Abstract
Colorectal cancer (CRC) is one of the most widely recognized and deadly malignancies worldwide. In spite of the fact that the death rates have declined over the previous decade, particularly because of enhanced screening or potential treatment alternatives, CRC still remains the third leading cause of cancer-related mortality in the world, with an estimated incidence of over 1 million new cases and approximately 600 000 deaths estimated yearly. Unlike prostate and lung cancer, CRC is not easily detectable in its early stage, which may also account for its high mortality rate. MicroRNAs (miRNAs) are a class of noncoding RNAs. The roles of these noncoding RNAs have been implicated in cancer pathogenesis, most especially CRC, due to their ability to posttranscriptionally regulate the expression of oncogenes and tumor suppressor genes. Dysregulated expression of many miRNAs regulates the expression of hundreds of growth regulatory genes and pathways that are important in the multistep model of colorectal carcinogenesis. If CRC is detected early, it is a largely treatable disease. Early diagnosis, including the identification of premalignant adenomas, is regarded a major concept for improving patient survival in CRC treatment. Several lines of research suggest that miRNAs are closely implicated in the metastatic process in CRC and some of these miRNAs could be useful as promising clinical tools for identifying specific stages of CRC due to their differential expression. This review discusses the correlation between CRC staging relative to the specific expression of miRNA for early detection, treatment, and disease management.
Keywords: microRNA, prognosis, colorectal cancer, diagnosis, biomarker, staging
Introduction
Colorectal cancer (CRC) is the most common malignancy in the gastrointestinal tract/bowel or large intestine, the third most commonly diagnosed cancer, and also the third cause of cancer-related demise worldwide.1 More often than not, CRC is thought of as a typical disease affecting old individuals, with most cases analyzed amid the fifth and sixth decades and a higher predominance among men.2 It is a multifactorial disease process, with etiology encompassing genetic factors, environmental exposures (including diet), and inflammatory conditions of the digestive tract. Colorectal cancer develops through a gradual accumulation of genetic and epigenetic changes, resulting in the transformation of normal colonic mucosa into invasive cancer.3 Over 90% of colorectal carcinomas are adenocarcinomas (adenoma–carcinoma sequence) arising from epithelial cells of the colorectal mucosa,4 and the neoplastic transformation time is considered to be 10 to 15 years, which represents the available time to detect and remove these adenomas before their progression.3 Based on the differentiation of colorectal adenocarcinoma specified by a group of gland forming cells, colorectal carcinomas can be divided into well, moderately, and poorly differentiated adenomas with varying gland formation. Over 95%, 50% to 95%, and <70%, respectively, of these adenocarcinomas are gland forming and are the basis for CRC diagnosis through histological grading. Also, approximately 70% of the diagnosed CRC are moderately differentiated, while others such as poor and well-differentiated CRCs are reportedly 20% and 10%, respectively. Some of the CRCs may also be undifferentiated.5 The epidemiology of CRC can be categorized into modifiable risk factors, which include age; family history of familial adenomatous polyposis (FAP), Lynch syndrome, and inflammatory bowel diseases; and nonmodifiable risk factors (red and processed meat consumption, obesity, alcohol, and smoking). The larger part of CRCs is sporadic (70%-80%), with age being the most critical risk factor. Other inherited forms of this disease are FAP (less than 1%), nonpolyposis hereditary CRC or Lynch syndrome (2%-5%), or MYH gene–associated polyposis (<1%), which constitute a small proportion of reported cases.6 Moreover, cases associated with hereditary components have been estimated to be 20% to 25% and are termed familial CRC.7 The ideal technique to precisely identify CRC and recurrence at the most punctual conceivable time is an exceedingly debatable concept in research. It is well known that most recurrences occur within 5 years.8 Although researchers have provided improved CRC diagnosis, good treatment option, and a suitable way to predict recurrence and/or prognosis in CRC in recent time, the proper staging of CRC noninvasively for effective diagnosis can also be a good lead to its management, thereby increasing the overall survival of patients suffering from this cancer subtype.9,10 The involvement of short oligonucleotide noncoding ribonucleic acid as biomarkers with specific attributes that are distinct for human processes are proven indicators for improved diagnosis and treatment intervention for CRC.11-14
MicroRNAs (miRNAs) are small, 18 to 25 noncoding nucleotide sequences of RNA. These sequences control the expression of several target genes at the same time either by translational repression or degradation of the messenger RNA (mRNA) transcript after targeting the 3′-Untranslaterd region (3′UTR).15 Many major cellular functions such as development, differentiation, growth, and metabolism are regulated by these miRNAs.16 Therefore, they play a central role in research and clinical settings as potential valuable biomarkers and novel therapeutics for cancer.17-19 A single miRNA has been reported to regulate up to several hundred mRNAs simultaneously and affects a number of target transcripts. As of 2010, approximately 2200 miRNA genes were suggested to exist in the mammalian genome16 and one-third of the human genome is estimated to be regulated by miRNAs.20 Knowing the expression, distribution, and longevity of these noncoding class of RNA in tissues is essential for the understanding of both physiological and pathological mechanisms. In addition, determination of the tissues that express specific miRNAs and their stages will help to develop a miRNA in biological samples into a biomarker for a specific disease. Recently, miRNA expression in multiple human tissues has been provided in an atlas (https://ccb-web.cs.uni-saarland.de/tissueatlas) for the elucidation of the role of miRNAs in tissue development and tissue-specific diseases such as CRC and has reported that these miRNAs have the half-life of about 1 to 14 days at 4°C.21 MicroRNAs have been found in an assortment of body liquids, where they are astoundingly stable.22-24 Extracellular miRNAs could serve as diagnostic biomarkers relevant to both prevention and treatment of human cancer. Notwithstanding, broad research is fundamental for distinguishing the attributes of extracellular miRNAs to portray their roles in tumorigenesis and prevention.22 Accordingly, there may be a poor correlation between cellular and extracellular miRNAs and between miRNAs detectable in various biological fluids.25,26 The tumorigenesis of CRC involves multistep genomic changes, including the activation of oncogenes and inactivation of tumor suppressor genes. Numerous miRNAs have been reported to play a role in cancer development, such as carcinogenesis, progression, and metastasis.27,28 Only a couple of studies have explored circulating miRNAs in patients with CRC.15,29 Also, there is extremely limited research on the identification of commonly and differentially expressed miRNA for CRC staging if there is any. Efforts to depict clinical, pathological, and molecular features in patients have reached disputable ends with respect to tumor grade and disease stage at diagnosis.30 Also, it is generally acknowledged that diagnosis in patients is always difficult because of the vulnerability of both patient and the specialist to the presenting symptoms, leading to a frequent unfavorable outcome of the disease. If specific miRNAs are expressed in a certain stage of CRC, then early detection of this disease will be largely treatable. The review aims to discuss the staging of CRC with respect to specific miRNAs for early detection, treatment, efficacy, and effective management of the disease.
Molecular Pathogenesis of CRC
Suppressor pathway or pathway of chromosomal instability (CIN) was first proposed as the mechanism of colorectal carcinogenesis.31 The accumulation of mutations leads to oncogene activation such as Kirsten rat sarcoma (KRAS) and inactivation of tumor suppressor genes such as Deleted in Colorectal Cancer (DCC), Total Protein-53 (TP-53), SMAD family member 4, Mothers against decapentaplegic homolog 4 (SMAD4), and Adenomatous polyposis coli (APC).32 Regardless of the order of this molecular alteration, their accumulation is responsible for neoplastic transformation.33 Mutations in the genes MSH2, MSH3, MSH6, Exo1, PMS1, PSM2, MLH1, and MLH3 responsible for DNA repair during replication are associated with the second mechanism of colorectal carcinogenesis. These mismatch repair (MMR) genes play a crucial role in the identification and repair of errors after replication in order to prepare them for cell division. Accumulation of errors in repetitive DNA fragments causes mutations in target genes.34 Approximately 20% of sporadic CRC and Lynch syndrome are reportedly caused by mutations in mismatch DNA repair genes, that is, defective DNA MMR system (microsatellite instability).32,35 The last pathway of aberrant hypermethylation was identified as a mechanism of gene function silencing in the field of epigenetics.36 The CpG island methylator, also known as CIMP, is referred to as dinucleotide methylation, which occurs in the transcription start site upstream of many genes. They are attributed to 15% to 20% of sporadic CRC.37 The hypermethylation of the promoter region of any gene is mainly carried on by the CpG island methylator phenotype. The positive tumor of this methylator methylates certain marker genes. Examples of these genes are the calcium voltage-gated channel subunit α1 G, the protein-coding gene, suppressor of cytokine signaling-1, Runt-related transcription factor-3, the induction of neuronal differentiation by the overexpression of NEUROG-1, and finally the insulin-like growth factor 2.38 More than 2 of these genes are targeted and methylated by CIMP. Histologically, the differentiation of these tumors is poorly defined. They also exhibit microsatellite instability and are known to be B-RAF mutation carriers.39 The precursor lesions of the methylator tumors are the sessile serrated adenomas.40 A superior comprehension of carcinogenesis pathways has allowed the improvement of diagnostic and prognostic biomarkers and furthermore the examination of new remedial targets and predictors of CRC treatment response.
MicroRNA
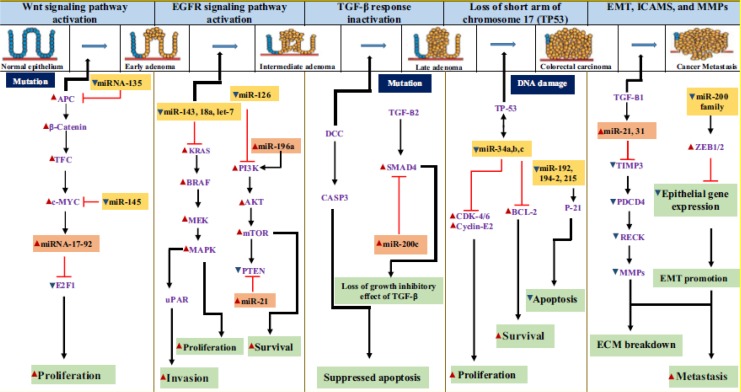
In our previous review, the overview of miRNA together with the synthesis, general functions, metabolic reprogramming, and their specific expression were discussed extensively.41 Furthermore, the mechanism of action underlying the initiation, progression, and metastasis of CRC with respect to miRNAs was also examined (Figure 1). Emerging evidence suggests the promising potential of these miRNAs as potential noninvasive biomarkers for CRC screening.42-48
Figure 1.
Involvement of microRNA (miRNA) in colorectal cancer.41 Red arrows—upregulation; blue arrows—downregulation. Experimentally validated miRNAs are shown alongside with their target genes in altered expression in CRC. CRC indicates colorectal cancer.
Biomarkers
Recently, interest has focused on the search for biomarkers in CRC. Tremendous research on CRC has revealed the 3 major pathways for carcinogenesis: chromosomal abnormalities, microsatellite instability pathway, and methylation pathway described by the epigenetic methylation of a large number of genes. Of the molecules associated with prognosis implicated in CIN pathway, only the epidermal growth factor receptor (EGFR) pathway as a biomarker is used for diagnosis due to its clinical relevance. This is because of the complexity and redundancy of several pathways occurring in cellular processes, as well as the lack of therapies that can effectively target various biomarkers.49 Epidermal growth factor receptor pathway has also been reported as the main target for the treatment of a specific type of CRC.50 Also, mutations observed in the pathways of the RAS family and the abnormal activation of the EGFR occur in a number of CRC cases.
The microsatellite instability status was also confirmed as the primary biomarker for stratification of stage II CRC. The CIMP pathway as reported is associated with a group of clinical and histological features involved with approximately 15% to 20% of CRC with MMR gene MLH. 51 The precursor lesions in CIMP cancers are serrated polyps, not adenomatous lesions, with the underlying genetic changes frequently occurring in the BRAF oncogene.52 Mutation in the Raf family B-Raf (B-Raf proto-oncogene, serine/threonine kinase) has been observed in the transformation of normal tissue layer or membrane into abnormal cell multiplication, such as crypt foci or sessile serrated polyps. The frequency of mutation in BRAF varies among human cancer, ranging from about 80% in skin cancer to around 0% to 18% among other cancers.53 About 1% to 3% and 5% were reported for lung and CRC, respectively. In nearly almost all the cases of BRAF mutation reported, thymine (T) is substituted with adenine (A) at the position 1799 nucleotide, thereby changing the amino acid valine (Val) to glutamic acid (Glu) at codon 600. This segment of activation has also been reported in various cancer types, including CRC.54-62 The methylation of BRAF gene promoter region causes loss of p16, leading to the cell progression to advanced polyps.63 Increase in activity also prompts the methylation of MutL homolog 1 gene, silencing transcription. Loss of function of this gene results in MMR deficiency and subsequently the high microsatellite instability in CRC phenotype.64,65
Moreover, aggregating evidence confirmed that cancer cells release some miRNAs into systemic circulation.66-68 This unique feature of miRNAs is one of the focal reasons behind the ongoing exploration and explosion of miRNA biomarker studies in cancer research. There are various types of biomarkers depending on their functions. Examples include diagnostic biomarkers (to identify/monitor or detect the type of tumor and/or reoccurrence, eg, carcinoembryonic antigen [CEA]), predictive biomarkers (to predict the efficacy or response to different treatments or therapeutic intervention), and prognostic biomarker (to indicate the progress of disease and to estimate the risk of disease recurrence, ie, estimation of survival outcome and treatment strategy).69
MicroRNAs have emanated as tumor-related biomarkers that reflect not only the existence of early-stage tumors but also the dynamics and status of advanced stage tumors, tumor recurrence, and drug sensitivities.66 Cancer-associated miRNAs are present in blood in a very stable and detectable form that is protected from endogenous ribonuclease activities and other conditions. Previous studies have demonstrated the ease of quantification of these circulating miRNAs using various methods.70-73 Several miRNA expressions have been implicated in various categories of a biomarker for the detection of tumor. High expression levels of miR-92a, miR-141, let-7a, miR-1229, miR-1246, miR-150, miR-21, miR-223, miR-23a, and miR-378 have been analyzed to be associated with diagnostic biomarkers, while high expression levels of miR-141, miR-320, miR-596, and miR-203 are majorly for prognosis, malignant potential, and tumor recurrence. High expression levels of miR-106a, miR-484, and miR-130b are associated with predictive biomarkers, and low expression levels of miR-106a, miR-484, and miR-130b are prognostic in nature.74
MiRNAs as CRC Diagnostic Tools
In CRC, abnormally expressed miRNAs disrupt cellular signal transduction and cell survival pathways, for example, Wnt signaling pathway, EGFR, and p53, connecting miRNA to known events in the pathway of cancer transformation.75 Accumulating evidence suggests that miRNAs may also have intense clinical applications. MicroRNA expression profiles have the ability to discriminate tumors from different cancer subtypes.76 Also, the expression of individual miRNAs may be used to predict patient survival, tumor stage, the presence of lymph node metastases, and the response to therapy in CRC.75,77,78 Studies investigated the differential expression of a panel of 95 miRNAs and also demonstrated that miR-92 was significantly elevated in the plasma of patients with CRC and that it has potential as a noninvasive molecular biomarker for CRC screening with high sensitivity and specificity.79 These researchers also showed that the discovery of miR-92a may differentiate CRC from other gastrointestinal cancers and inflammatory bowel diseases. Cheng et al80 proposed that plasma miR-141 may represent a novel biomarker that complements CEA in detecting CRC with distant metastasis and that high levels of miR-141 in plasma were associated with poor prognosis. Furthermore, 7 miRNAs (let-7a, miR-1229, miR-1246, miR-150, miR-21, miR-223, and miR-23a) were validated using quantitative real-time polymerase chain reaction (RT-PCR). These miRNAs were confirmed to be suitable biomarkers to detect CRC. They also possess high sensitivity and specificity.81 Another study also discovered miR-378 in biological fluid as a screening biomarker that can discriminate patients with CRC from a healthy individual.82
Colorectal Cancer Prognosis
Early detection of distant metastasis and selective criteria regarding which individuals would benefit most from invasive treatments is essential for improving long-term survival. The most important predictor of outcome is the stage of disease at diagnosis (Table 1). Therapeutic prognosis is an evaluative segment of medicine and research that includes the science of estimating the intricacy and recurrence of CRC and an anticipated survival of patients.83 A substantial number of variables, including tumor grade, tumor size and staging, and lymph node status including different viewpoints, may impact, influence, or correlate with prognosis for patients with CRC. Therefore, prognostication of CRC is an imperative element for providing compelling/effective treatment for patients with the colorectal tumor. However, survival studies have shown inconsistent results. Fu et al84 found out that younger patients tend to have a poorer prognosis compared to their older counterparts. Others studies did not agree with these findings and suggested that older patients have a poorer prognosis.30,85-88 Manjelievskaia et al89 reported that there is no survival difference between both patients. The use of biological markers to help prognostication is important. A good tumor biomarker should be less invasive, have a long half-life, and be estimated accurately and precisely by a simple and inexpensive blood test. It is also crucial to put into consideration how specific and sensitive they are to change so that it can be followed over time by serial measurements.90 A couple of biomarkers meet these criteria. MicroRNA is a flawless precedent. The only potentially curative modality employed in patients with stages I-III and selected stage IV patient with the oligometastatic disease is surgical resection.
Table 1.
Correlation of CRC TNM Stages With Prognosis (Modified From Cancer Therapy Advisor).a
| Stages | TNM | 5-Year Survival (%) |
|---|---|---|
| 0, I | Tis, T1, N0, M0 | >90 |
| I | T2, N0, M0 | 80-85 |
| II | T3-4, N0, M0 | 70-75 |
| III | T2, N1-3, M0 | 70-75 |
| III | T3, N1-3, M0 | 50-65 |
| III | T4, N1-2, M0 | 25-45 |
| IV | M1 | <3 |
Abbreviations: CRC, colorectal cancer; TNM, tumor–node–metastasis.
aTIS indicates carcinoma in situ intraepithelia or invasion of lamina propria; T1, tumor invasion of submucosa; T2, tumor invasion of muscularis propria; T3, tumor invasion through the muscularis propria into pericolorectal tissues; T4, penetration of tumor through the surface of the visceral peritoneum and further directly invading other organs.
Patients with prior phases of the disease, including stage I (5-year survival >90%) and stage II (5-year survival 70%-85%), do not require adjuvant treatment aside from those with high-risk stage II disease. Individuals with resectable CRC are at a higher risk of locoregional relapse and require chemoradiotherapy in addition to adjuvant chemotherapy for risk reduction. The majority of patients with metastatic CRC (stage IV) are treated with a palliative intent to prolong life while preserving the quality of life. With modern chemotherapy regimens, the median overall survival of patients with metastatic CRC is less than 3 years (5-year survival approximately 10%; Table 1).91 These numbers indicate clear improvements in outcomes for patients with CRC, and many promising novel therapies remain under development.92 Prognostic biomarkers have been described in CRC.80 BRAF mutations occur in 7% to 10% of patients and are associated with poor outcomes, especially in the metastatic setting. A study suggests that patients with primary tumors originating from the ascending colon have significantly worse overall survival compared to those with descending colon, including rectum, irrespective of the type of therapy used.93 MSI-H, found in 22% of stage II and 11% of stage III patients, is associated with better outcomes in the adjuvant setting.94
Staging and Grading of CRC
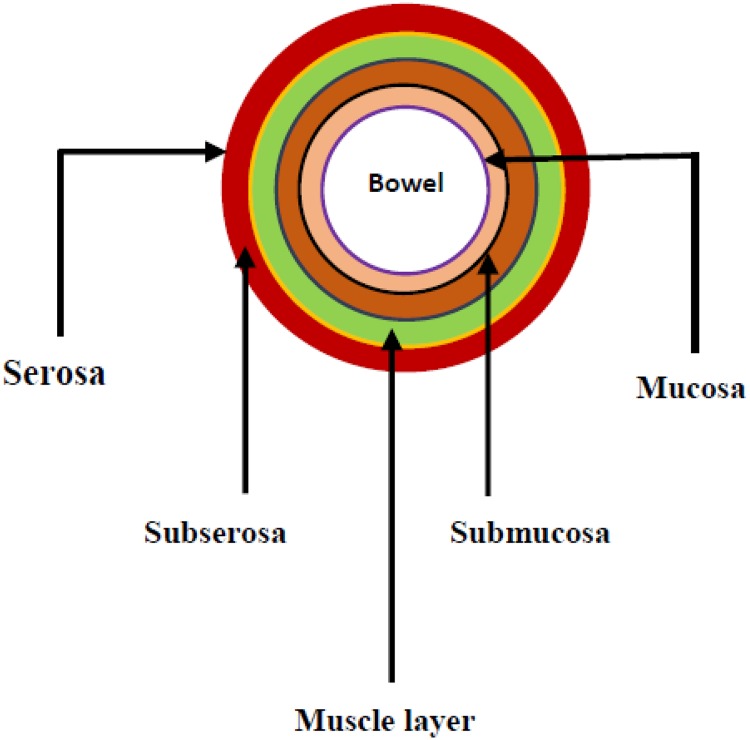
There were concerns regarding the stratification of patients with bowel cancer in order to establish an appropriate surgical treatment.95 Stage refers to the extent of cancer, that is, how large the tumor is, and the degree of metastasis. Knowing the stage of cancer helps to understand the degree and the chances of survival, plan the best treatment, and identify clinical trials that may be treatment options. The first clinical staging system is followed by Dukes’ monumental work, which creates in his first articles a purely pathological classification based on the extent of the primary tumor96 and highlights the implications of the histologic grading as a prognostic factor.97 Numerous staging systems have been proposed and have been used for the classifications of various cancer subtypes, while others may be specific to a particular type of cancer (Table 1). Most staging systems include information about tumor location, cell type, tumor type, the degree of metastasis, and tumor grade (Figure 2). The most common types of staging system aside the tumor–node–metastasis (TNM) is the Dukes’ staging system. As time progresses and new information unfolded, the Dukes’ staging was constantly modified by Kirklin, Astler-Coller, the Australian, and clinicopathological (mucinous adenocarcinoma) classification.
Figure 2.
Cross section of the colon.
The original description of cancer staging by Dukes is till date in use for the evaluation of prognosis and to a limited extent is used to determine the treatment for patients with CRC.98 This classification was formally limited to 3 classes A, B, and C, and finally, letter D as a class for stratification was added to infer the presence of metastasis. Table 2 represents the TNM classification as a universal system for CRC stratification. This system corresponds to Dukes’ mode of classification and is divided into 4 different categories.99 The TNM classification of staging used to classify the magnitude of cancer is established on the tumor’s anatomical information that is the size and degree (T), the node(s) involvement (N), and finally, whether or not the tumor has affected other organs through the blood stream (metastasis; M), grouping the cases with similar prognostic. The system is maintained collaboratively by the International Union for Cancer Control (IUCC) and the American Joint Committee for Cancer (AJCC), resulting in periodical and simultaneously publication of the TNM Classification of Malignant Tumours and the AJCC Cancer Staging Manual.100 Currently, despite some critics, it is the most used clinically.
Table 2.
TNM Classification of Colorectal Cancer.
| T: Primary tumor | N: Regional lymph node | M: Distant metastasis |
| Tx: Tumor cannot be assessed | Nx: Nodes cannot be assessed | Mx: Distant metastasis cannot be assessed |
| T0: No evidence of primary tumor | N0: No node metastasis | M0: No distant metastasis |
| Tis: Carcinoma in situ | N1: Metastasis in 1-3 nodes | M1: Distant metastasis |
| T2: Tumor invades muscularis propria | N2: Metastasis in 4 or more nodes | |
| T3: Tumor invades through into subserosa | ||
| T4: Tumor directly invades other organs |
Abbreviation: TNM, tumor–node–metastasis.
This system of classification was designed in such a way to prevent confusion and alleviate ambiguity by following physiopathological considerations after several repetitive revision of the Dukes’ procedure. Obrocea et al100 reported that research studies gave an improved understanding of cancer pathogenesis and focused on the significant role of more nonanatomical biomarkers in order to create the prognosis and response to treatment for patients with CRC in such extent that a staging of disease made only on anatomical ground no longer responds to the recent advances in clinical evaluation and therapeutic decisions.
The TNM mode of cancer classification presents a great advantage over the Dukes’ staging system (based on histopathology; Table 2). It allows the assessment of TNM categories by physical examination, imaging, endoscopy, and/or surgical exploration. Previously, data accumulated from pathologic staging were utilized essentially to determine prognosis. Current, the CRC staging has assumed additional roles, in particular, the determination of optimal therapy and assessment of response to treatment. The staging system is regularly used by cancer registries compared physicians. The staging system describes CRC as in situ (presence of abnormal cell without spread), localized (cancer is restricted to a particular location with no sign of spread), regional (spread of cancer to nearby lymph nodes, tissues, or organs), distant (spread of cancer to distance body parts), and unknown (limited information to discover the stage).
On a whole, for the first stage also known as Dukes A, the tumor growth captures the wall of the muscle, that is, the submucosa or muscular wall (T1 and/or T2). The second stage involved the lesions invasion stating from the propria (muscularis) through the subserosa and pericolic tissues (Dukes B, T3). The lesions could also penetrate and target other organs through the visceral peritoneum (T4). In the third stage, the tumors have metastasized, indicating their involvement in lymph nodes (N1, 1-3 nodes involved and N2 ≥4 nodes). Lastly, stage IV (Dukes’ D) lesions metastasize to other organs such as the liver, after perforation of a tumor into the peritoneal (Figure 2).
Grading
The most significant prognostic factor in CRC is the TNM staging established in accordance with the IUCC and AJCC, and therefore, it has crucial role in therapeutic decision-making in this cancer subtype101,102; regardless of its strong prognostic estimation of this staging system, it only indicates the anatomic degree of a tumor in some cases, without any correlation with patient survival.103 Poor histological differentiation is currently considered to be a major adverse prognostic factor in CRC. Therefore, histological grading is incorporated in the histopathological report of CRC in routine practice.104 Studies show that a 2-grade system can represent prognostic markers independent from TNM and with a better reproducibility.100,105 According to this system, low-grade CRC includes well-differentiated and moderately well-differentiated adenocarcinoma and high-grade CRC weakly differentiated adenocarcinoma, mucinous adenocarcinoma, signet-ring carcinoma, and medullary and undifferentiated carcinoma, accordingly.100,106-108 Tumor regression grade of the 4-grade system recommended are grade 0 (complete response)—no living cells; grade 1 (moderate response)—reduced number of cancer cells; grade 2 (minimal response)—insignificant cancer outgrown by fibrosis; and grade 3 (poor response)—minimal or no tumor kill, extensive residual cancer (Table 3).
Table 3.
Summary of CRC Classification System Based on TNM From AJCC, Modified Dukes’ Staging, and Dukes’ Staging System.a,109
| Stage | T | N | M | Dukes | MAC |
|---|---|---|---|---|---|
| 0 | TIS | N0 | M0 | - | - |
| I | T1 | N0 | M0 | A | A |
| T2 | N0 | M0 | A | B1 | |
| IIA | T3 | N0 | M0 | B | B2 |
| IIB | T4 | N0 | M0 | B | B3 |
| IIIA | T1-2 | N1 | M0 | C | C1 |
| IIIB | T3-4 | N1 | M0 | C | C2/C3 |
| IIIC | Any T | N2 | M0 | C | CI/C2/C3 |
| IV | Any T | Any N | M1 | - | D |
Abbreviations: AJCC, American Joint Committee for Cancer; CRC, colorectal cancer; TNM, tumor–node–metastasis.
aTIS indicates carcinoma in situ intraepithelia or invasion of lamina propria; T1, tumor invasion of submucosa; T2, tumor invasion of muscularis propria; T3, tumor invasion through the muscularis propria into pericolorectal tissues; T4, penetration of tumor through the surface of the visceral peritoneum and further directly invading other organs.
Specific miRNA Expression in CRC Initiation and Progression
The most imperative predictor of outcome is the stage of disease at diagnosis. In general, surgical resection is the main potential curative modality and is utilized in individuals with stages I-III and select stage IV patients with oligometastatic disease. Most CRCs emerge from adenomatous polyps over a time of years to decades by aggregation of serial physical changes (serial somatic mutations) because of basic acquired or gained CIN.110 As indicated by the adenoma-carcinoma sequence model, the initiating mutation is in the APC gene.111 Consequent changes incorporate KRAS and BRAF, with implications for treatment and prognosis, respectively. Different occasions incorporate p53 alterations and loss of chromosome 18q.112 Familial adenomatous polyposis is portrayed by germ line transformations in APC, leading to Wnt pathway activation.113 Hereditary nonpolyposis colorectal cancer (HNPCC) and around 15% of sporadic cases are portrayed by germ line or somatic DNA repair deformities or methylation changes in the MMR genes, which may prompt genomic instability because of the disabled capacity to correct DNA replication errors.114 This prompts mutations in malignancy-related genes subsequently driving carcinogenesis. Also, the contraction and expansion of microsatellites compared to the normal tissue are also implicated in carcinogenesis.115 The hypermethylation phenotype (CIMP+) is characterized by DNA methylation of CpG islands of numerous genes such as those involved in MMR, resulting in silencing of gene expression typically causing serrated adenomas.116
MicroRNAs Implicated in Each Stage of CRC Using the TNM Staging Classification
This section attempts to discuss both the differential miRNA expressions across various cancers, across all the stages of CRC, and also those that are commonly expressed to bring about good treatment outcome and better survival for patients with CRC since their expression levels in cancers may assist therapeutic decisions and have advantage as a therapeutic target through miRNA inhibition or replacement strategies. Several studies have examined the expression patterns of miRNA through various techniques (deep sequencing, quantitative RT-PCR, and microarray) and affirmed their reliability and reproducibly altered in CRC.76,78,117-119 All these studies revealed that miRNA is differentially expressed in CRC compared to normal tissues. This is in line with the hypothesis that aberrant miRNA expression is pivotal in colorectal carcinogenesis and development.120 Studies have affirmed that specific miRNAs have imperative oncogenic capacities while others have critical tumor suppressive capacities and that these capacities should be assessed for each miRNA independently with regard to the particular tissue or cancer type.
Cheng et al80 experimentally determined that miR-141 is differentially expressed in the late stage of CRC. This can, therefore, be used within colorectal tissue as a differential diagnostic biomarker for M1. Furthermore, miR-143 and miR-145 were suggested to play a tumor suppressive function in CRC.121 Wang et al122 built a diagnostic model for CRC by experimentally validating miR-21, miR-31, miR-203, miR-92a, miR-181b, miR-145, miR-143, miR-30c, miR-17, and let-7g and then identified a profile that combined 6 miRNAs, which can serve as a novel noninvasive biomarker for CRC diagnosis. MicroR-193a-3p was also predicted as a tumor-suppressive miRNA involved in the development of CRC (early stage of colorectal carcinogenesis) and also have an effect on the sensitivity of anti-EGFR therapy.123 Expression of miR-181c has been assessed to suggest the recurrence of stage II CRC.124 Both miR-17-3p and miR-221 were found to be commonly expressed in all the stages (stages I, II, III, and IV) of CRC, with a sensitivity of 64% and 86% and a specificity of approximately 70% and 41%, respectively, in plasma.79,125 In feces, miR-17 and 21 are as well been shown to be commonly expressed in all the stages of CRC.126,127 Also, miR-91a, miR-106a, miR-135a, and miR-135b were implicated, but their stages are not reported.128 Huang et al44 surveyed the expressions of 12 miRNAs in plasma samples from patients with advanced CRC and healthy controls utilizing RT-PCR and discovered that miR-29a and miR-92a possess significant diagnostic value for advanced neoplasia and proposed that these miRNAs have solid potential as novel noninvasive biomarkers for early CRC detection. From our ongoing research, 5 novel miRNAs have been discovered using in silico approaches and have been found to be linked/implicated in CRC but await molecular validation for the stratification of this disease at each stage of TNM.
Conclusions
The enthusiasm for biomarkers relating to CRC is obviously expanding. They shape another part of clinical and laboratory research, which helps interpret these ideas to more significant applications in disease management. MicroRNA biomarkers are an emerging field that can potentially assist in guiding the diagnosis, prognosis, treatment, and management of CRC. The potential miRNA advantage for clinical translation in CRC is a focal point for better understanding of staging and specific treatment efficacy in surgery for CRC. Staging supplies information regarding the prognosis and may suggest the requirement for other therapy. Accurate assessment of CRC with specific miRNA TNM classes is vital for deciding the best stage-specific management to improve the predictive and prognosis of the disease.
Future Perspective
Research has confirmed the increasing rate of incidence and mortality of CRC subtype worldwide, and as such, it has become a public health issue globally. The future perspective of this review is aimed at the provision of the current findings in the diagnosis and management of this disease as well as latest discoveries and future viewpoint in the field of oncology as a means to assist in the insight of the cancer subtype.
Since the major causes of CRC are both environmental factors and genetic factors, their exploitation can bring about new diagnosis and treatment strategies.
For CRC treatment, a therapeutic model that distinguishes individuals into various categories with clinical decisions, practices, mediations, and additionally items being custom-made to the individual patient depending on their anticipated reaction or risk of disease such as personalized medicine is fast becoming a significant tool. Therefore, it is noteworthy to carry out comprehensive research of the tumor features of individual patients to tailor the best therapy.
Finally, the greater part of current research in this field is largely dependent on the development of a new treatment that is noninvasive, less expensive, sensitive, specific, and more effective compared to the conventional therapies. MicroRNAs have proven to be widely distributed all over the body in terms of their abundance and their expression profiles have also been exploited to be different among cancer subtypes and are tissue-specific. The development of miRNAs as biomarkers will improve diagnosis as well as detection in the early stage of this disease since this disease is largely treatable when detected earlier. Discoveries in this area and their clinical significance will improve the overall survival and disease management of patients with CRC subtype in the nearest future.
Acknowledgments
The authors thank the Plant Omics Laboratory and the Bioinformatics Research Group of the University of the Western Cape for their usual support. Also, Dr Taiwo Akinsoji (MBBS, MPH) of the University of Illinois at Springfield for co-proof reading the final manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Adewale Oluwaseun Fadaka  https://orcid.org/0000-0002-3952-2098
https://orcid.org/0000-0002-3952-2098
References
- 1. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2016. doi:10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2. Brenner H, Altenhofen L, Hoffmeister M. Sex, age, and birth cohort effects in colorectal neoplasms: a cohort analysis. Ann Intern Med. 2010;152(11):697–703. [DOI] [PubMed] [Google Scholar]
- 3. Binefa G, Rodríguez-Moranta F, Teule À, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20(22):6786–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosman FT, Carneiro F, Hruban RH. Carcinoma of the colon and rectum In: Theise ND. ed. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Press; 2010:134–146. [Google Scholar]
- 5. Fleming M, Ravula S, Tatishchev SF, Wang HL. Colorectal carcinoma: pathologic aspects. J Gastrointest Oncol. 2012;3(3):153–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farrington SM, Tenesa A, Barnetson R, et al. Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am J Hum Genet. 2005;77(1):112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinol V, Castells A, Andreu M, et al. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA 2005;293(16):1986–1994. [DOI] [PubMed] [Google Scholar]
- 8. Walker AS, Johnson EK, Maykel JA, et al. Future directions for the early detection of colorectal cancer recurrence. J Cancer. 2014;5(4):272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sawyers CL. The cancer biomarker problem. Nature. 2008;452(7187):548–552. [DOI] [PubMed] [Google Scholar]
- 10. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 2015;25(1):16–27. [DOI] [PubMed] [Google Scholar]
- 11. Biomarkers Definitions Working Group, Atkinson AJ, Jr, Colburn WA, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. [DOI] [PubMed] [Google Scholar]
- 12. Aronson JK. Biomarkers and surrogate endpoints. Br J Clin Pharmacol. 2005;59(5):491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newton K, Newman W, Hill J. Review of biomarkers in colorectal cancer. Colorectal Dis. 2012;14(1):3–17. [DOI] [PubMed] [Google Scholar]
- 14. Rawson JB, Bapat B. Epigenetic biomarkers in colorectal cancer diagnostics. Expert Rev Mol Diagn. 2012;12(5):499–509. [DOI] [PubMed] [Google Scholar]
- 15. Schepeler T, Reinert JT, Ostenfeld MS, et al. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res. 2008;68(15):6416–6424. [DOI] [PubMed] [Google Scholar]
- 16. Ardekani AM, Naeini MM. The role of microRNAs in human diseases. Avicenna J Med Biotechnol. 2010;2(4):161–179. [PMC free article] [PubMed] [Google Scholar]
- 17. Keller A, Meese E. Can circulating miRNAs live up to the promise of being minimal invasive biomarkers in clinical settings? Wiley Interdiscip Rev RNA. 2016;7(2):148–156. [DOI] [PubMed] [Google Scholar]
- 18. Gambari R, Brognara E, Spandidos DA, Fabbri E. Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: new trends in the development of miRNA therapeutic strategies in oncology. Int J Oncol. 2016;49(1):5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fehlmann T, Ludwig N, Backes C, Meese E, Keller A. Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol. 2016;13(11):1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79(4):581–588. [DOI] [PubMed] [Google Scholar]
- 21. Ludwig N, Leidinger P, Becker K, et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016;44(8):3865–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deli Rev. 2015;81:75–93. [DOI] [PubMed] [Google Scholar]
- 23. Rehbein G, Schmidt B, Fleischhacker M. Extracellular microRNAs in bronchoalveolar lavage samples from patients with lung diseases as predictors for lung cancer. Clin Chim Acta. 2015;450:78–82. [DOI] [PubMed] [Google Scholar]
- 24. Izzotti A, Carozzo S, Pulliero A, Zhabayeva D, Ravetti JL, Bersimbaev R. Extracellular microRNA in liquid biopsy: applicability in cancer diagnosis and prevention. Am J Cancer Res. 2016;6(7):1461–1493. [PMC free article] [PubMed] [Google Scholar]
- 25. Molina-Pinelo S, Suárez R, et al. Association between the miRNA signatures in plasma and bronchoalveolar fluid in respiratory pathologies. Dis Markers. 2012;32(4):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Izzotti A, Balansky R, Ganchev G, et al. Blood and lung microRNAs as biomarkers of pulmonary tumorigenesis in cigarette smoke-exposed mice. Oncotarget. 2016;7(51):84758–84774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cho WC. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int J Biochem Biol. 2010;42(8):1273–1281. [DOI] [PubMed] [Google Scholar]
- 28. Aqeilan R, Calin G, Croce C. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2010;17(2):215–220. [DOI] [PubMed] [Google Scholar]
- 29. Wang L-G, Gu J. Serum microRNA-29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer Epidemiol. 2012;36(1):e61–e67. [DOI] [PubMed] [Google Scholar]
- 30. Campos FG, Figueiredo MN, Monteiro M, Nahas SC, Cecconello I. Incidence of colorectal cancer in young patients. Rev Col Bras Cir. 2017;44(2):208–215. [DOI] [PubMed] [Google Scholar]
- 31. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. [DOI] [PubMed] [Google Scholar]
- 32. Worthley DL, Leggett BA. Colorectal cancer: molecular features and clinical opportunities. Clin Biochem Rev. 2010;31(2):31–38. [PMC free article] [PubMed] [Google Scholar]
- 33. Rustgi AK. Hereditary gastrointestinal polyposis and nonpolyposis syndromes. N Engl J Med. 1994;331(25):1694–1702. [DOI] [PubMed] [Google Scholar]
- 34. Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001;61(8):3230–3239. [PubMed] [Google Scholar]
- 35. Nojadeh JN, Behrouz Sharif S, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6(2):107–116. [DOI] [PubMed] [Google Scholar]
- 37. Toyota M, Ohe-Toyota M, Ahuja N, Issa J-PJ. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc Natl Acad Sci U S A. 2000;97(2):710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38(7):787–793. [DOI] [PubMed] [Google Scholar]
- 39. Ogino S, Kawasaki T, Ogawa A, Kirkner GJ, Loda M, Fuchs CS. TGFBR2 mutation is correlated with CpG island methylator phenotype in microsatellite instability-high colorectal cancer. Hum Pathol. 2007;38(4):614–620. [DOI] [PubMed] [Google Scholar]
- 40. Nosho K, Irahara N, Shima K, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One 2008;3(11):e3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fadaka AO, Ojo BA, Adewale OB, Esho T, Pretorius A. Effect of dietary components on miRNA and colorectal carcinogenesis. Cancer Cell Int. 2018;18:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141(5):672–675. [DOI] [PubMed] [Google Scholar]
- 43. Heneghan HM, Miller N, Kerin MJ. MiRNAs as biomarkers and therapeutic targets in cancer. Curr Opin Pharmacol. 2010;10(5):543–550. [DOI] [PubMed] [Google Scholar]
- 44. Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127(1):118–126. [DOI] [PubMed] [Google Scholar]
- 45. Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu D-C, Li Q-G, Ding X-W, Ding Y-T. Circulating microRNAs: potential biomarkers for cancer. Int J Mol Sci. 2011;12(3):2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol. 2014;11(3):145–156. [DOI] [PubMed] [Google Scholar]
- 48. Toiyama Y, Okugawa Y, Goel A. DNA methylation and microRNA biomarkers for noninvasive detection of gastric and colorectal cancer. Biochem Biophys Res Commun. 2014;455(1-2):43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chand M, Keller DS, Mirnezami R, et al. Novel biomarkers for patient stratification in colorectal cancer: a review of definitions, emerging concepts, and data. World J Gastrointest Oncol. 2018;10(7):145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Plano D, Alcolea V, Sanmartín C, Sharma AK. Methods of selecting combination therapy for colorectal cancer patients: a patent evaluation of US20160025730A1. Expert Opin Ther Pat. 2017;27(5):527–538. [DOI] [PubMed] [Google Scholar]
- 51. Mojarad EN, Kuppen PJ, Aghdaei HA, Zali MR. The CpG island methylator phenotype (CIMP) in colorectal cancer. Gastroenterol Hepatol Bed Bench. 2013;6(3):120–128. [PMC free article] [PubMed] [Google Scholar]
- 52. Kambara T, Simms L, Whitehall V, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53(8):1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88(9):4393–4397. [DOI] [PubMed] [Google Scholar]
- 54. Tan YH, Liu Y, Eu KW, et al. Detection of BRAF V600E mutation by pyrosequencing. Pathology. 2008;40(3):295–298. [DOI] [PubMed] [Google Scholar]
- 55. Li WQ, Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Iacopetta B. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer. 2006;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Elisei R, Ugolini C, Viola D, et al. BRAFV600E mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–3949. [DOI] [PubMed] [Google Scholar]
- 57. Puxeddu E, Moretti S, Elisei R, et al. BRAFV599E mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89(5):2414–2420. [DOI] [PubMed] [Google Scholar]
- 58. Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. JNCI. 2003;95(24):1878–1890. [DOI] [PubMed] [Google Scholar]
- 59. Gear H, Williams H, Kemp EG, Roberts F. BRAF mutations in conjunctival melanoma. Invest Ophthalmol Vis Sci. 2004;45(8):2484–2488. [DOI] [PubMed] [Google Scholar]
- 60. Deng G, Bell I, Crawley S, et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res. 2004;10(1 pt 1):191–195. [DOI] [PubMed] [Google Scholar]
- 61. Benlloch S, Payá A, Alenda C, et al. Detection of BRAF V600E mutation in colorectal cancer: comparison of automatic sequencing and real-time chemistry methodology. J Mol Diagn. 2006;8(5):540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Qi Li W, Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Iacopetta B. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer 2006;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62(3):367–386. [DOI] [PubMed] [Google Scholar]
- 64. Mrkonjic M, Roslin NM, Greenwood CM, et al. Specific variants in the MLH1 gene region may drive DNA methylation, loss of protein expression, and MSI-H colorectal cancer. PLoS One. 2010;5(10):e13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vilar E, Gruber SB. Microsatellite instability in colorectal cancer—the stable evidence. Nat rev Clin Oncol. 2010;7(3):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Nati Acad Sci U S A. 2008;105(30):10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. [DOI] [PubMed] [Google Scholar]
- 68. Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39(16):7223–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jothimani G, Sriramulu S, Chabria Y, Sun X-F, Banerjee A, Pathak S. A review on theragnostic applications of microRNAs and long non-coding RNAs in colorectal cancer. Curr Top Med Chem. 2018;18(10):2614–2629. [DOI] [PubMed] [Google Scholar]
- 70. Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285(23):17442–17452. doi:10.1074/jbc. M110. 107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Nati Acad Sci U S A. 2011;108(12):5003–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ichikawa D, Komatsu S, Konishi H, Otsuji E. Circulating microRNA in digestive tract cancers. Gastroenterology. 2012;142(5):1074–1078.e1. [DOI] [PubMed] [Google Scholar]
- 74. Kawaguchi T, Komatsu S, Ichikawa D, et al. Circulating microRNAs: a next-generation clinical biomarker for digestive system cancers. Int J Mol Sci. 2016;17(9):1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Slaby O, Svoboda M, Michalek J, Vyzula R. MicroRNAs in colorectal cancer: translation of molecular biology into clinical application. Mol Cancer. 2009;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. [DOI] [PubMed] [Google Scholar]
- 77. Slaby O, Svoboda M, Fabian P, et al. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72(5-6):397–402. [DOI] [PubMed] [Google Scholar]
- 78. Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299(4):425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58(10):1375–1381. [DOI] [PubMed] [Google Scholar]
- 80. Cheng H, Zhang L, Cogdell DE, et al. Circulating plasma miR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS One. 2011;6:e17745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ogata-Kawata H, Izumiya M, Kurioka D, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One. 2014;9:e92921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zanutto S, Pizzamiglio S, Ghilotti M, et al. Circulating miR-378 in plasma: a reliable, haemolysis-independent biomarker for colorectal cancer. Br J Cancer. 2014;110(4):1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ohno-Machado L. Modeling medical prognosis: survival analysis techniques. J Biomed Inform. 2001;34(6):428–439. [DOI] [PubMed] [Google Scholar]
- 84. Fu J-F, Huang Y-Q, Yang J, Yi C-H, Chen H-L, Zheng S. Clinical characteristics and prognosis of young patients with colorectal cancer in Eastern China. World J Gastroenterol. 2013;19(44):8078–8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McKay A, Donaleshen J, Helewa RM, et al. Does young age influence the prognosis of colorectal cancer: a population-based analysis. World J Surg Oncol. 2014;12:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang M-J, Ping J, Li Y, et al. The prognostic factors and multiple biomarkers in young patients with colorectal cancer. Sci Rep. 2015;5:10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg. 2015;150(5):402–409. [DOI] [PubMed] [Google Scholar]
- 88. Jiang Z, Wang X, Tan X, Fan Z. Effect of age on survival outcome in operated and non-operated patients with colon cancer: a population-based study. PLoS One. 2016;11(1):e0147383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Manjelievskaia J, Brown D, McGlynn KA, Anderson W, Shriver CD, Zhu K. Chemotherapy use and survival among young and middle-aged patients with colon cancer. JAMA Surg. 2017;152(5):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McShane LM, Altman DG, Sauerbrei W. Identification of Clinically Useful Cancer Prognostic Factors: What are We Missing? Oxford, England: Oxford University Press; 2005. [DOI] [PubMed] [Google Scholar]
- 91. Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17(10):1225–1239. theoncologist. 2012-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Labianca R, Beretta GD, Kildani B, et al. Colon cancer. Critical reviews in oncology/hematology. Crit Rev Oncol Hematol. 2010;74:106–133. [DOI] [PubMed] [Google Scholar]
- 93. Tamas K, Walenkamp A, De Vries E, et al. Rectal and colon cancer: not just a different anatomic site. Cancer Treat Rev. 2015;41(8):671–679. [DOI] [PubMed] [Google Scholar]
- 94. Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46(15):2788–2798. [DOI] [PubMed] [Google Scholar]
- 95. Lockhart-Mummery JP. Two hundred cases of cancer of the rectum treated by perineal excision. British J Surg. 1926;14(53):110–124. [Google Scholar]
- 96. Dukes CE. The classification of cancer of the rectum. J Pathol Bacteriol. 1932;35:323–332. [Google Scholar]
- 97. Dukes C. Histological Grading of Rectal Cancer. Thousand Oaks, CA: Sage; 1937. [PMC free article] [PubMed] [Google Scholar]
- 98. Thebo JS, Senagore AJ, Reinhold DS, Stapleton SR. Molecular staging of colorectal cancer. Dis Colon Rectum. 2000;43:155–159. [DOI] [PubMed] [Google Scholar]
- 99. Hutter RV, Sobin LH. A universal staging system for cancer of the colon and rectum. Let there be light. Arch Pathol Lab Med. 1986;110(5):367–368. [PubMed] [Google Scholar]
- 100. Obrocea F, Sajin M, Marinescu EC, Stoica D. Colorectal cancer and the 7th revision of the TNM staging system: review of changes and suggestions for uniform pathologic reporting. Rom J Morphol Embryol. 2011;52(2):537–544. [PubMed] [Google Scholar]
- 101. Kantola T, Klintrup K, Väyrynen J, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107(10):1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Barresi V, Reggiani LB, Ieni A, Caruso RA, Tuccari G. Histological grading in colorectal cancer: new insights and perspectives. Histol Histopathol. 2015;30(9):1059–1067. [DOI] [PubMed] [Google Scholar]
- 103. Barresi V, Bonetti LR, Branca G, Di Gregorio C, De Leon MP, Tuccari G. Colorectal carcinoma grading by quantifying poorly differentiated cell clusters is more reproducible and provides more robust prognostic information than conventional grading. Virchows Arch. 2012;461(6):621–628. [DOI] [PubMed] [Google Scholar]
- 104. Gospodarowicz MK, Brierley JD, Wittekind C. TNM Classification of Malignant Tumours. Hoboken, NJ: John Wiley & Sons, 2017. [Google Scholar]
- 105. Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. Am J Surg Pathol. 2012;36(12):1761–1770. [DOI] [PubMed] [Google Scholar]
- 106. Bujanda L, Cosme A, Gil I, Arenas-Mirave JI. Malignant colorectal polyps. World J Gastroenterol. 2010;16(25):3103–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ghazi S, Lindforss U, Lindberg G, et al. Analysis of colorectal cancer morphology in relation to sex, age, location, and family history. J Gastroenterol. 2012;47(6):619–634. [DOI] [PubMed] [Google Scholar]
- 108. Kim JW, Shin MK, Kim BC. Clinicopathologic impacts of poorly differentiated cluster-based grading system in colorectal carcinoma. J Korean Med Sci. 2015;30(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wu JS. Rectal cancer staging. Clin Colon Rectal Surg. 2007;20(3):148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5(1):19–27. [PMC free article] [PubMed] [Google Scholar]
- 111. Matano M, Date S, Shimokawa M, et al. Modeling colorectal cancer using CRISPR-Cas9–mediated engineering of human intestinal organoids. Nat Med. 2015;21(3):256–262. [DOI] [PubMed] [Google Scholar]
- 112. Ogino S, Nosho K, Irahara N, et al. Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol. 2009;27(27):4591–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8(5):387–398. [DOI] [PubMed] [Google Scholar]
- 114. Jin B, Robertson KD. DNA methyltransferases, DNA damage repair, and cancer In: Epigenetic Alterations in Oncogenesis. Berlin, Germany: Springer, 2013:3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bunz F. Genetic instability and cancer In: Principles of Cancer Genetics. 2008:125–172. [Google Scholar]
- 116. Fu T, Pappou EP, Guzzetta AA, et al. CpG island methylator phenotype positive tumors in the absence of MLH1 methylation constitute a distinct subset of duodenal adenocarcinomas and are associated with poor prognosis. Clin Cancer Res. 2012;18(17):4743–4752. clincanres. 0707.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cummins JM, He Y, Leary RJ, et al. The colorectal microRNAome. Proc Natl Acad Sci U S A. 2006;103(10):3687–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Volinia S, Calin GA, Liu C-G, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103(7):2257–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Luo X, Burwinkel B, Tao S, Brenner H. MicroRNA signatures: novel biomarker for colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2011;20(7):1272–1286. [DOI] [PubMed] [Google Scholar]
- 120. Schetter AJ, Okayama H, Harris CC. The role of microRNAs in colorectal cancer. Cancer J (Sudbury, Mass.). 2012;18(3):244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Michael MZ, O’Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1(12):882–891. [PubMed] [Google Scholar]
- 122. Wang J, Huang S-K, Zhao M, et al. Identification of a circulating microRNA signature for colorectal cancer detection. PLoS One. 2014;9(4):e87451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Takahashi H, Takahashi M, Ohnuma S, et al. MicroRNA-193a-3p is specifically down-regulated and acts as a tumor suppressor in BRAF-mutated colorectal cancer. BMC Cancer. 2017;17(1):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yamazaki N, Koga Y, Taniguchi H, et al. High expression of miR-181c as a predictive marker of recurrence in stage II colorectal cancer. Oncotarget. 2017;8(4):6970–6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pu XX, Huang GL, Guo HQ, et al. Circulating miR-221 directly amplified from plasma is a potential diagnostic and prognostic marker of colorectal cancer and is correlated with p53 expression. J Gastroenterol Hepatol. 2010;25(10):1674–1680. [DOI] [PubMed] [Google Scholar]
- 126. Koga Y, Yasunaga M, Takahashi A, et al. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res. 2010;3(11). doi:10.1158/1940-6207. CAPR-10-0036. [DOI] [PubMed] [Google Scholar]
- 127. Link A, Balaguer F, Shen Y, et al. Fecal MicroRNAs as novel biomarkers for colon cancer screening. Cancer Epidemiol Biomark. 2010;19(7). doi:10.1158/1055-9965.EPI-10-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cheng Q, Feng F, Zhu L, et al. Circulating miR-106a is a novel prognostic and lymph node metastasis indicator for cholangiocarcinoma. Sci Rep. 2015;5:16103. [DOI] [PMC free article] [PubMed] [Google Scholar]