Abstract
Myoglobin (Mb) maturation involves heme incorporation as a final step. We investigated a role for heat shock protein (hsp) 90 in Mb maturation in C2C12 skeletal muscle myoblasts and cell lines. We found the following: 1) Hsp90 directly interacts preferentially with heme-free Mb both in purified form and in cells. 2) Hsp90 drives heme insertion into apoprotein-Mb in an ATP-dependent process. 3) During differentiation of C2C12 myoblasts into myotubes, the apo-Mb-hsp90 complex associates with 5 cell cochaperons, Hsp70, activator of hsp90 ATPase protein 1 (Aha1), alanyl-tRNA synthetase domain containing 1 (Aarsd1), cell division cycle 37 (Cdc37), and stress induced phosphoprotein 1 (STIP1) in a pattern that is consistent with their enabling Mb maturation. 4) Mb heme insertion was significantly increased in cells that had a functional soluble guanylyl cyclase (sGC)-cGMP signaling pathway and was diminished upon small interfering RNA knockdown of sGCβ1 or upon overexpression of a phosphodiesterase to prevent cGMP buildup. Together, our findings suggest that hsp90 works in concert with cochaperons (Hsp70, Aha1, Aarsd1, STIP1, and Cdc37) and an active sGC-cGMP signaling pathway to promote heme insertion into immature apo-Mb, and thus generate functional Mb during muscle myotube formation. This fills gaps in our understanding and suggests new ways to potentially control these processes.—Ghosh, A., Dai, Y., Biswas, P., Stuehr, D. J. Myoglobin maturation is driven by the hsp90 chaperone machinery and by soluble guanylyl cyclase.
Keywords: hemeprotein, myoblasts, myotubes
The hemeprotein myoglobin (Mb) is expressed in the muscle tissue of vertebrates (1–4), where it functions to store oxygen (2–8). A high level of muscle Mb expression allows certain mammals to hold their breath for longer time periods, as occurs in diving mammals like whales, seals, or dolphins (9–12). Genetic depletion of Mb in mice induces several compensatory mechanisms that partly overcome its loss and thus leads to viable and fertile mice (13). Mb was the first protein to be characterized by X-ray crystallography and is one of the most studied proteins in biology (14, 15). Despite this, our understanding of its maturation process is poor. In particular, we do not understand the mechanisms that enable and regulate its post-translational heme insertion, despite this being a critical step for generating mature and functional Mb.
Heat shock protein (hsp) 90 is a molecular chaperone that regulates the cellular stress response by maintaining the conformation, stability, and function of numerous client proteins (16–19). Hsp90 also assists in the ligand binding of diverse protein clients, including steroid-binding proteins (20, 21), the arylhydrocarbon receptor (22), kinases (23, 24), the hemeproteins NO synthase (25), soluble guanylyl cyclase (sGC) β subunit (26), and hemoglobins (Hbs) β and γ (27). Several lines of evidence also suggest important roles for hsp90 in muscle physiology, including myofibril assembly (28, 29), muscle fiber lineages (30), and myogenic differentiation (31). Our recent finding that hsp90 assists Hb maturation prompted us to study mechanisms of Mb maturation in differentiating myoblasts. Our current study shows that hsp90 does play an essential role by directly associating with immature apoprotein (apo)-Mb and then driving its heme insertion in an ATP-dependent process. Moreover, we identify several cochaperone proteins that associate with the hsp90-apo-Mb complex both prior to and during Mb maturation, and we reveal an unexpected involvement of the cell sGC-cGMP signaling pathway in promoting the post-translational heme insertion into apo-Mb. Together, the findings significantly improve our understanding of the cell biology and regulation of Mb maturation and suggest new avenues to potentially control the process.
MATERIAL AND METHODS
Reagents
All chemicals were purchased from MilliporeSigma (Burlington, MA, USA) and Thermo Fisher Scientific (Waltham, MA, USA). Hsp90 inhibitors (radicicol, novobiocin, and AUY-922), heme biosynthesis inhibitor succinyl acetone (SA), protein synthesis inhibitor (cycloheximide), hemin, NO donor, S-nitroso-N-acetyl-D,L-penicillamine, phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine, and horse (equine) skeletal muscle Mb were all purchased from MilliporeSigma. Human Mb was purchased from Lee Biosolutions (Maryland Heights, MO, USA). Cell culture medium and fetal bovine or horse serum that were required for culture and differentiation of skeletal muscle cells (C2C12) were purchased from Thermo Fisher Scientific and Atlanta Biologicals (Flowery Branch, GA, USA). Mouse skeletal muscle myoblasts (C2C12), human embryonic kidney cells (HEK)293T, COS-7, and RFL-6 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). Human airway smooth muscle cells were provided by Dr. Rey Panettieri (Rutgers, The State University of New Jersey, New Brunswick, NJ, USA). His-tag hsp90α bacterial expression (Escherichia coli) construct, mammalian expression wild-type (WT) hsp90, and dominant negative hsp90 mutant (D88N) constructs were gifts from Dr. Bill Sessa (Yale University, New Haven, CT, USA). Bacterial expression pet15MHL plasmids containing the coding sequences of full-length human hsp90β was obtained from Dr. Tomasz Religa (Case Western Reserve University, Cleveland, OH, USA). A myoglobin (Mb) construct expressing (E. coli) whale myoglobin was provided by Dr. John Olson (Rice University, Houston, TX, USA). sGCα1, β1 expression constructs, and sGCβ1 deletion constructs (sGC-β1Δ379–408, β1Δ379–436, β1Δ204–244, β1Δ204–303, and β1Δ204–244 + Δ379–408) were given by Dr. Andreas Papapetropolous (Athens University, Athens, Greece). Mouse phosphodiesterase 5A (PDE5A) and human skeletal muscle myoglobin expression constructs were purchased from OriGene (Rockville, MD, USA). Small interfering RNA (siRNA) specific to human sGCβ1 and mouse hsp90β was purchased from GE Healthcare Dharmacon (Lafayette, CO, USA). cGMP ELISA assay kits were obtained from Cell Signaling Technology (Danvers, MA, USA). Molecular mass markers were purchased from Bio-Rad (Hercules, CA, USA).
Antibodies
Antibodies were purchased from different sources. Supplemental Table S1 describes various types of antibodies used and its source.
Bacterial purification of tagged proteins
His-tag WT hsp90α was purified from E. coli following procedures as previously described by Young et al. (32). Briefly, his-tag hsp90α was expressed in BL21 (DE3) pLysS E. coli cells and purified by chromatography on NiNTA-agarose (Qiagen, Gilden, Germany) and Mono Q Superdex 200 columns (Amersham Biosciences, Little Chalfont, United Kingdom). His-tag Hsp90β was purified following similar procedures previously described by Sarkar et al. (33).
Purification of Mb from E. coli
Sperm whale Mb was purified following protocols previously reported by Carver et al. (34) and Springer and Sligar (35). The purified Mb was converted to apo-Mb by a process involving repeated extraction of heme by cold ethyl methyl ketone (36). The apo-Mb was then dialyzed against cold water, lyophilized, and stored at −80°C. In other cases, commercial horse (equine) or human Mb was used and converted to apo-Mb following similar procedures as outlined above.
In vitro binding assay
For in vitro binding assays, histidine-tagged hsp90α protein was bound to nickel beads and incubated at 4°C for 1 h with purified myoglobin or apo-myoglobin in 300 μl of binding buffer (20 mM Tris-HCl, pH 7.6, 100 mM NaCl, 1 mM EDTA, and 0.5% NP-40). The beads bound to proteins were then washed 6 times with 1 ml of the same buffer containing 250 mM of NaCl and the protein complexes boiled with SDS loading dye/buffer before SDS-PAGE and detection by Western blot.
Flouresence polarization assays
Apo-Mb and holo-Mb were dialyzed into 0.1 M sodium bicarbonate, pH 9.3, FITC was dissolved in the bicarbonate buffer and added to the proteins at a ratio of 20 g of FITC to 1 mg of protein. After incubation for 1 h at room temperature in the dark, the reaction mixture was passed through a PD-10 column (MilliporeSigma). to capture excess FITC and to exchange the protein sample into PBS buffer. FITC labeling efficiency was calculated based on a FITC extinction coefficient of 63,000 M−1 cm−1 at 495 nm (33). FITC-labeled proteins were diluted and mixed with different amount of full-length hsp90α/β to a final concentration of 0.5 µM. Samples containing 0.5 μM Mb and various amount of hsp90 were then transferred into black-coated wells of a 96-well plate. The fluorescence polarization of each well was read by a Flexstation 3 multimode microplate reader (Molecular Devices, San Jose, CA, USA). The resulting binding curves were fitted and plotted in Origin 8 (OriginLab, Northampton, MA, USA).
Cell culture, transient transfection, growth/differentiation of cells, and gene silencing by siRNA
All cell lines were grown and harvested as previously described (25, 26, 37, 38). Cultures (50–60% confluent) of C21C2 cells expressing basal levels of Mb were treated with SA for 72 h, pretreated with hsp90 inhibitors radicicol (10 µM) or novobiocin (250 µM) for 30 min along with cycloheximide (10 μg/ml), and then given hemin (5 µM) for additional 3 h before being harvested. Control untreated cultures not receiving SA were included in all experimental setups. Similar procedures were adopted for transient transfection of Myc-Mb in SA pretreated (48 h) HEK cells, which were then cultured for additional 42 h before incubation with hemin (5 µM for 3 h) in the presence or absence of hsp90 inhibitors as outlined above. Other experiments included transfection of Myc-Mb and HA-tagged WT-Hsp90 or D88N in HEK cells in various combinations, or Myc-Mb alone in COS-7 and HEK or cotransfection of Myc-Mb with Myc-PDE5A in HEK cells. Separate experiments included cotransfections of sGCα1β1 or β1 deletion constructs (sGC-β1Δ379–408, β1Δ379–436, β1Δ204–244, β1Δ204–303 & β1Δ204–244 + Δ379–408) in COS-7 cells.
Culture and differentiation of C2C12 cells
C2C12 cells were cultured following protocols as previously described by Wagatsuma et al. (39). C2C12 myoblasts were induced to differentiate into myotubes between 0 and 96 h by growing in medium containing 2% horse serum, with medium changed every 48 h. To investigate the effect of hsp90 inhibition on muscle differentiation and myoglobin maturation, hsp90 inhibitors (radicicol, 2 µM or AUY-922, 100 nM) were added to the differentiating cultures for longer durations between 0 and 96 h (with medium replenished every 48 h) or for shorter durations [i.e., after 48 h of differentiation to cultures (5 µM radicicol added to +SA or −SA conditions)] in the presence of 5 µM hemin for an additional 3 h before harvest. Cells in all stages were imaged every 24 h and parallel cultures harvested.
Gene silencing by siRNA
For silencing of sGCβ1 in HEK cells, 80 nM of human sGCβ1-specific siRNA (Dharmacon) was transiently transfected in HEK cells for 24–48 h prior to transfection of Myc-Mb for additional 42 h before being harvested. Mouse specific hsp90β siRNA (Dharmacon) was used to down-regulate hsp90β isoform expression in C2C12 cells. The C2C12 cells were transfected with 50 nM of hsp90β siRNA or control scramble siRNA for 48 h and were then induced to differentiate for between 0 and 96 h. The cells were imaged every 24 h before being harvested.
Western blots, heme staining, and immunoprecipitations
For Western blots, standard protocols were followed as previously mentioned in Ghosh et al. (26) and Ghosh et al. (37). Heme staining of Mb heme from C2C12 myoblasts/myotubes (250 µg) or from transfected COS-7/HEK (250 µg) cells was done as previously described (25, 38). For immunoprecipitations (IPs), 500 μg of the total cell supernatant was precleared with 20 µl of protein G-sepharose beads (Amersham Biosciences) for 1 h at 4°C, beads were pelleted, and the supernatants incubated overnight at 4°C with 3 μg of anti-Mb or sGCβ1 antibodies. Protein G-sepharose beads (20 µL) were then added and incubated for 1 h at 4°C. The beads were microcentrifuged (6000 rpm), washed 3 times with wash buffer (50 mM HEPES pH 7.6, 100 mM NaCl, 1 mM EDTA, and 0.5% NP-40), and then boiled with SDS-buffer and centrifuged. For IPs involving purified proteins, Mb or apo-Mb were mixed with purified Hsp90α or Hsp90β in 1:1 stoichiometry by using 10 µg of each protein. The buffer used was 20 mM Tris-HCl pH 7.4 containing 20 mM sodium molybdate. The proteins were allowed to bind to each other for 24 h at 4°C on a mixer. IP was performed by adding 2 µg of primary antibodies to each of the tubes and allowed to bind for 16 h at 4°C on the mixer. Protein G agarose beads (20 µl each) were used to pull down antibody-protein complexes. The beads were washed thrice with buffer, boiled with SDS-buffer, and centrifuged. The supernatants were then loaded on SDS-PAGE gels and Western blotted with specific antibodies. Band intensities on westerns were quantified using ImageJ quantification software (National Institutes of Health, Bethesda, MD, USA).
cGMP ELISA
The cGMP concentration in various cell supernatants made from intact cells that were either untreated or activated by giving NO donor S-nitroso-N-acetyl-dl-penicillamine was estimated using the cGMP ELISA assay kit (Cell Signaling Technology). The cGMP concentrations as determined by ELISA was a measure of sGC activity in the cells.
UV-visible absorption spectroscopy
UV-visible absorption spectra of cell supernatants were recorded at room temperature between 350 and 700 nm on a Shimadzu spectrophotometer (Shimadzu, Kyoto, Japan). Equal amounts of total protein supernatants were used for respective wavelength scans. The heme content for Mb was determined from the Soret absorption peak at 409 or the visible absorption at 555 nm by using the extinction coefficient of 179,000 M−1 cm−1 (409 nm) or 129,000 M−1 cm−1 (555 nm) and a manipulation to account for the variable absorbance contributions that were attributable to sample turbidity. This involved creating a baseline for each scan by drawing a line that connected the absorbance values at 380 and 470 nm or between 500 and 600 nm. The additional Soret absorbance at 409 nm or the visible at 555 nm above this baseline was then used to calculate the Mb heme content.
RESULTS
Hsp90 inhibition blocks heme insertion into muscle apo-Mb
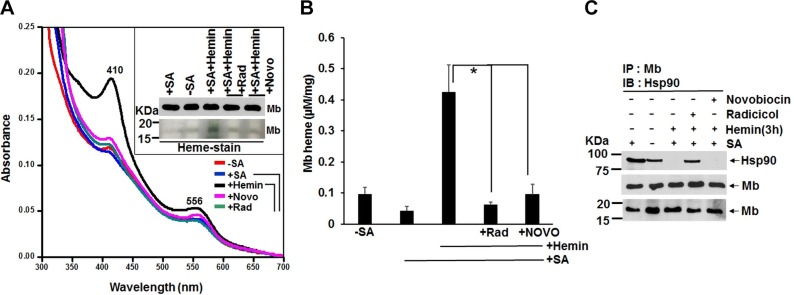
To investigate if heme insertion into muscle myoglobin is hsp90 dependent, we cultured C2C12 skeletal muscle cells with SA for 72 h to make them heme deficient and thus create a pool of apo-Mb within the cells, then added hemin for 3 h in the presence and absence of hsp90 inhibitors radicicol and novobiocin and determined the extent of heme incorporation into the apo-Mb by performing UV-visible spectroscopy and in-gel heme staining on the cell supernatants (Fig. 1A). The heme depletion treatment (+SA) diminished the Mb Soret absorbance by about half, whereas the hemin addition caused an 8-fold increase in the Soret absorbance (Fig. 1B). The increase in Soret heme absorbance correlated with an increase in the level of Mb-bound heme as detected by in-gel heme staining of the Mb protein band (Fig. 1A inset). Together, the results indicate that 1) the Soret peak absorbance in the cell supernatants primarily detects Mb heme, 2) the C2C12 myoblasts cultured under normal conditions contain a significant subpopulation of apo-Mb, and 3) the cells readily took up the provided exogenous hemin and incorporated it into their apo-Mb. The 3 h heme insertion into apo-Mb was severely inhibited by the concurrent presence of hsp90 inhibitors radicicol or novobiocin (Fig. 1A inset and Fig. 1B), without any change occurring in the Mb protein levels (Fig. 1A inset). IPs done on the supernatants (Fig. 1C) showed that hsp90 was associated with Mb under the normal culture condition (−SA) and that the hsp90 association increased under the heme-deficient condition (+SA). Radicicol and novobiocin caused either a partial or complete loss of hsp90 association to Mb, respectively, and hemin incorporation into Mb was associated with a complete loss of hsp90 association. Similar results were obtained with experiments involving transient expression of human Mb in SA pretreated HEK cells, which were then given hemin in the presence or absence of hsp90 inhibitors for 3 h (Supplemental Fig. S1). Our findings suggest that hsp90 associates with endogenous apo-Mb in the C2C12 muscle cells and with apo-Mb that is transiently expressed in HEK cells and helps to drive heme insertion into the apo-Mb in both cell types.
Figure 1.
Hsp90 inhibition blocks heme insertion into muscle Mb. C2C12 cells were heme deprived by treating with SA for 3 d, then were incubated for 3 h with 5 µM of hemin in the presence or absence of hsp90 inhibitors radicicol (5 µM) or novobiocin (250 µM), and cell supernatants (equal protein) were analyzed for absorption spectra, Mb expression, and IPs. A) Representative UV-visible spectra of the supernatants and the effect of hsp90 inhibition. The inset shows Mb protein expression levels and an in-gel heme stain of the Mb in the supernatants as indicated. B) The Mb heme content of supernatants like those depicted in A using the Soret absorbance at 410 nm. Results are means ± sd of n = 3 experiments. *P < 0.05, by 1-way ANOVA. C) Representative IP of Mb for each supernatant that compares levels of hsp90 associated to Mb (upper and middle panels; input 20% in all cases) and the total Mb in the supernatants (lower panel). Wherever applicable, MW markers (KDa) are depicted at the left of gel bands throughout the figure legends.
Hsp90 ATPase activity is critical for Mb heme insertion
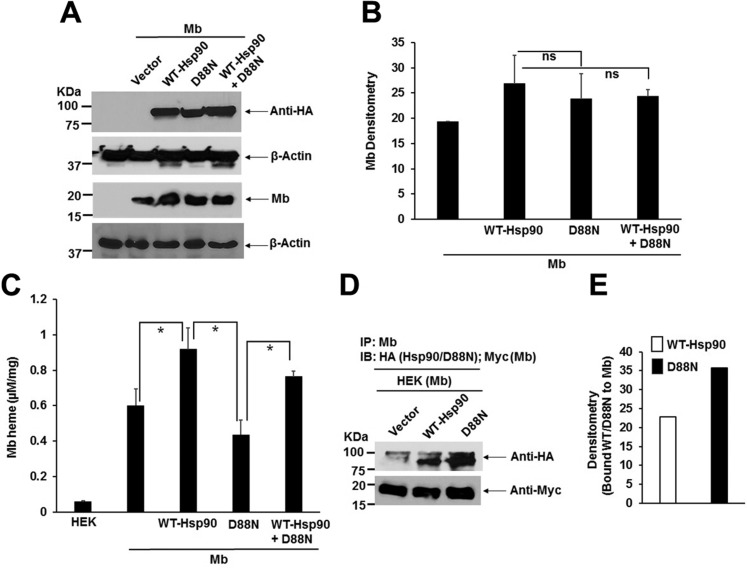
To further investigate the hsp90 dependence, HEK cells were transfected to express human skeletal muscle Mb either alone or along with HA-tagged versions of WT-Hsp90 or the ATPase-defective D88N hsp90 mutant. As shown in Fig 2A–C, the level of Mb expression did not change appreciably when it was coexpressed with WT-hsp90, but its heme content increased, suggesting this condition mildly stimulated Mb heme insertion. In contrast, coexpression of D88N hsp90 with Mb did not lead to a similar increase in the Mb heme content, and this negative effect was partly overcome by overexpressing WT hsp90 along with the D88N hsp90 mutant. IPs of the transfected HEK cell supernatants (Fig. 2D, E) showed that the HA-tagged D88N hsp90 associated with Mb to a similar or slightly greater extent than did HA-tagged WT-hsp90, consistent with there being more apo-Mb in the cells that expressed D88N hsp90. Our data indicate that hsp90 associates with apo-Mb independent of its ATPase activity and confirm that the ATPase activity of hsp90 is needed to support heme insertion into apo-Mb.
Figure 2.
An ATPase-defective hsp90 antagonizes Mb heme insertion. HEK cells were transiently transfected with Mb alone, or were cotransfected with Mb and HA-tagged WT-hsp90 or D88N hsp90 either individually or in combination, and cells were grown for 42 h before harvest and supernatant generation. The supernatants were analyzed for protein expression, absorption spectra, and IPs to determine the effect of D88N coexpression. A) Representative Western blot indicating protein expression levels. B) Densitometry of Mb expression in experiments as depicted in A. C) The calculated Mb heme content of the supernatants obtained from corresponding absorption spectra. Values are means ± sd of n = 3 experiments (*P < 0.05, by 1-way ANOVA, ns, not significant). D) Representative Western of an IP of Mb in each supernatant that compares levels of associated HA-tagged WT-hsp90 or D88N hsp90 or Mb (Myc-tagged) (input 20% in all cases). E) Densitometry of bound WT-hsp90 or D88N hsp90 associated with Mb as depicted in D (values are means of n = 2 experiments).
Hsp90 binds directly to apo-Mb to form a complex
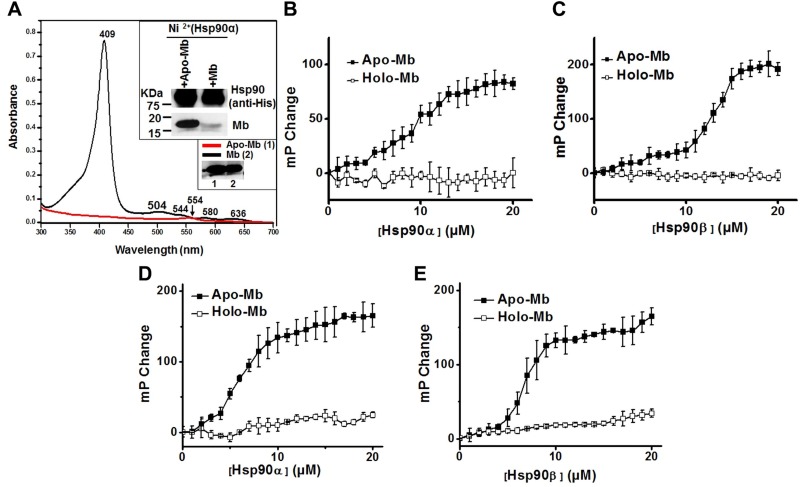
We next used purified proteins to investigate if hsp90 could directly associate with apo-Mb or with holo-Mb. Mb that was overexpressed and purified from E. coli showed a characteristic UV-visible absorbance spectrum for its bound heme, and the holo-Mb could be converted to apo-Mb by undergoing heme extraction (Fig. 3A). The apo-Mb, and to a much lesser extent the parent Mb, bound to hsp90 immobilized on Ni NTA beads (Fig. 3A inset). Hsp90α and hsp90β both bound to apo-Mb in a concentration-dependent and saturable manner when they were titrated into a constant amount of apo-Mb, but neither hsp90 form bound to holo-Mb under the same circumstance (Fig. 3B–E). This result was further confirmed in IP experiments using purified Hsp90α/β and the holo-Mb and apo-Mb proteins (Supplemental Fig. S2). These results show that apo-Mb, but not holo-Mb, can directly bind with free or immobilized hsp90α and hsp90β to form a complex.
Figure 3.
Apo-Mb but not holo-Mb binds directly to hsp90. The ability of purified Mb and hsp90 to interact directly was tested in 2 ways. A) UV-visible spectra of purified whale Mb and apo-Mb. Inset shows representative data for an assay comparing the binding of whale Mb or apo-Mb to purified human hsp90α that had been immobilized on Ni2+ affinity beads. B–E) Fluorescence polarization assays compare solution state binding of purified horse (B, C) or human (D, E) apo-Mb or Mb (1 µM) with increasing concentrations of purified human hsp90α or hsp90β, respectively. Values are means ± sd of 3 readings and representative of 2 independent trials.
Hsp90 binds to Mb in C21C2 myoblasts undergoing differentiation
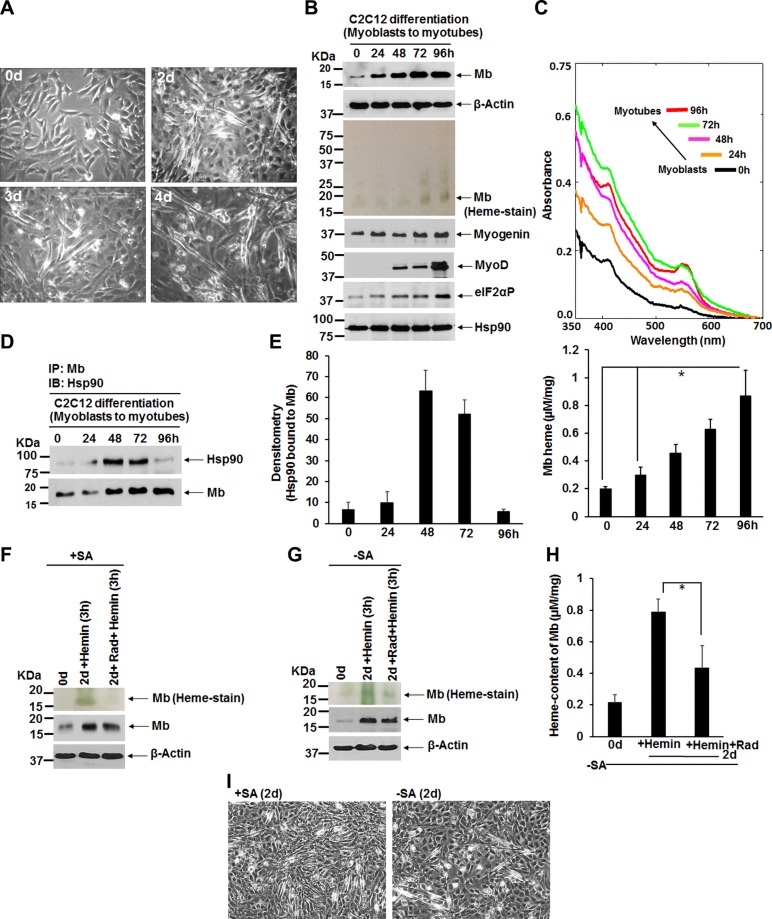
We next studied the hsp90-Mb interaction during C2C12 myoblast differentiation into myotubes. We cultured C2C12 cells in differentiation medium, which gradually transformed them over 96 h into myotubes, with an accompanying rise in Mb protein expression and Mb heme levels (Fig. 4A–C). Their differentiation was verified by the cells displaying expression of muscle-specific protein markers myogenin and increased myoD toward the end of the differentiation period (Fig. 4B) (39). Moreover, we found gradual elevated levels of phosphorylated-eIF2α (eIF2αP; Fig. 4B) during myoblast differentiation, which is indicative of stressful stimuli (40) as may prevail during the serum starved condition (2% horse serum used during myoblast differentiation). We performed IPs on cell supernatants to ascertain the level of hsp90 association with Mb during the differentiation time course, and found that the Mb-hsp90 interaction displayed a bell-shaped pattern, with the interaction peaking at 48 and 72 h and then diminishing by 96 h, corresponding with conversion of the cells into myotubes (Fig. 4A, D, E). This pattern of interaction is consistent with hsp90 interacting with newly formed apo-Mb and enabling its heme insertion over the course of the differentiation period.
Figure 4.
Hsp90 binding to Mb, Mb expression, and Mb heme content in differentiating C2C12 myoblasts and the effect of an acute period of hsp90 inhibition. C2C12 myoblasts were triggered to differentiate into myotubes by culturing in differentiating medium from 0 to 4 d. A subset of cells was pretreated for 48 h with SA (250 µM) to deplete heme stores and then allowed to differentiate. In some cases, after 2 d of differentiation the cells were treated for 3 h with 5 µM hemin in the presence or absence of hsp90 inhibitor radicicol (5 µM) before cell harvest. The cells were imaged each day, and the generated cell supernatants (equal protein) were analyzed for protein expression, Mb heme level, and IPs. A) Images (magnification value, ×10, bright field) show emergent myoblasts with a gradual increase in differentiated myotubes from 0 to 4 d. B, F, G) Western blots indicating various protein expression levels, and heme stain of Mb, in supernatants as indicated. C, H) Representative UV-visible spectra of differentiating cell supernatants (equal total protein) indicating their total Mb heme present (C, upper panel); content of total Mb heme in respective supernatants calculated using molar extinction coefficient of Mb at 555 nm (C, lower panel, and H). D) Representative IPs of Mb under differentiating conditions (0–96 h) that compare levels of associated Hsp90 and Mb (input 20% in all cases). E) Densitometry of bound hsp90 normalized to the bound Mb in experiments as depicted in D. I) Images (magnification value, ×10, bright field) show emergent myotubes at d 2 (2d) for both +SA or −SA culture conditions. All values are means ± sd of n = 3 experiments (*P < 0.05, by 1-way ANOVA).
Chronic hsp90 inhibition or knockdown of hsp90 expression blocks myoblast differentiation
To test if chronically inhibiting hsp90 would impact Mb maturation during myoblast differentiation, we treated C2C12 cells with the hsp90 inhibitors radicicol (2 µM) or AUY-922 (100 µM) over the course of a 4-d differentiation period. Unfortunately, such chronic hsp90 inhibition prevented cell differentiation, as evidenced by their not forming myotubes and their losing expression of the muscle-specific markers myogenin and MyoD (Supplemental Fig. S3A, B, D). The chronic hsp90 inhibition also prevented Mb protein expression and heme content from increasing (Supplemental Fig. S3B, C, E, F), which otherwise occurred in the myoblast cells during differentiation under normal culture conditions. We then tried an siRNA approach to test if knockdown of hsp90 expression in the C2C12 cells might influence their Mb maturation process without impacting their differentiation. Because C2C12 cells predominantly express the hsp90β isoform while their hsp90α isoform expression is further diminished during a 4-d differentiation period (Supplemental Fig. S4A), confirming a previous report (41), we focused on knockdown of hsp90β. Unfortunately, even a partial knockdown of hsp90β expression prevented differentiation by the C2C12 cells as assessed by several factors and also prevented cell accumulation of Mb (Supplemental Fig. S4B–D). Such broad effects of chronic hsp90 inhibition or of hsp90β knockdown prevented us from assessing an effect on Mb heme insertion under these circumstances.
Acute hsp90 inhibition blocks Mb heme insertion in differentiating myoblast cells
Given the above, we designed an experiment that could assess the effect of acute hsp90 inhibition on Mb heme insertion over a short period (3 h) in differentiating C2C12 cells. Normal or heme-deficient (SA pretreated) C2C12 cells were differentiated for 48 h and then the cultures were given hemin (5 µM) in the presence or absence of radicicol (5 µM) for an additional 3 h and then cells lysed for analyses. As shown in Fig. 4F–I, differentiation took place in both the normal and heme-depleted cells as judged by their myotube formation and an increase in Mb protein accumulation. Figure 4F–H shows that the 3 h hsp90 inhibition prevented the cells from inserting heme into their apo-Mb during this time window, consistent with hsp90 driving Mb heme insertion during active myoblast differentiation.
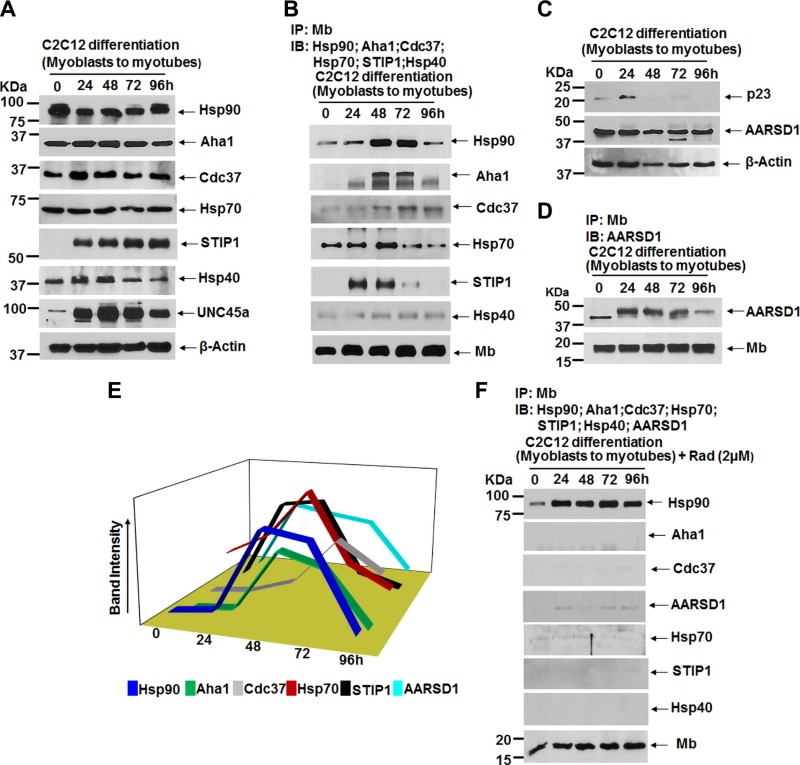
Cochaperones associate with hsp90 and Mb during maturation
Hsp90-dependent protein maturation typically involves several cochaperone proteins that complex with hsp90 during the process to enable its function (42–44). We determined the expression levels of several known cochaperones and their associations with Mb in C2C12 cells undergoing differentiation. Our analysis confirmed expression of activator of hsp90 ATPase protein 1 (Aha1), Cdc37, Hsp70, STIP1, Hsp40, and UNC-45 myosin chaperon A (UNC45a) (Fig. 5A). Of these, IP analysis showed that Mb associated with Aha1, Cdc37, Hsp70, and STIP1 over the 4-d period, whereas Hsp40 displayed only a feeble association (Fig. 5B). Binding of the hsp90 cochaperone UNC45a (28, 45) was also negative (unpublished results), despite its being expressed in the differentiating myoblasts (Fig. 5A). The association pattern of Aha1 most closely mimicked that of hsp90, where both were greatest at the 48 and 72 h time points. In comparison, the association patterns of Hsp70 and STIP1 overlapped with hsp90 but were relatively stronger at the 0 h (hsp70) and 24 h (hsp70 and STIP1) time points, and the association pattern of Cdc37 displayed less of a dropoff at the 96-h time point compared to hsp90.
Figure 5.
Cochaperons that associate with the hsp90-apo-Mb complex during its maturation and the effect of hsp90 inhibition. C2C12 cells were grown in differentiation medium in the presence and absence of hsp90 inhibitor radicicol (2 µM) from 0 to 4 d, and corresponding cell supernatants analyzed for protein expression and IPs. A, C) Representative Western blot indicating expression of hsp90 and the indicated cochaperons during C2C12 cell differentiation. B, D) Representative IPs of Mb taken at the indicated times during cell differentiation (0–96 h) that compare levels of associated hsp90 and cochaperons Aha1, cell division cycle 37 (Cdc37), Hsp70, stress induced phosphoprotein 1 (STIP1), Hsp40, and AARSD1 (input 20% in all cases). E) Densitometry of chaperons/cochaperons bound to Mb as depicted in B, D. F) IPs of Mb during cell differentiation as described for B and D but in the presence of radicicol (2 µM).
We also compared the expression levels of p23 and its homolog alanyl-tRNA synthetase domain containing 1 (Aarsd1) cochaperone because they typically play similar roles but are reported to be expressed and utilized differently in myoblasts (41). We saw an initial boost and then a loss in p23 expression during the cell differentiation period, as compared to a consistent expression of Aarsd1 (Fig. 5C). In addition, Aarsd1 associated with Mb in a pattern that was similar to the hsp90 association (Fig. 5D, E), consistent with the Aarsd1-hsp90 complex being essential for muscle differentiation (41).
We then performed a similar experiment with radicicol-treated C2C12 cells (Supplemental Fig. S3) to test the effect of hsp90 inhibition. Under this circumstance, the hsp90-Mb association remained stable during the 4-d differentiation period, but all of the cochaperone associations noted above were destabilized (Fig. 5F). This implies that a loss of cochaperone interactions is an additional consequence of inhibiting hsp90 in this system. Together, our data suggest that hsp90 and its cochaperone machinery including Aarsd1, Aha1, Cdc37, Hsp70, and STIP1 are involved in heme insertion into apo-Mb during myoblast differentiation (Fig. 5E).
Mb heme insertion is stimulated by an active sGC
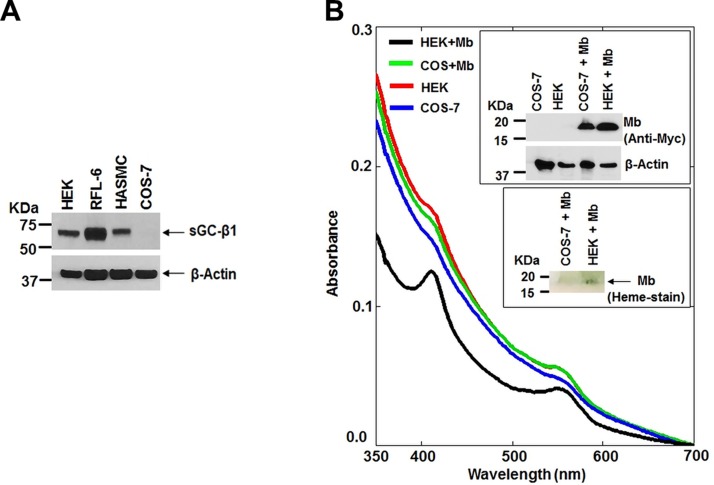
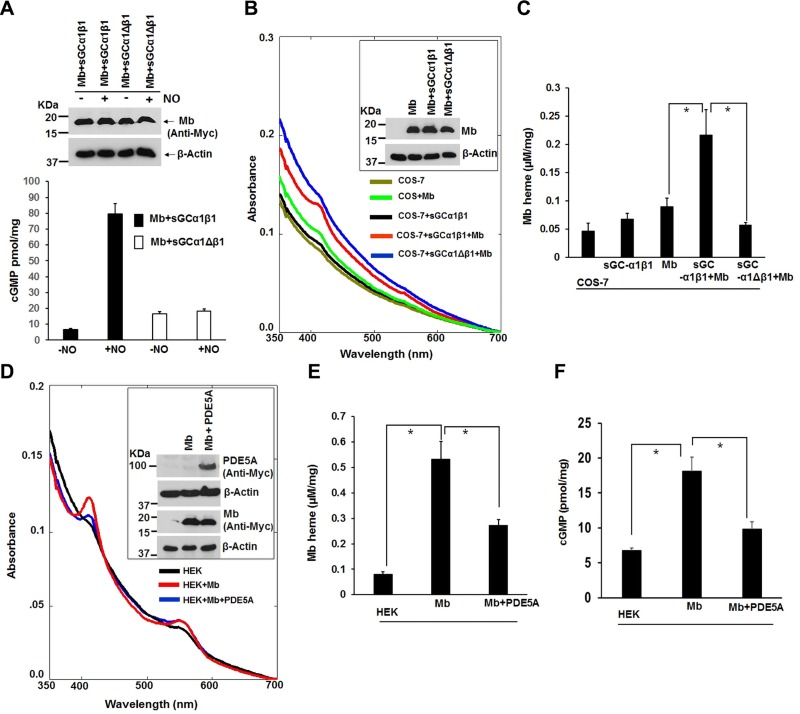
The sGC-cGMP signaling pathway is known to help drive Hb protein expression (46). We therefore investigated if sGC-cGMP signaling may influence Mb maturation. C2C12 cells contained measureable cGMP levels during their differentiation and had an increased cGMP level at the 24-h time point (Supplemental Fig. S5), indicating sGC activation. We next expressed human Mb in COS-7 or in HEK cells and compared the levels of Mb protein expression and heme content. The COS-7 cells, which lack an endogenous sGC activity (Fig. 6A) (47), supported a normal level of Mb protein expression but a poor Mb heme insertion relative to the HEK cells (Fig. 6B and insets), which do express an endogenous sGC (Fig. 6A). Analysis of hsp90 expression showed there was similar expression in COS-7 cells relative to HEK and other cell types (Supplemental Fig. S6A). Thus, despite a sufficient hsp90 expression in COS-7 cells, they supported relatively poor heme insertion into Mb. To test if a difference in sGC expression played a role, we attempted to silence sGCβ1 expression in HEK cells. Partial silencing of sGCβ1 expression in the HEK cells significantly reduced the heme content of their Mb without altering the level of Mb protein expression (Supplemental Fig. S6B–D). We next performed the reciprocal experiment by testing if supplying COS-7 cells with an active sGC would improve their Mb heme insertion capability. Cells were transiently transfected with Mb, sGCα1, and either the WT sGCβ1 or an ΔsGCβ1 mutant (Δ204–244 + Δ379–408) that does not interact with sGCα1 (Supplemental Fig. S7) and so cannot form an active sGC-α1β1 heterodimer in cells (48). We confirmed that the ΔsGCβ1 mutant (Δ204–244 + Δ379–408) could not form an active sGC-α1β1 heterodimer, as demonstrated by its lack of cGMP biosynthesis in response to NO (Fig. 7A, B). Coexpression of sGCα1 with WT sGCβ1 in COS-7 cells increased the heme content of Mb by 2 and a half-fold without causing any change in Mb protein expression, whereas coexpression with the mutant sGCβ1 did not support an increase in Mb heme content (Fig. 7C, D). Thus, provision of COS-7 cells with an active sGCα1β1 enabled them to support a more effective Mb heme insertion. To test this concept in a third way, we transiently expressed Mb in the HEK cells either alone or along with PDE5A, which is an enzyme that breaks down and inactivates cGMP, the product of sGC enzymatic activity. The proportion of heme-containing Mb was less when Mb was coexpressed with PDE5A relative to Mb alone (Fig. 7E, F), and this correlated with PDE5A expression inhibiting an increase in the cell cGMP level that otherwise occurred at 42 h during Mb expression (Fig. 7G). Together, our results reveal that sGC-cGMP signaling enables Mb maturation by somehow stimulating heme insertion into apo-Mb.
Figure 6.
Cells that express sGC support greater Mb heme content. Supernatants from COS-7 and HEK cells transiently expressing Myc-tagged human skeletal muscle Mb were analyzed to compare relative Mb and sGCβ1 protein levels and Mb heme levels. A) Western blot indicating that sGCβ1 is expressed in various cell types but not in COS-7 cells. B) UV-visible spectra of the indicated cell supernatants, with insets showing the expression level of Mb and the in-gel Mb heme stain.
Figure 7.
Mb heme insertion in cells is stimulated by an active sGC-cGMP signaling pathway. COS-7 cells were transiently transfected with the indicated combinations of Mb, sGCα1 plus sGCβ1, or sGCα1 plus the inactive Δ sGCβ1 (Δ204–244 + Δ379–408). Supernatants were analyzed for protein expression, sGC activity, and Mb heme content. A, B) Representative Western blot showing expression of Mb in the various transfected cells and the corresponding basal and NO-induced sGC activities (cGMP production). C) Representative UV-visible absorption spectra of transfected supernatants indicating their Mb heme content, with inset showing the Mb expression levels. D) Heme content of Mb in supernatants calculated from collected spectral traces. E–G) HEK cells were transiently transfected for 42 h to express Mb with or without a PDE5A, and supernatants were analyzed for protein expression, Mb heme content, and basal sGC activity (cGMP). E) Representative spectral traces indicating the relative heme content of Mb, inset shows the protein expression levels. F, G) Calculated Mb heme levels and basal cGMP levels in supernatants as indicated. All values in bar graphs are means ± sd of n = 3 experiments (*P < 0.05, by 1-way ANOVA).
DISCUSSION
A coordinate expression and maturation of Mb takes place during differentiation of myoblasts into myotubes (49, 50). Our study is the first to probe the mechanisms of Mb maturation during this process and reveals that the hsp90/cochaperone machinery associates with apo-Mb to ultimately drive heme insertion, which is an essential step for generating mature and functional Mb. Thus, Mb joins a growing list of hemeproteins whose heme insertion is hsp90-dependent, which at present includes the NO synthases (25), sGCβ1 (26), and Hb-β and γ (27).
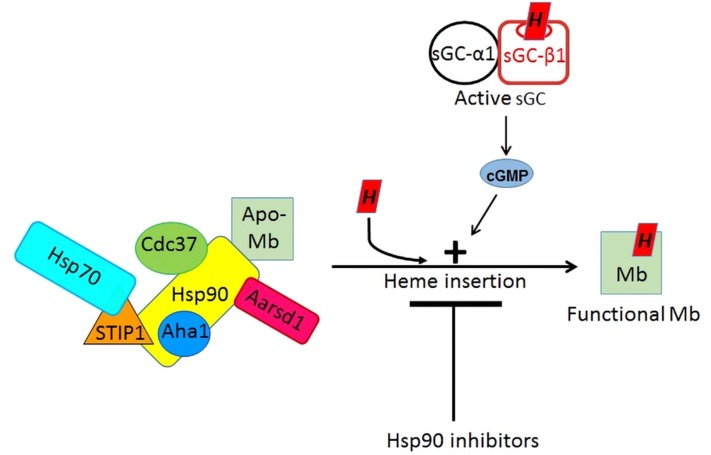
Mb is the first hemeprotein hsp90 client to have its cochaperones identified. Four of the cochaperones that we identified (STIP1, hsp70, Cdc37, and Aha-1) are all known to associate with hsp90 during the maturation of other client proteins, and in some cases the cochaperone binding site or their effect on hsp90 function has been studied (51, 52). The timing of the cochaperone associations that we observed for Mb in the differentiating C2C12 cells are consistent with their enabling hsp90 binding to apo-Mb and enabling the subsequent heme insertion step, after which hsp90 and the cochaperones dissociate from the mature Mb. For example, hsp70 and STIP1 were most strongly associated with the hsp90-apo-Mb complex in the early and middle stages of myoblast differentiation, consistent with STIP1 enabling hsp70 to form a complex with hsp90 so that it can transfer the partially folded client protein (in this case, apo-Mb) to hsp90 for further processing (53), which here would be heme insertion into the apo-Mb. Likewise, the association patterns of cochaperones Aha1 and CDC37 closely follow the hsp90 association pattern, and thus are consistent with their binding to hsp90 to regulate its ATP hydrolysis, which in turn regulates its conformational cycling and function (54, 55), presumably to help hsp90 drive heme insertion into apo-Mb. Of note, we confirmed that the hsp90 cochaperone p23 is poorly expressed in myoblasts during differentiation, whereas the homolog cochaperone Aarsd1 is strongly expressed (41), and in our case is found associated with the hsp90-apo-Mb complex, consistent with Aarsd1 taking the place of p23 and its being needed for myoblast differentiation (41). A model for hsp90/cochaperone assisted Mb maturation that is consistent with the results to date is presented in Fig. 8. Going forward, apo-Mb may prove to be particularly useful in elucidating the molecular mechanisms of hsp90-driven client maturation because its structural folding-unfolding transitions are robust and among the most well-studied in biology (56–58). Work with Mb could also guide investigations of how hsp90 aids maturation of more complex globins like Hb-β and γ, which unlike Mb form mixed tetrameric structures with an Hb-α partner during maturation.
Figure 8.
Model of the Mb maturation process. Immature apo-Mb is bound directly to hsp90 in cells and during its maturation is part of a dynamic protein complex that can also contain hsp70 and the cochaperones STIP1, Aha1, Cdc37, and Aarsd1. The heme insertion into apo-Mb is blocked by hsp90 inhibitors and is stimulated when cells contain an active sGC-cGMP signaling pathway.
Acute exposure to hsp90 inhibitors blocked heme insertion into apo-Mb in the C2C12 cells both when they were quiescent or were undergoing differentiation into myotubes. However, when the hsp90 inhibitors were chronically present, or when cell hsp90β expression was knocked down, it arrested the C2C12 cell differentiation into myotubes and led to a loss of muscle-specific protein marker expression. These effects are consistent with chronic hsp90 inhibition causing significant deviations in the myoblast gene expression profile during the 4-d differentiation (41).
Our study unexpectedly revealed that an active sGC-cGMP signaling pathway increases the extent of heme insertion into apo-Mb, and thereby stimulates this final step of Mb maturation. Stimulating heme insertion represents a new point of action for the sGC-cGMP pathway and distinguishes our finding from previous reports that showed NO activation of the sGC-cGMP pathway in cultured cells or in whole animals stimulated Mb and Hb protein biosynthesis at the transcriptional level (59, 60). In those studies, changes in the globin heme levels were not investigated. Our current findings imply that an additional, underappreciated role may exist for activating the sGC-cGMP signaling pathway, namely to support and improve heme insertion into apo-Mb and potentially into other hemeproteins during their maturation. This concept is incorporated into our model of Mb maturation that is illustrated in Fig. 8. Together, our findings broaden the current perspective on globin maturation and set the stage to investigate what are the molecular and biomedical implications of having both the NO-sGC-cGMP signaling pathway and the hsp90/cochaperone machinery involved in globin maturation.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Heart, Lung and Blood Institute Grants HL081064 (to D.J.S. and A.G.) and HL0103453 (to D.J.S.). The authors declare no conflicts of interest.
Glossary
- Aarsd1
alanyl-tRNA synthetase domain containing 1
- Aha1
activator of hsp90 ATPase protein 1
- Apo
apoprotein
- Cdc37
cell division cycle 37
- HEK
human embryonic kidney
- Hb
hemoglobin
- hsp
heat shock protein
- IP
immunoprecipitation
- Mb
myoglobin
- PDE5A
phosphodiesterase 5A
- SA
succinyl acetone
- sGC
soluble guanylyl cyclase
- siRNA
small interfering RNA
- STIP1
stress induced phosphoprotein 1
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. Ghosh and D. J. Stuehr designed the experiments, analyzed the data, and wrote the manuscript; A. Ghosh performed all cell culture and biochemical studies; and Y. Dai and P. Biswas performed experiments involving in vitro binding studies with purified proteins.
REFERENCES
- 1.Akaboshi E. (1985) Cloning of the human myoglobin gene. Gene 33, 241–249 [DOI] [PubMed] [Google Scholar]
- 2.Gros G., Wittenberg B. A., Jue T. (2010) Myoglobin’s old and new clothes: from molecular structure to function in living cells. J. Exp. Biol. 213, 2713–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ordway G. A., Garry D. J. (2004) Myoglobin: an essential hemoprotein in striated muscle. J. Exp. Biol. 207, 3441–3446 [DOI] [PubMed] [Google Scholar]
- 4.Wittenberg B. A., Wittenberg J. B. (1989) Transport of oxygen in muscle. Annu. Rev. Physiol. 51, 857–878 [DOI] [PubMed] [Google Scholar]
- 5.Collman J. P., Brauman J. I., Halbert T. R., Suslick K. S. (1976) Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proc. Natl. Acad. Sci. USA 73, 3333–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott E. E., Gibson Q. H., Olson J. S. (2001) Mapping the pathways for O2 entry into and exit from myoglobin. J. Biol. Chem. 276, 5177–5188 [DOI] [PubMed] [Google Scholar]
- 7.Wittenberg J. B., Wittenberg B. A. (2003) Myoglobin function reassessed. J. Exp. Biol. 206, 2011–2020 [DOI] [PubMed] [Google Scholar]
- 8.Wyman J. (1966) Facilitated diffusion and the possible role of myoglobin as a transport mechanism. J. Biol. Chem. 241, 115–121 [PubMed] [Google Scholar]
- 9.Kanatous S. B., Davis R. W., Watson R., Polasek L., Williams T. M., Mathieu-Costello O. (2002) Aerobic capacities in the skeletal muscles of Weddell seals: key to longer dive durations? J. Exp. Biol. 205, 3601–3608 [DOI] [PubMed] [Google Scholar]
- 10.Packer B. S., Altman M., Cross C. E., Murdaugh H. V., Jr., Linta J. M., Robin E. D. (1969) Adaptations to diving in the harbor seal: oxygen stores and supply. Am. J. Physiol. 217, 903–906 [DOI] [PubMed] [Google Scholar]
- 11.Panneton W. M. (2013) The mammalian diving response: an enigmatic reflex to preserve life? Physiology (Bethesda) 28, 284–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright T. J., Davis R. W. (2015) Myoglobin oxygen affinity in aquatic and terrestrial birds and mammals. J. Exp. Biol. 218, 2180–2189 [DOI] [PubMed] [Google Scholar]
- 13.Gödecke A., Flögel U., Zanger K., Ding Z., Hirchenhain J., Decking U. K., Schrader J. (1999) Disruption of myoglobin in mice induces multiple compensatory mechanisms. Proc. Natl. Acad. Sci. USA 96, 10495–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubbard S. R., Hendrickson W. A., Lambright D. G., Boxer S. G. (1990) X-ray crystal structure of a recombinant human myoglobin mutant at 2.8 A resolution. J. Mol. Biol. 213, 215–218 [DOI] [PubMed] [Google Scholar]
- 15.Kendrew J. C., Bodo G., Dintzis H. M., Parrish R. G., Wyckoff H., Phillips D. C. (1958) A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature 181, 662–666 [DOI] [PubMed] [Google Scholar]
- 16.Li J., Buchner J. (2013) Structure, function and regulation of the hsp90 machinery. Biomed. J. 36, 106–117 [DOI] [PubMed] [Google Scholar]
- 17.Makhnevych T., Houry W. A. (2012) The role of Hsp90 in protein complex assembly. Biochim. Biophys. Acta 1823, 674–682 [DOI] [PubMed] [Google Scholar]
- 18.Prodromou C. (2012) The ‘active life’ of Hsp90 complexes. Biochim. Biophys. Acta 1823, 614–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radli M., Rüdiger S. G. D. (2018) Dancing with the diva: Hsp90-client interactions. J. Mol. Biol. 430, 3029–3040 [DOI] [PubMed] [Google Scholar]
- 20.Echeverria P. C., Picard D. (2010) Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim. Biophys. Acta 1803, 641–649 [DOI] [PubMed] [Google Scholar]
- 21.Karagöz G. E., Rüdiger S. G. (2015) Hsp90 interaction with clients. Trends Biochem. Sci. 40, 117–125 [DOI] [PubMed] [Google Scholar]
- 22.Tsuji N., Fukuda K., Nagata Y., Okada H., Haga A., Hatakeyama S., Yoshida S., Okamoto T., Hosaka M., Sekine K., Ohtaka K., Yamamoto S., Otaka M., Grave E., Itoh H. (2014) The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open Bio 4, 796–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Citri A., Harari D., Shohat G., Ramakrishnan P., Gan J., Lavi S., Eisenstein M., Kimchi A., Wallach D., Pietrokovski S., Yarden Y. (2006) Hsp90 recognizes a common surface on client kinases. J. Biol. Chem. 281, 14361–14369 [DOI] [PubMed] [Google Scholar]
- 24.Mukai K., Shimizu T., Igarashi J. (2011) Phosphorylation of a heme-regulated eukaryotic initiation factor 2α kinase enhances the interaction with heat-shock protein 90 and substantially upregulates kinase activity. Protein Pept. Lett. 18, 1251–1257 [DOI] [PubMed] [Google Scholar]
- 25.Ghosh A., Chawla-Sarkar M., Stuehr D. J. (2011) Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. FASEB J. 25, 2049–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh A., Stuehr D. J. (2012) Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme. Proc. Natl. Acad. Sci. USA 109, 12998–13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh A., Garee G., Sweeny E. A., Nakamura Y., Stuehr D. J. (2018) Hsp90 chaperones hemoglobin maturation in erythroid and nonerythroid cells. Proc. Natl. Acad. Sci. USA 115, E1117–E1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barral J. M., Hutagalung A. H., Brinker A., Hartl F. U., Epstein H. F. (2002) Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 295, 669–671 [DOI] [PubMed] [Google Scholar]
- 29.Lele Z., Hartson S. D., Martin C. C., Whitesell L., Matts R. L., Krone P. H. (1999) Disruption of zebrafish somite development by pharmacologic inhibition of Hsp90. Dev. Biol. 210, 56–70 [DOI] [PubMed] [Google Scholar]
- 30.Sass J. B., Martin C. C., Krone P. H. (1999) Restricted expression of the zebrafish hsp90alpha gene in slow and fast muscle fiber lineages. Int. J. Dev. Biol. 43, 835–838 [PubMed] [Google Scholar]
- 31.Yun B. G., Matts R. L. (2005) Differential effects of Hsp90 inhibition on protein kinases regulating signal transduction pathways required for myoblast differentiation. Exp. Cell Res. 307, 212–223 [DOI] [PubMed] [Google Scholar]
- 32.Young J. C., Hoogenraad N. J., Hartl F. U. (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112, 41–50 [DOI] [PubMed] [Google Scholar]
- 33.Sarkar A., Dai Y., Haque M. M., Seeger F., Ghosh A., Garcin E. D., Montfort W. R., Hazen S. L., Misra S., Stuehr D. J. (2015) Heat shock protein 90 associates with the Per-Arnt-Sim domain of heme-free soluble guanylate cyclase: implications for enzyme maturation. J. Biol. Chem. 290, 21615–21628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carver T. E., Brantley R. E., Jr., Singleton E. W., Arduini R. M., Quillin M. L., Phillips G. N., Jr., Olson J. S. (1992) A novel site-directed mutant of myoglobin with an unusually high O2 affinity and low autooxidation rate. J. Biol. Chem. 267, 14443–14450 [PubMed] [Google Scholar]
- 35.Springer B. A., Sligar S. G. (1987) High-level expression of sperm whale myoglobin in Escherichia coli. Proc. Natl. Acad. Sci. USA 84, 8961–8965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teale F. W. (1959) Cleavage of the haem-protein link by acid methylethylketone. Biochim. Biophys. Acta 35, 543 [DOI] [PubMed] [Google Scholar]
- 37.Ghosh A., Stasch J. P., Papapetropoulos A., Stuehr D. J. (2014) Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content. J. Biol. Chem. 289, 15259–15271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waheed S. M., Ghosh A., Chakravarti R., Biswas A., Haque M. M., Panda K., Stuehr D. J. (2010) Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic. Biol. Med. 48, 1548–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagatsuma A., Shiozuka M., Kotake N., Takayuki K., Yusuke H., Mabuchi K., Matsuda R., Yamada S. (2011) Pharmacological inhibition of HSP90 activity negatively modulates myogenic differentiation and cell survival in C2C12 cells. Mol. Cell. Biochem. 358, 265–280 [DOI] [PubMed] [Google Scholar]
- 40.Taniuchi S., Miyake M., Tsugawa K., Oyadomari M., Oyadomari S. (2016) Integrated stress response of vertebrates is regulated by four eIF2α kinases. Sci. Rep. 6, 32886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Echeverría P. C., Briand P. A., Picard D. (2016) A remodeled Hsp90 molecular chaperone ensemble with the novel cochaperone Aarsd1 is required for muscle differentiation. Mol. Cell. Biol. 36, 1310–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J., Soroka J., Buchner J. (2012) The Hsp90 chaperone machinery: conformational dynamics and regulation by co-chaperones. Biochim. Biophys. Acta 1823, 624–635 [DOI] [PubMed] [Google Scholar]
- 43.Moran Luengo T., Mayer M. P., Rudiger S. G. D. (2018) The hsp70-hsp90 chaperone cascade in protein folding. Trends Cell Biol. 29, 164–177 [DOI] [PubMed] [Google Scholar]
- 44.Zuehlke A., Johnson J. L. (2010) Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers 93, 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L., Srikakulam R., Winkelmann D. A. (2008) Unc45 activates Hsp90-dependent folding of the myosin motor domain. J. Biol. Chem. 283, 13185–13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cokic V. P., Smith R. D., Beleslin-Cokic B. B., Njoroge J. M., Miller J. L., Gladwin M. T., Schechter A. N. (2003) Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J. Clin. Invest. 111, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuen P. S., Doolittle L. K., Garbers D. L. (1994) Dominant negative mutants of nitric oxide-sensitive guanylyl cyclase. J. Biol. Chem. 269, 791–793 [PubMed] [Google Scholar]
- 48.Zhou Z., Gross S., Roussos C., Meurer S., Müller-Esterl W., Papapetropoulos A. (2004) Structural and functional characterization of the dimerization region of soluble guanylyl cyclase. J. Biol. Chem. 279, 24935–24943 [DOI] [PubMed] [Google Scholar]
- 49.Graber S. G., Woodworth R. C. (1986) Myoglobin expression in L6 muscle cells. Role of differentiation and heme. J. Biol. Chem. 261, 9150–9154 [PubMed] [Google Scholar]
- 50.Kanatous S. B., Mammen P. P. (2010) Regulation of myoglobin expression. J. Exp. Biol. 213, 2741–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sahasrabudhe P., Rohrberg J., Biebl M. M., Rutz D. A., Buchner J. (2017) The plasticity of the Hsp90 co-chaperone system. Mol. Cell 67, 947–961.e5 [DOI] [PubMed] [Google Scholar]
- 52.Schopf F. H., Biebl M. M., Buchner J. (2017) The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 18, 345–360 [DOI] [PubMed] [Google Scholar]
- 53.Kirschke E., Goswami D., Southworth D., Griffin P. R., Agard D. A. (2014) Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 157, 1685–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Panaretou B., Siligardi G., Meyer P., Maloney A., Sullivan J. K., Singh S., Millson S. H., Clarke P. A., Naaby-Hansen S., Stein R., Cramer R., Mollapour M., Workman P., Piper P. W., Pearl L. H., Prodromou C. (2002) Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1. Mol. Cell 10, 1307–1318 [DOI] [PubMed] [Google Scholar]
- 55.Siligardi G., Panaretou B., Meyer P., Singh S., Woolfson D. N., Piper P. W., Pearl L. H., Prodromou C. (2002) Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J. Biol. Chem. 277, 20151–20159 [DOI] [PubMed] [Google Scholar]
- 56.Culbertson D. S., Olson J. S. (2010) Role of heme in the unfolding and assembly of myoglobin. Biochemistry 49, 6052–6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dantsker D., Roche C., Samuni U., Blouin G., Olson J. S., Friedman J. M. (2005) The position 68(E11) side chain in myoglobin regulates ligand capture, bond formation with heme iron, and internal movement into the xenon cavities. J. Biol. Chem. 280, 38740–38755 [DOI] [PubMed] [Google Scholar]
- 58.Samuel P. P., Smith L. P., Phillips G. N., Jr., Olson J. S. (2015) Apoglobin stability is the major factor governing both cell-free and in vivo expression of holomyoglobin. J. Biol. Chem. 290, 23479–23495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Flonta S. E., Arena S., Pisacane A., Michieli P., Bardelli A. (2009) Expression and functional regulation of myoglobin in epithelial cancers. Am. J. Pathol. 175, 201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rayner B. S., Hua S., Sabaretnam T., Witting P. K. (2009) Nitric oxide stimulates myoglobin gene and protein expression in vascular smooth muscle. Biochem. J. 423, 169–177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.