Abstract
Although the role of the Hippo signaling pathway in development and tumorigenesis has been extensively studied in multiple organs, its role in ovarian follicle development remains largely unknown. Here, we report that Yes-Associated Protein 1 (YAP1), the major effector of Hippo signaling, is spatiotemporally expressed in ovarian granulosa cells and plays a critical role in the regulation of follicle development. We found that the active form of YAP1 (nuclear YAP1) was predominantly expressed in proliferative granulosa cells, whereas the inactive form of YAP1 (cytoplasmic YAP1) was mainly detected in luteal cells (terminally differentiated granulosa cells). Pharmacological inhibition of YAP1 activity disrupted mouse ovarian follicle development in vitro and in vivo. Foxl2 promoter–driven knockout of Yap1 in ovarian granulosa cells resulted in increased apoptosis of granulosa cells, decreased number of corpora lutea, reduced ovarian size, and subfertility in transgenic mice. However, Cyp19a1 promoter–driven knockout of Yap1 in differentiated granulosa cells of preovulatory follicles and luteal cells of corpora lutea had no effect on ovarian morphology and fertility. Mechanistic studies demonstrated that YAP1 interacted with epidermal growth factor receptor and TGF-β signaling pathways to regulate granulosa cell proliferation, differentiation, and survival. Results from this study identify YAP1 as a critical regulator of granulosa cell proliferation and differentiation. Balanced expression and activation of YAP1 is essential for follicle development and successful reproduction. YAP1 is a promising target for treatment of subfertility associated with abnormal granulosa cell function.—Lv, X., He, C., Huang, C., Wang, H., Hua, G., Wang, Z., Zhou, J., Chen, X., Ma, B., Timm, B. K., Maclin, V., Dong, J., Rueda, B. R., Davis, J. S., Wang, C. Timely expression and activation of YAP1 in granulosa cells is essential for ovarian follicle development.
Keywords: Hippo pathway, Yes-Associated Protein 1, Steroidogenesis, fertility
The follicle is the basic functional unit of the ovary. Granulosa cells, somatic components of the follicle, make physical contact with oocytes to support their growth, maturation, and, ultimately, ovulation. Granulosa cells respond to stimuli from the pituitary and neighboring cells by promoting follicle growth and the secretion of sex hormones and growth factors that are vital for reproduction (1). In the growing follicle, granulosa cells proliferate rapidly to support follicle growth and oocyte maturation. Response to gonadotropin stimulation and acquisition of steroidogenic ability are hallmarks of granulosa cell differentiation. In preovulatory follicles, well-differentiated granulosa cells secrete large amounts of sex hormones and growth factors to ensure successful ovulation. Following ovulation, granulosa cells exit the cell cycle and become terminally differentiated steroidogenic cells in the corpus luteum, which produce progesterone and other hormones that are vital for embryo development and implantation during early stages of pregnancy (2). Although rapid progress has been made in the regulation of ovarian follicle development, the exact mechanisms underlying the regulation of granulosa cell proliferation and differentiation are not fully understood.
Studies in Drosophila melanogaster suggest that the Hippo pathway may play a role in ovarian development of the fruit fly (3–6). The Hippo signaling pathway was initially discovered as a critical regulator of organ size in Drosophila melanogaster (7). Later studies demonstrated that this pathway is a conserved organ size regulator across species (7). The core components of the Hippo pathway in mammals consist of the serine/threonine kinases mammalian Sterile 20 (STE20)-like protein kinase 1/2 and large tumor suppressor kinase (LATS) 1/2, the scaffold proteins Salvador family WW domain containing protein 1 and Mps one binder (MOB) kinase activator 1A, and the downstream effectors yes-associated protein (YAP) 1 and WW domain containing transcription regulator 1 (WWTR1) [commonly known as transcriptional coactivator with PDZ-binding motif (TAZ)], which are transcriptional coactivators. Activation of the upstream Hippo pathway kinases results in subsequent phosphorylation of YAP1 and TAZ proteins, leading to their translocation from the nucleus to the cytoplasm and inhibition of their activity (8). Numerous studies have indicated that YAP1 is a proto-oncogene that plays important roles in organ development and tumorigenesis by regulating cell proliferation (7, 9–12). For example, hyperactivation of YAP1 in the liver elicits widespread hepatocellular carcinoma in a tetracycline (Tet)-on–inducible transgenic mouse model (9). YAP activity is also involved in cell differentiation of progenitor cells in the liver, lung, intestine, and skin (11, 13–23). Activation of the Hippo pathway is required for the maintenance of the hepatocyte in a state of differentiation (19).
Several studies, including ours, demonstrated that the Hippo-YAP pathway may also be involved in the regulation of mammalian ovarian function. Recent studies from the Hsueh laboratory (24, 25) demonstrated that temporary activation of YAP1 by physical fragmentation of ovaries or use of chemical treatments could promote ovarian follicle growth. In a whole-body Lats1-knockout mouse model, LATS1 was found to play a role in maintaining the primordial follicle pool (26). However, Yu et al. (27) showed that knockout of Yap1 in the oocytes did not affect follicle development or oocyte maturation. Our previous study demonstrated that YAP1 is highly expressed in transformed granulosa cells and is able to promote granulosa cell tumor progression via regulating cell proliferation and migration (28). Despite this progress, much is unknown about the role of YAP1 in follicle development and granulosa cell proliferation and differentiation.
In the present study, we used in vitro and in vivo experimental models to evaluate the functional role of the Hippo-YAP pathway in human and mouse ovarian cells. We found that YAP1, the major effector of the Hippo pathway, is spatiotemporally expressed in the granulosa cells and plays a critical role in granulosa cell proliferation, differentiation, and survival. Knockout of Yap1 in granulosa cells disrupted ovarian follicle development, leading to severe subfertility. Further mechanistic studies demonstrated that YAP1 interacts with epidermal growth factor (EGF) receptor (EGFR), gonadotropin, and TGF-β signaling pathways to regulate granulosa cell proliferation and differentiation.
MATERIALS AND METHODS
Chemicals, antibodies, human ovarian cells, and tissues
YAP1 small interfering RNA (siRNA) was from Horizon Discovery (Cambridge, United Kingdom) and Thermo Fisher Scientific (Waltham, MA, USA). The RNeasy Mini Kit was from Qiagen (Hilden, Germany). PCR reagents were from Thermo Fisher Scientific, Qiagen, or Bio-Rad (Hercules, CA, USA). The SuperSignal West Femto Chemiluminescent Substrate Kit was from Thermo Fisher Scientific, and the Optitran nitrocellular transfer membrane was from Schleicher & Schuell BioScience (Dassel, Germany). Antibodies against YAP1, myc (avian myelocytomatosis viral oncogene homolog) proto-oncogene protein (MYC), cleaved caspase-3, mothers against decapentaplegic homolog (Smad)2, Smad3, phosphorylated (phospho)–YAP1 (S127), phospho-LATS1 (S909), phospho-ERK1/2 (T202/Y204), phospho–protein kinase B (AKT) (Ser473), phospho–cAMP responsive element binding protein (S133), phospho-Smad2 (S465/467), and phospho-Smad3 (S423/425) were from Cell Signaling Technology (Danvers, MA, USA). The antibody against β-actin was from MilliporeSigma (Burlington, MA, USA). The antibody against Ki67 (marker of proliferation Ki-67) was from Abcam (Cambridge, United Kingdom). The antibody against hydroxy-Δ5-steroid dehydrogenase, 3β (3β-HSD) was from Dr. Ian Mason (University of Texas Southwestern Medical Center, Dallas, TX, USA). Alexa-conjugated secondary antibodies were from Thermo Fisher Scientific. Peroxidase-conjugated secondary antibodies for Western blotting were from Jackson Immunoresearch Laboratories (West Grove, PA, USA).
HGrC1 [an immortalized human granulosa cell (hGC) line] and KGN (an ovarian granulosa cell–like tumor cell line) cells were obtained from the Riken Cell Bank (Riken BioResource Research Center, Tsukuba, Japan). Cell lines were validated using short tandem repeat polymorphism analysis performed by Genetica DNA Laboratories (Burlington, NC, USA). DMEM and other cell culture medium were purchased from MilliporeSigma. Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Flowery Branch, GA, USA). Normal human ovarian tissues were from Tianjin Medical University (Tianjin, China). The use of human samples was approved by the Institutional Review Board of the University of Nebraska Medical Center.
Creation of Yap1-knockout mouse models
The mouse strain with floxed Yap1 (Yap1flox/flox) was a kind gift from Dr. Eric N. Olson (University of Texas Southwestern Medical Center, Dallas, TX, USA). Mouse strains with a forkhead box L2 (Foxl2) promoter–driven Cre recombinase (CRE) (Foxl2-CRE-ERT2) and a cytochrome P450 (Cyp) 19–driven CRE (Cyp19-CRE) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Foxl2-CRE mice and Cyp19-CRE mice were crossed with Yap1flox/flox mice to generate Foxl2-CRE;Yap1flox/flox and Cyp19-CRE;Yap1flox/flox mice, respectively. Because Foxl2-CRE mice possess a tamoxifen-inducible CRE-mediated recombination system, tamoxifen (20 mg/ml in corn oil) was injected intraperitoneally (75 mg/kg body weight) for 5 d (d 25, 27, 29, 31, and 33) to induce CRE expression. The same dose of tamoxifen was given to control mice.
In vitro mouse ovary culture
Ovaries from postnatal d 12 CD-1 mice (Envigo, Huntington, United Kingdom) were harvested, washed with cold PBS, and loaded onto Costar Transwell culture inserts (Corning, Corning, NY, USA) with non–tissue culture–treated Nuclepore polycarbonate membranes (3.0-μm pore size; GE Healthcare, Waukesha, WI, USA). Tissues were cultured with Waymouth MB752/1 medium (0.5 ml/well; Millipore Sigma, St. Louis, MO) supplemented with 0.23 mM pyruvic acid, 3 mg/ml bovine serum albumin, 10% FBS, and 1% penicillin and streptomycin at 37°C, 5% CO2, and 95% air. Recombinant human follicle-stimulating hormone (FSH) (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, USA) was added to the medium (5 ng/ml) to support follicle development. Only 0.25 ml medium was replaced every other day to maintain the cell growth environment. Verteporfin (VP) or vehicle (DMSO) was added to the culture medium for treatments. After incubating for 7 d, ovarian tissues were collected, fixed with formalin, and processed for morphologic and immunohistochemical analyses.
Culture of human and mouse ovarian granulosa cells
Primary hGCs were harvested from follicular aspirates of reproductive-age women (26–34 yr old) who were undergoing oocyte retrieval for in vitro fertilization at the Heartland Center for Reproductive Medicine (Omaha, NE, USA). The use of hGCs was approved by the Institutional Review Board of the University of Nebraska Medical Center. The aspirates from a single donor were pooled and centrifuged at 500 g for 5 min under 4°C. Cells were resuspended in DMEM/F12 (Thermo Fisher Scientific) with 2% penicillin and streptomycin. This cell suspension was then carefully layered on 50% Percoll (MilliporeSigma, St. Louis, MO, USA) and centrifuged at 800 g for 15 min. hGCs were collected from the interphase of the medium and Percoll. After washing with DMEM/F12 3 times, cells were seeded into 10-cm dishes with DMEM/F12 supplemented with 1% Ultroser G serum substitute (Pall Corporation, Port Washington, NY, USA) and 1% penicillin and streptomycin. After incubation for 16 h, cells were trypsinized and plated into 6-well plates for treatment.
Primary mouse granulosa cells were harvested by punching follicles from ovaries of 12-wk-old female CD-1 mice. After washing with cold PBS, granulosa cells were resuspended, plated in 6-well cell culture plates, and incubated in Waymouth medium MB752/1 supplement with 0.23 mM pyruvic acid, 3 mg/ml bovine serum albumin, 10% FBS, and 1% penicillin and streptomycin.
Pharmacological inhibition of YAP1 activity in vivo
Pharmacological inhibition of YAP1 activity was performed by injection of VP, a selective antagonist of YAP1 activity (29). VP (50 mg/kg in 100 μl PBS with 5% DMSO) or vehicle (100 μl PBS with 5% DMSO) was injected peritoneally into 2-month-old female CD-1 mice daily for 5 d. Ovaries from each group were collected for preparation of RNA and protein for biochemical analyses, or paraffin or frozen sections for immunohistochemistry (IHC) and TUNEL analyses. The use of animals was approved by the Institutional Animal Care and Use Committees of both the University of Nebraska Medical Center and Massachusetts General Hospital.
Fluorescent and chromogenic IHC
Fluorescent IHC was used to examine the expression and localization of YAP1, Ki67, and 3β-HSD proteins in the mouse ovary. Briefly, frozen sections of 6-µm thickness were fixed in freshly prepared 4% paraformaldehyde and processed for staining YAP1, Ki67, and 3β-HSD proteins according to a protocol established in our laboratory (30). Images were captured using a Zeiss 710 Meta Confocal Laser Scanning Microscope and analyzed using Zeiss Zen 2010 software (Carl Zeiss, Oberkochen, Germany). Expression of YAP1, phospho-AKT1, and cleaved caspase-3 in human and mouse ovaries was detected using a peroxidase-based IHC assay as previously described by Wang et al. (31).
3-Dimensional cell culture
A 3-dimensional (3D) culture system was employed to determine the role of YAP1 in granulosa cell proliferation and cell-cell communication as previously described by He et al. (32). Briefly, an equal number (5 × 104) of KGN-MXIV, KGN-YAP, and KGN-YAPS127A cells were loaded onto an AlgiMatrix 3D Culture System (Thermo Fisher Scientific) according to the manufacturer’s instructions and incubated for 20 d. Spheroids were imaged and quantified under an Olympus inverted microscope equipped with a DP80 Color Camera (Olympus, Tokyo, Japan).
ELISA
Estradiol and progesterone levels in the cell culture medium were determined using ELISA. Medium estradiol levels were monitored using an E2 Enzyme Immunoassay Kit (Arbor Assays, Ann Arbor, MI, USA). Progesterone production was determined using a Progesterone(e) ELISA kit (DRG International, Springfield Township, NJ, USA).
YAP1 protein knockdown and overexpression
YAP1 protein knockdown was performed with human or mouse YAP1-specific siRNAs using protocols previously described by Wang et al. (31). At least 2 siRNAs with different sequences (indicated as siRNA#1 and siRNA#2) were used to verify knockdown specificity. Lipofactamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific) was used for siRNA transfection according to the manufacturer’s instructions.
Primary hGCs and the HGrC1 cell line were transfected with retrovirus-based YAP1 overexpression constructs to establish hGC-expressing wild-type YAP1 (hGC-YAP), HGrC1-expressing wild-type YAP1 (HGrC1-YAP), hGC-expressing constitutively active YAP1 (hGC-YAPS127A), and HGrC1-expressing constitutively active YAP1 (HGrC1-YAPS127A) cells. hGCs transfected with empty vector MXIV (hGC-MXIV) and HGrC1 transfected with empty vector MXIV (HGrC1-MXIV) cells were used as controls. YAP1 protein with a Serine-to-Alanine mutation at residue 127 (YAPS127A) prevents phosphorylation of YAP1, resulting in constitutive activation. KGN cell lines with different levels of YAP1 proteins were established in our laboratory, and the detailed transection protocols have been previously described by us (28).
Cell proliferation analysis
Cell numbers were monitored with a Thermo Fisher Scientific Countess automated cell counter as previously described by Wang et al. (31). Trypan blue (Thermo Fisher Scientific) was used to identify and quantify viable cells.
Western blot and RT-PCR analyses
Protein levels were determined by Western blot using a protocol established in our laboratory (31). Proteins were visualized using a Thermo Scientific SuperSignal West Femto Chemiluminescent Substrate Kit. The images were captured and analyzed with a UVP gel documentation system (Analytikjena, Jena, Germany). Relative mRNA levels were determined with semiquantitative PCR or real-time PCR using protocols previously established in our laboratory (32).
Statistical analysis
All experiments were repeated ≥3 times unless otherwise noted. Statistical analyses were conducted with Prism software (GraphPad Software, La Jolla, CA, USA). Data were analyzed for significance by Student’s t test or by 1-way ANOVA with Tukey’s post hoc test as indicated. A value of P < 0.05 was considered to be significant.
RESULTS
Expression and localization of YAP1 in human and mouse ovarian cells
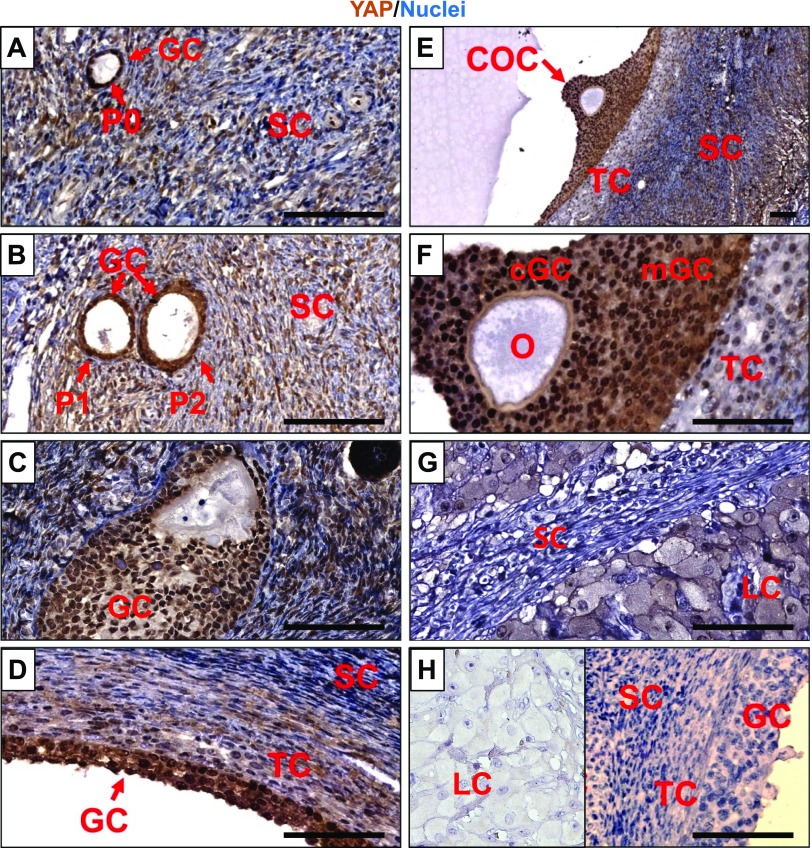
Our previous study showed that YAP1 is highly expressed in human granulosa tumor cells (28). However, the spatiotemporal expression of YAP1 in ovarian cells has not been thoroughly examined. Using chromogenic IHC, we found that YAP1 was mainly expressed in the granulosa cells and luteal cells (luteinized granulosa cells) of adult ovaries (Fig. 1). In adult human ovarian follicles, from the primary stage to preovulatory follicles, YAP1 protein was mainly localized to the nuclei of granulosa cells (Fig. 1A–F). In the corpus luteum, however, YAP1 protein was mainly localized to the cytoplasm of the luteal cells (derived from terminally differentiated granulosa and theca cells) (Fig. 1G, H). It is known that nuclear YAP1 serves as a coactivator of several transcription factors (e.g., TEA domain transcription factor 1–4) to drive expression of many proproliferation genes to promote cell proliferation (7). Translocation from the nucleus to cytoplasm will prevent YAP1 from binding to its transcription factors, leading to inactivation of YAP1 protein (9). The differential expression of YAP1 in the proliferative granulosa cells and differentiated luteal cells suggest that YAP1 activity may contribute to granulosa cell proliferation and differentiation.
Figure 1.
Expression and localization of YAP1 protein in human ovarian cells. A–G) The chromogenic IHC showing YAP1 protein expression in primordial follicles (A), primary and secondary follicles (B), growing follicles (C), preovulatory follicles (D–F), and corpus luteum (G) of human ovaries. H) Antibody control showing negative staining of YAP1 in granulosa cells, theca cells, and stromal cells in normal ovarian tissues and luteal cells in a corpus luteum (insert). cGC, cumulus granulosa cell; COC, cumulus-oocyte complex; LC, luteal cell; GC, granulosa cell; mGC, mural granulosa cell; O, oocyte; P0, primordial follicle; P1, primary follicle; P2, secondary follicle; SC, stromal cell; TC, theca cell. Scale bars, 100 μm.
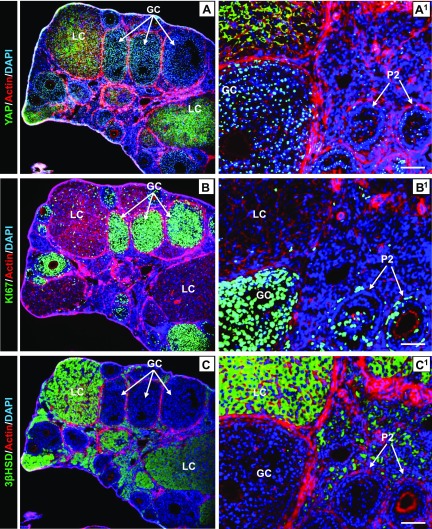
Consistent with observations in human ovaries, Yap1 protein was also detected in the granulosa cells and luteal cells of the mouse ovary by fluorescent IHC. Yap1 was predominantly localized to nuclei of proliferative granulosa cells of the growing follicle and cytoplasm of luteal cells (luteinized granulosa cells) in the corpus luteum (Fig. 2A). The proliferative granulosa cells of growing follicles coexpressed nuclear Yap1 (active form) and Ki67, a cell proliferation marker (Fig. 2A, B). Similarly, in the terminally differentiated luteal cells, cytoplasmic Yap1 (inactive form) was coexpressed with 3β-Hsd, a marker for granulosa cell differentiation (33) (Fig. 2C, C1). Consistent with IHC and immunofluorescence results, Western blot analysis showed that mouse granulosa cells had significantly higher expression of total Yap1 protein and lower expression of phospho-Yap1 compared with luteal cells in corpora lutea (Supplemental Fig. S1). The distinct expression and distribution of Yap1 in mouse ovarian cells confirm the notion that the Hippo-YAP signaling pathway may play a role in granulosa cell proliferation and differentiation.
Figure 2.
Expression and localization of Yap1 protein in mouse ovarian cells. A–C) Fluorescent IHC and confocal microscopy showing expression and localization of Yap1 (A), Ki67 (B), and 3β-Hsd (C) in ovarian cells using series sections of mouse ovarian tissues. The green color in A–C represents expression and localization of Yap1, Ki67, and 3β-HSD in mouse ovarian cells, respectively. A1–C1) Corresponding high-resolution images. Actin filaments were stained with rhodamine-phalloidin. Nuclei were stained with DAPI. GC, granulosa cell; LC, luteal cell; P2, secondary follicle. Scale bars, 100 μm.
Inactivation of Yap1 disrupts mouse ovarian follicle development
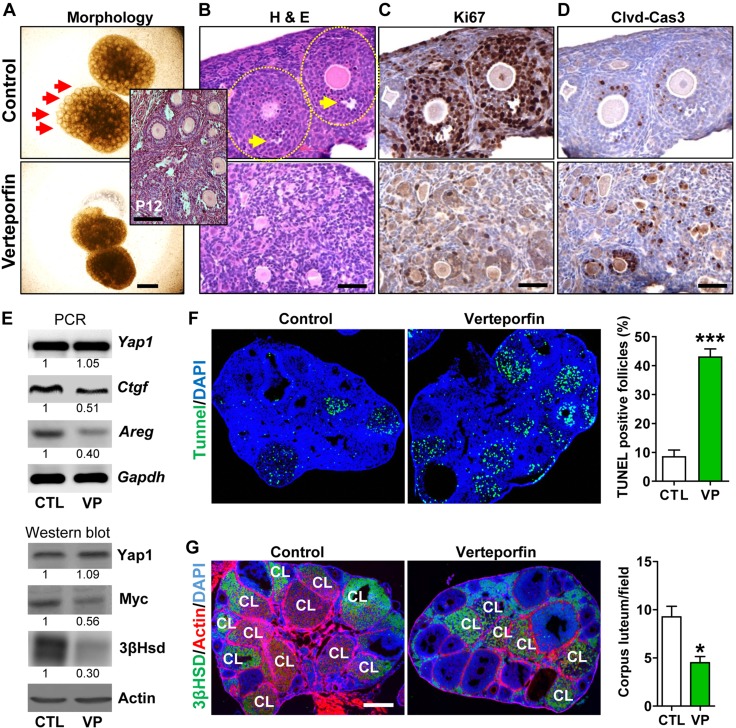
We first used an in vitro ovary culture system to examine the role of YAP1 in ovarian follicle development. Mouse ovaries were collected on postnatal day 12 and cultured in the presence or absence of verteporfin, a selective YAP1 antagonist (29). After incubating for 7 days, growing follicles were clearly evident in ovaries in the control (CTL) group, observed under a light microscope, whereas ovaries in the VP-treated group did not show obvious follicle growth (Fig. 3A). Histologic analyses showed the formation of antral follicles in the control ovaries, but no growing follicles were observed in the VP-treated group (Fig. 3B). Granulosa cells in control ovaries displayed high levels of Ki67 and low levels of cleaved caspase-3. In contrast, granulosa cells in VP-treated ovaries contained little Ki67 and high levels of cleaved caspase-3, indicating that pharmacological inactivation of Yap1 in the mouse ovary suppressed granulosa cell growth and disrupted follicle development (Fig. 3C, D).
Figure 3.
Pharmacological inhibition of Yap1 disrupts ovarian follicle development in vitro and in vivo. A) Representative images showing the morphology of postnatal d 12 mouse ovaries incubated for 7 d in the presence or absence of VP (Verteporfin, a selective YAP1 antagonist). Scale bar, 500 μm. B) Hematoxylin and eosin (H&E) stain showing histology of control (CTL) and VP-treated ovaries. Scale bar, 50 μm. The insert image shows the histology of ovaries in postnatal d 12 mice. Please note that only early stage growing follicles (<5 layers of GCs) are present in these ovaries. C, D) Representative IHC images showing expression of Ki67 and cleaved Caspase-3 (Clvd-Cas3) in serial sections of cultured CTL and VP-treated ovarian tissues. Scale bars, 50 μm. E) Representative images showing expression of Yap1, Yap1-targeting genes [connective tissue growth factor (Ctgf), Myc, and Areg], and 3β-Hsd in the CTL and VP-treated ovaries determined by RT-PCR and Western blot. F) TUNEL assay showing the apoptosis of granulosa cells and atresia of follicles in CTL and VP-treated ovarian tissues. Scale bar, 500 μm. The percentages of TUNEL-positive follicles in CTL and VP-treated groups are also presented in a graph next to images. ***P < 0.001 compared with CTL. G) Inhibition of Yap1 decreases the number of corpora lutea in the mouse ovary. 3β-Hsd staining was used to identify the corpus luteum (CL). Scale bar, 500 μm. *P < 0.05 compared with CTL.
To further evaluate the role of Yap1 in mouse follicle development in vivo, VP (50 mg/kg) or vehicle was injected daily into the peritoneal cavity of C57B/6 mice for 5 days to block YAP1 activity. RT-PCR and Western blot analyses showed that VP treatment suppressed expression of YAP1-targeting genes such as connective tissue growth factor, amphiregulin (Areg), and Myc (Fig. 3E and Supplemental Fig. S2) in ovarian tissues, suggesting that VP successfully blocked Yap1 activity. A TUNEL assay demonstrated that VP treatment significantly induced apoptosis in granulosa cells (Fig. 3F; P < 0.001 compared with controls). In addition, 3β-Hsd (corpus luteum marker) staining showed that VP treatment significantly reduced the number of corpora lutea in the ovary (Fig. 3G; P < 0.05 compared with controls). Western blot analysis confirmed that VP treatment significantly reduced 3β-Hsd protein levels in the ovaries (Fig. 3E). These results further suggest that YAP1 is critical for granulosa cell proliferation, survival, and follicle development.
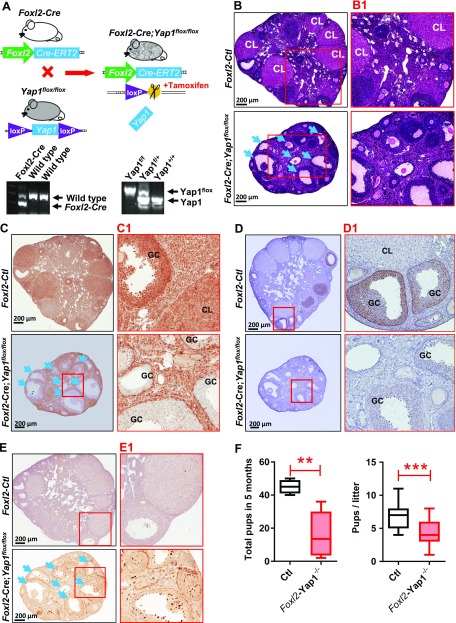
Knockout of Yap1 in granulosa cells blocks follicle development in mice
To further understand the role of YAP1 in follicle development, we crossed the Foxl2-CRE/ERT2 mouse strain (Foxl2-CRE), which has a Foxl2 promoter–driven CRE, with the Yap1flox/flox mouse strain to generate the Foxl2-CRE;Yap1flox/flox mice (Fig. 4A). Because Foxl2 is predominantly expressed in ovarian granulosa cells, activation of the Foxl2 promoter in granulosa cells will lead to CRE expression (CRE nuclear localization induced by tamoxifen administration) and Yap1 deletion in these cells. Morphologic and histologic analyses of ovaries from 6-month-old Foxl2-CRE;Yap1flox/flox mice and age-matched control mice (Foxl2-CRE or Yap1flox/flox) after tamoxifen treatment showed that granulosa cell–specific knockout of Yap1 resulted in reduced ovarian size, decreased number of corpora lutea, and increased number of atretic follicles (Fig. 4B, B1). IHC analysis confirmed that Yap1 was successfully deleted from the majority of granulosa cells of growing follicles in tamoxifen-treated Foxl2-CRE;Yap1flox/flox mice (Fig. 4C, C1). Although small follicles were morphologically normal, the majority of growing follicles were abnormal in structure, and many follicles had no viable oocytes (Fig. 4B, B1). Previous studies showed that AKT1 signaling pathway is critical for granulosa cell proliferation and survival. In Yap1-knockout granulosa cells, including granulosa cells in some morphologically normal large antral follicles, phosphorylation of Akt1 was significantly inhibited (Fig. 4D, D1). Cleaved caspase-3, a biomarker for cell apoptosis, was highly expressed in granulosa cells of abnormal follicles, indicating granulosa cells in these follicles were undergoing apoptosis (Fig. 4E, E1). After mating Foxl2-CRE;Yap1flox/flox mice with normal experienced male mice, the litter size and accumulated pup number in the tamoxifen-treated Foxl2-CRE;Yap1flox/flox group was reduced significantly (Fig. 4F; P < 0.001 compared with the age-matched tamoxifen-treated control groups). These results provide strong evidence that Yap1 plays a critical role in normal granulosa cell function and follicle development.
Figure 4.
Knockout of Yap1 in granulosa cells disrupts ovarian follicle development. A) Top panel: schematic cartoon showing the generation of Foxl2 promoter–driven granulosa cell–specific Yap1-knockout mouse model (Foxl2-CRE;Yap1flox/flox). Lower panel: Representative gel images showing the genotyping PCR products. B) Representative hematoxylin and eosin (H&E) staining images showing the ovarian morphology of control (Ctl) and Foxl2-CRE;Yap1flox/flox mice. Scale bars, 200 μm. C) Representative images showing expression of Yap1 in ovarian cells of the Ctl and Foxl2-CRE;Yap1flox/flox mice. Yap1 protein was determined by IHC staining. Scale bars, 200 μm. D) Representative images showing the phosphorylation of Akt1 determined by IHC in ovarian granulosa cells of Ctl and Foxl2-CRE;Yap1flox/flox mice. Scale bars, 200 μm. E) Representative IHC images showing expression of cleaved caspase-3 in ovarian cells of the Ctl and Foxl2-CRE;Yap1flox/flox mice. Serial sections from ovaries of the Ctl and Foxl2-CRE;Yap1flox/flox mice were used for morphologic and IHC analyses. B1–E1 are high-resolution images of B–E, respectively. Arrows indicate follicles with granulosa cell–specific Yap1 gene knockout. Scale bars, 200 μm. F) Accumulated pups and pups/litter in Ctl and Foxl2-CRE;Yap1flox/flox mice. GC, granulosa cell; CL, corpus luteum. **P < 0.01, ***P < 0.001, compared with Ctl.
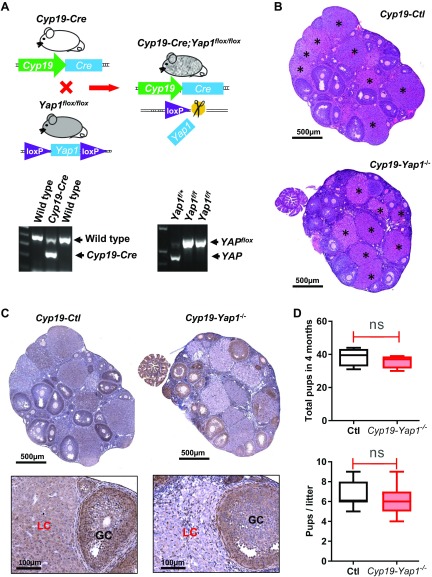
We also crossed Cyp19-CRE mice with Yap1flox/flox mice to generate Cyp19-CRE;Yap1flox/flox mice (Fig. 5A). Because Cyp19 is mainly expressed in the differentiated granulosa cells, we expected that Yap1 in the well-differentiated granulosa cells and luteal cells would be deleted in the Cyp19-CRE;Yap1flox/flox mice, which would help us examine whether cytoplasmic Yap1 protein observed in luteal cells also plays a role in luteal cell function and animal fertility. The morphologic and histologic analyses of ovaries from 4-month-old control (Cyp19-CRE or Yap1flox/flox) and Cyp19-CRE;Yap1flox/flox mice indicated that knockout of Yap1 in luteal cells had no significant effect on ovarian morphology (Fig. 5B). IHC staining shows that Yap1 was knocked out in some granulosa cells of preovulatory follicles and in the majority of luteal cells of the corpora lutea (Fig. 5C). Moreover, the litter size of the Cyp19-CRE;Yap1flox/flox mice and the control mice were comparable after mating to the experienced healthy adult male (Fig. 5D), suggesting that knockout Yap1 in the well-differentiated granulosa cells and luteal cells had no effect on animal fertility.
Figure 5.
Knockout of Yap1 in luteinized granulosa cells has limited effect on mouse ovarian follicle development and fertility. A) Top panel: schematic cartoon showing the generation of Cyp19 promoter–driven GC-specific Yap1-knockout mouse model (Cyp19-CRE;Yap1flox/flox). Lower panel: Representative gel images showing the genotyping PCR products. B) Representative images [hematoxylin and eosin (H&E) staining] showing the ovarian morphology of control (Ctl) and Cyp19-CRE;Yap1flox/flox mice. Each astrisk marks a corpus luteum. Scale bars, 500 μm. C) Top panel: representative images showing Yap1 expression in ovarian tissues of Ctl and Cyp19-CRE;Yap1flox/flox mice. Yap1 was stained using IHC. Nuclei were counterstained with hematoxylin. Scale bars, 500 μm. Lower panel: high-resolution images showing expression of Yap1 protein in ovarian tissues of the Ctl and Cyp19-CRE;Yap1flox/flox mice. Scale bars, 100 μm. D) Accumulated pups and litter size in Ctl and Cyp19-CRE;Yap1flox/flox mice. GC, granulosa cell; LC, luteal cell; ns, no significance. P>0.05.
YAP1 interacts with EGFR signaling pathway to stimulate granulosa cell proliferation
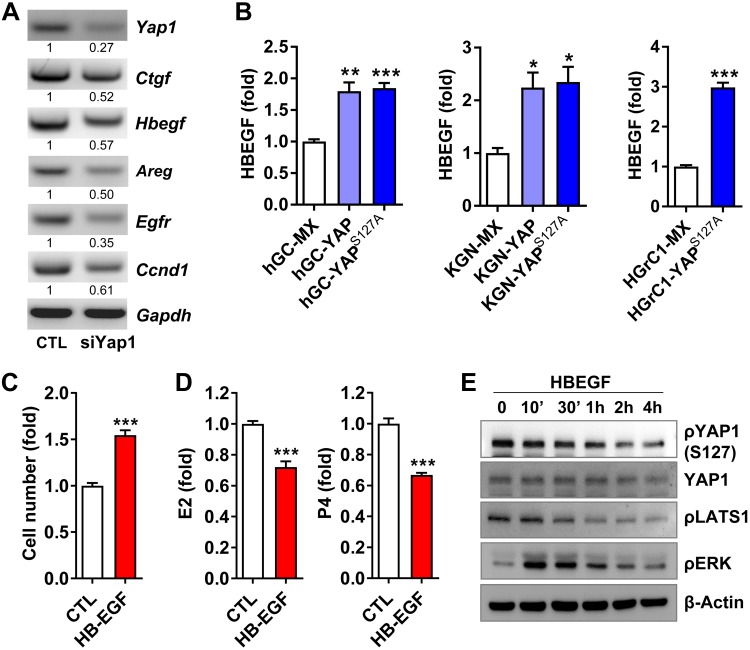
To investigate the possible molecular mechanisms underlying YAP1-regulated granulosa cell proliferation and survival, we screened expression of key molecules related to cell proliferation and differentiation using control and Yap1-knockdown mouse granulosa cells. Among the many genes and pathways that were affected by Yap1 knockdown was the EGF-EGFR signaling pathway. Knockdown of Yap1 dramatically suppressed expression of heparin-binding EGF-like growth factor (Hbegf), Areg, and Egfr in granulosa cells (Fig. 6A and Supplemental Fig. S3). Cyclin D1, an important gene for granulosa cell proliferation and a downstream target gene of the EGFR signaling pathway, was also decreased by Yap1 knockdown (Fig. 6A). To verify the biologic role of YAP1-EGFR crosstalk, we first examined the expression of HBEGF in primary cultures of hGCs. We found that, compared with HBEGF mRNA expression in hGC-MX cells (hGCs transfected with empty vectors as control), HBEGF mRNA levels were almost doubled in both hGC-YAP (hGCs transfected with vectors expressing wild-type YAP1) cells and hGC-YAPS127A (hGCs transfected with vectors expressing a constitutively active YAP1, YAPS127A) cells (Fig. 6B). Similar results were also observed in the KGN and HGrC1 cell lines (Fig. 6B). We then examined the role of HBEGF in granulosa cell proliferation and differentiation and found that HBEGF stimulated proliferation of hGCs and suppressed production of both estradiol and progesterone (Fig. 6C, D). Interestingly, we found that HBEGF activated YAP1 by suppressing an upstream suppressor, which was indicated by the significant dephosphorylation of LATS1 at serine 909 and YAP1 in serine 127 (Fig. 6E). These results indicated that YAP1 and the EGFR signaling pathway form a feedforward loop in the granulosa cells to drive cell proliferation and control cell differentiation.
Figure 6.
YAP1 interacts with EGFR signaling pathway to drive granulosa cell proliferation. A) Knockdown of Yap1 in mouse granulosa cells reduced mRNA levels of Egfr, EGF-like ligands (Hbegf and Areg), and cyclin D1 (Ccnd1). Relative mRNA levels were determined using RT-PCR. The experiment was repeated ≥3 times, and representative images are presented. B) Expression of HBEGF in hGCs, KGN cells, and HGrC1 cells transfected with empty vectors [MX as control (CTL)], vectors expressing wild-type YAP1 (YAP), or constitutively active YAP1 (YAPS127A). Relative mRNA levels were quantified using real-time PCR. Each bar represents the mean ± sem (n = 4). C, D) HBEGF treatment (50 ng/ml, 3 d) stimulated cell proliferation (C), but suppressed estradiol (E2) and progesterone (P4) production (D) in cultured granulosa cells. Each bar represents the mean ± sem (n = 4). E) HBEGF (50 ng/ml) induced rapid dephosphorylation of LATS1 (S909) and YAP1 (S127) and phosphorylation of ERK1/2 in cultured granulosa cells. β-Actin protein was used as loading CTL. The relative levels of the total proteins and phosphoproteins were determined by Western blot. Ctgf, connective tissue growth factor; Gapdh, glyceraldehyde 3-phosphate dehydrogenase; p, phosphorylated. Experiments were repeated ≥3 times, and representative images are presented. *P < 0.05, **P < 0.01, ***P < 0.001, compared with corresponding CTL groups (MX groups).
YAP1 promotes proliferation but suppresses differentiation of granulosa cells
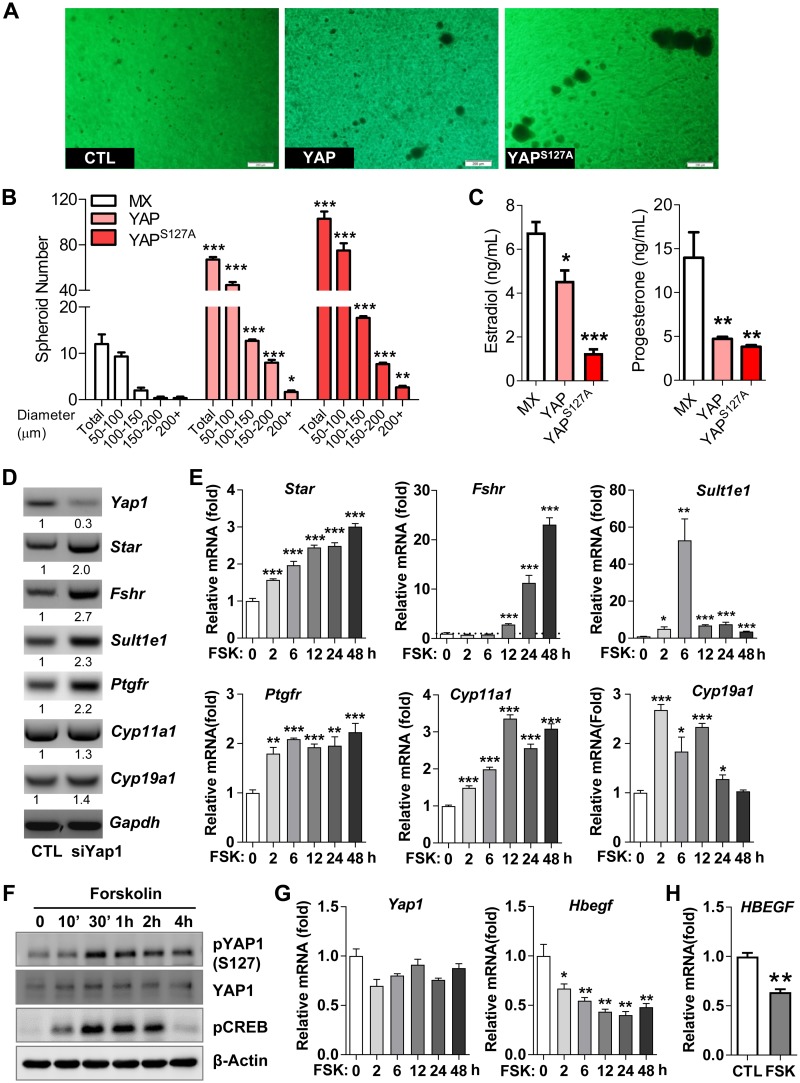
To better mimic the physiologic conditions observed in the follicle, we used a 3D culture system to study the role of YAP1 in the proliferation and differentiation of granulosa cells. Compared with KGN-MX cells, KGN-YAP and KGN-YAPS127A cells formed significantly more and larger spheroids, indicating that YAP1 promoted granulosa cell proliferation (Fig. 7A, B). However, ectopic expression of YAP or constitutively active YAP1 (YAPS127A) significantly suppressed the production of estradiol and progesterone (Fig. 7C). Because expression of steroidogenic enzymes and production of estrogen and progesterone are markers of granulosa cell differentiation, these observations suggest that hyperactivation of YAP1 suppress granulosa cell differentiation. Consistently, knockdown of Yap1 in mouse granulosa cells stimulated expression of granulosa cell differentiation genes such as FSH receptor (Fshr), steroidogenic acute regulatory protein (Star), sulfotransferase family 1E member 1 (Sult1e1), prostaglandin F receptor (Ptgfr), Cyp11a1, and Cyp19a1 (34, 35) (Fig. 7D and Supplemental Fig. S4A). Forskolin, an agonist of adenylyl cyclase, is widely used to mimic FSH and LH in activating differentiation signaling in granulosa cells (36). Forskolin treatment induced all examined granulosa cell differentiation–associated genes in both KGN and mouse granulosa cells (Fig. 7E and Supplemental Fig. S4B). Treatment of hGCs and mouse granulosa cells with forskolin induced phosphorylation of YAP1 protein at serine 127 and suppressed the expression of Hbegf/HBEGF, further suggesting that inactivation of YAP1 is critical for the activating differentiation signaling in granulosa cells (Fig. 7F–H). These results indicated that YAP1 promotes proliferation but suppresses differentiation of granulosa cells.
Figure 7.
YAP1 stimulates proliferation but suppresses differentiation of granulosa cells. A) Representative images showing spheroids formed in KGN-MX, KGN-YAP, and KGN-YAPS127A cells in a 3D culture system. Scale bars, 200 μm. B) Numbers of spheroids with different diameters formed by KGN-MX, KGN-YAP, and KGN-YAPS127A cells in the 3D culture system in A. Each bar represents the mean ± sem (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 compared with corresponding control (CTL). C) Production of E2 and progesterone in KGN-MX, KGN-YAP, and KGN-YAPS127A cells in the 3D culture system. *P < 0.05, **P < 0.01, ***P < 0.001 compared with MX control. D) Knockdown of Yap1 with Yap1-specific siRNA (siYap) in cultured mouse granulosa cells up-regulated the expression of granulosa cell differentiation–associated genes. The relative mRNA levels of Star, Fshr, Sult1e1, Ptgfr, cyp11A1, cyp19a1, and Gapdh were determined by RT-PCR. E) Forskolin (FSK) induced expression of differentiation-associated genes in cultured primary mouse granulosa cells. The relative mRNA levels of Yap1, Star, Fshr, Sult1e1, Ptgfr, cyp11a1, and cyp19a1 in mouse granulosa cells treated with 10 μM FSK for the indicated time points were determined by quantitative PCR. *P < 0.05, **P < 0.01, ***P < 0.001 compared with CTL. F) FSK (10 μM) induced phosphorylation of YAP1 in cultured HGrC1 cells. Please note that phosphorylation results in inactivation of YAP1 protein. The relative levels of the total proteins and phosphoproteins were determined by Western blot. All experiments were repeated ≥3 times, and representative images were presented. G) FSK (10 μM) suppressed the expression of Hbegf mRNA in cultured primary mouse granulosa cells in a time-dependent manner. Each bar represents the mean ± sem (n = 4). *P < 0.05, **P < 0.01 compared with CTL. H) FSK (10 μM, 72 h) suppressed HBEGF mRNA expression in cultured primary hGCs. CREB, cAMP responsive element binding protein; Gapdh, glyceraldehyde 3-phosphate dehydrogenase; p, phosphorylated. Each bar represents the mean ± sem (n = 4). **P < 0.01 compared with CTL.
Inactivation of YAP1 compromises TGF-β signaling in granulosa cells
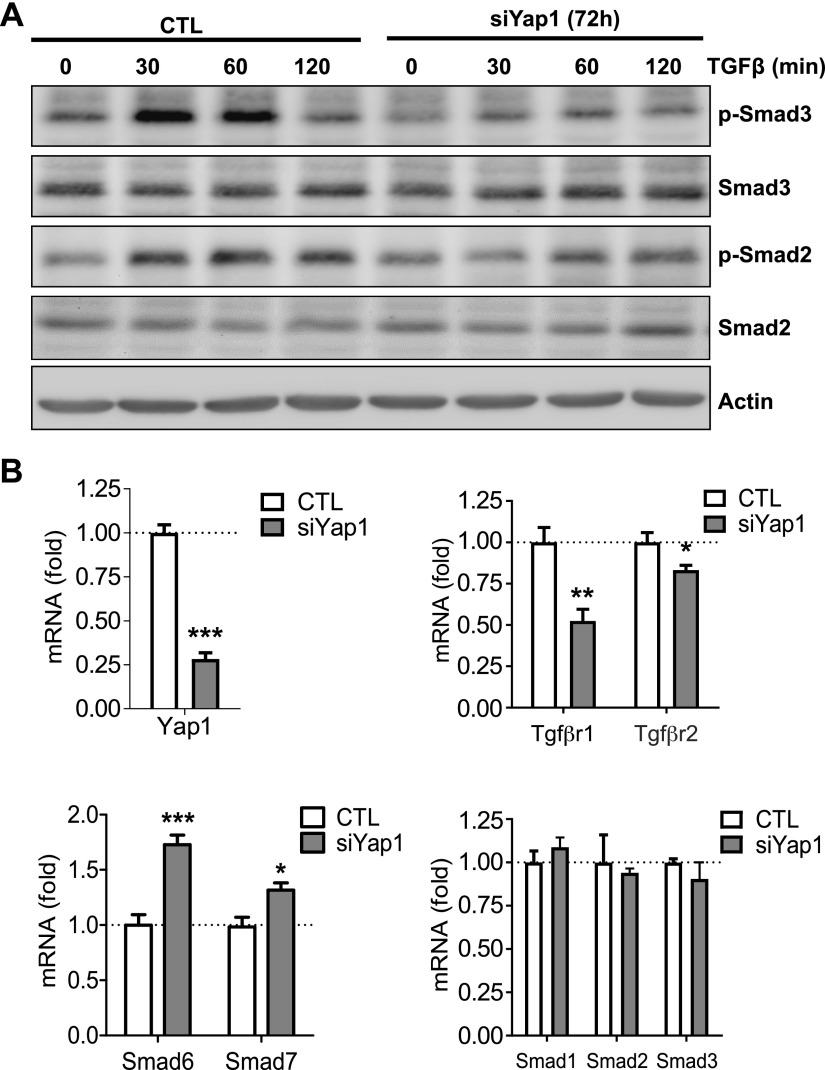
TGF-β signaling plays a critical role in ovarian follicle development (37). Western blot analyses showed that knockdown of Yap1 compromised TGF-β1–induced phosphorylation of Smad2 and Smad3 in cultured primary mouse ovarian cells (Fig. 8A). Quantitative PCR results also indicated that knockdown of Yap1 in mouse granulosa cells suppressed mRNA expression of Tgfbr1 and Tgfbr2 (Fig. 8B). Interestingly, knockdown of Yap1 also increased mRNA levels of Smad6 and Smad7, 2 inhibitory Smads that suppress the activity of receptor-regulated Smads such as Smad2 and Smad3 (Fig. 8B). Taken together, YAP1 controls the TGF-β signaling pathway in mouse ovarian granulosa cells via regulating the transcription of TGF-β1 receptors and inhibitory Smads.
Figure 8.
Knockdown of Yap1 compromises Tgf-β signaling in mouse ovarian granulosa cells. A) Representative blots showing alteration of TGF-β1–induced phosphorylation of Smad2 and Smad3 in mouse granulosa cells with or without Yap1 knockdown. The relative levels of the total proteins and phosphoproteins were determined by Western blot. Experiments were repeated ≥3 times. B) Relative mRNA levels of Tgfßr1, Tgfßr2, Smad6, Smad7, Smad1, Smad2, and Smad3 in ovarian granulosa cells with or without Yap1 knockout. p, phosphorylated; siYap, Yap1-specific siRNA. Each bar represents the mean ± sem (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 compared with corresponding control (CTL).
DISCUSSION
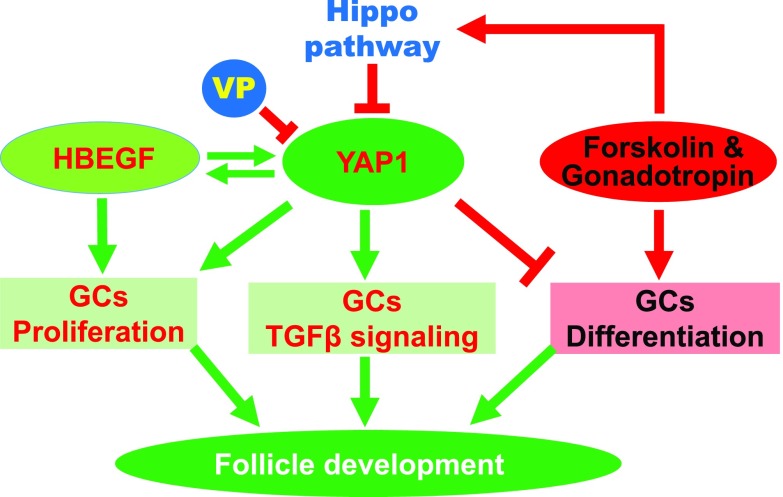
The Hippo signaling pathway controls organ size by regulating YAP1 activity. Activation of the Hippo pathway by growth-suppressive signals retains YAP1 in the cytoplasm and blocks its interaction with transcription factors in the nucleus, which leads to decreased expression of growth-promoting genes, suppressed cell proliferation, and, eventually, reduced organ size. In contrast, cell growth signals suppress the Hippo pathway and induce the translocation of YAP1 from the cytoplasm into the nucleus to drive expression of proliferative genes, resulting in cell proliferation and organ growth. The roles of the Hippo pathway in development and tumorigenesis have been extensively studied (7, 10, 38–41). However, its role in ovarian follicle development is largely unknown. In the present study, we found that YAP1, the major effector of the Hippo signaling pathway, interacts with several different signaling pathways to control granulosa cell proliferation and differentiation (Fig. 9). Our in vitro and in vivo results indicate that timely expression and activation of YAP1 is essential for granulosa cell function and ovarian follicle development.
Figure 9.
A schematic illustration showing the interactions between YAP1 and HBEGF, gonadotropin signaling transduction, and TGF-β signaling pathways in regulating granulosa cell (GC) proliferation and differentiation and, thereby, follicle development. In the growing follicle, YAP1 and HBEGF form a feedforward loop to drive GC proliferation. Activation of gonadotropin signaling triggers the Hippo pathway and phosphorylates YAP1, leading to differentiation of GCs. YAP1 activity is also essential for appropriate TGF-β signal transduction in GCs, which is critical for follicle development and oocyte maturation. Therefore, our study suggests that timely expression and activation of YAP1 is essential for GC function and ovarian follicle development.
The expression of nuclear YAP1 in granulosa cells of growing follicles in both mouse and human ovaries implies a role of YAP1 in granulosa cell physiology. The experimental results from our cellular models suggest that activation of YAP1 promotes proliferation but suppresses differentiation and steroidogenesis of cultured granulosa cells. Foxl2-driven knockout of Yap1 in granulosa cells resulted in abnormal ovarian function, which was indicated by smaller ovarian size, increased apoptosis of granulosa cells, inactivation of the AKT signaling pathway, reduced number of corpora lutea, and significantly increased numbers of atretic follicles when compared with the age-matched control mice. These data strongly support our hypothesis that the Hippo-YAP pathway plays a critical role in granulosa cell physiology and follicle development. Interestingly, the breeding experiments showed that granulosa cell–specific knockout of Yap1 protein induced subfertility but not infertility. This is consistent with the presence of few preovulatory follicles in some Yap1-knockout mice. This phenotype could be attributed to a potential compensative effect from TAZ, another effector protein of the Hippo pathway. Previous studies have shown that WWTR1 partially compensates for loss of YAP1 (42–44). Currently, we are generating Yap1/Wwtr1–double knockout mouse model to verify this speculation.
As previously mentioned, YAP1 protein was observed in the cytoplasm of the terminally differentiated luteal cells. To examine whether high levels of cytoplasmic YAP1 also have a function in animal fertility, we created a Cyp19 promoter–driven Yap1-knockout mouse model to delete YAP1 protein in the luteinized granulosa cells. Surprisingly, we found that deletion of YAP1 in luteal cells did not affect the morphology of ovaries, the number of corpora lutea, or the fertility of transgenic mice. It seems that cytoplasmic YAP1 in the luteinized granulosa cells has a minimal effect on mouse fertility. The differential ovarian phenotypes between the Foxl2-CRE;Yap1flox/flox mice and Cyp19-CRE;Yap1flox/flox mice may be attributed to the differential expression of Foxl2 and Cyp19 in granulosa cells. Because Foxl2 is predominantly expressed in granulosa cells of growing follicles, Foxl2-driven deletion of Yap1 may affect follicle development and oocyte maturation, leading to subfertility. However, in the luteinized granulosa cells, YAP1 becomes inactivated (predominantly in the cytoplasm). Cyp19-driven deletion of Yap1 mainly occurred in the luteinized cells, which have minimal effect on follicle development and oocyte maturation. However, the exact mechanisms underlying this phenotypical disparity need further investigation.
The transition of YAP1 protein from the nucleus to the cytoplasm during granulosa cell differentiation indicates that inactivation of YAP1 may be a necessary step for granulosa cell differentiation. It is known that the terminal differentiation of granulosa cells into nondividing luteal cells is triggered by high levels of LH (2). We observed that forskolin, mediated by PKA-cAMP signaling, activated the Hippo pathway and suppressed YAP1 activity in cultured granulosa cells. These data support our hypothesis that inactivation of YAP1 is a critical step for the terminal differentiation of granulosa cells. Moreover, hyperactivation of YAP1 in cultured ovarian granulosa cells suppressed the expression of steroidogenic enzymes and inhibited basal and FSH-induced production of estrogen and progesterone, indicating that inactivation of YAP1 is important for proper differentiation of granulosa cells. Obviously, timely and balanced expression and activation of YAP1 is essential for granulosa cell proliferation, differentiation, and follicle development.
An important finding in this study is that the Hippo signaling pathway may regulate follicle development via crosstalk with the EGFR pathway. EGF and EGFR and their downstream signaling pathways are key regulators of granulosa cell proliferation, follicle development, and ovulation (45–48). Both in vivo and in vitro studies have identified EGF and EGF-like ligands and their downstream signaling pathways as critical suppressors of apoptosis and potent mitogens of granulosa cells (49–51). Importantly, EGF-like growth factors were shown to recapitulate the biochemical events triggered by LH, including cumulus expansion and oocyte maturation in incubated mouse germinal vesicle–stage follicles (45). In the present study, we found that knockdown of YAP1 resulted in a significant reduction of HBEGF, AREG, and EGFR expression, suggesting that regulation of the EGFR pathway is an important mechanism for YAP1 to regulate follicle development. Consistently, we found that ectopic expression of YAP1 induced HBEGF expression in granulosa cells. High HBEGF in turn suppressed the Hippo pathway and activated YAP1, indicating that YAP1 and HBEGF formed a feedforward loop to drive granulosa cell proliferation in the growing follicles. YAP1 activity in granulosa cells of the preovulatory follicles was significantly reduced. Interestingly, Conti et al. (45) showed that HBEGF levels in the preovulatory follicle were reduced, and this reduction was critical for ovulation. Intriguingly, previous studies showed that down-regulation of HBEGF expression was necessary for the final maturation of follicles (52, 53). Therefore, YAP1 activity is associated with HBEGF expression and function during follicle development and ovulation. Crosstalking to the EGFR signaling pathway is an important mechanism for the Hippo-YAP signaling pathway to control granulosa cell function and follicle development.
Previous studies have shown that TGF-β1 may act as a local regulator of granulosa cell proliferation and differentiation, granulosa cell and oocyte communication, and follicle development in the mouse (54–56). Compared with age-matched control mice, Tgfb1–null mice had significantly reduced ovarian weight and number of corpora lutea and suffered from severe subfertility (57). In the present study, we found that knockout of Yap1 also results in reduced ovarian size, decreased number of corpora lutea, and severe subfertility. In the cultured mouse ovarian granulosa cells, knockdown of Yap1 suppressed Tgfbr1/2 expression and interrupted Tgfb1 signaling in the granulosa cells. These data indicate that the Hippo-YAP pathway may crosstalk with the TGF-β signaling pathway to regulate granulosa cell function and follicle development in the mouse. Further studies are needed to explore the detailed molecular mechanisms underlying their crosstalk and the importance of their interaction on reproductive physiology and pathology.
In summary, results from both in vitro and in vivo experimental models in the present study provided convincing evidence that balanced YAP1 activity in granulosa cells is essential for granulosa cell proliferation, differentiation, and survival, as well as follicle development. The Hippo-YAP pathway contributes significantly to female fertility by cooperating with multiple signaling pathways in the granulosa cells to nurture a viable oocyte.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Eric N. Olson (University of Texas Southwestern Medical Center, Dallas, TX) for providing the Yap1-floxed mouse model. The authors also thank Janice A. Taylor and James R. Talaska of the Advanced Microscopy Core Facility at the University of Nebraska Medical Center for providing assistance with confocal microscopy. This work was supported by a University of Nebraska Medical Center Fellowship; the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5R00HD059985); The National Cancer Institute and U.S. National Institutes of Health (NIH) (1R01CA197976, 1R01CA201500), the Vincent Center for Reproductive Biology, Department of Obstetrics and Gynecology, Massachusetts General Hospital–Harvard Medical School; the Olson Center for Women’s Health at the University of Nebraska Medical Center; The Department of Veteran’s Affairs, and the Colleen’s Dream Foundation. X.L., C. He, and C. Huang are co-first authors. The authors declare no conflicts of interest.
Glossary
- 3β-HSD
hydroxy-Δ5-steroid dehydrogenase, 3β
- 3D
3 dimensional
- AKT
protein kinase B
- AREG
amphiregulin
- CRE
Cre recombinase
- Cyp
cytochrome P450
- EGF
epidermal growth factor
- EGFR
EGF receptor
- FBS
fetal bovine serum
- Foxl2
forkhead box L2
- FSH
follicle-stimulating hormone
- FSHR
FSH receptor
- HBEGF
heparin-binding EGF-like growth factor
- hGC
human granulosa cell
- IHC
immunohistochemistry
- Ki67
marker of proliferation Ki-67
- LATS
large tumor suppressor kinase
- MYC
myc proto-oncogene protein
- phospho
phosphorylated
- Ptgfr
prostaglandin F receptor
- siRNA
small interfering RNA
- Smad
mothers against decapentaplegic
- Star
steroidogenic acute regulatory protein
- Sult1e1
sulfotransferase family 1E member 1
- VP
verteporfin
- WWTR1
WW domain containing transcription regulator 1
- TAZ
transcriptional coactivator with PDZ-binding motif
- YAP1
yes-associated protein 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X. Lv, C. He, and C. Wang designed research; X. Lv, C. He, C. Huang, H. Wang, G. Hua, Z. Wang, J. Zhou, X. Chen, and B. Ma performed research; B. K. Timm, V. Maclin, and J. Dong contributed new reagents and cells; X. Lv, C. He, C. Huang, B. R. Rueda, J. S. Davis, and C. Wang analyzed data; X. Lv, B. R. Rueda, J. S. Davis, and C. Wang contributed to the manuscript revision; X. Lv and C. Wang wrote the manuscript; and C. Wang supervised the studies.
REFERENCES
- 1.Hsueh A. J., Kawamura K., Cheng Y., Fauser B. C. (2015) Intraovarian control of early folliculogenesis. Endocr. Rev. 36, 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stocco C., Telleria C., Gibori G. (2007) The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 28, 117–149 [DOI] [PubMed] [Google Scholar]
- 3.Meignin C., Alvarez-Garcia I., Davis I., Palacios I. M. (2007) The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr. Biol. 17, 1871–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polesello C., Tapon N. (2007) Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 17, 1864–1870 [DOI] [PubMed] [Google Scholar]
- 5.Huang J., Kalderon D. (2014) Coupling of Hedgehog and Hippo pathways promotes stem cell maintenance by stimulating proliferation. J. Cell Biol. 205, 325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarikaya D. P., Extavour C. G. (2015) The Hippo pathway regulates homeostatic growth of stem cell niche precursors in the Drosophila ovary. PLoS Genet. 11, e1004962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan D. (2010) The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu F. X., Guan K. L. (2013) The Hippo pathway: regulators and regulations. Genes Dev. 27, 355–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S. A., Gayyed M. F., Anders R. A., Maitra A., Pan D. (2007) Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B., Li L., Lei Q., Guan K. L. (2010) The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 24, 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry E. R., Camargo F. D. (2013) The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr. Opin. Cell Biol. 25, 247–253 [DOI] [PubMed] [Google Scholar]
- 12.Halder G., Johnson R. L. (2011) Hippo signaling: growth control and beyond. Development 138, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lian I., Kim J., Okazawa H., Zhao J., Zhao B., Yu J., Chinnaiyan A., Israel M. A., Goldstein L. S., Abujarour R., Ding S., Guan K. L. (2010) The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 24, 1106–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beverdam A., Claxton C., Zhang X., James G., Harvey K. F., Key B. (2013) Yap controls stem/progenitor cell proliferation in the mouse postnatal epidermis. J. Invest. Dermatol. 133, 1497–1505 [DOI] [PubMed] [Google Scholar]
- 15.Zhao R., Fallon T. R., Saladi S. V., Pardo-Saganta A., Villoria J., Mou H., Vinarsky V., Gonzalez-Celeiro M., Nunna N., Hariri L. P., Camargo F., Ellisen L. W., Rajagopal J. (2014) Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev. Cell 30, 151–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H., Pasolli H. A., Fuchs E. (2011) Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc. Natl. Acad. Sci. USA 108, 2270–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahoney J. E., Mori M., Szymaniak A. D., Varelas X., Cardoso W. V. (2014) The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev. Cell 30, 137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu F. X., Zhang Y., Park H. W., Jewell J. L., Chen Q., Deng Y., Pan D., Taylor S. S., Lai Z. C., Guan K. L. (2013) Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 27, 1223–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yimlamai D., Christodoulou C., Galli G. G., Yanger K., Pepe-Mooney B., Gurung B., Shrestha K., Cahan P., Stanger B. Z., Camargo F. D. (2014) Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musah S., Wrighton P. J., Zaltsman Y., Zhong X., Zorn S., Parlato M. B., Hsiao C., Palecek S. P., Chang Q., Murphy W. L., Kiessling L. L. (2014) Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc. Natl. Acad. Sci. USA 111, 13805–13810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lange A. W., Sridharan A., Xu Y., Stripp B. R., Perl A. K., Whitsett J. A. (2015) Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. J. Mol. Cell Biol. 7, 35–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlegelmilch K., Mohseni M., Kirak O., Pruszak J., Rodriguez J. R., Zhou D., Kreger B. T., Vasioukhin V., Avruch J., Brummelkamp T. R., Camargo F. D. (2011) Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo E., Basu-Roy U., Gunaratne P. H., Coarfa C., Lim D. S., Basilico C., Mansukhani A. (2013) SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Reports 3, 2075–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamura K., Cheng Y., Suzuki N., Deguchi M., Sato Y., Takae S., Ho C. H., Kawamura N., Tamura M., Hashimoto S., Sugishita Y., Morimoto Y., Hosoi Y., Yoshioka N., Ishizuka B., Hsueh A. J. (2013) Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 110, 17474–17479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Y., Feng Y., Jansson L., Sato Y., Deguchi M., Kawamura K., Hsueh A. J. (2015) Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP. FASEB J. 29, 2423–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun T., Pepling M. E., Diaz F. J. (2015) Lats1 deletion causes increased germ cell apoptosis and follicular cysts in mouse ovaries. Biol. Reprod. 93, 22 [DOI] [PubMed] [Google Scholar]
- 27.Yu C., Ji S. Y., Dang Y. J., Sha Q. Q., Yuan Y. F., Zhou J. J., Yan L. Y., Qiao J., Tang F., Fan H. Y. (2016) Oocyte-expressed yes-associated protein is a key activator of the early zygotic genome in mouse. Cell Res. 26, 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu D., Lv X., Hua G., He C., Dong J., Lele S. M., Li D. W., Zhai Q., Davis J. S., Wang C. (2014) YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors. Endocr. Relat. Cancer 21, 297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu-Chittenden Y., Huang B., Shim J. S., Chen Q., Lee S. J., Anders R. A., Liu J. O., Pan D. (2012) Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C., Lv X., He C., Hua G., Tsai M. Y., Davis J. S. (2013) The G-protein-coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian cancer cells by blocking tubulin polymerization. Cell Death Dis. 4, e869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C., Lv X., Jiang C., Cordes C. M., Fu L., Lele S. M., Davis J. S. (2012) Transforming growth factor alpha (TGFα) regulates granulosa cell tumor (GCT) cell proliferation and migration through activation of multiple pathways. PLoS One 7, e48299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He C., Lv X., Hua G., Lele S. M., Remmenga S., Dong J., Davis J. S., Wang C. (2015) YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression. Oncogene 34, 6040–6054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenz S., Pöhland R., Becker F., Vanselow J. (2004) Expression of the bovine aromatase cytochrome P450 gene (Cyp19) is primarily regulated by promoter 2 in bovine follicles and by promoter 1.1 in corpora lutea. Mol. Reprod. Dev. 67, 406–413 [DOI] [PubMed] [Google Scholar]
- 34.Puri P., Little-Ihrig L., Chandran U., Law N. C., Hunzicker-Dunn M., Zeleznik A. J. (2016) Protein kinase A: a master kinase of granulosa cell differentiation. Sci. Rep. 6, 28132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amsterdam A., Selvaraj N. (1997) Control of differentiation, transformation, and apoptosis in granulosa cells by oncogenes, oncoviruses, and tumor suppressor genes. Endocr. Rev. 18, 435–461 [DOI] [PubMed] [Google Scholar]
- 36.Ranta T., Knecht M., Darbon J. M., Baukal A. J., Catt K. J. (1984) Induction of granulosa cell differentiation by forskolin: stimulation of adenosine 3′,5′-monophosphate production, progesterone synthesis, and luteinizing hormone receptor expression. Endocrinology 114, 845–850 [DOI] [PubMed] [Google Scholar]
- 37.Knight P. G., Glister C. (2006) TGF-beta superfamily members and ovarian follicle development. Reproduction 132, 191–206 [DOI] [PubMed] [Google Scholar]
- 38.Yu F. X., Zhao B., Guan K. L. (2015) Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvey K. F., Zhang X., Thomas D. M. (2013) The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 [DOI] [PubMed] [Google Scholar]
- 40.Badouel C., Garg A., McNeill H. (2009) Herding Hippos: regulating growth in flies and man. Curr. Opin. Cell Biol. 21, 837–843 [DOI] [PubMed] [Google Scholar]
- 41.Ramos A., Camargo F. D. (2012) The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 22, 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plouffe S. W., Lin K. C., Moore J. L., III, Tan F. E., Ma S., Ye Z., Qiu Y., Ren B., Guan K. L. (2018) The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J. Biol. Chem. 293, 11230–11240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finch-Edmondson M. L., Strauss R. P., Passman A. M., Sudol M., Yeoh G. C., Callus B. A. (2015) TAZ protein accumulation is negatively regulated by YAP abundance in mammalian cells. J. Biol. Chem. 290, 27928–27938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Q., Zhang N., Xie R., Wang W., Cai J., Choi K. S., David K. K., Huang B., Yabuta N., Nojima H., Anders R. A., Pan D. (2015) Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev. 29, 1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conti M., Hsieh M., Park J. Y., Su Y. Q. (2006) Role of the epidermal growth factor network in ovarian follicles. Mol. Endocrinol. 20, 715–723 [DOI] [PubMed] [Google Scholar]
- 46.Jamnongjit M., Gill A., Hammes S. R. (2005) Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc. Natl. Acad. Sci. USA 102, 16257–16262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richani D., Gilchrist R. B. (2018) The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update 24, 1–14 [DOI] [PubMed] [Google Scholar]
- 48.Yamashita Y., Shimada M. (2012) The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J. Reprod. Dev. 58, 510–514 [DOI] [PubMed] [Google Scholar]
- 49.Markström E., Svensson E. Ch., Shao R., Svanberg B., Billig H. (2002) Survival factors regulating ovarian apoptosis -- dependence on follicle differentiation. Reproduction 123, 23–30 [DOI] [PubMed] [Google Scholar]
- 50.Vitale A. M., Abramovich D., Peluffo M. C., Meresman G., Tesone M. (2006) Effect of gonadotropin-releasing hormone agonist and antagonist on proliferation and apoptosis of human luteinized granulosa cells. Fertil. Steril. 85, 1064–1067 [DOI] [PubMed] [Google Scholar]
- 51.Tilly J. L., Billig H., Kowalski K. I., Hsueh A. J. (1992) Epidermal growth factor and basic fibroblast growth factor suppress the spontaneous onset of apoptosis in cultured rat ovarian granulosa cells and follicles by a tyrosine kinase-dependent mechanism. Mol. Endocrinol. 6, 1942–1950 [DOI] [PubMed] [Google Scholar]
- 52.Pan B., Sengoku K., Goishi K., Takuma N., Yamashita T., Wada K., Ishikawa M. (2002) The soluble and membrane-anchored forms of heparin-binding epidermal growth factor-like growth factor appear to play opposing roles in the survival and apoptosis of human luteinized granulosa cells. Mol. Hum. Reprod. 8, 734–741 [DOI] [PubMed] [Google Scholar]
- 53.Pan B., Sengoku K., Takuma N., Goishi K., Horikawa M., Tamate K., Ishikawa M. (2004) Differential expression of heparin-binding epidermal growth factor-like growth factor in the rat ovary. Mol. Cell. Endocrinol. 214, 1–8 [DOI] [PubMed] [Google Scholar]
- 54.Roy S. K. (1993) Epidermal growth factor and transforming growth factor-beta modulation of follicle-stimulating hormone-induced deoxyribonucleic acid synthesis in hamster preantral and early antral follicles. Biol. Reprod. 48, 552–557 [DOI] [PubMed] [Google Scholar]
- 55.Adashi E. Y., Resnick C. E., Hernandez E. R., May J. V., Purchio A. F., Twardzik D. R. (1989) Ovarian transforming growth factor-beta (TGF beta): cellular site(s), and mechanism(s) of action. Mol. Cell. Endocrinol. 61, 247–256 [DOI] [PubMed] [Google Scholar]
- 56.Vanderhyden B. C., Macdonald E. A., Nagyova E., Dhawan A. (2003) Evaluation of members of the TGFbeta superfamily as candidates for the oocyte factors that control mouse cumulus expansion and steroidogenesis. Reprod. Suppl. 61, 55–70 [PubMed] [Google Scholar]
- 57.Ingman W. V., Robker R. L., Woittiez K., Robertson S. A. (2006) Null mutation in transforming growth factor beta1 disrupts ovarian function and causes oocyte incompetence and early embryo arrest. Endocrinology 147, 835–845 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.