Abstract
The success of immune checkpoint receptor blockade has brought exciting promises for the treatment of head and neck squamous cell carcinoma (HNSCC). While patients who respond to checkpoint inhibitors tend to develop a durable response, <15% of patients with HNSCC respond to immune checkpoint inhibitors, underscoring the critical need to alleviate cancer resistance to immunotherapy. Major advances have been made to elucidate the intrinsic and adaptive resistance mechanisms to immunotherapy. Central genomic events in HNSCC have been found to possess previously unknown roles in suppressing immune sensing. Such inhibitory function affects both the innate and adaptive arms of tumor-specific immunity. While checkpoint blockade effectively reinvigorates adaptive T-cell responses, additional targeting of the oncogenic inhibitors of innate immune sensing likely informs a novel and potent strategy for immune priming. This review discusses the recent advances on the identification of key HNSCC oncogenes that impair antitumor immunity and emerging immune-priming approaches that sensitize poorly immunogenic HNSCCs to checkpoint blockade. These approaches include but are not limited to cancer vaccine systems utilizing novel type I interferon agonists as immune adjuvants, radiation, DNA damage-inducing agents, and metabolic reprogramming. The goal of these multipronged approaches is to expand tumor-specific effector T-cells, break checkpoint receptor-mediated tolerance, and metabolically support sustained T-cell activation. The translation of therapeutics that reverses oncogenic inhibition of immune sensing requires thorough characterization of the HNSCC regulators of innate immune sensors, development of additional immunocompetent HNSCC mouse models, as well as engineering of more robust immune adjuvant delivery systems. Built on the success of checkpoint blockade, validation of novel immune-priming approaches holds key promises to expand the pool of responders to immunotherapy.
Keywords: head and neck cancer, immunotherapy, innate immunity, cancer vaccines, type I interferon, glycolysis
Introduction
Head and neck cancer is the sixth-leading cause of cancer-related death globally, with >500,000 new cases diagnosed each year (Bray et al. 2018). Head and neck squamous cell carcinoma (HNSCC), the major subtype of this disease, is responsible for >90% of new cases (Merhi et al. 2018). Current standard-of-care treatments are often associated with significant morbidity that limits patient quality of life (Maxwell et al. 2016; Yom et al. 2017).
Immunotherapy has emerged as a paradigm shift in cancer therapy, with unprecedented durability in patient response and substantially improved patient quality of life. Cancer immunotherapy approaches rely on the pivotal role of the immune system in recognizing and eliminating transformed malignant cells.
Fitting with the well-established observation that the incidence of squamous cell carcinomas is higher in immunocompromised patients (Herman et al. 2007; Gonzalez et al. 2019), the alleviation of cancer-potentiated immune suppression has shown potential in reducing HNSCC tumor burden and improving patient quality of life (Ferris et al. 2016; Ferris et al. 2018; Cohen et al. 2019). The success of monoclonal antibodies blocking immune checkpoint receptor (ICR) signaling has transformed the landscape of emerging cancer therapeutic pipelines. This line of treatment aims to enhance the function of CD8+ cytotoxic T lymphocytes (CTLs), which play a crucial role in recognizing and eliminating tumor cells. Two signals are needed to activate CD8+ T-cells: T-cell receptor interaction with the major histocompatibility complex (MHC)–peptide complex (signal 1) and CD28-mediated costimulation (signal 2). To prevent excessive immune activation that is often linked to autoimmunity, ICRs are employed to fine-tune the magnitude of immune activation. The characterization of 2 pivotal members of the ICR family—programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte–associated protein 4 (CTLA-4)—informed the clinical advance in ICR blockade therapy. PD-1 pathway dampens signal 1, and CTLA-4 tunes down signal 2 (Sharma and Allison 2015). Thus, blocking ICRs can reinvigorate the effector function of CTLs.
Although <15% of the patients responded to ICR blockade, the responders developed a durable response (Ferris et al. 2016; Ferris et al. 2018; Cohen et al. 2019). This important observation is similar to results from phase III immunotherapy trials for other cancer types (Hamid et al. 2013; Tumeh et al. 2014). In this review, we discuss recently identified pathways that can be further exploited to enhance immune priming for ICR blockade.
Genetic Basis for Oncogenic Suppression of Immune Sensing of HNSCC
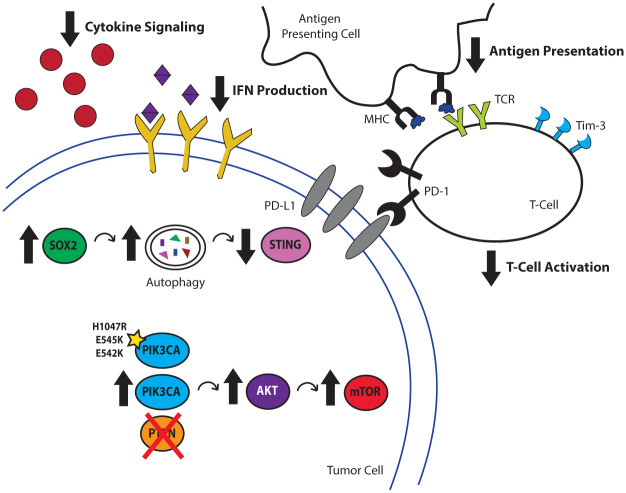
It is well established that the efficacy of ICR blockade is dictated, at least in part, by a sufficient number of infiltrating CTLs. Somatic mutations give rise to a pool of neoantigens that could be potentially perceived by CTLs. Higher mutation load is found to be associated with better response to immunotherapy (Rizvi et al. 2015). Compared with many cancer subtypes, HNSCCs have a high mutational load (Alexandrov et al. 2013). However, their response rates to ICR blockade remain modest, which raises the possibility that other mechanisms also contribute to HNSCC immunogenicity. The expansion of tumor-specific CTLs depends on proper tumor recognition and antigen processing by the innate immune system, which employs an array of sensors, also known as pattern recognition receptors, to become the first responders to abnormal cells. The collective effort in The Cancer Genome Atlas (TCGA) revealed cancer-specific molecular circuitry with unprecedented details. Emerging evidence suggests that certain genetic and genomic alterations specifically interfere with innate and adaptive immune sensing of tumors (Fig. 1).
Figure 1.
Genetic abnormalities in HNSCC contribute to poor tumor immunogenicity. Aberrant signaling resulting from genes controlling processes, ranging from self-renewal to metabolism, can lead to deficits in cancer immunogenicity. The transcription factor SOX2 has been recently implicated in negatively regulating IFN-I-mediated antitumor immune responses by promoting the autophagosome-mediated degradation of the endoplasmic reticulum–resident protein STING, leading to decreased immune infiltration. PIK3CA is commonly coamplified with SOX2 in HNSCC, leading to the activation of the mTOR pathway, which reduces CTL infiltration into the tumor microenvironment. CTL, cytotoxic T lymphocyte; HNSCC, head and neck cancer squamous cell carcinoma; IFN, interferon; MHC, major histocompatibility complex; PD-1, programmed cell death protein 1; TCR, T-cell receptor.
SOX2 Dampens Type I Interferon–Mediated Immune Sensing of HNSCC
One such recurrent genetic event, 3q amplification, which occurs in 16% of HNSCC patients from TCGA and contains transcription factor SRY-box 2 (SOX2), has emerged as a pivotal oncogenic driving event that promotes the initiation, proliferation, and malignant transformation of squamous cell carcinomas, in addition to its well-known role in maintaining stemness. SOX2 is amplified in a range of squamous cell carcinomas, and its expression in HNSCC is correlated with decreased patient survival (Wuebben and Rizzino 2017), higher incidences of nodal metastasis, and a higher cancer stage at the time of initial diagnosis (Tan et al. 2018). Among HNSCCs, SOX2 has been demonstrated to promote disease pathogenesis by driving tumor initiation and self-renewal of cancer stem cell populations (Liu et al. 2013; Boumahdi et al. 2014; Lee et al. 2014; Siegle et al. 2014).
In addition to these critical functions, recent work has uncovered a surprising role for SOX2 in the potentiation of cancer immune escape in HNSCCs. In particular, SOX2 emerged from an RNA-Seq-based screen selecting for cancer cell–intrinsic genes associated with the development of resistance to immune killing. Interestingly, in an immunocompetent mouse model of HNSCC, Sox2-overexpressing tumors displayed diminished CD8+ CTL infiltration and enhanced tumor growth, suggesting that SOX2 dampens antitumor immunity in vivo. Further investigation revealed that these effects are mediated by SOX2-mediated suppression of the stimulator of interferon genes (STING)–dependent type I interferon (IFN-I) signaling pathway. STING senses cytoplasmic DNA, which is frequently present in cancer cells that are sustaining DNA damage due to unstable genome and treatments, and it triggers the production of IFN-I to promote antigen-presenting cell maturation. The centricity of the STING-IFN-I signaling axis has been corroborated in preclinical models of several cancer types, including HNSCC (Gajewski et al. 2013; Deng, Liang, Xu, et al. 2014; Leach et al. 2018; Tan et al. 2018). Effective delivery of STING agonists expands tumor-specific CTLs and synergizes with ICR blockade (Leach et al. 2018; Tan et al. 2018). Not surprising, suppression of this pathway has emerged as a common strategy used by cancer cells to potentiate immune escape, which is evidenced by frequent loss of STING expression in the tumor cells (Xia et al. 2016; Song et al. 2017). As a previously unknown mechanism, SOX2 promotes autophagy-dependent turnover of STING, suppressing IFN-I activation. In agreement, SOX2-high HNSCCs exhibit increased regulatory T cells and decreased M1-like macrophage infiltration, a phenotype that is commonly seen with a deficiency in STING signaling (Tan et al. 2018).
Activation of the PI3K Pathway Promotes Adaptive Resistance to ICR Blockade
Frequent SOX2 amplification is a defining feature of a major subset of HNSCC. Notably, SOX2 and PIK3CA genes are both located at the 3q26.3 locus and frequently coamplified in HNSCCs. Aberrant phosphatidylinositol 3-kinase (PI3K) pathway activation, particularly via mutation or amplification of the gene PIK3CA, is central for the transformation of HNSCC. Data from TCGA HNSCC cohort indicate that the majority of patients with this tumor type display genetic alterations in ≥1 PI3K pathway members and that over half of these patients with PI3K alteration harbor mutations or copy number alterations in PIK3CA (Cerami et al. 2012; Cancer Genome Atlas Network 2015). HNSCC with multiple concurrent PI3K mutations is all advanced, suggesting its critical role in tumor development (Lui et al. 2013). PIK3CA activates mTOR signaling and promotes HNSCC growth and resistance to EGFR-targeted therapy (Wang et al. 2014). In addition to its well-characterized role in promoting cancer cell proliferation, emerging evidence suggests that targeting PI3K represents a promising strategy to improve immunotherapy. Such improvement is likely achieved by the pleiotropic effects of PI3K inhibition on cancer cells and CTLs.
Innate immune priming expands CTLs, and ICR blockade helps to alleviate CTL exhaustion. However, sustained CTL activation entails rapid genome replication, active migration to come in proximity to target tumor cells, production of large amounts of cytokines de novo, and establishment of immunologic synapses. All of these processes are metabolically demanding and require bioenergetics support. Indeed, extracellular glucose is a key nutrient source to maintain CTL effector function, and deprivation of extracellular glucose leads to rapid CTL exhaustion (Delgoffe and Powell 2015; Siska and Rathmell 2015; Palucka and Coussens 2016; Topalian et al. 2016; Sugiura and Rathmell 2018). However, a hallmark of cancer is its prioritization of the aerobic glycolysis pathway over oxidative phosphorylation, a phenomenon coined as the Warburg effect (Hanahan and Weinberg 2011). PI3K promotes glucose uptake and enhances glycolysis via the mTOR-AKT pathway (Courtnay et al. 2015). Tumor cells with activating mutations or amplifications of the PIK3CA gene may directly compete with CTLs in the microenvironment for the limited glucose supply. Thus, inhibiting the PI3K pathway is a promising approach to reprogram cancer metabolism in the tumor microenvironment (TME) to favor sustained immune effector activation.
In addition to promoting the Warburg effect in tumor cells, the PI3K pathway directly enhances CTL exhaustion in HNSCCs. A potential adaptive resistance mechanism to ICR blockade is the compensatory upregulation of other ICR members. Utilizing clinical HNSCC specimens, a recent study demonstrated that the ICRs PD-1 and T-cell Ig and mucin domain 3 protein (TIM-3) are coexpressed by the most exhausted and dysfunctional CTLs. Interestingly, patients with PD-1 blockade-treated HNSCC exhibit upregulated TIM-3 expression by CTLs, and such upregulation is dependent on the activation of the PI3K/AKT pathway (Shayan et al. 2017). Thus, targeting the PI3K pathway can also directly prevent compensatory induction of additional ICR signaling to maintain CTL activation.
Other Oncogenic Pathways That Modulate Host Immune Responses to HNSCC
The amplification of the 3q26.3 locus is not the only event that engages the host-tumor interface. For example, active β-catenin signaling was initially characterized to be associated with a T cell–poor tumor microenvironment among 266 patients with metastatic cutaneous melanomas. Interestingly, SOX2 was also discovered in this patient cohort as a significant factor driving T-cell exclusion (Spranger et al. 2015), in agreement with another unbiased whole transcriptome screen that utilizes HNSCC cells (Tan et al. 2018). Activated WNT/β-catenin drives melanoma resistance to checkpoint blockade (Spranger et al. 2015). In a more recent pan-cancer-type bioinformatics analysis of TCGA database, mutations of β-catenin signaling components were also more frequently found in non–T cell inflamed specimens (Luke et al. 2019).
The human papillomavirus (HPV) is another oncogenic factor that emerges as a regulator of the immune microenvironment. The distinction of HPV+ HNSCC was initially made prominent through a retrospective analysis of the prognostic potential of HPV status. The HPV+ HNSCC subset shows a significantly lower hazard ratio for death (Ang et al. 2010). However, the impact of HPV on the tumor immune environment and response to immunotherapy is more complex, with interesting findings from randomized phase III trials. Two such trials are CheckMate 141 and KEYNOTE-040. Based on the initial report of CheckMate 141 and a 2-y follow-up of the same cohort, the response rates between HPV- and HPV+ groups were similar, with almost identical hazard ratios (Ferris et al. 2016; Bauman et al. 2017; Ferris et al. 2018). Similarly, the results from KEYNOTE-040 suggest that the hazard ratio for pembrolizumab versus standard of care was 0.77, with a 95% CI of 0.61 to 0.97 in the HPV- group. Interestingly, the hazard ratio for pembrolizumab versus standard of care was 0.97 with a 95% CI of 0.63 to 1.49 in the HPV+ (p16+) group (Cohen et al. 2019), suggesting that pembrolizumab did not significantly reduce hazard risk of death in this group. In-depth examinations of the T-cell receptor repertoire within HNSCC revealed either similar or worse T-cell clonal expansion in the HPV+ subset (Saloura et al. 2017; Kansy et al. 2018). Thus, despite the more favorable clinical response to standard of care, HPV+ tumors may present not-yet fully understood challenges that smolder treatment-induced immune activation.
Strategies to Improve Innate Immune Priming to Sensitize Cold HNSCC
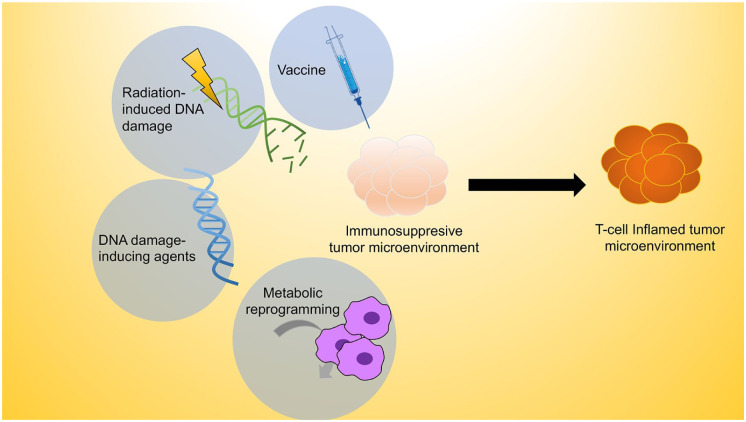
One of the key goals of immune priming is to polarize the immunologically “cold” tumor, which lacks sufficient T-cell infiltration and is resistant to checkpoint protein blockade, toward an immunologic milieu that is deemed “hot” or highly T cell inflamed (Haanen 2017; Fig. 2). The innate immune system constitutes the first line of defense against cancer. The innate immune sensors capture conserved molecular patterns that are associated with tissue damage to alert the adaptive arm of immunity. As discussed earlier, cytoplasmic DNA is a recently identified damage-associated molecular pattern that is commonly present in cancer cells. However, DNA-induced STING-mediated IFN-I activation is often suppressed in a subset of HNSCC by oncogenes. Thus, strategies to bypass oncogenic inhibition of innate immune sensors inform a major class of immune-priming therapies.
Figure 2.
Strategies to sensitize cold HNSCC to ICR blockade. A multipronged approach is needed to most effectively prime the tumor microenvironment for ICR blockade. The goal of immune priming is to release frequent oncogenic inhibitors of the innate and adaptive immune signaling, to expand the pool of tumor-specific CTLs, and to metabolically support the activation of antigen-presenting cells and effectors. Some immune-priming approaches have shown promises in HNSCC immunotherapy, including but not limited to cancer vaccines, radiotherapy, DNA damage–inducing chemotherapy, and metabolic reprograming agents. CTL, cytotoxic T lymphocyte; HNSCC, head and neck cancer squamous cell carcinoma; ICR, immune checkpoint receptor.
Utilize IFN-I Agonists as Vaccine Adjuvant
From an unbiased transcriptome-wide screen, the IFN-I signaling-centered defense response emerges as the most critical pathway regulating HNSCC cell sensitivity to immune effector-mediated cytotoxicity (Tan et al. 2018). In addition, IFN-I agonists potently create a TH1-skewed cytokine milieu to activate macrophages and dendritic cells (Dunn et al. 2006; Sistigu et al. 2014). Indeed, IFN-I-inducing agents show remarkable efficacy in various tumor models. A prototypic experimental vaccine adjuvant is CpG, which induces IFN-I in a Tlr9-dependent fashion. CpG-based vaccines can significantly expand tumor-specific CD8+ CTLs and improve the tumor response to ICR blockade (Kuai et al. 2017). However, the TLR9 expression profile in humans is drastically different from that in mice. Tlr9 is broadly expressed by the myeloid compartment in mice, which underpins the success of CpG-based formulations in polarizing the antigen-presenting cells toward a productive antitumor immune response. However, TLR9 expression is largely absent in the human myeloid compartment except for plasmacytoid dendritic cells (Hornung et al. 2002; Edwards et al. 2003). Thus, novel IFN-I-inducing adjuvants that are evolutionarily conserved in tissue distribution are explored to improve cancer immunotherapy.
One of these adjuvants is STING agonist, cGAMP. As cGAMP is a highly polar molecule, several independent groups have reported different delivery systems to improve its pharmacokinetic properties. One such model uses a peptide hydrogel-based system known as “STINGel” for delivering the STING agonist intratumorally (Leach et al. 2018). This platform achieves extended release of drug due to the semisolidified state that the drug conforms when injected into the tumor. This technology increases the survival rate of the mice bearing an ICR blockade–resistant HNSCC model (Leach et al. 2018).
Emerging nanoparticle-based approaches for immunotherapy have shown remarkable efficacy in preclinical HNSCC models. Nanoparticles are composed of versatile carriers for an array of treatments and, as a whole, can deliver high-density antigens to the tumor microenvironment (Wu and Zhou 2015). Nanoparticles can be also designed to accumulate in the lymph nodes, increasing APC uptake of antigen and improving cross-priming (Peer et al. 2007). One such example is a system coined the nanosatellite. The nanosatellite vaccine significantly enhances the efficacy of cGAMP, accumulates in the draining lymph nodes, improves APC maturation, expands tumor-specific CTL, and improves HNSCC response to ICR blockade. Interestingly, the nanoparticle-based STING agonist delivery system shows better efficacy than that of Montanide, one of the strongest clinical-grade adjuvants (Tan et al. 2018). Overall, new technologies and targeted therapies to bypass oncogenic suppression of innate immune sensors likely substantially improve HNSCC response to ICR inhibitors.
Radiation Can Prime HNSCC for ICR Blockade
Radiation therapy (RT) is a critical component of the standard of care for patients with HNSCC. Due to the more favorable response to RT among patients with HPV+ HNSCC, randomized phase III trials have assessed potential options for treatment de-escalation. Recently, 2 such studies confirmed that RT plus cisplatin remains the standard of care (Gillison et al. 2019; Mehanna et al. 2019). Conventionally, these responses to RT are found to be dependent on the production of reactive oxygen species, resulting in DNA damage and endoplasmic reticulum stress, and on the induction of apoptosis. Interestingly, recent evidence suggests that the efficacy of RT depends on an intact immune response, as RT loses its efficacy in immunocompromised hosts (Deng, Liang, Xu, et al. 2014). As a mechanism, following RT-induced DNA damage, the resulting DNA fragments in the cytoplasm likely engage the STING pathway to prime the tumors for ICR blockade therapy (Deng, Liang, Burnette, et al. 2014; Deng, Liang, Xu, et al. 2014). MHC class I genes are downstream targets of IFN-I signaling. It is known that HNSCC exhibits reduced expression of MHC class I molecules, as a mechanism of immune escape. RT can directly increase MHC class I expression (Reits et al. 2006; Oweida et al. 2017; Miyauchi et al. 2019), possibly through the STING-IFN-I axis. Improved responses to RT combined with PD-L1 blockade have been reported with an orthotopic mouse model of HNSCC (Oweida et al. 2017). The authors of these studies showed that effects were dependent on infiltration of CD8+ T cells (Verbrugge et al. 2012; Oweida et al. 2017). Targeting additional ICRs such as Tim-3 can further enhance responses (Wirsdorfer et al. 2014; Sharabi et al. 2015; Oweida et al. 2018).
Despite these promising preclinical data, the most effective combination of RT with immunotherapy remains incompletely understood. Clinically, several factors require further investigation and may necessitate patient- or subgroup-specific treatment protocols. These factors include the patient population of interest (early stage/curative vs. recurrent/metastatic), the timing of RT treatment (before or after surgery as well as neoadjuvant, concurrent, or adjuvant with immunotherapy), and the specific dosing and fractionation of radiation treatment. HPV positivity may be also an important biomarker for patient stratification. These considerations are being evaluated in a variety of ongoing clinical trials, which were recently reviewed elsewhere (Miyauchi et al. 2019).
DNA Damage-Inducing Agents Reduces Tumor-Potentiated Immunosuppression
Cytotoxic chemotherapies, in particular DNA-damaging agents such as cisplatin and 5-fluorouracil (5-FU), are commonly used in the treatment of HNSCC. Platinum-based chemotherapies bind to DNA and result in the formation of inter- and intrastrand cross-links, while 5-FU is a pyrimidine analogue that inhibits thymidylate synthase. Although through different mechanisms, both inhibit DNA replication. These agents are administered widely, given alone or with radiation, surgery, or other cytotoxic agents or targeted therapies.
Although DNA damage agents have conventionally been considered immunosuppressive, recent evidence suggests that they may also have immunostimulatory effects (Hato et al. 2014; Miyauchi et al. 2019). HMGB1 and calreticulin are released following the administration of platinum therapies and activate TLR4-mediated tumor immune responses, although these effects may be specific to oxaliplatin (Apetoh et al. 2007; Tesniere et al. 2010). Cisplatin has shown both immunosuppressive and immunostimulatory roles, depending on dosing. A recent study found that sublethal cisplatin helps to increase the expression levels of antigen-presenting machinery and immunogenic killing. Higher doses of cisplatin dampen the production of IFN-γ by T cells (Tran et al. 2017). Due to the different pharmacokinetics of cisplatin between murine models and human, additional studies would be informative to determine the optimal dosing of cisplatin when designing a combination trial. Several DNA damage agents have been shown to decrease the number and/or function of myeloid-derived suppressor cells (MDSCs; Suzuki et al. 2005; Vincent et al. 2010; Huang et al. 2015; Kim and Kim 2019); effects on MDSCs, however, may not be specific to this drug class (Ko et al. 2009; Kodumudi et al. 2010; Alizadeh and Larmonier 2014) and can in other cases occur in the opposite direction (Bruchard et al. 2013). Platinum-based therapies can also activate cytotoxic T cells via STAT6-dependent reductions in PD-L2 expression (Lesterhuis et al. 2011) and/or increased permeability to granzyme B (Ramakrishnan et al. 2010). Further supporting the potential role of DNA-damaging agents in improving responses to immunotherapy, inhibition of ATR, a critical component of the DNA damage response, can promote T-cell killing by preventing the PD-1/PD-L1 interaction (Sun et al. 2018). An inhibitor of ATR can prevent RT-induced PD-L1 upregulation and decrease Tregs in the implantable tumors (Vendetti et al. 2018). Finally, in vivo data recently published by Tran et al. (2017) demonstrated the benefit of combining low-dose cisplatin with PD-L1 inhibitors in an immunogenic model of HNSCC. Ongoing clinical trials, such as NCT02358031, will further inform rational design of combinatorial strategies to expand the pool of responders to ICR blockade.
Metabolic Reprogramming Enhances Immune Effector Function
Aberrant metabolic rewiring is a hallmark of cancer. Emerging evidence suggests that cancer-associated metabolites have a potent impact on intratumoral immune cell function. For example, high levels of lactate, generated as a by-product of increased glycolysis in cancer cells, increases the acidity of the TME. This increased acidity subsequently polarizes macrophages toward an immunosuppressive M2-like phenotype and impairs the activity of CTLs to emit cytokines such as IFN-γ (Choi et al. 2013; Lyssiotis and Kimmelman 2017). In addition, the depletion of amino acids from the TME leads to nutrient restriction, aiding the dysregulated activity of CTLs (Le Bourgeois et al. 2018). Recently, findings in melanoma indicate that pH neutralization of the TME both increases immune cell infiltration and improves the efficacy of ICR inhibitors anti-PD1 and anti-CTLA-4 (Pilon-Thomas et al. 2016).
Increased glycolysis is driven by a number of genetic alterations in HNSCC, including the PI3K-mTOR pathway. The activation of mTOR activity has been shown to promote the expression of glucose transporter proteins such as GLUT1 and GLUT2, thus boosting glycolytic flux (Robey and Hay 2009). Interestingly, in addition to the well-characterized function in inhibiting tumor proliferation, mTOR inhibitors have been found to exhibit a previously unknown effect on immune activation. Inhibition of the mTOR pathway may reduce MDSCs and increase the ratio of M1-/M2-like macrophages in HNSCC models (Cash et al. 2015). A combination of mTOR inhibitor with ICR blockade results in improved survival in an immunogenic HNSCC model (Moore et al. 2016).
Challenges and Future Directions
We have discussed a number of promising immune-priming approaches to sensitize cold tumors to ICR blockade. Due to the profound impact of inhibition of innate immune sensors on the antitumor immune response, there are likely multiple oncogenic inhibitors of the IFN-I pathway in addition to the published literature. Additional identification of these critical checkpoints for host innate immune response not only helps identify cold cancers but also sheds light on the design of novel adjuvants to maximally prime tumors for immune response.
While therapeutic vaccines are a highly promising approach to synergize with ICR blockade, many previous cancer vaccine trials have failed to yield promising results. There are several important considerations in the design of the next-generation cancer vaccines. 1) The choice of vaccine antigens is critical. Tumor-associated antigens are also expressed by normal tissue, albeit at lower levels. These antigens may be less immunogenic than tumor-specific antigens, such as HPV oncoproteins and somatic mutation–elicited neoantigens, due to the naturally developed central tolerance. 2) Novel and robust vaccine adjuvants are crucial to improve efficacy. The extended characterization of the oncogenic inhibitors of innate immune sensors may reveal previously unknown classes of adjuvants to prime the immune system. 3) The development of next-generation delivery may overcome many common challenges with an emulsion-based vaccine, such as rapid components degradation and inefficient uptake by the antigen-presenting cells. Thus, developing additional nanoparticle and controlled release systems will likely bring transformative changes to immune-priming strategies.
Novel rational combinations need to be tested in a spectrum of immunocompetent animal models. HNSCC is a molecularly heterogenous disease; thus, no single model can capture the full spectrum of key genetic alterations. New murine HNSCC cell lines that capture distinct genomic features of human disease would be highly desirable to develop the most robust immunotherapeutic. In full appreciation of the critical importance of implantable models, these cell lines are already transformed and able to surpass host-intrinsic immunosurveillance. Thus, they cannot recapitulate the transformation process, as premalignant cells adopt key genomic events to suppress innate and adaptive immune response and establish an immune-privileged niche. New genetically defined transgenic models can further complement the implantable models to rigorously test novel combinatorial immunotherapies.
Overall, exciting breakthroughs have been made by elucidating the mutation landscape in HNSCC and characterizing the efficacy of ICR blockade among this group of patients (Cancer Genome Atlas Network 2015). Built on the advances in HNSCC ICR blockade immunotherapy, novel priming strategies are central to further improving patient outcomes and quality of life.
Author Contributions
B.R. Heath, D. Sun, contributed to design, drafted the manuscript; N.L. Michmerhuizen, contributed to conception, drafted and critically revised the manuscript; C.R. Donnelly, K. Sansanaphongpricha, contributed to design, drafted and critically revised the manuscript; J.C. Brenner, contributed to design, critically revised the manuscript; Y.L. Lei, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work is supported by National Institutes of Health grants R01 DE026728, R00 DE024173, R03 DE027399, U01 DE025184, and F31 DE028740 and a Rogel Cancer Center Research Committee grant.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, et al. 2013. Signatures of mutational processes in human cancer. Nature. 500(7463):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh D, Larmonier N. 2014. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 74(10):2663–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, et al. 2010. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. 2007. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 13(9):1050–1059. [DOI] [PubMed] [Google Scholar]
- Bauman JE, Cohen E, Ferris RL, Adelstein DJ, Brizel DM, Ridge JA, O’Sullivan B, Burtness BA, Butterfield LH, Carson WE, et al. 2017. Immunotherapy of head and neck cancer: emerging clinical trials from a National Cancer Institute Head and Neck Cancer Steering Committee Planning Meeting. Cancer. 123(7):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, et al. 2014. Sox2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 511(7508):246–250. [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424. [DOI] [PubMed] [Google Scholar]
- Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. 2013. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 19(1):57–64. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. 2015. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 517(7536):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash H, Shah S, Moore E, Caruso A, Uppaluri R, Van Waes C, Allen C. 2015. mTOR and MEK1/2 inhibition differentially modulate tumor growth and the immune microenvironment in syngeneic models of oral cavity cancer. Oncotarget. 6(34):36400–36417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. 2012. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Collins CC, Gout PW, Wang Y. 2013. Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? J Pathol. 230(4):350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, et al. 2019. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 393(10167):156–167. [DOI] [PubMed] [Google Scholar]
- Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC. 2015. Cancer metabolism and the warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep. 42(4):841–851. [DOI] [PubMed] [Google Scholar]
- Delgoffe GM, Powell JD. 2015. Feeding an army: the metabolism of T cells in activation, anergy, and exhaustion. Mol Immunol. 68(2 Pt C):492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. 2014. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 124(2):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al. 2014. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 41(5):843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Koebel CM, Schreiber RD. 2006. Interferons, immunity and cancer immunoediting. Nat Rev. 6(11):836–848. [DOI] [PubMed] [Google Scholar]
- Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e, Sousa C. 2003. Toll-like receptor expression in murine dc subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 33(4):827–833. [DOI] [PubMed] [Google Scholar]
- Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. 2016. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE, Even C, et al. 2018. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 81:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. 2013. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 25(2):268–276. [DOI] [PubMed] [Google Scholar]
- Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, Sturgis EM, Burtness B, Ridge JA, Ringash J, et al. 2019. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 393(10166):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JL, Reddy ND, Cunningham K, Silverman R, Madan E, Nguyen BM. 2019. Multiple cutaneous squamous cell carcinoma in immunosuppressed vs immunocompetent patients. JAMA Dermatol. 155(5):625–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanen J. 2017. Converting cold into hot tumors by combining immunotherapies. Cell. 170(6):1055–1056. [DOI] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. 2013. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 369(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell. 144(5):646–674. [DOI] [PubMed] [Google Scholar]
- Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. 2014. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. 20(11):2831–2837. [DOI] [PubMed] [Google Scholar]
- Herman S, Rogers HD, Ratner D. 2007. Immunosuppression and squamous cell carcinoma: a focus on solid organ transplant recipients. Skinmed. 6(5):234–238. [DOI] [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 168(9):4531–4537. [DOI] [PubMed] [Google Scholar]
- Huang X, Guan D, Shu YQ, Liu LK, Ni F. 2015. Effect of cisplatin on the frequency and immuno-inhibitory function of myeloid-derived suppressor cells in A375 melanoma model. Asian Pac J Cancer Prev. 16(10):4329–4333. [DOI] [PubMed] [Google Scholar]
- Kansy BA, Shayan G, Jie HB, Gibson SP, Lei YL, Brandau S, Lang S, Schmitt NC, Ding F, Lin Y, et al. 2018. T cell receptor richness in peripheral blood increases after cetuximab therapy and correlates with therapeutic response. Oncoimmunology. 7(11):e1494112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NR, Kim YJ. 2019. Oxaliplatin regulates myeloid-derived suppressor cell-mediated immunosuppression via downregulation of nuclear factor-κβ signaling. Cancer Med. 8(1):276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, et al. 2009. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 15(6):2148–2157. [DOI] [PubMed] [Google Scholar]
- Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. 2010. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 16(18):4583–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. 2017. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 16(4):489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourgeois T, Strauss L, Aksoylar HI, Daneshmandi S, Seth P, Patsoukis N, Boussiotis VA. 2018. Targeting T cell metabolism for improvement of cancer immunotherapy. Front Oncol. 8:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach DG, Dharmaraj N, Piotrowski SL, Lopez-Silva TL, Lei YL, Sikora AG, Young S, Hartgerink JD. 2018. STINGel: controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials. 163:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Oh SY, Do SI, Lee HJ, Kang HJ, Rho YS, Bae WJ, Lim YC. 2014. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br J Cancer. 111(11):2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, Schreibelt G, de Boer A, Van Herpen CM, Kaanders JH, et al. 2011. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 121(8):3100–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Jiang M, Lu Y, Chen H, Sun J, Wu S, Ku WY, Nakagawa H, Kita Y, Natsugoe S, et al. 2013. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell. 12(3):304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui VW, Hedberg ML, Li H, Vangara BS, Pendleton K, Zeng Y, Lu Y, Zhang Q, Du Y, Gilbert BR, et al. 2013. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 3(7):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. 2019. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res. 25(10):3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssiotis CA, Kimmelman AC. 2017. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27(11):863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JH, Grandis JR, Ferris RL. 2016. HPV-associated head and neck cancer: unique features of epidemiology and clinical management. Annu Rev Med. 67:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, Dalby M, Mistry P, Sen M, O’Toole L, et al. 2019. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (de-escalate HPV): an open-label randomised controlled phase 3 trial. Lancet. 393(10166):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhi M, Raza A, Inchakalody VP, Nashwan AJJ, Allahverdi N, Krishnankutty R, Uddin S, Zar Gul AR, Al Homsi MU, Dermime S. 2018. Squamous cell carcinomas of the head and neck cancer response to programmed cell death protein-1 targeting and differential expression of immunological markers: a case report. Front Immunol. 9:1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi S, Kim SS, Pang J, Gold KA, Gutkind JS, Califano J, Mell LK, Cohen EEW, Sharabi AB. 2019. Immune modulation of head and neck squamous cell carcinoma and the tumor microenvironment by conventional therapeutics. Clin Cancer Res. 25(14):4211–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EC, Cash HA, Caruso AM, Uppaluri R, Hodge JW, Van Waes C, Allen CT. 2016. Enhanced tumor control with combination mTOR and PD-L1 inhibition in syngeneic oral cavity cancers. Cancer Immunol Res. 4(7):611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oweida A, Hararah MK, Phan A, Binder D, Bhatia S, Lennon S, Bukkapatnam S, Van Court B, Uyanga N, Darragh L, et al. 2018. Resistance to radiotherapy and PD-L1 blockade is mediated by TIM-3 upregulation and regulatory T-cell infiltration. Clin Cancer Res. 24(21):5368–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oweida A, Lennon S, Calame D, Korpela S, Bhatia S, Sharma J, Graham C, Binder D, Serkova N, Raben D, et al. 2017. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology. 6(10):e1356153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka AK, Coussens LM. 2016. The basis of oncoimmunology. Cell. 164(6):1233–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. 2007. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2(12):751–760. [DOI] [PubMed] [Google Scholar]
- Pilon-Thomas S, Kodumudi KN, El-Kenawi AE, Russell S, Weber AM, Luddy K, Damaghi M, Wojtkowiak JW, Mule JJ, Ibrahim-Hashim A, et al. 2016. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 76(6):1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, Altiok S, Celis E, Gabrilovich DI. 2010. Chemotherapy enhances tumor cell susceptibility to ctl-mediated killing during cancer immunotherapy in mice. J Clin Invest. 120(4):1111–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al. 2006. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 203(5):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. 2015. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RB, Hay N. 2009. Is Akt the “Warburg kinase”? Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 19(1):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloura V, Fatima A, Zewde M, Kiyotani K, Brisson R, Park JH, Ikeda Y, Vougiouklakis T, Bao R, Khattri A, et al. 2017. Characterization of the T-cell receptor repertoire and immune microenvironment in patients with locoregionally advanced squamous cell carcinoma of the head and neck. Clin Cancer Res. 23(16):4897–4907. [DOI] [PubMed] [Google Scholar]
- Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, Deweese TL, Drake CG. 2015. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 3(4):345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Allison JP. 2015. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 161(2):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. 2017. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 6(1):e1261779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, Rendl M, Tsirigos A, Carucci JA, Schober M. 2014. Sox2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun. 5:4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siska PJ, Rathmell JC. 2015. T cell metabolic fitness in antitumor immunity. Trends Immunol. 36(4):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, et al. 2014. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 20(11):1301–1309. [DOI] [PubMed] [Google Scholar]
- Song S, Peng P, Tang Z, Zhao J, Wu W, Li H, Shao M, Li L, Yang C, Duan F, et al. 2017. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci Rep. 7:39858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Bao R, Gajewski TF. 2015. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 523(7559):231–235. [DOI] [PubMed] [Google Scholar]
- Sugiura A, Rathmell JC. 2018. Metabolic barriers to T cell function in tumors. J Immunol. 200(2):400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LL, Yang RY, Li CW, Chen MK, Shao B, Hsu JM, Chan LC, Yang Y, Hsu JL, Lai YJ, et al. 2018. Inhibition of ATR downregulates PD-L1 and sensitizes tumor cells to T cell-mediated killing. Am J Cancer Res. 8(7):1307–1316. [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. 2005. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 11(18):6713–6721. [DOI] [PubMed] [Google Scholar]
- Tan YS, Sansanaphongpricha K, Xie Y, Donnelly CR, Luo X, Heath BR, Zhao X, Bellile E, Hu H, Chen H, et al. 2018. Mitigating SOX2-potentiated immune escape of head and neck squamous cell carcinoma with a STING-inducing nanosatellite vaccine. Clin Cancer Res. 24(17):4242–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. 2010. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 29(4):482–491. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Taube JM, Anders RA, Pardoll DM. 2016. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 16(5):275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Allen CT, Xiao R, Moore E, Davis R, Park SJ, Spielbauer K, Van Waes C, Schmitt NC. 2017. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res. 5(12):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 515(7528):568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendetti FP, Karukonda P, Clump DA, Teo T, Lalonde R, Nugent K, Ballew M, Kiesel BF, Beumer JH, Sarkar SN, et al. 2018. ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation. J Clin Invest. 128(9):3926–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, Duret H, Yagita H, Johnstone RW, Smyth MJ, et al. 2012. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 72(13):3163–3174. [DOI] [PubMed] [Google Scholar]
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 2010. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell–dependent antitumor immunity. Cancer Res. 70(8):3052–3061. [DOI] [PubMed] [Google Scholar]
- Wang Z, Martin D, Molinolo AA, Patel V, Iglesias-Bartolome R, Degese MS, Vitale-Cross L, Chen Q, Gutkind JS. 2014. mTOR co-targeting in cetuximab resistance in head and neck cancers harboring PIK3CA and RAS mutations. J Natl Cancer Inst. 106(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsdorfer F, Cappuccini F, Niazman M, de Leve S, Westendorf AM, Ludemann L, Stuschke M, Jendrossek V. 2014. Thorax irradiation triggers a local and systemic accumulation of immunosuppressive CD4+ FoxP3+ regulatory T cells. Radiat Oncol. 9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TT, Zhou SH. 2015. Nanoparticle-based targeted therapeutics in head-and-neck cancer. Int J Med Sci. 12(2):187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuebben EL, Rizzino A. 2017. The dark side of SOX2: cancer—a comprehensive overview. Oncotarget. 8(27):44917–44943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Konno H, Ahn J, Barber GN. 2016. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 14(2):282–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yom SS, Mallen-St Clair J, Ha PK. 2017. Controversies in postoperative irradiation of oropharyngeal cancer after transoral surgery. Surg Oncol Clin N Am. 26(3):357–370. [DOI] [PubMed] [Google Scholar]