Abstract
The sensors of the unfolded protein response react to endoplasmic reticulum (ER) stress by transient activation of their enzymatic activities, which initiate various signaling cascades. In addition, the sensor IRE1α exhibits stress-induced clustering in a transient time frame similar to activation of its endoRNase activity. Previous work had suggested that the clustering response and RNase activity of IRE1α are functionally linked, but here we show that they are independent of each other and have different behaviors and modes of activation. Although both clustering and the RNase activity are responsive to luminal stress conditions and to depletion of the ER chaperone binding protein, RNase-inactive IRE1α still clusters and, conversely, full RNase activity can be accomplished without clustering. The clusters formed by RNase-inactive IRE1α are much larger and persist longer than those induced by ER stress. Clustering requires autophosphorylation, and an IRE1α mutant whose RNase domain is responsive to ligands that bind the kinase domain forms yet a third type of stress-independent cluster, with distinct physical properties and half-lives. These data suggest that IRE1α clustering can follow distinct pathways upon activation of the sensor.—Ricci, D., Marrocco, I., Blumenthal, D., Dibos, M., Eletto, D., Vargas, J., Boyle, S., Iwamoto, Y., Chomistek, S., Paton, J. C., Paton, A. W., Argon, Y. Clustering of IRE1α depends on sensing ER stress but not on its RNase activity.
Keywords: differential ER Stress, autophosphorylation, BiP, luteolin
Endoplasmic reticulum (ER) stress is associated with many pathologic processes, from inflammation to cancer and neurodegeneration. Experimental ER stress is induced with different chemicals, most commonly inhibitors of glycosylation [e.g., tunicamycin (Tm)], disulfide bond formation (e.g., DTT) or calcium pumps [e.g., thapsigargin (Tg)], all of which inhibit normal folding of proteins in the ER (1, 2). Induction of ER stress typically initiates a signaling pathway called the unfolded protein response (UPR) in order to restore the cellular homeostasis and survive.
Apart from responding to chemical stress, the UPR is triggered by physiologic changes such as glucose stimulation in pancreatic β cells or antigen-induced differentiation of antibody-secreting cells (3–7). Importantly, the UPR is induced by accumulation of misfolded proteins, such as the null Hong Kong (NHK) truncated variant of α1-antitrypsin (α1AT) (8–10).
In both pathologic and physiologic conditions, the prosurvival function of the UPR involves transcriptional up-regulation of multiple genes, such as luminal ER chaperones, components of the ER-associated degradation system, and lipid synthesis enzymes (11). If the stress exceeds a tolerable threshold, the UPR initiates programmed cell death (12–16).
To initiate the proper responses the cell needs to sense ER stress as soon as it occurs. Three trans-membrane ER proteins are dedicated to sensing stress in the ER lumen and transducing the information to trigger cytosolic effector functions: serine/threonine-protein kinase/endoRNase inositol-requiring enzyme 1 α (IRE1α), protein kinase R–like ER kinase (PERK), and activating transcription factor 6 (ATF6) (17). This work focuses on the activation of IRE1α.
In the absence of ER stress, IRE1α is maintained in an inactive state through the binding of the chaperone binding protein (BiP)/glucose-regulated protein 78 kDa (18) to the luminal domain of IRE1α. Following ER stress, this binding partner dissociates and IRE1α dimerizes and oligomerizes, leading to activation of its cytosolic kinase domain and auto-trans phosphorylation. A conformational change is then transmitted from the kinase domain to the RNase domain, activating RNase activity (19–22).
The best-known activity of the IRE1α RNase domain is an unconventional splicing of a 26 nucleotides intron in the X box protein 1 (XBP1) mRNA that leads to generation of the XBP1s isoform (3, 23). This isoform is a transcription factor that translocates to the nucleus and promotes the expression of many targets, mostly prosurvival genes (11). The IRE1α RNase domain also performs a second activity, called regulated IRE1-dependent decay (RIDD), where IRE1α cleaves a number of RNA transcripts and micro-RNAs (24–27). The degradation of these RNA molecules either helps restore ER homeostasis or induces cell death programs, depending on the RIDD target and the quality of the initiating stimulus.
An early event in the activation of IRE1α is dimerization and oligomerization (28), which is required for the activation of the kinase domain. IRE1α activation shares these features with many other trans-membrane receptors (29–31). In addition to the biochemical evidence for oligomerization, live cell microscopy with IRE1α tagged with fluorescent proteins shows it to cluster in the ER membrane in response to ER stress (13, 19, 32–34). Remarkably, the IRE1α clusters disperse over time, even while the stress remains unmitigated (19, 32–34). The time course of this clustering behavior mirrors those of the phosphorylation of IRE1α and its XBP1/HAC1 splicing activity (33). The responsiveness to stress and its transient nature strongly suggest that clustering is related to activation of IRE1α. Work in yeast suggested that it promotes IREα activation, for example by recruiting HAC1 to the ER membrane (32). Other data suggested, however, that clusters are reservoirs of inactive IRE1α molecules (35). It has further been suggested that the clustering acts as a timer switch, signaling either cytoprotective or proapoptotic outcomes to IRE1α activation (33).
Here we provide several lines of evidence that IRE1α clustering is a distinct response to ER stress from the activation of the RNase domain. Although both depend on IRE1α ability to sense ER stress and on autophosphorylation by the IRE1α kinase domain, clustering is uncoupled from either the XBP1 splicing or RIDD activities. RNase-deficient IRE1α still clusters, bypass activation of the RNase domain does not allow normal clustering, and a pseudo-kinase IRE1α mutant impacts both activities differentially. Furthermore, we distinguish 3 types of IRE1α clusters that differ in their sizes, molecular packing, cellular distribution, and half-lives, indicating that IRE1α clusters can arise from distinct molecular conformers.
MATERIALS AND METHODS
Cell culture and reagents
HAP1 IRE1α KO (HAP1KO) is a near-haploid human cell line (HZGHC000742c006) derived from the male chronic myelogenous leukemia cell line KBM-7 (Horizon Discovery, Cambridgeshire, United Kingdom) by clustered regularly interspaced short palindromic repeats(CRISPR)/CRISPR asociated protein 9 (Cas9) editing, to contain an 8 bp deletion in a coding exon of the endogenous IRE1α gene. HAP1 and HAP1KO cells were maintained in Iscove’s Modified Dulbecco’s Medium (IMDM) (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 5% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA, USA), 1% penicillin/streptomycin (Thermo Fisher Scientific) and 1% sodium pyruvate (Corning, Corning, NY, USA). MEF XBP1 KO cells were a kind gift of Dr. Andrew Hu (The Wistar Institute, Philadelphia, PA, USA) and were grown in DMEM (Mediatech USA, Mooresville, NC, USA) supplemented with 5% fetal bovine serum, 1% penicillin/streptomycin, 1% sodium pyruvate, and 0.11 mM β-mercaptoethanol (MilliporeSigma, Burlington, MA, USA). HEK 293T cells [CRL-3216; American Type Culture Collection (ATCC), Manassas, VA, USA] were maintained in DMEM supplemented as above but without β-mercaptoethanol.
HAP1, HAP1KO, and MEF cells were transduced with both Tet-On (G418 resistant) and pTIGHT-IRE1-GFP-HA (IRE1GFP) (puromycin resistant) lentiviruses and selected for antibiotic resistance (G418 1 mg/ml, puromycin 2 μg/ml). IRE1GFP contained the human IRE1α sequence and a superfolded GFP grafted in-frame between codons 494 and 495, plus an HA peptide at the C terminus of IRE1α. IRE1GFP expression was induced with 1 μg/ml doxycycline (dox; MilliporeSigma) for 16–24 h. HEK 293T cells were transfected with plasmids encoding the same fusion proteins using lipofectamine 2000 and scored after 36–48 h.
Tm and 4μ8c were purchased from MilliporeSigma, Tg was purchased from MP Biomedicals (Solon, OH, USA), and luteolin was from MilliporeSigma. 1NM-PP1 was from Santa Cruz Biotechnology (Dallas, TX, USA) and used at 4 μM. Wild-type (WT) subtilase cytotoxin (SubAB) and its S272A mutant were purified as previously described by Paton et al. (36). GFP-Trap_A and RFP-Trap_A beads were obtained from Chromotek (Munich, Germany) and Lipofectamine from Thermo Fisher Scientific.
pCDNA-NHK-GFP, N1-BiP-mCherry, and pCDNA-α1AT plasmid were kindly provided by Dr. Christianson, Dr. Hebert, and Dr. Snapp, respectively. pEGFP-mCherry-Sec61β was obtained from Addgene (Watertown, MA, USA). NHK and α1AT plasmids were tagged with mCherry at the C-terminal end.
Mutagenesis
IRE1GFP WT plasmid was used as template for site-directed mutagenesis according to Kunkel et al. (37). Pfu Ultra II Fusion HS polymerase was purchased from Agilent Technologies (Santa Clara, CA, USA). All mutations were validated by Sanger sequencing. Primers used for K907A: 5′-AGATCTCCTCCGAGCCATGAGAAATGCCAAGCACCACTACCG-3′; D123P: 5′-CTCTACATGGGTAAAAAGCAGCCCATCT-3′; C148S: 5′-CCTTTGCAGATAGTCTCAGCCCATCAACCTCTCT-3′T; K599A: 5′-CGACGTGGCCGTGGCGAGGATCCTCCCC-3′.
RNA extraction, PCR, and quantitative PCR
Total RNA was isolated with the Trizol reagent (Thermo Fisher Scientific), following the manufacturer’s instructions. Two hundred ng of RNA were retrotranscribed to cDNA by priming with oligo(dT)12–18 and Superscript II retrotranscriptase (Thermo Fisher Scientific). Primers to detect unspliced/spliced Xbp1: forward: 5′-AAACAGAGTAGCAGCTCAGACTGC-3′; reverse: 5′-TCCTTCTGGGTAGACCTCTGGGAG-3′.
XBP1 splicing was assayed as described in Calfon et al. (3). Quantitative PCR was performed using SYBR green reagent (Thermo Fisher Scientific) and the reaction run on Applied Biosystems StepOne Plus machine. Data were analyzed using ΔΔCt method.
Quantitative PCR primers: Rpl19 (Ribosomal Protein L19): forward: 5′-AAAACAAGCGGATTCTCATGGA-3′; reverse: 5′-TGCGTGCTTCCTTGGTCTTAG-3′; Bloc1s1: forward: 5′-CAAGGAGCTGCAGGAGAAGA-3′; reverse: 5′-GCCTGGTTGAAGTTCTCCAC-3′; CHOP: forward: 5′-GGAGCTGGAAGCCTGGTATG-3′; reverse: 5′-AAGCAGGGTCAAGAGTGGTG-3′; BiP: forward: 5′-GTGATCAAGATACAGGTGACCTG-3′; reverse: 5′-GTCTTTTGTCAGGGGTCTTTCAC-3′.
Immunoprecipitation
Cells were lysed in lysis buffer [50 mM Tris-HCl pH 8, 150 mM NaCl, 5 mM KCl, 5 mM MgCl2, 1% NP-40, 20 mM iodoacetamide, 1 time protease inhibitors (Roche, Basel, Switzerland)]. Five percent of the volume of the lysate was saved as input in sample buffer and the rest were diluted in Tris-NaCl-NP40-BSA (TNNB) buffer (50 mM Tris pH 7.5, 250 mM NaCl, 0.5% NP-40, 0.1% BSA, 0.02% NaN3). GFP-Trap_A or RFP- Trap_A beads were added and incubated for 1 h at 4°C. After washing, beads were resuspended in sample buffer, boiled for 5 min, and the proteins were analyzed by SDS-PAGE and Western blot.
Western blots
Cells were lysed in lysis buffer [50 mM Tris-HCl pH 8, 150 mM NaCl, 5 mM KCl, 5 mM MgCl2, 1% NP-40, 20 mM iodoacetamide, 1 × protease inhibitors (Roche)]. Protein content was determined by BCA assay (Pierce, Rockford, IL, USA) and proteins were separated by SDS-PAGE and transferred onto nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Membranes were blocked, probed with primary and secondary antibodies, and scanned on an Odyssey Infrared imager (Li-Cor Biosciences, Lincoln, NE, USA).
Primary antibodies used: anti-phospho-IRE1α (Ser724) (Novus Biologicals, Centennial, CO, USA); anti-IRE1α (Cell Signaling Technology, Danvers, MA, USA); anti-PERK (Cell Signaling Technology); anti-HA (Covance, Princeton, NJ, USA); anti-tubulin (MilliporeSigma); anti-mCherry (Thermo Fisher Scientific); anti-BiP; anti-GFP (Thermo Fisher Scientific). IRDye-conjugated secondary antibodies were from Li-Cor.
Microscopy and image analysis
HAP1 cells were plated on 35-mm microscopy-grade plastic dishes (Ibidi, Gräfelfing, Germany). Expression of the exogenous IRE1GFP was induced with dox as previously described. The next day the cells were stained with NucBlue Live ReadyProbes Reagent (Thermo Fisher Scientific), the medium was replaced with IMDM with no phenol red containing ER stressors, and the cells were imaged over 8 h using a Marianas fluorescence microscope equipped with an OKO Lab CO2 enclosure (Okolab USA, San Francisco, CA, USA) on a Zeiss inverted platform (Carl Zeiss, Oberkochen, Germany), with a ×63 Plan-Apochromat 1.4 NA objective. Images were collected using an ORCA-ER camera (Hamamatsu Photonics, Iwata City, Japan) using the Volocity acquisition software (V5.5 and 6; PerkinElmer, Waltham, MA, USA). At each time point, a 4.5 µm Z-stack of images was collected with SlideBook v.6 (Intelligent Imaging Innovations, Denver, CO, USA) at intervals of 0.5 µm. Exposure times varied between 0.1 and 0.5 s, depending on sample intensity, unless otherwise specified.
Confocal images were taken with a Zeiss Axiovert 200 M inverted microscope (Carl Zeiss) equipped with a spinning disk confocal system (UltraView ERS 6; PerkinElmer) and a ×63 Plan-Apochromat 1.4 NA objective (Carl Zeiss). Images were collected with an ORCA-ER camera and the Volocity software, analyzed with Volocity and ImageJ (National Institutes of Health, Bethesda, MD, USA), and adapted for presentation with Adobe Illustrator (Adobe, San Jose, CA).
Cluster analysis was done with a homemade algorithm for Image J v.1.50i with Java v.1.8.0_77 (Oracle, Redwood Shores, CA, USA). The algorithm was used to extract the number of clusters, their size (area), intensity, and percentage of total cellular fluorescence for each image series (e.g., different treatments and mutants). Briefly, projection image of all in-focus planes of a Z-stack was created. Background intensity was corrected by measuring the mean intensity of an intranuclear area and subtracting the resulting value from each pixel in the image. The algorithm distinguishes between clusters and acquisition noise, and extracts cluster parameters by executing 2 consecutive procedures on each region of interest (ROI) within a single cell. First, an autocorrelation approach was used to estimate the mean cluster size (area) in all ROIs within a cell. Each ROI (Supplemental Fig. S1A) is divided into 32 × 32 pixel subregions (Supplemental Fig. S1B), and a typical object size is defined for each subregion by extracting the full width at half maximum from a Gaussian fit of the autocorrelation product (Supplemental Fig. S1D, E). A distribution of mean cluster sizes from all subregions produces the mean and sd cluster size for the entire cell. This approach is described in more detail in Blumenthal et al. (38). Specific clusters are defined by fitting all potential clusters with a 2-dimensional Gaussian fit, and selecting those that meet a set of user-defined constraining parameters. 1) Allowed cluster size range: defined as the number of sem cluster size. For our analysis, we allowed for 4.5 sem. 2) Minimal allowed amplitude fold increase: the minimal allowed intensity increase above local background. Here, this parameter varied from 10 to 350, depending on the intensity of the sample. 3) Minimal allowed goodness of fit: a measure for how well the cluster intensity profile fits a Gaussian model. We used a value of 0.75. 4) Fitting window size: defines the area used for the Gaussian fit as a function of estimated cluster size. This parameter was set to 1.2 in order to distinguish between close clusters. 5) Minimal allowed cluster size: used to eliminate false-positives due to peak noise, was set to 3 pixels.
Because the algorithm only detects round clusters, when nonsymmetric clustering was observed (as in the case of K907A IRE1GFP or in WT cells treated with 4µ8c or SubAB), cluster areas were selected manually using the ImageJ software. The analysis procedure was performed as previously described.
Statistical analyses
To enumerate cells containing clusters, >200 cells were counted per condition in 2 or more independent experiments. Statistically significant differences were evaluated by Student’s t test. Size and intensity of individual clusters were enumerated on 100–1100 clusters from multiple cells in distinct microscope fields. Because the data sets were distributed log normally, ANOVA was used to compare them by using Prism software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Reconstitution of IRE1GFP in HAP1 IRE1KO cells allows studying the dynamics of ER stress-induced clusters
Given the linkage between the dynamic clustering of IRE1α and the response to ER stress, we sought to determine the structural requirements and the functional significance of IRE1α clustering. Because overexpression of IRE1α leads to spontaneous activation even without deliberate stress [(21, 39, 40) and Supplemental Fig. S2F], we stably expressed WT and mutant versions of IRE1GFP under the transcriptional control of the TetON system in a HAP1-derived cell line that lacks the endogenous IRE1α gene (Supplemental Fig. S2A, B, termed below HAP1KO). When induced by dox, IRE1GFP is expressed at 1–6 times the endogenous IRE1α level in the parental HAP1 cells, depending on the mutant used, which is a much lower level than that observed in 293T cells when IRE1α expression is under the control of a strong promoter (Supplemental Fig. S2C). The spontaneous IRE1α activity in our expression system is similar to that seen in cells with endogenous IRE1α (Supplemental Fig. S2B, E), and we chose to accept a 4–6-fold overexpression compared to the endogenous IRE1α level in order to gain improved sensitivity in the clustering assays.
Importantly, IRE1GFP expression restores XBP1 splicing and RIDD activities to the HAP1KO cells when chemical ER stress is imposed (Supplemental Fig. S2B, E). Although our IRE1GFP construct is similar to the IRE1α-3F6HGFP used in (13, 33), IRE1GFP can support essentially complete, transient splicing of XBP1 transcripts and RIDD activity in HAP1KO cells (Supplemental Fig. S2).
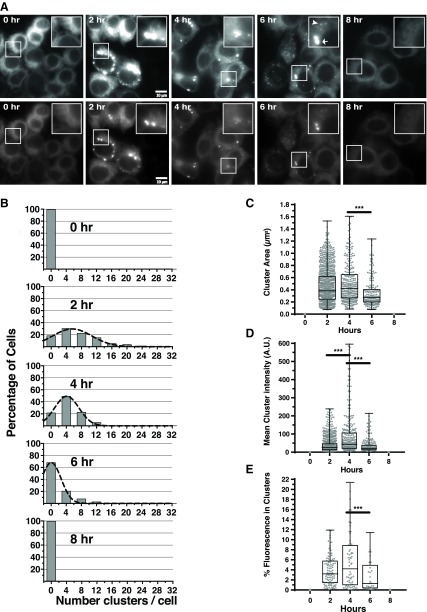
When stably expressed in HAP1KO cells, WT IRE1GFP exhibited a reticular distribution typical of ER proteins (Fig. 1A). After applying ER stress (Tm), IRE1GFP became progressively clustered, with a peak at 4 h and then returned to a reticular pattern (Fig. 1A–E and Supplemental Video S1). We quantified several parameters of IRE1α clustering in order to infer the underlying dynamics. Within 2 h of Tm treatment, ∼60% of the cells contained clusters, with a wide distribution of cluster number per cell (Fig. 1B). The mean cluster size changed little between 2 and 4 h of Tm treatment (Fig. 1C), but the distribution of cluster number per cell narrowed, and there were more intense clusters in each cell (Fig. 1B, D). To ensure that the level of IRE1GFP remained constant during the course of the experiments, dox was washed out before the initiation of ER stress, IRE1GFP levels were confirmed by a Western blot (Supplemental Fig. S2D), and quantitation of total cell fluorescence showed that it remained constant over 8 h (unpublished results). Taken together, these measurements are consistent with initial formation of smaller clusters of IRE1α that later coalesce into larger ones, and time-lapse analysis indeed captured a number of apparent fusion events (Supplemental Videos S1 and S2). These observations also suggest that the molecular density of IRE1α in the clusters changed as the clusters aged. The source of the added IRE1GFP molecules must have been the large population of dispersed IRE1α that was present even in the most clustered cells (e.g., Fig. 1A). In fact, <20% of the total IRE1GFP was included in the clusters, with the rest remaining dispersed throughout the ER (Fig. 1A, E).
Figure 1.
IRE1GFP clusters in HAP1KO cells in a transient fashion in response to ER stress. A) IRE1GFP clusters reversibly in response to the ER stressor Tm. Representative fields of HAP1KO cells expressing WT IRE1GFP that were exposed to Tm treatment for the indicated times. Each field is shown at 2 exposures, with the top series emphasizing the overall distribution of fluorescence and the bottom series keeping the exposure of clusters at the linear range of the camera. Insets: selected areas with IRE1GFP clusters. The cells had been incubated with 1 μg/ml dox for 24 h to induce WT IRE1GFP expression; dox was then removed, Tm (4 μg/ml) added to the medium, and the cells imaged. Zero hour: Reticular distribution of IRE1GFP. Two, four, and six hours: Clustered distribution of WT IRE1GFP. Arrow and arrowhead in the 6 h inset denote examples of large and small clusters. Eight hours: representative image of cells after 8 h of continuous exposure to Tm, when the clusters dispersed and returned to a reticular distribution. Scale bars, 10 μm. B) The time course of clustering. Time course of clustering displayed as histogram analyses of cell images taken at 0, 2, 4, 6, and 8 h Tm continuous treatment. At each time point, the numbers of clusters/cell was scored using the ImageJ algorithm (see Materials and Methods), binned, and the percentage of cells with each bin of clusters was plotted. Between 86 and 124 cells were analyzed per time point, taken from 3 independent time courses. All quantitations were performed on images within the linear range of the camera. C) Distribution of cluster sizes during the Tm time course. Each dot represents an individual cluster, and at each time point from 183 to 1056 clusters were counted. ***P < 0.01; n = 3. D) Distribution of cluster fluorescence intensities at each time point. Each dot is the integrated fluorescence intensity of a single cluster. E) Percentage of total cell fluorescence intensity found in clusters at each time point. Each dot represents a cell.
After the peak time, the percentage of cells with clustered IREGFP declined, even under continuous exposure to the stressor (Fig. 1A, B). By 6 h of Tm stress, the clusters started to disperse and during the next 2 h, IRE1GFP distribution in the vast majority of the cells reverted to the initial reticular fluorescence (Fig. 1A, B). We conclude that IRE1α clustering in HAP1 cells is qualitatively and quantitatively similar to the clustering described before in 293T-Rx cells (13, 33, 41). IRE1α clustering is dynamic, with a time course that parallels the transient nature of XBP1 splicing (42, 43).
Clustering of IRE1α under mild ER stress
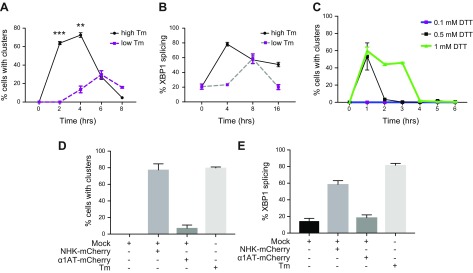
The usual stimuli that have been used to induce IRE1α clustering, such as the glycosylation inhibitor Tm or the reductant DTT, impart severe stress via global proteomic changes that were shown to differ dramatically from expression of a misfolded protein (44). Therefore, we sought to determine if and how milder stresses induce IRE1α clustering. One form of milder stress was a 40-fold reduction in the dose of Tm (Fig. 2A), which lowered the fraction of cells displaying IRE1α clusters from 75 to 35%, but more remarkably, delayed the clustering response and the timing of XBP1 splicing (Fig. 2B). Similarly, using 10-fold less DTT also proved sufficient to induce clustering; again, the delay in the timing was more remarkable than the change in amplitude (Fig. 2C).
Figure 2.
Clustering of IRE1GFP in response to mild ER stress. A) Time course of WT IRE1GFP clustering in response to high and low Tm doses. HAP1KO cells stably expressing WT IRE1GFP were treated with 4 μg/ml Tm (high Tm) or 0.125 μg/ml Tm (low Tm), and the distribution of IRE1GFP was monitored over the subsequent 8 h. Cells were defined to have clusters if they had more than 3 clusters per cell. B) Time course of XBP1 splicing in response to high and low Tm doses. Cells were treated as in A, and the efficacy of splicing was determined by an RT-PCR assay whose amplicons were analyzed by agarose gel electrophoresis. Bands were quantified with ImageJ. C) Time course of IRE1GFP clustering in response to doses of DTT. Cells were treated and observed as in A, but with 1, 0.5 or 0.1 mM DTT. D) NHK induces IRE1GFP clustering. 293T cells stably expressing Tet-inducible WT IRE1GFP were transiently transfected with either NHK-mCherry or α1AT-mCherry using the lipofectamine method or treated with Tm (4 μg/ml 4 h). Cells were imaged and the quantitation of the percentage of double-positive cells (mCherry and GFP) with clusters is shown (Mock, lipofectamine only). E) NHK induces XBP1 splicing. 293T cells were transiently transfected with either NHK-mCherry or α1AT-mCherry using lipofectamine or treated with Tm (4 μg/ml 4 h). XBP1 mRNA splicing was determined by RT-PCR and % XBP1 splicing was calculated and plotted.
Another form of milder, more physiologic ER stress is expression of a misfolded protein. Bakunts et al. (41) showed that accumulation of unassembled Ig μ chain induced IRE1α clustering and we quantified the clustering behavior in response to a permanently misfolded enzyme, the NHK variant of α1AT (8, 9). We coexpressed mCherry-tagged NHK or WT α1AT in 293T cells, that stably expressed the dox-inducible IRE1GFP. Overexpression of NHK-mCherry, but not equivalent overexpression of secreted α1AT-mCherry, induced IRE1α clustering to a similar extent as the clustering induced by Tm (Fig. 2D). As expected, NHK-mCherry expression also induced XBP1 splicing, whereas expression of α1AT-mCherry did not increase XBP1 splicing beyond that of mock transfection (Fig. 2E). We conclude that IRE1α clustering is not just a response to excessive stress but is induced by mild ER stress that approximates physiologic levels.
BiP cleavage induces massive, persistent IRE1α clustering
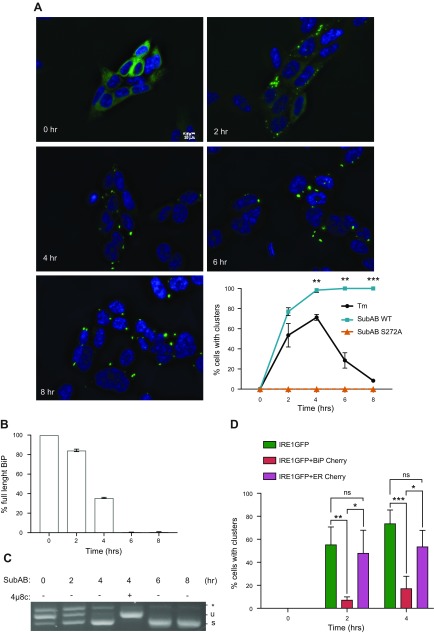
The binding of the chaperone BiP to the luminal domain of IRE1α plays a key role in tuning the activation of IRE1α (35, 45–47). Therefore, ablation of BiP is a particularly effective mode of activating IRE1α. To test whether ablation of BiP also activates the clustering response, we used the highly specific bacterial serine protease SubAB, which cleaves mammalian BiP (48) and decreases its inhibitory effect on IRE1α activation (35, 49). Treatment with SubAB indeed induced IRE1α clustering within 2 h (Fig. 3A). This response was due to the BiP-cleaving activity of SubAB because the inactive SubAB mutant S272A, which does not cleave BiP (48), did not induce IRE1α clustering (Fig. 3A). The induction of IRE1α clustering with SubAB coincided with induction of XBP1 splicing, which was maximal after 4 h (Fig. 3C). The sharp dependence of clustering on BiP levels was remarkable because 2 h treatment with 0.1 μg/ml SubAB only cleaved ∼15% of BiP (Supplemental Fig. S4A and Fig. 3B), yet this was sufficient to cause appearance of IRE1α clusters in ∼80% of the cells (Fig. 3A, C). These data show that ablation of even a fraction of cellular BiP is sufficient to induce massive IRE1α clustering, whereas deliberate expression of exogenous BiP has the reverse effect (Fig. 3D), reflecting either the presumed ratio of active BiP to total misfolded protein (41) or the sensitivity of a special pool of BiP that binds IRE1α.
Figure 3.
Formation of large clusters after BiP cleavage. A) BiP cleavage induces massive and irreversible clustering. HAP1KO cells expressing WT IRE1GFP were treated with SubAB WT or S272A mutant (0.1 μg/ml) for the indicated times. Representative fields recorded at the indicated times are shown together with a quantitation of the percentage of cells with clusters over the time course. At least 100 cells were evaluated per time point and statistically significant differences between Tm and SubAB WT time points are indicated. **P < 0.01, ***P < 0.001. Scale bar, 10 μm. B) Time course of SubAB cleavage of BiP. Cells expressing IRE1GFP WT were treated with SubAB (0.1 μg/ml) for the indicated times. Proteins were then extracted and analyzed by Western blot with anti-BiP antibody. One of the blots is shown in Supplemental Fig. S4A. 14.3.3 was used as housekeeping. The full-length (∼78 kDa) and cleaved BiP (∼28 kDa) bands were quantified using ImageJ and the percentage of full-length BiP is plotted. C) SubAB induces XBP1 splicing. HAP1KO cells expressing WT IRE1GFP were treated with SubAB (0.1 μg/ml) for the indicated times. XBP1 mRNA splicing was determined by RT-PCR, % XBP1 splicing was calculated and plotted. Note that the activation of IRE1α persists throughout the experiment and is inhibited by 16 μM 4μ8c treatment, demonstrating IRE1α dependence. D) Overexpression of BiP inhibits Tm-inducible clustering of WT IRE1GFP. HEK 293T cells expressing Tet-inducible IRE1GFP WT were transiently transfected with either BiP-mCherry or ER-mCherry and treated with Tm (4 μg/ml) for the indicated times. Cells with clusters that were positive for mCherry were counted and graphed. At least 88 cells were evaluated, and experiments were performed at least 3 times. Ns, not significant; P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001.
The SubAB-induced clustering differed from that induced by the more physiologic ER stress in 2 important ways: first, the clusters were much longer lived and did not disperse over 8 h (Fig. 3 and Supplemental Fig. S4B). This suggests that the transcriptional up-regulation of BiP late in the response is not sufficient to counteract the depletion of the protein. Second, the SubAB-induced clusters were much larger than Tm-induced clusters and their fluorescent intensity increased progressively after they formed; at the peak time, the SubAB-induced clusters were 6 times bigger and 11 times more intense than Tm-induced clusters (Table 1). The progressive increase in the 2 parameters indicated continual migration of IRE1α molecules into these clusters.
TABLE 1.
Physical parameters of IRE1α clusters
| IRE1GFP | Treatment | Sizea | Intensityb | Stability |
|---|---|---|---|---|
| WT | Tm | 0.42 ± 0.02 | 41,771 ± 323 | Transient |
| WT | SubAB | 2.47 ± 3.24 | 465,096 ± 4968 | Persistent |
| WT | 4μ8c | 1.88 ± 0.33 | 365,858 ± 5789 | Persistent |
| K907A | Tm | 1.84 ± 0.32 | 556,822 ± 5514 | Persistent |
| I642G | Tm | 0.32 ± 0.01 | 10,436 ± 21 | Persistent |
| I642G | 1NM-PP1 | 0.37 ± 0.01 | 15,398 ± 42 | Persistent |
| I642G | 1NM-PP1+ Tm | 0.29 ± 0.02 | 7809 ± 15 | Persistent |
Cluster size (diameter) measured at peak time and given in micrometers.
Integrated cluster fluorescent intensity in arbitrary units.
A functional RNase domain is not needed for IRE1α clustering
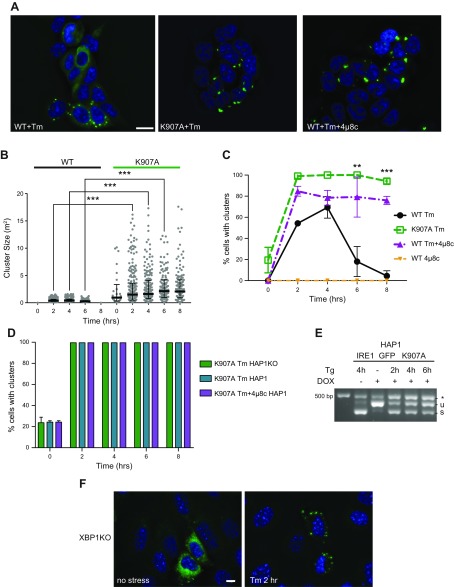
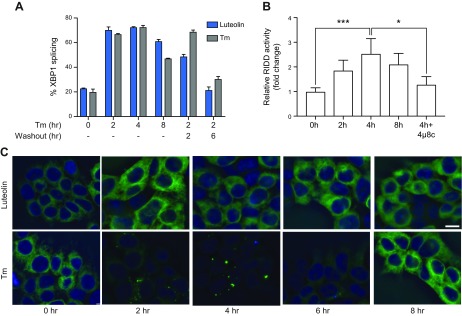
To understand whether clustering and RNase activity are mechanistically related, we tested this directly by inactivating the RNase activity both pharmacologically and by mutation. IRE1α is inactivated selectively by treatment with the inhibitor 4μ8c, which forms a Schiff base with lysine 907 in the RNase-active site (50). 4μ8c-inactivated IRE1α could not splice XBP1 mRNA (Supplemental Fig. S3A) but still clustered in response to ER stress, forming large and bright clusters (Fig. 4A–C) that persisted over 8 h (Fig. 4C).
Figure 4.
A functional IRE1α RNase domain is not needed for cluster formation but affects cluster dispersal. A) RNase-inhibited IRE1α still clusters. HAP1KO cells expressing K907A or WT IRE1GFP were induced with dox and treated with either Tm for 2 h, or 4μ8C (16 μM) plus Tm for 2 h. Representative pictures are shown. B) Clusters of K907A are bigger than WT IRE1GFP clusters. Histogram distribution of cluster size (square micrometers) following Tm treatment at the indicated times. At least 119 cells were evaluated for each condition. ***P < 0.001. C) RNase-inhibited IRE1α displays persistent clustering. Percentages of K907A or WT IRE1GFP cells that clustered following Tm (4 μg/ml), 4μ8c (16 μM), or Tm (4 μg/ml) plus 4μ8c (16 μM) treatments were graphed. At least 291 cells were evaluated for each condition. **P < 0.01, ***P < 0.001. D) Hyperclustering is intrinsic to K907A IRE1α. HAP1KO cells or parental HAP1 cells, which expressed K907A IRE1GFP were induced with dox, treated with Tm or with Tm plus 4μ8c, as in C. The percentages of cells with clusters are graphed. E) K907A IRE1α affects WT IRE1α XBP1 splicing. Parental HAP1 cells expressing K907A IRE1GFP were treated with Tg (0.2 μM) for the indicated times and XBP1 mRNA splicing was determined by RT-PCR. F) Absence of the XBP1 gene does not affect the ability of IRE1α to cluster. XBP1KO MEFs were transiently transfected with WT IRE1GFP and TetON plasmids and induced with dox as before. The cells were then treated with Tm (4 μg/ml) and imaged at 0 and 2 h. Representative pictures are shown. The exposure time for GFP was 1 s. Scale bars, 10 μm.
A second way to inactivate IRE1α is through the mutant K907A, where the catalytic lysine 907 in the RNase-active site is substituted with Alanine and is unable to splice XBP1 or cleave RIDD substrate RNAs (51). When HAP1KO cells expressing K907A IRE1GFP were treated with Tm, they responded to the stress by persistent clustering, like the chemically inhibited IRE1α (Fig. 4A–C). Therefore, formation of IRE1α clusters, while induced by ER stress, does not require the RNase activity of IRE1α.
Like the SubAB-induced clusters (Fig. 3), the clusters formed by RNase-deficient IRE1α are larger and brighter than clusters of WT IRE1GFP (Fig. 4A–C and Supplemental Fig. S3B, C) (Table 1) and contain a larger fraction of the total IRE1α population (unpublished results). These measurements indicate that IRE1α molecules are recruited into the clusters after the induction of ER stress, even when they are splicing-impaired. In addition, the large clusters formed by inactive IRE1α molecules or by active molecules under SubAB treatment are not transient, persist for at least 8 h, and are less mobile than stress-induced WT clusters (Fig. 4B, C and Supplemental Fig. S3C).
To understand if the hyperclustering is an intrinsic property of the RNase-inactive IRE1α, we next asked whether the large clusters of inactive IRE1α are affected in the presence of WT IRE1α. We found that cluster number and kinetics of formation by K907A IRE1GFP were maintained even in the presence of the endogenous WT IRE1α in the parental HAP1 cells (Fig. 4D). Conversely, the presence of the inactive K907A decreased the total splicing activity of the endogenous WT IRE1 (Fig. 4E). Whether this effect is due to codimerization of WT and mutant IRE1α, without any effect on clustering, or to competition for substrates or cofactors is yet to be determined.
All these observations suggest that inactive IRE1GFP not only oligomerizes but also has a higher propensity to do so. Furthermore, the RNase activity seems to restrict the clustering and to promote their dissociation with time. The dissociation of clusters under continuous Tm treatment is likely dependent on increased concentration of BiP in the ER as a result of a transcriptional response, as suggested by Bakunts et al. (41).
If clustering does not involve RNA cleavage activity, does it require the presence of IRE1α substrates? To explore this question, we expressed IRE1GFP in XBP1KO MEFs that lack either spliced or unspliced XBP1 transcripts. IRE1GFP responded to Tm treatment in these cells just as it did in XBP1-sufficient cells (Fig. 4F). The level of IRE1GFP expression was insufficient to ascertain the life time of these clusters. This suggests that the binding of the main substrate of IRE1α, XBP1, does not affect clustering.
Clustering of IRE1α is dispensable for its RNase activities
If IRE1α clustering does not require the RNase activity, does the RNase activity require clustering? Enhancement of IREα activity was indeed one of the functions proposed for clusters (32). To address this question, we took advantage of the ability of the membrane-permeable flavonoid luteolin to activate the RNase domain of IRE1α without ER stress (52) and asked if this bypass activation requires clustering. The treatment with luteolin induced both maximal XBP1 splicing activity, which was reversible once luteolin was washed out (Fig. 5A), and strong RIDD activity (Fig. 5B). Yet, this strong activation of IRE1GFP by luteolin did not lead to any discernible IRE1α clustering in contrast to its activation by Tm treatment (Fig. 5C). Thus, our results define clustering and RNase activities as distinct manifestations of activation: IRE1α clustering is not dependent on RNase activity nor is RNase activity, or even maximal activity, dependent on clustering.
Figure 5.
Clustering of IRE1α is dispensable for its RNase activities. A) Luteolin reversibly induces XBP1 splicing. XBP1 mRNA splicing in HAP1KO cells expressing WT IRE1GFP was induced by incubation with luteolin (50 μM) or Tm (4 μg/ml) for the indicated times. Washout: Cells were treated for 2 h and then incubated without the drugs for 2 or 6 h. Percentage of XBP1 splicing was determined from RT-PCR and quantification of bands was performed with ImageJ. B) Luteolin induces RIDD activity. HAP1KO cells expressing WT IRE1GFP were incubated for the indicated times with luteolin (50 μM) and RIDD activity was assessed by quantitative RT-PCR measurements of IRE1α-dependent Biogenesis Of Lysosomal Organelles Complex 1 Subunit 1 (BLOC1S1) mRNA. Dependence on IRE1α was shown by the sensitivity to 4μ8c (16 μM). Experiments were performed 3 times. ***P < 0.001, *P < 0.05. C) Luteolin does not induce IRE1α clustering. HAP1KO cells expressing WT IRE1GFP were treated with luteolin (50 μM) or Tm (4 μg/ml) and imaged at the indicated times. Representative pictures are shown. The exposure time was 1 s. Scale bar, 10 μm.
Active kinase domain is required for stress-induced IRE1α clustering
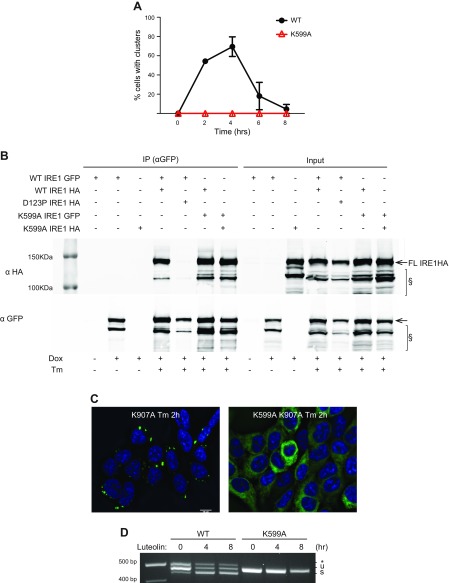
Because induction of the IRE1α RNase activity is preceded by activation of the IRE1α kinase domain (19), we asked if autophosphorylation is required to induce clusters. When expressed in HAP1KO cells, the kinase-impaired mutant K599A (39) was unable to cluster during Tm stress (Fig. 6A), showing instead a dispersed, reticular ER distribution over the 8 h time course. Importantly, the K599A mutant still dimerized, either with itself or with WT IRE1GFP, as shown by an immunoprecipitation assay (Fig. 6B). Because the kinase activity of IRE1α was required for normal IRE1GFP clustering, we next asked if it was also required for the hyperclustering of RNase-inactive IRE1α molecules. The double IRE1GFP mutant K599A K907A was unable to cluster in response to Tm treatment (Fig. 6C), suggesting that not only is the kinase activity required for clustering but also that kinase impairment is dominant over the hyperclustering phenotype of K907A IRE1α. Interestingly, the kinase-defective mutant K599A also did not respond to the bypass activation of the RNase-dependent XBP1 splicing activity by luteolin (Fig. 6D). This demonstrates that both the RNase activity and the clustering depend on prior initiation of the kinase activity, whether during the normal activation of IRE1α or its activation by luteolin.
Figure 6.
Active kinase domain is needed for formation of clusters. A) Kinase-deficient IRE1GFP does not cluster under ER stress. HAP1KO cells expressing WT or K599A IRE1GFP were treated with Tm (4 μg/ml) and imaged at the indicated times. Shown are the percentages of cells with IRE1GFP clusters. B) IRE1GFP K599A mutant is able to dimerize. HAP1KO cells expressing IRE1HA WT, D123P, or K599A and coexpressing IRE1GFP WT or K599A were treated with Tm (4 μg/ml) for 4 h when indicated. Cells were collected, lysed, and subjected to immunoprecipitation with GFP-Trap beads. Beads-bound proteins were analyzed by Western blot. Input: 5%. §: lower MW bands that appear to be IRE1α specific and size-sensitive to Tm treatment. C) The K599A mutation suppresses the clustering induced by the K907A mutation. Cells expressing either the K907A mutant or the K599AK907A double mutant were treated with Tm (4 μg/ml) for 2 h and clustering was visualized as in Fig. 1. Scale bar, 10 μm. D) The K599A mutant is refractive to the stimulation of RNase activity via luteolin. HAP1KO cells expressing WT or K599A IRE1GFP were treated with luteolin (50 μM) for the indicated times and RT-PCR was performed as previously described.
The finding that K599A can dimerize, although it does not cluster, might explain why K599A acts as a trans-dominant negative mutant (39). However, the observed failure to cluster is at odds with Oikawa et al. (34), who found that K599A does cluster. We believe that overexpression conditions in the cells used by Oikawa et al. might have caused this discrepancy. Our finding that the K599A mutant can homo-dimerize, presumably through its luminal domain (Fig. 6B), gives credence to this explanation.
Clustering dispersal is not dependent on phosphorylation in the kinase activation loop
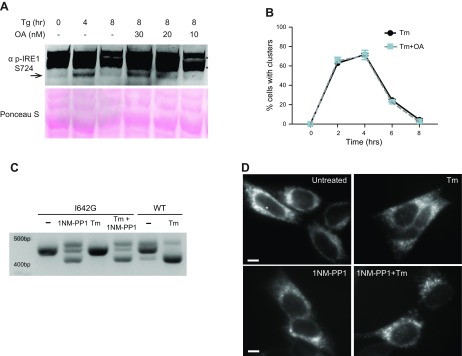
Because the WT kinase activity is required to initiate clustering, we further asked if it was also necessary to maintain the clusters. For that purpose, we treated HAP1KO cells expressing WT IRE1GFP with the phosphatase inhibitor okadaic acid, which is known to inhibit dephosphorylation of IRE1α (53). As shown by immunoblotting with an antibody to phospho-Ser 724 in the activation loop of the kinase domain (54), cotreatment of the cells with okadaic acid plus Tm significantly prolonged IRE1α phosphorylation in comparison to Tm alone (Fig. 7A). However, the prolonged phosphorylation had no effect on the duration of clustering, which decreased as usual after the peak time (Fig. 7B). Because the kinase activity is not required for cluster dispersal, initiation and dispersal of IRE1α clusters are dictated by different events.
Figure 7.
Effects of persistent phosphorylation and nucleotide binding on clustering. A) Okadaic acid treatment prolongs phosphorylation of IRE1α. HAP1 cells were treated with Tg (0.2 μM) and okadaic acid (OA) (30, 20, or 10 nM) for the indicated times. Cells were lysed and subjected to Western blot analysis with anti-phospho-serine 724 in the activation loop of IRE1α. Arrow, specific phosphoS724 species; asterisk, nonspecific bands. B) Sustained phosphorylation of IRE1α does not affect the kinetics of clustering. HAP1KO cells expressing WT IRE1GFP were treated with 4 μg/ml Tm alone or Tm+ 30 nM OA. Cells were imaged at the indicated times and the percentage of cells with clusters was graphed. C) The XBP1 splicing activity of HAP1KO cells expressing I642G IRE1GFP is induced by treatment with 1NM-PP1 but not Tm. HAP1KO cells expressing I642G IREGFP were treated with 1NM-PP1 (4 μM) for 4 h. RT-PCR was performed as before. D) Intracellular distribution of I642G IREGFP in HAP1KO with and without the ATP mimetic ligand 1NM-PP1 and with and without Tm. HAP1KO cells expressing I642G IREGFP were treated with 1NM-PP1 (4 μM) and Tm (4 μg/ml) for 4 h and imaged as previously. Scale bars, 10 μm.
The pseudo-kinase mutant I642G IRE1 oligomerizes along a distinct pathway
It is possible that the K599A mutant is deficient in nucleotide binding and therefore stabilizes an IRE1α conformation that cannot cluster. To address this possibility, we used a second phosphorylation-deficient mutant, I642G, which does not bind ATP and does not autophosphorylate but can bind the ATP mimetic 1NM-PP1 and responds to it by transmitting a conformational change that activates the RNase domain (19, 21, 55). Treatment of cells expressing I642G IRE1α with 1NM-PP1, alone or with Tm, activated the RNase-dependent XBP1 splicing reaction (Fig. 7C), as described by Papa et al. (21). However, I642G IRE1α exhibited an unusual clustering behavior: half the cells showed dispersed IRE1GFP and the other half had small, uniform clusters measuring 0.3 μm in diameter, even without applied ER stress (Fig. 7D and Table 1). In addition, these small clusters tended to be asymmetrically distributed within the cell, with most clusters appearing on one side of the nucleus (Fig. 7D). When treated with the ligand 1NM-PP1, essentially all I642G IRE1α cells displayed these small clusters and there was no increase in cluster size over time (unpublished results). Treatment with Tm plus 1NM-PP1 did not change the clustering frequency, the number of clusters per cell, or the size of the I642G clusters any further (Table 1). Therefore, I642G IRE1α clusters in a distinct manner from WT IRE1α: it is independent of the splicing activity, is initiated spontaneously, unaffected by ER stress, and the clusters are small and uniform in size. We conclude that binding of the nucleotide analog is not needed for clustering of IRE1α per se, but ATP hydrolysis and the accompanying conformational change are needed.
DISCUSSION
Dimerization and oligomerization of IRE1α have long been known to be part of the activation sequence of this sensor (28, 56), but more recently, live cell imaging revealed larger dynamic oligomers of IRE1α, here termed clusters, which form in response to ER stress (19, 32, 33). The induction of clustering, its time course, and its reversibility indicated that this type of oligomerization is related to the stress sensing function of IRE1α, but it is not yet clear how clustering relates to either the RNase or the kinase activities of IRE1α.
One main conclusion from our data is that although clustering is an ER stress-induced behavior whose time course parallels the RNase activities of IRE1α, the 2 are mechanistically separate. RNase-inactive IRE1α can still cluster and, conversely, clustering does not predict RNase activity. Our second main conclusion is that IRE1α clustering can be initiated by distinct molecular forms, likely reflecting distinct conformations of IRE1α. RNase-inactive IRE1α clusters, either due to mutation or to binding of inhibitor, but clusters differently from WT IRE1α. A third form that can also give rise to clusters is the pseudo-kinase mutant I642G, but it forms clusters with properties distinct from the other 2 types. The third conclusion from our work is that clustering depends on the kinase activity and clusters normally form only after the trans-phosphorylation of monomers.
IRE1α clustering is a response to many forms of ER stress because it is induced by chemical stressors as well as by the presence of misfolded, nonsecreted protein, as has also been shown by Bakunts et al. (41). Importantly, IRE1α clusters in response to mild, not only severe stress, so it is likely a physiologically relevant response. IRE1α clustering is visible within the first hour after the onset of stress, and it often reaches >30 clusters per cell. Over the next 2–3 h, up to ∼80% of the cells develop a clustered appearance. The number of clusters per cell actually drops during this time, whereas the average fluorescent intensity of clusters increases; this is consistent with fusion of smaller clusters to form larger ones, a process that is observed directly in time-lapse sequences. During the fusion of clusters, the calculated density of IRE1α molecules increases, suggesting that its molecular packing is initially relatively loose and then increases. Importantly, even at the peak response, <20% of the total IRE1α in each cell is clustered. After the peak, clustering decreases and most IRE1α returns to the dispersed and reticular distribution, even in the continued presence of stress. Thus, the packing of IRE1α in clusters is dynamic and undoubtedly is regulated by the rather large set of IRE1α interacting proteins (57).
Sundaram et al. (58) used native gels to resolve oligomeric complexes of IRE1α and showed, surprisingly, that even unstressed cells contain oligomeric IRE1α, primarily of ∼480 and 720 kDa. Moreover, they found only minor changes in these complexes in stressed cells, whereas the sizes of PERK complexes increased with stress (59). Our method of analysis is obviously quite different, and we have not calibrated our fluorescence images to allow estimation of the number of IRE1α molecules in individual clusters. Nevertheless, based on quantitation of IRE1α expression by mass spectrometry in U2OS (59) and HeLa cells (60), we estimate 2500–5000 IRE1α molecules per cell. Our data show that after 2 h of stress, roughly 2.5% of IRE1α molecules are within clusters, and the mean cluster number is 6 clusters per cell (Fig. 1), indicating that by the lower estimate clusters consist of 10 IRE1α molecules or more. This is similar to the estimate from analytical ultracentrifugation that oligomers in clusters are tetramers or larger (33), with most of the clusters being much larger. Aside from the inference that our fluorescent clusters are larger than the oligomers resolved by native gels, we see changes in the fluorescent clusters that are proportional to the level of stress and to the availability of BiP, which the analysis of complexes by native gels did not resolve. Thus, the apparently distinct behaviors will require experiments that bridge the 2 experimental approaches.
Because IRE1α clusters in response to ER stress and exhibits a time course similar to that of the RNase activity, the 2 have been considered related to each other. However, WT IRE1GFP molecules cluster in response to stress even when their RNase activity is inhibited chemically or by substitution of the catalytic residue K907. Not only can RNase-inactive IRE1α initiate clustering but clusters are bigger and persist over time compared to RNase-capable molecules. Our data suggest that RNase-inactive IRE1α can still sense ER stress through its luminal and trans-membrane domains, go through conformational changes, and oligomerize, but it fails to finally perform its enzymatic activity.
That a functional RNase domain is not required for clustering and for splicing of HAC1 transcripts had been previously shown for yeast IRE1 (60, 61). Nonetheless, because mammalian IRE1α differs from the yeast sensor in important aspects of substrate recognition, binding, and RNase activity (19, 26, 32), it is important that we demonstrate here that clustering of mammalian IRE1α is divorced from the RNase activities and also from the availability of its XBP1 substrate. Interestingly, clustering and RNase activity are also uncoupled by activation of IRE1α via membrane lipid saturation (62), underscoring the importance of distinct conformations of IRE1α.
The second type of IRE1α clusters defined in this work differs from the activation induced clusters in 2 respects: the clusters are much larger, and once formed, they persist longer. This type of clusters is observed not only for RNase-inactive IRE1α but also active IRE1α that is induced to cluster by ablation of BiP. The large increase in cluster size when BiP is depleted or when IRE1α is inactivated raises the question of what normally caps cluster size. Perhaps only a fraction of total IRE1α population is normally clustering-competent and is released from the inhibitory interaction with BiP? Although all cells express far more BiP than IRE1α, even a partial depletion of BiP by SubAB is sufficient to trigger massive IRE1α clustering. It is possible that the BiP molecules associated with IRE1α are more susceptible to the toxin than the bulk BiP population and once they are cleaved, the barrier to self-association of IRE1α is reduced. Interestingly, Hu et al. (63) observed that newly synthesized BiP is more susceptible to SubAB, raising the possibility that the IRE1α-bound BiP is indeed the newly synthesized protein. Another relevant mechanism maybe the recruitment of a complex of BiP with the cochaperone ERdj4, which was shown to mediate monomerization of IRE1α (64). The availability of this complex may be altered when some BiP is ablated, or perhaps its recruitment to inactive IRE1α is insufficient to limit cluster size.
The fact that both RNase-inactive (WT + 4μ8c and K907A IRE1α) and RNase-active (WT IRE1α + SubAB) molecules show similar clustering characteristics argues that cluster persistence is not simply due to the inability to cleave RNA substrates. Persistent clustering is also not likely due to insufficient recovery of BiP levels because BiP transcription (and presumably protein) recovered after 6 and 8 h of stress, when the mega-clusters are still observed. This suggests that the mechanisms dictating the persistent clustering are different between SubAB and RNase-impaired IRE1α and consistent with the data that the activating transcription factor 6 branch of the UPR is important for BiP induction (65, 66).
One common denominator between the persistent clustering induced by SubAB, 4μ8c, and IRE1α K907A is the heightened and unrelieved ER stress. In fact, cleavage of BiP should provoke an irreversible perturbation in the ER homeostasis as well as an impairment in the ratio between BiP and client protein levels (41), and, similarly, the absence of spliced XBP1 after ER stress induction should reduce the folding ability of the cell. It is therefore possible that increased levels of misfolded proteins and possibly their binding to IRE1α (67–69) promote the sustained clustering.
It has been suggested for yeast IRE1 that docking of multiple RNA molecules may strengthen its oligomeric assembly by tethering several IRE1α monomers (60). We find that the main substrate of IRE1α RNase activity does not affect the formation of clusters because clusters form in XBP1KO MEF cells. It is possible, however, that RIDD substrates affect cluster initiation. Furthermore, clustering occurs even in RNase-impaired IRE1α, showing that the endoRNase activity is not required for formation of clusters. However, it is possible that the binding of RNA targets, even without processing, induces clustering. Future biochemical experiments will test this possibility.
Furthermore, our data show that the products of IRE1α RNase activities have no effect on dispersal of clusters. In fact: persistent clustering happens in both the absence (IRE1α K907A; WT + 4μ8c) or the presence (SubAB) of XBP1 splicing; K907A IRE1α clusters even in the presence of a WT functional IRE1α. The expression of K907A in the parental HAP1 cells, which express functional endogenous, untagged IRE1α, did not change the clustering parameters measured when K907A was the only form of IRE1α in the cell (Fig. 6 and unpublished results). One possibility is that the processing and release of IRE1α RNA targets provoke a conformational change in IRE1α that favors dispersal of clusters and restores IRE1α to its monomeric form.
Unlike the RNase activity, we find that IRE1α kinase activity is essential for the normal, stress-responsive formation of clusters. The kinase inactive mutant K599A, which is capable of dimerization, does not support clustering, contrary to the data of Oikawa et al. (34). We do not know the reason for this discrepancy, but we have verified our observation with an independently derived double mutant K599A-K907A that was also clustering defective. Whether the discrepancy is due to subtle differences in the IRE1α coding sequences used, the placement of the fluorescent protein in the coding sequence, or the expression level in the host cells used remains to be determined. Our conclusion about phosphorylation was further supported by the finding that a mutated nucleotide-binding pocket version of IRE1α, which can activate its RNase activity upon binding of the ATP-competitive ligand 1NM-PP1 (21), forms small distinct clusters that do not respond to ER stress in absence of phosphorylation.
Our data on oligomerization of the I642G mutant of IRE1GFP differ from that of Ghosh et al. (13) in that in HAP1KO cells I642G IRE1GFP form clusters constitutively, even without the nucleotide mimetic ligand. The clusters formed by I642G IRE1GFP define a third mode of clustering with different physical properties and cellular dynamics compared to clusters formed by either WT IRE1α oligomers or the mega-clusters. We conclude that IRE1α clusters can be formed not only during the activation sequence of IRE1α but also from the inactive RNase or kinase conformers. The active and inactive conformations display distinct rotational orientations when crystalized, either face-to-face or back-to-back, where different molecular surfaces mediate interactions between monomers (20, 70). The ability to oligomerize into distinct structures starting from different conformations is reminiscent of distinct aggregation pathways that are often found in protein-conformation diseases.
Given that IRE1α kinase activity is essential for cluster formation, we explored the possibility that persistent phosphorylation in the activation loop prevents dissociation of clusters. However, the failure of okadaic acid treatment to prolong cluster half-life argues against this possibility. Our data therefore suggest that IRE1α phosphorylation is required only for cluster formation and that cluster dispersal is regulated by a distinct mechanism.
Luteolin is known to bypass the usual activation sequence of IRE1α by engaging a pocket at the cytosolic dimer interface of IRE1α (52), and we find that it induces XBP1 splicing and RIDD without inducing IRE1α clustering. This suggests that clustering is not a requirement for IRE1α RNase activities. This also supports the idea that more than one molecular conformation leads to activation of IRE1α because it reveals distinct post-translationally modified forms. It was previously reported that IRE1α is phosphorylated on several serine residues (54) and can also be ubiquitinated on lysine residues 545 and 828 (71). Luteolin-activated IRE1α exhibits at least 1 phospho-isoform that is not found after ER stress activation (unpublished results). Therefore, it is possible that depending on how it is activated, IRE1α exposes different phosphorylation or other post-translational modification sites, reflecting distinct conformations and also different ability to self-associate but preserving its RNase activities.
We conclude that clustering is a distinct response of IRE1α that can be triggered not only by chemically induced ER stress but also by stress imposed by the presence of misfolded proteins. We find that the kinase activity is required for a specific conformational change (19–21) that not only induces dimerization of the RNase domains but also independently triggers the clustering behavior. Our data show that IRE1α clustering depends on the progression of IRE1α activation from the ER lumen to the RNase domain of the molecule. If this progression is interrupted by mutations, small molecules, or perturbation in cofactors binding, clustering would be impaired either in the presence or absence of RNase activity.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Nathan Roy and Dr. Ed Williamson (Both from the Children's Hospital of Philadelphia) for help with microscopy; Erik Snapp (Albert Einstein College of Medicine, New York, NY, USA), Peter Walter (University of California–San Francisco, San Francisco, CA, USA), John Christianson (Oxford University, Oxford, United Kingdom), and Daniel Hebert (University of Massachusetts–Amherst, Amherst, MA, USA) for providing plasmids; Andrew Hu (Wistar Institute, Philadelphia, PA, USA) and David Ron (University of Cambridge, United Kingdom) for MEFs, and our colleagues Tali Gidalevitz (Drexel University, Philadelphia, PA, USA) and Janis Burkhardt (University of Pennsylvania, Philadelphia, PA, USA) for critical reading of this manuscript. This work was funded by U.S. National Institutes of Health (NIH) National Institute on Aging Grant AG18001 and NIH National Institute of General Medical Sciences Grant GM077480 to Y.A. D.E. was supported by a postdoctoral fellowship from the Italian-American Cancer Foundation. D.R. was supported by NIH training Grant 5 T32 HL 7954-18. The authors declare no conflicts of interest.
Glossary
- α1AT
α1-antitrypsin
- BiP
binding protein
- dox
doxycycline
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- HA
human influenza hemagglutinin
- HAP1KO
HAP1 IRE1α KO
- HEK
human embryonic kidney
- IRE1α
inositol-requiring enzyme 1α
- IRE1GFP
pTIGHT-IRE1-GFP-HA
- KO
knockout
- MEF
mouse embryonic fibroblasts
- NHK
null Hong Kong
- PERK
protein kinase R–like endoplasmic reticulum kinase
- RIDD
regulated IRE1-dependent decay
- ROI
region of interest
- SubAB
subtilase cytotoxin
- Tg
thapsigargin
- Tm
tunicamycin
- UPR
unfolded protein response
- WT
wild type
- XBP1
X box protein 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHORS CONTRIBUTIONS
D. Ricci, I. Marrocco, D. Blumenthal, M. Dibos, D. Eletto, J. Vargas, S. Boyle, Y. Iwamoto, and S. Chomistek designed and performed experiments; J. C. Paton and A. W. Paton contributed the SubAB toxins; and D. Ricci and Y. Argon wrote the manuscript.
REFERENCES
- 1.Kaufman R. J. (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13, 1211–1233 [DOI] [PubMed] [Google Scholar]
- 2.Schuck S., Prinz W. A., Thorn K. S., Voss C., Walter P. (2009) Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J. Cell Biol. 187, 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calfon M., Zeng H., Urano F., Till J. H., Hubbard S. R., Harding H. P., Clark S. G., Ron D. (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96; erratum: 420, 202 [DOI] [PubMed] [Google Scholar]
- 4.Iwakoshi N. N., Lee A. H., Vallabhajosyula P., Otipoby K. L., Rajewsky K., Glimcher L. H. (2003) Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 4, 321–329 [DOI] [PubMed] [Google Scholar]
- 5.Lipson K. L., Fonseca S. G., Ishigaki S., Nguyen L. X., Foss E., Bortell R., Rossini A. A., Urano F. (2006) Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 4, 245–254 [DOI] [PubMed] [Google Scholar]
- 6.Scheuner D., Kaufman R. J. (2008) The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr. Rev. 29, 317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eletto D., Eletto D., Boyle S., Argon Y. (2016) PDIA6 regulates insulin secretion by selectively inhibiting the RIDD activity of IRE1. FASEB J. 30, 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greene C. M., Marciniak S. J., Teckman J., Ferrarotti I., Brantly M. L., Lomas D. A., Stoller J. K., McElvaney N. G. (2016) α1-antitrypsin deficiency. Nat. Rev. Dis. Primers 2, 16051; erratum: 4, 40 [DOI] [PubMed] [Google Scholar]
- 9.Sifers R. N., Brashears-Macatee S., Kidd V. J., Muensch H., Woo S. L. (1988) A frameshift mutation results in a truncated alpha 1-antitrypsin that is retained within the rough endoplasmic reticulum. J. Biol. Chem. 263, 7330–7335 [PubMed] [Google Scholar]
- 10.Brodbeck R. M., Brown J. L. (1992) Secretion of alpha-1-proteinase inhibitor requires an almost full length molecule. J. Biol. Chem. 267, 294–297 [PubMed] [Google Scholar]
- 11.Travers K. J., Patil C. K., Wodicka L., Lockhart D. J., Weissman J. S., Walter P. (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101, 249–258 [DOI] [PubMed] [Google Scholar]
- 12.Han D., Lerner A. G., Vande Walle L., Upton J. P., Xu W., Hagen A., Backes B. J., Oakes S. A., Papa F. R. (2009) IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138, 562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh R., Wang L., Wang E. S., Perera B. G., Igbaria A., Morita S., Prado K., Thamsen M., Caswell D., Macias H., Weiberth K. F., Gliedt M. J., Alavi M. V., Hari S. B., Mitra A. K., Bhhatarai B., Schürer S. C., Snapp E. L., Gould D. B., German M. S., Backes B. J., Maly D. J., Oakes S. A., Papa F. R. (2014) Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158, 534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu M., Lawrence D. A., Marsters S., Acosta-Alvear D., Kimmig P., Mendez A. S., Paton A. W., Paton J. C., Walter P., Ashkenazi A. (2014) Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 345, 98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H. P., Ron D. (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 [DOI] [PubMed] [Google Scholar]
- 16.Cunha D. A., Hekerman P., Ladrière L., Bazarra-Castro A., Ortis F., Wakeham M. C., Moore F., Rasschaert J., Cardozo A. K., Bellomo E., Overbergh L., Mathieu C., Lupi R., Hai T., Herchuelz A., Marchetti P., Rutter G. A., Eizirik D. L., Cnop M. (2008) Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J. Cell Sci. 121, 2308–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter P., Ron D. (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 [DOI] [PubMed] [Google Scholar]
- 18.Bertolotti A., Zhang Y., Hendershot L. M., Harding H. P., Ron D. (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326–332 [DOI] [PubMed] [Google Scholar]
- 19.Korennykh A. V., Egea P. F., Korostelev A. A., Finer-Moore J., Zhang C., Shokat K. M., Stroud R. M., Walter P. (2009) The unfolded protein response signals through high-order assembly of Ire1. Nature 457, 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali M. M., Bagratuni T., Davenport E. L., Nowak P. R., Silva-Santisteban M. C., Hardcastle A., McAndrews C., Rowlands M. G., Morgan G. J., Aherne W., Collins I., Davies F. E., Pearl L. H. (2011) Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response. EMBO J. 30, 894–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papa F. R., Zhang C., Shokat K., Walter P. (2003) Bypassing a kinase activity with an ATP-competitive drug. Science 302, 1533–1537 [DOI] [PubMed] [Google Scholar]
- 22.Rubio C., Pincus D., Korennykh A., Schuck S., El-Samad H., Walter P. (2011) Homeostatic adaptation to endoplasmic reticulum stress depends on Ire1 kinase activity. J. Cell Biol. 193, 171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidrauski C., Walter P. (1997) The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90, 1031–1039 [DOI] [PubMed] [Google Scholar]
- 24.Hollien J., Weissman J. S. (2006) Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 [DOI] [PubMed] [Google Scholar]
- 25.Hollien J., Lin J. H., Li H., Stevens N., Walter P., Weissman J. S. (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 186, 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurel M., Chevet E., Tavernier J., Gerlo S. (2014) Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 39, 245–254 [DOI] [PubMed] [Google Scholar]
- 27.Moore K., Hollien J. (2015) Ire1-mediated decay in mammalian cells relies on mRNA sequence, structure, and translational status. Mol. Biol. Cell 26, 2873–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamu C. E., Walter P. (1996) Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 15, 3028–3039 [PMC free article] [PubMed] [Google Scholar]
- 29.Neefjes J. J., Hengeveld T., Tol O., Ploegh H. L. (1990) Intracellular interactions of transferrin and its receptor during biosynthesis. J. Cell Biol. 111, 1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jing S., Tapley P., Barbacid M. (1992) Nerve growth factor mediates signal transduction through trk homodimer receptors. Neuron 9, 1067–1079 [DOI] [PubMed] [Google Scholar]
- 31.Angers S., Salahpour A., Joly E., Hilairet S., Chelsky D., Dennis M., Bouvier M. (2000) Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc. Natl. Acad. Sci. USA 97, 3684–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aragón T., van Anken E., Pincus D., Serafimova I. M., Korennykh A. V., Rubio C. A., Walter P. (2009) Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature 457, 736–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H., Korennykh A. V., Behrman S. L., Walter P. (2010) Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc. Natl. Acad. Sci. USA 107, 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oikawa D., Kitamura A., Kinjo M., Iwawaki T. (2012) Direct association of unfolded proteins with mammalian ER stress sensor, IRE1β. PLoS One 7, e51290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pincus D., Chevalier M. W., Aragón T., van Anken E., Vidal S. E., El-Samad H., Walter P. (2010) BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 8, e1000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paton A. W., Srimanote P., Talbot U. M., Wang H., Paton J. C. (2004) A new family of potent AB(5) cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 200, 35–46; erratum: 1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunkel T. A., Roberts J. D., Zakour R. A. (1987) Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 154, 367–382 [DOI] [PubMed] [Google Scholar]
- 38.Blumenthal D., Edidin M., Gheber L. A. (2016) Trafficking of MHC molecules to the cell surface creates dynamic protein patches. J. Cell Sci. 129, 3342–3350 [DOI] [PubMed] [Google Scholar]
- 39.Tirasophon W., Welihinda A. A., Kaufman R. J. (1998) A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 12, 1812–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X. Z., Harding H. P., Zhang Y., Jolicoeur E. M., Kuroda M., Ron D. (1998) Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 17, 5708–5717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakunts A., Orsi A., Vitale M., Cattaneo A., Lari F., Tadè L., Sitia R., Raimondi A., Bachi A., van Anken E. (2017) Ratiometric sensing of BiP-client versus BiP levels by the unfolded protein response determines its signaling amplitude. eLife 6, e27518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J. H., Li H., Yasumura D., Cohen H. R., Zhang C., Panning B., Shokat K. M., Lavail M. M., Walter P. (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318, 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eletto D., Eletto D., Dersh D., Gidalevitz T., Argon Y. (2014) Protein disulfide isomerase A6 controls the decay of IRE1α signaling via disulfide-dependent association. Mol. Cell 53, 562–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergmann T. J., Fregno I., Fumagalli F., Rinaldi A., Bertoni F., Boersema P. J., Picotti P., Molinari M. (2018) Chemical stresses fail to mimic the unfolded protein response resulting from luminal load with unfolded polypeptides. J. Biol. Chem. 293, 5600–5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardner B. M., Pincus D., Gotthardt K., Gallagher C. M., Walter P. (2013) Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 5, a013169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimata Y., Oikawa D., Shimizu Y., Ishiwata-Kimata Y., Kohno K. (2004) A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J. Cell Biol. 167, 445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oikawa D., Kimata Y., Kohno K., Iwawaki T. (2009) Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp. Cell Res. 315, 2496–2504 [DOI] [PubMed] [Google Scholar]
- 48.Paton A. W., Beddoe T., Thorpe C. M., Whisstock J. C., Wilce M. C., Rossjohn J., Talbot U. M., Paton J. C. (2006) AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443, 548–552 [DOI] [PubMed] [Google Scholar]
- 49.Hetz C. (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 [DOI] [PubMed] [Google Scholar]
- 50.Cross B. C., Bond P. J., Sadowski P. G., Jha B. K., Zak J., Goodman J. M., Silverman R. H., Neubert T. A., Baxendale I. R., Ron D., Harding H. P. (2012) The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Natl. Acad. Sci. USA 109, E869–E878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tirasophon W., Lee K., Callaghan B., Welihinda A., Kaufman R. J. (2000) The endoribonuclease activity of mammalian IRE1 autoregulates its mRNA and is required for the unfolded protein response. Genes Dev. 14, 2725–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiseman R. L., Zhang Y., Lee K. P., Harding H. P., Haynes C. M., Price J., Sicheri F., Ron D. (2010) Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Mol. Cell 38, 291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu Y., Mao T., Zhang Y., Shao M., You J., Ding Q., Chen Y., Wu D., Xie D., Lin X., Gao X., Kaufman R. J., Li W., Liu Y. (2010) A crucial role for RACK1 in the regulation of glucose-stimulated IRE1alpha activation in pancreatic beta cells. Sci. Signal. 3, ra7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prischi F., Nowak P. R., Carrara M., Ali M. M. (2014) Phosphoregulation of Ire1 RNase splicing activity. Nat. Commun. 5, 3554; erratum: 4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin J. H., Li H., Zhang Y., Ron D., Walter P. (2009) Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One 4, e4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welihinda A. A., Kaufman R. J. (1996) The unfolded protein response pathway in Saccharomyces cerevisiae. Oligomerization and trans-phosphorylation of Ire1p (Ern1p) are required for kinase activation. J. Biol. Chem. 271, 18181–18187 [DOI] [PubMed] [Google Scholar]
- 57.Hetz C., Glimcher L. H. (2009) Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol. Cell 35, 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sundaram A., Plumb R., Appathurai S., Mariappan M. (2017) The Sec61 translocon limits IRE1α signaling during the unfolded protein response. eLife 6, e27187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sundaram A., Appathurai S., Plumb R., Mariappan M. (2018) Dynamic changes in complexes of IRE1α, PERK, and ATF6α during endoplasmic reticulum stress. Mol. Biol. Cell 29, 1376–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Anken E., Pincus D., Coyle S., Aragón T., Osman C., Lari F., Gómez Puerta S., Korennykh A. V., Walter P. (2014) Specificity in endoplasmic reticulum-stress signaling in yeast entails a step-wise engagement of HAC1 mRNA to clusters of the stress sensor Ire1. eLife 3, e05031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimata Y., Ishiwata-Kimata Y., Ito T., Hirata A., Suzuki T., Oikawa D., Takeuchi M., Kohno K. (2007) Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J. Cell Biol. 179, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitai Y., Ariyama H., Kono N., Oikawa D., Iwawaki T., Arai H. (2013) Membrane lipid saturation activates IRE1α without inducing clustering. Genes Cells 18, 798–809 [DOI] [PubMed] [Google Scholar]
- 63.Hu C. C., Dougan S. K., Winter S. V., Paton A. W., Paton J. C., Ploegh H. L. (2009) Subtilase cytotoxin cleaves newly synthesized BiP and blocks antibody secretion in B lymphocytes. J. Exp. Med. 206, 2429–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amin-Wetzel N., Saunders R. A., Kamphuis M. J., Rato C., Preissler S., Harding H. P., Ron D. (2017) A J-protein co-chaperone recruits BiP to monomerize IRE1 and repress the unfolded protein response. Cell 171, 1625–1637.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baumeister P., Luo S., Skarnes W. C., Sui G., Seto E., Shi Y., Lee A. S. (2005) Endoplasmic reticulum stress induction of the Grp78/BiP promoter: activating mechanisms mediated by YY1 and its interactive chromatin modifiers. Mol. Cell. Biol. 25, 4529–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen J., Chen X., Hendershot L., Prywes R. (2002) ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 [DOI] [PubMed] [Google Scholar]
- 67.Gardner B. M., Walter P. (2011) Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333, 1891–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karagöz G. E., Acosta-Alvear D., Nguyen H. T., Lee C. P., Chu F., Walter P. (2017) An unfolded protein-induced conformational switch activates mammalian IRE1. eLife 6, e30700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kopp M. C., Nowak P. R., Larburu N., Adams C. J., Ali M. M. (2018) In vitro FRET analysis of IRE1 and BiP association and dissociation upon endoplasmic reticulum stress. eLife 7, e30257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korennykh A., Walter P. (2012) Structural basis of the unfolded protein response. Annu. Rev. Cell Dev. Biol. 28, 251–277 [DOI] [PubMed] [Google Scholar]
- 71.Zhu X., Zhang J., Sun H., Jiang C., Dong Y., Shan Q., Su S., Xie Y., Xu N., Lou X., Liu S. (2014) Ubiquitination of inositol-requiring enzyme 1 (IRE1) by the E3 ligase CHIP mediates the IRE1/TRAF2/JNK pathway. J. Biol. Chem. 289, 30567–30577 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.