: alkaline phosphatase; bFGF: basic fibroblast growth factor; BFP1: bone‐forming peptide‐1; BMSCs: bone marrow stromal cells; Ca: calcium; e‐PTFE: expanded tetrafluoroethylene; MNA: metronidazole; MSCs: Mesenchymal stem cells; nHA: nanohydroxyapatite; PA66: polyamide‐66; PCL: polycaprolactone; PDLFs: periodontal ligament fibroblasts; PLA: polylactic acid; PLGA: poly(lactide‐co‐glycolide); PLLA: poly(l‐lactic acid); ROS: rat osteogenesis sample; SDF‐1α: stromal cell‐derived factor‐1; TCP: tissue culture polystyrene; TiO(2): titanium dioxide; UVECs: umbilical vein endothelial cells
In vivo animal experiments have compared the bone regenerative effects of native membranes and membranes immobilized or loaded with recombinant human BMP‐2 (rhBMP‐2) (Shim et al., 2014), rhBMP‐2 with a collagen‐binding domain (Lai, Zhou, Wang, Lu, & Gao, 2013), BMP‐7 (Jo et al., 2015), a combination of BMP‐2 and BMP‐7 (Jo et al., 2015), bFGF/FGF‐2 (Lee et al., 2015), FGF‐2 (Hong et al., 2010), PDGF (Park, Ku, Chung, & Lee, 1998) and bone‐forming peptide‐1 (BFP1) (Lee et al., 2013) (a peptide sequence from the immature region of BMP‐7) (Kim et al., 2008) (examples are provided in Table 3). Most experimental in vivo studies have been performed with degradable membranes/materials in rodent calvarial defects. Compared with native membranes, membranes immobilized or loaded with these different growth factors/peptides demonstrate increased in vivo bone regeneration (Hong et al., 2010; Jo et al., 2015; Lai et al., 2013; Lee et al., 2013, 2015; Park et al., 1998; Shim et al., 2014) (Table 3). Moreover, a polycaprolactone (PCL)/gelatin membrane functionalized with CXCL12/SDF‐1α, which stimulated bone marrow stromal cell (BMSC) chemotaxis in vitro, promoted a sixfold increase in bone formation compared to that afforded by the native membrane after 8 weeks in vivo (Ji et al., 2013). The immobilization of proteins such as collagen, decorin and fibronectin derived from rat BMSC conditioned medium on a poly(lactide‐co‐glycolide) (PLGA) membrane enhanced cell proliferation and ALP activity in rat BMSCs in vitro and promoted more bone formation than the PLGA membrane in vivo (Tsuchiya et al., 2015). Further, dexamethasone has been shown to induce bone regeneration when immobilized onto the surface of a collagen membrane (Piao et al., 2014).
Table 3.
In vivo studies evaluating the performance of membranes with incorporated biological/antimicrobial factors
| Modification | Experimental model | Experimental groups (membrane and/or graft materials) | Main findings | References |
|---|---|---|---|---|
| Incorporation of growth factors and other biological molecules | Calvarial defect (rabbit) | PCL/PLGA/β‐TCP membranePCL/PLGA/β‐TCP membrane loaded with rhBMP‐2 | More bone formation was observed in association with the rhBMP‐2 loaded membrane than the native membrane after 4 and 8 weeks of implantation | Shim et al. (2014) |
| Calvarial defect (rat) |
PLLA membrane PDGF‐BB‐loaded PLLA membrane |
The PDGF‐BB‐loaded membrane showed markedly increased new bone formation in rat calvarial defects, and bony reunion was completed after 2 weeks of implantation | Park et al. (1998) | |
| Calvarial defect (rat) |
Sham Collagen membrane Collagen‐nBG membrane Collagen‐nBG‐FGF‐2 membrane |
The presence of nBG in the collagen membrane enhanced the level of bone regeneration, which was further improved after the addition of FGF‐2 | Hong et al. (2010) | |
| Titanium cylinder fixed into calvarial bone (rabbit) |
MBM without a membrane Collagen membrane + MBM rhBMP‐2‐collagen membrane + MBM rhBMP‐2/CBD‐collagen membrane + MBM |
Loading the membrane with rhBMP‐2/CBD strongly induced vertical bone formation after 6 weeks of implantation, whereas the presence of rhBMP‐2 alone in the collagen membrane did not show any added beneficial effect | Lai et al. (2013) | |
| Calvarial defect (mouse) |
Sham PLGA membrane Polydopamine‐coated PLGA membrane BFP1‐immobilized PLGA membrane |
Incorporation of polydopamine or BFP1 into the PLGA improved membrane integration with the host tissue and enhanced the level of bone formation after 2 months of healing | Lee et al. (2013) | |
| Calvarial defect (rat) |
Collagen membrane BMP‐2‐collagen membrane BMP‐7‐heparinized collagen membrane BMP‐7/BMP‐2‐heparinized collagen membrane |
The presence of BMP‐2 or BMP‐7 in the collagen membrane enhanced the level of bone regeneration at 2 and 8 weeks of healing The BMP‐7/BMP‐2‐heparinized collagen membrane (combination of both growth factors) showed the highest level of bone regeneration |
Jo et al. (2015) | |
| Calvarial defect (mouse) |
PCL/gelatin membrane PCL/gelatin membrane immobilized with 50 or 100 ng/mL bFGF |
The presence of bFGF in the membrane enhanced bone formation after 2 weeks The increase in the bone quantity was dependent on the bFGF dose |
Lee et al. (2015) | |
| Calvarial defect (rat) | PCL/gelatin membraneSDF‐1α‐loaded PCL/gelatin membrane | Combining SDF‐1α with the membrane promoted a sixfold increase in the amount of bone formation after 8 weeks of healing | Ji et al. (2013) | |
| Calvarial defect (rat) |
PLGA membrane treated with: PBS PBS and NaOH Bone marrow stromal cell (BMSC) conditioned medium (CM) PBS, NaOH and CM |
The membrane treated with PBS, NaOH and CM showed the highest bone formation, which was attributed to the higher level of immobilized proteins (e.g. collagen, decorin, and fibronectin) on the membrane after the hydrophilic treatment | Tsuchiya et al. (2015) | |
| Calvarial defect (rat) |
Collagen membrane Collagen membrane combined with DEX‐loaded microparticles |
The presence of DEX‐loaded microparticles in the collagen membrane enhanced the volume and quality of new bone formation | Piao et al. (2014) | |
| Incorporation of antibiotics or antimicrobial agents | Tibial defect (bacteria‐contaminated) (rat) |
Collagen membrane + bone graft Doxycycline‐collagen membrane + bone graft |
The use of a doxycycline‐releasing membrane reduced bacterial overgrowth in the contaminated defect and led to significantly higher bone formation | Kutan et al. (2016) |
| Calvarial defect (rat) |
PLLA membrane Tetracycline‐PLLA membrane |
Tetracycline‐loaded membranes markedly increased new bone formation after 2 weeks of implantation | Park et al. (2000) | |
| Subcutaneous pocket (rabbit) |
PCL PCL incorporated with 30% MNA |
The MNA‐loaded membrane invoked a lower inflammatory response than the pure PCL membrane | Xue et al. (2014) | |
| Calvarial defect (rat) |
Sham Polyamide 66 membrane nHA‐PA66 membrane Silver‐nHA/TiO(2)/PA66 membrane |
Incorporation of nHA with or without Ag and TiO(2) resulted in higher bone formation in the treated defect than sham and polyamide membrane | Li et al. (2012) | |
|
Subcutaneous pocket (rat) Calvarial defect (rat) |
e‐PTFE membrane nAg‐HA‐TiO(2)/PA |
The nAg‐HA‐TiO(2)/PA membrane showed less granulation tissue and a higher serum ALP level compared to the e‐PTFE membrane No differences were observed between the two membranes in the amount and optical density of newly formed bone |
Zhang et al. (2010) |
β‐TCP: beta‐tricalcium phosphate; bFGF: basic fibroblast growth factor; BFP1: bone‐forming peptide‐1; BMP: bone morphogenetic protein; CBD: collagen‐binding domain; DEX: dexamethasone; FGF‐2: fibroblast growth factor‐2; MBM: mineralized bone matrix; MNA: metronidazole; NaOH: sodium hydroxide; nBG: nano‐bioactive glass.; nHA: nanohydroxyapatite; PA66: polyamide 66; PBS: phosphate‐buffered saline; PCL: Polycaprolactone; PDGF‐BB: platelet‐derived growth factor‐BB; PLGA: poly(lactide‐co‐glycolide); PLLA: poly(l‐lactic acid); rhBMP‐2: recombinant bone morphogenetic protein BMP‐2; SDF‐1α: stromal cell‐derived factor‐1; TiO(2): titanium dioxide
Incorporation of antimicrobial agents and antibiotics in the membrane
Antibiotics and silver may change the cellular and tissue responses as well as the propensity of bacteria to adhere to and colonize the membrane. The incorporation of antimicrobial agents, for example tetracyclines (Kutan et al., 2016; Park et al., 2000), metronidazole (Xue et al., 2014) and silver ions (Li et al., 2012; Tokuda, Obata, & Kasuga, 2009; Ye et al., 2011; Zhang et al., 2010), into the membrane may inhibit bacterial infection, for example in conjunction with membrane exposure. In vitro studies (Table 2) demonstrate a reduction in S. aureus and similar proliferation of osteoblast cell lines after exposure to silver‐loaded poly(lactic acid) (PLA)/siloxane/calcium carbonate composite membranes compared with the S. aureus population and osteoblast proliferation with native membranes (Tokuda et al., 2009). A reduced viability but similar osteogenic differentiation of an osteoblast cell line has also been shown for nanoHA/titanium dioxide (TiO)/polyamide 66 (PA66) composite membranes loaded with silver (Ye et al., 2011).
In vivo studies (Table 3) demonstrate increased bone regeneration with membranes containing tetracyclines (Kutan et al., 2016; Park et al., 2000), whereas compared to non‐silver‐containing control (nanoHA‐PA66) membranes, silver‐loaded membranes do not reduce bone growth (Li et al., 2012). Similar bone filling of cranial defects in rats has been shown for silver‐HA‐titanium/polyamide nanocomposite membranes and e‐PTFE membranes (Zhang et al., 2010). In addition, reduced inflammation in soft tissues has been demonstrated for PCL membranes with incorporated metronidazole (Xue et al., 2014). However, few membrane‐targeted antibacterial strategies have been evaluated for efficacy in reducing microbial adhesion and infection in vivo. One exception is a study showing that the placement of a doxycycline‐releasing collagen membrane in a model of a bacteria‐contaminated rat tibial defect significantly reduced bacterial growth at the defect site and promoted osteogenesis (Kutan et al., 2016). In addition to the relatively scant experimental evidence that a local antibacterial strategy would work for GBR, there are general concerns about an excessive use of antibiotics, for example, broad‐spectrum antibiotics, and the risk for the development of multi‐resistant bacterial strains. In contrast to several studies on infected periodontal defects locally treated with antibiotics and GTR membranes, no specific antimicrobial strategy for GBR has yet been evaluated clinically.
Bone regeneration and restitution of defects and the subsequent integration of implants is today an accepted clinical treatment, merely by using non‐resorbable or resorbable GBR materials, without exogenously administered cells or bone inductive cues. The incorporation of growth factors, cells or antibacterial agents in the membrane is yet a promising approach to promote bone formation, especially for challenging clinical situations (e.g. large defects or sites prone to exposure and subsequent bacterial contamination) as well as in patients with bone compromising conditions. However, despite the promising results observed in vitro and in preclinical animal models, the clinical value of incorporating biological cues for bone promotion and antibacterial strategies has not yet been demonstrated. Barriers to clinical translation may relate to the need for cost‐effective protocols that meet the regulatory requirements. It is urgent to facilitate technology spread and enable effective and reproducible clinical outcomes. Randomized, clinical trials are required in order to determine the safety and clinical efficacy of novel innovative GBR procedures.
4.3.2. Incorporation of biological cues, natural elements and synthetic bioactive materials into the defect in combination with the membrane
The administration of growth factors or cells into the defect compartment in conjunction with the placement of a GBR membrane is based on the hypothesis that the presence of biological cues in the secluded space can trigger the development of a favourable recipient bone microenvironment and promote bone regeneration. Hence, growth factors have not only been incorporated into membranes to enhance membrane bioactivity (see Incorporation of biological cues, natural elements and synthetic bioactive materials in the membrane) but have also been delivered locally to the defect via injection or in combination with a biocompatible carrier (e.g. bone graft or substitute). The administration of growth factors or cells into the defects has been explored with both non‐resorbable and resorbable GBR membrane.
Non‐resorbable membranes
One of the earliest experiments was conducted by Becker et al. (1992) in a dog model. The study compared the bone promotion around titanium implants, which were inserted in intentionally created defects in extraction sockets simultaneously with the placement of e‐PTFE membranes alone or e‐PTFE membranes in combination with a mixture of platelet‐derived growth factor‐BB (PDGF) and insulin‐like growth factor‐I (PDGF/IGF‐I) (Becker et al., 1992). The results showed that combination of the e‐PTFE membrane plus PDGF‐BB/IGF‐I (administered into the defect) promoted higher bone regeneration around the implant (bone area and bone–implant contact) compared to defects receiving e‐PTFE membranes alone. Similarly, in rat mandibular defect model, the combination of rhBMP‐2 (delivered into the defect) with an e‐PTFE membrane (covering the defect) demonstrated better bone healing as well as maintenance of the bone contour compared with e‐PTFE membrane alone at both 12 and 24 days of healing (Linde & Hedner, 1995). The added bone promotive effect of rhBMP‐2 has also been demonstrated in another study using dog mandible model (Wikesjo et al., 2004). In the latter study, rhBMP‐2 was loaded on acellular collagen sponge (ACS) carrier and administrated into surgical defect around titanium implant and covered with macro‐porous e‐PTFE membrane, whereas the control was the ACS covered with similar membrane (Wikesjo et al., 2004). The study revealed significantly enhanced bone formation under the membrane in defects receiving rhBMP‐2/ACS compared to ACS (without rhBMP‐2). Moreover, the efficacy of synthetic biphasic calcium phosphate (BCP) in promoting peri‐implant defect regeneration was increased after a coating of rhBMP‐2 under titanium mesh membrane compared to BCP only under the membrane (Shin et al., 2014).
The transplantation of progenitor cells to the defect site to enhance GBR is another interesting strategy that could be implemented with either non‐resorbable or resorbable membranes and with bioactive factors. For instance, there is experimental evidence that the injection of a mixture of autologous MSCs and platelet‐rich fibrin into the bone defect, in conjunction with the placement of porous high‐density polyethylene membrane, increases bone formation compared to the membrane alone (Liao, Chen, Chen, Chen, & Tsai, 2011). Moreover, the delivery of adipose‐derived stem cells with anorganic bovine bone beneath calvarial titanium dome barriers has been shown to increase vertical bone regeneration and implant osseointegration as compared to the dome barriers with anorganic bovine bone alone (Pieri et al., 2010). Similarly, in a secluded space created by the placement of a gold dome on a rat calvarial bone and filled with synthetic β‐TCP, the administration of peripheral blood‐derived endothelial progenitor cells and/or bone marrow‐derived MSCs significantly promoted bone regeneration (Zigdon‐Giladi, Bick, Lewinson, & Machtei, 2014, 2015; Zigdon‐Giladi, Bick, Morgan, Lewinson, & Machtei, 2015; Zigdon‐Giladi, Lewinson, Bick, & Machtei, 2013).
Resorbable membranes
As observed with the non‐resorbable membranes, there are existing data showing bone promotive effect of growth factors administered into the defects in conjunction with the placement of resorbable GBR membranes. For example, mandibular ridge defects in dogs filled with rhPDGF‐loaded BCP and covered with a collagen membrane exhibited a higher degree of angiogenesis and bone formation compared to that observed in other defects filled with BCP and covered with the same membrane, but without rhPDGF (Schwarz, Sager, Ferrari, Mihatovic, & Becker, 2009). It has also been shown that the local administration of basic fibroblast growth factor (bFGF) with collagen sponge into dog mandible defect and covered with poly(lactic acid‐co‐glycolic acid‐co‐ε‐caprolactone) membrane promotes higher bone volume compared with defect treated with similar membrane and collagen sponge without bFGF (Matsumoto et al., 2012). Moreover, the positive data related to BMP‐2 agree with clinical findings showing that the addition of rhBMP‐2 to deproteinized bovine bone improves GBR for lateral ridge augmentation under collagen membrane in human jawbone defects (Jung et al., 2013). In fact, experimental study in dogs showed that the horizontal GBR was as effective in bone defect treated with a rhBMP‐2‐loaded bovine hydroxyapatite/collagen graft and covered with collagen membrane as in defects treated with bovine hydroxyapatite/collagen graft and covered with a rhBMP‐2‐loaded collagen membrane (Chang et al., 2015). Interestingly, in the latter study, while more bone appeared in contact with the graft material in the rhBMP‐2‐loaded graft group, more bone projected and approached the membrane in the rhBMP‐2‐loaded collagen membrane group.
Likewise, the application of cell therapy in defects treated with resorbable membranes appears to promote the regenerative outcome of GBR. For example, in a rabbit model, compared with cell‐free control, tibial periosteal cells loaded in a collagen scaffold and transplanted to a calvarial defect under polymeric dome barrier were shown to induce higher proportion of mineralized bone under the polymeric barrier (Miyamoto, Tsuboi, Takahashi, Hyon, & Iizuka, 2004). In another GBR model in minipigs, the addition of porcine MSCs to a platelet‐rich plasma‐fluorohydroxyapatite bone substitute enhanced bone formation in collagen membrane‐treated defects compared to similar treatment, but without the MSCs (Pieri et al., 2009). Interestingly, a cell therapeutic approach has recently been applied for the regeneration of craniofacial bone in humans. Kaigler et al. (2013) performed the first human trial to evaluate stem cell therapy or the transplantation of tissue repair cells vs. the GBR procedure for the treatment of localized peri‐implant defects. The extraction sockets were treated with collagen sponges with or without autologous bone marrow‐derived progenitor cells, the implants were placed, and all defects were covered with collagen membranes. The clinical and histomorphometric analyses at 6 and 12 weeks following treatment showed that the cell therapy approach reduced the implant bony dehiscence exposure (residual bone defects) and accelerated bone regeneration (Kaigler et al., 2013).
The strategies of administering growth factors and cells into the bone defect in conjunction with the GBR membrane have provided very interesting and promising experimental results. Clinical evidence for and the exploitation of the growth factor strategy in conjunction with GBR have yet to be realized. For example, this strategy is largely hampered by potential long‐term and serious adverse events and by regulatory and financial constraints. Similarly, the cell therapy approach needs additional experimental and clinical investigation. Practical considerations, the occurrence of adverse events, and regulatory and financial constraints may slow the development of this approach.
5. SUMMARY AND FUTURE PERSPECTIVES
The application of a membrane to exclude the entry of soft tissue cells to the underlying bone defect is an established principle that has been successfully implemented clinically. Careful scrutiny of the published literature on GBR indicates that biological events in both the membrane and the underlying defect are important for bone regeneration. The bone‐promoting environments in the two compartments can likely be optimized by several strategies targeting both material aspects and host tissue responses (Figure 4). The membrane, the main component of GBR, can be modified differently according to the functional requirements and the involved biological mechanism. These modifications include the following: (a) optimizing the physicochemical and mechanical properties, for example the porosity, structure, thickness, rigidity and plasticity; (b) incorporating biological factors (e.g. bFGF, BMP‐2, BMP‐7, PDGF, BFP1 and SDF‐1α) and synthetic bioactive materials (e.g. HA, β‐TCP and bioactive glass); (c) incorporating antibacterial agents (e.g. silver) and antibiotics (e.g. tetracycline and metronidazole); and furthermore, in order to stimulate a bone‐promoting environment during GBR, targeting the bone defect with (d) osteoconductive and osteoinductive materials, with or without natural elements (e.g. strontium and zinc) introduced into be bone defect or (e) by delivering biological cues (e.g. growth factors and progenitor cells) into the bone defect. It is envisioned that several of the above strategies can be combined.
Figure 4.
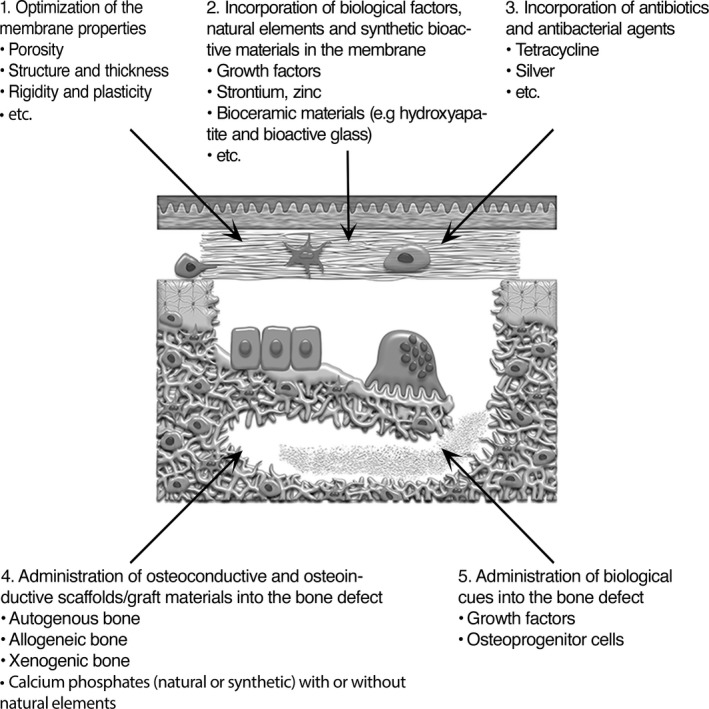
Schematic showing the membrane and bone defect compartments, both of which are amenable to potential strategies to enhance the clinical results of the GBR technique. The strategies include (1) the optimization of membrane material properties, (2) the incorporation of biological factors, natural elements and synthetic bioactive materials in the membrane, (3) the incorporation of antibiotic and antibacterial agents in the membrane, (4) the administration of osteoconductive and osteoinductive scaffolds/graft materials into the bone defect, and (5) the administration of biological cues into the bone defect. The figure is adapted and reprinted from Eur J Oral Sci, 125 (5), Elgali I, Omar O, Dahlin C, Thomsen P, Guided bone regeneration: materials and biological mechanisms revisited., 315–337, copyright (2017), published by John Wiley & Sons Ltd. under a Creative Commons licence (CC‐BY‐NC‐ND): https://creativecommons.org/licenses/by-nc-nd/4.0/
The relative importance of the barrier and the bioactive membrane compartment has yet to be determined. Further, the relative importance of the barrier and bioactive membrane compared with exogenously administered grafts and bioactive compounds in the bone defect remains to be established. From scientific, developmental and clinical perspectives, the challenge of the barrier concept by new scientific data on the mechanisms of GBR as well as results from tissue engineering and drug delivery approaches stimulates new research questions and may expand future clinical opportunities for GBR.
CONFLICT OF INTEREST
Omar Omar received grants from Osteology Foundation , grants from Hjalmar Svensson Foundation, during the conduct of the study. Ibrahim Elgali received a grant from Hjalmar Svensson Foundation, during the conduct of the study.Peter Thomsen received grants from Swedish Research Council , grants from ALF/LUA Research Grant , grants from IngaBritt and Arne Lundberg Foundation, grants from Vilhelm and Martina Lundgren Vetenskapsfond , grants from Area of Advance Materials of Chalmers and GU Biomaterials within the Strategic Research Area initiative launched by the Swedish Government, during the conduct of the study. Christer Dahlin received a grant from Osteology Foundation, during the conduct of the study.
Omar O, Elgali I, Dahlin C, Thomsen P . Barrier membranes: More than the barrier effect?. J Clin Periodontol. 2019;46(Suppl. 21):103–123. 10.1111/jcpe.13068
Funding Information
This work was supported by the Swedish Research Council (K2015‐52X‐09495‐28‐4), the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG‐725641), the Osteology Foundation (project grants 14‐049 and 15‐103), the IngaBritt and Arne Lundberg Foundation, the Hjalmar Svensson Foundation, the Vilhelm and Martina Lundgren Vetenskapsfond and the Area of Advance Materials of Chalmers and GU Biomaterials within the Strategic Research Area initiative launched by the Swedish government.
REFERENCES
- Aghaloo, T. L. , & Moy, P. K. (2007). Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? International Journal of Oral & Maxillofacial Implants, 22(Suppl), 49–70. [PubMed] [Google Scholar]
- Ahn, Y. S. , Kim, S. G. , Kim, C. S. , Oh, J. S. , & Lim, S. C. (2012). Effect of guided bone regeneration with or without pericardium bioabsorbable membrane on bone formation. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 114, S126–S131. 10.1016/j.oooo.2011.08.008 [DOI] [PubMed] [Google Scholar]
- Al‐Hezaimi, K. , Rudek, I. , Al‐Hamdan, K. S. , Javed, F. , Nooh, N. , & Wang, H. L. (2013). Efficacy of using a dual layer of membrane (dPTFE placed over collagen) for ridge preservation in fresh extraction sites: A micro‐computed tomographic study in dogs. Clinical Oral Implants Research, 24, 1152–1157. 10.1111/j.1600-0501.2012.02526.x [DOI] [PubMed] [Google Scholar]
- Al‐Kattan, R. , Retzepi, M. , Calciolari, E. , & Donos, N. (2017). Microarray gene expression during early healing of GBR‐treated calvarial critical size defects. Clinical Oral Implants Research, 28, 1248–1257. 10.1111/clr.12949 [DOI] [PubMed] [Google Scholar]
- Amar, S. , Chung, K. M. , Nam, S. H. , Karatzas, S. , Myokai, F. , & Van Dyke, T. E. (1997). Markers of bone and cementum formation accumulate in tissues regenerated in periodontal defects treated with expanded polytetrafluoroethylene membranes. Journal of Periodontal Research, 32, 148–158. 10.1111/j.1600-0765.1997.tb01397.x [DOI] [PubMed] [Google Scholar]
- Artzi, Z. , Kozlovsky, A. , Nemcovsky, C. E. , Moses, O. , Tal, H. , Rohrer, M. D. , … Weinreb, M. (2008). Histomorphometric evaluation of natural mineral combined with a synthetic cell‐binding peptide (P‐15) in critical‐size defects in the rat calvaria. International Journal of Oral & Maxillofacial Implants, 23, 1063–1070. [PubMed] [Google Scholar]
- Azuma, Y. , Kaji, K. , Katogi, R. , Takeshita, S. , & Kudo, A. (2000). Tumor necrosis factor‐alpha induces differentiation of and bone resorption by osteoclasts. Journal of Biological Chemistry, 275, 4858–4864. 10.1074/jbc.275.7.4858 [DOI] [PubMed] [Google Scholar]
- Becker, W. , Lynch, S. E. , Lekholm, U. , Becker, B. E. , Caffesse, R. , Donath, K. , & Sanchez, R. (1992). A comparison of ePTFE membranes alone or in combination with platelet‐derived growth factors and insulin‐like growth factor‐I or demineralized freeze‐dried bone in promoting bone formation around immediate extraction socket implants. Journal of Periodontology, 63, 929–940. 10.1902/jop.1992.63.11.929 [DOI] [PubMed] [Google Scholar]
- Benic, G. I. , Thoma, D. S. , Munoz, F. , Sanz Martin, I. , Jung, R. E. , & Hammerle, C. H. (2015). Guided bone regeneration of peri‐implant defects with particulated and block xenogenic bone substitutes. Clinical Oral Implants Research, 27, 567–576. 10.1111/clr.12625 [DOI] [PubMed] [Google Scholar]
- Bernabe, P. F. , Melo, L. G. , Cintra, L. T. , Gomes‐Filho, J. E. , Dezan, E. Jr , & Nagata, M. J. (2012). Bone healing in critical‐size defects treated with either bone graft, membrane, or a combination of both materials: A histological and histometric study in rat tibiae. Clinical Oral Implants Research, 23, 384–388. 10.1111/j.1600-0501.2011.02166.x [DOI] [PubMed] [Google Scholar]
- Binder, N. B. , Niederreiter, B. , Hoffmann, O. , Stange, R. , Pap, T. , Stulnig, T. M. , … Redlich, K. (2009). Estrogen‐dependent and C‐C chemokine receptor‐2‐dependent pathways determine osteoclast behavior in osteoporosis. Nature Medicine, 15, 417–424. 10.1038/nm.1945 [DOI] [PubMed] [Google Scholar]
- Bocker, W. , Docheva, D. , Prall, W. C. , Egea, V. , Pappou, E. , Rossmann, O. , … Schieker, M. (2008). IKK‐2 is required for TNF‐alpha‐induced invasion and proliferation of human mesenchymal stem cells. Journal of Molecular Medicine (Berlin, Germany), 86, 1183–1192. 10.1007/s00109-008-0378-3 [DOI] [PubMed] [Google Scholar]
- Bornstein, M. M. , Halbritter, S. , Harnisch, H. , Weber, H. P. , & Buser, D. (2008). A retrospective analysis of patients referred for implant placement to a specialty clinic: Indications, surgical procedures, and early failures. International Journal of Oral & Maxillofacial Implants, 23, 1109–1116. [PubMed] [Google Scholar]
- Busenlechner, D. , Kantor, M. , Tangl, S. , Tepper, G. , Zechner, W. , Haas, R. , & Watzek, G. (2005). Alveolar ridge augmentation with a prototype trilayer membrane and various bone grafts: A histomorphometric study in baboons. Clinical Oral Implants Research, 16, 220–227. 10.1111/j.1600-0501.2004.01103.x [DOI] [PubMed] [Google Scholar]
- Buser, D. , Hoffmann, B. , Bernard, J. P. , Lussi, A. , Mettler, D. , & Schenk, R. K. (1998). Evaluation of filling materials in membrane–protected bone defects. A comparative histomorphometric study in the mandible of miniature pigs. Clinical Oral Implants Research, 9, 137–150. 10.1034/j.1600-0501.1998.090301.x [DOI] [PubMed] [Google Scholar]
- Calciolari, E. , Mardas, N. , Dereka, X. , Anagnostopoulos, A. K. , Tsangaris, G. T. , & Donos, N. (2018). Protein expression during early stages of bone regeneration under hydrophobic and hydrophilic titanium domes. A pilot study. Journal of Periodontal Research, 53, 174–187. 10.1111/jre.12498 [DOI] [PubMed] [Google Scholar]
- Calciolari, E. , Ravanetti, F. , Strange, A. , Mardas, N. , Bozec, L. , Cacchioli, A. , … Donos, N. (2018). Degradation pattern of a porcine collagen membrane in an in vivo model of guided bone regeneration. Journal of Periodontal Research, 53, 430–439. 10.1111/jre.12530 [DOI] [PubMed] [Google Scholar]
- Ceradini, D. J. , Kulkarni, A. R. , Callaghan, M. J. , Tepper, O. M. , Bastidas, N. , Kleinman, M. E. , … Gurtner, G. C. (2004). Progenitor cell trafficking is regulated by hypoxic gradients through HIF‐1 induction of SDF‐1. Nature Medicine, 10, 858–864. 10.1038/nm1075 [DOI] [PubMed] [Google Scholar]
- Chang, Y. Y. , Lee, J. S. , Kim, M. S. , Choi, S. H. , Chai, J. K. , & Jung, U. W. (2015). Comparison of collagen membrane and bone substitute as a carrier for rhBMP‐2 in lateral onlay graft. Clinical Oral Implants Research, 26, e13–e19. 10.1111/clr.12320 [DOI] [PubMed] [Google Scholar]
- Cho, K. S. , Choi, S. H. , Han, K. H. , Chai, J. K. , Wikesjo, U. M. , & Kim, C. K. (1998). Alveolar bone formation at dental implant dehiscence defects following guided bone regeneration and xenogeneic freeze‐dried demineralized bone matrix. Clinical Oral Implants Research, 9, 419–428. 10.1034/j.1600-0501.1996.090607.x [DOI] [PubMed] [Google Scholar]
- Clementini, M. , Morlupi, A. , Canullo, L. , Agrestini, C. , & Barlattani, A. (2012). Success rate of dental implants inserted in horizontal and vertical guided bone regenerated areas: A systematic review. International Journal of Oral and Maxillofacial Surgery, 41, 847–852. 10.1016/j.ijom.2012.03.016 [DOI] [PubMed] [Google Scholar]
- Dahlin, C. (2010). Optimal implant placement in the esthetic zone by the use of Guided Bone Regeneration In Andersson L., Kahnberg K.‐E., & Pogrel M. A. (Eds.), Oral and maxillofacial surgery (pp. 357–366). Chicago, IL: John Wiley & Sons Ltd.. [Google Scholar]
- Dahlin, C. , Alberius, P. , & Linde, A. (1991). Osteopromotion for cranioplasty. An experimental study in rats using a membrane technique. Journal of Neurosurgery, 74, 487–491. 10.3171/jns.1991.74.3.0487 [DOI] [PubMed] [Google Scholar]
- Dahlin, C. , Andersson, L. , & Linde, A. (1991). Bone augmentation at fenestrated implants by an osteopromotive membrane technique. A controlled clinical study. Clinical Oral Implants Research, 2, 159–165. 10.1034/j.1600-0501.1991.020401.x [DOI] [PubMed] [Google Scholar]
- Dahlin, C. , Linde, A. , Gottlow, J. , & Nyman, S. (1988). Healing of bone defects by guided tissue regeneration. Plastic and Reconstructive Surgery, 81, 672–676. 10.1097/00006534-198805000-00004 [DOI] [PubMed] [Google Scholar]
- Dahlin, C. , Sennerby, L. , Lekholm, U. , Linde, A. , & Nyman, S. (1989). Generation of new bone around titanium implants using a membrane technique: An experimental study in rabbits. International Journal of Oral & Maxillofacial Implants, 4, 19–25. [PubMed] [Google Scholar]
- De Angelis, N. , Felice, P. , Pellegrino, G. , Camurati, A. , Gambino, P. , & Esposito, M. (2011). Guided bone regeneration with and without a bone substitute at single post‐extractive implants: 1‐year post‐loading results from a pragmatic multicentre randomised controlled trial. European Journal of Oral Implantology, 4, 313–325. [PubMed] [Google Scholar]
- De Marco, A. C. , Jardini, M. A. , Modolo, F. , Nunes, F. D. , & de Lima, L. A. (2012). Immunolocalization of bone morphogenetic protein 2 during the early healing events after guided bone regeneration. Oral Surgery, Oral Medicine, Oral Pathology, and Oral Radiology, 113, 533–541. 10.1016/j.oooo.2011.09.016 [DOI] [PubMed] [Google Scholar]
- Dies, F. , Etienne, D. , Abboud, N. B. , & Ouhayoun, J. P. (1996). Bone regeneration in extraction sites after immediate placement of an e‐PTFE membrane with or without a biomaterial. A report on 12 consecutive cases. Clinical Oral Implants Research, 7, 277–285. 10.1034/j.1600-0501.1996.070310.x [DOI] [PubMed] [Google Scholar]
- Dimitriou, R. , Mataliotakis, G. I. , Calori, G. M. , & Giannoudis, P. V. (2012). The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Medicine, 10, 81 10.1186/1741-7015-10-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donos, N. , Graziani, F. , Mardas, N. , & Kostopoulos, L. (2011). The use of human hypertrophic chondrocytes‐derived extracellular matrix for the treatment of critical‐size calvarial defects. Clinical Oral Implants Research, 22, 1346–1353. 10.1111/j.1600-0501.2010.02120.x [DOI] [PubMed] [Google Scholar]
- Donos, N. , Kostopoulos, L. , & Karring, T. (2002). Alveolar ridge augmentation using a resorbable copolymer membrane and autogenous bone grafts. An experimental study in the rat. Clinical Oral Implants Research, 13, 203–213. 10.1034/j.1600-0501.2002.130211.x [DOI] [PubMed] [Google Scholar]
- Donos, N. , Mardas, N. , & Chadha, V. (2008). Clinical outcomes of implants following lateral bone augmentation: Systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). Journal of Clinical Periodontology, 35, 173–202. 10.1111/j.1600-051X.2008.01269.x [DOI] [PubMed] [Google Scholar]
- Dupoirieux, L. , Neves, M. , & Pourquier, D. (2000). Comparison of pericranium and eggshell as space fillers used in combination with guided bone regeneration: An experimental study. Journal of Oral and Maxillofacial Surgery, 58, 40–46; discussion 47–48. 10.1016/S0278-2391(00)80013-0 [DOI] [PubMed] [Google Scholar]
- Elgali, I. , Omar, O. , Dahlin, C. , & Thomsen, P. (2017). Guided bone regeneration: Materials and biological mechanisms revisited. European Journal of Oral Sciences, 125, 315–337. 10.1111/eos.12364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgali, I. , Turri, A. , Xia, W. , Norlindh, B. , Johansson, A. , Dahlin, C. , … Omar, O. (2016). Guided bone regeneration using resorbable membrane and different bone substitutes: Early histological and molecular events. Acta Biomaterialia, 29, 409–423. 10.1016/j.actbio.2015.10.005 [DOI] [PubMed] [Google Scholar]
- Fei, Y. , Gronowicz, G. , & Hurley, M. M. (2013). Fibroblast growth factor‐2, bone homeostasis and fracture repair. Current Pharmaceutical Design, 19, 3354–3363. 10.2174/1381612811319190002 [DOI] [PubMed] [Google Scholar]
- Fernandes, P. G. , Novaes, A. B. Jr , de Queiroz, A. C. , de Souza, S. L. , Taba, M. Jr , Palioto, D. B. , & Grisi, M. F. (2011). Ridge preservation with acellular dermal matrix and anorganic bone matrix cell‐binding peptide P‐15 after tooth extraction in humans. Journal of Periodontology, 82, 72–79. 10.1902/jop.2010.100241 [DOI] [PubMed] [Google Scholar]
- Fu, X. , Han, B. , Cai, S. , Lei, Y. , Sun, T. , & Sheng, Z. (2009). Migration of bone marrow‐derived mesenchymal stem cells induced by tumor necrosis factor‐alpha and its possible role in wound healing. Wound Repair and Regeneration, 17, 185–191. 10.1111/j.1524-475x.2009.00454.x [DOI] [PubMed] [Google Scholar]
- Ge, Y. , Feng, H. , & Wang, L. (2011). Application of a novel resorbable membrane in the treatment of calvarial defects in rats. Journal of Biomaterials Science. Polymer Edition, 22, 2417–2429. 10.1163/092050610x540477 [DOI] [PubMed] [Google Scholar]
- Gerstenfeld, L. C. , Cho, T. J. , Kon, T. , Aizawa, T. , Cruceta, J. , Graves, B. D. , & Einhorn, T. A. (2001). Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor‐alpha signaling. Cells, Tissues, Organs, 169, 285–294. 10.1159/000047893 [DOI] [PubMed] [Google Scholar]
- Guarnieri, R. , Stefanelli, L. , De Angelis, F. , Mencio, F. , Pompa, G. , & Di Carlo, S. (2017). Extraction socket preservation using porcine‐derived collagen membrane alone or associated with porcine‐derived bone. Clinical Results of Randomized Controlled Study. Journal of Oral & Maxillofacial Research, 8, e5 10.5037/jomr.2017.8305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri, R. , Testarelli, L. , Stefanelli, L. , De Angelis, F. , Mencio, F. , Pompa, G. , & Di Carlo, S. (2017). Bone healing in extraction sockets covered with collagen membrane alone or associated with porcine‐derived bone graft: A comparative histological and histomorphometric analysis. Journal of Oral & Maxillofacial Research, 8, e4 10.5037/jomr.2017.8404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guda, T. , Walker, J. A. , Singleton, B. M. , Hernandez, J. W. , Son, J. S. , Kim, S. G. , … Wenke, J. C. (2013). Guided bone regeneration in long‐bone defects with a structural hydroxyapatite graft and collagen membrane. Tissue Engineering. Part A, 19, 1879–1888. 10.1089/ten.TEA.2012.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerle, C. H. , Chiantella, G. C. , Karring, T. , & Lang, N. P. (1998). The effect of a deproteinized bovine bone mineral on bone regeneration around titanium dental implants. Clinical Oral Implants Research, 9, 151–162. 10.1034/j.1600-0501.1998.090302.x [DOI] [PubMed] [Google Scholar]
- Hammerle, C. H. , Schmid, J. , Lang, N. P. , & Olah, A. J. (1995). Temporal dynamics of healing in rabbit cranial defects using guided bone regeneration. Journal of Oral and Maxillofacial Surgery, 53, 167–174. 10.1016/0278-2391(95)90396-8 [DOI] [PubMed] [Google Scholar]
- Hermann, J. S. , & Buser, D. (1996). Guided bone regeneration for dental implants. Current Opinion in Periodontology, 3, 168–177. [PubMed] [Google Scholar]
- Hess, K. , Ushmorov, A. , Fiedler, J. , Brenner, R. E. , & Wirth, T. (2009). TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF‐kappaB signaling pathway. Bone, 45, 367–376. 10.1016/j.bone.2009.04.252 [DOI] [PubMed] [Google Scholar]
- Hong, K. S. , Kim, E. C. , Bang, S. H. , Chung, C. H. , Lee, Y. I. , Hyun, J. K. , … Kim, H. W. (2010). Bone regeneration by bioactive hybrid membrane containing FGF2 within rat calvarium. Journal of Biomedical Materials Research. Part A, 94, 1187–1194. 10.1002/jbm.a.32799 [DOI] [PubMed] [Google Scholar]
- Jardini, M. A. , De Marco, A. C. , & Lima, L. A. (2005). Early healing pattern of autogenous bone grafts with and without e‐PTFE membranes: A histomorphometric study in rats. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 100, 666–673. 10.1016/j.tripleo.2005.03.020 [DOI] [PubMed] [Google Scholar]
- Jensen, S. S. , & Terheyden, H. (2009). Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone‐substitute materials. International Journal of Oral & Maxillofacial Implants, 24(Suppl), 218–236. [PubMed] [Google Scholar]
- Ji, W. , Yang, F. , Ma, J. , Bouma, M. J. , Boerman, O. C. , Chen, Z. , … Jansen, J. A. (2013). Incorporation of stromal cell‐derived factor‐1alpha in PCL/gelatin electrospun membranes for guided bone regeneration. Biomaterials, 34, 735–745. 10.1016/j.biomaterials.2012.10.016 [DOI] [PubMed] [Google Scholar]
- Jo, J. Y. , Jeong, S. I. , Shin, Y. M. , Kang, S. S. , Kim, S. E. , Jeong, C. M. , & Huh, J. B. (2015). Sequential delivery of BMP‐2 and BMP‐7 for bone regeneration using a heparinized collagen membrane. International Journal of Oral and Maxillofacial Surgery, 44, 921–928. 10.1016/j.ijom.2015.02.015 [DOI] [PubMed] [Google Scholar]
- Jung, R. E. , Kokovic, V. , Jurisic, M. , Yaman, D. , Subramani, K. , & Weber, F. E. (2011). Guided bone regeneration with a synthetic biodegradable membrane: A comparative study in dogs. Clinical Oral Implants Research, 22, 802–807. 10.1111/j.1600-0501.2010.02068.x [DOI] [PubMed] [Google Scholar]
- Jung, U. W. , Lee, J. S. , Lee, G. , Lee, I. K. , Hwang, J. W. , Kim, M. S. , … Chai, J. K. (2013). Role of collagen membrane in lateral onlay grafting with bovine hydroxyapatite incorporated with collagen matrix in dogs. Journal of Periodontal & Implant Science, 43, 64–71. 10.5051/jpis.2013.43.2.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaigler, D. , Pagni, G. , Park, C. H. , Braun, T. M. , Holman, L. A. , Yi, E. , … Giannobile, W. V. (2013). Stem cell therapy for craniofacial bone regeneration: A randomized, controlled feasibility trial. Cell Transplantation, 22, 767–777. 10.3727/096368912x652968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp, J. M. , & Leng Teo, G. S. (2009). Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell, 4, 206–216. 10.1016/j.stem.2009.02.001 [DOI] [PubMed] [Google Scholar]
- Katagiri, T. , & Takahashi, N. (2002). Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Diseases, 8, 147–159. 10.1034/j.1601-0825.2002.01829.x [DOI] [PubMed] [Google Scholar]
- Khojasteh, A. , Kheiri, L. , Motamedian, S. R. , & Khoshkam, V. (2017). Guided bone regeneration for the reconstruction of alveolar bone defects. Annals of Maxillofacial Surgery, 7, 263–277. 10.4103/ams.ams_76_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M. , Kim, J. H. , Lee, J. Y. , Cho, K. , Kang, S. S. , Kim, G. , … Choi, S. H. (2008). Effect of bone mineral with or without collagen membrane in ridge dehiscence defects following premolar extraction. In vivo (Athens, Greece), 22, 231–236. [PubMed] [Google Scholar]
- Kitaori, T. , Ito, H. , Schwarz, E. M. , Tsutsumi, R. , Yoshitomi, H. , Oishi, S. , … Nakamura, T. (2009). Stromal cell‐derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis and Rheumatism, 60, 813–823. 10.1002/art.24330 [DOI] [PubMed] [Google Scholar]
- Koerdt, S. , Ristow, O. , Wannhoff, A. , Kubler, A. C. , & Reuther, T. (2014). Expression of growth factors during the healing process of alveolar ridge augmentation procedures using autogenous bone grafts in combination with GTR and an anorganic bovine bone substitute: An immunohistochemical study in the sheep. Clinical Oral Investigations, 18, 179–188. 10.1007/s00784-013-0938-y [DOI] [PubMed] [Google Scholar]
- Kohal, R. J. , Mellas, P. , Hurzeler, M. B. , Trejo, P. M. , Morrison, E. , & Caffesse, R. G. (1998). The effects of guided bone regeneration and grafting on implants placed into immediate extraction sockets. An experimental study in dogs. Journal of Periodontology, 69, 927–937. 10.1902/jop.1998.69.8.927 [DOI] [PubMed] [Google Scholar]
- Kostopoulos, L. , & Karring, T. (1994). Guided bone regeneration in mandibular defects in rats using a bioresorbable polymer. Clinical Oral Implants Research, 5, 66–74. 10.1034/j.1600-0501.1994.050202.x [DOI] [PubMed] [Google Scholar]
- Kuchler, U. , Rybaczek, T. , Dobask, T. , Heimel, P. , Tangl, S. , Klehm, J. , … Gruber, R. (2018). Bone‐conditioned medium modulates the osteoconductive properties of collagen membranes in a rat calvaria defect model. Clinical Oral Implants Research, 29, 381–388. 10.1111/clr.13133 [DOI] [PubMed] [Google Scholar]
- Kuru, L. , Griffiths, G. S. , Petrie, A. , & Olsen, I. (1999). Alkaline phosphatase activity is upregulated in regenerating human periodontal cells. Journal of Periodontal Research, 34, 123–127. 10.1111/j.1600-0765.1999.tb02231.x [DOI] [PubMed] [Google Scholar]
- Kuru, L. , Parkar, M. H. , Griffiths, G. S. , & Olsen, I. (2001). Flow cytometry analysis of guided tissue regeneration‐associated human periodontal cells. Journal of Periodontology, 72, 1016–1024. 10.1902/jop.2001.72.8.1016 [DOI] [PubMed] [Google Scholar]
- Kutan, E. , Duygu‐Capar, G. , Ozcakir‐Tomruk, C. , Dilek, O. C. , Ozen, F. , Erdogan, O. , … Gurel, A. (2016). Efficacy of doxycycline release collagen membrane on surgically created and contaminated defects in rat tibiae: A histopathological and microbiological study. Archives of Oral Biology, 63, 15–21. 10.1016/j.archoralbio.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Lai, C. H. , Zhou, L. , Wang, Z. L. , Lu, H. B. , & Gao, Y. (2013). Use of a collagen membrane loaded with recombinant human bone morphogenetic protein‐2 with collagen‐binding domain for vertical guided bone regeneration. Journal of Periodontology, 84, 950–957. 10.1902/jop.2012.120415 [DOI] [PubMed] [Google Scholar]
- Lee, J. H. , Lee, Y. J. , Cho, H. J. , Kim, D. W. , & Shin, H. (2015). The incorporation of bFGF mediated by heparin into PCL/gelatin composite fiber meshes for guided bone regeneration. Drug Delivery and Translational Research, 5, 146–159. 10.1007/s13346-013-0154-y [DOI] [PubMed] [Google Scholar]
- Lee, Y. J. , Lee, J. H. , Cho, H. J. , Kim, H. K. , Yoon, T. R. , & Shin, H. (2013). Electrospun fibers immobilized with bone forming peptide‐1 derived from BMP7 for guided bone regeneration. Biomaterials, 34, 5059–5069. 10.1016/j.biomaterials.2013.03.051 [DOI] [PubMed] [Google Scholar]
- Li, J. , Zuo, Y. , Man, Y. , Mo, A. , Huang, C. , Liu, M. , … Li, Y. (2012). Fabrication and biocompatibility of an antimicrobial composite membrane with an asymmetric porous structure. Journal of Biomaterials Science. Polymer Edition, 23, 81–96. 10.1163/092050610x543159 [DOI] [PubMed] [Google Scholar]
- Liao, H. T. , Chen, C. T. , Chen, C. H. , Chen, J. P. , & Tsai, J. C. (2011). Combination of guided osteogenesis with autologous platelet‐rich fibrin glue and mesenchymal stem cell for mandibular reconstruction. Journal of Trauma, 70, 228–237. 10.1097/TA.0b013e3181e12b56 [DOI] [PubMed] [Google Scholar]
- Lima, L. L. , Goncalves, P. F. , Sallum, E. A. , Casati, M. Z. , & Nociti, F. H. Jr (2008). Guided tissue regeneration may modulate gene expression in periodontal intrabony defects: A human study. Journal of Periodontal Research, 43, 459–464. 10.1111/j.1600-0765.2008.01094.x [DOI] [PubMed] [Google Scholar]
- Linde, A. , & Hedner, E. (1995). Recombinant bone morphogenetic protein‐2 enhances bone healing, guided by osteopromotive e‐PTFE membranes: An experimental study in rats. Calcified Tissue International, 56, 549–553. 10.1007/BF00298588 [DOI] [PubMed] [Google Scholar]
- Liu, J. , & Kerns, D. G. (2014). Mechanisms of guided bone regeneration: A review. The Open Dentistry Journal, 8, 56–65. 10.2174/1874210601408010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczyszyn, S. M. , Papalexiou, V. , Novaes, A. B. Jr , Grisi, M. F. , Souza, S. L. , & Taba, M. Jr (2005). Acellular dermal matrix and hydroxyapatite in prevention of ridge deformities after tooth extraction. Implant Dentistry, 14, 176–184. 10.1097/01.id.0000165082.77499.41 [DOI] [PubMed] [Google Scholar]
- Lundgren, D. , Lundgren, A. K. , Sennerby, L. , & Nyman, S. (1995). Augmentation of intramembraneous bone beyond the skeletal envelope using an occlusive titanium barrier. An experimental study in the rabbit. Clinical Oral Implants Research, 6, 67–72. 10.1034/j.1600-0501.1995.060201.x [DOI] [PubMed] [Google Scholar]
- Martinez, A. , Balboa, O. , Gasamans, I. , Otero‐Cepeda, X. L. , & Guitian, F. (2015). Deproteinated bovine bone vs. beta‐tricalcium phosphate as bone graft substitutes: Histomorphometric longitudinal study in the rabbit cranial vault. Clinical Oral Implants Research, 26, 623–632. 10.1111/clr.12349 [DOI] [PubMed] [Google Scholar]
- Matsumoto, G. , Hoshino, J. , Kinoshita, Y. , Sugita, Y. , Kubo, K. , Maeda, H. , … Kinoshita, Y. (2012). Alveolar bone regeneration using poly‐(lactic acid‐co‐glycolic acid‐co‐epsilon‐caprolactone) porous membrane with collagen sponge containing basic fibroblast growth factor: An experimental study in the dog. Journal of Biomaterials Applications, 27, 485–493. 10.1177/0885328211414940 [DOI] [PubMed] [Google Scholar]
- Matsuzaka, K. , Shimono, M. , & Inoue, T. (2001). Characteristics of newly formed bone during guided bone regeneration: Observations by immunohistochemistry and confocal laser scanning microscopy. The Bulletin of Tokyo Dental College, 42, 225–234. 10.2209/tdcpublication.42.225 [DOI] [PubMed] [Google Scholar]
- Mattout, P. , & Mattout, C. (2000). Conditions for success in guided bone regeneration: Retrospective study on 376 implant sites. Journal of Periodontology, 71, 1904–1909. 10.1902/jop.2000.71.12.1904 [DOI] [PubMed] [Google Scholar]
- Mattout, P. , Nowzari, H. , & Mattout, C. (1995). Clinical evaluation of guided bone regeneration at exposed parts of Branemark dental implants with and without bone allograft. Clinical Oral Implants Research, 6, 189–195. 10.1034/j.1600-0501.1995.060308.x [DOI] [PubMed] [Google Scholar]
- Meijndert, L. , Raghoebar, G. M. , Schupbach, P. , Meijer, H. J. , & Vissink, A. (2005). Bone quality at the implant site after reconstruction of a local defect of the maxillary anterior ridge with chin bone or deproteinised cancellous bovine bone. International Journal of Oral and Maxillofacial Surgery, 34, 877–884. 10.1016/j.ijom.2005.04.017 [DOI] [PubMed] [Google Scholar]
- Melo, L. G. , Nagata, M. J. , Bosco, A. F. , Ribeiro, L. L. , & Leite, C. M. (2005). Bone healing in surgically created defects treated with either bioactive glass particles, a calcium sulfate barrier, or a combination of both materials. A histological and histometric study in rat tibias. Clinical Oral Implants Research, 16, 683–691. 10.1111/j.1600-0501.2005.01090.x [DOI] [PubMed] [Google Scholar]
- Miyamoto, I. , Tsuboi, Y. , Takahashi, K. , Hyon, S. H. , & Iizuka, T. (2004). Enhancement of bone volume in guided bone augmentation by cell transplants derived from periosteum: An experimental study in rabbit calvarium bone. Clinical Oral Implants Research, 15, 308–314. 10.1111/j.1600-0501.2004.01011.x [DOI] [PubMed] [Google Scholar]
- Okazaki, K. , Shimizu, Y. , Xu, H. , & Ooya, K. (2005). Blood‐filled spaces with and without deproteinized bone grafts in guided bone regeneration. A histomorphometric study of the rabbit skull using non‐resorbable membrane. Clinical Oral Implants Research, 16, 236–243. 10.1111/j.1600-0501.2004.01095.x [DOI] [PubMed] [Google Scholar]
- Omar, O. , Dahlin, A. , Gasser, A. , & Dahlin, C. (2018). Tissue dynamics and regenerative outcome in two resorbable non‐cross‐linked collagen membranes for guided bone regeneration: A preclinical molecular and histological study in vivo . Clinical Oral Implants Research, 29, 7–19. 10.1111/clr.13032 [DOI] [PubMed] [Google Scholar]
- Omar, O. , Trobos, M. , Johansson, A. , Emanuelsson, L. , Sahlin, H. , & Dahlin, C. (2018) PR586: Molecular events in bone and soft tissue at different non‐resorbable PTFE membranes during GBR. Journal of Clinical Periodontology, 45, 320–320. 10.1111/jcpe.588_12915 [DOI] [Google Scholar]
- Park, J. Y. , Jung, I. H. , Kim, Y. K. , Lim, H. C. , Lee, J. S. , Jung, U. W. , & Choi, S. H. (2015). Guided bone regeneration using 1‐ethyl‐3‐(3‐dimethylaminopropyl) carbodiimide (EDC)‐cross‐linked type‐I collagen membrane with biphasic calcium phosphate at rabbit calvarial defects. Biomaterials Research, 19, 15 10.1186/s40824-015-0038-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, Y. J. , Ku, Y. , Chung, C. P. , & Lee, S. J. (1998). Controlled release of platelet‐derived growth factor from porous poly (L‐lactide) membranes for guided tissue regeneration. Journal of Controlled Release, 51, 201–211. 10.1016/S0168-3659(97)00169-7 [DOI] [PubMed] [Google Scholar]
- Park, Y. J. , Lee, Y. M. , Park, S. N. , Lee, J. Y. , Ku, Y. , Chung, C. P. , & Lee, S. J. (2000). Enhanced guided bone regeneration by controlled tetracycline release from poly(L‐lactide) barrier membranes. Journal of Biomedical Materials Research, 51, 391–397. 10.1002/(ISSN)1097-4636 [DOI] [PubMed] [Google Scholar]
- Piao, Z. G. , Kim, J. S. , Son, J. S. , Lee, S. Y. , Fang, X. H. , Oh, J. S. , … Kim, S. G. (2014). Osteogenic evaluation of collagen membrane containing drug‐loaded polymeric microparticles in a rat calvarial defect model. Tissue Engineering. Part A, 20, 3322–3331. 10.1089/ten.TEA.2013.0717 [DOI] [PubMed] [Google Scholar]
- Pieri, F. , Lucarelli, E. , Corinaldesi, G. , Aldini, N. N. , Fini, M. , Parrilli, A. , … Marchetti, C. (2010). Dose‐dependent effect of adipose‐derived adult stem cells on vertical bone regeneration in rabbit calvarium. Biomaterials, 31, 3527–3535. 10.1016/j.biomaterials.2010.01.066 [DOI] [PubMed] [Google Scholar]
- Pieri, F. , Lucarelli, E. , Corinaldesi, G. , Fini, M. , Aldini, N. N. , Giardino, R. , … Marchetti, C. (2009). Effect of mesenchymal stem cells and platelet‐rich plasma on the healing of standardized bone defects in the alveolar ridge: A comparative histomorphometric study in minipigs. Journal of Oral and Maxillofacial Surgery, 67, 265–272. 10.1016/j.joms.2008.06.036 [DOI] [PubMed] [Google Scholar]
- Poniatowski, Ł. A. , Wojdasiewicz, P. , Gasik, R. , & Szukiewicz, D. (2015). Transforming growth factor beta family: Insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediators of Inflammation, 2015, 10.1155/2015/137823 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz, T. P. , Hochuli‐Vieira, E. , Gabrielli, M. A. , & Cancian, D. C. (2006). Use of bovine bone graft and bone membrane in defects surgically created in the cranial vault of rabbits. Histologic comparative analysis. International Journal of Oral & Maxillofacial Implants, 21, 29–35. [PubMed] [Google Scholar]
- Ramalingam, S. , Al‐Rasheed, A. , ArRejaie, A. , Nooh, N. , Al‐Kindi, M. , & Al‐Hezaimi, K. (2016). Guided bone regeneration in standardized calvarial defects using beta‐tricalcium phosphate and collagen membrane: A real‐time in vivo micro‐computed tomographic experiment in rats. Odontology, 104, 199–210. 10.1007/s10266-015-0211-8 [DOI] [PubMed] [Google Scholar]
- Retzepi, M. , & Donos, N. (2010). Guided Bone Regeneration: Biological principle and therapeutic applications. Clinical Oral Implants Research, 21, 567–576. 10.1111/j.1600-0501.2010.01922.x [DOI] [PubMed] [Google Scholar]
- Schenk, R. K. , Buser, D. , Hardwick, W. R. , & Dahlin, C. (1994). Healing pattern of bone regeneration in membrane‐protected defects: A histologic study in the canine mandible. International Journal of Oral & Maxillofacial Implants, 9, 13–29. [PubMed] [Google Scholar]
- Schlegel, A. K. , Donath, K. , & Weida, S. (1998). Histological findings in guided bone regeneration (GBR) around titanium dental implants with autogenous bone chips using a new resorbable membrane. Journal of Long‐Term Effects of Medical Implants, 8, 211–224. [PubMed] [Google Scholar]
- Schmid, J. , Hammerle, C. H. , Fluckiger, L. , Winkler, J. R. , Olah, A. J. , Gogolewski, S. , & Lang, N. P. (1997). Blood‐filled spaces with and without filler materials in guided bone regeneration. A comparative experimental study in the rabbit using bioresorbable membranes. Clinical Oral Implants Research, 8, 75–81. 10.1034/j.1600-0501.1997.080201.x [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Mihatovic, I. , Golubovic, V. , Hegewald, A. , & Becker, J. (2012). Influence of two barrier membranes on staged guided bone regeneration and osseointegration of titanium implants in dogs: Part 1. Augmentation using bone graft substitutes and autogenous bone. Clinical Oral Implants Research, 23, 83–89. 10.1111/j.1600-0501.2011.02187.x [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Sager, M. , Ferrari, D. , Mihatovic, I. , & Becker, J. (2009). Influence of recombinant human platelet‐derived growth factor on lateral ridge augmentation using biphasic calcium phosphate and guided bone regeneration: A histomorphometric study in dogs. Journal of Periodontology, 80, 1315–1323. 10.1902/jop.2009.090034 [DOI] [PubMed] [Google Scholar]
- Shah, P. , Keppler, L. , & Rutkowski, J. (2014). A review of platelet derived growth factor playing pivotal role in bone regeneration. Journal of Oral Implantology, 40, 330–340. 10.1563/aaid-joi-d-11-00173 [DOI] [PubMed] [Google Scholar]
- Shim, J.‐H. , Yoon, M.‐C. , Jeong, C.‐M. , Jang, J. , Jeong, S.‐I. , Cho, D.‐W. , & Huh, J.‐B. (2014). Efficacy of rhBMP‐2 loaded PCL/PLGA/β‐TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomedical Materials, 9, 065006 10.1088/1748-6041/9/6/065006 [DOI] [PubMed] [Google Scholar]
- Shin, Y. S. , Seo, J. Y. , Oh, S. H. , Kim, J. H. , Kim, S. T. , Park, Y. B. , & Moon, H. S. (2014). The effects of ErhBMP‐2‐/EGCG‐coated BCP bone substitute on dehiscence around dental implants in dogs. Oral Diseases, 20, 281–287. 10.1111/odi.12109 [DOI] [PubMed] [Google Scholar]
- Simion, M. , Dahlin, C. , Rocchietta, I. , Stavropoulos, A. , Sanchez, R. , & Karring, T. (2007). Vertical ridge augmentation with guided bone regeneration in association with dental implants: An experimental study in dogs. Clinical Oral Implants Research, 18, 86–94. 10.1111/j.1600-0501.2006.01291.x [DOI] [PubMed] [Google Scholar]
- Stavropoulos, F. , Dahlin, C. , Ruskin, J. D. , & Johansson, C. (2004). A comparative study of barrier membranes as graft protectors in the treatment of localized bone defects. An experimental study in a canine model. Clinical Oral Implants Research, 15, 435–442. 10.1111/j.1600-0501.2004.01029.x [DOI] [PubMed] [Google Scholar]
- Stavropoulos, A. , Kostopoulos, L. , Mardas, N. , Nyengaard, J. R. , & Karring, T. (2001). Deproteinized bovine bone used as an adjunct to guided bone augmentation: An experimental study in the rat. Clinical Implant Dentistry and Related Research, 3, 156–165. 10.1111/j.1708-8208.2001.tb00136.x [DOI] [PubMed] [Google Scholar]
- Stavropoulos, A. , Kostopoulos, L. , Nyengaard, J. R. , & Karring, T. (2003). Deproteinized bovine bone (Bio‐Oss) and bioactive glass (Biogran) arrest bone formation when used as an adjunct to guided tissue regeneration (GTR): An experimental study in the rat. Journal of Clinical Periodontology, 30, 636–643. 10.1034/j.1600-051X.2003.00093.x [DOI] [PubMed] [Google Scholar]
- Stetzer, K. , Cooper, G. , Gassner, R. , Kapucu, R. , Mundell, R. , & Mooney, M. P. (2002). ffects of fixation type and guided tissue regeneration on maxillary osteotomy healing in rabbits. Journal of Oral and Maxillofacial Surgery, 60, 427–436; discussion 436‐427. 10.1053/joms.2002.31232 [DOI] [PubMed] [Google Scholar]
- Sverzut, C. E. , Faria, P. E. , Magdalena, C. M. , Trivellato, A. E. , Mello‐Filho, F. V. , Paccola, C. A. , … Salata, L. A. (2008). Reconstruction of mandibular segmental defects using the guided‐bone regeneration technique with polylactide membranes and/or autogenous bone graft: A preliminary study on the influence of membrane permeability. Journal of Oral and Maxillofacial Surgery, 66, 647–656. 10.1016/j.joms.2007.06.664 [DOI] [PubMed] [Google Scholar]
- Taga, M. L. , Granjeiro, J. M. , Cestari, T. M. , & Taga, R. (2008). Healing of critical‐size cranial defects in guinea pigs using a bovine bone‐derived resorbable membrane. International Journal of Oral & Maxillofacial Implants, 23, 427–436. [PubMed] [Google Scholar]
- Taguchi, Y. , Amizuka, N. , Nakadate, M. , Ohnishi, H. , Fujii, N. , Oda, K. , … Maeda, T. (2005). A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials, 26, 6158–6166. 10.1016/j.biomaterials.2005.03.023 [DOI] [PubMed] [Google Scholar]
- Tanaka, S. , Matsuzaka, K. , Sato, D. , & Inoue, T. (2007). Characteristics of newly formed bone during guided bone regeneration: Analysis of cbfa‐1, osteocalcin, and VEGF expression. Journal of Oral Implantology, 33, 321–326. 10.1563/1548-1336(2007)33[321:confbd]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Tehemar, S. , Hanes, P. , & Sharawy, M. (2003). Enhancement of osseointegration of implants placed into extraction sockets of healthy and periodontally diseased teeth by using graft material, an ePTFE membrane, or a combination. Clinical Implant Dentistry and Related Research, 5, 193–211. 10.1111/j.1708-8208.2003.tb00202.x [DOI] [PubMed] [Google Scholar]
- Thomaidis, V. , Kazakos, K. , Lyras, D. N. , Dimitrakopoulos, I. , Lazaridis, N. , Karakasis, D. , … Agrogiannis, G. (2008). Comparative study of 5 different membranes for guided bone regeneration of rabbit mandibular defects beyond critical size. Medical Science Monitor, 14, Br67–Br73. [PubMed] [Google Scholar]
- Tokuda, S. , Obata, A. , & Kasuga, T. (2009). Preparation of poly(lactic acid)/siloxane/calcium carbonate composite membranes with antibacterial activity. Acta Biomaterialia, 5, 1163–1168. 10.1016/j.actbio.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Tsuchiya, S. , Ohmori, M. , Hara, K. , Fujio, M. , Ikeno, M. , Hibi, H. , & Ueda, M. (2015). An Experimental study on guided bone regeneration using a polylactide‐co‐glycolide membrane‐immobilized conditioned medium. International Journal of Oral & Maxillofacial Implants, 30, 1175–1186. 10.11607/jomi.3915 [DOI] [PubMed] [Google Scholar]
- Turri, A. , Elgali, I. , Vazirisani, F. , Johansson, A. , Emanuelsson, L. , Dahlin, C. , … Omar, O. (2016). Guided bone regeneration is promoted by the molecular events in the membrane compartment. Biomaterials, 84, 167–183. 10.1016/j.biomaterials.2016.01.034 [DOI] [PubMed] [Google Scholar]
- Ueyama, Y. , Ishikawa, K. , Mano, T. , Koyama, T. , Nagatsuka, H. , Suzuki, K. , & Ryoke, K. (2002). Usefulness as guided bone regeneration membrane of the alginate membrane. Biomaterials, 23, 2027–2033. 10.1016/S0142-9612(01)00332-5 [DOI] [PubMed] [Google Scholar]
- Wakabayashi, R. C. , Iha, D. K. , Niu, J. J. , & Johnson, P. W. (1997). Cytokine production by cells adherent to regenerative membranes. Journal of Periodontal Research, 32, 215–224. 10.1111/j.1600-0765.1997.tb00527.x [DOI] [PubMed] [Google Scholar]
- Weng, D. , Poehling, S. , Pippig, S. , Bell, M. , Richter, E. J. , Zuhr, O. , & Hurzeler, M. B. (2009). The effects of recombinant human growth/differentiation factor‐5 (rhGDF‐5) on bone regeneration around titanium dental implants in barrier membrane‐protected defects: A pilot study in the mandible of beagle dogs. International Journal of Oral & Maxillofacial Implants, 24, 31–37. [PubMed] [Google Scholar]
- Wikesjo, U. M. , Qahash, M. , Thomson, R. C. , Cook, A. D. , Rohrer, M. D. , Wozney, J. M. , & Hardwick, W. R. (2004). rhBMP‐2 significantly enhances guided bone regeneration. Clinical Oral Implants Research, 15, 194–204. 10.1111/j.1600-0501.2004.00971.x [DOI] [PubMed] [Google Scholar]
- Williams, D. F. (2017). Biocompatibility pathways: Biomaterials‐induced sterile inflammation, mechanotransduction, and principles of biocompatibility control. ACS Biomaterials Science & Engineering, 3, 2–35. 10.1021/acsbiomaterials.6b00607 [DOI] [PubMed] [Google Scholar]
- Xing, Z. , Lu, C. , Hu, D. , Yu, Y. Y. , Wang, X. , Colnot, C. , … Marcucio, R. S. (2010). Multiple roles for CCR2 during fracture healing. Disease Models & Mechanisms, 3, 451–458. 10.1242/dmm.003186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, J. , He, M. , Niu, Y. , Liu, H. , Crawford, A. , Coates, P. , … Zhang, L. (2014). Preparation and in vivo efficient anti‐infection property of GTR/GBR implant made by metronidazole loaded electrospun polycaprolactone nanofiber membrane. International Journal of Pharmaceutics, 475, 566–577. 10.1016/j.ijpharm.2014.09.026 [DOI] [PubMed] [Google Scholar]
- Ye, J. , Yao, Q. , Mo, A. , Nie, J. , Liu, W. , Ye, C. , & Chen, X. (2011). Effects of an antibacterial membrane on osteoblast‐like cells in vitro . International Journal of Nanomedicine, 6, 1853–1861. 10.2147/ijn.s17749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Xu, Q. , Huang, C. , Mo, A. , Li, J. , & Zuo, Y. (2010). Biological properties of an anti‐bacterial membrane for guided bone regeneration: An experimental study in rats. Clinical Oral Implants Research, 21, 321–327. 10.1111/j.1600-0501.2009.01838.x [DOI] [PubMed] [Google Scholar]
- Zigdon‐Giladi, H. , Bick, T. , Lewinson, D. , & Machtei, E. E. (2014). Mesenchymal stem cells and endothelial progenitor cells stimulate bone regeneration and mineral density. Journal of Periodontology, 85, 984–990. 10.1902/jop.2013.130475 [DOI] [PubMed] [Google Scholar]
- Zigdon‐Giladi, H. , Bick, T. , Lewinson, D. , & Machtei, E. E. (2015). Co‐transplantation of endothelial progenitor cells and mesenchymal stem cells promote neovascularization and bone regeneration. Clinical Implant Dentistry and Related Research, 17, 353–359. 10.1111/cid.12104 [DOI] [PubMed] [Google Scholar]
- Zigdon‐Giladi, H. , Bick, T. , Morgan, E. F. , Lewinson, D. , & Machtei, E. E. (2015). Peripheral blood‐derived endothelial progenitor cells enhance vertical bone formation. Clinical Implant Dentistry and Related Research, 17, 83–92. 10.1111/cid.12078 [DOI] [PubMed] [Google Scholar]
- Zigdon‐Giladi, H. , Lewinson, D. , Bick, T. , & Machtei, E. E. (2013). Mesenchymal stem cells combined with barrier domes enhance vertical bone formation. Journal of Clinical Periodontology, 40, 196–202. 10.1111/jcpe.12044 [DOI] [PubMed] [Google Scholar]
- Zubery, Y. , Goldlust, A. , Alves, A. , & Nir, E. (2007). Ossification of a novel cross‐linked porcine collagen barrier in guided bone regeneration in dogs. Journal of Periodontology, 78, 112–121. 10.1902/jop.2007.060055 [DOI] [PubMed] [Google Scholar]


