Abstract
Aims
Longitudinal change in left atrial (LA) structure and function could be helpful in predicting risk for incident atrial fibrillation (AF). We used cardiac magnetic resonance (CMR) imaging to explore the relationship between change in LA structure and function and incident AF in a multi-ethnic population free of clinical cardiovascular disease at baseline.
Methods and results
In the Multi-Ethnic Study of Atherosclerosis (MESA), 2338 participants, free at baseline of clinically recognized AF and cardiovascular disease, had LA volume and function assessed with CMR imaging, at baseline (2000–02), and at Exam 4 (2005–07) or 5 (2010–12). Free of AF, 124 participants developed AF over 3.8 ± 0.9 years (2015) following the second imaging. In adjusted Cox regression models, an average annualized change in all LA parameters were significantly associated with an increased risk of AF. An annual decrease of 1-SD unit in total LA emptying fractions (LAEF) was most strongly associated with risk of AF after adjusting for clinical risk factors for AF, baseline LA parameters, and left ventricular mass-to-volume ratio (hazard ratio per SD = 1.91, 95% confidence interval = 1.53–2.38, P < 0.001). The addition of change in total LAEF to an AF risk score improved model discrimination and reclassification (net reclassification improvement = 0.107, P = 0.017; integrative discrimination index = 0.049, P < 0.001).
Conclusion
In this multi-ethnic study population free of clinical cardiovascular disease at baseline, a greater increase in LA volumes and decrease in LA function were associated with incident AF. The addition of change in total LAEF to risk prediction models for AF improved model discrimination and reclassification of AF risk.
Keywords: atrial fibrillation, left atrium
Introduction
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia, is increasing in prevalence as populations age.1 It affects up to 9–10% of those aged over 80,1,2 and contributes to significant morbidity, such as increased stroke rates,1,2 thrombo-embolic events,3 cognitive decline,4 and left ventricular (LV) dysfunction.5
Previous studies have shown that greater volumes and impaired function of the left atrium (LAm) are modestly associated with incident AF, independent of traditional risk factors.6–9 Adverse remodelling of the LAm has been proposed to facilitate both initiation and maintenance of AF by promoting ectopic triggers, and altering the wavelength of the re-entrant circuit,10 while recent studies have shown left atrial (LA) parameters to be useful in stratifying the risk of incident stroke in patients with AF.11,12 Thus, change in LA parameters may be helpful in risk stratification of incident AF in asymptomatic individuals.13
To date, few prospective studies have investigated the association between longitudinal change of the LAm with the development of AF, neither have they shown incremental benefit to existing AF risk prediction models.7,8 Furthermore, most studies utilized speckle-tracking echocardiography to analyse the LAm, which has proven challenging given the anatomic locations of the LAm, pulmonary veins and thin atrial wall.14 We used multimodality tissue tracking (MTT) on cardiac magnetic resonance (CMR) imaging, the gold-standard reference in atrial and ventricular volume measurement, and a well-established method for the assessment of cardiac deformation,15–17 to explore the relationship between change in LA parameters and incident AF. It is described as being superior to echocardiography in delineating static LA volumes, given its excellent ability to define the spatial resolution of endocardial and epicardial borders.15–17 In a population free of clinical cardiovascular disease at baseline, we hypothesized that an increase in LA volumes and decrease in LA function are associated with a greater risk of incident AF, after adjusting for known risk factors.
Methods
Study design
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective, population-based multi-ethnic (White, African-American, Chinese, and Hispanic) cohort study of subclinical cardiovascular disease. The study design of MESA has been described in detail previously.18 In summary, between 2000–02 (Exam 1), 6814 men and women aged 45–84 years, free of clinical cardiovascular disease at enrolment, were recruited from six US field centres (Baltimore, MD; Chicago, IL, USA; Forsyth County, NC, USA; Los Angeles County, CA, USA; Northern Manhattan, NY; St Paul, MN, USA). Exam 1 was followed by Exam 2 (2002–04); Exam 3 (2004–05); Exam 4 (2005–07) and Exam 5 (2010–12). Approximately every 9 months, each participant was contacted by a telephone interviewer, as follow-up, to inquire about hospital admissions, cardiovascular outpatient diagnoses and mortality. Medical records and information were successfully obtained in 98% of reported hospitalized cardiovascular events and 95% of reported outpatient cardiovascular diagnostic encounters. The methodology of risk factor and outcome collection is detailed in Supplementary data online. All participants provided written informed consent. All study protocols were approved by the institutional review boards of each participating field centre.
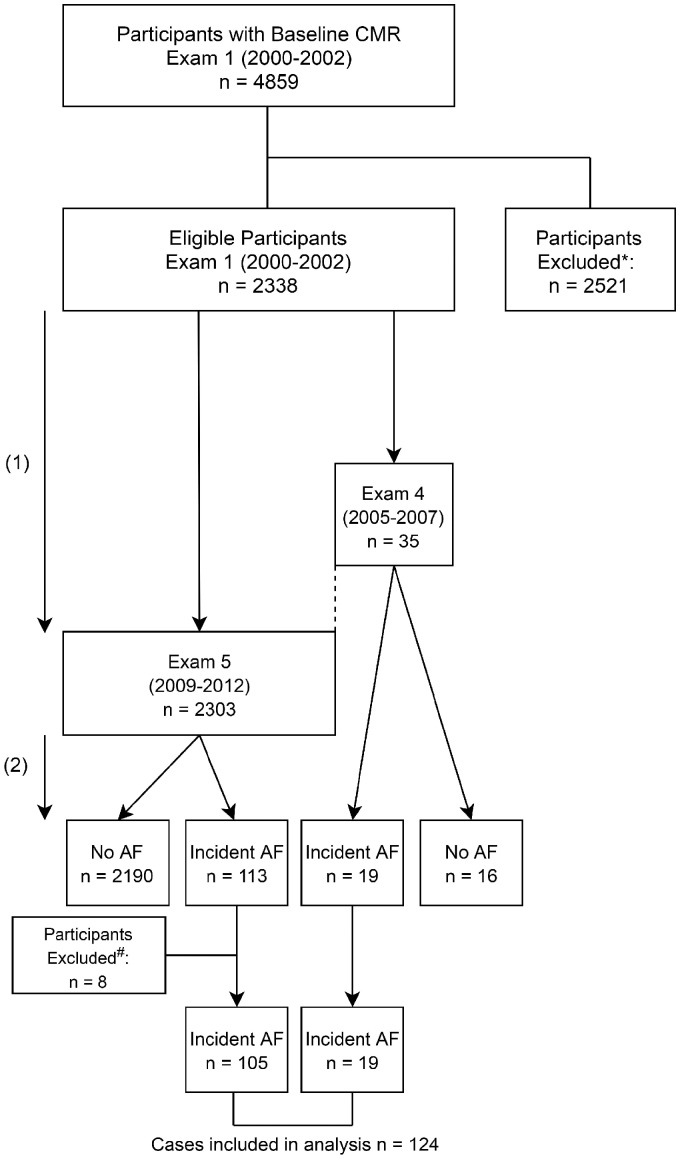
The flowchart of the MESA population investigated in this study is illustrated in Figure 1. A baseline CMR study (Exam 1) was conducted in 4859 participants, measuring LA volumes, EF, and global peak longitudinal strain. Of these, 2338 eligible participants returned for a second CMR study (Exam 4/5) on average 9.4 ± 0.5 years later. Exam 5 measures were used in participants who had CMR at both Exams 4 and (n = 457), unless they developed AF in between Exams 4 and 5 (dotted line of Figure 1), whereby Exam 4 measures were used (n = 19). Participants were excluded if (i) they did not have at least two CMR studies (baseline and Exam 4/5) or (ii) due to unavailability or poor quality of images (n = 2421), or (iii) if they developed AF before the second study (Exam 4: n = 8; Exam 5: n = 92). Over a mean follow-up period of 3.8 ± 0.9 years after the second study, 132 participants developed incident AF (Exam 4: n = 19; Exam 5: n = 113). However, due to unavailability or poor quality of images (n = 8), only 124 AF cases were included in the event analysis.
Figure 1.
Flowchart of study. (1): Baseline to second CMR study mean time: 9.4 ± 0.5years (2): AF follow-up mean time: 3.8 ± 0.9 years after second CMR study *Exclusion: (1) did not have at least 2 CMR studies (baseline and Exam 4/5) or (2) images were unavailable or of poor quality (n = 2421), or (3) developed clinically recognized AF before the second CMR study (Exam 4, n = 8; Exam 5, n = 92). #Exclusion due to unavailability or poor quality of images (n = 8). AF, atrial fibrillation; CMR, cardiac magnetic resonance.
Identification of AF cases
Incident cases of AF during the follow-up period (2007/2012 (Exam 4/5)–2015) were identified through MESA surveillance and, for participants enrolled in fee-for-service Medicare, from inpatient and outpatient Medicare claims data. As a part of standard event surveillance procedures, all hospitalizations were identified during follow-up calls to study participants or a proxy. Discharge diagnosis and procedure codes from those hospitalizations were abstracted. AF was documented as present if an International Classification of Diseases, Ninth Revision diagnosis code 427.31 (AF) or 427.32 (atrial flutter) was present. If the first AF claim occurred before the baseline study, the participant was considered to have prevalent AF and therefore was excluded from the analysis.
CMR and MTT image analysis
Baseline CMR images were acquired using 1.5 T MR scanners: Signa LX or CVi (GE Medical Systems, Waukesha, WI, USA) or Symphony or Sonata (Siemens Medical Systems, Erlangen, Germany). Long axis cine images were obtained from 2-chamber and 4-chamber views, using electrocardiogram-gated fast gradient-echo pulse sequence. All cine images were acquired with a temporal resolution of ∼50 ms. A stack of short-axis images recorded at end diastole was obtained for the assessment of LV mass. A detailed description of the MESA MRI protocol has been published.19
Multimodality Tissue Tracking software (MTT, v6.0, Toshiba, Japan) was used to quantify LA volume, EF, and strain from 2- and 4- chamber cine CMR images. This method has been validated previously with good-excellent [intraclass correlation (ICC); 0.88–0.98, P < 0.001] intra- and inter-reader reproducibility, and good (ICC; 0.44–0.82, P < 0.05–0.001) inter-study reproducibility.20,21
A single experienced operator blinded to the case status of the participant defined endocardial and epicardial borders of the LAm at end systole. Using the marked points, the software creates endocardial and epicardial borders, then tracks LA tissue in subsequent frames. The endocardial and epicardial contours generated by the software are then followed by the operator during the cardiac cycle for quality control. Maximum, pre-atrial, and minimum contraction LA volumes were extracted from volume curves that were created using the area-length method from apical 2- and 4-chamber views, using the following formula for Biplane calculation: . All LA volumes were indexed to body surface area (mL/m2), while LA emptying fractions (LAEF) were derived from LA volumes (Supplementary data online). Biplanar volume and function assessment on MTT had a strong positive linear correlation and concordance to other manual methods (e.g. Simpson’s method).21
Strain measurement
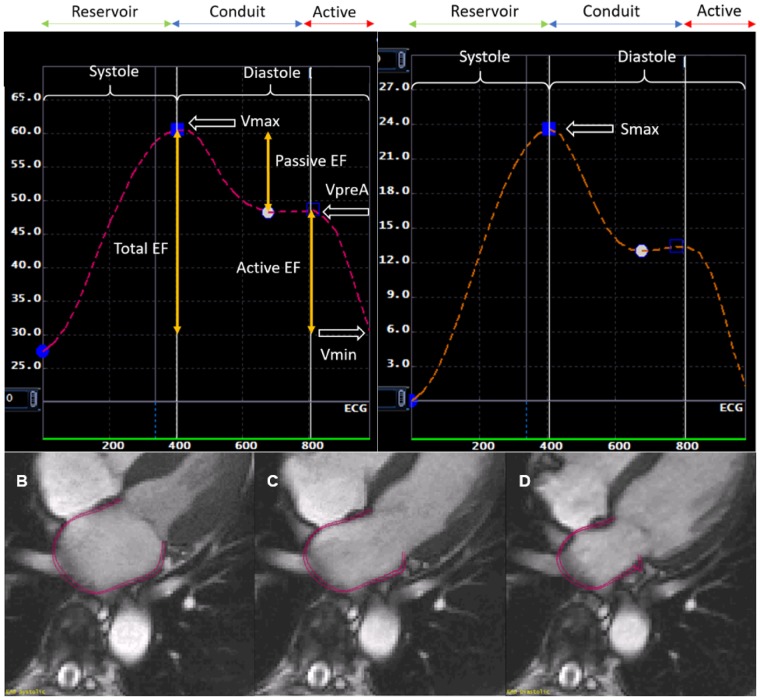
MTT software calculates global longitudinal atrial strain by averaging longitudinal strain of all LA segments, determined by the software’s automatic division of the LA wall into equal segments, in 2- and 4-chamber views during each cardiac cycle. Peak longitudinal LA strain (LASmax) was extracted from the global longitudinal strain curve (Figure 2). Calibration for strain measurements was accounted for in the MTT software as Exam 1 used Fast Gradient Recalled Echo (FGRE) images, while Exams 4 and 5 used Steady State Free Precision (SSFP) cine images.22
Figure 2.
Phasic left atrial volumes, EF, and peak longitudinal strain corresponding to one cardiac cycle. Volumes: maximum (Vmax), pre-atrial contraction (VpreA), minimum (Vmin); MTT CMR imaging in different phases of the cardiac cycle (A): end systole (B), pre-atrial contraction (C), end diastole (D). EF, emptying fractions; Smax, peak longitudinal strain.
Statistical analysis
Baseline characteristics of study participants are presented as mean ± SD for continuous variables in Table 1. Annual change in LA variables was determined by averaging the change over time (years) between both studies, presented as mean ± SD in Table 2. We used Cox proportional hazards regression models to study associations between annual change in LA variables and incident AF. The assumption of proportionality of hazards was confirmed for each model. The AF risk prediction score used was the CHARGE-AF (Cohort for Heart and Aging Research in Genomic Epidemiology-atrial fibrillation) risk model, previously validated using the Age, Gene and Environment-Reykjavik Study (AGES) and Rotterdam Study (RS).23
Table 1.
Population demographics
| Baseline (Exam 1) | Second study (Exam 4/5) after 9.4 ± 0.5 years |
||
|---|---|---|---|
| n = 2338 | No AF n = 2206 | Incident AF n = 132 | |
| Age | 59.3 ± 9.22 | 68.2 ± 8.97 | 75.1 ± 7.13 |
| Male gender | 1096 (47%) | 1028 (47%) | 68 (52%) |
| Race | |||
| White, Caucasian | 1005 (43%) | 935 (42%) | 70 (53%) |
| Chinese-American | 275 (12%) | 263 (12%) | 12 (9%) |
| African-American | 575 (24%) | 545 (25%) | 30 (23%) |
| Hispanic | 483 (21%) | 463 (21%) | 20 (15%) |
| BMI | 27.8 ± 4.95 | 28.1 ± 5.16 | 28.2 ± 5.12 |
| Systolic blood pressure (mmHg) | 123 ± 20.1 | 122.4 ± 19.8 | 125.2 ± 20.0 |
| Diastolic blood pressure (mmHg) | 71.8 ± 10.2 | 68.3 ± 9.8 | 67.5 ± 10.5 |
| Antihypertensive medications | 722 (31%) | 1123 (51%) | 94 (71%) |
| Glycaemic status | |||
| Normal | 1821 (79%) | 1360 (62%) | 75 (57%) |
| Impaired fasting glucose | 261 (11%) | 453 (21%) | 28 (21%) |
| Diabetes mellitus | 232 (10%) | 376 (17%) | 29 (22%) |
| Smoking | |||
| Never | 1221 (53%) | 1035 (47%) | 49 (37%) |
| Former | 832 (36%) | 992 (45%) | 74 (56%) |
| Current | 267 (11%) | 166 (8%) | 9 (7%) |
| Events | |||
| Myocardial infarction | 0 | 35 (2%) | 4 (3%) |
| Congestive heart failure | 0 | 19 (1%) | 3 (2%) |
AF, atrial fibrillation; BMI, body mass index.
Table 2.
Annual change in LA variables
| Annual change (over 9.4 ± 0.5 years) |
|||
|---|---|---|---|
| LA variable | No AF n = 2206 | Incident AF n = 132 | P-value |
| ΔLAVImax (mL/m2/year) | 0.58 ± 1.14 | 1.06 ± 1.51 | <0.001 |
| ΔLAVIpreA (mL/m2/year) | 0.56 ± 0.96 | 0.97 ± 1.26 | <0.001 |
| ΔLAVImin (mL/m2/year) | 0.46 ± 0.77 | 1.05 ± 1.23 | <0.001 |
| ΔTotal LAEF (%/year) | −0.69 ± 1.33 | −1.32 ± 1.56 | <0.001 |
| ΔPassive LAEF (%/year) | −0.36 ± 1.09 | −0.59 ± 1.07 | 0.015 |
| ΔActive LAEF (%/year) | −0.65 ± 1.56 | −1.22 ± 1.71 | <0.001 |
| ΔPeak LA strain (%/year) | −0.59 ± 1.66 | −1.13 ± 1.59 | <0.001 |
Δ, Annual change; AF, atrial fibrillation; EF, emptying fractions; Indexed volumes: maximum (VImax), pre-atrial (VIpreA), minimum (VImin); LA, left atrial.
Three models were generated to examine the associations between annual LA change and AF. In model 1, we adjusted for CHARGE-AF risk factors at the second CMR study (Exam 4/5): age, race, height, weight, systolic and diastolic blood pressure, antihypertensive medication use, smoking status, diabetes, and the development of myocardial infarction and congestive heart failure.23 In model 2, we added baseline LA measures to account for baseline differences when measuring change, and potential measurement error bias.24 Model 3 additionally adjusted for LV mass-to-volume ratio (MVR), a measure of LV diastolic dysfunction. The cumulative risk of AF over the follow-up years for the cohort, stratified by tertiles of LA parameters, was determined using the Kaplan–Meier curves, censoring at last follow-up. Differences across tertiles were compared using the log-rank test.
We compared model discrimination using the C-statistic for post-survival analysis. The additional predictive value of annual LA change was calculated by the increment in the C-statistic, the categorical net reclassification improvement (NRI), and the integrative discrimination index (IDI).25 Risk categories for NRI were defined a priori as <2.5%, 2.5–5.0%, and >5.0%, as estimated by the CHARGE-AF risk prediction model.23 We assessed model calibration using Grønnesby and Borgan’s modified Hosmer–Lemeshow 2 statistic for survival analysis.26 A P-value of <0.05 in two-tailed tests was considered statistically significant. All statistical analyses were performed using Stata software (version 12.0; Stata Corp, TX, USA).
Results
Population demographics of eligible participants (n = 2338) at baseline and the second CMR (9.4 ± 0.5 years after baseline), divided into those who developed AF (n = 132), and those who did not (n = 2206), are summarized in Table 1.
Annual change in LA structure and function
The annual change in LA variables over 9.4 ± 0.5 years are summarized in Table 2. Participants who developed incident AF had a greater increase in LA volumes (ΔLAVImax 1.06 ± 1.51 vs. 0.58 ± 1.14 mL/m2/year; P < 0.001;ΔLAVImin 1.05 ± 1.23 vs. 0.46 ± 0.77 mL/m2/year; P < 0.001) (Table 2). Similarly, peak LA strain and all LAEFs (total, passive, and active) decreased to a greater extent in AF cases (all P < 0.001; ΔPassive LAEF P = 0.015).
Association of change in LA variables with incident AF
The association of change in LA variables with incident AF over 3.8 ± 0.9 years of follow-up, analysed with multivariable Cox regression models, is summarized in Table 3. In the unadjusted model, all LA variables were significantly associated with incident AF, whereby an annual increase of 1-SD unit of ΔLAVImin was associated with the greatest increase in risk of incident AF [hazard ratio (HR) per SD = 1.47, 95% confidence interval (CI) (1.31–1.64); P < 0.001] (Supplementary data online, Table S4).
Table 3.
Association of annual LA change with incident AF in Cox regression models
| A. Model 1 |
B. Model 2 |
C. Model 3 |
||||
|---|---|---|---|---|---|---|
| Clinical risk (CHARGE-AF)a + ΔLA variable |
Model 1 + Baseline LA variable |
Model 2 + LV MVR |
||||
| Variable | HR (CI) | P-value | HR (CI) | P-value | HR (CI) | P-value |
| ΔLAVImax (mL/m2/year) | 1.24 | 0.003 | 1.36 | <0.001 | 1.39 | <0.001 |
| (1.08–1.42) | (1.19–1.56) | (1.21–1.60) | ||||
| ΔLAVIpreA (mL/m2/year) | 1.26 | 0.009 | 1.41 | <0.001 | 1.42 | <0.001 |
| (1.06–1.50) | (1.19–1.67) | (1.20–1.69) | ||||
| ΔLAVImin (mL/m2/year) | 1.54 | <0.001 | 1.64 | <0.001 | 1.64 | <0.001 |
| (1.30–1.83) | (1.40–1.91) | (1.40–1.92) | ||||
| ΔTotal LAEF (%/year) | 0.84 | 0.008 | 0.63 | <0.001 | 0.62 | <0.001 |
| (0.75–0.96) | (0.54–0.74) | (0.53–0.73) | ||||
| ΔPassive LAEF (%/year) | 0.96 | 0.632 | 0.63 | 0.001 | 0.60 | <0.001 |
| (0.79–1.15) | (0.48–0.81) | (0.45–0.79) | ||||
| ΔActive LAEF (%/year) | 0.87 | 0.018 | 0.67 | <0.001 | 0.67 | <0.001 |
| (0.77–0.98) | (0.57–0.79) | (0.57–0.79) | ||||
| ΔPeak LA strain (%/year) | 0.90 | 0.048 | 0.72 | <0.001 | 0.70 | <0.001 |
| (0.81–0.99) | (0.62–0.85) | (0.59–0.83) | ||||
| ΔLAVImin (per 1-SD) | 1.42 | <0.001 | 1.49 | <0.001 | 1.49 | <0.001 |
| (1.24–1.63) | (1.31–1.69) | (1.31–1.69) | ||||
| −ΔTotal LAEF (per 1-SD) | 1.26 | 0.008 | 1.86 | <0.001 | 1.91 | <0.001 |
| (1.06–1.49) | (1.50–2.31) | (1.53–2.38) | ||||
| −ΔPeak LA strain (per 1-SD) | 1.20 | 0.048 | 1.71 | <0.001 | 1.81 | <0.001 |
| (1.01–1.43) | (1.31–2.24) | (1.36–2.41) | ||||
Δ, Annual change; AF, atrial fibrillation; EF, emptying fractions; Indexed volumes: maximum (VImax), pre-atrial (VIpreA), minimum (VImin); LA, left atrial.
CHARGE-AF risk model: age, race, height, weight, systolic and diastolic blood pressure, antihypertensive medication, smoking status, diabetes, myocardial infarction, and congestive heart failure by the second study.24
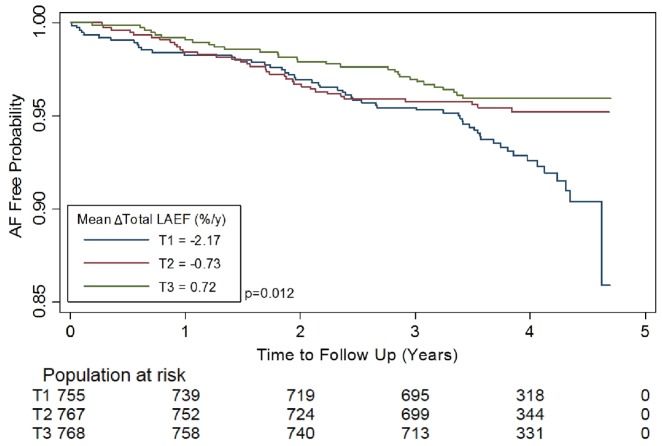
After adjusting for CHARGE-AF risk factors in model 1, the association remained significant for all variables except for passive LAEF. After additionally adjusting for the baseline value of each LA variable in model 2, and LV MVR in model 3, all LA variables were significantly associated with incident AF (P < 0.001). We calculated the decrease in 1-SD unit of total LAEF(−ΔTotal LAEF) and peak LA strain (−ΔPeak LA strain) to compare the increase in hazards per 1-SD unit for incident AF across the categories of volume (ΔLAVImin), EF and strain, as they showed the greatest magnitude of association with AF risk. An annual decrease of 1-SD unit in total LAEF was associated with the greatest increase in risk of incident AF after adjusting for clinical risk factors, baseline LA measures and LV MVR (HR per SD = 1.91, 95% CI 1.53–2.38, P < 0.001) (Table 3C). The Kaplan–Meier curves were plotted, stratified by tertiles of unadjusted ΔTotal LAEF, statistically significant on the log-rank test (all P < 0.05) (Figure 3).
Figure 3.
The Kaplan–Meier curves depicting the probability of freedom from AF stratified by tertiles of unadjusted ΔTotal LAEF. Δ, annual change; AF, atrial fibrillation; EF, emptying fractions; LA, left atrial; LAEF, LA emptying fraction.
Improvement in risk prediction with addition of annual change in LA variables
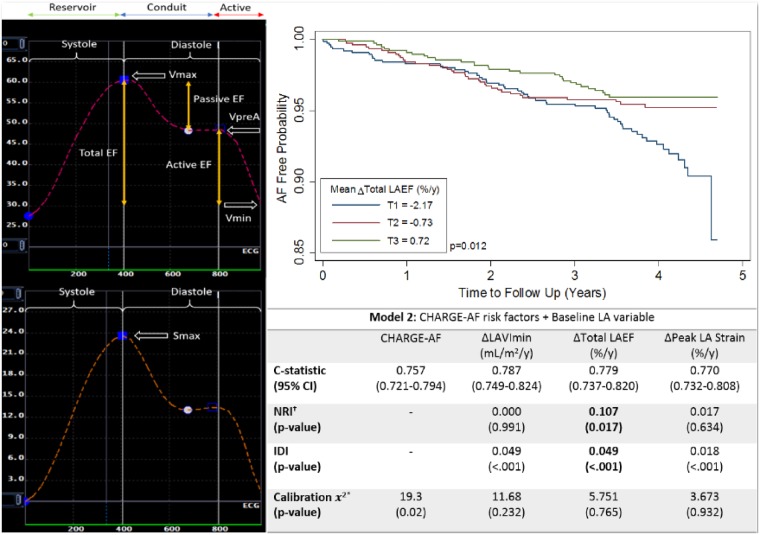
The CHARGE-AF score demonstrated excellent performance for predicting AF in our population (C-statistic = 0.757, 95% CI 0.721–0.794). We added ΔLAVImin, ΔTotal LAEF, and ΔPeak LA strain separately to the CHARGE-AF model, as model 1, and additionally adjusted for baseline LA values in model 2, to investigate the additional discriminatory value. In model 2, ΔLAVImin had the highest discriminatory value (C-statistic = 0.787, 95% CI 0.747–0.824). Model 2 with ΔTotal LAEF showed significant improvement to model discrimination and reclassification compared to model 2 alone (NRI = 0.107, P = 0.017; IDI = 0.049, P < 0.001) (Table 4).
Table 4.
Model discrimination, calibration, NRI, and IDI
| Model 2: CHARGE-AF risk factors + Baseline LA variable | ||||
|---|---|---|---|---|
| CHARGE-AF | ΔLAVImin (mL/m2/year) | ΔTotal LAEF (%/year) | ΔPeak LA strain (%/year) | |
| C-statistic (95% CI) | 0.757 (0.721–0.794) | 0.787 (0.749–0.824) | 0.779 (0.737–0.820) | 0.770 (0.732–0.808) |
| NRIa (P-value) | 0.000 (0.99) | 0.107 (0.017) | 0.017 (0.63) | |
| IDI (P-value) | 0.049 (<0.001) | 0.049 (<0.001) | 0.018 (<0.001) | |
| Calibration 2 (P-value) | 19.3 (0.02) | 11.68 (0.23) | 5.751 (0.77) | 3.673 (0.93) |
Δ, Annual change; AF, atrial fibrillation; EF, emptying fractions; IDI, integrative discrimination index; LA, left atrial; NRI, net reclassification improvement; VImin, minimum indexed volume.
Categories of NRI:<2.5%, 2.5–5.0%, and >5.0%.24
Discussion
This study showed a relationship between total LAEF and incident AF: an average annual change in total LAEF showed the greatest association with incident AF risk in a multi-ethnic cohort, and improved model discrimination and reclassification in predicting risk of incident AF, after adjusting for clinical risk factors and baseline total LAEF. To our knowledge, the incremental model discrimination of LAEF to clinical AF risk scores has not been reported before. Our findings suggest that total LAEF may detect subtle and earlier structural, as well as functional, LA derangements in high-risk individuals, making it a more valuable risk predictor compared to structural LA remodelling (volume) alone (Figure 4). Validation of these findings should be replicated in an independent cohort. Similar findings were seen in a recent investigation from the Framingham Offspring Study by Sardana et al. on LA Functional Index, a composite echocardiographic measure of LA structure and function, demonstrating a strong risk association with incident AF even in patients with normal LA volume; however, not significantly improving model discrimination on addition to AF prediction models.7
Figure 4.
Change in total left atrial emptying fractions, derived by multimodality tissue tracking on CMR imaging, showed incremental risk prediction and reclassification of incident AF on addition to an AF risk prediction model.
The association of structural and functional LA remodelling and incident AF has been extensively researched previously.6–9 Our findings of increasing LA volumes and decreasing LA function being associated with an increased risk of AF in community-based samples are consistent with current literature, in particular, showing minimum LA volume to have a higher risk association with AF compared to maximum LA volume, as seen in the study by Fatema et al., which found minimum LA volume to be marginally superior to maximum LA volume in predictive ability of AF over a mean follow-up of 1.9 years.9
A previous investigation involving participants in the original Framingham Heart Study cohort, showed that a 5-mm increase in LA diameter was associated with a 39% increase in the HR for incident AF over a median follow-up of 7.2 years.8 Our study shows that an annual decrease of 1-SD unit in total LAEF was associated with a 91% increase in incident AF risk, after adjusting for AF risk factors, baseline LA values, and LV remodelling, differing from existing studies by providing an annualized rate of LA change and HRs per 1-SD unit. Annual LA change was averaged across 9 years, thus assumed to be linear over time, which may not have fully captured the noise in year-to-year measurements, providing precedence for further investigations. The concept of dynamic change in risk profile, as patients age and accumulate exposure to risk factors, has been explored using other prediction models like the CHA2DS2-VASc, and HAS-BLED risk score, showing risk profile change to be superior to single baseline determinations.27–29 In this study, we investigated the predictive power of changes in parameters of LA structure and function to detect incident AF over and above their baseline magnitude.
The observation that annual LA change precedes the development of AF, warrants further investigation into the pathophysiology of AF. It has previously been suggested that LA structural and functional remodelling both lead to, and are caused by, AF. The resultant atrial deformation and fibrosis plays a role in altering electrical conduction properties, which may predispose to the development of arrhythmogenic micro-re-entrant circuits and triggered ectopic activity.10 However, this is but one of the many pathophysiological factors contributing to AF. Electrical remodelling, autonomic nervous system changes, and calcium-handling abnormalities all result in a re-entry prone substrate and atrial ectopic activity, contributing to the initiation and maintenance of AF.10 While it is possible that impaired total LAEF may be an indicator of any of the changes listed above, evidence that it precedes the development of AF does not necessarily imply causality. Abnormalities in LA volume or total LAEF may simply be markers for unidentified factors that are causally related to the development of AF. How functional and structural LA remodelling contributes to these known mechanistic processes, or confers directionality to the development of AF, will require further studies for elucidation.
Limitations
We used linear, instead of 3D, methods to measure LA volumes, which may underestimate true volumes by 11.5–20%.30 However, this method has been widely used and validated in research studies.20,21 Diagnosing incident AF based on hospital discharge codes meant a possible underestimation of subclinical AF that did not result in hospitalization, while subclinical episodes of AF between both CMR measures may have contributed to LA remodelling prior to clinical AF detection. However, a validation sub-study of 45 MESA participants with the diagnosis of AF based on hospital discharge codes showed that AF was confirmed in 93% on review of Medicare, implying a high specificity for the diagnosis.31 We also acknowledge that missing data from participants who did not participate in Exams 4 or 5 could have introduced bias in the study, however, as they tended to be older and had more risk factors, we hypothesize that their inclusion would be more likely to increase the strength of our associations.
Conclusion
In this multi-ethnic study population free of clinical cardiovascular disease at baseline, a greater annual increase in LA volume and annual decrease in LAEFs and strain, measured on CMR imaging, were associated with increased incident AF risk during follow-up. The addition of change in total LAEF to risk prediction models for AF showed improvement to model discrimination and reclassification of AF risk. Future studies should validate our findings to better understand this contributory role to the pathophysiology of AF.
Supplementary Material
Acknowledgements
We thank all the investigators, staff, and participants of MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://mesa-nhlbi.org (Clinical Trial Registration: NCT00005487). The views expressed in this abstract are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Funding
This work was supported by the National Heart, Lung and Blood Institute [HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, R01-HL-127659]; and the National Center for Advancing Translational Sciences, National Institutes of Health [UL1-TR-000040, UL1-TR-001079].
Conflict of interest: none declared.
References
- 1. Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS.. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042–6. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV. et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370. 5. [DOI] [PubMed] [Google Scholar]
- 3. Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C. et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014;129:2094–9. [DOI] [PubMed] [Google Scholar]
- 4. Thacker EL, McKnight B, Psaty BM, Longstreth WT Jr., Sitlani CM, Dublin S. et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology 2013;81:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anter E, Jessup M, Callans DJ.. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation 2009;119:2516–25. [DOI] [PubMed] [Google Scholar]
- 6. Habibi M, Lima JA, Khurram IM, Zimmerman SL, Zipunnikov V, Fukumoto K. et al. Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study. Circ Cardiovasc Imaging 2015;8:e002769.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sardana M, Lessard D, Tsao CW, Parikh NI, Barton BA, Nah G. et al. Association of left atrial function index with atrial fibrillation and cardiovascular disease: the Framingham Offspring Study. J Am Heart Assoc 2018;7:e008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaziri SM, Larson MG, Benjamin EJ, Levy D.. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 1994;89:724–30. [DOI] [PubMed] [Google Scholar]
- 9. Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB. et al. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr 2009;10:282–6. [DOI] [PubMed] [Google Scholar]
- 10. Nattel S, Burstein B, Dobrev D.. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 2008;1:62–73. [DOI] [PubMed] [Google Scholar]
- 11. Shih JY, Tsai WC, Huang YY, Liu YW, Lin CC, Huang YS. et al. Association of decreased left atrial strain and strain rate with stroke in chronic atrial fibrillation. J Am Soc Echocardiogr 2011;24:513–9. [DOI] [PubMed] [Google Scholar]
- 12. Leung M, van Rosendael PJ, Abou R, Ajmone Marsan N, Leung DY, Delgado V. et al. Left atrial function to identify patients with atrial fibrillation at high risk of stroke: new insights from a large registry. Eur Heart J 2018;39:1416–25. [DOI] [PubMed] [Google Scholar]
- 13. Thomas L, Abhayaratna WP.. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging 2017;10:65–77. [DOI] [PubMed] [Google Scholar]
- 14. Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S. et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 2010;3:231–9. [DOI] [PubMed] [Google Scholar]
- 15. Hoit B. Evaluation of left atrial function: current status. Struct Heart 2017;1:109–20. [Google Scholar]
- 16. Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ.. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12:65.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodevan O, Bjornerheim R, Ljosland M, Maehle J, Smith HJ, Ihlen H, Left AVA, By T. et al. Echocardiography compared to MRI estimates. Int J Card Imaging 1999;15:397–410. [DOI] [PubMed] [Google Scholar]
- 18. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR. et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 19. Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M. et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. Am J Roentgenol 2006;186:S357–65. [DOI] [PubMed] [Google Scholar]
- 20. To AC, Flamm SD, Marwick TH, Klein AL.. Clinical utility of multimodality LA imaging: assessment of size, function, and structure. JACC Cardiovasc Imaging 2011;4:788–98. [DOI] [PubMed] [Google Scholar]
- 21. Zareian M, Ciuffo L, Habibi M, Opdahl A, Chamera EH, Wu C. et al. Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment. J Cardiovasc Magn Reson 2015;17:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eng J, Bluemke D. Calibration of longitudinal measurements of cardiac anatomy and function in the multiethnic study of atherosclerosis. MESA Website2009. https://www.mesa-nhlbi.org/Mesainternal/committees/MRI/documents/Calibration%20plan%203-16-09.pdf (23 October 2018, date last accessed).
- 23. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB. et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yanez ND, Kronmal RA 3rd, Shemanski LR, Psaty BM, Cardiovascular HS.. A regression model for longitudinal change in the presence of measurement error. Ann Epidemiol 2002;12:34–8. [DOI] [PubMed] [Google Scholar]
- 25. Pencina MJ, D' Agostino RB, D'Agostino RB, Vasan RS.. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statist Med 2008;27:157–72; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 26. Gronnesby JK, Borgan O.. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal 1996;2:315–28. [DOI] [PubMed] [Google Scholar]
- 27. Chao T-F, Lip GYH, Liu C-J, Lin Y-J, Chang S-L, Lo L-W. et al. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. J Am Coll Cardiol 2018;71:122–32. [DOI] [PubMed] [Google Scholar]
- 28. Yoon M, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS. et al. Dynamic changes of CHA2DS2-VASc score and the risk of ischaemic stroke in Asian patients with atrial fibrillation: a nationwide cohort study. Thromb Haemost 2018;118:1296–304. [DOI] [PubMed] [Google Scholar]
- 29. Chao TF, Lip GYH, Lin YJ, Chang SL, Lo LW, Hu YF. et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow-up and delta HAS-BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemost 2018;118:768–77. [DOI] [PubMed] [Google Scholar]
- 30. Vardoulis O, Monney P, Bermano A, Vaxman A, Gotsman C, Schwitter J. et al. Single breath-hold 3D measurement of left atrial volume using compressed sensing cardiovascular magnetic resonance and a non-model-based reconstruction approach. J Cardiovasc Magn Reson 2015;17:47.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G. et al. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart 2013;99:1832–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.