Abstract
Rac-GTPases are major regulators of cytoskeletal remodeling and their deregulation contributes to numerous pathologies. Whether or how Rac promotes tubulointerstitial fibrosis and chronic kidney disease (CKD) is currently unknown. We showed that the major profibrotic cytokine, TGF-β1 promoted rapid Rac1-GTP loading in human kidney 2 (HK-2) human renal epithelial cells. A Rac-specific chemical inhibitor, EHT 1864, blocked TGF-β1–induced fibrotic reprogramming in kidney epithelial cells and fibroblasts. Stable Rac1 depletion in HK-2 cells, moreover, eliminated TGF-β1–mediated non-SMAD pathway activation [e.g., Src, epidermal growth factor receptor (EGFR), p53] and subsequent plasminogen activator inhibitor-1 (PAI-1), connective tissue growth factor, fibronectin, and p21 induction. Rac1 and p22phox knockdown abrogated free radical generation by TGF-β1 in HK-2 cells, consistent with the role of Rac1 in NAPD(H). TGF-β1–induced renal epithelial cytostasis was also completely bypassed by Rac1, p22phox, p47phox, and PAI-1 silencing. Rac1b isoform expression was robustly induced in the fibrotic kidneys of mice and humans. Intraperitoneal administration of EHT 1864 in mice dramatically attenuated ureteral unilateral obstruction–driven EGFR, p53, Rac1b, yes-associated protein/transcriptional coactivator with PDZ-binding motif activation/expression, dedifferentiation, cell cycle arrest, and renal fibrogenesis evident in vehicle-treated obstructed kidneys. Thus, the Rac1-directed redox response is critical for TGF-β1–driven epithelial dysfunction orchestrated, in part, via PAI-1 up-regulation. Rac pathway inhibition suppressed renal oxidative stress and maladaptive repair, identifying Rac as a novel therapeutic target against progressive CKD.—Patel, S., Tang, J., Overstreet, J. M., Anorga, S., Lian, F., Arnouk, A., Goldschmeding, R., Higgins, P. J., Samarakoon, R. Rac-GTPase promotes fibrotic TGF-β1 signaling and chronic kidney disease via EGFR, p53, and Hippo/YAP/TAZ pathways.
Keywords: Rac1, renal fibrosis, NADPH oxidases, CTGF, PAI-1
Chronic kidney disease (CKD) continues to remain a major public health burden, affecting >15% of the US population and costing hundreds of billion dollars to treat worldwide (1–3). Regardless of the underlying etiology, kidney injury leads to tubular dysfunction, tissue inflammation and interstitial expansion, secretion of fibrotic stimuli (e.g., angiotensin and TGF-β1), and nephron loss, culminating in progressive fibrosis (1–9). The resulting end stage renal disease requires kidney replacement therapy to sustain life. Inadequate availability of dialysis and renal transplantation as well as lack of approved therapies to halt progressing fibrotic disease are major unmet and pressing clinical needs (1–3). Identification of novel pathways causally involved in the development of renal failure would be a significant step in the design of new CKD therapies (10).
Rac is a member of the Rho-family of GTPases, which play important roles in cytoskeletal remodeling, cell motility, and cell cycle transit and exists in 2 confirmations: an active GTP-bound version (Rac-GTP) and an inactive GDP-bound form (Rac-GDP). These reversible states of Rho-GTPase activity are regulated by guanine nucleotide exchange factors and guanine nucleotide dissociation inhibitors, which orchestrate downstream signaling (11, 12). Rac1/2 are essential components of the reactive oxygen species (ROS)-generating NOX1 and NOX2 NADP(H) oxidase system. During phagocytosis, activated Rac interacts with the p67Phox subunit, facilitating p67Phox localization to the plasma membrane and subsequent assembly of multisubunit NOX1 and NOX2 complexes crucial for superoxide generation (13). Increased oxidative stress is associated with various neoplastic and fibrotic pathologies where NADP(H) oxidases transmit growth factor-driven signaling pathways (14). Expression of p22Phox and p47Phox are elevated during obstructive and diabetic renal injury and p47Phox gene ablation ameliorated development of glomerular damage and diabetic kidney disease, establishing a role for free radicals in progressive renal injury (15, 16). Involvement of NOX proteins in pulmonary, hepatic, and cardiovascular fibrosis is tissue-specific and well characterized (14), although the role of NADP(H) oxidase proteins in renal disease remains controversial. In this regard, some studies demonstrated that pharmacological inhibition of NOX1/4 mitigated fibrosis progression (17, 18), while others showed that genetic ablation of NOX4 in the kidney worsened injury (19). In view of these contradictory findings, continued definition of the molecular basis of NADP(H) oxidases involvement is required to specifically address consequences of free radical generation in the kidney.
Aberrant Rac-GTPase activity is linked to various pathologies including cancer and organ fibrosis (20–22). Fibroblast-specific Rac-1 ablation affords protection from bleomycin-driven skin fibrosis (21), while Rac activation in the lung in response to asbestos exposure promotes pulmonary fibrosis (22). Although Rac-GTPase involvement tubulointerstitial fibrosis remains to be established, Rac-1 is critical for mineralcorticoid receptor (MR) activation and subsequent sodium retention, leading to renal hypertension and glomerular injury. Indeed, high-salt diets promote Rac1 activation, and hypertension-prone Tsukuba transgenic mice have elevated renal Rac1 loading and nuclear MR levels. Pharmacological inhibition of Rac-1 and MR in these animals attenuated inflammation and glomerular sclerosis (23, 24). Whether or how Rac-GTPases contributes to renal epithelial dysfunction, maladaptive repair, and tubulointerstitial fibrosis, a major contributor to CKD, affecting >850 million people worldwide, is currently not clear.
It is conceivable that Rac targeting could provide a new approach to manipulate the activity of certain NADP(H) oxidases without impacting protective effects of certain NOX members (i.e., NOX4) in the context of kidney injury. These observations prompted us to evaluate Rac inhibition as a potential approach to attenuate tubulointerstitial fibrosis and test the hypothesis that Rac-GTPases, particularly Rac1, is a therapeutically relevant non-SMAD transducer of the TGF-β1 pathway involved in CKD progression.
MATERIALS AND METHODS
Creation of stable cell lines
Human kidney 2 (HK-2) human proximal tubular epithelial cells and NRK-49F rat renal fibroblasts were grown in DMEM supplemented with 5–10% fetal bovine serum (FBS). To generate stable Rac1, p22Phox, p47Phox, p53, transcriptional coactivator with PDZ-binding motif (TAZ), and plasminogen activator inhibitor-1 (PAI-1) knockdowns, semiconfluent HK-2 cells were infected with Rac1 short hairpin RNA (shRNA), p22Phox shRNA, p47Phox shRNA, p53 shRNA, TAZ shRNA, or PAI-1 shRNA or the corresponding empty vector (control shRNA) lentiviral particles (all from Santa Cruz Biotechnology, Dallas, TX, USA) using 5 μg/ml Polybrene in 10% FBS/DMEM for 24 h. Cells were allowed to recover for 24 h and then selected in 10% FBS/DMEM/5 μg/ml puromycin; medium was changed every 3 d. Rac1, p22Phox, p47Phox, p53, TAZ, and PAI-1 depletion was confirmed by Western blot analysis. The Rac inhibitor, EHT 1864 was purchased from Tocris Biosciences (Bristol, United Kingdom).
Luciferase assay for PAI-1 promoter activation
Mink lung epithelial cells stably transduced with an 800 bp PAI-1 promoter fragment linked to a luciferase reporter (Mv1Lu-800 bp-Luc) were used for assessments of Rac involvement in PAI-1 transcriptional induction by TGF-β1. Cells were treated with the Rac inhibitor, EHT 1864 for 60 min prior to TGF-β1 stimulation for 24 h. The Dual-Luciferase Reporter Assay Kit (Promega, Madison, WI, USA) was used for PAI-1 promoter activity measurements (of equivalent cells) in accordance with the manufacturer’s recommendations. Luciferase determinations were done with a Turner Designs TD-20/20 luminometer (Turner Designs, Sunnyvale, CA, USA).
Unilateral ureteral obstruction model
C57Bl/6 mice were randomized into 2 groups prior to unilateral ureteral obstruction (UUO) surgery. One group (n = 5) received intraperitoneal injections of H2O 4 times [1 d before ureteral ligation (−1), and 1-, 3-, and 5-d postobstruction], while the second group (n = 5) was identically administered the Rac inhibitor, EHT 1864 at a dose of 50 mg/kg [based on a recommendation by a published study (24)] on 4 occasions as above. Following anesthetization, a small incision was made in the flank under aseptic conditions, the left ureter exposed and ligated with two 5-0 silk sutures. On d 7 postsurgery, all mice were euthanized and the obstructed (UUO) as well as contralateral (contra) kidneys from both groups harvested for biochemical and immunohistochemical analysis. Individual body weight of mice was recorded daily; animal survival and behavior were similarly monitored. All animal experiments were conducted by the contract research organization SMC Laboratories (Tokyo, Japan).
Western blotting
Renal cell cultures were lysed in sample buffer containing 5% beta-mercaptoethanol and kidney extracts prepared in 2% SDS/PBS; samples were vortexed, homogenized, and boiled for 5 min. Following electrophoretic separation, proteins were transferred to nitrocellulose membranes, blocked in 5% milk in 0.05% Triton X-100/PBS and incubated overnight with the following primary antibodies at indicated dilutions; pATMSer1981 (1:1000; ab81292), pSMAD3 (1:1000; ab52903), and fibronectin (1:10000; ab2413) from Abcam (Cambridge, MA, USA). pEGFRY845 (1:1000; 2231), p-p53Ser15 (1:1000; 9284), pSrcY418 (1:1000; 6943), yes-associated protein/TAZ (1:2000; 8418), pHistoneH3Ser10 (1:1000; 9701), p53 (1:1000; 2524), and p21 (1:1000; 2947) obtained from Cell Signaling Technology (Danvers, MA, USA). Vimentin (1:5000; cs-5565), TAZ (1:1000; sc-48805), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5000; sc-25778), p22Phox (1:1000; sc-20781), p47Phox (1:1000; sc-14015), and connective tissue growth factor (CTGF; 1:500; sc-14939) purchased from Santa Cruz Biotechnology. E-Cadherin (1:1000; 610181) was from BD Biosciences (San Jose, CA, USA); Rac1 (1:1000; 05-389) and Rac1b (1:2000; 09-271) were from MilliporeSigma (Burlington, MA, USA). Membranes were washed 3 times in 0.05% Triton X-100/PBS prior to incubation with appropriate secondary antibodies for 45 min. Following three 15 min washes in 0.05% Triton X-100/PBS, immunoreactive proteins visualized with enhanced chemiluminescence reagent and quantitated by densitometry.
Immunohistochemistry
Kidney sections were deparaffinized prior to antigen retrieval, endogenous peroxidase activity quenched and tissues blocked in 10% normal goat serum for incubation (30 min) with primary rabbit antibodies to Rac1b (1 μg/ml: 09-271 from MilliporeSigma) in 1% bovine serum albumin followed by appropriate secondary biotinylated antibodies (Vector Laboratories, Burlingame, CA, USA) for 30 min. Tissue sections were scanned with a semiautomated digital microscope (NanoZoomer 2.0-RS; Hamamatsu, Bridgewater Township, NJ, USA) and images analyzed with the Nanozoomer Digital Pathology viewer software (NDP.view; Hamamatsu).
Assessments of Rac1 activity
Rac-GTPase activity was measured with a kit from MilliporeSigma (17-441) as recommended. Briefly, confluent serum-deprived HK-2 cells, stimulated with TGF-β1 for various times with or without the Rac inhibitor EHT 1864, were washed in cold TBS/PBS and extracted in 1 ml of Magnesium Lysis/Wash Buffer (MLB) buffer supplemented with proteinase cocktail inhibitor and Na3VO4. Following lysate preclearing in protein A/G agarose (Santa Cruz Biotechnology), supernatants were collected by centrifugation. Ten microliters of PAK1-PBD-Agarose was added to 500 ml of each supernatant (treated with 50 µl of 0.5 M EDTA) and rocked for 1 h at 4°C. Agarose beads, collected by centrifugation after 2 washes in MLB buffer, were resuspended in 100 μl of sample buffer for subsequent Western blotting. Active Rac1 (PAK-PBD-bound) and total Rac1 levels (GTP-Rac1+ GDP-Rac1) were determined by Western blotting with a Rac1-specific antibody.
Assessment of ROS
The carboxy derivative of fluorescein, 2′,7′-dichlorofluorescein (C400; Molecular Probes, Eugene, OR, USA) was used to determine ROS generation in response to TGF-β1 according to the manufacturer’s recommendations. Briefly, HK-2 cells or transgenic epithelial cells stably expressing Rac shRNA, p22Phox shRNA, or control shRNA were stimulated with TGF-β1 for the times indicated. Following removal of medium, cells were incubated with 5 μM 2′, 7′-dichlorofluorescin diacetate (DCFDA) in PBS for 15 min prior to scrape harvest. An equivalent number of cells was used to assess baseline fluorescence (unstimulated) and response to TGF-β1 stimulation with a multidetection microplate reader (Synergy HT; BioTek Instruments, Winooski, VT, USA) at an excitation wavelength of 495 nm.
Growth analysis HK-cells with stable Rac1, p22Phox, p47Phox, and PAI-1 depletion
Control shRNA, Rac1 shRNA, p22Phox shRNA, p47Phox shRNA, and PAI-1 shRNA stably expressing HK-2 cells maintained at similar density (30–40%) were serum starved for 1 d. Cells were stimulated with TGF-β1 for 24 h prior to addition of 1% FBS/DMEM for 3–5 d to promote growth. Total cell number was assessed by evaluation of phase contrast images and with the Sceptor 2.0 Handheld Automated Cell Counter (MilliporeSigma) according to the manufacturer’s recommendations.
Human renal tissue samples
A diabetic renal lysate was derived from a human donor kidney, which was not transplanted due to extensive diabetic nephropathy (i.e., Kimmelstiel Wilson nodules). Lysates of normal human kidney were derived from nontumor part of a nephrectomy for renal cell carcinoma.
Statistical analysis
Two-tailed Student’s t test and ANOVA with Tukey post hoc analysis were used to assess statistical differences. A P value of <0.05 was considered significant.
RESULTS
Critical role of Rac1-GTPases in renal TGF-β1 signaling
TGF-β1, a master regulator of renal disease progression via both the canonical (SMAD-dependent) and non-SMAD pathways [e.g., NOX, p53, ataxia telangiectasia mutated (ATM), epidermal growth factor receptor (EGFR), c-Src, YAP/TAZ] (14, 16, 25–29), induces rapid Rac1-GTP loading (4-fold increase at 5 min poststimulation; P < 0.05) in HK-2 human renal tubular epithelial cells (Fig. 1A, B). Rac activation was completely inhibited by preincubation of HK-2 cells with a specific inhibitor, EHT 1864 (Fig. 1A, B; P < 0.05), which prevents Rac activation by binding to Rac1/2/3, dissociating guanine nucleotide and preventing GTP exchange or loading (30). TGF-β1–induced transcriptional (i.e., promoter) activation of the prototypic target gene PAI-1 was dose-dependently inhibited by EHT 1864 pretreatment with complete ablation of the inductive response at a concentration of 12 μM (Fig. 1C; P < 0.01). Activation of PAI-1 (Fig. 1D, E), fibronectin (Fig. 1F), and p21(Fig. 1D, G) expression by TGF-β1 stimulation was effectively eliminated by EHT 1864 in HK-2 cells. Similar responses were also observed for PAI-1 and fibronectin up-regulation by TGF-β1 in NRK-49F renal fibroblasts (Fig. 1H–J), suggesting that TGF-β1–mediated Rac activation in renal epithelial cells and fibroblasts mediates fibrotic responses.
Figure 1.
Rac-GTPases involvement in TGF-β1 signaling in renal epithelial cells and fibroblasts. A) HK-2 cells were stimulated with TGF-β1 for various times with or without preincubation with the Rac inhibitor EHT 1864 (12 μM) and extracts subjected to Rho-GTPase assay to assess active Rac1 levels relative to total Rac1 protein. B) Histogram illustrates relative Rac1-GTP levels (mean ± sd) for 3 independent experiments setting expression levels in unstimulated (−) HK-2 cells as 1. *P < 0.05. C) Data depicts relative luciferase measurements (mean ± sd) of PAI-1 promoter activity in Mv1Lu-800 bp PAI-1-Luc cells stimulated with TGF-β1 (1 ng/ml; 18 h) with or without EHT 1864 pretreatment for 1 h at the indicated doses (3–12 μM). There were triplicate determinations for each group and experiments were repeated multiple times. **P < 0.01 as indicated. D–G) Serum-deprived HK-2 cultures remained untreated (−) or stimulated with TGF-β1 for 24 h with or without EHT 1864 at indicated doses and protein lysates processed by Western analysis for PAI-1 (D, E), fibronectin (F), p21 (D, G), and GAPDH (a loading marker) expression (D). Histograms (E–G) depict relative protein levels of several markers for 3 separate studies setting protein expression in untreated (−) HK-2 cells as 1 in each case. *P < 0.05, **P < 0.01. H–J) Serum-deprived normal rat kidney (NRK)-49F renal fibroblasts were stimulated with TGF-β1 for 24 h with or without EHT 1864 at the indicated doses and lysates immune blotted for fibronectin (H, I), PAI-1 (H, J), and GAPDH expression (H). T = TGF-β1, E = EHT 1864.
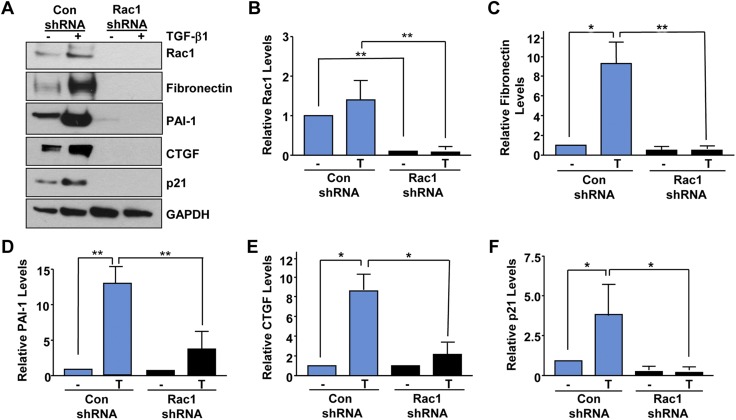
We investigated the potential involvement of Rac1 in fibrotic cytokine signaling via lentiviral shRNA-directed gene silencing approaches. Stable depletion of Rac1 (>95%; Fig. 2A, B; P < 0.01) in HK-2 cells completely inhibited the TGF-β1–mediated induction of fibronectin (Fig. 2A, C; P < 0.01), PAI-1 (Fig. 2A, D; P < 0.01), CTGF (Fig. 2A, E; P < 0.05), and p21 (Fig. 2A, F; P < 0.05) expression evident in vector-transduced control epithelial cultures (control shRNA), identifying Rac1 as an important transducer of fibrotic signaling.
Figure 2.
Stable silencing of Rac1 in renal epithelial cells ablates TGF-β1–mediated fibrotic reprogramming. Confluent control shRNA or Rac1 shRNA stably expressing cultures were untreated (−) or stimulated with TGF-β1 for 24 h and extracts processed by Western analysis for Rac1 (A, B), fibronectin (A, C), PAI-1 (A, D), CTGF (A, E), p21 (A, F), and GAPDH expression (A). Histograms (B–F) illustrate relative protein levels (mean ± sd) for 3 separate studies setting expression levels in untreated (−) control shRNA cells as 1 for each. Con, control. *P < 0.05, **P < 0.01.
Rac1 activates several non-SMAD pathways downstream of TGF-β1
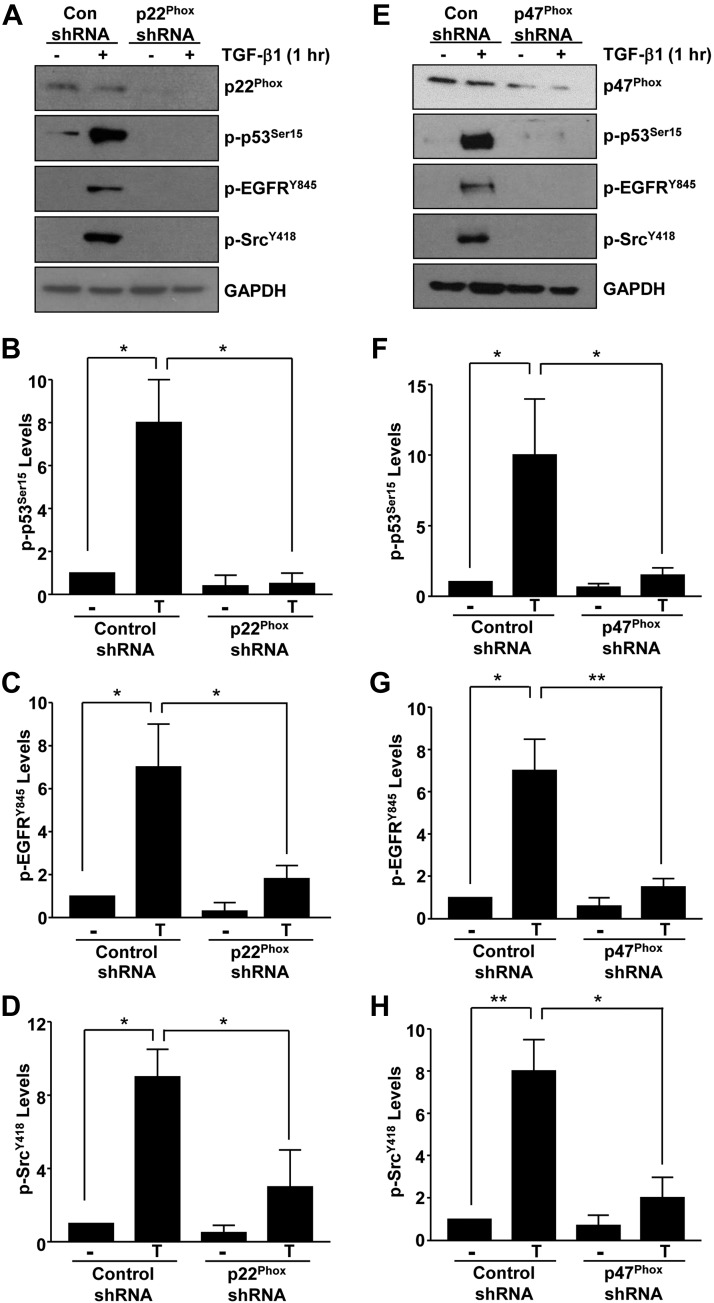
Recent studies have identified several non-SMAD effectors (e.g., p53, ATM, EGFR, and c-Src) activated by TGF-β1 that contribute to fibrosis progression (16, 25–29). TGF-β1–induced phosphorylation of p53Ser15 (Fig. 3A, B), ATMSer1981 (Fig. 3A, C), EGFRY845 (Fig. 3A, D), and c-SrcY418 (Fig. 3A, E) evident in control shRNA–expressing HK-2 cells were eliminated in Rac1-depleted epithelial cultures (P < 0.05 for all times points tested). These data suggest that Rac1 is a crucial upstream regulator of several downstream noncanonical pathways required for TGF-β1–mediated fibrotic responses. Because Rac1 is a major component of the NOX1/2 NADP(H) oxidases, we tested whether genetic silencing of p22Phox or p47Phox (subunits of NOX1/2) also impacts non-SMAD–mediated signaling. Stable suppression of p22Phox (via lentiviral mediated shRNA expression in HK-2 cells; Fig. 4A) similarly eliminated p53 (Fig. 4A, B), EGFR (Fig. 4A, C), and c-Src (Fig. 4A, D) activation by TGF-β1. Stable shRNA lentiviral transduction of 47Phox in HK-2 cells also led to a significant attenuation of p53 (Fig. 4E, F), EGFR (Fig. 4E, G), and c-Src (Fig. 4E, H) phosphorylation, suggesting that Rac1-GTPase is a functional component of ROS-generating NOX1/2 complexes (Fig. 5A). Consistent with the obligatory role of p53, p22Phox, and TAZ in TGF-β1–induced signal propagation, stable depletion of these non-SMAD entities in HK-2 renal epithelial cells virtually eliminated cytokine-directed fibrotic reprogramming (Supplemental Fig. S1A–C).
Figure 3.
Rac1 is required for TGF-β1 to activate several non-SMAD pathways critical for fibrogenesis. Confluent control shRNA and Rac1 shRNA stably expressing cell cultures (maintained in low serum medium) were stimulated with TGF-β1 for 1–2 h. Cell extracts were immunoblotted with antibodies to p-p53Ser15 (A, B), pATMSer1981 (A, C), pEGFRY845 (A, D), p-cSrcY418 (A, E), and GAPDH (A). Plots (B–E) summarize the relative expression (mean ± sd) of the indicated markers setting the expression in control shRNA stable cells without TGF-β1 stimulation (−) as 1 (n = 3). Con, control. *P < 0.05, **P < 0.01, as indicated.
Figure 4.
Upstream role of p22Phox and p47Phox NOX subunits in promoting TGF-β1 noncanonical signaling. Serum-starved confluent control shRNA and p22Phox shRNA (A) or p47Phox shRNA (E) stably transduced HK-2 cells were treated with TGF-β1. Cellular extracts were immunoblotted with antibodies to p-p53Ser15, pEGFRY845, p-cSrcY416, and GAPDH following confirmation of p22Phox and p47Phox depletion compared to their respective vector control cells (A, E). Histograms in B–D and F–H represent the relative expression (mean ± sd) of indicated phospho-proteins for 3 separate studies. Con, control. *P < 0.05, **P < 0.01.
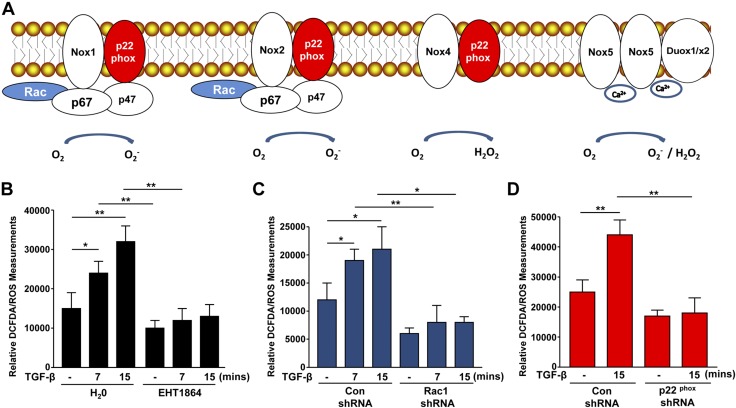
Figure 5.
Rac1 contributes to TGF-β1–mediated ROS generation via NADP(H) oxidases. A) The composition of different NOX proteins. Rac and p47Phox are common subunits of NOX1 and NOX2, whereas p22Phox is present in NOX1/2/4. Confluent HK-2 cells were stimulated with TGF-β1 for the times indicated with or without the Rac inhibitor EHT 1864 then incubated with 5 μM DCFDA for 15 min prior to scrape harvest. B) Histogram depicts relative DCFDA measurements (mean ± sd) in triplicate studies for each experimental condition. An equal number of cells were used to assess baseline fluorescence in the unstimulated (−) state and in response to TGF-β1; all measurements were done at the same time. *P < 0.05, **P < 0.01. Serum-deprived control shRNA and Rac1 shRNA stable HK-2 transductants were stimulated with TGF-β1 (15 min) for DCFDA analysis. C) Data illustrate relative free radical levels (mean ± sd) for each experimental condition for triplicate cultures. *P < 0.05, **P < 0.01. D) Plot depicts relative DCFDA measurements (mean ± sd) of serum-deprived control shRNA and p22Phox shRNA stably expressing HK-2 cells, which remained untreated (−) or stimulated with TGF-β1 (15 min); data plotted are for triplicate replicates for each condition in each of 3 separate experiments. Con, control. **P < 0.01.
Indeed, DCFDA analysis confirmed that rapid free radical generation induced by TGF-β1 in HK-2 cells was completely blocked by the Rac inhibitor, EHT 1864 (Fig. 5B; P < 0.01). Furthermore, stable suppression of Rac1 (Fig. 5C; P < 0.05) or p22Phox (Fig. 5D; P < 0.01) similarly eliminated TGF-β1–induced ROS generation evident in control vector-transduced HK-2 cells, confirming a role of Rac1/NOX proteins in redox-dependent signaling.
Rac1-NOX-PAI-1 signaling are major determinants of TGF-β1–induced renal tubular epithelial cytostasis
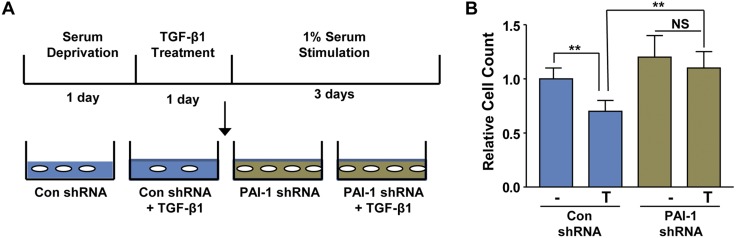
Renal tubular G2/M cell cycle arrest has emerged as an important determinant of progression of acute renal injury to fibrotic disease; secretion of paracrine fibrotic factors including CTGF and TGF-β1 by growth inhibited epithelial cells promotes the acquisition of fibroproliferative phenotype in the interstitial compartment (31). Although recent studies demonstrate that the TGF-β1–mediated proliferative arrest in renal epithelial cells occurs through ATM activation (29), upstream regulators of this response are not clear. Because Rac1 silencing ablated ATM phosphorylation by TGF-β1 (Fig. 3A, C), we evaluated Rac1 as a potential regulator of epithelial growth inhibition. Similarly seeded control shRNA–expressing or Rac1 shRNA–expressing epithelial cells were stimulated with TGF-β1 for 24 h followed by incubation in low serum (1% FBS) for 3 d to promote cell growth (Fig. 6A). Rac1 stable-depleted HK-2 cells completely bypassed TGF-β1–induced proliferative inhibition (>35% decrease; P < 0.05) observed in control shRNA transductants (Fig. 6A, B). Because p22Phox and p47Phox subunits (along with Rac-GTPases) are key components of the NOX1 and NOX2 NADP(H) oxidases (13, 14), we evaluated their involvement in TGF-β1–directed cytostasis using gene silencing methods. HK-2 cells stably expressing either p22Phox shRNA (Fig. 6C, D; P < 0.05) or p47Phox shRNA (Fig. 6E, F; P < 0.05) also bypassed the TGF-β1–induced epithelial proliferative blockade evident in the respective control shRNA–expressing counterparts. Collectively, these studies establish Rac1 and NADP(H) oxidases as critical upstream determinants of TGF-β1–induced cytostasis.
Figure 6.
Rac1 and NADP(H) oxidases are key effectors of renal epithelial growth inhibition in response to TGF-β1. A) A schematic representation of study design for Rac1 involvement in renal epithelial cell cycle arrest. Briefly, subconfluent control shRNA or Rac1 shRNA stably transduced HK-2 epithelial cells at a similar density were serum-deprived for 1 d then incubated with TGF-β for 1 d followed by serum addition (1%) for 3 d to stimulate cell growth. B) Relative epithelial cell counts (mean ± sd) are plotted in setting the cell number in untreated (−) control shRNA cultures as 1. *P < 0.05 as indicated. N.S., not significant. C–F) To investigate the potential involvement of p22Phox and p47Phox subunits of the NADP(H) oxidases in TGF-β1–induced epithelial growth inhibition, study designs are adopted similar to above and the schematics (C, E) illustrate the experimental approaches. Semiconfluent and serum-starved control shRNA and p22Phox or p47Phox shRNA stably expressing renal epithelial cells at a similar density were incubated with TGF-β for 1 d followed by 3 d of 1% serum stimulation. Relative cell counts (mean ± sd) provided the comparisons of cell growth between control shRNA and p22Phox shRNA stable transductants (D) or control shRNA and p47Phox shRNA (F) stably expressing HK-2 cells setting the cell number in untreated control shRNA as 1 in each case. Con, control; NS, not significant; n = 3. *P < 0.05 as indicated.
PAI-1 is an established fibrotic factor and a target gene of the TGF-β1 pathway downstream of Rac activation. Moreover, PAI-1 induction upon phosphatase and tensin homolog (PTEN) and protein phosphatase magnesium-/manganese- dependent 1A (PPM1A) silencing in renal epithelial cells is critical for G1 cell cycle arrest and fibrotic tubular dysfunction (32, 33). Therefore, it is worthy to determine whether PAI-1 induction regulates TGF-β1–mediated epithelial cell cycle inhibition in HK-2 cells. PAI-1 stable depletion via shRNA lentiviral transduction in HK-2 cells resulted in the complete bypass of TGF-β1–stimulated cytostasis evident in control vector-transduced renal epithelial cells (Fig. 7; P < 0.01), establishing a role for PAI-1 in maladaptive cell cycle control and epithelial dysfunction downstream of TGF-β1/Rac1/NOX signaling.
Figure 7.
TGF-β1–induced PAI-1 up-regulation mediates renal epithelial cell proliferative restriction. A) Subconfluent and serum-starved control shRNA and PAI-1 shRNA stably expressing HK-2 cells were treated with TGF-β for 1 d prior to serum stimulation for 3 d to promote cell growth. B) Data depict relative cell counts (mean ± sd) setting cell number in untreated (−) control shRNA cultures as 1. Con, control; NS, not significant; n = 3. **P < 0.01.
Elevated renal Rac1b expression is associated with renal maladaptive repair and fibrosis
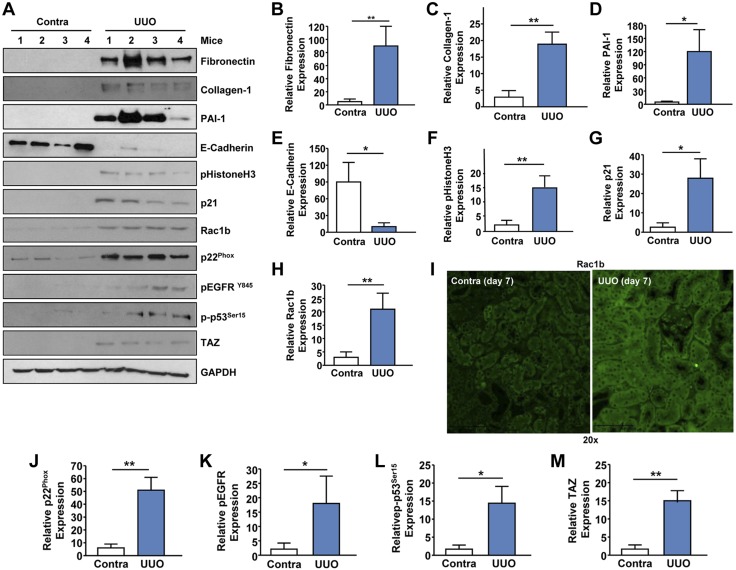
UUO in mice mimics obstructive uropathies (i.e., uropelvic junction obstruction in children), which can lead to renal injury, fibrosis, and organ failure (34). UUO is also a highly reproducible model for inducing renal fibrosis in mice, as evidenced by increased expression of fibronectin (Fig. 8A, B; P < 0.01), collagen-1 (Fig. 8A, C), and PAI-1 (Fig. 8A, D; P < 0.05) in the ligated kidneys (at d 7 postobstruction) compared to contralateral controls. Epithelial dedifferentiation/partial-EMT (marked by decreased E-cadherin expression and gain of mesenchymal markers such as vimentin) during renal injury leads to G2/M cell cycle arrest, contributing to progressive fibrosis (35, 36). Reduced E-cadherin expression (Fig. 8A, E; P < 0.05) and elevated levels of pHistoneH3Ser10 (a G2/M arrest marker; Fig. 8A, F; P < 0.01) and p21 (a cell cycle arrest gene; Fig. 8A, G; P < 0.05) are further indicative of epithelial dedifferentiation and cell cycle inhibition occurring in the fibrotic kidney relative to unligated controls. Expression of the Rac1b isoform, a constitutively active and a truncated version of Rac1 (37), is highly induced (>6-fold) in the UUO mouse kidneys compared to contralateral controls (Fig. 8A, H; P < 0.01). Elevated SMAD3 phosphorylation (evidence for active TGF-β1 signaling) and fibronectin, CTGF and collagen-1 levels (indicative of a state of fibrosis) as well as induction Rac1b expression are also evident in a human diabetic kidney compared to control renal tissue, suggesting that our observations in animal models may be pertinent to human CKD progression (Supplemental Fig. S2). Immunohistochemistry further confirmed elevated Rac1b levels in the renal tubules of the fibrotic mouse kidneys (Fig. 8I). Robust increases (>8-fold) of p22Phox expression in the obstructed kidneys (Fig. 8A, J; P < 0.01) reflect increased NOX signaling in the injured kidney. Obstructive nephropathy is associated with increased activation of the EGFR (Fig. 8A, K; P < 0.05) and p53 (Fig. 8A, L; P < 0.05) as well as up-regulation of the Hippo pathway nuclear effector, TAZ (Fig. 8A, M; P < 0.01). p53, EGFR, and YAP/TAZ are causative factors of CKD (38–43) and prominent non-SMAD control elements of the TGF-β1 pathway in progressive renal disease (16, 25–29).
Figure 8.
Up-regulation of the Rac1b isoform in UUO-induced renal fibrosis. Extracts derived from contralateral (Contra) and UUO kidneys at d 7 postsurgery were immunoblotted for fibronectin (A, B), Col-1 (A, C), PAI-1 (A, D), E-cadherin (A, E), pHistoneH3Ser10 (A, F), p21 (A, G), Rac1b (A, H), p22Phox (A, J), pEGFR (A, K), p53Ser15 (A, L), TAZ (A, M), and GAPDH (A) expression. I) Immunohistochemical staining of paraffin sections of Contra and UUO kidneys with anti-Rac1b antibodies. Original magnification, ×20. B–H, J–M) Histograms demonstrate relative levels (mean ± sd) of indicated markers between ligated and contralateral kidneys; n = 4–5 mice per group. *P < 0.05, **P < 0.01.
Inhibition of Rac activity in mice attenuates renal epithelial dedifferentiation, G2/M arrest, and fibrosis
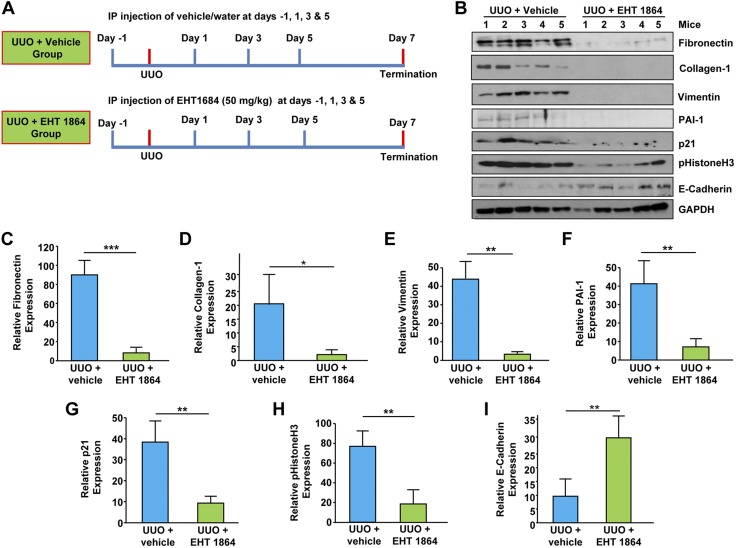
TGF-β1 is a major contributor to progressive kidney disease irrespective of injury (i.e., obstruction, diabetes) (4–10). Therefore, we investigated the involvement of Rac-GTPases in CKD using in vivo pharmacological inhibition as an approach because EHT 1864 blocked Rac activation by TGF-β1 in renal cells (Fig. 1A). EHT1864 (at a dose of 50 mg/kg) or control vehicle (DMSO) was intraperitoneally administered (every other day as outlined in Fig. 9A) to mice undergoing ureteral ligation to determine its impact on renal fibrosis on d 7 post-UUO. Renal expression of fibronectin (Fig. 9B, C; P < 0.01), collagen-1 (Fig. 9B, D; P < 0.05), vimentin (Fig. 9B, E; P < 0.01), PAI-1 (Fig. 9B, F; P < 0.01), p21 (Fig. 9B, G; P < 0.01), and pHistoneH3 at Ser10 (Fig. 9B, H; P < 0.01) readily evident in vehicle-treated UUO kidneys are each markedly decreased in the obstructed renal tissue isolated from EHT 1864–treated mice. Moreover, low E-cadherin expression observed in the ligated kidney (Fig. 9B, I; P < 0.01) of control animals was significantly rescued (>3-fold increase in E-cadherin levels) in the UUO kidneys following EHT 1864 administration. These studies demonstrate that pharmacological intervention of Rac activation reverses epithelial dysfunction (dedifferentiation/plasticity and G2/M cell cycle arrest) and induction of fibrotic factors associated with CKD progression.
Figure 9.
Rac pathway inhibition attenuates maladaptive fibrotic responses in the kidney. A) Schematic of the study design for Rac pharmacological inhibition in mice undergoing obstructive nephropathy. Briefly, C57BL/6 mice received (via intraperitoneal injection) either vehicle (water) or EHT 1864 (50 mg/kg) 1 d prior to UUO surgery. Similar treatments continued at d 1, 3, and 5 postsurgery to suppress Rac activity. B–I) Renal extracts from UUO kidneys (d 7) from vehicle and EHT 1864–injected mice were immune blotted for fibronectin (B, C), collagen-1 (B, D), vimentin (B, E), PAI-1 (B, F), p21 (B, G), pHistoneH3Ser10 (B, H), E-Cadherin (B, I), and GAPDH expression. Histograms (C–I) illustrate relative renal expression (mean ± sd) for each protein; n = 5 mice per experimental condition. *P < 0.05, **P < 0.01.
Rac signaling inhibition during obstructive nephropathy leads to attenuation of NOX, YAP/TAZ, EGFR, and p53 signaling
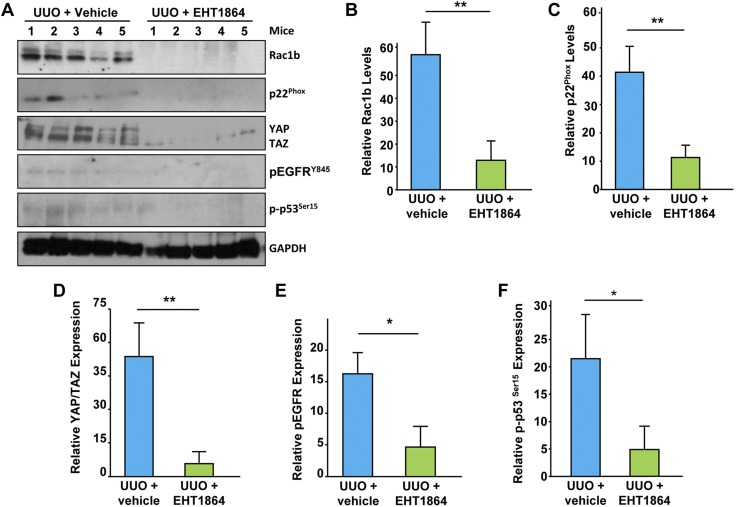
Downstream effectors of Rac during progressive kidney fibrosis are currently unknown. This study demonstrated that p53, EGFR, and c-Src are downstream targets of Rac-1/TGF-β1 pathway (Fig. 3). Increased expression of Rac1b (Fig. 10A, B; P < 0.01), p22Phox (Fig. 10A, C; P < 0.01), and YAP/TAZ (Fig. 10A, D; P < 0.01) as well as EGFR (Fig. 10A, E; P < 0.05) and p53 (Fig. 10A, F; P < 0.05) phosphorylation readily observed in the vehicle-treated obstructed kidneys were significantly diminished in UUO kidneys isolated from EHT 1864–treated mice. This strongly suggests that Rac inhibition during renal injury blocked activation of several known fibrotic effectors including key oxidative stress response mediators (such as p22Phox) and p53, EGFR, and YAP/TAZ causatively linked to the CKD development (38–43).
Figure 10.
Rac inhibition results in decreased Rac1b, NOX, YAP/TAZ, p53 and EGFR signaling. Obstructed kidney extracts from EHT 1864–treated or vehicle-treated mice were Western blotted with antibodies against Rac1b (A, B), p22Phox (A, C), YAP/TAZ (A, D), pEGFRY845 (A, E), p-p53Ser15 (A, F), and GAPDH. Histograms illustrate renal levels (mean ± sd) of each protein between the (UUO + vehicle) vs. (UUO + EHT 1864) experimental groups (B–F); n = 5 mice per group. *P < 0.05, **P < 0.01.
DISCUSSION
This study establishes that Rac1, critical for ROS generation by TGF-β1, serves as an upstream regulator of several noncanonical pathways that facilitate the activation of a battery of fibrotic genes including PAI-1, fibronectin, and CTGF. Rac1-NOX–mediated free radical generation, in such circumstances, directs ATM, p53, EGFR, and c-Src phosphorylation. TGF-β1–stimulated SMAD3 and non-SMAD cooperation result in the formation of p53-SMAD2/3 transcriptional complexes that initiate the expression of target genes including PAI-1 in renal cells (16, 26–29). Furthermore, Rac-1/NOX–mediated PAI-1 induction orchestrates the TGF-β1–induced epithelial cytostasis. ATM activates p53 and stimulates fibrotic factor induction and secretion (i.e., CTGF, PAI-1, TGF-β1), which mediate pathologic renal epithelial-fibroblast communications during TGF-β1–driven maladaptive repair responses (27–29).
Our in vivo studies with the Rac inhibitor, EHT 1684 further demonstrate a causative role for Rac-GTPase in renal fibrosis. p53, EGFR, and YAP/TAZ, activated during renal injury, are direct targets of the Rac pathway in CKD progression because EHT 1864 administration not only reversed fibrotic factor induction and epithelial dysfunction associated with obstructive nephropathy but dramatically attenuated p53, EGFR, and YAP/TAZ activation compared to vehicle-treated ligated kidneys. Previous studies using either chemical blockade and global or kidney-specific gene silencing in mice identified the crucial role of p53, EGFR, c-Src, and YAP/TAZ in the progression of kidney injury. p53 proximal tubular ablation in mice significantly retards ischemia reperfusion- and obstruction-induced acute kidney injury and subsequent development of fibrosis, confirming the participation of this tumor suppressor in CKD (38–40). Moreover, TGF-β1 promotes p53 phosphorylation and p53-SMAD3 transcriptional complex assembly, which are crucial for fibrogenic reprogramming and subsequent epithelial maladaptive repair involving both transcriptional-dependent and independent mechanisms (28, 29, 40). Mice with EGFR proximal tubular ablation similarly exhibits diminished fibrosis in response to angiotensin infusion (41). EGFR transactivation by c-Src downstream TGF-β1 is also necessary for subsequent fibrogenesis. TGF-β1–induced c-Src activation promotes extracellular matrix deposition in renal fibroblasts and pharmacological inhibition of c-Src attenuated UUO-driven fibrogenesis (16, 42). The Hippo nuclear effectors YAP/TAZ are activated (e.g., increased expression and nuclear accumulation) in various kidney injuries (27). Pharmacological inhibition of YAP/TAZ with verteporfin attenuates UUO-driven CKD in mice (27, 43). Genetic silencing studies confirmed that YAP/TAZ are indispensable for TGF-β1–driven and mechano-orchestrated renal fibrogenic responses (27, 43).
Expression of the constrictively-active Rac1 splice variant, Rac1b is significantly elevated in the UUO kidneys compared to contralateral controls. Rac1b also forms complexes with NADP(H) oxidases to induce ROS generation, consistent with the role this Rac1 isoform in promoting oxidative stress responses (44). Rac pharmacological inhibition significantly attenuates both p22Phox and Rac1b expression and oxidative stress during obstructive nephropathy.
Our demonstration of a causative role of Rac activation in renal fibrosis is in accord with the involvement of this GTPase in chronic injury in other organs. Overexpression of constitutively active Rac1 in the heart (driven by the myosin heavy chain promoter) leads to increased NOX activity, excessive tissue remodeling, and the development of fibrosis compared to wild-type control mice (45). Mice with RacV12 active mutant expression (under the control of α−smooth muscle actin promoter) also exhibits more severe hepatic fibrosis compared to control animals following carbon tetrachloride exposure (46). Although the precise mechanisms are not clear, these studies indicate that sustained Rac1 signaling aggravates tissue injury predisposing toward fibrosis.
We established that Rac1 is a major redox-dependent upstream regulator of several non-SMAD/TGF-β1 signaling cascades critical for epithelial dysfunction as evident by a proliferative arrest and fibrotic reprogramming (Fig. 11). Obstructive renal injury and subsequent fibrosis are associated with increased Rac1b expression; inhibition of Rac activation in the UUO kidney markedly attenuates associated cellular dedifferentiation and maladaptive fibrotic tissue remodeling. Therefore, Rac1 targeting is a novel and likely clinically adaptable strategy to limit renal injury and development of CKD.
Figure 11.
A model for Rac1 involvement in TGF-β1–driven renal fibrosis. Rac1 is rapidly activated in response to TGF-β1 stimulation and likely facilitates the assembly of NOX1 and NOX2 signaling complexes in the renal cellular plasma membrane. NADP(H)-mediated generation of free radicals in response to TGF-β1 induces ATM and p53 phosphorylation as well as EGFR and c-Src activation. The resulting accumulation of p-p53Ser15 and pSMAD3 transcriptional complexes on the promoter of TGF-β1 target genes mediates fibrotic reprogramming and PAI-1–dependent cell cycle arrest. Rac1b and NOX subunit expression is dramatically increased in UUO-driven renal injury. Chemical blockade of Rac attenuated progressive renal fibrosis and Rac1b, NOX, YAP/TAZ, p53, and EGFR activation in the kidney. Therefore, Rac1 is a new non-SMAD control element of the TGF-β1 pathway and a novel therapeutic target against CKD.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
R.G. was previously employed (2008–2009) by, performs contract research for, and receives reagents for CTGF-related research from FibroGen, a company involved in the development of anti-CTGF therapies. This study was supported by U.S. National Institutes of Health (NIH), National Institute of General Medical Sciences Grant GM057242 (to P.J.H.), a Capital Region Medical Research Institute grant (to R.S.), the Graver Family Endowment, and the Friedman Family Fund (to P.J.H.). The authors declare no conflicts of interest.
Glossary
- ATM
ataxia telangiectasia mutated
- CKD
chronic kidney disease
- CTGF
connective tissue growth factor
- DCFDA
2',7'-dichlorofluorescin diacetate
- EGFR
epidermal growth factor receptor
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HK-2
human kidney 2
- MR
mineralcorticoid receptor
- NRK
normal rat kidney
- PAI-1
plasminogen activator inhibitor-1
- ROS
reactive oxygen species
- shRNA
short hairpin RNA
- TAZ
transcriptional coactivator with PDZ-binding motif
- UUO
unilateral ureteral obstruction
- YAP
yes-associated protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. J. Higgins and R. Samarakoon conceived and supervised the experiments; S. Patel, J. Tang, J. M. Overstreet, S. Anorga, F. Lian, A. Arnouk, and R. Samarakoon performed the experiments; all authors were involved in data analysis; R. Goldschmeding contributed key reagents and materials; R. Samarakoon wrote and R. Samarakoon and P. J. Higgins edited the manuscript; and all authors agreed on the manuscript prior to submission.
REFERENCES
- 1.Couser W. G., Remuzzi G., Mendis S., Tonelli M. (2011) The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 80, 1258–1270 [DOI] [PubMed] [Google Scholar]
- 2.Jha V., Garcia-Garcia G., Iseki K., Li Z., Naicker S., Plattner B., Saran R., Wang A. Y., Yang C. W. (2013) Chronic kidney disease: global dimension and perspectives. Lancet 382, 260–272 [DOI] [PubMed] [Google Scholar]
- 3.Perico N., Remuzzi G. (2012) Chronic kidney disease: a research and public health priority. Nephrol. Dial. Transplant. 27(Suppl 3), iii19–iii26 [DOI] [PubMed] [Google Scholar]
- 4.Ferenbach D. A., Bonventre J. V. (2015) Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 11, 264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macconi D., Remuzzi G., Benigni A. (2014) Key fibrogenic mediators: old players. Renin-angiotensin system. Kidney Int. Suppl. (2011) 4, 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rüster C., Wolf G. (2011) Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J. Am. Soc. Nephrol. 22, 1189–1199 [DOI] [PubMed] [Google Scholar]
- 7.Boor P., Ostendorf T., Floege J. (2010) Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 6, 643–656 [DOI] [PubMed] [Google Scholar]
- 8.Kramann R., DiRocco D. P., Humphreys B. D. (2013) Understanding the origin, activation and regulation of matrix-producing myofibroblasts for treatment of fibrotic disease. J. Pathol. 231, 273–289 [DOI] [PubMed] [Google Scholar]
- 9.Strutz F., Neilson E. G. (2003) New insights into mechanisms of fibrosis in immune renal injury. Springer Semin. Immunopathol. 24, 459–476 [DOI] [PubMed] [Google Scholar]
- 10.Friedman S. L., Sheppard D., Duffield J. S., Violette S. (2013) Therapy for fibrotic diseases: nearing the starting line. Sci. Transl. Med. 5, 167sr [DOI] [PubMed] [Google Scholar]
- 11.Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 [DOI] [PubMed] [Google Scholar]
- 12.Jaffe A. B., Hall A. (2005) Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 13.Abo A., Pick E., Hall A., Totty N., Teahan C. G., Segal A. W. (1991) Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353, 668–670 [DOI] [PubMed] [Google Scholar]
- 14.Samarakoon R., Overstreet J. M., Higgins P. J. (2013) TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell. Signal. 25, 264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Chen X., Su Y., Paueksakon P., Hu W., Zhang M. Z., Harris R. C., Blackwell T. S., Zent R., Pozzi A. (2015) p47(phox) contributes to albuminuria and kidney fibrosis in mice. Kidney Int. 87, 948–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samarakoon R., Dobberfuhl A. D., Cooley C., Overstreet J. M., Patel S., Goldschmeding R., Meldrum K. K., Higgins P. J. (2013) Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species. Cell. Signal. 25, 2198–2209 [DOI] [PubMed] [Google Scholar]
- 17.Jha J. C., Gray S. P., Barit D., Okabe J., El-Osta A., Namikoshi T., Thallas-Bonke V., Wingler K., Szyndralewiez C., Heitz F., Touyz R. M., Cooper M. E., Schmidt H. H., Jandeleit-Dahm K. A. (2014) Genetic targeting or pharmacologic inhibition of NADPH oxidase nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. 25, 1237–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sedeek M., Gutsol A., Montezano A. C., Burger D., Nguyen Dinh Cat A., Kennedy C. R., Burns K. D., Cooper M. E., Jandeleit-Dahm K., Page P., Szyndralewiez C., Heitz F., Hebert R. L., Touyz R. M. (2013) Renoprotective effects of a novel Nox1/4 inhibitor in a mouse model of Type 2 diabetes. Clin. Sci. (Lond.) 124, 191–202 [DOI] [PubMed] [Google Scholar]
- 19.Nlandu Khodo S., Dizin E., Sossauer G., Szanto I., Martin P. Y., Feraille E., Krause K. H., de Seigneux S. (2012) NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J. Am. Soc. Nephrol. 23, 1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radisky D. C., Levy D. D., Littlepage L. E., Liu H., Nelson C. M., Fata J. E., Leake D., Godden E. L., Albertson D. G., Nieto M. A., Werb Z., Bissell M. J. (2005) Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S., Kapoor M., Shi-wen X., Kennedy L., Denton C. P., Glogauer M., Abraham D. J., Leask A. (2008) Role of Rac1 in a bleomycin-induced scleroderma model using fibroblast-specific Rac1-knockout mice. Arthritis Rheum. 58, 2189–2195 [DOI] [PubMed] [Google Scholar]
- 22.Osborn-Heaford H. L., Ryan A. J., Murthy S., Racila A. M., He C., Sieren J. C., Spitz D. R., Carter A. B. (2012) Mitochondrial Rac1 GTPase import and electron transfer from cytochrome c are required for pulmonary fibrosis. J. Biol. Chem. 287, 3301–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata S., Mu S., Kawarazaki H., Muraoka K., Ishizawa K., Yoshida S., Kawarazaki W., Takeuchi M., Ayuzawa N., Miyoshi J., Takai Y., Ishikawa A., Shimosawa T., Ando K., Nagase M., Fujita T. (2011) Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J. Clin. Invest. 121, 3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawarazaki W., Nagase M., Yoshida S., Takeuchi M., Ishizawa K., Ayuzawa N., Ueda K., Fujita T. (2012) Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J. Am. Soc. Nephrol. 23, 997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng X. M., Nikolic-Paterson D. J., Lan H. Y. (2016) TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 [DOI] [PubMed] [Google Scholar]
- 26.Samarakoon R., Overstreet J. M., Higgins S. P., Higgins P. J. (2012) TGF-β1→ SMAD/p53/USF2 →PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis. Cell Tissue Res. 347, 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anorga S., Overstreet J. M., Falke L. L., Tang J., Goldschmeding R. G., Higgins P. J., Samarakoon R. (2018) Deregulation of Hippo-TAZ pathway during renal injury confers a fibrotic maladaptive phenotype. FASEB J. 32, 2644–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overstreet J. M., Samarakoon R., Meldrum K. K., Higgins P. J. (2014) Redox control of p53 in the transcriptional regulation of TGF-β1 target genes through SMAD cooperativity. Cell. Signal. 26, 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Overstreet J. M., Samarakoon R., Cardona-Grau D., Goldschmeding R., Higgins P. J. (2015) Tumor suppressor ataxia telangiectasia mutated functions downstream of TGF-β1 in orchestrating profibrotic responses. FASEB J. 29, 1258–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shutes A., Onesto C., Picard V., Leblond B., Schweighoffer F., Der C. J. (2007) Specificity and mechanism of action of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. J. Biol. Chem. 282, 35666–35678 [DOI] [PubMed] [Google Scholar]
- 31.Yang L., Besschetnova T. Y., Brooks C. R., Shah J. V., Bonventre J. V. (2010) Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543, 1p, 143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samarakoon R., Helo S., Dobberfuhl A. D., Khakoo N. S., Falke L., Overstreet J. M., Goldschmeding R., Higgins P. J. (2015) Loss of tumour suppressor PTEN expression in renal injury initiates SMAD3- and p53-dependent fibrotic responses. J. Pathol. 236, 421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samarakoon R., Rehfuss A., Khakoo N. S., Falke L. L., Dobberfuhl A. D., Helo S., Overstreet J. M., Goldschmeding R., Higgins P. J. (2016) Loss of expression of protein phosphatase magnesium-dependent 1A during kidney injury promotes fibrotic maladaptive repair. FASEB J. 30, 3308–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chevalier R. L., Forbes M. S., Thornhill B. A. (2009) Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 75, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 35.Lovisa S., LeBleu V. S., Tampe B., Sugimoto H., Vadnagara K., Carstens J. L., Wu C. C., Hagos Y., Burckhardt B. C., Pentcheva-Hoang T., Nischal H., Allison J. P., Zeisberg M., Kalluri R. (2015) Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 21, 998–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grande M. T., Sánchez-Laorden B., López-Blau C., De Frutos C. A., Boutet A., Arévalo M., Rowe R. G., Weiss S. J., López-Novoa J. M., Nieto M. A. (2015) Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat. Med. 21, 989–997; erratum: 22, 217 [DOI] [PubMed] [Google Scholar]
- 37.Singh A., Karnoub A. E., Palmby T. R., Lengyel E., Sondek J., Der C. J. (2004) Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene 23, 9369–9380 [DOI] [PubMed] [Google Scholar]
- 38.Ying Y., Kim J., Westphal S. N., Long K. E., Padanilam B. J. (2014) Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury. J. Am. Soc. Nephrol. 25, 2707–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang D., Liu Y., Wei Q., Huo Y., Liu K., Liu F., Dong Z. (2014) Tubular p53 regulates multiple genes to mediate AKI. J. Am. Soc. Nephrol. 25, 2278–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang R., Xu X., Li H., Chen J., Xiang X., Dong Z., Zhang D. (2017) p53 induces miR199a-3p to suppress SOCS7 for STAT3 activation and renal fibrosis in UUO. Sci. Rep. 7, 43409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J., Chen J. K., Nagai K., Plieth D., Tan M., Lee T. C., Threadgill D. W., Neilson E. G., Harris R. C. (2012) EGFR signaling promotes TGFβ-dependent renal fibrosis. J. Am. Soc. Nephrol. 23, 215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan Y., Ma L., Zhou X., Ponnusamy M., Tang J., Zhuang M. A., Tolbert E., Bayliss G., Bai J., Zhuang S. (2016) Src inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis. Kidney Int. 89, 68–81; erratum: 92, 770–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szeto S. G., Narimatsu M., Lu M., He X., Sidiqi A. M., Tolosa M. F., Chan L., De Freitas K., Bialik J. F., Majumder S., Boo S., Hinz B., Dan Q., Advani A., John R., Wrana J. L., Kapus A., Yuen D. A. (2016) YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J. Am. Soc. Nephrol. 27, 3117–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee K., Chen Q. K., Lui C., Cichon M. A., Radisky D. C., Nelson C. M. (2012) Matrix compliance regulates Rac1b localization, NADPH oxidase assembly, and epithelial-mesenchymal transition. Mol. Biol. Cell 23, 4097–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavall D., Schuster P., Jacobs N., Kazakov A., Böhm M., Laufs U. (2017) Rac1 GTPase regulates 11β hydroxysteroid dehydrogenase type 2 and fibrotic remodeling. J. Biol. Chem. 292, 7542–7553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi S. S., Sicklick J. K., Ma Q., Yang L., Huang J., Qi Y., Chen W., Li Y. X., Goldschmidt-Clermont P. J., Diehl A. M. (2006) Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology 44, 1267–1277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.