Abstract
Two kidney transplant recipients from a single donor became infected with HTLV-1 (human T-lymphotropic virus type 1) in Spain. One developed myelopathy 8 months following surgery despite early prescription of antiretroviral therapy. The allograft was removed from the second recipient at month 8 due to rejection and immunosuppressors discontinued. To date, 3 years later, this patient remains infected but asymptomatic. HTLV-1 infection was recognized retrospectively in the donor, a native Spaniard who had sex partners from endemic regions. Our findings call for a reappraisal of screening policies on donor–recipient organ transplantation. Based on the high risk of disease development and the large flux of persons from HTLV-1 endemic regions, pre-transplant HTLV-1 testing should be mandatory in Spain.
Keywords: antiretroviral drugs, HTLV-1, myelopathy, screening, transplantation
Introduction
Human T-lymphotropic virus type 1 (HTLV-1) infection is a neglected disease despite there being 10–15 million people infected worldwide.1,2 As in HIV infection, chronicity uniformly occurs following acute HTLV-1 acquisition and there are no self-limited infections. However, less than 10% of HTLV-1 carriers may develop lifelong clinical manifestations, including two life-threatening illnesses, namely a subacute invalidating myelopathy known as tropical spastic paraparesis (TSP)3 and an acute T-cell leukemia/lymphoma (ATLL).4
HTLV-1 is efficiently transmitted perinatally (breastfeeding), sexually (more from male to female) and parenterally (transfusions, injection drug use and transplants).2 The diagnosis is based on the demonstration of specific serum HTLV-1 antibodies. A high HTLV-1 proviral load predicts the risk of disease development5 and sexual transmission.6 To date there is neither prophylactic vaccine nor effective antiviral therapy.7
Spain is one of the world leading countries performing solid organ transplants. The annual figure is steadily rising, and is currently nearly 5300 transplants per year. To date, more than 110,000 individuals have benefited from organ donations. More than 60% are kidney transplants, with liver being the second most frequent allograft. Heart, lung and pancreas are transplanted less frequently. On the other hand, of the nearly 47 million people currently living in Spain, around 4.5 million are foreigners. In addition, the country is a frequent destination for tourists, with 75 million visitors during 2016, more than 10% from HTLV-1 endemic regions.8
Rapid-onset subacute myelopathy and T lymphomas associated with HTLV-1 infection have both been reported following solid organ transplantation. At least three different scenarios have been described for HTLV-1 acquisition and disease in the transplantation setting, including infection from the organ donor, contaminated blood transfusions during surgery, and baseline carriage of HTLV-1 by the recipient.9,10 It seems that the immunosuppression used to avoid organ rejection (e.g. corticoids, tacrolimus, cyclosporine, mycophenolate, etc.) plays a major role in frequent and rapid-onset disease development in this population, particularly for HTLV-1 associated myelopathy (HAM).9,10
Case reports
Patient 1
During 2015 a 54-year-old woman underwent kidney transplantation in Spain. She was a white native Spaniard and denied any risk factors for HTLV-1 infection. To date, HTLV testing of blood and tissues in Spain is only recommended for ‘donors that came from or live in highly endemic regions, or have either sex partners or parents from those regions’.11 However, many transplantation centers do HTLV screening in all donor-recipients when possible. In this case, results of the donor HTLV testing are received after surgery, informing of reactivity for HTLV antibodies using a commercial enzyme immunoassay. No organs other than the two kidneys were transplanted from the same donor.
Positivity for HTLV-1 in the first kidney recipient was confirmed thereafter using immunoblot. Soon after this became known she was informed and antiretroviral therapy with zidovudine, lamivudine plus raltegravir was introduced within the first week following transplantation. Antiretrovirals were used for at least 18 months. In a retrospective interview of the deceased donor’s relatives, it became apparent that he most likely had acquired HTLV-1 from a Brazilian heterosexual partner.
Eight months later the recipient developed difficulty walking, along with unsteady gait, followed by progressive lower extremity weakness, all suggestive of HAM/TSP.12 Her HTLV-1 proviral load had been high in sequential longitudinal samples collected soon after transplantation, in the range of 320–350 HTLV-1 DNA copies per 10,000 peripheral blood mononuclear cells/ml. At the time of presentation of the initial neurological symptoms, the proviral load in the cerebrospinal fluid was also very high (2340 HTLV-1 DNA copies per 10,000 mononuclear cells/ml).
Patient 2
A 65-year-old male was the second kidney transplant recipient from the same infected donor. Like the other recipient, he was a white native Spaniard who denied any risk factor for HTLV-1 infection. He also became infected with HTLV-1 after transplantation but to date, 3 years later, has not developed any disease. He received antiretroviral drugs, including zidovudine, lamivudine plus raltegravir during the first 2 months following transplantation. It should be highlighted that immunosuppressive therapy (mycophenolate and tacrolimus) dosing had to be reduced from the second month due to hematological toxicity. Finally, rejection of the allograft forced its removal 8 months following transplantation. The patient is no longer undergoing immunosuppressant treatment and is currently undergoing hemodialysis. His proviral load has always been low, in the range of 25 HTLV-1 DNA copies per 10,000 peripheral blood mononuclear cells/ml.
Discussion
Similar cases of HAM/TSP shortly after transplantation have been reported in the literature (Table 1).13–25 Myelopathy was originally reported in a heart transplant recipient in France following HTLV-1 acquisition from contaminated blood transfusions during the surgery.13 In Spain, two kidney recipients and one liver transplant recipient who received organs from a single deceased HTLV-1 donor developed HAM/TSP within 2 years.16 More recently, in the USA two kidney transplant recipients from separate infected donors developed HAM/TSP.19,20
Table 1.
Reports of HTLV-1 associated diseases in solid organ transplant recipients.HAM/TSP developed in recipients negative for HTLV-1 who received organs from infected donors or, more occasionally, contaminated blood transfusions during surgery. In contrast, most recipients who developed ATLL were HTLV-1 before transplantation and immunosuppression.
| Country | Year | Age (years) | Gender | Organ | Interval | Reference | |
|---|---|---|---|---|---|---|---|
| HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) | |||||||
| 1 | France | 1990 | 41 | Male | Heart | 5 months | Gout et al.13 |
| 2 | Japan | 1992 | 32 | Male | Kidney | 11 months | Kuroda et al.14 |
| 3 | Japan | 2000 | 50 | Male | Kidney | 4 years | Nakatsuji et al.15 |
| 4 | Spain | 2003 | 44 | Female | Liver | 18 months | Toro et al.16 |
| 5 | Spain | 2003 | 54 | Female | Kidney | <3 months | Toro et al.16 |
| 6 | Spain | 2003 | 57 | Male | Kidney | 20 months | Toro et al.16 |
| 7 | Japan | 2008 | 58 | Male | Liver | 20 months | Soyama et al.17 |
| 8 | Japan | 2010 | 51 | Male | Kidney | 10 months | Inose et al.18 |
| 9 | USA | 2014 | 56 | Male | Kidney | 5 months | Ramanan et al.19 |
| 10 | USA | 2015 | 59 | Female | Kidney | 8 years | Younger20 |
| 11 | Germany | 2016 | 46 | Female | Kidney | 4 years | Gövert et al.21 |
| 12 | Japan | 2015 | 38 | Female | Kidney | 2 months | Nagamine et al.22 |
| 13 | Japan | 2016 | 42 | Female | Kidney | 3 years | Tajima et al.23 |
| 14 | Japan | 2016 | 65 | Male | Kidney | 8 months | Tajima et al.23 |
| 15 | Japan | 2016 | ? | ? | Liver | 15 months | Yoshizumi et al.24 |
| 16 | Japan | 2016 | ? | ? | Liver | 46 months | Yoshizumi et al.24 |
| 17 | Ecuador | 2016 | 40 | Male | Kidney | 2 years | Montesdeoca et al.25 |
| 18 | Spain | 2016 | 54 | Female | Kidney | 8 months | Current case |
| Adult T-cell leukemia/lymphoma (ATLL) | |||||||
| 1 | Canada | 1989 | 58 | Female | Kidney | 11 years | Zanke et al.26 |
| 2 | UK | 1995 | ? | Male | Kidney | ? | Jenks et al.27 |
| 3 | Japan | 2001 | 32 | Male | Kidney | 5 months | Hoshida et al.28 |
| 4 | Japan | 2001 | 32 | Male | Kidney | 11 months | Hoshida et al.28 |
| 5 | Japan | 2001 | 43 | Male | Kidney | 4 years | Hoshida et al.28 |
| 6 | Japan | 2001 | 56 | Male | Kidney | 18 months | Hoshida et al.28 |
| 7 | Japan | 2001 | 47 | Female | Kidney | <3 months | Hoshida et al.28 |
| 8 | Japan | 2006 | 39 | Female | Liver | 6 months | Kawano et al.29 |
| 9 | Japan | 2006 | 67 | Male | Liver | 9 months | Kawano et al.29 |
| 10 | Japan | 2006 | 45 | Male | Liver | 25 months | Kawano et al.29 |
| 11 | Germany | 2008 | 59 | Male | Liver | 2 years | Glowacka et al.30 |
| 12 | Germany | 2009 | 28 | Male | Kidney | 3 years | Glowacka et al.30 |
| 13 | Japan | 2016 | 39 | Female | Liver | 181 days | Yoshizumi et al.24 |
| 14 | Japan | 2016 | 45 | Male | Liver | 291 days | Yoshizumi et al.24 |
| 15 | Japan | 2016 | 67 | Male | Liver | 257 days | Yoshizumi et al.24 |
| 16 | Japan | 2016 | 48 | Male | Liver | 823 days | Yoshizumi et al.24 |
| 17 | Japan | 2016 | 59 | Female | Liver | 1315 days | Yoshizumi et al.24 |
In Japan, where HTLV-1 is endemic, cases of HAM/TSP have been reported following either kidney or liver transplants.14,15,17,22–24 More interesting, however, is the diagnosis of ATLL shortly following solid organ transplants, mostly among native Japanese recipients already infected with HTLV–1.28,29 Similar cases have been reported in other places.26,27 This is somewhat unique since most post-transplant lymphoproliferative disorders are of B lymphocytes and linked to Epstein–Barr virus infection. Anecdotally, in Germany, transplanted kidneys and a liver resulted in primary cutaneous T-cell lymphoma in at least two patients infected from a single HTLV-1 donor.30 Table 1 summarizes the major features of ATLL cases reported so far following solid organ transplantation.
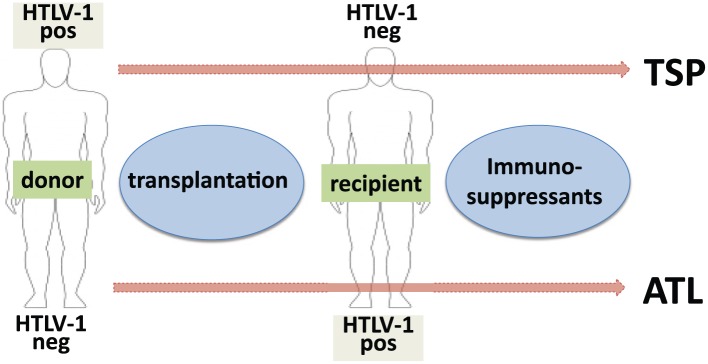
These findings call into question the current view that anti-HTLV screening of donated organs is not needed or is only recommended when there is suspicion. This opinion is based on the assumption that HTLV-1 associated diseases will develop only in a small proportion of carriers and that progression to disease is slow compared with the average lifespan of humans and therefore poses no major threats to public health.31 We postulate that in the transplant setting the very high risk of transmission and the high rate along with the rapidity of developing HTLV-1 disease is the result of immunosuppressive therapy. Interestingly, it seems to be a divergent predominant clinical manifestation depending on whether the allograft derives from an HTLV-1 infected donor or it is the recipient who actually carries the virus (Figure 1). When the recipient is already infected, transplantation might lead more frequently to ATLL, whereas when HTLV-1 is acquired from the donor, the recipient’s greater risk is for HAM/TSP.
Figure 1.
Model for HTLV-1 disease development in transplant recipients.
In a recent large retrospective study conducted in Japan including over 180 kidney transplants that involved HTLV-1 either in donors or recipients, 4 out of 10 HTLV-negative recipients that became infected following transplantation of a positive donor developed HAM/TSP within 4 years. In contrast, only 1 of 59 HTLV-1 positive recipients developed complications, with the only exception being a patient who developed both TSP/HAM and ATLL at 8 and 10 years, respectively, after transplantation.32
More than 35 years after the discovery of HTLV-1,33 donor/recipient screening for the virus remains sporadic or nonexistent in most countries.34 In 1993, the American CDC recommended that persons infected with HTLV be counseled ‘not to donate blood, semen, body organs or other tissues’.35 Nevertheless, in 2009 the recommendation for universal HTLV screening in deceased organ donors was dropped in the USA because of the perception of low HTLV-1 prevalence and low positive predictive value of serologic screening tests.36 Alongside this recommendation, most international transplant society guidelines currently do not provide any advice on HTLV-1 screening and use of donated organs.
High rates of HTLV-1 have been found in specific groups in nonendemic regions of North America37,38 and Europe,39 and decades of migration/immigration and tourism/traveling have altered the demographics of many Western countries. For example, the ongoing refugee migration from the Middle East and Africa would rapidly change the prevalence of HTLV-1 in many European countries. For a while this has been the case with Latin Americans in Spain, given the large influx of immigration facilitated by strong cultural and ancestral links.8 Since 1988 there has been a national register of HTLV-1 cases in Spain. As of December 2018, a total of 369 cases have been reported. Most cases are concentrated around the largest urban areas (Madrid and Barcelona), where the greatest immigrant populations are living. However, HTLV-1 infected persons have been identified across the whole Spanish geography.
In response to the worldwide evolving foci of HTLV-1 infections and the very poor prognosis of post-transplant TSP and ATLL, there are urgent calls for broader HTLV-1 screening of live and –more difficult – deceased organ donors. Organ procurement organizations and transplant programs should determine local prevalence to guide HTLV-1 screening efforts.37 Targeted screening of potential high-risk living (and deceased) donors for HTLV-1 has been recommended by some experts.19 Suggestions have also been put forward for national or international registries of all HTLV-1 affected transplants.10 Alerted to the dangers of rapid-onset TSP following HTLV-1 infected organ transplants, Japan begun in 2014 HTLV-1 screening of all kidney donations. Similarly, the UK issued new transplantation guidance on HTLV-1 screening of cadaveric solid organs in 2011. Finally, the Global Virus Network has recently called for more systematic HTLV-1 screening before solid organ transplantation everywhere.31
It has been highlighted that ‘whereas not screening donors . . . for HIV infection would be considered unethical, the same is not the case for HTLV-1, another human retrovirus, where risk assessments are made based on the predicted prevalence of cases among donors, the probable risk of transmission, and the subsequent likelihood of disease’.10 Yet, those risk estimates are based on inadequate national epidemiology, ignoring changing demographics in many countries,1,2 and a poor understanding of the transplant-acquired HTLV-1 disease risk.9,10 The screening costs for HTLV-1 are small in comparison with the cost of post-transplant illness and/or death associated with TSP or ATLL following HTLV-1 infection.40 For all these reasons, the American and European CDCs, along with other health agencies, should urgently update their policy recommendations on organ transplant HTLV-1 screening. In the meantime, some transplant centers around the world, including a few in Spain, have already implemented ‘rapid’ (or ‘urgent’) HTLV-1 testing of all deceased organ donors. In parallel, diagnostic companies should improve the specificity of HTLV-1 screening tests and design rapid tools (i.e. point-of-care, or PIC, tests) to minimize unwanted organ discharge.
Acknowledgments
We would like to thank all members of the HTLV Spanish Study Group.
C. Rodríguez, M. Vera and J. del Romero (Centro Sanitario Sandoval, Madrid); G. Marcaida and M.D. Ocete (Hospital General Universitario, Valencia); E. Caballero and I. Molina (Hospital Vall d’Hebró, Barcelona); A. Aguilera, J.J. Rodríguez-Calviño, D. Navarro, C. Rivero and M.D. Vilariño (Hospital Conxo-CHUS, Santiago); R. Benito, S. Algarate and J. Gil (Hospital Clínico Universitario Lozano Blesa, Zaragoza); R. Ortiz de Lejarazu and S. Rojo (Hospital Clínico Universitario, Valladolid); J.M. Eirós and A. San Miguel (Hospital Rio Hortega, Valladolid); C. Manzardo and J.M. Miró (Hospital Clínic-IDIBAPS, Barcelona); J. García and I. Paz (Hospital Cristal-Piñor, Orense); E. Poveda (INIBIC-Complejo Hospitalario Universitario, A Coruña); E. Calderón (Hospital Virgen del Rocío and CIBERESP, Sevilla); D. Escudero (Hospital Germans Trias i Pujol, Barcelona); M. Trigo, J. Diz and M. García-Campello (Complejo Hospitalario, Pontevedra); M. Rodríguez-Iglesias (Hospital Universitario, Puerto Real); A. Hernández-Betancor and A.M. Martín (Hospital Insular Hospital Universitario, Las Palmas de Gran Canaria); J.M. Ramos and A. Gimeno (Hospital Universitario, Alicante); F. Gutiérrez, J.C. Rodríguez and V. Sánchez (Hospital General, Elche); C. Gómez-Hernando (Complejo Hospitalario Virgen de la Salud, Toledo); G. Cilla and E. Pérez-Trallero (Hospital Donostia, San Sebastián); J. López-Aldeguer (Hospital La Fe, Valencia); L. Fernández-Pereira (Hospital San Pedro de Alcántara, Cáceres); J. Niubó (Ciudad Sanitaria de Bellvitge, Barcelona); M. Hernández, A.M. López-Lirola and J.L. Gómez-Sirvent (Hospital Universitario La Laguna, Tenerife); L. Force (Hospital General, Mataró); C. Cifuentes (Hospital Son Llátzer, Palma de Mallorca); S. Pérez and L. Morano (Hospital do Meixoeiro, Vigo); C. Raya (Hospital del Bierzo, Ponferrada); A. González-Praetorius (Hospital Universitario, Guadalajara); J.L. Pérez and M. Peñaranda (Hospital Son Espases, Mallorca); S. Hernáez-Crespo (Hospital de Basurto, Bilbao); J.M. Montejo (Hospital de Cruces, Bilbao); L. Roc and A. Martínez-Sapiña (Hospital Miguel Servet, Zaragoza); I. Viciana (Hospital Virgen de la Victoria, Málaga); T. Cabezas, A. Lozano and J.M. Fernández (Hospital de Poniente, Almería); I. García-Bermejo and G. Gaspar (Hospital Universitario, Getafe); R. García, M. Górgolas, C. Vegas and J. Blas (Fundación Jiménez Díaz, Madrid); P. Miralles, M. Valeiro and T. Aldamiz (Hospital Gregorio Marañón, Madrid); N. Margall (Hospital Santa Creu i Sant Pau, Barcelona); C. Guardia and E. do Pico (ICS, Barcelona); I. Polo, A. Aguinaga and C. Ezpeleta (Complejo Hospitalario Navarra, Pamplona); S. Sauleda and M. Pirón (Banco de Sangre and Tejidos, Barcelona); R. González and L. Barea (Centro de Transfusiones, Madrid); A. Jiménez and L. Blanco (Centro de Hemoterapia y Hemodonación de Castilla y León, Valladolid); A. Suárez and I. Rodríguez-Avial (Hospital Clínico San Carlos, Madrid); A. Pérez-Rivilla, P. Parra and M. Fernández (Hospital Universitario 12 de Octubre, Madrid); M. Fernández-Alonso y G. Reina (Clínica Universitaria, Pamplona); A. Treviño, S. Requena, L. Benítez-Gutiérrez, V. Cuervas-Mons and C. de Mendoza (IIS Hospital Universitario Puerta de Hierro, Majadahonda); P. Barreiro (La Paz University Hospital, Madrid); V. Soriano, O. Corral and F. Gómez-Gallego (UNIR Health Sciences School, Madrid).
Footnotes
Conflict of interest statement: The author(s) declare that there is no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical approval: This study was approved by the Puerta de Hierro Research Institute, where the coordinator of the HTLV Spanish study group is based.
Informed consent: Data recorded were collected anonymously through the national registry.
ORCID iD: Vicente Soriano  https://orcid.org/0000-0002-4624-5199
https://orcid.org/0000-0002-4624-5199
Contributor Information
Lourdes Roc, Microbiology Department, Hospital Miguel Servet, Zaragoza.
Carmen de Mendoza, Puerta de Hierro Research Institute and University Hospital, Majadahonda, Madrid, Spain.
Miriam Fernández-Alonso, Microbiology Department, Clinica Universitaria, Pamplona.
Gabriel Reina, Microbiology Department, Clinica Universitaria, Pamplona.
Vicente Soriano, UNIR Health Sciences School, Calle Almansa 101, Madrid, 28040, Spain.
on behalf of the Spanish HTLV Network:
C. Rodríguez, M. Vera, J. del Romero, G. Marcaida, M.D. Ocete, E. Caballero, I. Molina, A. Aguilera, J.J. Rodríguez-Calviño, D. Navarro, C. Rivero, M.D. Vilariño, R. Benito, S. Algarate, J. Gil, R. Ortiz de Lejarazu, S. Rojo, J.M. Eirós, A. San Miguel, C. Manzardo, J.M. Miró, J. García, I. Paz, E. Poveda, E. Calderón, D. Escudero, M. Trigo, J. Diz, M. García-Campello, M. Rodríguez-Iglesias, A. Hernández-Betancor, A.M. Martín, J.M. Ramos, A. Gimeno, F. Gutiérrez, J.C. Rodríguez, V. Sánchez, C. Gómez-Hernando, G. Cilla, E. Pérez-Trallero, J. López-Aldeguer, L. Fernández-Pereira, J. Niubó, M. Hernández, A.M. López-Lirola, J.L. Gómez-Sirvent, L. Force, C. Cifuentes, S. Pérez, L. Morano, C. Raya, A. González-Praetorius, J.L. Pérez, M. Peñaranda, S. Hernáez-Crespo, J.M. Montejo, L. Roc, A. Martínez-Sapiña, I. Viciana, T. Cabezas, A. Lozano, J.M. Fernández, I. García-Bermejo, G. Gaspar, R. García, M. Górgolas, C. Vegas, J. Blas, P. Miralles, M. Valeiro, T. Aldamiz, N. Margall, C. Guardia, E. do Pico, I. Polo, A. Aguinaga, C. Ezpeleta, S. Sauleda, M. Pirón, R. González, L. Barea, A. Jiménez, L. Blanco, A. Suárez, I. Rodríguez-Avial, A. Pérez-Rivilla, P. Parra, M. Fernández, M. Fernández-Alonso, G. Reina, A. Treviño, S. Requena, L. Benítez-Gutiérrez, V. Cuervas-Mons, C. de Mendoza, P. Barreiro, V. Soriano, O. Corral, and F. Gómez-Gallego
References
- 1. Hlela C, Shepperd S, Khumalo Net al. The prevalence of HTLV type 1 in the general population is unknown. AIDS Rev 2009; 11: 205–214. [PubMed] [Google Scholar]
- 2. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 2012; 3: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gessain A, Barin F, Vernant Jet al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 1985; 2: 407–410. [DOI] [PubMed] [Google Scholar]
- 4. Yoshida M, Seiki M, Yamaguchi Ket al. Monoclonal integration of HTLV provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA 1984; 81: 2534–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martins M, Guimarães J, Ribas Jet al. Long-term follow-up of HTLV-1 proviral load in asymptomatic carriers and in incident cases of HAM/TSP: what is its relevance as a prognostic marker for neurologic disease? J Neurovirol 2017; 23: 125–133. [DOI] [PubMed] [Google Scholar]
- 6. Paiva A, Smid J, Haziot Met al. High risk of heterosexual transmission of human T-cell lymphotropic virus type 1 infection in Brazil. J Med Virol 2017; 89: 1287–1294. [DOI] [PubMed] [Google Scholar]
- 7. Willems L, Hasegawa H, Accolla Ret al. Reducing the global burden of HTLV-1 infection: an agenda for research and action. Antiviral Res 2017;137: 41–48. [DOI] [PubMed] [Google Scholar]
- 8. De Mendoza C, Caballero E, Aguilera Aet al. HTLV-1 infection and disease in Spain. AIDS 2017; 31: 1653–1663. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong M, Corbett C, Rowe Iet al. HTLV-1 in solid organ transplantation: current challenges and future management strategies. Transplantation 2012; 94: 1075–1084. [DOI] [PubMed] [Google Scholar]
- 10. Taylor G. Lessons on transplant-acquired HTLV infection. Clin Infect Dis 2013; 57: 1425–1426. [DOI] [PubMed] [Google Scholar]
- 11. Boletin Oficial del Estado (BOE). 2014, November 5, pp.90536–90538. [Google Scholar]
- 12. Moreno D, Yuste JR, Martín Pet al. HTLV-1 myelopathy after renal transplant and antiviral prophylaxis: the need for screening. J Neurovirol 2018; 24: 523–525. [DOI] [PubMed] [Google Scholar]
- 13. Gout O, Baulac M, Gessain Aet al. Rapid development of myelopathy after HTLV-I infection acquired by transfusion during cardiac transplantation. N Engl J Med 1990; 322: 383–388. [DOI] [PubMed] [Google Scholar]
- 14. Kuroda Y, Takashima H, Yukitake Met al. Development of HTLV-1-associated myelopathy after blood transfusion in a patient with aplastic anemia and a recipient of a renal transplant. J Neurol Sci 1992; 109: 196–199. [DOI] [PubMed] [Google Scholar]
- 15. Nakatsuji Y, Sugai F, Watanabe Set al. HTLV-I associated myelopathy manifested after renal transplantation. J Neurol Sci 2000; 177: 154–156. [DOI] [PubMed] [Google Scholar]
- 16. Toro C, Rodés B, Poveda Eet al. Rapid development of subacute myelopathy in three organ transplant recipients after transmission of HTLV type I from a single donor. Transplantation 2003; 75: 102–104. [DOI] [PubMed] [Google Scholar]
- 17. Soyama A, Eguchi S, Takatsuki Met al. HTLV type 1-associated myelopathy following living donor liver transplantation. Liver Transpl 2008; 14: 647–650. [DOI] [PubMed] [Google Scholar]
- 18. Inose Y, Akiyama S, Mochizuki Aet al. Case report of HTLV-1 associated myelopathy (HAM) manifested after renal transplantation. Rinsho Shinkeigaku 2010; 50: 241–245. [DOI] [PubMed] [Google Scholar]
- 19. Ramanan P, Deziel P, Norby Set al. Donor-transmitted HTLV-1-associated myelopathy in a kidney transplant recipient: case report and literature review. Am J Transplant 2014; 14: 2417–2421. [DOI] [PubMed] [Google Scholar]
- 20. Younger D. HTLV-1-associated myelopathy/tropical spastic paraparesis and peripheral neuropathy following live-donor renal transplantation. Muscle Nerve 2015; 51: 455–456. [DOI] [PubMed] [Google Scholar]
- 21. Gövert F, Krumbholz A, Witt Ket al. HTLV-1 associated myelopathy after renal transplantation. J Clin Virol 2015; 72: 102–105. [DOI] [PubMed] [Google Scholar]
- 22. Nagamine Y, Hayashi T, Kato Yet al. HTLV-1-associated myelopathy manifesting shortly after living-donor renal transplantation. Intern Med 2015; 54: 75–78. [DOI] [PubMed] [Google Scholar]
- 23. Tajima Y, Matsumura M, Yaguchi Het al. Two cases of HAM/TSP caused by living-donor renal transplantation. Case Rep Neurol Med 2016; 2016: 4203079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshizumi T, Takada Y, Shirabe Ket al. Impact of human T-cell leukemia virus type 1 on living donor liver transplantation: a multi-center study in Japan. J Hepatobiliary Pancreat Sci 2016; 23: 333–341. [DOI] [PubMed] [Google Scholar]
- 25. Montesdeoca MJ, Correa Diaz E, Buestán ME. HTLV-1-associated myelopathy in a solid organ transplant recipient. BMJ Case Rep 2016. Epub ahead of print 6 June 2016. DOI: 10.1136/bcr-2016-215243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zanke B, Rush D, Jeffery Jet al. HTLV-1 T cell lymphoma in a cyclosporine-treated renal transplant patient. Transplantation 1989; 48: 695–696. [PubMed] [Google Scholar]
- 27. Jenks P, Barrett W, Raftery Met al. Development of HTLV-1-associated ATL during immunosuppressive treatment following renal transplantation. Clin Infect Dis 1995; 21: 992–993. [DOI] [PubMed] [Google Scholar]
- 28. Hoshida Y, Li T, Dong Zet al. Lymphoproliferative disorders in renal transplant patients in Japan. Int J Cancer 2001; 91: 869–875. [DOI] [PubMed] [Google Scholar]
- 29. Kawano N, Shimoda K, Ishikawa Fet al. Adult T-cell leukemia development from a HTLV-1 carrier after a living-donor liver transplantation. Transplantation 2006; 82: 840–843. [DOI] [PubMed] [Google Scholar]
- 30. Glowacka I, Korn K, Potthoff Set al. Delayed seroconversion and rapid onset of lymphoproliferative disease after transmission of HTLV type 1 from a multiorgan donor. Clin Infect Dis 2013; 57: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 31. Gallo R, Willems L, Hasegawa Het al. Screening transplant donors for HTLV-1 and -2. Blood 2016; 128: 3029–3031. [DOI] [PubMed] [Google Scholar]
- 32. Yamauchi J, Yamano Y, Yuzawa K. Risk of human T-cell leukemia virus type 1 infection in kidney transplantation. N Engl J Med 2019; 380: 296–298. [DOI] [PubMed] [Google Scholar]
- 33. Poiesz B, Ruscetti F, Gazdar Aet al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA 1980; 77: 7415–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taylor G. HTLV-1 and HTLV-1-associated myelopathy/tropical spastic paraparesis. Clin Infect Dis 2015; 61: 57–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention and the U.S.P.H.S. Working Group. Guidelines for counseling persons infected with HTLV type I and type II. Ann Intern Med 1993; 118: 448–454. [DOI] [PubMed] [Google Scholar]
- 36. Stramer S, Notari E, Zou Set al. HTLV antibody screening of blood donors: rates of false positive results and evaluation of a potential donor re-entry algorithm. Transfusion 2011; 51: 692–701. [DOI] [PubMed] [Google Scholar]
- 37. Chang Y, Kaidarova Z, Hindeset al. Seroprevalence and demographic determinants of HTLV-1 and 2 infections among first-time blood donors: United States, 2000–2009. J Infect Dis 2014; 209: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cook L, Taylor G. HTLV-1 and HTLV-2 prevalence in the United States. J Infect Dis 2014; 209: 486–487. [DOI] [PubMed] [Google Scholar]
- 39. Mowbray J, Mawson S, Chawira Aet al. Epidemiology of HTLV-1 infections in a subpopulation of Afro-Caribbean origin in England. J Med Virol 1989; 29: 289–295. [DOI] [PubMed] [Google Scholar]
- 40. Tedla F, Brar A, John Det al. Risk of transmission of HTLV through transplant. Am J Transplant 2015; 15: 1123–1124. [DOI] [PubMed] [Google Scholar]