Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic disorder causing renal failure. Mutations of polycystic kidney disease 1 (PKD1) account for most ADPKD cases. Defective ciliary localization of polycystin-1 (PC1), a large integral membrane protein encoded by PKD1, underlies the pathogenesis of a subgroup of patients with ADPKD. However, the mechanisms by which PC1 and other ciliary proteins traffic to the primary cilium remain poorly understood. A ciliary targeting sequence (CTS) that resides in ciliary receptors is considered to function in the process. It has been reported that the VxP motif in the intracellular C-terminal tail of PC1 functions as a CTS in an ADP ribosylation factor 4 (Arf4)/ArfGAP with SH3 domain, ankyrin repeat and PH domain 1 (ASAP1)–dependent manner. However, other recent studies have revealed that this motif is dispensable for PC1 trafficking to cilia. In this study, we identified a novel CTS consisting of 8 residues (RHKVRFEG) in the PC1 C tail. We found that this motif is sufficient to bind protein phosphatase 1 (PP1)α, a ubiquitously expressed phosphatase in the phosphoprotein phosphatase (PPP) family. Mutations in this CTS motif disrupt binding with PP1α and impair ciliary localization of PC1. Additionally, short hairpin RNA–mediated knockdown of PP1α results in reduced ciliary localization of PC1 and elongated cilia, suggesting a role for PP1α in the regulation of ciliary structure and function.—Luo, C., Wu, M., Su, X., Yu, F., Brautigan, D. L., Chen, J., Zhou, J. Protein phosphatase 1α interacts with a novel ciliary targeting sequence of polycystin-1 and regulates polycystin-1 trafficking.
Keywords: PP1, primary cilium, CTS
As a microtubule-based hair-like structure, the primary cilium is a specialized and highly conserved nonmotile cellular organelle protruding from almost every vertebrate cell. Numerous studies have suggested that the primary cilium has diverse functions, including signaling transduction as well as mechanosensation, that are crucial for development and tissue homeostasis (1). Ciliary defects underlie a wide range of human diseases such as autosomal dominant polycystic kidney disease (ADPKD), Bardet-Biedl syndrome, Joubert’s syndrome, and nephronophthisis, which are collectively termed ciliopathies (2).
ADPKD is 1 of the most common genetic disorders, affecting 1 in 400–1000 individuals worldwide, and is characterized by adult-onset, progressive development of fluid-filled cysts in both kidneys, resulting in 7–10% of end-stage renal disease cases (3, 4). ADPKD is genetically heterogeneous, with 2 genes identified to date: polycystic kidney disease (PKD)-1 (chromosome region 16p13.3; around 85% of cases) and PKD2 (4q21; ∼15% of cases) (5–8). Polycystin-1 (PC1), encoded by PKD1, is a large integral membrane protein (∼4300 aa) consisting of 11 membrane-spanning domains, a large extracellular domain, and a small intracellular C-terminal tail (CTT) (∼200 aa). It has been shown that PC1 colocalizes with polycystin-2 (PC2; encoded by PKD2) on the primary cilium where it functions as an atypical GPCR and mediates mechanosensation (9–11). Recent studies have implied that impaired ciliary trafficking of polycystins contributes to the pathogenesis of ADPKD (12–15). However, the mechanisms by which PC1 is targeted to cilia remain poorly understood. A ciliary targeting sequence (CTS) is considered to be involved (16, 17). Previous studies have reported that the PC1 CTT contains the VxP motif that targets rhodopsin to cilia (18), and this VxP motif also targets a small fragment of PC1 to cilia in an ADP ribosylation factor 4 (Arf4)–dependent manner (19). However, this VxP motif is not required for full-length PC1 targeting to cilia (15).
Protein phosphatase 1 (PP1) is a major eukaryotic protein serine-threonine phosphatase of the phosphoprotein phosphatase (PPP) family that participates in the dephosphorylation of as much as 30% of all proteins in eukaryotic cells and thus regulates a broad range of cellular functions, including intracellular trafficking, cell cycle progression, glycogen metabolism, and protein synthesis (20–22). In mammals, there are 4 PP1 isoforms (PP1α, PP1β, PP1γ1, and PP1γ2) encoded by 3 genes (PPP1CA, PPP1CB, and PPP1CC). PP1γ2 is enriched in the testis, whereas the other 3 are ubiquitously expressed (22). The PP1 holoenzyme is typically composed of a catalytic subunit and a regulatory subunit, and the function of each holoenzyme is dictated by 1 of the >200 regulatory subunits that are structurally and functionally diverse (23). A majority of the PP1 regulatory subunits contain an RVxF docking motif, which binds to a hydrophobic groove of PP1 remote from the catalytic site (24–26). Recent studies have suggested that PC1 is a regulatory protein as well as a substrate of PP1α, and PP1α recognizes PKA-phosphorylated PC1 through the RVxF motif in the PC1 CTT and mediates PC1 and PC2 dephosphorylation (27, 28).
In this study, we report the identification of a novel 8-residue CTS, including the RVxF PP1 docking motif at the PC1 CTT that regulates ciliary trafficking in both small chimeric and full-length PC1. Knockdown of PP1α isoform led to aberrant PC1 ciliary localization and elongated cilia in inner medullary collecting duct (IMCD3) cells, suggesting a requirement for PP1α in ciliary structure and function.
MATERIALS AND METHODS
Cell culture and transfection
Mouse IMCD3 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) (CRL-2123) and cultured in DMEM with F12 medium (50:50) supplemented with 10% fetal bovine serum (FBS). HEK293 cells were cultured in DMEM complemented with 10% FBS. PC2 wild-type (WT) and knockout (KO) cells were generated by our group from kidneys of Pkd2 WT and KO mice via temperature-sensitive simian virus 40 viral immortalization. These cell lines were selected using collecting tubular marker dolichos biflorus agglutinin and cultured in DMEM/F12 medium supplemented with 10% FBS and 5 U/ml IFN-γ. For analysis of ciliary trafficking of PC1 and mini-constructs, each construct was mixed with polyethylenimine (Thermo Fisher Scientific, Waltham, MA, USA), diluted in minimum essential medium (Corning, Corning, NY, USA), and incubated at room temperature for 30 min. After incubation, the mixture was added to cells at ∼60% confluence. After overnight culture, cells were serum starved for 36–40 h in DMEM/F12 for immunostaining. For the glycogen synthase kinase 3 (GSK3) inhibitor 6-bromoindirubin-3′-oxime (MilliporeSigma, Burlington, MA, USA) experiment, transfected IMCD3 cells were treated with 1 μM inhibitor for 36 h starting at the time of starvation. Cells treated with DMSO or without any treatment were used as controls.
Lentiviral knockdown of PP1α
For lentiviral knockdown experiments, plasmid targeting against PP1α was purchased from MilliporeSigma (TRCN0000012373). Lentiviral particles were assembled in HEK293 cells following the manufacturer’s instructions. IMCD3 cells were infected with viruses overnight followed by selection with puromycin at a concentration of 2 μg/ml. Knockdown efficiency in stable cells was tested by quantitative RT-PCR, and primer sequences are listed in Table 1.
TABLE 1.
Primers sequences for quantitative RT-PCR
| Primers (5′-3′) | ||
|---|---|---|
| Gene | Forward | Reverse |
| Ppp1ca | GGAGAGTTTGACAATGCTGG | GGTTCAGGCCGCTGAACTGC |
| Ppp1cb | TGCCCCAAATTACTGTGGCG | CAGCCCACCATACTGGTACT |
| Ppp1cc | ATGATGAGTGTGGATGAGACC | GCTTTGTGATCATACCCCGTG |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Plasmids and antibodies
The YFPPC1-HA–encoding plasmid was generated by inserting enhanced yellow fluorescent protein (YFP) after the first 34 residues downstream from the predicted signal peptide and a triple–human influenza hemagglutinin (HA) tag to the C terminus of full-length PC1 as we previously reported (13). The ADPKD mutation database (http://pkdb.mayo.edu/) was used to search for the human PKD1 missense mutations. All subsequent mutations derived from YFPPC1-HA were performed using PCR-based mutagenesis. All CD16.7 PC1 chimeric mini-constructs were amplified by recombinant PCR and then cloned into the pcDNA3.1 plasmid. To produce glutathione S-transferase (GST)–fusion constructs, the regions encoding the C-terminal 42 aa of mouse PC1 or the 8 aa and its mutations were cloned into the EcoRI and NotI sites of pGEX4T1. PP1α, PP1β, and PP1γ1 were expressed in pKHA3. All plasmids in this study were confirmed by DNA sequencing analysis.
For immunofluorescence, the following primary antibodies were used: anti–green fluorescent protein (GFP) rabbit pAb (1:20,000; Abcam, Cambridge, United Kingdom), anti–acetylated α-tubulin mouse mAb (1:50,000; MilliporeSigma), anti–acetylated α-tubulin rabbit pAb (1:1200; Cell Signaling Technology, Danvers, MA, USA), and anti-CD16 mouse mAb (1:200; Santa Cruz Biotechnology, Dallas, TX, USA).
For Western blotting and GST pulldown, the following primary antibodies were used: anti-PC2 rabbit pAb (96525, generated by our group; 1:1000) (10), anti-PC1 (7e12) mouse mAb (1:200; Santa Cruz Biotechnology), anti-HA rat mAb (1:1000; Roche, Basel, Switzerland), anti–pan-PP1 chicken IgY (generous gift from D.L.B.; 1:1000), anti-PP1α rabbit pAb (1:500; Cell Signaling Technology), anti-GFP rabbit pAb (1:2000; Abcam), anti–glyceraldehyde 3-phosphate dehydrogenase mouse mAb (1:50,000; Thermo Fisher Scientific), and anti α-tubulin mouse mAb (1:1000; MilliporeSigma). Horseradish peroxidase–linked anti-rabbit IgG (1:2000; Cell Signaling Technology), anti-mouse IgG (1:2000; Cell Signaling Technology), and goat anti-chicken IgY (1:2000; Santa Cruz Biotechnology) antibodies were used as secondary antibodies.
Glycosylation analysis
Cell lysates were denatured using glycoprotein denature buffer (New England Biolabs, Ipswich, MA, USA) for 1 min at 99°C and then chilled on ice. The denatured glycoprotein was incubated with either reaction buffer alone, peptide:N-glycosidase F, or endoglycosidase H according to the manufacturer’s instructions.
Western blotting
Western blotting was performed as previously reported by Wang et al. (29). Briefly, proteins were electrophoresed on SDS-PAGE gels and transferred onto Hybond ECL nitrocellulose membranes (GE Healthcare, Waukesha, WI, USA). Blots were blocked with 5% nonfat dry milk in PBS with 0.05% Tween 20, incubated with the primary antibody for 3 h at room temperature or overnight in a cold room, and washed 3 times with PBS with 0.05% Tween 20. The blots were finally incubated with a horseradish peroxidase–linked secondary antibody for 1 h at room temperature, washed thoroughly, and detected with the ECL Western blot analysis system.
GST-pulldown assay
For GST-pulldown analysis, extracts containing 500 μg protein from HEK293 cells expressing different HA-tagged PP1 isoforms or 500 μg protein from IMCD3 cells were incubated with purified GST fusion proteins immobilized on glutathione-Sepharose beads (GE Healthcare, Waukesha, WI, USA) at room temperature for 2 h. The beads were then centrifuged and washed 4 times with ice-cold PBS washing buffer containing 5% Triton X-100 and Complete protease inhibitor cocktail (Roche). The washed beads were suspended in an equal volume of 2 times SDS sample buffer. Proteins were eluted by boiling for 5 min and then analyzed by Western blotting.
Immunostaining
Cells were fixed with 4% paraformaldehyde for 10 min and then permeabilized with 0.5% Triton X-100 in PBS for 5 min. After 3 washes, cells were blocked with 5% bovine serum albumin for 1 h and then incubated with the desired primary antibodies for 3 h at room temperature or overnight in a cold room. After thorough washing, secondary antibodies with appropriate labeling were added for 1 h of incubation. After 3 washes, slides were either mounted with ProLong Gold antifade reagent with DAPI (Thermo Fisher Scientific) directly or stained with bisBenzimide H 33342 trihydrochloride (Hoechst; MilliporeSigma) at room temperature for 5 min and then mounted with ProLong Gold antifade reagent without DAPI (Invitrogen).
Image acquisition and quantification
Fluorescence images were acquired with a Nikon 1000 epifluorescence microscope (Nikon, Tokyo, Japan). All images in the same set of experiments were taken with identical settings and contrast adjusted equally in PowerPoint (Microsoft, Redmond, WA, USA). For each experiment, the number of cells with ciliary expression of recombinant proteins (YFPPC1-HA and its variants or chimeric proteins) was counted in ≥10 randomly selected microscopic fields and then divided by the number of transfected and ciliated cells.
Statistical analyses
Data are presented as mean values ± sd; a Student’s t test was used for statistical analysis. A value of P < 0.05 was considered significant. All analyses were carried out using Prism (GraphPad Software, La Jolla, CA, USA).
RESULTS
The 42-residue fragment in the PC1 C-terminal cytoplasmic tail harbors a novel CTS
Although the PC1 CTT is <5% of the whole PC1 sequence, we have previously shown that it plays a fundamental role in regulating full-length PC1 protein trafficking to the primary cilium (13). Through a systematic analysis, we have further identified multiple sequences in the PC1 C tail, including the coiled-coil domain, that are involved in the regulation of PC1 ciliary trafficking. Notably, the first identified CTS for PC1, the VxP motif at the end of PC1 C tail, is completely dispensable for full-length PC1 trafficking, although we found that it is capable of driving CD16.7 to cilia as previously described by Su et al. (15). CD16.7 is a well-characterized chimeric protein consisting of the human CD16 extracellular domain and the human CD7 transmembrane domain, and is frequently used for identifying functional motifs, domains, and sequences (19, 30–32). Because the coiled-coil domain cannot target chimeric protein to cilia, we hypothesized that the full-length PC1 and chimeric protein might traffic to cilia via different mechanisms (15). Based on this hypothesis, we next examined whether PC2 regulates chimeric protein trafficking by expressing these chimeric constructs in both WT and PC2-KO cells and costaining the chimeric protein with a cilium marker. These constructs are referred to as mini-constructs in this study to distinguish them from the full-length PC1 constructs. Unlike full-length PC1, which requires PC2 to reach cilia (14, 15), we found that the ciliary trafficking of chimeric PC1 proteins was independent of PC2 (Supplemental Fig. S1).
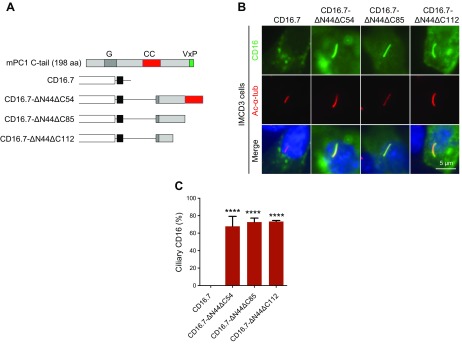
The properties of CD16.7 chimeric proteins did not fully represent those of the full-length PC1; however, a chimeric system like CD16.7 is important and necessary for at least 2 reasons: for dissecting functional sequences within a protein, especially large proteins with structural complexity like PC1; and for studies to evaluate whether a motif is sufficient to serve a particular function. We have recently found that a fragment of PC1 C tail consisting of 100 aa (including the entire coiled-coil domain) can drive CD16.7 (CD16.7-ΔN44ΔC54) to cilia efficiently (15). Because the coiled-coil domain could not target the chimeric protein to cilia, we speculated that the sequences upstream the coiled-coil domain were responsible for ciliary targeting of the chimeric protein. To identify the functional sequences in this region, we generated 2 additional truncation constructs by fusing either 69 or 42 residues in the 100-residue fragment to CD16.7 (CD16.7-ΔN44ΔC85 and CD16.7-ΔN44ΔC112) and tested for their function (Fig. 1A). We found that CD16.7-ΔN44ΔC112 could also localize to cilia as efficiently as the other 2 in mouse IMCD3 cells, indicating a potential PC2-independent CTS is located within the 42-residue fragment (Fig. 1B, C).
Figure 1.
A 42-residue fragment at the C terminus of PC1 likely harbors a new CTS. A) Schematic representation of CD16.7 with and without different PC1 C-terminal fragment fusions. B) Expression of indicated constructs in A in the primary cilia of IMCD3 cells. Cells were stained by antibodies against CD16 (green) and acetylated α-tubulin (Ac-α-tub; red). Scale bar, 5 μm. C) Quantification of percentage of CD16-positive cilia in cells. A total of ≥50 ciliated, transfected cells were counted for the presence of CD16 signal on cilia under each condition. Error bars represent the sd between microscope fields from ≥3 independent experiments. G, G-protein activation domain; CC, coiled-coil domain. ****P < 0.0001 compared with CD16.7 control.
Identification of a novel 8-residue CTS
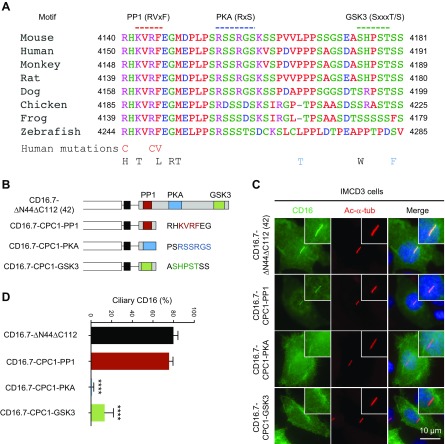
Multiple-species sequence alignment of the 42-residue region suggests that the N terminus of this segment in PC1 is highly conserved in vertebrates (Fig. 2A) and other portions of this segment are moderately conserved in mammals. This short fragment contains several motifs, including a PP1 docking motif at the N terminus (27), which overlaps with the last 5 aa of the G-protein activation domain (33) and a nuclear localization sequence (34). There are 2 potential PKA phosphorylation sites in the middle (35) and 1 potential GSK3 phosphorylation site at the end. To determine whether any mutations found in human patients with ADPKD are located in this region, we searched in the ADPKD mutation database (http://pkdb.mayo.edu/). A total of 11 missense mutations have been identified within this region so far, including 1 highly likely pathogenic mutation at R4150 (R4150C) and 2 likely pathogenic substitutions at R4154 (R4154C) and F4155 (F4155V). Interestingly, all 3 pathogenic mutations are located at the N terminus of the 42-residue fragment, suggesting the importance of this region. In addition, there are 2 mutations or sequence changes located within the GSK3 motif; 1 is indeterminate (S4185W) and the other is likely neutral (S4190F). No missense mutations have been reported within the PKA motif (Fig. 2A).
Figure 2.
An 8-residue sequence including a PP1 binding motif in the CTT of PC1 serves as a novel CTS. A) Amino acid sequence alignment of the 42 residues in PC1 C tail from 8 species. The 3 putative motifs are underlined in color. The 11 reported human mutations within the 42 residues are listed below the sequences. Red, definitely, highly likely, or likely pathogenic mutations; black, indeterminate mutations; blue, likely neutral mutations. B) Schematic representation of CD16.7-ΔN44ΔC112 and CD16.7 fused with each of the 3 motifs. C) Expression of indicated constructs in B in the primary cilia of IMCD3 cells. Cells were stained by antibodies against CD16 (green) and acetylated α-tubulin (Ac-α-tub; red). Scale bar, 10 μm. D) Quantification of percentage of CD16-positive cilia in cells. Error bars represent the sd between microscope fields from ≥3 independent experiments. ****P < 0.0001 compared with CD16.7-ΔN44ΔC112 control.
In order to identify the sequence motif within the 42-residue fragment that mediates ciliary targeting of CD16.7, we constructed 3 mini-constructs by fusing smaller portions (8 residues) of the 42-residue fragment that contained ≥1 entire motif with CD16.7 (CD16.7-CPC1-PP1, CD16.7-CPC1-PKA, and CD16.7-CPC1-GSK3, respectively) (Fig. 2B). We found that CD16.7-CPC1-PKA failed to localize to cilia, suggesting that this 8-residue fragment consisting of 2 potential PKA phosphorylation sites lacks CTS activity. CD16.7-CPC1-GSK3 could traffic to cilia but with a much lower efficiency compared with CD16.7-ΔN44ΔC112, indicating that the GSK3 motif–containing 8-residue sequence has milder CTS activity (Fig. 2C, D). Consistently, ciliary localization of CD16.7-ΔN44ΔC112 was slightly reduced after GSK3 inhibitor BIO treatment, whereas CD16.7-VxP was not affected (Supplemental Fig. S2). Intriguingly, CD16.7-CPC1-PP1 could traffic to cilia almost as efficiently as CD16.7-ΔN44ΔC112, thus supporting the idea that the 8-residue sequence containing a PP1 docking motif acts as a novel CTS (Fig. 2C, D).
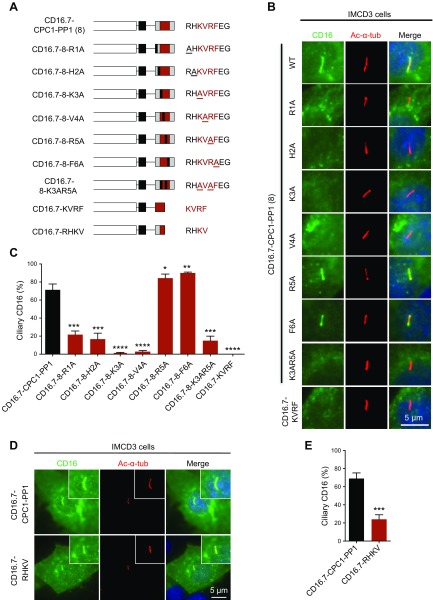
Alanine-scanning mutagenesis identifies crucial residues for CTS function
To further define the CTS, we next performed alanine-scanning mutagenesis to individually mutate each amino acid from R4150 to F4155 (R1 to F6 in mini-constructs) and tested whether the CTS activity was impaired (Fig. 3A). We found that mutations R4150A (R1A in mini-construct) and H4151A (H2A in mini-construct) dramatically reduced ciliary trafficking but did not completely hinder function, whereas mutations K4152A (K3A) and V4153A (V4A) blocked CTS function, indicating these 2 residues are most critical for the CTS to drive CD16.7 to cilia. At the other extreme, mutations R4154A (R5A) and F4155A (F6A) did not reduce CTS activity. Instead, the ciliary localization of these 2 chimeric proteins was even slightly increased compared with the WT control. The ciliary localization efficiency of another chimeric protein with double point mutations in K4152 and R4154 (CD16.7-8-K3AR5A) was reduced, although it was not as low as the single mutation CD16.7-8-K3A (Fig. 3B, C).
Figure 3.
Multiple residues in the novel CTS are crucial for chimeric proteins targeting to primary cilia. A) Schematic representation of CD16.7 fused with the 8-residue CTS bearing different point mutations or 2 4-residue sequences within the CTS. B) Expression of indicated constructs in A in the primary cilia of IMCD3 cells. Cells were stained by antibodies against CD16 (green) and acetylated α-tubulin (Ac-α-tub; red). Scale bar, 5 μm. C) CD16-positive cilia were quantified for all constructs in B. D) Expression of CD16.7 fused with either the novel 8-residue CTS or the first 4 key residues of the CTS in the primary cilia of IMCD3 cells. Scale bar, 5 μm. E) Quantification of percentage of CD16-positive cilia in cells. Error bars represent the sd between microscope fields from ≥3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with CD16.7-CPC1-PP1 control.
We also constructed a chimeric protein fusing CD16.7 with the 4-residue PP1 docking motif KVRF. However, this chimeric protein did not traffic to cilia (Fig. 3A–C), indicating these 4 residues, including the 2 key residues (K3 and V4) of the CTS, are not sufficient for ciliary targeting, and other residues flanking this motif are likely required. Consistent with this finding, CD16.7 fused with the first 4 residues (RHKV), which are all essential for ciliary targeting based on the alanine-scanning mutagenesis results, also had lower ciliary trafficking efficiency compared with CD16.7-CPC1-PP1 (Fig. 3D, E).
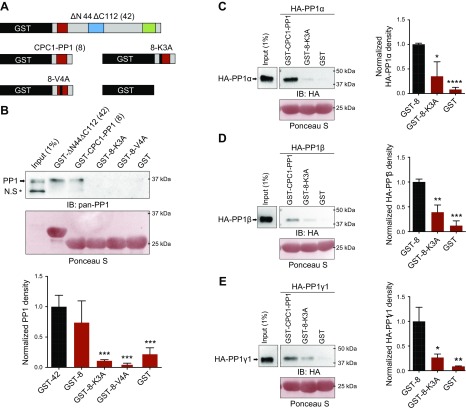
The newly identified CTS interacts with PP1
Mounting evidence has suggested that the function of PP1 regulatory proteins is related to binding to PP1, and point mutation within the PP1 anchoring motif is often sufficient to reduce the binding affinity with an apparent loss of association in pulldown assays (24, 36). To test if the CTS interacts with PP1, we next performed a GST-pulldown assay. We found that both GST-ΔN44ΔC112 and GST-CPC1-PP1 were able to interact with endogenous PP1 of IMCD3 cells, and mutations in 2 residues (Lys and Val) (GST-8-K3A, GST-8-V4A), which are critical for ciliary trafficking activity, disrupted the association between PP1 and the CTS (Fig. 4A, B).
Figure 4.
The novel CTS of PC1 interacts with PP1. A) Schematic representation of different GST fusion proteins used in this study. B) Endogenous PP1 in IMCD3 cell lysates pulled down by different GST fusion proteins in A. The Ponceau S–stained GST fusion proteins were used as loading controls. Anti–pan-PP1 antibody detects all isoforms of PP1 (indicated with arrow). IB, immunoblot; N.S, nonspecific band. Below the Western blot images is the quantification result of normalized endogenous PP1 density from ≥3 independent pulldown assays. ***P < 0.001 compared with GST-ΔN44ΔC112 (GST-42). C–E) The GST and GST fusion protein beads were used to pull down HA-PP1α (C), -β (D), and -γ1 (E), which are exogenously expressed in HEK293 cells. Exogenous PP1 proteins were blotted with an antibody against HA. The input and pulldown lanes are from a single gel but were cropped because of discontinuous loading. Quantification results of normalized density of different PP1 isoforms from 3 independent pulldown assays are shown to the right of respective Western blot images. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 compared with GST-CPC1-PP1 (GST-8).
We next asked which isoforms were able to interact with the 8-residue CTS. We overexpressed HA-tagged PP1 isoforms in HEK293 cells and used cell extracts for pulldown assays. We found that all 3 ubiquitous PP1 isoforms were able to bind with the newly identified CTS, and point mutation at the critical Lys residue (GST-8-K3A) dramatically reduced the binding affinity (Fig. 4C–E).
Mutations in the newly identified CTS impair ciliary trafficking of full-length PC1
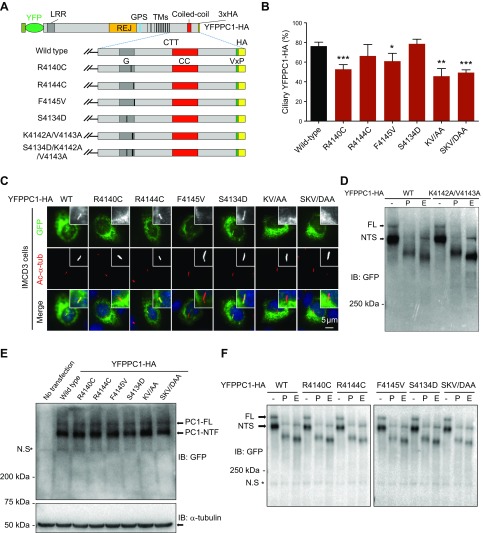
The new CTS is sufficient to drive a foreign protein (CD16.7) to cilia; does it still function in regulating ciliary trafficking of full-length PC1? As discussed above, the full-length PC1 traffics to cilia via a mechanism different from chimeric PC1 proteins. Thus, we introduced several point mutations within this CTS to YFP- and HA-tagged full-length mouse PC1 and tested the functionality of the newly identified CTS by quantifying their ciliary trafficking efficiency (Fig. 5A). We first tested the mouse equivalents (YFPPC1-R4140C-HA, YFPPC1-R4144C, and YFPPC1-F4145V-HA, respectively) of the 3 naturally occurring human pathogenic mutations (R4150C, R4154C and F4155V). A total of 2 of these mutations (not R4144C) had slightly reduced ciliary localization (Fig. 5B, C), further supporting the idea that ciliary trafficking can be used as a functional assay for a subgroup of pathogenic PC1 mutations (12, 15). We also investigated the consequence of disrupting PC1-PP1 binding by mutating the 2 key residues, Lys and Val (YFPPC1-K4142A/V4143A-HA). We found that YFPPC1-K4142A/V4143A-HA also had significant reduction in ciliary localization (Fig. 5B, C), and this is not due to reduced PC1 expression level or abnormal GPS cleavage (Fig. 5D). These data suggest that this CTS, unlike the VxP motif, functions in full-length PC1 trafficking, and PP1 might regulate this process.
Figure 5.
Ciliary targeting of full-length PC1 is compromised by mutations that affect PC1-PP1 binding. A) Schematic representation of full-length YFPPC1-HA and various point mutant variants. B) Respective constructs in A were individually expressed in IMCD3 cells. Ciliary expression of these constructs was evaluated by staining with antibody against GFP (green), and cilia were marked with acetylated α-tubulin (Ac-α-tub; red). Representative images of each construct to function are shown. Scale bar, 5 μm. C) Quantification of percentage of YFPPC1-HA–positive cilia in cells. A total of ≥50 ciliated, PC1-transfected cells were counted for the presence of the YFPPC1-HA signal on cilia under each condition. Error bars represent the sd between microscope fields from ≥3 independent experiments. D) Deglycosylation analysis of YFPPC1-HA WT or K4142A/V4143A mutant exogenously expressed in HEK293 cells. Representative blots are shown from 3 independent experiments. E) Western blots of recombinant PC1 expressed in HEK293 cells, PC1 was detected using an antibody against GFP, and α-tubulin was used as loading control. NTF, N-terminal fragment. F) Deglycosylation analysis of WT and mutant full-length PC1. All constructs were transfected into HEK293 cells, and cell lysates were incubated with either reaction buffer alone (−), peptide:N-glycosidase F (P), or endoglycosidase H (E) and detected with an antibody against GFP. CC, coiled-coil domain; FL, full-length; G, G-protein activation domain; IB, immunoblot; KV/AA, K4142A/V4143A; SKV/DAA, S4134D/K4142A/V4143A; LRR, leucine-rich repeat; NTS, N-terminal fragment, endoglycosidase H sensitive; N.S, non-specific band; REJ, receptor for egg jelly; TMs, transmembrane domains. *P < 0.05, **P < 0.01, ***P < 0.001 compared with WT control.
Several studies have revealed that PP1 can form a complex with calmodulin, and this complex is essential for intracellular trafficking (20, 37). Interestingly, the PC1 CTT also contains a calmodulin-binding site overlapping with the G-protein activation domain, which is close to the PP1 docking motif, and point mutation S4144D (mouse equivalent, S4134D) impairs PC1-calmodulin interaction (38). To test whether PP1 and calmodulin function together to regulate PC1 trafficking, we generated 2 more mouse full-length PC1 constructs, YFPPC1-S4134D-HA and YFPPC1-S4134D/K4142A/V4143A-HA, to disrupt the calmodulin-PC1 binding or calmodulin-PC1 and PP1-PC1 binding simultaneously. Consistent with a previous study (38), the S4134D mutation did not impair PC1 ciliary localization. Triple point mutations in PC1 significantly reduced its ciliary localization, whereas the efficiency is similar to that of YFPPC1-K4142A/V4143A-HA (Fig. 5B, C), indicating that calmodulin does not act synergistically with PP1 to regulate PC1 trafficking. Similar to K4142A/V4143A mutation, the other mutations did not affect the protein expression or GPS cleavage of PC1 either. However, in the glycosylation assay, we could only detect the FL and immature glycoform of PC1 N-terminal fragment (PC1-NTS) in all exogenously expressed PC1, including the WT (Fig. 5D–F). The probable explanation for the absence of detectable mature glycoform (PC1-NTR) is that the endogenous PC2 level in HEK293 is very low (unpublished results) and the PC1-NTR is not evident at a short exposure, which is similar to a previous observation from Gainullin et al. (39).
PP1α regulates cilium length and PC1 trafficking
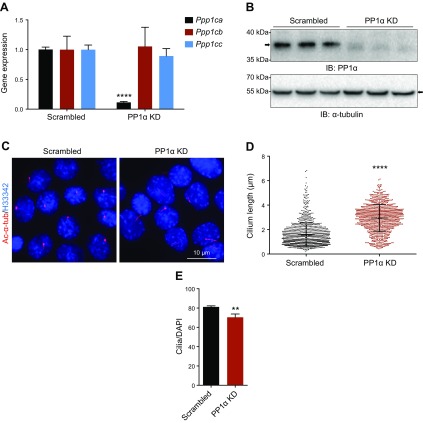
To further delineate the role of PP1 in PC1 ciliary trafficking, we focused on PP1α for more detailed investigation. We used short hairpin RNA to selectively knock down endogenous PP1α expression in IMCD3 cells (Fig. 6A, B). Compared with control cells, we found that depletion of PP1α led to a significant increase in cilium length (Fig. 6C, D) and the ciliation rate in knockdown cells was slightly reduced (Fig. 6E), suggesting a role of PP1α in regulating cilium length and ciliogenesis.
Figure 6.
PP1α regulates cilium length and ciliation rate. A) quantitative RT-PCR analysis of PP1α depletion by lentiviral short hairpin RNA (shRNA) in IMCD3 cells. The relative knockdown (KD) efficiency of Ppp1ca was around 90%, whereas the expression of Ppp1cb and Ppp1cc was not affected compared with scrambled control. ****P < 0.0001 compared with the scrambled group. B) Western blot analysis confirms the KD of PP1α in IMCD3 cells. C) Depletion of PP1α in IMCD3 cells affected cilium length as evaluated by bisBenzimide H 33342 trihydrochloride (H33342) and acetylated α-tubulin (Ac-α-tub; red) staining. Scale bar, 10 μm. D) The length of cilia in scrambled and PP1α-KD IMCD3 cells at 36 h post serum starvation was measured (n > 1000). Error bars represent sd. ****P < 0.0001 compared with scrambled control. E) Quantification of the percentage of ciliated cells in control and PP1α-KD IMCD3 cells. Error bars represent the sd between microscope fields from ≥3 independent experiments. IB, immunoblot. **P < 0.01 compared with scrambled control.
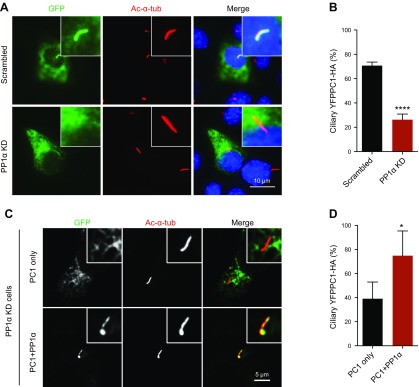
We next evaluated the effect of PP1α knockdown on PC1. We found that the GPS cleavage and maturation of endogenous PC1 in these cells were not affected (Supplemental Fig. S3); nevertheless, the ciliary localization of PC1 was significantly reduced in knockdown cells and can be rescued by overexpression of exogenous PP1α (Fig. 7), further confirming that PP1α is responsible for regulating PC1 trafficking to cilia.
Figure 7.
PP1α regulates PC1 ciliary localization. A) Depletion of PP1α affected PC1 trafficking to cilia in IMCD3 cells. YFPPC1-HA was visualized with an antibody against GFP (green). Cilia were marked with acetylated α-tubulin (Ac-α-tub; red). Scale bar, 10 μm. B) Percentage of YFPPC1-HA–positive cilia in A. A total of ≥50 ciliated, PC1-transfected cells were counted under each condition. Error bars represent the sd between microscope fields from ≥3 independent experiments. ****P < 0.0001 compared with scrambled control. C) PP1α–knockdown (KD) cells were transiently transfected with YFPPC1-HA alone or together with HA-PP1α. Ciliary expression of PC1 was evaluated by staining with antibody against GFP (green), and cilia were marked with acetylated α-tubulin (Ac-α-tub; red). Representative images of each construct to function are shown. Scale bar, 5 μm. D) Quantification of percentage of YFPPC1-HA–positive cilia in cells. *P < 0.05 compared with the YFPPC1-HA single-transfection group.
DISCUSSION
The primary cilium harbors hundreds of proteins that function in diverse cellular pathways (40). How most of these proteins traffic to cilia after being synthesized in the endoplasmic reticulum remains obscure. One explanation is that the ciliary proteins contain unique sequences that can be recognized by specific ciliary trafficking machineries (41). The VxP motif is 1 of the most comprehensively studied CTSs and has been found in several ciliary proteins, including rhodopsin, a 7-transmembrane protein that is highly concentrated in the rod outer segments of retinal photoreceptors (42). It has been reported that the VxP motif drives rhodopsin to cilia via interaction with the small GTPase Arf4 (18).
Previous studies have revealed that both PC1 and PC2 contain a VxP CTS motif similar to rhodopsin. This motif in PC1 is located in its extreme C terminus, whereas the 1 in PC2 appears in the first 15 residues of the cytoplasmic domain at the N terminus (19, 43). Recognition of the VxP motif by Arf4 has been proposed to regulate PC1 ciliary trafficking. Nonetheless, we found that the VxP motif was not required for full-length PC1 targeting to cilia. Knockdown of Arf4 did not affect PC1 ciliary localization (15). Endogenous full-length PC1 does not bind with Arf4 in collecting duct cells (14). Moreover, depletion of Arf4 in vivo results in neither ciliary defects nor cystic kidneys, and it was concluded that the VxP motif is not a predictor of ciliary targeting (44). These results have raised the possibility that there are other functional CTSs in full-length PC1 used for intracellular trafficking.
We have identified a new 8-residue functional CTS in PC1. This CTS does not share any consensus with other known CTSs (41, 45, 46). The CTSs of several ciliary proteins have been shown to directly interact with several regulators such as the BBSome that modulates intracellular trafficking, including ciliary trafficking and export (47, 48). In this study we showed the 8-residue RHKV motif (named based on the first 4 key amino acids), which contains a PP1 RVxF docking site, could bind with PP1, a protein that is implicated in regulating the terminal step of intracellular membrane fusion (20). Notably, the RHKV motif is sufficient to bind all 3 major PP1 isoforms. This is reasonable because the PP1 isoforms are about 90% identical in mammals, and the differences between these isoforms mainly reside in the C termini. Some of the PP1 regulatory proteins interact with PP1 in an isoform-specific manner (49, 50) but most regulatory proteins do not exclusively bind with certain PP1 isoforms. Mutations in the PP1 RVxF binding motif usually disturb the interaction between PP1 and its regulatory proteins (24, 51). Our GST-pulldown assays confirmed that mutations of the key residues in the CTS severely reduced the binding affinity between the CTS and all 3 PP1 isoforms. The fact that the PP1 binding ability of WT or mutant CTSs correlated well with their respective CTS activity supports the idea that the PC1-PP1 interaction is critical for PC1 trafficking to cilia.
Unlike what we observed with the chimeric protein, mutations that disturb PC1-PP1 binding did not completely block full-length PC1 ciliary trafficking. There may be several reasons accounting for this observation. Firstly, the mutations only reduced the binding affinity between PC1 and PP1; thus, some PP1 is still bound to mutant PC1 to regulate its trafficking, and, similarly, the residual level of PP1α in knockdown cells may function in promoting PC1 trafficking. Secondly, we have shown that PC1 interacts with PP1α, PP1β, and PP1γ1. The β and γ1 isoforms may also be able to regulate PC1 trafficking, particularly when the PP1α expression level is low. Thirdly, we and others have shown that multiple sequences, including the coiled-coil domain in the PC1 CTT, are important for full-length PC1 targeting to cilia. The coiled-coil domain is required for PC1-PC2 complex formation and is a determinant of PC1 ciliary localization (15). This domain is also essential for PC1 to bind with RAB GTPase binding effector protein 1, which forms a complex with golgi-localized, gamma adaptin ear-containing, ARF-binding 1 and ARF-like GTPase 3 to function as a ciliary sorting module of PC1 (14). These results suggest that PP1 is 1 of the underlying mechanisms regulating PC1 ciliary trafficking.
Phosphorylation and dephosphorylation are important post-translational modifications of a protein that affect protein functions such as altering protein assembly, targeting, or activity (52). Both PC1 and PC2 can be phosphorylated or dephosphorylated by several kinases (28, 35, 53–55) or phosphatases (27, 28, 56). It has been reported that in ADPKD cells, PC1 is highly phosphorylated and mislocalized compared with control cells (57). Surface localization of PC2 has also been shown to be regulated by GSK3-mediated phosphorylation at the N terminus (55). A recent study suggests that the PC1, lipoxygenase, alpha-toxin domain in the first intracellular loop of PC1 is involved in regulating PC1 trafficking and phosphorylation of a serine in this domain by PKA reduces PC1 localization on the plasma membrane as well as primary cilia, whereas a phosphodeficient mutation has normal cilia localization (58). Former studies have implied an association between PC1 phosphorylation status and its intracellular localization. A previous study has shown that PKA can phosphorylate mouse PC1 at S4159, which can be then dephosphorylated by PP1α (27). To determine whether PP1α affects PC1 ciliary localization by modulating its phosphostatus, we mutated S4159 and another potential PKA phosphorylation site of full-length PC1 (S4156A, S4156D, S4159A, and S4159D). Intriguingly, none of these mutations affected PC1 trafficking to cilia (unpublished results), indicating that PP1α-mediated PC1 dephosphorylation might not be related to PC1 ciliary trafficking, but we cannot rule out the possibility that PP1α can dephosphorylate PC1 at other unknown sites that might affect PC1 trafficking. In addition, it is possible that PP1α indirectly regulates PC1 ciliary trafficking via altering the phosphorylation status of other PC1 binding proteins, which are responsible for PC1 localization. We and other groups have previously shown that PC2, the most well-known PC1 binding protein, is a prerequisite for PC1 targeting to cilia (12, 14, 15). Interestingly, PP1α can also dephosphorylate PC2 at S829 via binding with PC1 because PP1α and PC2 do not directly interact, and endogenous PC2 with phosphorylation at S829 is absent from cilia (28). Thus, we speculate that the reduced ciliary localization of mutated PC1 in IMCD3 cells or WT PC1 in PP1α-knockdown cells in our study might be due to weakened PP1α-PC1 interaction, which leads to an alteration of PC2 phosphorylation and eventually affecs PC2 function and regulation in PC1 localization to cilia.
The primary cilium is a highly dynamic structure related to the cell cycle (8, 59, 60), and PP1 plays a vital role in cell cycle regulation (21, 22). However, we did not observe an obvious cell cycle change in PP1α-knockdown cells (unpublished results); this is probably due to the residual PP1α or existence of other PP1 isoforms. Reduced expression of several ciliary proteins has been shown to cause elongated cilia (61–63). Therefore, we speculate that the lengthened cilia in PP1α-knockdown cells might be an indirect effect of reduced ciliary PC1, even through the underlying mechanism remains obscure.
In summary, we have identified a novel 8-residue CTS, RHKV, that is highly evolutionarily conserved in multiple species from zebrafish to humans. This CTS regulates the trafficking of a foreign protein and the full-length ADPKD protein PC1. We have also demonstrated that this CTS interacts with a protein phosphatase, PP1, and knockdown of PP1α leads to impaired ciliary trafficking of PC1 and elongated cilia. It remains to be investigated whether PP1 is a modifier of polycystic kidney disease.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank the members of the Zhou laboratory and Harvard Center for Polycystic Kidney Disease Research for scientific discussions and support. This work was supported by grants from the U.S. National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (RO1DK099532 and R37DK51050 to J.Z.) and by grants from National Natural Science Foundation of China (81470938, 81770687, 81770752, 81770750). The authors declare no conflicts of interest.
Glossary
- ADPKD
autosomal dominant polycystic kidney disease
- Arf4
ADP ribosylation factor 4
- CTS
ciliary targeting sequence
- CTT
C-terminal tail
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- GSK3
glycogen synthase kinase 3
- GST
glutathione S-transferase
- HA
human influenza hemagglutinin
- IMCD3
inner medullary collecting duct
- KO
knockout
- PC1
polycystin-1
- PC2
polycystin-2
- PKD
polycystic kidney disease
- PP1
protein phosphatase 1
- PPP
phosphoprotein phosphatase
- WT
wild type
- YFP
yellow fluorescent protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
C. Luo contributed to the design of the project and wrote the manuscript; C. Luo, M. Wu, X. Su, and F. Yu performed experiments; D. L. Brautigan provided important reagents, contributed to the design of the project and data analysis, and edited the manuscript; J. Chen contributed to directing the project; and J. Zhou conceived, designed and directed the project, and edited the manuscript.
REFERENCES
- 1.Malicki J. J., Johnson C. A. (2017) The cilium: cellular antenna and central processing unit. Trends Cell Biol. 27, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun D. A., Hildebrandt F. (2016) Ciliopathies. Cold Spring Harb. Perspect. Biol. 9, a028191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres V. E., Harris P. C., Pirson Y. (2007) Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 [DOI] [PubMed] [Google Scholar]
- 4.Wilson P. D. (2004) Polycystic kidney disease. N. Engl. J. Med. 350, 151–164 [DOI] [PubMed] [Google Scholar]
- 5.Hughes J., Ward C. J., Peral B., Aspinwall R., Clark K., San Millán J. L., Gamble V., Harris P. C. (1995) The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 10, 151–160 [DOI] [PubMed] [Google Scholar]
- 6.The International Polycystic Kidney Disease Consortium (1995) Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell 81, 289–298 [DOI] [PubMed] [Google Scholar]
- 7.Mochizuki T., Wu G., Hayashi T., Xenophontos S. L., Veldhuisen B., Saris J. J., Reynolds D. M., Cai Y., Gabow P. A., Pierides A., Kimberling W. J., Breuning M. H., Deltas C. C., Peters D. J., Somlo S. (1996) PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339–1342 [DOI] [PubMed] [Google Scholar]
- 8.Zhou J. (2009) Polycystins and primary cilia: primers for cell cycle progression. Annu. Rev. Physiol. 71, 83–113 [DOI] [PubMed] [Google Scholar]
- 9.Delmas P., Nomura H., Li X., Lakkis M., Luo Y., Segal Y., Fernández-Fernández J. M., Harris P., Frischauf A. M., Brown D. A., Zhou J. (2002) Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J. Biol. Chem. 277, 11276–11283 [DOI] [PubMed] [Google Scholar]
- 10.Nauli S. M., Alenghat F. J., Luo Y., Williams E., Vassilev P., Li X., Elia A. E., Lu W., Brown E. M., Quinn S. J., Ingber D. E., Zhou J. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 [DOI] [PubMed] [Google Scholar]
- 11.Nauli S. M., Kawanabe Y., Kaminski J. J., Pearce W. J., Ingber D. E., Zhou J. (2008) Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117, 1161–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Y., Fedeles S. V., Dong K., Anyatonwu G., Onoe T., Mitobe M., Gao J. D., Okuhara D., Tian X., Gallagher A. R., Tang Z., Xie X., Lalioti M. D., Lee A. H., Ehrlich B. E., Somlo S. (2014) Altered trafficking and stability of polycystins underlie polycystic kidney disease. J. Clin. Invest. 124, 5129–5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su X., Driscoll K., Yao G., Raed A., Wu M., Beales P. L., Zhou J. (2014) Bardet-Biedl syndrome proteins 1 and 3 regulate the ciliary trafficking of polycystic kidney disease 1 protein. Hum. Mol. Genet. 23, 5441–5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H., Xu H., Yao Q., Li W., Huang Q., Outeda P., Cebotaru V., Chiaravalli M., Boletta A., Piontek K., Germino G. G., Weinman E. J., Watnick T., Qian F. (2014) Ciliary membrane proteins traffic through the Golgi via a Rabep1/GGA1/Arl3-dependent mechanism. Nat. Commun. 5, 5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su X., Wu M., Yao G., El-Jouni W., Luo C., Tabari A., Zhou J. (2015) Regulation of polycystin-1 ciliary trafficking by motifs at its C-terminus and polycystin-2 but not by cleavage at the GPS site. J. Cell Sci. 128, 4063–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsiao Y. C., Tuz K., Ferland R. J. (2012) Trafficking in and to the primary cilium. Cilia 1, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhopadhyay S., Badgandi H. B., Hwang S. H., Somatilaka B., Shimada I. S., Pal K. (2017) Trafficking to the primary cilium membrane. Mol. Biol. Cell 28, 233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazelova J., Astuto-Gribble L., Inoue H., Tam B. M., Schonteich E., Prekeris R., Moritz O. L., Randazzo P. A., Deretic D. (2009) Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 28, 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward H. H., Brown-Glaberman U., Wang J., Morita Y., Alper S. L., Bedrick E. J., Gattone V. H., II, Deretic D., Wandinger-Ness A. (2011) A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol. Biol. Cell 22, 3289–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters C., Andrews P. D., Stark M. J., Cesaro-Tadic S., Glatz A., Podtelejnikov A., Mann M., Mayer A. (1999) Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science 285, 1084–1087 [DOI] [PubMed] [Google Scholar]
- 21.Lesage B., Qian J., Bollen M. (2011) Spindle checkpoint silencing: PP1 tips the balance. Curr. Biol. 21, R898–R903 [DOI] [PubMed] [Google Scholar]
- 22.Rebelo S., Santos M., Martins F., da Cruz e Silva E. F., da Cruz e Silva O. A. (2015) Protein phosphatase 1 is a key player in nuclear events. Cell. Signal. 27, 2589–2598 [DOI] [PubMed] [Google Scholar]
- 23.Heroes E., Lesage B., Gornemann J., Beullens M., Van Meervelt L., Bollen M. (2013) The PP1 binding code: a molecular-lego strategy that governs specificity. FEBS. J. 280, 584–595 [DOI] [PubMed] [Google Scholar]
- 24.Egloff M. P., Johnson D. F., Moorhead G., Cohen P. T., Cohen P., Barford D. (1997) Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 16, 1876–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terrak M., Kerff F., Langsetmo K., Tao T., Dominguez R. (2004) Structural basis of protein phosphatase 1 regulation. Nature 429, 780–784 [DOI] [PubMed] [Google Scholar]
- 26.Shi Y. (2009) Serine/threonine phosphatases: mechanism through structure. Cell 139, 468–484 [DOI] [PubMed] [Google Scholar]
- 27.Parnell S. C., Puri S., Wallace D. P., Calvet J. P. (2012) Protein phosphatase-1α interacts with and dephosphorylates polycystin-1. PLoS One 7, e36798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streets A. J., Wessely O., Peters D. J., Ong A. C. (2013) Hyperphosphorylation of polycystin-2 at a critical residue in disease reveals an essential role for polycystin-1-regulated dephosphorylation. Hum. Mol. Genet. 22, 1924–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S., Wu M., Yao G., Zhang J., Zhou J. (2014) The cytoplasmic tail of FPC antagonizes the full-length protein in the regulation of mTOR pathway. PLoS One 9, e95630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu C., Rossetti S., Jiang L., Harris P. C., Brown-Glaberman U., Wandinger-Ness A., Bacallao R., Alper S. L. (2007) Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am. J. Physiol. Renal Physiol. 292, F930–F945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chernova M. N., Vandorpe D. H., Clark J. S., Alper S. L. (2005) Expression of the polycystin-1 C-terminal cytoplasmic tail increases Cl channel activity in Xenopus oocytes. Kidney Int. 68, 632–641 [DOI] [PubMed] [Google Scholar]
- 32.Chebib F. T., Sussman C. R., Wang X., Harris P. C., Torres V. E. (2015) Vasopressin and disruption of calcium signalling in polycystic kidney disease. Nat. Rev. Nephrol. 11, 451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parnell S. C., Magenheimer B. S., Maser R. L., Rankin C. A., Smine A., Okamoto T., Calvet J. P. (1998) The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem. Biophys. Res. Commun. 251, 625–631 [DOI] [PubMed] [Google Scholar]
- 34.Chauvet V., Tian X., Husson H., Grimm D. H., Wang T., Hiesberger T., Igarashi P., Bennett A. M., Ibraghimov-Beskrovnaya O., Somlo S., Caplan M. J. (2004) Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J. Clin. Invest. 114, 1433–1443; erratum: 115, 788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parnell S. C., Magenheimer B. S., Maser R. L., Calvet J. P. (1999) Identification of the major site of in vitro PKA phosphorylation in the polycystin-1 C-terminal cytosolic domain. Biochem. Biophys. Res. Commun. 259, 539–543 [DOI] [PubMed] [Google Scholar]
- 36.Hendrickx A., Beullens M., Ceulemans H., Den Abt T., Van Eynde A., Nicolaescu E., Lesage B., Bollen M. (2009) Docking motif-guided mapping of the interactome of protein phosphatase-1. Chem. Biol. 16, 365–371 [DOI] [PubMed] [Google Scholar]
- 37.Peters C., Mayer A. (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396, 575–580 [DOI] [PubMed] [Google Scholar]
- 38.Doerr N., Wang Y., Kipp K. R., Liu G., Benza J. J., Pletnev V., Pavlov T. S., Staruschenko A., Mohieldin A. M., Takahashi M., Nauli S. M., Weimbs T. (2016) Regulation of polycystin-1 function by calmodulin binding. PLoS One 11, e0161525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gainullin V. G., Hopp K., Ward C. J., Hommerding C. J., Harris P. C. (2015) Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner. J. Clin. Invest. 125, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa H., Thompson J., Yates J. R., III, Marshall W. F. (2012) Proteomic analysis of mammalian primary cilia. Curr. Biol. 22, 414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berbari N. F., Johnson A. D., Lewis J. S., Askwith C. C., Mykytyn K. (2008) Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol. Biol. Cell 19, 1540–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deretic D., Schmerl S., Hargrave P. A., Arendt A., McDowell J. H. (1998) Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc. Natl. Acad. Sci. USA 95, 10620–10625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geng L., Okuhara D., Yu Z., Tian X., Cai Y., Shibazaki S., Somlo S. (2006) Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J. Cell Sci. 119, 1383–1395 [DOI] [PubMed] [Google Scholar]
- 44.Pearring J. N., San Agustin J. T., Lobanova E. S., Gabriel C. J., Lieu E. C., Monis W. J., Stuck M. W., Strittmatter L., Jaber S. M., Arshavsky V. Y., Pazour G. J. (2017) Loss of Arf4 causes severe degeneration of the exocrine pancreas but not cystic kidney disease or retinal degeneration. PLoS Genet. 13, e1006740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loktev A. V., Jackson P. K. (2013) Neuropeptide Y family receptors traffic via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia. Cell Reports 5, 1316–1329 [DOI] [PubMed] [Google Scholar]
- 46.Mukhopadhyay S., Wen X., Ratti N., Loktev A., Rangell L., Scales S. J., Jackson P. K. (2013) The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell 152, 210–223 [DOI] [PubMed] [Google Scholar]
- 47.Badgandi H. B., Hwang S. H., Shimada I. S., Loriot E., Mukhopadhyay S. (2017) Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J. Cell Biol. 216, 743–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu P., Lechtreck K. F. (2018) The Bardet-Biedl syndrome protein complex is an adapter expanding the cargo range of intraflagellar transport trains for ciliary export. Proc. Natl. Acad. Sci. USA 115, E934–E943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colbran R. J., Bass M. A., McNeill R. B., Bollen M., Zhao S., Wadzinski B. E., Strack S. (1997) Association of brain protein phosphatase 1 with cytoskeletal targeting/regulatory subunits. J. Neurochem. 69, 920–929 [DOI] [PubMed] [Google Scholar]
- 50.MacMillan L. B., Bass M. A., Cheng N., Howard E. F., Tamura M., Strack S., Wadzinski B. E., Colbran R. J. (1999) Brain actin-associated protein phosphatase 1 holoenzymes containing spinophilin, neurabin, and selected catalytic subunit isoforms. J. Biol. Chem. 274, 35845–35854 [DOI] [PubMed] [Google Scholar]
- 51.Moorhead G. B., Trinkle-Mulcahy L., Nimick M., De Wever V., Campbell D. G., Gourlay R., Lam Y. W., Lamond A. I. (2008) Displacement affinity chromatography of protein phosphatase one (PP1) complexes. BMC Biochem. 9, 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen P. (2000) The regulation of protein function by multisite phosphorylation--a 25 year update. Trends Biochem. Sci. 25, 596–601 [DOI] [PubMed] [Google Scholar]
- 53.Li H. P., Geng L., Burrow C. R., Wilson P. D. (1999) Identification of phosphorylation sites in the PKD1-encoded protein C-terminal domain. Biochem. Biophys. Res. Commun. 259, 356–363 [DOI] [PubMed] [Google Scholar]
- 54.Geng L., Burrow C. R., Li H. P., Wilson P. D. (2000) Modification of the composition of polycystin-1 multiprotein complexes by calcium and tyrosine phosphorylation. Biochim. Biophys. Acta 1535, 21–35 [DOI] [PubMed] [Google Scholar]
- 55.Streets A. J., Moon D. J., Kane M. E., Obara T., Ong A. C. (2006) Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum. Mol. Genet. 15, 1465–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boucher C. A., Ward H. H., Case R. L., Thurston K. S., Li X., Needham A., Romero E., Hyink D., Qamar S., Roitbak T., Powell S., Ward C., Wilson P. D., Wandinger-Ness A., Sandford R. N. (2011) Receptor protein tyrosine phosphatases are novel components of a polycystin complex. Biochim. Biophys. Acta 1812, 1225–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roitbak T., Ward C. J., Harris P. C., Bacallao R., Ness S. A., Wandinger-Ness A. (2004) A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol. Biol. Cell 15, 1334–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y., Streets A. J., Hounslow A. M., Tran U., Jean-Alphonse F., Needham A. J., Vilardaga J. P., Wessely O., Williamson M. P., Ong A. C. (2016) The polycystin-1, lipoxygenase, and α-toxin domain regulates polycystin-1 trafficking. J. Am. Soc. Nephrol. 27, 1159–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerdes J. M., Davis E. E., Katsanis N. (2009) The vertebrate primary cilium in development, homeostasis, and disease. Cell 137, 32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phua S. C., Chiba S., Suzuki M., Su E., Roberson E. C., Pusapati G. V., Setou M., Rohatgi R., Reiter J. F., Ikegami K., Inoue T. (2017) Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell 168, 264–279.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartman T. R., Liu D., Zilfou J. T., Robb V., Morrison T., Watnick T., Henske E. P. (2009) The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum. Mol. Genet. 18, 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DiBella L. M., Park A., Sun Z. (2009) Zebrafish Tsc1 reveals functional interactions between the cilium and the TOR pathway. Hum. Mol. Genet. 18, 595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonnet C. S., Aldred M., von Ruhland C., Harris R., Sandford R., Cheadle J. P. (2009) Defects in cell polarity underlie TSC and ADPKD-associated cystogenesis. Hum. Mol. Genet. 18, 2166–2176 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.