Abstract
Healthy cardiomyocytes are electrically coupled at the intercalated discs by gap junctions. In infarcted hearts, adverse gap-junctional remodeling occurs in the border zone, where cardiomyocytes are chemically and electrically influenced by myofibroblasts. The physical movement of these contacts remains unquantified. Using scanning ion conductance microscopy, we show that intercellular contacts between cardiomyocytes and myofibroblasts are highly dynamic, mainly owing to the edge dynamics (lamellipodia) of the myofibroblasts. Decreasing the amount of functional connexin-43 (Cx43) at the membrane through Cx43 silencing, suppression of Cx43 trafficking, or hypoxia-induced Cx43 internalization attenuates heterocellular contact dynamism. However, we found decreased dynamism and stabilized membrane contacts when cellular coupling was strengthened using 4-phenylbutyrate (4PB). Fluorescent-dye transfer between cells showed that the extent of functional coupling between the 2 cell types correlated with contact dynamism. Intercellular calcein transfer from myofibroblasts to cardiomyocytes is reduced after myofibroblast-specific Cx43 down-regulation. Conversely, 4PB-treated myofibroblasts increased their functional coupling to cardiomyocytes. Consistent with lamellipodia-mediated contacts, latrunculin-B decreases dynamism, lowers physical communication between heterocellular pairs, and reduces Cx43 intensity in contact regions. Our data show that heterocellular cardiomyocyte–myofibroblast contacts exhibit high dynamism. Therefore, Cx43 is a potential target for prevention of aberrant cardiomyocyte coupling and myofibroblast proliferation in the infarct border zone.—Schultz, F., Swiatlowska, P., Alvarez-Laviada, A., Sanchez-Alonso, J. L., Song, Q., de Vries, A. A. F., Pijnappels, D. A., Ongstad, E., Braga, V. M. M., Entcheva, E., Gourdie, R. G., Miragoli, M., Gorelik, J. Cardiomyocyte–myofibroblast contact dynamism is modulated by connexin-43.
Keywords: heart failure, myocardial infarction, Cx43, heterocellular coupling
Communication between cells is necessary to maintain proper organ development and function. Coupling between both cells of the same cell type as well as between different cell types (heterocellular coupling) occurs ubiquitously throughout numerous tissues (1–5). Formation of cell–cell contacts via stable junctions is the basis of cellular crosstalk. Twelve connexin-hemichannel proteins form a stable gap junction between 2 cells. This coupling has been shown to be present in different cell types, such as neurons, vascular smooth muscle cells, and cardiomyocytes (1–7). Independently of the tissue and composition of different connexin subunits, these gap junctions perform the same electrical and chemical functions.
Intercellular communication controls critical physiologic processes, facilitates dynamic tissue responses, and promotes tissue remodeling triggered by pathologic events. In the healthy heart, operating at the highest level of multicellular synchronicity of action, cellular packing and strong electrical communication are essential for its operation. Interdispersed among the highly coupled adult ventricular cardiomyocytes are a large number of fibroblasts and myofibroblasts (8, 9). These mesenchymal cells are 2 to 3 times more abundant than cardiomyocytes, but because of their smaller size, they only comprise ∼20% of the cardiac muscle tissue (8). Interactions between cardiomyocytes and fibroblasts occur in the heart at different levels. The paracrine functions of fibroblasts have been characterized, including secretion of TGF-β and other soluble factors (10, 11). Of particular note, fibroblasts synthesize extracellular matrix fibers, such as collagen and fibronectin, which form the 3-dimensional scaffold of the heart that supports the cardiomyocytes. It has also been shown that cardiomyocytes and fibroblasts in the heart are mechanically coupled either via the extracellular matrix or via adherens junctions; however, little is known about the importance of such interactions (12). Finally, electrical coupling may occur between the 2 cell types (9), although to what extent this occurs and what the role of heterocellular coupling in the normal heart is remains poorly understood. In contrast to cardiomyocytes, fibroblasts are motile cells that constantly form new contacts with neighboring cells and that remodel the extracellular matrix; both of these processes depend on the actin cytoskeleton motility of cardiac fibroblasts. The potential impact of the motility of fibroblasts on the coupling between cardiomyocytes and fibroblasts has not yet been investigated.
After an ischemic event, such as myocardial infarction, myofibroblasts proliferate, specifically in the region in which the injury occurred, as well as in the surrounding area. Fibroblasts are replaced by, or differentiate into, myofibroblasts, which are characterized by a number of distinct markers, including de novo expression of α-smooth muscle actin (α-SMA) (13). Myofibroblasts have been implicated in the formation of myocardial scars and scar expansion, which in turn has been linked to an increase in arrhythmogenesis (14–18). A narrow atypical zone of myocardial tissue that surrounds the scar is where close contacts between myofibroblasts and cardiomyocytes occur; this is in contrast to the scar proper, where few cardiomyocytes survive because of necrosis (19).
Transformation or infiltration of myofibroblasts is thought to be triggered by a number of signals, including local inflammatory reactions, mechanical stress, and various cytokines and biochemical factors (9, 17). Cultured myofibroblasts have been shown to couple to cardiomyocytes through gap junction-based interactions (14). However, it is not clear whether gap-junctional coupling occurs between myofibroblasts and cardiomyocytes in the diseased myocardium in vivo, although a recent study has shown that myofibroblasts can electrically couple to cardiomyocytes in the infarct border zone of isolated, Langendorff-perfused mouse hearts ex vivo (20). Camelliti et al. (21) demonstrated that the main connexin subtypes that are expressed by resident fibroblasts in infarct scars are connexin-43(Cx43) and connexin-45 (Cx45) (14, 21, 22). It is thought that when an ischemic event occurs, cardiomyocytes undergo structural remodeling, with Cx43 moving from the intercalated discs at the polar ends of the cell to the lateral surfaces. This lateralization of Cx43 increases the likelihood of cardiomyocyte-to-myofibroblast (CM–MFB) coupling and the associated risk of arrhythmia (21).
Myofibroblasts appear to have complex roles, as, depending on the setting, they have been implicated in both pro- or antiarrhythmic modulation. Heterocellular electrotonic communication has been shown to be detrimental to cardiac function in computer-modeling and experimental studies because myofibroblasts are thought to directly depolarize cardiomyocytes, slowing action potential conduction and promoting ectopic electrical activity (14, 23–26). On the other hand, myofibroblast–cardiomyocyte coupling by gap junctions can have a positive impact on conduction across discontinuities in networks of cardiomyocytes (15). In a study of recipient and donor atria, where a scar formed in the region of the suture, evidence of successful restoration of electrical activity was found (27). By acting as passive electrical conduits for current flow, myofibroblasts were thus shown to contribute to the electrical synchronization between host and graft tissue following heart transplantation. Similarly, expression of exogenous Cx43 in infarct scar myofibroblasts has been demonstrated to improve conduction and stabilize the electrical rhythm in preclinical animal models of cardiac injury (28). In addition to the modulation of electrical properties, there is growing evidence that heterocellular mechanical interactions have a pivotal role in the structure and function of the CM–MFB coupling zone and scar (29, 30).
Therefore, myofibroblasts appear to have complex regulatory roles; depending on the setting, they are implicated in either proarrhythmic or antiarrhythmic modulation of the heart after a pathologic event. Nevertheless, the detailed cellular and molecular mechanisms by which physical heterocellular contacts mediate effects in cardiac disease remain poorly characterized. In this study, we developed an in vitro model of heterocellular contacts that are formed during heart failure and we investigated whether electrical and mechanical coupling are independent processes or whether they act in synergy during CM–MFB zone expansion. We show that dynamism of interactions with this model depends on gap-junctional Cx43 levels.
MATERIALS AND METHODS
Neonatal rat cells used for contact motility studies
Isolation of neonatal rat ventricular cardiomyocytes and myofibroblasts from 1–2-d-old Sprague-Dawley rats was done in accordance with the guidelines of the Home Office Animal (Scientific Procedures) Act of 1986 of the United Kingdom. Cardiomyocytes and myofibroblasts were isolated and cultured as previously described (31). Monocultures of cardiomyocytes or myofibroblasts were seeded into MatTek dishes (13 cm2; MatTek, Ashland, MA, USA) or plastic-bottom dishes (35 mm, CytoOne; USA Scientific, Ocala, FL, USA). For the coculture experiments, cardiomyocytes were also plated in dishes, with myofibroblasts seeded on top on the subsequent day. The myofibroblasts were detached as previously described (31), spun, and resuspended in 1 ml HBSS per 1 million cells. The myofibroblasts were then labeled by incubating for 20 min at 37°C and 1% CO2 with either Vybrant DiI (excitation/emission: 549/565 nm; Thermo Fisher Scientific, Waltham, MA, USA) or WGA-488 (wheat germ agglutinin-488, excitation/emission: 495/519 nm; Thermo Fisher Scientific) for recognition afterward. The myofibroblasts were then seeded in with the cardiomyocytes and were left to make gap junctions for 24 h before imaging.
Adult rat fibroblasts used for contact motility studies
Studies involving adult rats were performed in accordance with the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), Bethesda, MD, USA]. For the myocardial infarction model, male adult Sprague-Dawley rats (250–300 g) were anesthetized and underwent proximal coronary ligation to induce chronic myocardial infarction. Rats were allowed to recover for 16 wk to induce heart failure. Time-matched control animals that did not undergo surgery were used as control. After 16 wk, in vivo pressure–volume analysis was performed before the hearts were explanted, weighed, and prepared for cell isolation as previously described (32–34). Fibroblasts were collected from the supernatant of the cardiomyocyte isolation. Cardiomyocytes were spun at 400 g for 1 min, after which the supernatant containing the fibroblasts was collected. This supernatant was then spun at 1000 g for 10 min to pellet the fibroblasts. The supernatant was removed, and the pellet was resuspended in DMEM containing 10% fetal bovine serum, 1% antibiotic/antimycotic solution, and 1% l-glutamine. Myofibroblasts were plated in 25-cm2 flasks and grown until confluency.
Modulation of gap junctions and Cx43
Various methods to modulate gap junctions were used. Overexpression of Cx43 fused at its C terminus to enhanced green-fluorescent protein (eGFP) was achieved using a first-generation adenovirus vector (Cx43–eGFP) and knockdown (KD) was carried out using small interfering RNA (siRNA) (199927; Thermo Fisher Scientific, Waltham, MA, USA) or vesicular stomatitis virus G-protein-pseudotyped lentiviral vectors encoding GFP and a Cx43-specific short hairpin RNA (shRNA) (35, 36). Cx43 modulation by zonula occludens-1 (ZO-1) was inhibited by a membrane-permeable peptide inhibitor that contains the Cx43 C-terminal postsynaptic density-95/disks-large/ZO-1 (PDZ)-binding domain (αCT1 peptide) (37). Cardiomyocytes and myofibroblasts were incubated, separately or in cocultures, for ∼4 h with the αCT1 peptide (100 μM). Increase in cellular coupling was also achieved using a small molecule, 4PB (Calbiochem, San Diego, CA, USA), which was previously published to act as a gap-junction agonist, as well as shown to increase Cx43 expression in cardiomyocytes (38). We therefore added 1 mM 4PB to myofibroblasts alone or both cardiomyocytes and myofibroblasts for 48 h at 37°C. 1-Heptanol was used to completely uncouple gap junctions (39). Hypoxia leads to internalization of Cx43 (40). Hypoxia was induced by incubation at 37°C, 1% CO2, and 8% O2 overnight (∼15 h). Single cultures were seeded at least 24 h before hypoxia, whereas in cocultures, myofibroblasts were seeded into 1–2-d-old cardiomyocytes ∼4 h before hypoxia treatment. For the dynamism experiments with hypoxia, cells were first treated with hypoxia, after which the closed dish was taken out of the incubator and rapidly placed in the small, self-contained chamber that is necessary for recording. We used each dish for a maximum of 1 h and did not find any differences in speed between the start and end. Oxygen levels were measured at the end of the session and were found to not have returned to normoxia before the end of the set of scans. Cocultures were treated with latrunculin-B (100 mM), at 37°C for 24 h in order to disrupt actin stress fibers (41).
Scanning ion conductance microscopy
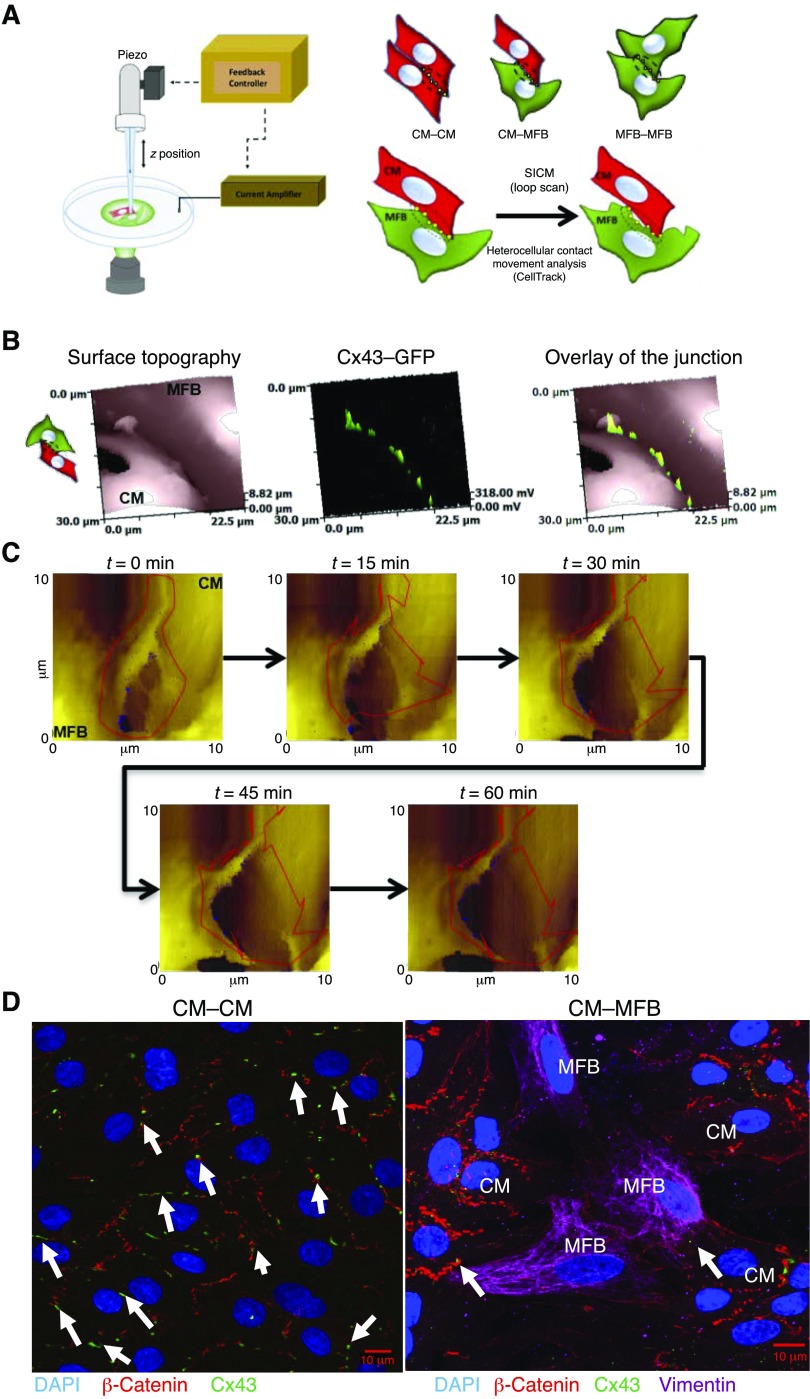
Scanning ion conductance microscopy (SICM) was used to image the contact area between cells (Fig. 1A). In brief, SICM provides continuous topographical images in hopping mode via a feedback-controlled nanopipette scan with minimal spatial drift (a few nm) (23). SICM is a noncontact method that allows the imaging of the topography of intact cells (23, 42, 43). Surface topographical images of cardiomyocytes and myofibroblasts were acquired by SICM at 25°C in a low-calcium physiologic solution (144 mM NaCl (MilliporeSigma, Burlington, MA, USA), 5 mM KCl (MilliporeSigma), 1 mM MgCl2 (MilliporeSigma), 10 mM HEPES, in 2 l dH2O, pH 7.4) or in HBSS, in order to avoid contraction. A large scan (60–100 × 60–100 μm) was carried out to identify the contact areas between cells. After that, a smaller, higher resolution scan of ∼15 × 15 μm of the contact area was carried out and repeated for 1 h (loop) to visualize changes in the contact area between 2 cells (Fig. 1A, C). Afterward, the images (15 × 15 μm) were processed in chronological order using the CellTrack software (44) (http://bio.cse.ohio-state.edu/CellTrack/; Fig. 1A, C). The program returns the average of the speed (pixel per frame) from the randomly selected points within the selected contact area (Fig. 1C). By knowing the time of each acquired image (hh:mm:ss) and the number of pixels for each image (512 × 512 pixels), it was possible to evaluate the average movement velocity (μm/min) of a selected area.
Figure 1.
SICM and the analysis of contact movement. A) Schematic of the SICM setup (left), cartoons representing the different cell configurations used here (top right), and the analysis using CellTrack (44) (bottom right). CellTrack (44) selects a set of random points (yellow squares) in the contact area that is selected (circled) and follows these throughout the scans to calculate the movement of the cell contact. B) Representative images of a CM–MFB contact, first scanned with SICM (left) and subsequently with the laser confocal (middle); an overlay showing the junction (SICM scan) and GFP-Cx43 in the junction (green) is shown on the right. C) Representative set of scans of the looped scans at representative time points. Each scan takes 4–6 min, generating between 10 and 20 images, although some may not be used as debris on the cell may interfere with the SICM scan. t, time; z, cell height. D) Representative image of CM–CM and CM–MFB Cx43 distribution at the cell–cell contacts. Red, β-catenin; green, Cx43; pink, vimentin; blue, DAPI. Cx43 was expressed at the CM–MFB interface. The white arrows indicate the presence of Cx43 at the cell–cell contacts. Scale bars, 10 μm.
SICM in combination with confocal microscopy
Surface topographical images of cardiomyocytes and myofibroblasts were acquired by SICM at 25°C in low-calcium buffer. For the visualization of Cx43 at the junctions, cells were incubated for 24 h with the first-generation adenovirus vector encoding Cx43–GFP (Adeno-X Expression System; Takara, Kyoto, Japan) at a multiplicity of infection of 9. Cx43–GFP was excited at 473 nm with a Stradus 473 laser (Vortran, Sacramento, CA, USA) and confocal images were made at ×100 magnification using a Photomultiplier Detection System (PTI). Using the SICM setup, the tip of the pipette was aligned with the laser beam. The surface of the cell was then scanned as a conventional SCIM image. The same area was subsequently scanned with the laser to visualize the Cx43. The resulting images could be overlapped with the corresponding topography image of the surface (Fig. 1B).
Immunofluorescence microscopy
Immunofluorescence microscopy of vimentin (anti-chicken PA1-16759, 1:3,000; Thermo Fisher Scientific), α-SMA (anti-mouse MA511547, 1:500; Thermo Fisher Scientific), Cx43 (anti-rabbit C6219, 1:1000; MilliporeSigma), and DAPI (33342, 1:1000; Thermo Fisher Scientific) were used to assess the internalization of Cx43 after hypoxia and the effect of latrunculin-B on the heterocellular contact.
Protein extraction and Western blots
Western blots for the gap-junctional protein, Cx43, were carried out as previously described (45); β-actin was used as a loading control. In brief, cells were plated in 35-mm dishes (0.5 million cells per well) and cultured for 72 h with or without treatment. The cells were subsequently washed with cold PBS and the protein was extracted using lysis buffer containing protein inhibitors. Collected lysates were sonicated for 1 min at 0°C, incubated on a shaker for 15 min on ice, and centrifuged for 15 min at 4°C, 14,000 g. Protein concentrations were determined using a BCA Protein Assay according to the manufacturer’s protocol (Pierce, Rockford, IL, USA). For Western blotting, each protein sample was fractioned in denaturating conditions by SDS–PAGE electrophoresis. A Trans-Blot Turbo System (Bio-Rad, Hercules, CA, USA) was used to transfer the proteins to the membrane (Bio-Rad). Membranes were blocked for 1 h in 5% milk (MilliporeSigma) and then incubated overnight at 4°C in primary antibody (Cx43, C6219, anti-rabbit 1:1000, MilliporeSigma; β-actin, A3854, anti-mouse 1:50,000; MilliporeSigma) diluted in 5% milk. The next day membranes were washed 3 times in Tris-buffered saline with Tween-20 (TBST; 1 M Tris pH 8.0, NaCl diluted in distilled water) and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (7074, donkey anti-rabbit 1:1000, Cell Signaling Technology, Danvers, MA, USA; 7076, donkey anti-mouse 1:1000; Cell Signaling Technology) diluted in TBST for 1 h at room temperature. Blots were visualized using Luminata Forte Western HRP substrate (MilliporeSigma). ImageJ software (NIH) was used to quantify bands that were normalized to β-actin.
Parachute assays
Myofibroblasts were plated on 35-mm dishes and, upon confluency, treated with the indicated treatments. For 4PB experiments, myofibroblasts were treated for 48 h to increase Cx43 expression. To achieve Cx43 KD, myofibroblasts were either transfected with Cx43-specific siRNA or transduced with lentiviral vector particles expressing a Cx43-specific shRNA to silence Cx43 and eGFP to detect the transfected cells, prior to the dye staining.
Cells were incubated with 5 µM Cell Tracker Orange/FarRed (Thermo Fisher Scientific) and 1 µM Calcein/Calcein Orange (Thermo Fisher Scientific) for 30 min at 37°C, trypsinized and plated on cardiomyocytes in a 1:2 ratio to form heterocellular contacts. Cells were cocultured for 24 h. Live-cell imaging was performed on a Zeiss Laser Scanning Microscope 780 Confocal. Only cells that were calcein-positive and that were directly adjacent to dually labeled fibroblasts (transferring cells) were counted.
Cell boundary measurement
Quantification of images was performed using a custom-made computer vision program to identify cell–cell contact (courtesy of V.B.). In brief, the cell boundary was selected based on the α-SMA protein staining and manually calibrated. The threshold and dilation cycle measurements were set for the interface area to pick up only Cx43 expressed at the cell boundary, and these were kept constant throughout all image analyses. Cx43 labeling was identified and calculated as the intensity in Cx43 area (arbitrary units/pixel2). Data are presented as means ± sem.
Statistical analysis
A statistical analysis was carried out in Prism 7 (GraphPad, La Jolla, CA, USA) for all the scanning data and Western blots. All data are represented as means ± sem. All data were tested for normality before a Kruskal–Wallis test with post hoc tests (scans, Western blotting) or Student’s t test (parachute assays) was carried out. For all scanning data, n represents the number of 1 h scan sets that were used. More image sets were carried out but may not have been used for various reasons, including noise in the scan due to debris on the cell, which interferes with the scan of the membrane. All data were carried out with at least 1 dish of cells; dishes were changed after 1–2 scan sets, especially for the hypoxia work, as the scans were carried out at normal oxygen tension, which meant that changes induced by hypoxia may have reverted back after 1–2 h. Most experiments were carried out with cells from at least 2 separate isolations (10–15 pups/isolation).
RESULTS
Dynamic measurement of cell–cell contacts
We used neonatal rat cardiomyocytes and myofibroblasts to establish a baseline (control) for the dynamism. After an area was identified to contain a cluster of both myofibroblasts (identified by Vibrant DiI or WGA-488) and cardiomyocytes (in the heterocellular cultures), or clusters of either cell type (in homocellular cultures), a large scan was obtained using SICM, followed by recording a set of scans for 60 min. We then followed the contact over time and analyzed the dynamism (see Materials and Methods). The presence of gap junctions at the cellular interfaces was confirmed using cardiomyocytes expressing Cx43 with GFP fused to its C terminus (Cx43–GFP; Fig. 1). First, a topographical image was obtained and subsequently, using confocal microscopy, the same area was imaged and the Cx43–GFP was visualized. Each scan took ∼5 min, representative SICM and confocal images of Cx43–GFP at the junction of a cardiomyocyte and myofibroblast (CM–MFB) are shown in Fig. 1B. An example of the distribution of Cx43 at the cardiomyocyte–cardiomyocyte (CM–CM) and CM–MFB contacts is presented in Fig. 1D.
Cx43-mediated coupling has implications for cell–cell border zone dynamism
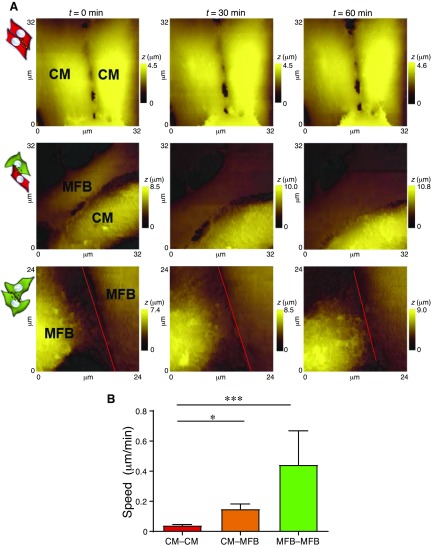
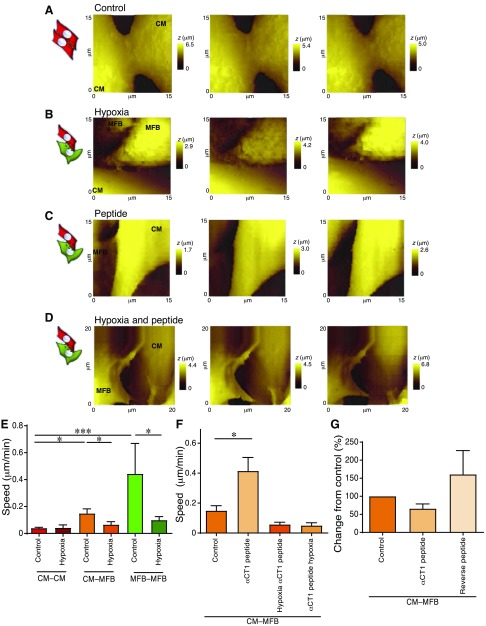
We evaluated the dynamism of neonatal rat ventricular CM–CM, CM–MFB, and myofibroblast–myofibroblast (MFB–MFB) contacts (Fig. 2). The CM–CM pairs, known to be coupled by the electrical gap-junctional protein Cx43, displayed minimal movement [0.04 ± 0.005 μm/min, n = 14 scan sets (data were obtained from ≥2 dishes per isolation from n ≥ 3 isolations, unless otherwise indicated)], whereas the border zone, modeled by the coculture of cardiomyocytes and myofibroblasts, showed a significant increase in dynamism (0.14 ± 0.033 μm/min, n = 11 scan sets, P ≤ 0.001). Interestingly, MFB–MFB contacts displayed a considerable level of dynamism in culture (0.44 ± 0.22 μm/min, n = 7 scan sets, P ≤ 0.05), compared to heterocellular cell pairs.
Figure 2.
Dynamism in control CM–CM, CM–MFB, and MFB–MFB contacts. A) Representative set of scans for each configuration. Cartoons indicate the configuration, as shown in Fig. 1. The red line is included for the contact between MFB–MFB for clarity. t, time; z, cell height. B) Movement speed of the contacts. All data are represented as means ± sem. *P ≤ 0.05, ***P ≤ 0.001.
Membrane Cx43-deficient cells show limited dynamism
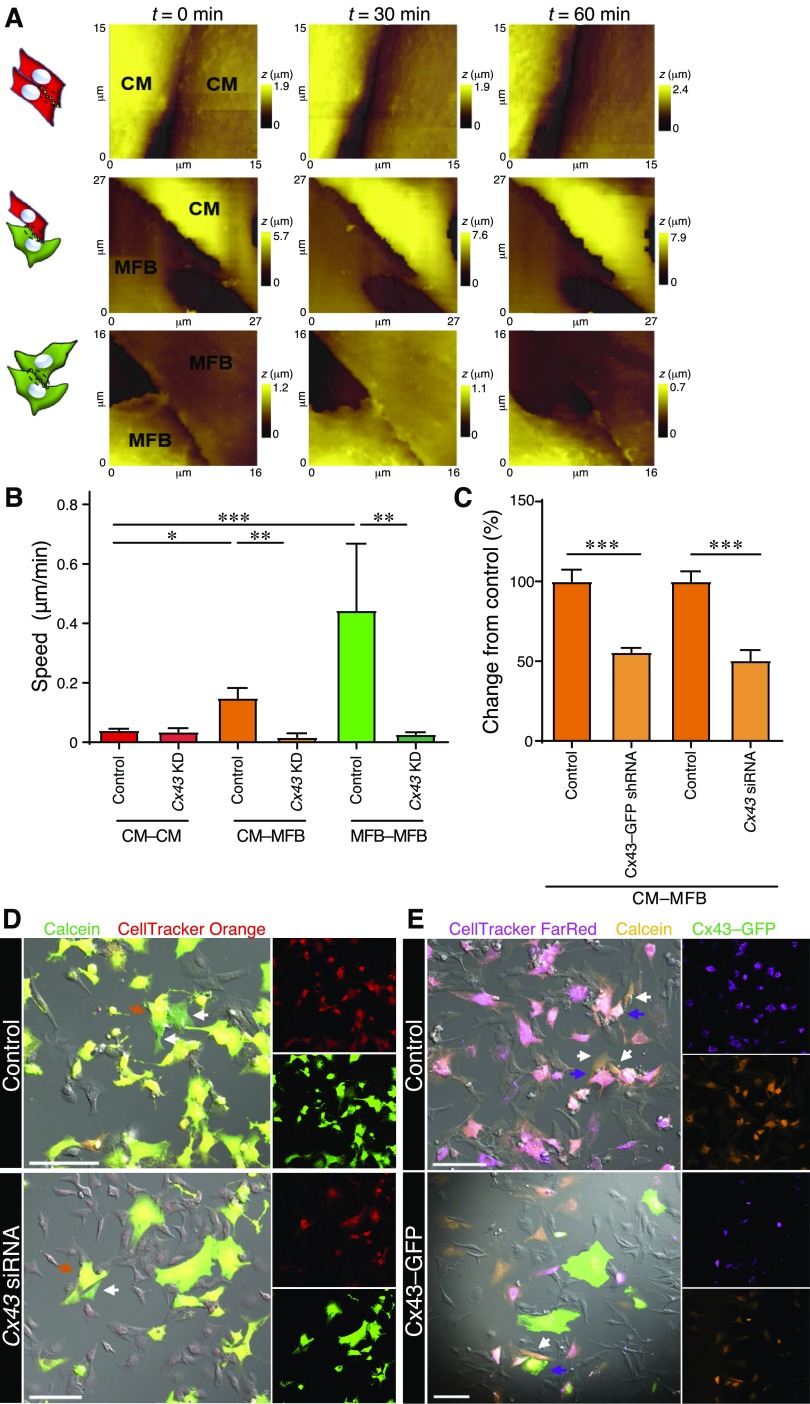
The involvement of Cx43 in the CM–MFB and MFB–MFB dynamism was elucidated by infecting myofibroblasts (or cardiomyocytes in CM–CM control experiments) with lentiviral vector particles encoding GFP and a Cx43-specific shRNA (Cx43–GFP shRNA) or by using siRNA against Cx43 (Fig. 3). Green fluorescence was used to identify myofibroblasts in which Cx43 was successfully silenced. The myofibroblast membrane was also labeled with a lipophilic membrane dye (Vybrant DiI, Thermo Fisher Scientific; see Supplemental Fig. S1). Cx43 KD significantly slowed the cell–cell dynamism of contacts involving myofibroblasts: CM–MFB (0.017 ± 0.013 μm/min, n = 6 scan sets, n = 2 isolations, P ≤ 0.01) and MFB–MFB (0.027 ± 0.007 μm/min, n = 7 scan sets, n = 2 isolations, P ≤ 0.01) but did not significantly affect cardiomyocytes alone [0.035 ± 0.012, n = 5 scan sets (from a single isolation)] (Fig. 3A, B). By contrast, 50 μM heptanol (a gap-junctional uncoupler) did not slow the dynamism in CM–MFB (0.047 ± 0.010, n = 7 scan sets, n = 2 isolations) and MFB–MFB (0.059 ± 0.016, n = 6 scan sets, n = 2 isolations), suggesting that the presence of gap junctions on the myofibroblast membrane, and not their function, is required to activate the dynamic coupling (Supplemental Fig. S2).
Figure 3.
Cx43 KD leads to decreased dynamism and reduced dye transfer. A) Representative set of scans for each configuration. Illustrations indicate the configuration, as shown in Fig. 1. t, time; z, cell height. B) Movement speed of the contacts after Cx43 KD using siRNA in only the myofibroblasts (in CM–MFB cultures) or in either cell type in the monocultures. C–E) Cx43 KD using Cx43-specific siRNA or Cx43–GFP shRNA in only the myofibroblasts leads to decreased dye transfer and therefore a reduced number of functional gap junctions. C) Analysis of the parachute assay. Cx43 siRNA, n = 52 images (for all parachute assays: the total number of images from 2 dishes per isolation for n = 3 isolations are indicated); control, n = 61 images. Cx43–GFP shRNA, n = 29 images; control, n = 29 images. D) Representative images of control and Cx43 siRNA. Calcein (green) is used as a cell-permeable dye, whereas CellTracker Orange (red) is a cell-impermeable dye. E) Representative images of control and Cx43–GFP shRNA. GFP (green) is used to identify myofibroblasts with Cx43 KD (only shown in the shRNA-treated images). Calcein Orange (orange) is used as a cell-permeable dye, whereas CellTracker FarRed (magenta) is a cell-impermeable dye. Scale bars, 100 μm. All data are represented as means ± sem. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
To confirm that the gap junctions between cardiomyocytes and myofibroblasts were functional and to investigate the extent of direct physical coupling, parachute assays were carried out (Fig. 3C, D). Control acceptor cells (cardiomyocytes) were plated and control or Cx43-deficient myofibroblasts [Cx43 siRNA (Fig. 3D) or Cx43–GFP shRNA (Fig. 3E)] were added to cultures of cardiomyocytes. Prior to addition, myofibroblasts had been dually labeled with permeable (Calcein or Calcein Orange) and impermeable (CellTracker Orange or CellTracker FarRed) dyes. Myofibroblasts with reduced Cx43 expression due to treatment with either Cx43 siRNA [50.50 ± 6.457%, n = 52 images (for all parachute assays: the total number of images from 2 dishes per isolation for n = 3 isolations are indicated); control, 100 ± 6.236%, n = 61 images; P ≤ 0.001] or Cx43–GFP shRNA (55.63 ± 2.662%, n = 29 images; control, 100 ± 7.333%, n = 29 images; P ≤ 0.001) showed significantly attenuated calcein transfer compared to the respective control conditions (normalized to 100%).
Increased cellular coupling induced by 4PB
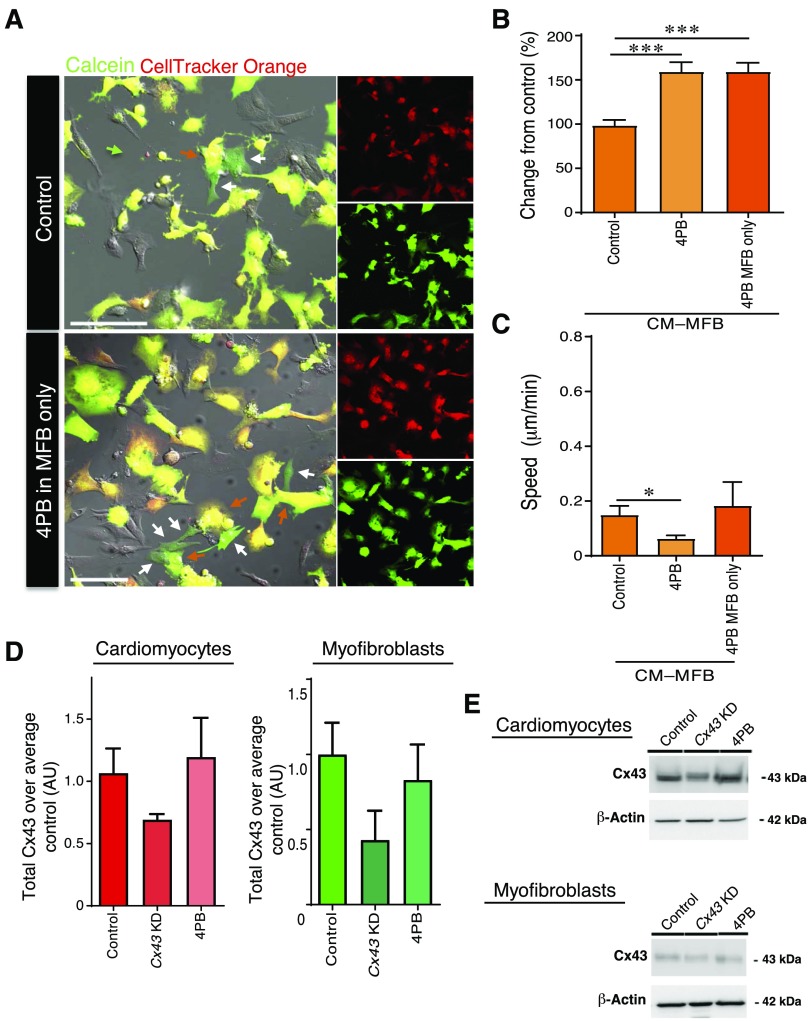
A small molecule (4PB) has previously been shown to act as an epigenetic modulator that increases cellular functional coupling between cardiomyocytes (38). We used this molecule as a method to strengthen Cx43 coupling between cardiomyocytes and myofibroblasts (Fig. 4). 4PB induced a significant reduction in dynamism in the CM–MFB configuration (Fig. 4C), when both cell types were incubated with the small molecule (0.063 ± 0.012, n = 29, P ≤ 0.05), but not when only the myofibroblasts were incubated with 4PB (0.183 ± 0.086, n = 55). Using an in vitro assay of direct cell–cell communication in the parachute assay, there is a significant increase in dye transfer in both cases; 4PB CM–MFB: 159.3 ± 10.64%, n = 29 vs. control (100 ± 8.479%, n = 33) and myofibroblasts only treated with 4PB: 159.3 ± 9.961%, n = 55 vs. control (100 ± 7.425%, n = 53) (Fig. 4A, B).
Figure 4.
Increased cellular coupling induced by 4PB. A) Representative images of control cocultures and cocultures with 4PB-treated myofibroblasts. Calcein (green) is used as a cell-permeable dye, whereas CellTracker Orange (red) is a cell-impermeable dye. Scale bars, 100 μm. B) Analysis of the parachute assay. 4PB treatment of cocultured cardiomyocytes and myofibroblasts (n = 29 images, n = 3 isolations) or myofibroblasts alone (n = 55 images, n = 3 isolations) leads to increased calcein transfer. C) Treatment of both cardiomyocytes and myofibroblasts, but not myofibroblasts alone, leads to reduced movement speed in the contact. D, E) Western blot analysis of Cx43 (n = 3–4 independent isolations). Quantification (D) and representative blots (E) are shown. AU, arbitrary units. All data are represented as means ± sem. *P ≤ 0.05, ***P ≤ 0.001.
To confirm KD of Cx43 and to investigate whether 4PB has a similar effect on myofibroblasts as was previously published in cardiomyocytes (38), Western blotting was carried out (Fig. 4D, E). 4PB treatment did not lead to a significant increase in Cx43 levels in cardiomyocytes or myofibroblasts, in contrast to previous findings in cardiomyocytes (38). However, this may be caused by differences in incubation time or technical repeats (n = 3–4) and a potentially lower baseline level of Cx43 expression in myofibroblasts compared to cardiomyocytes. Following Cx43 KD, Cx43 protein levels were reduced in cardiomyocytes to 69% of control (P = 0.057), and although not significant, a trend toward reduction was found in myofibroblasts; Cx43 expression was reduced to 41% of control values (n = 3–4, P = 0.094; Fig. 4D, E). As Cx43 expression is generally lower in myofibroblasts compared to cardiomyocytes (35, 46), this reduction was found to be sufficient to induce the observed changes in membrane motility and calcein transfer.
Hypoxia-induced internalization of Cx43 induced a decrease in dynamism
The presence of Cx43 on the membranes of isolated cardiomyocytes and myofibroblasts was assessed by culturing the cells in hypoxic conditions (8% O2) for 12–16 h before measuring cell–cell dynamism (Fig. 5B). Hypoxia is known to internalize the connexons in the plasmalemma of cardiomyocytes (40) and myofibroblasts (Fig. 5B, E and Supplemental Fig. S3C). As postulated, for both CM–MFB [0.067 ± 0.021, n = 7 scan sets (3 dishes each from 2 isolations), P ≤ 0.05] and MFB–MFB (0.100 ± 0.025, n = 13 scan sets, P ≤ 0.05) models, limited dynamism was measured when cells were cultured in hypoxic conditions compared to normoxic (control) conditions. This indicates that the presence of Cx43, and thus the capacity to establish cell–cell electrical coupling, is necessary for cell–cell border zone movement. In terms of functional coupling, chronic hypoxia also caused a reduction in dye transfer from neonatal myofibroblasts to neonatal cardiomyocytes (Supplemental Fig. S3A, B).
Figure 5.
Removing Cx43 by hypoxia and forcing Cx43 to the membrane by αCT1 peptide. A–D) Representative scans of control and treatments. z, cell height. E) Hypoxia reduces the dynamism between cells. F) αCT1 treatment leads to increased dynamism in control, but not in hypoxic, conditions. G) Treatment with the αCT1, but not the reverse, peptide leads to decreased dye transfer, showing that, although the plaque size increases, the number of functional gap junctions decreases. All data are represented as means ± sem. *P ≤ 0.05, ***P ≤ 0.001. For representative Western blots and quantification of Cx43 protein expression in cardiomyocytes and myofibroblasts treated with hypoxia or αCT1, see Fig. 7A, B.
Inducing recruitment of Cx43 to the cell membrane using the mimetic αCT1 peptide
A second approach was used to examine the effects of increased levels of Cx43-mediated contact. The mimetic Cx43 peptide αCT1 has previously been shown to promote the extent of Cx43 gap junction-mediated contacts between cells (37), as well as reducing inducible arrhythmias at the CM–MFB zone following ventricular cryoinjury (19). We therefore investigated the effect of the peptide in our cellular heterocellular model (Fig. 5). In both cardiomyocyte and myofibroblast monocultures, as well as in cocultures of these cells, the αCT1 peptide induced the occurrence of Cx43 at the contact regions between cells. The increased presence of Cx43-mediated contacts increased CM–MFB dynamism [0.415 ± 0.090 μm/min, n = 5 scan sets (1–2 dishes from 3 isolations)], indicating a novel “mechanical” role for an “electrical” intercellular connection (Fig. 5C, F). By contrast, hypoxic conditions, in the presence of the αCT1 peptide [0.057 ± 0.015 μm/min, n = 6 scan sets (3 dishes each from 2 isolations)], or hypoxia after a 4-h preincubation with the αCT1 peptide [0.050 ± 0.018 μm/min, n = 3 (3 dishes from a single isolation)], significantly reduced dynamism (Fig. 5) despite Cx43 localization at heterocellular junctions (data not shown).
Therefore, we investigated whether enhanced Cx43-mediated coupling had a functional role in intercellular communication between cardiomyocytes and myofibroblasts (Fig. 5G). Myofibroblasts were dually labeled with the cell-permeable dye Calcein and cell-impermeable dye CellTracker Orange and treated with either the αCT1 or reverse (control) peptide. The myofibroblasts were then parachuted onto cardiomyocytes, which were also treated with either the αCT1 or reverse peptide (Fig. 5G; n = 4 isolations/treatment). Although not significant, a small decrease in gap junction intercellular communication, after treatment with the αCT1 peptide, was observed (P = 0.12) with respect to control.
Actin filament-based modification of Cx43
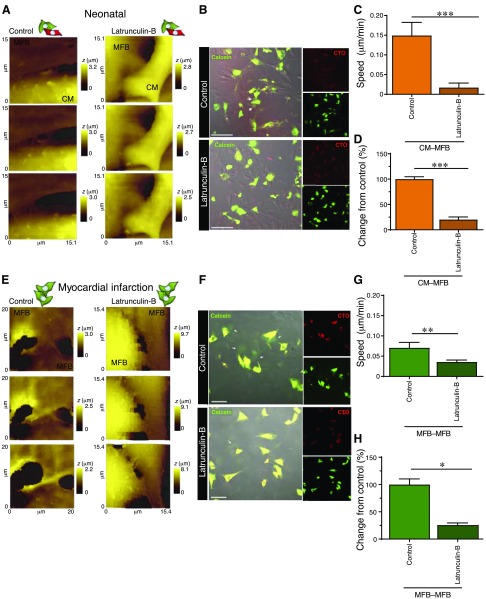
Myofibroblasts are known to promote conduction slowing and ectopic activity in cardiac tissue (25, 47). As previously shown (41), these arrhythmogenic properties can be suppressed by disruption of α-SMA stress fibers using pharmacological manipulation. Moreover, trafficking of Cx43 to the membrane is mediated by α-actin filaments. To investigate the role of α-SMA in cell membrane mobility and to prevent intercellular gap-junctional communication and functional Cx43 connexon expression on the sarcolemma, neonatal CM–MFB cocultures were treated with latrunculin-B, an inhibitor of actin polymerization. After incubation for 24 h, the myofibroblast dynamism was significantly decreased [0.03 ± 0.006 μm/min, n = 9 scan sets (2 and 4 dishes from 2 isolations), P ≤ 0.05; Fig. 6A, C]. Cell membrane mobility was then examined in myofibroblasts isolated from a rat model of myocardial infarction (16 wk postinfarction) (32). α-SMA ablation resulted in lower cell dynamism (0.03 ± 0.004 μm/min, n = 13 scan sets) compared to untreated myofibroblasts from the same model (0.12 ± 0.041 μm/min, P ≤ 0.01; Fig. 6E, G). We assessed the functionality of CM–MFB contacts in both models using a parachute assay and observed a significant reduction in calcein transfer following treatment with latrunculin-B in cocultures of neonatal cardiomyocytes and myofibroblasts (latrunculin-B, 20.18 ± 5.113%, n = 38 images, n = 3; control, 100 ± 4.744%, n = 35 images, n = 3; P ≤ 0.001; Fig. 6B, D) and among adult rats with myocardial infarction (latrunculin-B, 25.8 ± 3.8%, n = 14 images, n = 3; control, 100 ± 10.36%, n = 14 images, n = 3; P ≤ 0.001) (Fig. 6F, H). Similar to the decrease in calcein transfer seen after latrunculin-B treatment of myofibroblast cultures derived from adult rats with myocardial infarction, control (sham) rat myofibroblast cultures also showed a reduction (42%, P < 0.001) in dye transfer after latrunculin-B treatment (Supplemental Fig. S4C, D); this was accompanied by a decrease in cell dynamism (Supplemental Fig. S4A, B). This indicates that myofibroblasts derived from both adult rats with myocardial infarction and sham-treated adult rats are sensitive to the effects of latrunculin-B. Significant effects of both condition [sham vs. myocardial infarction (P = 0.0006)] and treatment [control vs. latrunculin-B (P = 0.0364)] on contact dynamism was found (2-way ANOVA), but the interaction between treatment and condition was not significant.
Figure 6.
Treatment with latrunculin-B abolishes contact dynamism and dye transfer. A, B) Representative SICM scans (A) and images of parachute assays (B) of control CM–MFB cultures and CM–MFB cultures treated with latrunculin-B from neonatal rats. z, cell height. C, D) Treatment with latrunculin-B leads to reduced dynamism (C) and a reduction in the number of functional contacts (D). B, D, Control, n = 35 images, n = 3 isolations; Latrunculin-B, n = 39 images, n = 3 isolations. For representative Western blots and quantification of Cx43 protein expression in cardiomyocytes and myofibroblasts treated with latrunculin-B, see Fig. 7A, B. E, F) Representative SICM scans (E) and images of parachute assays (F) of myofibroblasts cultures derived from adult rats with myocardial infarction that were treated with latrunculin-B or vehicle control (MFB–MFB). For experiments carried out using myofibroblasts from sham-treated adult rats, see Supplemental Fig. S4. G, H) Treatment with latrunculin-B leads to reduced dynamism (G) and a reduction in the number of functional contacts (H). F, H, Control, n = 24 images, n = 3 isolations; Latrunculin-B, n = 25 images, n = 3 isolations. CTO, CellTracker Orange; LatB, latrunculin-B. All data are represented as means ± sem. Scale bars, 100 μm. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Changes in the level of Cx43 expression in the different treatment groups
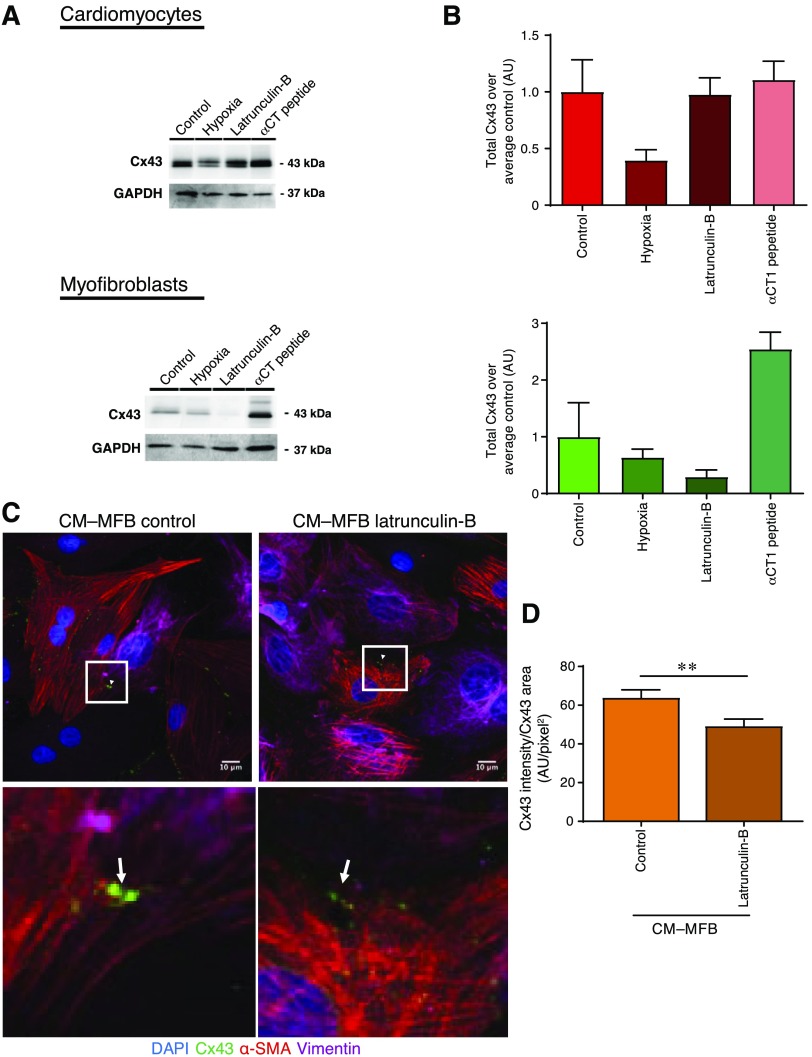
In order to further validate that the changes seen were due to the functional effects of Cx43, protein levels were assessed in separate cultures of neonatal cardiomyocytes and fibroblasts using Western blot. Hypoxic conditions reduce the levels of Cx43 in both cell types. A marked drop in Cx43 expression is also observed in the myofibroblast cultures treated with latrunculin-B. By contrast, αCT1 treatment notably increased the level of the Cx43 expression in myofibroblast samples (Fig. 7A, B).
Figure 7.
Quantification of Cx43 expression in control, hypoxia-, latrunculin-B-, and αCT1-treated samples. A) Cx43 and loading control [glyceraldehyde 3-phosphate dehydrogenase (GAPDH)] intensity bands in neonatal cardiomyocyte and myofibroblast cultures (n = 2–4 independent isolations). B) Quantification of Western blots shown in A. All data are represented as means ± sem; n = 2–4 independent isolations. C) Latrunculin-B treatment significantly decreases the Cx43 intensity in the Cx43 clusters (n = 15 images, n = 3 isolations). All data are represented as means ± sem. **P ≤ 0.01. D) Representative images of immunostaining of CM–MFB contacts. Red, α-SMA; green, Cx43; pink, vimentin; blue, DAPI. Cx43 was expressed at the CM–MFB interface. Scale bars, 10 μm. AU, arbitrary units; latrunculin-B, LatB.
Immunofluorescence staining was performed to visualize the contacts that had formed. Heterocellular connections were further quantified using a custom-made cell boundary measurement tool (V.B. laboratory). Quantifying the Cx43 intensity in the Cx43 area, we observed that the disruption of α-SMA reduced the functionality of CM–MFB Cx43-based coupling compared to the untreated sample (n = 15 images, n = 3 isolations, P ≤ 0.01; Fig. 7C, D), which is consistent with the parachute assay data of cultures treated with latrunculin-B.
DISCUSSION
Pathologic events, such as myocardial infarction, trigger complex changes and dynamic remodeling of the cardiac tissue, which occurs over time. Through coordinated regulation of the gap-junctional proteins and the extracellular matrix in the affected region, the irreversibly damaged myocardium becomes electrically quarantined from the rest of the heart to restrict the spread of adverse effects; the border zone area between the infarct and the healthy tissue undergoes highly dynamic changes in cell–cell communication. It has been reported that myofibroblasts coupled to cardiomyocytes show an increase in Cx43 expression and an associated increase in gap junction formation (21). Cx43-based contact between myofibroblasts and cardiomyocytes is thought to contribute to ectopic activity and arrhythmias (25, 47). In the present study, we show a novel Cx43-dependent aspect of myofibroblast behavior that may contribute to heart failure pathology. Specifically, myofibroblasts demonstrate highly motile contacts with each other and with cardiomyocytes. This unique dynamism is characterized by the rapid formation of new areas of contact and similarly efficient remodeling of existing interactions. By contrast, cardiomyocytes in culture form stable gap junctions with each other and have very low movement in zones of mutual Cx43-based interactions—consistent with the idea that myocardial cell-to-cell coupling is important for the propagation of electrical impulse (48).
This dynamic ability to remodel cell–cell contacts appears to be necessary for the supportive roles of myofibroblasts. An interesting question arises as to what the relationship between these physical contact dynamics and the drivers of electrical coupling, Cx43 gap junctions, is. In this study, using a variety of methods to modulate assembly of Cx43 and actin, we aimed to address this question. Our results indicate that this relationship (contact dynamism and electrical coupling) is not simple and monotonic. The levels of gap-junctional interaction require tight control to ensure that, while sufficient levels of intercellular coupling are in place, gap junction-mediated adhesions are not so strong as to restrict the dynamism of heterocellular contacts. Following myocardial infarction, myofibroblasts showed an increase in Cx43 expression and associated level of gap junction formation (21).
In our experiments, CM–MFB cultures exhibited much higher membrane contact dynamics than cardiomyocyte monocultures. This difference in the vigor of cell–cell membrane contact dynamics was associated with differences in Cx43 expression and the stability of intercellular Cx43 connections. We show that, although myofibroblasts had lower Cx43 expression (35) than cardiomyocytes, Cx43 functionally couples cardiomyocytes and myofibroblasts, as indicated by intercellular calcein transfer. It appears that Cx43 serves both as an affector and as a sensor of contact dynamism between cells. Furthermore, we determined that decreasing the level of Cx43 through Cx43 silencing, by preventing Cx43 trafficking, or through Cx43 internalization reduced contact dynamism.
This is correlated with a reduction in dye transfer and, thus, a reduction in gap-junctional coupling between myofibroblasts and cardiomyocytes. By contrast, increasing membrane Cx43 levels did not necessarily lead to an increase in coupling and dynamism. We found that 4PB increased coupling between cardiomyocytes and myofibroblasts without changing Cx43 protein levels (Fig. 4); however, interestingly, it decreased dynamism.
In human glioblastoma cells, it was shown that levels of unphosphorylated Cx43 were increased after treatment with 4PB, whereas the phosphorylated form remained stable (49). We assume that the stabilization at the membrane that we have previously shown (38) occurs through a combined increase of both forms. Notably, 4PB affects Cx43 epigenetically; therefore, there is not necessarily a change in expression. 4PB is known to exert multiple effects. 4PB is an epigenetic modulator that increases cellular functional coupling between 2 cardiomyocytes (unpublished data), 2 myofibroblasts or between a cardiomyocyte and myofibroblast. Among the nonepigenetic effects of 4PB is its recognized action as a chemical chaperone, which can reduce endoplasmic reticulum stress and can attenuate the unfolded protein response (50, 51). Better folding and improved trafficking to the membrane can give rise to better and more stable (low dynamism) electrical coupling even if Cx43 transcription is not significantly changed, which is probably the case here.
This suggests that there may be an optimal level of Cx43-mediated electrical coupling that allows for high dynamism of CM–MFB contacts. When Cx43 levels are too low in the contact area, the edge motility of myofibroblasts and heterocellular contact dynamics are reduced. When the electrical coupling is too high in the contact area, this becomes too restrictive and dynamism is also reduced. Myofibroblasts are normally only lightly coupled to each other and to cardiomyocytes, with contacts breaking and reforming continuously; they are highly mobile and show a lot of plasticity. For instance, in vitro studies have shown that they are capable of bridging gaps among cardiomyocytes of up to 300 μm (15, 21). Increasing the number of Cx43 connexons on the myofibroblast membrane that are effectively coupled to their counterparts in the cardiomyocyte membrane increases the strength of the CM–MFB contacts, and as the pair starts behaving as one, the motility of the myofibroblast is reduced.
Treatment with the αCT1 peptide has been shown to lead to an increase in Cx43 plaque size and a decrease in Cx43 hemichannels (37, 52). Although treatment with the αCT1 peptide led to an increase in dynamism, this was associated with a small decrease in dye transfer. This can be explained by the fact that, after treatment of the cells with the αCT1 peptide, the phosphorylation status of Cx43 is likely to have changed, as phosphorylation is one of the main ways in which Cx43 channels are regulated through post-translational modification (53–55). We have previously reported that αCT1 increased Cx43 phosphorylation at S368 at the interface between cardiomyocyte and myofibroblast in vivo (19), a post-translational modification known to be associated with decreased Cx43 channel activity (56). We have observed that αCT1 similarly increased S368 phosphorylation in our in vitro CM–MFB model (57). The observed reduction in dye transfer between coupled cells is therefore likely to be explained by this change in Cx43 phosphorylation status, despite an increase in Cx43 expression and plaque size. In addition, with recruitment of Cx43 to the membrane, myofibroblasts treated with the αCT1-peptide showed increased lamellipodia formation, which may indicate their search for stable connections with neighboring cells.
It may be hypothesized that other modifications, such as ubiquitination (which would decrease the amount of protein at the junctions) are reduced or changed (for example, acetylation or methylation) after treatment with our interventions; however, it is beyond the scope of this study to analyze these modifications further.
It has been shown that Cx43 trafficking to the sarcolemma of cardiomyocytes is dependent on both the microtubular network (58) and actin cytoskeleton (59). Previously, the role of α-SMA stress fibers in myofibroblasts has been investigated, and it has been shown that pharmacological ablation of these cytoskeletal elements eliminates the arrhythmogenic effects of myofibroblasts on cardiomyocytes (12, 41, 59). We therefore expanded on this research by studying the effect of pharmacological ablation of the actin cytoskeleton in cocultures of cardiomyocytes and myofibroblasts to investigate the impact on heterocellular contact dynamism. Disruption of the actin cytoskeleton significantly reduced CM–MFB contact movement in cocultures of neonatal rat cardiomyocytes with either neonatal rat myofibroblasts or myofibroblasts derived from adult rats with myocardial infarction. Subsequently, we investigated the diminishing heterocellular contact functionally. Using a custom-made cell–cell measurement software (V.B. laboratory), we found a partial drop in Cx43 intensity in the Cx43 area after α-SMA stress fiber removal, which could be related to the actin-dependent Cx43 pool that has previously been described (59). These results indicate that Cx43 not only affects contact dynamism, but also that the opposite is true—Cx43 coupling senses and reacts to actin dynamics.
How the Cx43-dependent myofibroblast dynamism characterized in our study affects the formation of proarrhythmic substrates at the heterocellular coupling area should be investigated further. The irregularity of the interface between surviving cardiac muscle and the scar tissue of myocardial infarction is a well-characterized determinant and originator of re-entrant arrhythmias (60, 61). Modeling studies have shown that microstructural variations in cardiac tissue facilitate the formation of isolated sites of wavefront breakthrough that may enable abnormal electrical activity; these small regions of heterogeneous diseased tissue can develop more widespread re-entrant activity (62, 63). The relationship between Cx43 expression and level of heterocellular contact dynamism that we report here likely has implications for the disposition of mechanical forces imparted by myofibroblasts on cardiomyocytes. How Cx43-based mechano-interactions at the microscale shape the macrostructure of the extended heterocellular interface thus may be of key importance to understanding the formation and pathogenesis of arrhythmic re-entrant circuits.
In summary, here we reveal the complex relationship between Cx43-mediated heterocellular electrical coupling and physical contact dynamism (Fig. 8). We show that Cx43 is not only involved in electrical coupling between cardiomyocytes and myofibroblasts, but is also important for the mechanical properties of the contacts. We provide new insights into Cx43-mediated coupling between cardiomyocytes and nanoscale evidence of the formation and dynamism of heterocellular contacts. Mechanistically, we demonstrate that Cx43 is necessary for both the dynamism of structural membrane contacts and for direct physical coupling of myofibroblast and cardiomyocytes in an in vitro infarct border zone model.
Figure 8.
Schematic of our current view on the role of Cx43 in CM–MFB dynamism. Max., maximum; min., minimum.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank P. O’Gara (Imperial College London, London, United Kingdom) for the assistance with the isolation of adult rat ventricular fibroblasts and M. Sikkel (Imperial College London, London) and C. Mansfield (Imperial College London, London) for generating rat models of myocardial infarction. This work was supported by British Heart Foundation (RG/17/13/33173 to J.G.), Medical Research Council (MR/L006855/1 to J.G.), National Heart, Lung, and Blood Institute (NHLBI), U.S. National Institutes of Health Grant-1R01HL141855-01 (to R.G., J.G. and A.A.-L.), Heart Research UK, and by Italian Ministry of Education, Universities and Research (FFABR-MIUR-2017 to M.M.). M.M. and J.G. jointly supervised this work. The authors declare no conflicts of interest.
Glossary
- 4PB
4-phenylbutyrate
- CM–CM
cardiomyocyte–cardiomyocyte
- CM–MFB
cardiomyocyte–myofibroblast
- Cx43
connexin-43
- KD
knockdown
- MFB–MFB
myofibroblast–myofibroblast
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- SICM
scanning ion conductance microscopy
- α-SMA
α-smooth muscle actin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. Miragoli and J. Gorelik conceived the study; F. Schultz, P. Swiatlowska, A. Alvarez-Laviada, M. Miragoli, and J. Gorelik designed experiments; F. Schultz, P. Swiatlowska, A. Alvarez-Laviada, and M. Miragoli isolated, cultured, and treated the cells; F. Schultz, P. Swiatlowska, and M. Miragoli carried out scanning ion conductance microscopy (SICM) experiments; A. Alvarez-Laviada and J. L. Sanchez-Alonso carried out SICM/confocal experiments; A. Alvarez-Laviada and E. Ongstad performed parachute assays; F. Schultz, P. Swiatlowska, A. Alvarez-Laviada, Q. Song, and M. Miragoli analysed the data; F. Schultz, P. Swiatlowska, and M. Miragoli carried out Western blots; A. A. F. de Vries, D. A. Pijnappels, V. M. M. Braga, E. Entcheva, and R. G. Gourdie provided advice or supervision, assisted in analysis, provided reagents and commented on the manuscript; and F. Schultz wrote the manuscript with input from P. Swiatlowska, A. Alvarez-Laviada, J. L. Sanchez-Alonso, V. M. M. Braga, E. Entcheva, R. G. Gourdie, M. Miragoli, and J. Gorelik.
REFERENCES
- 1.Kohl P., Gourdie R. G. (2014) Fibroblast-myocyte electrotonic coupling: does it occur in native cardiac tissue? J. Mol. Cell. Cardiol. 70, 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fróes M. M., Correia A. H., Garcia-Abreu J., Spray D. C., Campos de Carvalho A. C., Neto M. V. (1999) Gap-junctional coupling between neurons and astrocytes in primary central nervous system cultures. Proc. Natl. Acad. Sci. USA 96, 7541–7546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Maubecin V., Garcia-Hernandez F., Williams J. T., Van Bockstaele E. J. (2000) Functional coupling between neurons and glia. J. Neurosci. 20, 4091–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haefliger J. A., Nicod P., Meda P. (2004) Contribution of connexins to the function of the vascular wall. Cardiovasc. Res. 62, 345–356 [DOI] [PubMed] [Google Scholar]
- 5.Truskey G. A. (2010) Endothelial cell vascular smooth muscle cell co-culture assay for high throughput screening assays for discovery of anti-angiogenesis agents and other therapeutic molecules. Int. J. High Throughput Screen. 2010, 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirschi K. K., Rohovsky S. A., D’Amore P. A. (1998) PDGF, TGF-β, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol. 141, 805–814; erratum: 1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee S. (2009) Fibroblasts in three dimensional matrices: cell migration and matrix remodeling. Exp. Mol. Med. 41, 858–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto A. R., Ilinykh A., Ivey M. J., Kuwabara J. T., D’Antoni M. L., Debuque R., Chandran A., Wang L., Arora K., Rosenthal N. A., Tallquist M. D. (2016) Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gourdie R. G., Dimmeler S., Kohl P. (2016) Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat. Rev. Drug Discov. 15, 620–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwahara F., Kai H., Tokuda K., Kai M., Takeshita A., Egashira K., Imaizumi T. (2002) Transforming growth factor-β function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 106, 130–135 [DOI] [PubMed] [Google Scholar]
- 11.Shinde A. V., Frangogiannis N. G. (2014) Fibroblasts in myocardial infarction: a role in inflammation and repair. J. Mol. Cell. Cardiol. 70, 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smyth J. W., Hong T.-T., Gao D., Vogan J. M., Jensen B. C., Fong T. S., Simpson P. C., Stainier D. Y. R., Chi N. C., Shaw R. M. (2010) Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J. Clin. Invest. 120, 266–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foo I. T., Naylor I. L., Timmons M. J., Trejdosiewicz L. K. (1992) Intracellular actin as a marker for myofibroblasts in vitro. Lab. Invest. 67, 727–733 [PubMed] [Google Scholar]
- 14.Miragoli M., Gaudesius G., Rohr S. (2006) Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ. Res. 98, 801–810 [DOI] [PubMed] [Google Scholar]
- 15.Gaudesius G., Miragoli M., Thomas S. P., Rohr S. (2003) Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ. Res. 93, 421–428 [DOI] [PubMed] [Google Scholar]
- 16.Biernacka A., Frangogiannis N. G. (2011) Aging and cardiac fibrosis. Aging Dis. 2, 158–173 [PMC free article] [PubMed] [Google Scholar]
- 17.Rohr S. (2009) Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm 6, 848–856 [DOI] [PubMed] [Google Scholar]
- 18.McAnulty R. J. (2007) Fibroblasts and myofibroblasts: their source, function and role in disease. Int. J. Biochem. Cell Biol. 39, 666–671 [DOI] [PubMed] [Google Scholar]
- 19.O’Quinn M. P., Palatinus J. A., Harris B. S., Hewett K. W., Gourdie R. G. (2011) A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ. Res. 108, 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubart M., Tao W., Lu X.-L., Conway S. J., Reuter S. P., Lin S.-F., Soonpaa M. H. (2018) Electrical coupling between ventricular myocytes and myofibroblasts in the infarcted mouse heart. Cardiovasc. Res. 114, 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camelliti P., Devlin G. P., Matthews K. G., Kohl P., Green C. R. (2004) Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc. Res. 62, 415–425 [DOI] [PubMed] [Google Scholar]
- 22.Van Veen A. A., van Rijen H. V. M., Opthof T. (2001) Cardiac gap junction channels: modulation of expression and channel properties. Cardiovasc. Res. 51, 217–229 [DOI] [PubMed] [Google Scholar]
- 23.Miragoli M., Moshkov A., Novak P., Shevchuk A., Nikolaev V. O., El-Hamamsy I., Potter C. M. F., Wright P., Kadir S. H. S. A., Lyon A. R., Mitchell J. A., Chester A. H., Klenerman D., Lab M. J., Korchev Y. E., Harding S. E., Gorelik J. (2011) Scanning ion conductance microscopy: a convergent high-resolution technology for multi-parametric analysis of living cardiovascular cells. J. R. Soc. Interface 8, 913–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miragoli M., Salvarani N., Rohr S. (2007) Myofibroblasts induce ectopic activity in cardiac tissue. Circ. Res. 101, 755–758 [DOI] [PubMed] [Google Scholar]
- 25.Mahoney V. M., Mezzano V., Mirams G. R., Maass K., Li Z., Cerrone M., Vasquez C., Bapat A., Delmar M., Morley G. E. (2016) Connexin43 contributes to electrotonic conduction across scar tissue in the intact heart. Sci. Rep. 6, 26744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salvarani N., Maguy A., De Simone S. A., Miragoli M., Jousset F., Rohr S. (2017) TGF-β1 (transforming growth factor-β1) plays a pivotal role in cardiac myofibroblast arrhythmogenicity. Circ. Arrhythm. Electrophysiol. 10, e004567 [DOI] [PubMed] [Google Scholar]
- 27.Lefroy D. C., Fang J. C., Stevenson L. W., Hartley L. H., Friedman P. L., Stevenson W. G. (1998) Recipient-to-donor atrioatrial conduction after orthotopic heart transplantation: surface electrocardiographic features and estimated prevalence. Am. J. Cardiol. 82, 444–450 [DOI] [PubMed] [Google Scholar]
- 28.Roell W., Klein A. M., Breitbach M., Becker T. S., Parikh A., Lee J., Zimmermann K., Reining S., Gabris B., Ottersbach A., Doran R., Engelbrecht B., Schiffer M., Kimura K., Freitag P., Carls E., Geisen C., Duerr G. D., Sasse P., Welz A., Pfeifer A., Salama G., Kotlikoff M., Fleischmann B. K. (2018) Overexpression of Cx43 in cells of the myocardial scar: correction of post-infarct arrhythmias through heterotypic cell-cell coupling. Sci. Rep. 8, 7145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson S. A., Copeland C. R., Reich D. H., Tung L. (2011) Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell monolayers. Circulation 123, 2083–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P., Su J., Mende U. (2012) Cross talk between cardiac myocytes and fibroblasts: from multiscale investigative approaches to mechanisms and functional consequences. Am. J. Physiol. Heart Circ. Physiol. 303, H1385–H1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz F., Hasan A., Alvarez-Laviada A., Miragoli M., Bhogal N., Wells S., Poulet C., Chambers J., Williamson C., Gorelik J. (2016) The protective effect of ursodeoxycholic acid in an in vitro model of the human fetal heart occurs via targeting cardiac fibroblasts. Prog. Biophys. Mol. Biol. 120, 149–163 [DOI] [PubMed] [Google Scholar]
- 32.Lyon A. R., MacLeod K. T., Zhang Y., Garcia E., Kanda G. K., Lab M. J., Korchev Y. E., Harding S. E., Gorelik J. (2009) Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc. Natl. Acad. Sci. USA 106, 6854–6859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis C. J., Gong H., Brown M. J., Harding S. E. (2004) Overexpression of β 1-adrenoceptors in adult rat ventricular myocytes enhances CGP 12177A cardiostimulation: implications for ‘putative’ β 4-adrenoceptor pharmacology. Br. J. Pharmacol. 141, 813–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorelik J., Yang L. Q., Zhang Y., Lab M., Korchev Y., Harding S. E. (2006) A novel Z-groove index characterizing myocardial surface structure. Cardiovasc. Res. 72, 422–429 [DOI] [PubMed] [Google Scholar]
- 35.Askar S. F., Bingen B. O., Swildens J., Ypey D. L., van der Laarse A., Atsma D. E., Zeppenfeld K., Schalij M. J., de Vries A. A., Pijnappels D. A. (2012) Connexin43 silencing in myofibroblasts prevents arrhythmias in myocardial cultures: role of maximal diastolic potential. Cardiovasc. Res. 93, 434–444 [DOI] [PubMed] [Google Scholar]
- 36.Majumder R., Engels M. C., de Vries A. A. F., Panfilov A. V., Pijnappels D. A. (2016) Islands of spatially discordant APD alternans underlie arrhythmogenesis by promoting electrotonic dyssynchrony in models of fibrotic rat ventricular myocardium. Sci. Rep. 6, 24334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter A. W., Barker R. J., Zhu C., Gourdie R. G. (2005) Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol. Biol. Cell 16, 5686–5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Z., Bien H., Shiferaw Y., Entcheva E. (2012) Cardiac cellular coupling and the spread of early instabilities in intracellular Ca2+. Biophys. J. 102, 1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takens-Kwak B. R., Jongsma H. J., Rook M. B., Van Ginneken A. C. (1992) Mechanism of heptanol-induced uncoupling of cardiac gap junctions: a perforated patch-clamp study. Am. J. Physiol. 262, C1531–C1538 [DOI] [PubMed] [Google Scholar]
- 40.Danon A., Zeevi-Levin N., Pinkovich D. Y., Michaeli T., Berkovich A., Flugelman M., Eldar Y. C., Rosen M. R., Binah O. (2010) Hypoxia causes connexin 43 internalization in neonatal rat ventricular myocytes. Gen. Physiol. Biophys. 29, 222–233 [DOI] [PubMed] [Google Scholar]
- 41.Rosker C., Salvarani N., Schmutz S., Grand T., Rohr S. (2011) Abolishing myofibroblast arrhythmogeneicity by pharmacological ablation of α-smooth muscle actin containing stress fibers. Circ. Res. 109, 1120–1131 [DOI] [PubMed] [Google Scholar]
- 42.Nikolaev V. O., Moshkov A., Lyon A. R., Miragoli M., Novak P., Paur H., Lohse M. J., Korchev Y. E., Harding S. E., Gorelik J. (2010) β2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327, 1653–1657 [DOI] [PubMed] [Google Scholar]
- 43.Novak P., Li C., Shevchuk A. I., Stepanyan R., Caldwell M., Hughes S., Smart T. G., Gorelik J., Ostanin V. P., Lab M. J., Moss G. W. J., Frolenkov G. I., Klenerman D., Korchev Y. E. (2009) Nanoscale live-cell imaging using hopping probe ion conductance microscopy. Nat. Methods 6, 279–281; erratum: 935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacan A., Ferhatosmanoglu H., Coskun H. (2008) CellTrack: an open-source software for cell tracking and motility analysis. Bioinformatics 24, 1647–1649 [DOI] [PubMed] [Google Scholar]
- 45.Thompson S. A., Blazeski A., Copeland C. R., Cohen D. M., Chen C. S., Reich D. M., Tung L. (2014) Acute slowing of cardiac conduction in response to myofibroblast coupling to cardiomyocytes through N-cadherin. J. Mol. Cell. Cardiol. 68, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y., Kanter E. M., Laing J. G., Aprhys C., Johns D. C., Kardami E., Yamada K. A. (2008) Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun. Adhes. 15, 289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahoney V. M., Mezzano V., Morley G. E. (2016) A review of the literature on cardiac electrical activity between fibroblasts and myocytes. Prog. Biophys. Mol. Biol. 120, 128–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhett J. M., Gourdie R. G. (2012) The perinexus: a new feature of Cx43 gap junction organization. Heart Rhythm 9, 619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asklund T., Appelskog I. B., Ammerpohl O., Ekström T. J., Almqvist P. M. (2004) Histone deacetylase inhibitor 4-phenylbutyrate modulates glial fibrillary acidic protein and connexin 43 expression, and enhances gap-junction communication, in human glioblastoma cells. Eur. J. Cancer 40, 1073–1081 [DOI] [PubMed] [Google Scholar]
- 50.Fu H. Y., Sanada S., Matsuzaki T., Liao Y., Okuda K., Yamato M., Tsuchida S., Araki R., Asano Y., Asanuma H., Asakura M., French B. A., Sakata Y., Kitakaze M., Minamino T. (2016) Chemical endoplasmic reticulum chaperone alleviates doxorubicin-induced cardiac dysfunction. Circ. Res. 118, 798–809 [DOI] [PubMed] [Google Scholar]
- 51.Takatori O., Usui S., Okajima M., Kaneko S., Ootsuji H., Takashima S. I., Kobayashi D., Murai H., Furusho H., Takamura M. (2017) Sodium 4-phenylbutyrate attenuates myocardial reperfusion injury by reducing the unfolded protein response. J. Cardiovasc. Pharmacol. Ther. 22, 283–292 [DOI] [PubMed] [Google Scholar]
- 52.Rhett J. M., Jourdan J., Gourdie R. G. (2011) Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol. Biol. Cell 22, 1516–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aasen T., Johnstone S., Vidal-Brime L., Lynn K. S., Koval M. (2018) Connexins: synthesis, post-translational modifications, and trafficking in health and disease. Int. J. Mol. Sci. 19, E1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnstone S. R., Billaud M., Lohman A. W., Taddeo E. P., Isakson B. E. (2012) Posttranslational modifications in connexins and pannexins. J. Membr. Biol. 245, 319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pogoda K., Kameritsch P., Retamal M. A., Vega J. L. (2016) Regulation of gap junction channels and hemichannels by phosphorylation and redox changes: a revision. BMC Cell Biol. 17 (Suppl 1), 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lampe P. D., TenBroek E. M., Burt J. M., Kurata W. E., Johnson R. G., Lau A. F. (2000) Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J. Cell Biol. 149, 1503–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang J., Palatinus J. A., He H., Iyyathuraia J., Jordan J., McGowan F. X., Schey K., Bultynck G., Zhang Z., Gourdie R. G. (2016) Abstract 16380: phosphorylation of connexin43 at serine368 is necessary for induction of cardioprotection by a connexin43 carboxyl-terminal mimetic peptide. Circulation 134, A16380 [Google Scholar]
- 58.Lauf U., Giepmans B. N. G., Lopez P., Braconnot S., Chen S.-C., Falk M. M. (2002) Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl. Acad. Sci. USA 99, 10446–10451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smyth J. W., Vogan J. M., Buch P. J., Zhang S. S., Fong T. S., Hong T. T., Shaw R. M. (2012) Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ. Res. 110, 978–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connolly A., Trew M. L., Smaill B. H., Plank G., Bishop M. J. (2015) Local gradients in electrotonic loading modulate the local effective refractory period: implications for arrhythmogenesis in the infarct border zone. IEEE Trans. Biomed. Eng. 62, 2251–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rutherford S. L., Trew M. L., Sands G. B., LeGrice I. J., Smaill B. H. (2012) High-resolution 3-dimensional reconstruction of the infarct border zone: impact of structural remodeling on electrical activation. Circ. Res. 111, 301–311 [DOI] [PubMed] [Google Scholar]
- 62.Gokhale T. A., Asfour H., Verma S., Bursac N., Henriquez C. S. (2018) Microheterogeneity-induced conduction slowing and wavefront collisions govern macroscopic conduction behavior: a computational and experimental study. PLOS Comput. Biol. 14, e1006276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hubbard M. L., Henriquez C. S. (2012) Microscopic variations in interstitial and intracellular structure modulate the distribution of conduction delays and block in cardiac tissue with source-load mismatch. Europace 14 (Suppl 5), v3–v9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.