Abstract
The purpose of this study was to examine the role of Elovl3 gene in meibogenesis and the impact of ELOVL3 protein ablation on the physiology of the mouse ocular surface and Meibomian glands (MGs). Elovl3 knockout, ELOVL3-ablated (E3hom) mice and their wild type littermates (E3wt) were studied side by side. E3hom mice had abnormal ocular phenotypes such as delayed eye opening, weeping eyes, crusty eyelids, eyelid edema, highly vascularized cornea and tarsal plates (TPs), slit eye, and increased tearing that resemble symptoms observed in human subjects with various forms of dry eye, MG dysfunction and blepharitis. Lipid profiling of E3hom TPs was conducted using chromatography and mass spectrometry. The analyses revealed that the lipid composition of E3hom TPs was strikingly different from that of their E3wt littermates. The mutation affected major classes of meibomian lipids – cholesteryl esters, wax esters, and cholesteryl esters of (O)-acylated w-hydroxy fatty acids. The studies illuminated the central role of ELOVL3 in producing C21:0-C29:0 fatty acids, including odd-chain and branched ones. Ablation of ELOVL3 leads to selective changes in the lipid composition of meibum, making E3hom mice instrumental in studying the mechanisms of the biosynthesis of meibum and modeling various pathologies of human ocular surface and adnexa.—Butovich, I. A., Wilkerson, A., Bhat, N., McMahon, A., Yuksel, S. On the pivotal role of Elovl3/ELOVL3 in meibogenesis and ocular physiology of mice.
Keywords: meibomian glands, tarsal plates, fatty acid elongation, meibum
Meibomian glands (MGs) produce a lipid-enriched secretion [meibum (1)] that plays a central role in ocular surface protection. Results of comparative analyses of the lipid composition of human and animal meibum have been reported in our previous publications (2–5), but little information is available with regard to the mechanisms of its biosynthesis [termed meibogenesis (3, 5, 6)]. Recently, we reported results of our pilot studies of meibogenesis in mice and humans (2, 3, 5, 6). Close similarities between the 2 species in terms of biochemistry and physiology of their MGs were observed, which made mice a credible and convenient animal model for our studies. From our previously published lipidomic and genomic data (3, 5, 6) and a recent publication of Sassa et al. (7), one can infer that enzymes of the fatty acid (FA) elongation cycle [elongase of very long-chain (VLC) FAs (ELOVL) 1 through 7] are critically important players in meibogenesis. The enzymes are encoded by 7 genes that carry the same names in humans and mice: ELOVL1/Elovl1 through ELOVL7/Elovl7. All 7 genes are expressed in MGs of humans and mice (3, 5, 6), but their expression levels differ from gene to gene. One of the genes (ELOVL3/Elovl3) is among those that are highly expressed in the MGs of both species. This gene encodes an enzyme, ELOVL3, that was reported to catalyze the elongation of C18 saturated and monounsaturated FAs to make VLC-FA of the C20–C24 family (8). The roles of this enzyme in human and animal physiology have been discussed in a number of previous publications. ELOVL3 has been shown to regulate skin barrier function (9), cold-induced adipose tissue browning (10, 11), and resistance to diet-induced obesity (12). Down-regulation of ELOVL3 was observed in human psoriatic plaques (13), whereas in a mouse model of nonalcoholic fatty liver disease, Elovl3 was up-regulated (14). Overall, the enzyme seems to play an important role in various lipid-related metabolic processes. However, its role in meibogenesis, though anticipated (3, 6), has not been evaluated in direct experiments yet. The purpose of this study was to examine the role of Elovl3, which is highly expressed in MGs, in meibogenesis and the impact of ELOVL3 ablation on the physiology of the mouse ocular surface and MGs.
MATERIALS AND METHODS
Reagents and instrumentation
Lipid standards were purchased from Nu-Chek Prep (Elysian, MN, USA), Matreya (State College, PA, USA), and MilliporeSigma (Burlington, MA, USA). HPLC and mass spectrometry (MS)–grade organic solvents (chloroform, methanol, iso-propanol, hexane, and water) were from MilliporeSigma, Burdick & Jackson (Muskegon, MI, USA), and Thermo Fisher Scientific (Waltham, MA, USA). Other reagents were of analytical or HPLC grade from MilliporeSigma and Thermo Fisher Scientific.
The following instrumentation has been used in the study: a setup for normal-phase isocratic HPLC-MS experiments consisted of a LCQ Fleet ion trap mass spectrometer from Thermo Fisher Scientific, a Waters 1525 binary HPLC pump, a column heater module, a temperature control module, and a 717 Plus autosampler (all from Waters, Milford, MA, USA). Reverse-phase gradient chromatographic experiments were conducted using a Synapt G2-Si high-resolution quadrupole time-of-flight (ToF) mass spectrometer with an atmospheric pressure chemical ionization (APCI) IonSabre ion source, a LockSpray, and a Binary Acquity M-Class ultra-high-performance-liquid chromatography (UPLC) system (all from Waters).
Source of mice
The founder stock mutant Elovl3burf/GrsrJ mice [also known as buttery rumpled fur (Burf) mice] were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) (stock no. 024182). The mutation had originally been characterized as a spontaneous G-to-A transition at chromosome 19 position 46,133,123 bp (GRCm38) (https://www.jax.org/strain/024182). The transition was predicted (but not proven) to inactivate a splice acceptor site upstream of exon 2, yielding Elovl3−/−, ELOVL3-ablated mutant mice. To genotype mice, a 606-bp PCR fragment, amplified from genomic tail DNA of all mice using forward primer E3-F11 and reverse primer E3-R11, was subjected to Sanger DNA sequencing using primer E3-F10. Mutant mice were identified by the presence of the Burf G-to-A transition in the intron splice sequence located on the 5′ side of the exon 2 sequence.
Accompanying mutations in the study mice
All animal procedures used in the study had been approved by the University of Texas Southwestern Medical Center’s Institutional Animal Care and Use Committee and were conducted in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
The Elovl3burf mice were cryo-recovered at The Jackson Laboratory from the frozen sperm of Elovl3burf mice on a predominantly SWR/J background and oocytes of BALB/cByJ mice. The BALB/cByJ line of mice is known to carry an inactivating mutation in the acyl-CoA dehydrogenase, short chain (Acads) gene (Acadsdel-J) (15). ACADS is a dehydrogenase that is responsible for metabolism of short-chain FA (16). The Acadsdel-J mutation results in a truncated protein with no enzymatic activity. Mice were genotyped for the presence or absence of Elovl3burf and Acadsdel-J mutations using the primers shown in Supplemental Table S1.
As the Acads gene is at least moderately expressed in MGs of mice [∼6.5 on a log2 scale from 0 to 20; (unpublished results); analyzed using Clariom D mRNA microarrays (from Thermo Fisher Scientific) as previously described (3, 5)] and could play a role in meibogenesis, we bred out the mutation from the Elovl3burf mice through heterozygous × heterozygous breeding schemes (Fig. 1). This tactic allowed for a successful separation of the Elovl3 mutation from the Acadsdel-J mutation and resulted in strains homozygous for each of them (i.e., Elovl3−/−/Acads+/+and Elovl3+/+/Acads−/− only), as well as all possible combinations of these 2 mutations, including a control strain of Elovl3+/+Acads+/+ mice. Our subsequent experiments described in this paper were conducted with Elovl3+/+Acads+/+ [Elovl3–wild type mice (E3wt)], Elovl3+/−Acads+/+ [Elovl3-knockout mice (heterozygous) (E3het)], and Elovl3−/−Acads+/+ [Elovl3-knockout mice (homozygous) (E3hom)] mice, leaving other variants for another project.
Figure 1.
The overall design of the Elovl3 experiment. GC, gas chromatography; RP, reverse phase.
All animals used in this study were housed in ventilated microisolator cages on a 12-h light/dark cycle and fed a standard commercial laboratory diet (Teklad 2016; Envigo, Somerset, NJ, USA) with free access to water.
A retinal degeneration mutation rd1 is another mutation that had to be considered while working with Elovl3burf mice. This mutation is known to affect the Pde6b gene in the SWR/J line of mice. This gene encodes the β subunit of heterotrimeric enzyme cyclic guanosine monophosphate phosphodiesterase 6B, which is highly concentrated in retinal photoreceptors. Fortunately, the expression level of this gene was found to be extremely low in the MGs of mice, at ≤3.7 on the log2 scale (unpublished results). Also, this enzyme is not known for being involved in lipid metabolism. However, all mice used in this study were confirmed to be homozygous for the rd1 mutation.
Elovl3 mRNA transcript characterization
Tarsal plates (TPs) were dissected from E3wt, E3het, and E3hom mice and stored in RNAlater solution (Qiagen, Germantown, MD, USA) at −80°C until processed. Total RNA was isolated and reverse transcribed using Superscript IV (Thermo Fisher Scientific) and Oligo dT primers. Specific Elovl3 oligonucleotide pairs were used to amplify cDNA sequence, spanning 5′ untranslated sequence through 3′ untranslated sequence for both Elovl3 mRNA reference sequence transcripts (NM_007703 amplified with E3 F12/R12 and XM_006526624.3, coding for Elovl3 transcript variant ×1 with alternate exon 1 usage, amplified with E3 F14/R15; Supplemental Table S1). The resulting PCR products for all genotypes were subjected to Sanger DNA sequencing.
Evaluation of mouse phenotypes
Male (n = 8) and female (n = 10) 2–4-mo-old E3hom mice were evaluated by slit lamp and phenol red thread test. In total, 17 female and 12 E3wt mice, who were littermates of the evaluated E3hom mice, were used as controls.
To measure blink rates and ellipticity of eye openings, mice were videotaped for 1–2 min, and the frequency of blinking in the right eye was calculated. The ellipticity (also known as eccentricity) of the eye was calculated using still photographs of the mouse eye analyzed using the Axiovision software (Carl Zeiss, Oberkochen, Germany).
To examine the corneal staining, mice were lightly anesthetized with a ketamine/xylazine cocktail and evaluated using a slit lamp. The ocular surface and eyelids were observed. Sodium fluorescein staining was performed by lowering the bottom eyelids in both eyes to administer a maximum of 0.25 μl of a 0.2% ophthalmic solution of the dye (Fluorescein-PF 2% from Greenpark Compounding Pharmacy, Houston, TX, USA) into the conjunctival sac. Then, the eyelids were gently closed to distribute the dye across the eye. Each mouse was then placed in the light path of a slit lamp with a cobalt blue filter, which allows visualization of stained surfaces, and photographed using an attached Canon EOS Rebel T6i digital camera (Canon, Melville, NY, USA). After the completion of the examination, the mice were returned to the colony. One week later, tear production was measured using phenol red thread (from Zone-Quick, Tokyo, Japan). The thread was placed in the inferior conjunctival fornix near the lateral canthus for 15 s using a jeweller’s forceps. The length of the wetted portion of the thread was read by the examiner using the millimeter scale provided on the packaging of the thread.
Histology
Structural features of mouse eyes were examined by hematoxylin and eosin staining. TPs and whole eye globes of 6 mice were removed, fixed for at least 24 h in buffered Carson’s formalin, and then dehydrated by successive passage through 50, 70, 95, and 100% ethanol before paraffin embedding. Tissue was sectioned, rehydrated through decreasing ethanol concentrations, and stained with hematoxylin and eosin (StatLab Medical Products, McKinney, TX, USA).
Extraction of mouse TP lipids
Surgical excision of TPs of freshly euthanized mice was conducted using a surgical microscope as previously described (3, 5). The specimens (2 or 4 TPs from each animal) were initially placed in glass vials with 0.5 ml of 2:1 (v/v) chloroform:methanol solvent mixture and kept at −20°C to preserve lipids. Then, mouse TP lipids (TPLs) were extracted from TPs at room temperature thrice with 1 ml of the solvent mixture for 15 min each time. Because of the very small size of mouse TPs, no homogenization of the tissues was necessary. The initial storage solution and the 3 extracts were pooled, the solvent was evaporated at 36°C under a stream of nitrogen, and the oily residue was redissolved in 1 ml of iso-propanol. The TPL samples were stored at −20°C in glass HPLC vials with crimped Teflon-lined caps until the analysis. No noticeable deterioration of the samples was observed during at least 1-yr storage under these conditions.
Lipid analysis
Screening and initial characterization of the samples were performed using isocratic normal-phase HPLC on a Diol column and APCI-MS in positive ion mode (PIM). A 717 Plus autosampler, a Waters 1525 binary HPLC pump, a column heater module, a temperature control module, and an LCQ Fleet ion trap mass spectrometer were used as previously described (17, 18). Precautions were taken to avoid any contact of samples with plasticware (except Teflon) and inadvertent contamination of samples with chemical extractives such as oleamide, phthalates, plastic oligomers, etc. (19).
After screening the study samples for contaminants, samples were analyzed using reverse-phase C18-UPLC and high-resolution ToF MS. Separation of analytes was achieved on an Acquity UPLC C18 BEH column (1 × 100 mm, 1.7 μm). A Waters Acquity M-Class UPLC system was used to create a binary gradient of iso-propanol/water and acetonitrile/water solvent mixtures (all with 5% of aqueous 10 mM ammonium formate as additive). The experiments were conducted in both PIM and negative ion mode (NIM). The experimental procedures have been previously described in detail in a recent publication (5). For the readers’ convenience, typical major lipids of mouse TPs that are discussed in this manuscript are listed in Supplemental Table S2.
Reproducibility of the analyses was estimated in an experiment with serial injections of the same sample (Supplemental Fig. S1). Both total ion chromatograms (TICs) and extracted ion chromatograms (EICs) of selected ions were evaluated. The overall reproducibility of the analyses was found to be high. The retention times of analytes remained, on average, within a ±5-s range. The m/z values were typically within a ±2-mDa range, whereas peak areas for EIC were within ±3% of the corresponding mean values. The linearity of the response of the MS detector was checked as previously described (5).
Note that accurate m/z values (routinely better than ±4 mDa of the theoretical values) were obtained for all analytes. However, they were rounded up in the text of the manuscript for clarity. As signal intensities of lipids are known to be dependent on their molecular masses and degree of unsaturation, a range of standard lipids was used to correct for these effects, as previously described (20).
Intact wax esters (WEs) of meibum were additionally analyzed using high-temperature gas chromatography-ion trap MS as previously described (20). These experiments were primarily necessary to detect and characterize saturated WE, which are poorly ionizable in liquid chromatography/MS experiments. The overall design of the experiment is summarized in Fig. 1.
RESULTS
Elovl3 mRNA transcript characterization
Exome sequencing of Elovl3 in Elovl3burf/GrsrJ mice identified a G-to-A transition at chromosome 19 position 46,133,123 bp (GRCm38) (http://www.informatics.jax.org/reference/J:223995). The identified G residue is located in an intron-exon splice sequence located at the 5′ end of exon 2 in the Elovl3 gene (Fig. 2). To define the molecular consequences of the Burf mutation for Elovl3 mRNA, PCR primers were used to amplify full-length coding sequence from reverse-transcribed tarsus RNA isolated from wild-type, hetero-, and homozygous Elovl3burf/GrsrJ mice (Fig. 2). Two different primer sets were used: F12/R12 to target the Elovl3 transcript NM_007703 and F14/R15 to target the Elovl3 alternative Xl1 transcript, XM_006526624.3. Both primer pairs amplified discrete DNA fragments that were detected in TPs of all genotypes (Fig. 2). Thus, both Elovl3 mRNA transcripts are expressed in mouse tarsus tissue, and the presence of the Burf mutation does not cause loss of Elovl3 transcripts. DNA sequencing of the amplified DNA fragments revealed that the consequence of the presence of the Burf genomic G-to-A transition was that the splice junction was shifted by 1 nt downstream, resulting in a frame shift mutation in exon 2 coding sequence, as shown in Fig. 2. In both Elovl3 transcripts, this frame shift inserted a stop codon at the beginning of exon 2 coding sequence, thus causing a function null (i.e., no full length intact ELOVL3 protein translation in the mutant mice).
Figure 2.
Characterization of tarsus Elovl3 transcripts in Burf mice. A) Elovl3 transcripts corresponding to Elovl3 NM_007703 (amplified with primers E3 F12/R12) and XM_006526624.3 (amplified with primers E3 F14/R15) were amplified from reverse-transcribed RNA isolated from the TPs of wild-type (WT) (+/+), heterozygous (+/−), and homozygous (−/−) Burf mice. In the absence of reverse transcriptase (no RT), no PCR products were observed in homozygous (−/−) samples. B) Schematic of the 4 exons in Elovl3 showing the location of the Burf G-to-A transition located in the intronic sequence adjacent to exon 2 (reside shown in red). Sequence analysis of RT-PCR products revealed that for both Elovl3 transcripts the Burf mutation shifted the intron-exon splice site downstream by 1 nt, resulting in loss of 1 nt from the mRNA transcript and a frame shift, which introduced a stop codon. KO, knockout.
Mouse phenotypes, development, and ocular features
The E3het and E3hom mice were viable and fertile. Unlike E3wt mice, E3hom mice had abnormal eye and fur phenotypes, such as delayed eye opening, weeping eyes, crusty eyelids, eyelid edema, highly vascularized cornea and TPs, a slit eye appearance, and greasy fur (Fig. 3). These features have been studied in more details.
Figure 3.
Phenotyping the Elovl3-mutant mice. A) A 2-mo-old E3wt male mouse with round eyes (normal phenotype). B) A 2-mo-old E3hom male mouse with an elliptical (slit) eye appearance (abnormal phenotype). C) Accumulation of ocular secretions was noted in the corner of the eye along the eyelid margin (double red arrow) with crust formation on the lashes (labeled with a red arrow). D, E) A 2-mo-old male E3hom mouse with extensive vascularization of the cornea [blood vessels are labeled with yellow triangles (D) and red marks (E)] with no other noticeable changes in the cornea. F, G) No vascularization was observed in the vast majority of E3wt mice. H–K) The E3hom MG (panels I and K) shows extensive accumulation of proteinaceous material within the connecting ductuli leading into the central duct (red arrows; H, I) compared with the E3wt (panels H and J), which shows a light eosinophilic material that completely disappears by the time it reaches the central duct (J, K). Central ducts are labeled with red “D”. L) Tear production in wild-type and mutant mice measured using the phenol red thread (PRT) test. The difference was statistically significant (P < 0.001). M) Ellipticity of the eye opening in E3hom mice was much higher than that in E3wt mice (P < 0.001). A.u., arbitrary units, unitless. N) Blink rates in E3hom and E3wt mice differed significantly (P < 0.05).
Upon initial examination, the E3hom mice could easily be distinguished from their E3het or E3wt littermates because of the formers’ disheveled, greasy-appearing coat. They displayed excessive grooming behavior, sometimes resulting in extensive hair loss. Whereas both the E3het mice and the E3wt mice showed the appearance of hair around the same age, the E3hom mice were easily identified even at young ages, as the hair growth was extremely sparse and the fur was greasy. Some of the E3hom pups, in fact, appeared hairless at the time of weaning but continued to develop a fuller coat as they progressed toward adulthood. As E3hom mice aged, the greasiness of the coat decreased, and adult mice tended to be less greasy when compared with young mice. The E3wt mice, on the other hand, had no developmental delays and exhibited a full, shiny coat.
Eye opening in the E3hom mice was delayed by up to 4–6 wk, though this varied among different mice, even if they belonged to the same litter. When the eyes finally opened, they continued to have a squinting appearance and had crusting and excessive mucoid and/or lipid discharge around the eyelid margins and canthal and adjacent areas (Fig. 3). In some cases, the E3hom eyelids appeared to be stuck together due to dried up discharge. In these cases, the eyes could be reopened after softening the crust with PBS. In general, the E3hom mice were reluctant to fully open their eyes, which was apparent by the overall slit appearance compared with the round, wide-open eyes of E3wt mice. The difference in ellipticity between the wild-type and knockout mice was statistically significant (P < 0.001; Fig. 3). There was no statistically significant difference in ellipticity between males and females of the same genotype.
Slit lamp evaluation of E3wt mouse eyelids demonstrated no abnormalities: the eyelids appeared normal and healthy. They did not have any lash loss or misdirected lashes. E3hom mice, on the other hand, had scant short eyelashes.
Elovl3 mutation induced considerable (histo)pathologic changes in TPs of mice. The eyelid margins of E3hom mice were swollen and inflamed with increased vascularization and erythema. The E3hom mice showed hyperplasia of the ductal epithelium, and the central ducts appeared narrower than the norm. Also, large amounts of an eosinophilic proteinaceous material within the ductules leading to the central duct and the orifice were observed in TPs of E3hom mice. The latter could impact the natural efflux of meibum and cause MG plugging.
To determine if the mutation affected other genes of the Elovl1–Elovl7 family, the expression levels of Elovl1 and Elovl7 genes were measured using RNA microarrays as previously described (5) and shown to remain unchanged in E3hom vs. E3wt mice: 18.2 in females and 17.4 in males for Elovl1 and 14.7 in females and 15.9 in males for Elovl7 (on a 0– 20 log2 scale).
The Elovl3 mutation also affected the mouse cornea. Of the 10 E3hom female mice evaluated, 3 eyes of 2 mice developed corneal neovascularization (Fig. 3). These corneal blood vessels were first noted at the age of 2 mo, with the mean age at discovery being 3.5 mo. In the E3hom male mouse group, 8 mice were examined, and corneal neovascularization was observed in 7 eyes of 5 mice. In total, 17 female and 12 male E3wt mice were also evaluated. Corneal neovascularization with increased corneal opacity were observed in only 1 female E3wt mouse at the age of 4 mo. None of the male E3wt mice developed corneal vessels. Overall, slit lamp evaluation revealed corneal neovascularization in 20% of female and about 60% of male mice in the E3hom group. This corneal vascularization could be, in part, a reaction to the altered composition of the ocular secretions. Another possibility is that the cornea in E3hom mice might be in a hypoxic state because of excessive accumulation of the discharge, decreased eye opening, and the eye being shut for extended periods. Further examination will be required to understand the reasons for cornea neovascularization.
Both E3wt and E3hom mice exhibited variable degrees of corneal staining with fluorescein. There was no significant difference between the study groups. However, the results were deemed too random and variable, even for the same animal, to be further pursued.
Finally, the E3hom mice had excessive tearing, which was evident as an overall increase in length of wetness of the phenol red threads compared with E3wt mice (P < 0.001). No differences in the tear production levels between males and females were observed.
Lipid analyses: WEs
Representative TICs and observation mass spectra of TPL of E3wt and E3hom mice are shown in Fig. 4. The impact of the mutation was complex but reproducible from animal to animal. Let’s discuss the effects of the mutation on each type of TPL in some detail.
Figure 4.
Initial characterization of E3wt and E3hom mouse TPL using liquid chromatography and MS. A1) TIC of a total lipid extract from TPs of a E3wt mouse. The analysis was conducted as previously described using C18-UPLC and high-resolution time-of-flight MS. Detection of the analytes was conducted using APCI ion source operated in PIM. Two TPs were processed, and the lipid extract was redissolved in 1 ml of i-propanol. Injection volume 0.5 μl. B1) TIC of total lipid extract from TPs of an E3hom mouse. A2) Observation high-resolution mass spectrum of a E3wt TP sample (APCI, PIM). Note that different scaling (magnification) factors (×2 and ×20) were used for different areas of the spectrum to visualize weaker signals. B2) Observation high-resolution mass spectrum of a E3hom TP sample (APCI, PIM). AP+ - atmopsheric pressure ionization, positive ion mode.
The TIC of E3wt and E3hom mice were found to be consistently different from each other, and so were their observation mass spectra. The mass spectra of TPL of E3wt mice demonstrated expected signals of free cholesterol (Chl) with m/z 369.35 detected as (M – H2O + H)+ ion, a range of WEs (m/z values from 500 to ∼750), triacylglycerols (m/z from 800 to 950), cholesteryl esters (CEs; m/z from 600 to 875), CEs of (O)-acylated ω-hydroxy FAs (Chl-OAHFAs, m/z from 1100 to 1200), and some other lipids and lipid families. The overall profile of E3wt TPL was very similar to the one described in our previous publication for wild-type C57BL/6J mice (5). The E3hom mice, on the other hand, had a markedly altered balance of these lipids. Several global differences were noted.
The first major trend was an increase in total unsaturated WEs at the expense of saturated WEs in TPLs of E3hom mice compared with E3wt mice (Fig. 5A, B). In general, the molar fraction of monounsaturated WEs (MUWEs) in TPs of E3hom mice declined, whereas those of di- and triunsaturated WEs (DUWEs and TUWEs, respectively) rose (Fig. 5C). Interestingly, the effects were dependent on the molecular masses of WE. Most affected were WEs with C40 to C50 total carbon chain lengths. Saturated WEs demonstrated a decline in all species from C40:0 to C46:0 (Fig. 5D), whereas the ratios of longer saturated WEs remained unchanged. The most affected among unsaturated WEs were major waxes of mouse meibum with m/z 603.53 and 605.63 (C41:2 and C41:1), 617.62 and 619.64 (C42:2 and C42:1), 633.65 (C43:1), 645.66 and 647.67 (C44:2 and C44:1), and 661.69 (C45:1), whereas their shorter and longer counterparts did not change much, or increased slightly, such as compounds with m/z 703.73 and 731.76 (C48:1 and C50:1) (Fig. 5D, E). The deepest reduction in molecular ratios (>50%) was observed for odd-numbered compounds (such as C41:1, C43:1, and C45:1 WEs), whereas even-numbered WEs (C42:1 and C44:1) underwent an ∼25% decline. The trend reversed itself at compounds C46:1 and longer, apparently because of the activity of other ELOVLs (see Discussion).
Figure 5.
Effects of the Elovl3 mutation on WEs of the mouse TPs. A) Representative observation high-resolution mass spectrum of the WE fraction of E3wt TPLs. B) Representative observation high-resolution mass spectrum of the WE fraction of E3hom TPLs. C) The overall balance of total saturated WEs (SWEs), MUWEs, DUWEs, and TUWEs in E3wt and E3hom mice. D) Molecular distribution of the SWE species of the TPLs of experimental animals. E) Molecular distribution of the MUWE species of the TPLs of experimental animals. F) Molecular distribution of the DUWE species of the TPLs of experimental animals. G) The overall balance of WE species TPLs of experimental animals.
A strong effect of the Elovl3 mutation was observed for DUWEs (Fig. 5F). Waxes with even-numbered carbon chains (C40:2 to C48:2) increased by almost 100%, on average, whereas the odd-numbered WEs remained virtually unchanged. This increase was one of the primary causes of the overall shift of TPLs toward less saturated WEs.
The overall amount of TUWEs was very low in TPs of both types of mice (about 1% of the total WE fraction in E3wt mice and 2% in E3hom). Because of their low abundance, only major species with m/z values of 699.70 (C48:3), 713.72 (C49:3), and 727.73 (C50:3) were possible to be accounted for. Again, as with DUWEs, the largest increase was observed for the even-numbered WEs C48:3 and C50:3, whereas the C49:3 wax ester was not affected. A general overview of major MUWEs, DUWEs, and TUWEs detected in TPs of E3wt and E3hom mice is provided in Fig. 5G.
Lipid analyses: CEs
CEs of mouse TPs is a major and diverse group of lipids that is based on saturated (both straight chain and branched) and unsaturated straight-chain FAs, with the chain lengths ranging from roughly C14 to C34 (3). This could make them susceptible to the effects of inactivating mutations of Elovls in general and Elovl3 specifically. Thus, their profiles in E3wt and E3hom TPL samples needed to be evaluated.
As our first step, we attempted to detect the intact CEs in mouse TPL samples. The inherent instability of CEs in the conditions of mass spectrometric analysis led to low but still detectable levels of their (M + H)+ and some other adducts (Supplemental Fig. S2 and Supplemental Table S2). Importantly, the MS behavior of saturated CEs (SCEs) differed from that of monounsaturated CEs (MUCEs) in 2 respects: SCEs showed much weaker signals than MUCEs of the equivalent carbon chain length and concentration, and the ability of SCEs to form (M + H)+ adducts did not depend on their FA length, whereas MUCE demonstrated an almost exponential increase in the (M + H)+ signal intensity with the increase in the unsaturated FA chain length. In the case of SCE, the relative insensitivity of the instrument’s response to the length of esters allowed for a (cautious) extrapolation of the data beyond the tested range. However, a limited number of available authentic MUCE standards, and a steep increase in their signals, made extrapolation of the data beyond the C16:1 to C24:1 range unreliable. On the contrary, the virtual linearity of the SCE response curve could be used to (cautiously) extrapolate the results to longer-chain CEs. Notably, the retention times of most SCEs from both wild-type and mutant mice were systematically shorter than those of authentic isobaric straight chain CE standards, whereas MUCEs coeluted with their straight chain standards (unpublished results).
The distribution of MUCE did not significantly change in response to the Elovl3 mutation (Fig. 6). Noted were an incremental increase in C16:1- and C18:1-CEs, and a minimal decline of C22:1-, C24:1-, and C26:1-CEs. At the same time, CEs in the C28:1 to C36:1 range remained unchanged. These alterations in the MUCE profiles were not nearly as dramatic as changes in the SCE profiles, in which a sharp increase in C16:0- to C19:0-CEs was observed, along with a simultaneous obliteration of SCEs with C21:0 and longer FA moieties. The tipping point in this change was the C20:0-CE.
Figure 6.
Characterization of CEs of the mouse E3hom TPs. A) Representative EIC of an analytical ion m/z 369.35 specific to Chl and all CEs. B) Representative EIC of an analytical (M + H)+ ion m/z 791.76 specific to a C28:1-FA–based cholesterol ester with MW of 792 Da. Insert: mass spectrum of the analyte with retention time of 32.7 min produced 2 major signals with m/z values of 369.3531 and 791.7630 specific to the ester. C) Molecular distribution of the SCE species in the TPL extracts of homozygous and wild-type mice. The compounds were detected as (M + H)+ adducts. D) Molecular distribution of the MUCE species in the TPL extracts of homozygous and wild-type mice. The compounds were detected as (M + H)+ adducts.
The pairwise comparison of identical (M + H)+ adducts of CEs of E3wt and E3hom mice provided important information on the changes in the CE profiles. However, direct comparison of SCE and MUCE pairs was hampered by the nonlinear response of the mass spectrometric detector to the elongation of the FA chains of MUCEs. Conveniently, all CEs could also be observed and monitored in UPLC experiments using their common analytical ion with m/z value of 369.35 (Chl – H2O + H)+, which was formed due to spontaneous in-source fragmentation of CEs with a neutral loss of their FA moieties. Because the intensity of the signal m/z 369.35 in chosen conditions is virtually independent of the length and degree of unsaturation of the FA moieties of CEs (5, 21, 22), the UPLC peak areas of the corresponding CE analytes could be used to determine their relative abundances in the samples. However, because of the very close retention times, or virtual coelution, of CE pairs Cn:0/C(n+2):1 (i.e., SCEs and MUCEs differing by 2 carbons), separate quantitation of individual esters within each pair was impossible, and their combined peak areas had to be used instead.
Representative EIC of CEs of E3wt and E3hom TPLs are shown in Fig. 7. The first major observation was a shift in the elongation pattern of mouse CEs: the E3hom TPL samples revealed a large increase in shorter-chain CEs (C16–C19) and a decrease in VLC-CEs (C20–C27) compared with E3wt and E3het mice. By integrating the chromatographic peaks of the common ion m/z 369.35 (Fig. 7) and those of proton adducts of individual CEs of the (M + H)+ type (Fig. 6), the apparent abundances of various CE species were determined. It became clear that the homozygous Elovl3 mutation affected mostly SCEs, with an almost complete loss of products with C21:0 and above: no compounds of this nature were observed in E3hom samples above the noise level. Importantly, extremely long-chain (ELC) MUCEs in the C24:1 to C34:1 range remained virtually unaffected. A C20:0-CE/C22:1-CE pair (Fig. 7) seemed to be a tipping point for the change. A comparative analysis of E3wt, E3het, and E3hom samples demonstrated that the effect of the E3het mutation on meibomian lipids was about half of that of the E3hom mutation.
Figure 7.
Effects of the Elovl3 mutation on CEs of the mouse TPs. A) Representative EIC of ion m/z 369.35 detected in a E3wt mouse TPL extract. The chemical nature of analytes in chromatographic peaks was identified as Chl (1); C14:0- and C16:1-CE (2); C16:0- and C18:1-CE (3); C17:0-CE (4); C18:0- and C20:1-CE (5); C19:0-CE (6); C20:0- and C22:1-CE (7); C21:0-CE (8); C22:0- and C24:1-CE (9); C23:0-CE (10); C24:0- and C26:1-CE (11); C25:0-CE (12); C26:0- and C28:1-CE (13); C27:0-CE (14); C28:0- and C30:1-CE (15); C30:0- and C32:1-CE (16); and C34:1-CE (17). B) Representative EIC of ion m/z 369.35 detected in a E3hom mouse TPL extract. Note the change in the balance of CEs that led to the accumulation of shorter-chain CEs with shorter retention times and a decline in longer-chain compounds with longer retention times. C) The overall balance of CE species in TPLs of homozygous, heterozygous, and wild-type mice.
Lipid analyses: Chl-OAHFAs
Chl-OAHFA is the second major type of CE in human meibum and animal meibum and TP extracts (2–5, 23, 24). Chl-OAHFA can be monitored similarly to CE, either as (M + H)+ ions or as the analytical ion m/z 369.35. However, apparently more favorable ionization conditions in the IonSabre APCI ion source of the Synapt G2-Si quadropole ToF instrument used in this study compared with the LCQ Deca XP Max utilized in our earlier projects, led to a weaker spontaneous fragmentation of Chl-OAHFA in our current experiments. Consequently, this effect led to a higher abundance of (M + H)+ adducts, which facilitated their direct evaluation (Fig. 8 and Supplemental Table S2), though at the expense of a lower abundance of ions m/z 369.35. The following observations were made. First, Chl-OAHFAs were easily detectable in all study samples as their (M + H)+ adducts. Also, visible were products of their spontaneous in-source fragmentation, such as fragments of corresponding (O)-acylated ω-hydroxy FAs (OAHFAs), such as major ions 731.69, 759.72, and 787.74, and their corresponding dehydration products 713.67, 741.71, and 769.74. Second, the relative balance of major Chl-OAHFA changed in response to the Elovl3 mutation. Specifically, the abundance of saturated Chl-OAHFA in the entire pool of saturated and unsaturated Chl-OAHFA of the same length dropped from ∼7.5 to 1%, whereas the intergroup ratios of mono-, di-, and triunsaturated compounds remained virtually unchanged (Fig. 8D). This result duplicated the effect of the Elovl3 mutation on SCEs and WEs.
Figure 8.
Effects of the Elovl3 mutation on CEs of OAHFA of the mouse TPs. A1, A2) EIC of a diunsaturated Chl-OAHFA with m/z 1100.03 and its corresponding mass spectrum with characteristic fragments 369.35, 713.68, and 731.69 formed in-source because of the spontaneous fragmentation of the large, unstable molecule of Chl-OAHFA. B1, B2) EIC of a diunsaturated Chl-OAHFA with m/z 1128.06 and its corresponding mass spectrum with characteristic fragments 369.35, 741.71, and 759.72. C1, C2) EIC of a diunsaturated Chl-OAHFA with m/z 1156.09 and its corresponding mass spectrum with characteristic fragments 369.35, 769.74, and 787.75. D1–D4) The overall balance of Chl-OAHFA species in TPLs of mutant and wild-type mice. Saturated (D1); monounsaturated (D2); diunsaturated (D3); triunsaturated (D4) Chl-OAHFA.
Lipid analyses: amphiphilic lipids
ELC OAHFAs (25, 26) have been found in meibum of humans (2, 3, 17, 25–27), rabbits (2), canines (4), and mice (2, 3, 5, 28). This diverse group of compounds is present in meibum in at least 2 forms: as free acids and as Chl-OAHFA. The latter group has been described in the previous section of the paper. Here, we will compare the OAHFA composition of E3wt and E3hom TPL.
Being free FAs (i.e., anionogenic compounds), this group of lipids produces 5–10 times stronger signals of (M – H)− type than those of (M + H)+ type. An additional benefit of running NIM MS is a much lower number of MS signals because most of meibomian lipids are nonionic compounds that easily form proton adducts, which results in very crowded spectra in PIM. Thus, running experiments in the NIM was a method of choice for analyzing OAHFA.
The general molecular formula of OAHFA is CnH2n-mO4, where n typically is in the 38 to 56 range, whereas m = 2, 4, 6, 8, or 10 (mono, di-, tri, tetra-, and pentaunsaturated OAHFAs, respectively). The vast majority of OAHFAs are of a mono-, di-, and triunsaturated nature. Fully saturated OAHFAs, in our experiments, were not detected. Two of the most common unsaturated OAHFAs in TPL are compounds with m/z values of 783.72 and 811.75 (Fig. 9). Together, these compounds represent up to 40% of the entire OAHFA pool in TPL. Importantly, the Elovl3 mutation did not affect the molecular distribution of OAHFA in the TPs; the abundances of various OAHFA in mutant mice were the same as in wild-type animals. Notable was the rather high parallelism in the distribution patterns of all tested OAHFA species except for the compound with m/z 835.75 (molecular formula C56H100O4), which varied considerably from sample to sample.
Figure 9.
Effects of the Elovl3 mutation on OAHFAs of the mouse TPs. A1, A2) EIC of a triunsaturated OAHFA with m/z 811.75 and its mass spectrum obtained in NIM. Conditions: APCI, detected as (M − H)− ion. B1, B2) EIC of a triunsaturated OAHFA with m/z 783.72 and its mass spectrum obtained in NIM. Conditions: APCI, detected as (M − H)− ion. C) Molecular distribution of OAHFA species in TPLs of mutant and wild-type mice.
DISCUSSION
A generalized diagram that sums up our lipidomic findings is shown in Fig. 10. Based on the lipid analyses of study samples, it is obvious that Elovl3 mutation has a rather selective impact on meibogenesis in mice, affecting only certain groups of compounds within WE, CE, OAHFA, and Chl-OAHFA families of meibomian lipids. Let’s discuss these changes and their probable causes and consequences in more detail.
Figure 10.
Effects of the Elovl3 mutation on metabolism of WEs, CEs, OAHFAs, and CEs of OAHFAs in the MGs of mice.
ELOVL3 has been proposed to be responsible for elongation of saturated and unsaturated C20–C24-FAs (8, 12, 29). Interestingly, Kihara considered ELOVL3 to elongate C18:1 oleic acid to make a C20:1 gondoic acid, leaving the function of making a C22:1 erucic acid to ELOVL1 and assigning elongation of saturated C16:0 –C22:0 to both enzymes (30). However, in a recent study by Sassa et al. (7) this idea was modified, now stating that ELOVL1 was responsible primarily for production of saturated VLC-FA, whereas ELOVL1, ELOVL3, and ELOVL4 were responsible for production of monounsaturated ones. Thus, these controversial possibilities needed to be re-evaluated in direct experiments using Elovl3-knockout mice.
The most direct effect of the Elovl3 mutation on meibogenesis was expected to be seen in the family of CEs (Fig. 11). The main reasons for this assumption were as follows: 1) the high abundance of CEs in meibum in general; 2) the fact that CEs are direct products of FA esterification to cholesterol; and 3) the fact that the CE family is formed of CEs ranging from C14 to C36, making them ideal subjects for evaluating the effects of the mutation.
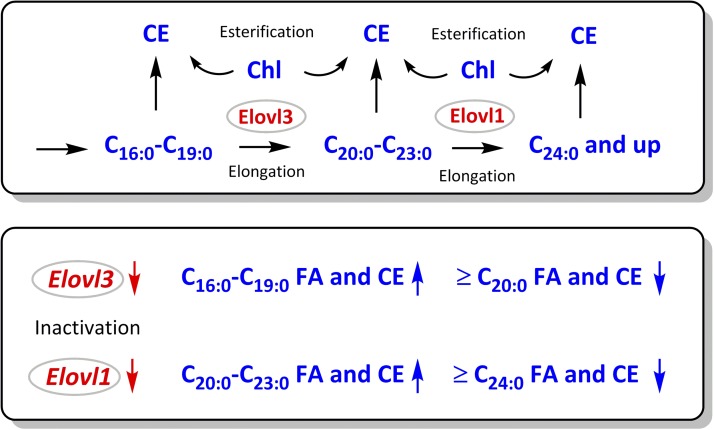
Figure 11.
Deciphering the roles of ELOVL1 and ELOVL3 in elongation of FAs in the MGs of mice using CEs as an example. Upper panel: the sequence of events in FA elongation in MGs of mice. Lower panel: specific effects of Elovl3 and Elovl1 inactivation on lipid profiles in MGs.
Indeed, our results clearly demonstrated a rather minor effect of the mutation on the intrasample balance of MUCEs and an almost complete obliteration of SCEs longer than C20:0 (Figs. 6 and 7). This observation was more in line with the reports of Westerberg et al. (29) and Zadravec et al. (12) than with the conclusions of Sassa et al. (7).
Another group of major meibomian lipids, Chl-OAHFA, demonstrated the same response to the mutation: saturated members of the Chl-OAHFA family were severely suppressed, whereas mono-, di-, and triunsaturated compounds remained unchanged (Fig. 8). The overall effect of the mutation on this group of lipids was an obvious increase in the degree of unsaturation of Chl-OAHFA.
Importantly, the retention times of many mouse SCEs, including those affected by the Elovl3 mutation, were systematically shorter than the retention times of authentic isobaric straight-chain SCEs. This effect can be explained only by assuming that mouse CEs were branched. This observation makes it possible that ELOVL3 elongates not only straight-chain lipids but also branched ones because branching of FA occurs upstream of the ELOVL3-catalyzed reactions. As no standards of branched SCEs are available, we were unable to test this hypothesis in our current experiments.
A similar observation with regard to branching was made while studying WE (Fig. 5), though the effect of the Elovl3 mutation on WE was partially masked by the fact that these lipids are based on rather short FAs of the C12 to C20–FA family (with the major members being C16, C17, and C18 ones) and ELC fatty alcohols (FAls) with 20–34 carbons (please note that both shorter and longer FA and FAl fragments may exist in some minor members of the WE family). Thus, the end effect of Elovl3 mutation may be mediated (or modulated) by other enzymes that are downstream of ELOVL3 in meibogenesis or by those that have (partially) overlapping substrate and product specificities. One of these reactions is the enzymatic reduction of FA carboxyls by, presumably, a fatty acyl-CoA reductase (30, 31), which is a separate step shown in Fig. 10 whose mechanism has not been evaluated for ELC lipids yet.
Another complicating factor in the analysis of the WE data is the variable degree of (un)saturation of WEs: their FA and FAl can be (and are) found as saturated, mono-, and diunsaturated moieties, in different combinations. Thus, the actual effects of the Elovl3 mutation on the degree of (un)saturation of TPL might be obscured by some other (unidentified at this time) factors. However, fully saturated WEs (Fig. 5D) demonstrated a clear and systematic decline in the C38:0–C45:0 group (total carbon chain length) and remained relatively unchanged in the C47:0–C50:0 group. Taking into account that the major saturated FAs in WE are C16:0-, C17:0-, and C18:0-FAs, the mutation severely impaired the biosynthesis of FAls with chain lengths between C20:0 and C29:0 or so.
DUWEs demonstrated a different trend, with an almost 2-fold increase in the fraction regardless of the total carbon chain length, but only for even-chained members; the odd-chain compounds were not affected by the mutation and were a minor group of the subfamily (Fig. 5F). Interestingly, MUWEs showed a combination of both of the above trends: a decline in C40:1 to C45:1 compounds and an increase in C46:1 and above (Fig. 5E). Finally, TUWEs, though detected, were such a minor subfamily of the total pool of WEs (Fig. 5C) that their detailed characterization was deemed unnecessary at this time. The observed changes resulted in an overall increase in the degree of unsaturation of the WE fraction of TPLs (Fig. 5C). We attribute the shift in product specificity of the elongation reactions to the activity of other enzymes, which may possibly utilize the same precursors as ELOVL3, to make other types of products, such as DUWE and TUWE.
Unfortunately, a direct comparison of our data with the results of Sassa et al. (7), which would help in establishing the roles of Elovl1/ELOVL1 in these processes, was not possible because of the methodology chosen in the latter study: Sassa et al. assumed that the main FAs in WEs of mouse meibum were based on a C18:1 (oleic) acid and designed their experimental protocols to detect those compounds only, although in fact, mouse meibomian WEs are based primarily on a C16:1-FA (presumably, palmitoleic acid), as well as smaller amounts of C15:0-C18:0– and C18:1-FA (3, 5, 6). All of those compounds (except for oleic acid-based WE) remained undetected in the experiments of Sassa et al. Thus, the results of Sassa et al. with regard to meibomian WE are incomplete, and the conclusions with regard to the roles of Elovl1/ELOVL1 need to be revisited in future studies.
Finally, the makeup of the OAHFA family remained relatively unchanged (Fig. 9), apparently because this group had no detectable fully saturated members in either mutant or wild-type lines of mice, which would be expected to change if they were present in quantities above the low limit of detection. This observation was in line with data of Sassa et al. (7), who reported no saturated OAHFA either.
The results presented in this paper shed new light on the functions of ELOVL3 in MGs and its substrate and product specificity. It seems that ELOVL3 is involved in the elongation of saturated FAs (including branched and odd-chain ones) much more than in the biosynthesis of unsaturated FAs. The Elovl3-inactivating mutation leads to the accumulation of CEs, which are directly based on the most probable ELOVL3 substrates (FAs with C16:0 to C19:0 carbon chain lengths). Simultaneously, the expected products of ELOVL3 (such as C20:0-FA and above) are either reduced or obliterated in the CE and Chl-OAHFA groups of TPLs (Figs. 6–8 and 11). The relative inability of the Elovl3 mutation to suppress formation of MUCEs and Chl-OAHFAs as well as di- and triunsaturated OAHFAs, a rather muted effect on the production of MUWEs, and an increase in production of DUWEs (Figs. 5–9) imply the involvement of other enzymes of the ELOVL family in the process of their synthesis.
Our observations regarding the effects of Elovl3 mutation on mouse meibum should be considered together with observations of Sassa et al. (7) concerning the Elovl1 mutation (5, 7). It seems that ELOVL3 acts upstream of ELOVL1 in terms of its effects on FA elongation in MGs. Using CEs as marker compounds (Fig. 6 of this manuscript and Fig. 2 of the paper of Sassa et al.), one can see that inactivation of Elovl3 leads to accumulation of C16:0- to C19:0-CEs, whereas inactivation of Elovl1 results in an increase in C20:0- to C23:0-CEs, a generalized mechanism of which is illustrated in Fig. 11. Contrary to the findings of Sassa et al., ablation of ELOVL3 does not inhibit the biosynthesis of monounsaturated VLC-FA and ELC-FA, as profiles of unsaturated CEs, Chl-OAHFAs, and OAHFAs were not altered, whereas the effects of the mutation on WEs were moderate and, with all likelihood, mediated by other enzymes involved in their biosynthesis. A certain specificity of the effects (such as a clearly stronger suppression of odd-chain WEs of the C41:1, C43:1, and C45:1 types, Fig. 5) needs to be evaluated in future studies, but it is likely to be related not only to the ELOVL3 enzyme but also to other enzymes that synthesize and esterify FAls.
In summary, Elovl3/ELOVL3 play central roles in meibogenesis by regulating the lengths and degree of unsaturation of meibomian lipids. Both factors may have a direct effect on the quality of MG secretions: shorter lipids and lipids with a higher degree of unsaturation have lower melting temperatures and, if present in abnormally high ratios, may change the fluidity and other properties of meibum beyond normal ranges, thus destabilizing the tear film and causing the loss of its protective properties. Inactivation of Elovl3 has serious physiologic consequences, as it leads to an abnormal ocular phenotype such as the increased blink rate and tear production, accumulation of lipid discharge (crusting) around the eyes of E3hom mice, and eyelid edema. These conditions in mutant mice seem to resemble those in human subjects with various forms of dry eye, MG dysfunction, and blepharitis. Additional studies are needed to determine the causative relationships between the observed changes, but we are confident that Elovl3/ELOVL3-deficient mice can be instrumental in studying the fundamentals of lipid metabolism in the eye and adnexa, as well as various human ocular surface pathologies.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported, in part, by U.S. National Institutes of Health, National Eye Institute Grant R01EY024324 (to I.A.B.), an unrestricted grant from Research to Prevent Blindness (New York, NY, USA), and also, in part, by a 2219–International Postdoctoral Research Scholarship from the Scientific and Technological Research Council of Turkey (TÜBITAK; Ankara, Turkey) to S.Y. The authors declare no conflicts of interest.
Glossary
- ACADS
acyl-CoA dehydrogenase, short chain
- APCI
atmospheric pressure chemical ionization
- Burf
buttery rumpled fur
- CE
cholesteryl ester
- Chl
free cholesterol
- Chl-OAHFA
CE of (O)-acylated ω-hydroxy FAs
- DUWE
diunsaturated WE
- E3het
Elovl3-knockout mice (heterozygous)
- E3hom
Elovl3-knockout mice (homozygous)
- E3wt
Elovl3–wild type mice
- EIC
extracted ion chromatogram
- ELC
extremely long chain
- ELOVL
elongase of VLC-FAs
- FA
fatty acid
- FAl
fatty alcohol
- LLoD
lower limit of detection
- MG
Meibomian gland
- MS
mass spectrometry
- MUCE
monounsaturated CE
- MUWE
monounsaturated WE
- NIM
negative ion mode
- OAHFA
(O)-acylated ω-hydroxy FA
- PIM
positive ion mode
- SCE
saturated CE
- TIC
total ion chromatogram
- ToF
time-of-flight
- TP
tarsal plate
- TPL
TP lipid
- TUWE
triunsaturated WE
- UPLC
ultra-high-performance liquid chromatography
- VLC
very long chain
- WE
wax ester
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
I. A. Butovich designed the project, planned and performed research, analyzed data, and wrote the manuscript; A. Wilkerson, N. Bhat, and A. McMahon planned and performed research; S. Yuksel (a postdoctoral trainee) helped I. A. Butovich with HPLC/MS experiments; A. Wilkerson, N. Bhat, and A. McMahon participated in writing the manuscript; and all authors reviewed the final version of the manuscript.
REFERENCES
- 1.Nicolaides N., Kaitaranta J. K., Rawdah T. N., Macy J. I., Boswell F. M., III, Smith R. E. (1981) Meibomian gland studies: comparison of steer and human lipids. Invest. Ophthalmol. Vis. Sci. 20, 522–536 [PubMed] [Google Scholar]
- 2.Butovich I. A., Lu H., McMahon A., Eule J. C. (2012) Toward an animal model of the human tear film: biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Invest. Ophthalmol. Vis. Sci. 53, 6881–6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butovich I. A., McMahon A., Wojtowicz J. C., Lin F., Mancini R., Itani K. (2016) Dissecting lipid metabolism in meibomian glands of humans and mice: an integrative study reveals a network of metabolic reactions not duplicated in other tissues. Biochim. Biophys. Acta 1861, 538–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butovich I. A., Borowiak A. M., Eule J. C. (2011) Comparative HPLC-MS analysis of canine and human meibomian lipidomes: many similarities, a few differences. Sci. Rep. 1, 24 DOI: 10.1038/srep00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butovich I. A., McMahon A., Wojtowicz J. C., Bhat N., Wilkerson A. (2019) Effects of sex (or lack thereof) on meibogenesis in mice (Mus musculus): comparative evaluation of lipidomes and transcriptomes of male and female tarsal plates. [E-pub ahead of print] Ocul. Surf. 10.1016/j.jtos.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butovich I. A. (2017) Meibomian glands, meibum, and meibogenesis. Exp. Eye Res. 163, 2–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sassa T., Tadaki M., Kiyonari H., Kihara A. (2018) Very long-chain tear film lipids produced by fatty acid elongase ELOVL1 prevent dry eye disease in mice. FASEB J. 32, 2966–2978 [DOI] [PubMed] [Google Scholar]
- 8.Guillou H., Zadravec D., Martin P. G., Jacobsson A. (2010) The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog. Lipid Res. 49, 186–199 [DOI] [PubMed] [Google Scholar]
- 9.Fehrenschild D., Galli U., Breiden B., Bloch W., Schettina P., Brodesser S., Michels C., Günschmann C., Sandhoff K., Niessen C. M., Niemann C. (2012) TCF/Lef1-mediated control of lipid metabolism regulates skin barrier function. J. Invest. Dermatol. 132, 337–345 [DOI] [PubMed] [Google Scholar]
- 10.Defour M., Dijk W., Ruppert P., Nascimento E. B. M., Schrauwen P., Kersten S. (2018) The Peroxisome Proliferator-Activated Receptor α is dispensable for cold-induced adipose tissue browning in mice. Mol. Metab. 10, 39–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westerberg R., Månsson J. E., Golozoubova V., Shabalina I. G., Backlund E. C., Tvrdik P., Retterstøl K., Capecchi M. R., Jacobsson A. (2006) ELOVL3 is an important component for early onset of lipid recruitment in brown adipose tissue. J. Biol. Chem. 281, 4958–4968 [DOI] [PubMed] [Google Scholar]
- 12.Zadravec D., Brolinson A., Fisher R. M., Carneheim C., Csikasz R. I., Bertrand-Michel J., Borén J., Guillou H., Rudling M., Jacobsson A. (2010) Ablation of the very-long-chain fatty acid elongase ELOVL3 in mice leads to constrained lipid storage and resistance to diet-induced obesity. FASEB J. 24, 4366–4377 [DOI] [PubMed] [Google Scholar]
- 13.Gudjonsson J. E., Ding J., Johnston A., Tejasvi T., Guzman A. M., Nair R. P., Voorhees J. J., Abecasis G. R., Elder J. T. (2010) Assessment of the psoriatic transcriptome in a large sample: additional regulated genes and comparisons with in vitro models. J. Invest. Dermatol. 130, 1829–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu C., Wang G., Hao Y., Zhi J., Zhang L., Chang C. (2011) Correlation analysis between gene expression profile of rat liver tissues and high-fat emulsion-induced nonalcoholic fatty liver. Dig. Dis. Sci. 56, 2299–2308 [DOI] [PubMed] [Google Scholar]
- 15.Amendt B. A., Freneaux E., Reece C., Wood P. A., Rhead W. J. (1992) Short-chain acyl-coenzyme A dehydrogenase activity, antigen, and biosynthesis are absent in the BALB/cByJ mouse. Pediatr. Res. 31, 552–556 [DOI] [PubMed] [Google Scholar]
- 16.Kelly C. L., Wood P. A. (1996) Cloning and characterization of the mouse short-chain acyl-CoA dehydrogenase gene. Mamm. Genome 7, 262–264 [DOI] [PubMed] [Google Scholar]
- 17.Butovich I. A., Uchiyama E., McCulley J. P. (2007) Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J. Lipid Res. 48, 2220–2235 [DOI] [PubMed] [Google Scholar]
- 18.Butovich I. A. (2009) Lipidomic analysis of human meibum using HPLC-MSn. Methods Mol. Biol. 579, 221–246 [DOI] [PubMed] [Google Scholar]
- 19.Butovich I. A. (2008) On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest. Ophthalmol. Vis. Sci. 49, 3779–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butovich I. A., Arciniega J. C., Lu H., Molai M. (2012) Evaluation and quantitation of intact wax esters of human meibum by gas-liquid chromatography-ion trap mass spectrometry. Invest. Ophthalmol. Vis. Sci. 53, 3766–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butovich I. A. (2009) Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J. Lipid Res. 50, 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butovich I. A. (2010) Fatty acid composition of cholesteryl esters of human meibomian gland secretions. Steroids 75, 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J., Green-Church K. B., Nichols K. K. (2010) Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Invest. Ophthalmol. Vis. Sci. 51, 6220–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolaides N., Santos E. C. (1985) The di- and triesters of the lipids of steer and human meibomian glands. Lipids 20, 454–467 [DOI] [PubMed] [Google Scholar]
- 25.Butovich I. A., Wojtowicz J. C., Molai M. (2009) Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J. Lipid Res. 50, 2471–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butovich I. A. (2010) On the presence and role of polar lipids in meibum. Invest. Ophthalmol. Vis. Sci. 51, 6908–6910, author reply 6910–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butovich I. A. (2011) On the presence of (O-acyl)-omega-hydroxy fatty acids and of their esters in human meibomian gland secretions. Invest. Ophthalmol. Vis. Sci. 52, 639–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butovich I. A., Lu H., McMahon A., Ketelson H., Senchyna M., Meadows D., Campbell E., Molai M., Linsenbardt E. (2014) Biophysical and morphological evaluation of human normal and dry eye meibum using hot stage polarized light microscopy. Invest. Ophthalmol. Vis. Sci. 55, 87–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westerberg R., Tvrdik P., Undén A. B., Månsson J. E., Norlén L., Jakobsson A., Holleran W. H., Elias P. M., Asadi A., Flodby P., Toftgård R., Capecchi M. R., Jacobsson A. (2004) Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J. Biol. Chem. 279, 5621–5629 [DOI] [PubMed] [Google Scholar]
- 30.Kihara A. (2012) Very long-chain fatty acids: elongation, physiology and related disorders. J. Biochem. 152, 387–395 [DOI] [PubMed] [Google Scholar]
- 31.Cheng J. B., Russell D. W. (2004) Mammalian wax biosynthesis. I. Identification of two fatty acyl-Coenzyme A reductases with different substrate specificities and tissue distributions. J. Biol. Chem. 279, 37789–37797 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.