This editorial refers to ‘Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency’, by K.L. Ho et al., pp. 1606–1616.
Growing evidence suggests that the utilization of ketone bodies increases in the heart during hypertrophy and heart failure; however, how the increased utilization of ketone bodies affects cardiac metabolism and function remains to be elucidated. The Lopaschuk group1 demonstrates that an increased supply of β-hydroxybutyrate (β-OHB), a major ketone body, enhances total energy production through increases in ketone body and glucose oxidation, which, however, fails to improve either cardiac work or efficiency in the failing hearts. These findings redefine the role of ketone body oxidation in cardiac function as a fuel source.
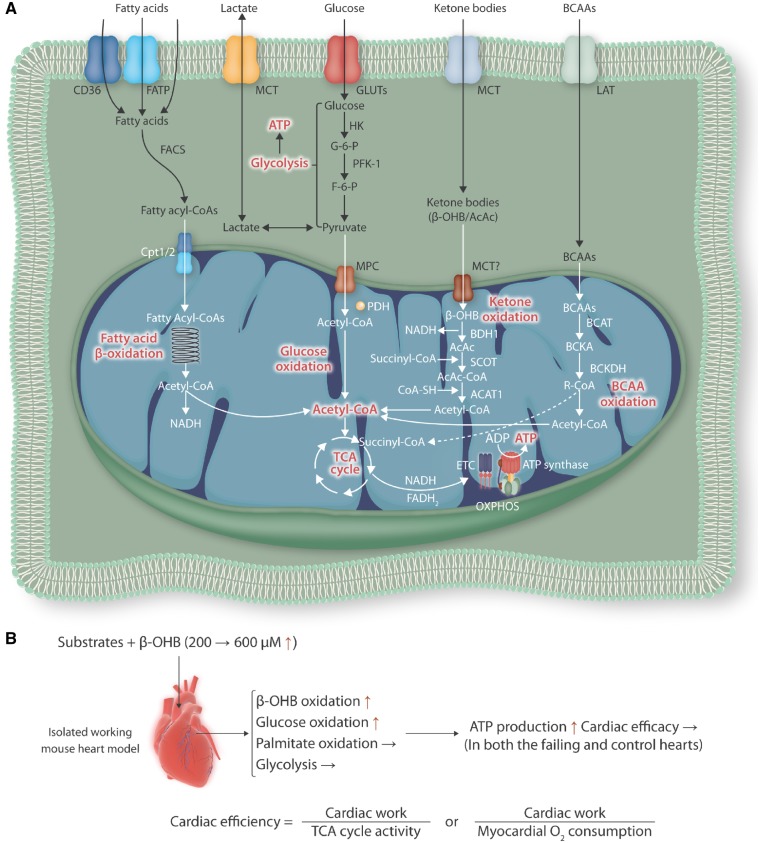
Major fuel sources in the heart, an oxidative and highly energy-demanding organ, are fatty acids and glucose.2 Under physiological conditions, however, the heart flexibly utilizes a variety of substrates, including ketones, amino acids, and lactate, based on availability to generate large quantities of adenosine triphosphate (ATP) (Figure 1A). Pathological stresses, including increased afterload (e.g. hypertension and aortic valve stenosis), volume overload (e.g. mitral valve regurgitation and arteriovenous shunt), and ischaemia, are thought to reduce the metabolic flexibility of the heart, with decreases in fatty acid oxidation and total energy production that may be partially compensated for by increased glycolysis and other types of oxidation. Increasing lines of evidence suggest that this metabolic remodelling towards substrate utilization similar to that of the foetal heart may contribute to the development of cardiomyocyte and cardiac hypertrophy and heart failure with systolic and/or diastolic dysfunction.3 Therefore, it is important to understand the underlying mechanisms by which pathological stimuli affect cardiac metabolism and how altered metabolism contributes to cardiac pathology.
Figure 1.
(A) Substrate utilization for energy production in the heart. (B) An isolated working heart model using either failing hearts 4 weeks post-pressure overload or intact hearts with sham operation. β-hydroxybutyrate (β-OHB) was used as a source of ketone body at two different concentrations (200 and 600 μM). β-OHB, β-hydroxybutyrate; AcAc, acetoacetate; ACAT1, acetyl-CoA C-acetyltransferase, mitochondrial; ADP, adenosine diphosphate; ATP, adenosine triphosphate; BCAAs, branched-chain amino acids; BCAT, branched-chain amino acid aminotransferase; BCKA, branched-chain alpha-keto acid; BCKDH, branched-chain alpha-keto acid dehydrogenase; BDH1, D-β-hydroxybutyrate dehydrogenase, mitochondrial; Cpt1/2, carnitine palmitoyl transferase-1/2; ETC, electron transport chain; F-6-P, fructose-6-phosphate; FACS, fatty-acyl-CoA synthase; FATP, fatty acid transport protein; G-6-P, glucose-6-phosphate; GLUTs, glucose transporters; HK, hexokinase; LAT, large neutral amino acid transporter; MCT, monocarboxylase transporter; MPC, mitochondrial pyruvate carrier; OXPHOS, oxidative phosphorylation; PDH, pyruvate dehydrogenase; PFK-1, phosphofructose kinase-1; SCOT, succinyl CoA: 3-ketoacid CoA transferase 1, mitochondrial; TCA, tricarboxylic acid.
Ketone bodies comprise three water-soluble molecules, β-OHB, acetoacetate (AcAc), and acetone, which are produced predominantly in the liver during intake of a low-carbohydrate high-fat (ketogenic) diet, fasting, prolonged exercise, or untreated diabetes.4 Ketone bodies play pivotal roles in various cellular processes under physiological conditions. In the heart, ketone bodies are catabolized into acetyl-CoA (termed ketolysis) in the mitochondria, which enters the tricarboxylic acid (TCA) cycle and produces ATP via oxidative phosphorylation (Figure 1A).4 Ketone bodies serve not only as an alternative fuel source but also as a signalling molecule, through chromatin modification with lysine acetylation (protein post-translational modification), modulation of inflammation and oxidative stress, and regulation of the sympathetic nervous system via G protein-coupled receptors when carbohydrates are abundant.4,5
The circulating level of ketone bodies is increased under pathological conditions as well, including diabetes and heart failure.4,6 Uncontrolled diabetes leads to overproduction of ketone bodies due to a lack of insulin action, inducing ketoacidosis, a life-threatening complication of diabetes. Heart failure is associated with insulin resistance, accompanied by enhanced lipolysis in adipose tissue,7 which in part contributes to increased plasma levels of ketone bodies. Patients with heart failure exhibit increased expression of ketolysis-related proteins, including D-β-hydroxybutyrate dehydrogenase, mitochondrial (BDH1), and succinyl CoA: 3-ketoacid CoA transferase 1, mitochondrial (SCOT), accompanied by an increase in ketone body oxidation in the heart.6,8 The plasma level of ketone bodies is negatively associated with cardiac function in patients with congestive heart failure,9 and is positively associated with worse prognosis in patients with haemodialysis.10 Furthermore, an isolated working heart model using intact rat hearts showed that ketone bodies inhibit the TCA cycle by sequestering CoA and induce acute contractile dysfunction that is reversed by supplementation with glucose or TCA cycle intermediates.11 In contrast, genetic deletion of BDH1 or SCOT in the mouse heart exacerbates cardiac dysfunction and remodelling in response to pressure overload with12 or without ischaemia,13 respectively, suggesting adaptive roles of enhanced ketone metabolism in hypertrophy and heart failure. However, much less is known about how increased ketone body utilization affects overall cardiac metabolism and efficiency in established heart failure. The study by Ho et al.1 addresses this question.
In this study, failing hearts with established hypertrophy 4 weeks after pressure overload and control hearts with sham operation were perfused in an isolated working heart model with two different concentrations of radiolabeled β-OHB (200 and 600 µM) (Figure 1B). This state-of-the-art study clearly demonstrates that an increased supply of β-OHB enhances overall ATP production through increases in ketone body and glucose oxidation without improving cardiac work and efficiency in both the failing and control hearts. Furthermore, the authors show that fatty acid oxidation, which is believed to be suppressed in the failing heart, is not reduced or even enhanced after normalization by cardiac work independently of β-OHB concentrations in their failing heart model. These results suggest that increased oxidation of ketone body and glucose may not sufficiently compensate for the reduced energy production or cardiac efficiency during heart failure. Given that patients with a higher plasma concentration of ketone bodies have more severe cardiac dysfunction, the persistent reliance on β-OHB-mediated ATP production may even contribute to the progression of heart failure in the long term. Interestingly, the authors show that the level of fatty acid oxidation is preserved in the failing heart, in contrast with the general belief that fatty acid oxidation is decreased in the failing heart. The discrepancy may be in part due to differences in the perfusates used in working heart models, and suggests that the failing heart may retain ability to oxidize fatty acids at a similar level to that of the intact heart when perfusates contain sufficient fatty acids, such as 0.8 mM of albumin-bound palmitate used in this study.
The authors’ findings are quite novel and significant but important questions remain. First, the authors’ findings were obtained from an ex vivo working heart model and the in vivo evidence has yet to be shown. A recent paper published by the Kelly group showed that direct chronic infusion of β-OHB into the right ventricle in vivo improves cardiac function, efficiency, and remodelling in the tachypacing-induced heart failure model.12 Thus, it would be important to determine whether these discrepancies stem from differences in duration, concentration, or initiation time of β-OHB or from the nature of stress. Second, ketone bodies act as an anti-inflammatory by suppressing activation of NLRP3 inflammasome formation14 and an endogenous Class I histone deacetylase inhibitor.15 Thus, it would be interesting to test whether β-OHB treatment can serve as primary prevention for developing fibrosis and hypertrophy, which in turn secondarily affect metabolism. Finally, it is important to clarify the underlying mechanisms by which β-OHB increases glucose oxidation without improving efficiency. Similarly, the mechanism by which increased acetylation of mitochondrial proteins, such as long chain acyl-CoA dehydrogenase, modulates ATP production despite the absence of a change in fatty acid oxidation remains to be elucidated.
In summary, Lopaschuk et al. clearly demonstrate that the failing heart with established hypertrophy retains the ability to enhance ketone body and glucose oxidation in response to an increased supply of β-OHB, which are accompanied by increased ATP production, although these mechanisms may not sufficiently contribute to cardiac efficiency.
Acknowledgements
We thank Daniela Zablocki for critical reading of the manuscript.
Conflict of interest: none declared.
Funding
This work was supported by grants from the NIH (to J.S.), the Foundation Leducq Transatlantic Network of Excellence (to J.S.), and an American Heart Association Scientist Development Grant (17SDG33660358 to M.N).
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1. Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, Kelly DP, Lopaschuk GD.. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res 2019;115:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC.. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207–258. [DOI] [PubMed] [Google Scholar]
- 3. Nakamura M, Sadoshima J.. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 2018;15:387–407. [DOI] [PubMed] [Google Scholar]
- 4. Puchalska P, Crawford PA.. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 2017;25:262–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Newman JC, Verdin E.. Ketone bodies as signaling metabolites. Trends Endocrinol Metab 2014;25:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE.. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016;133:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakamura M, Sadoshima J.. Cardiomyopathy in obesity, insulin resistance and diabetes. J Physiol 2019;doi:10.1113/JP276747. [DOI] [PubMed] [Google Scholar]
- 8. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP.. The failing heart relies on ketone bodies as a fuel. Circulation 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lommi J, Kupari M, Koskinen P, Naveri H, Leinonen H, Pulkki K, Harkonen M.. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol 1996;28:665–672. [DOI] [PubMed] [Google Scholar]
- 10. Obokata M, Negishi K, Sunaga H, Ishida H, Ito K, Ogawa T, Iso T, Ando Y, Kurabayashi M.. Association between circulating ketone bodies and worse outcomes in hemodialysis patients. J Am Heart Assoc 2017;6. doi:10.1161/JAHA.117.006885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taegtmeyer H, Hems R, Krebs HA.. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J 1980;186:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, Recchia FA, Kelly DP.. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019;4. doi:10.1172/jci.insight.124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schugar RC, Moll AR, André d’Avignon D, Weinheimer CJ, Kovacs A, Crawford PA.. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab 2014;3:754–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD.. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015;21:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E.. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013;339:211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]