Abstract
Coenzyme Q10 (CoQ10) is an essential component of the mitochondrial respiratory chain and an important scavenger of reactive oxygen species. Low levels are found in individuals with reduced energy expenditure, cardiac and skeletal muscle dysfunction, and mitochondrial disorders, many of these manifestations are seen in individuals with Prader-Willi syndrome (PWS). In addition, CoQ10 supplementation frequently is given to individuals with this syndrome. To determine if CoQ10 levels are decreased in PWS, we studied plasma CoQ10 levels in 16 subjects with PWS, 13 with obesity of unknown cause, and 15 subjects without obesity but of similar age and compared with body composition. Plasma CoQ10 levels were significantly decreased (P < 0.05), using several statistical approaches in subjects with PWS (0.45 ± 0.16 μg/ml), compared to subjects without obesity (0.93 ± 0.56 μg/ml), but not different from subjects with obesity (0.73 ± 0.53 μg/ml). When plasma CoQ10 was normalized relative to cholesterol, triglyceride, and creatinine levels and fat and lean mass [determined by dual energy X-ray absorptiometry (DEXA)] in the subjects with either PWS or obesity, no significant differences were observed. However, a lower muscle mass was found in the PWS subjects.
Keywords: plasma coenzyme Q10, low energy expenditure, simple obesity, Prader-Willi syndrome, fat mass, lean mass
INTRODUCTION
Prader-Willi syndrome (PWS) is characterized by infantile hypotonia, hypogonadism, feeding difficulties in infancy, mental deficiency, small hands and feet, behavior problems, early onset of childhood obesity, short stature, and a characteristic facial appearance [Bray et al., 1983; Cassidy, 1984; Butler et al., 1986; Butler, 1990; Holm et al., 1993; Butler and Thompson, 2000]. About 70% of subjects with PWS have a de novo paternally derived chromosome 15q11-q13 deletion, while the remaining subjects have uniparental maternal disomy 15 (both 15s from the mother) or genetic imprinting defects of chromosome 15. PWS is recognized as the most common genetic cause of marked obesity in humans and occurs in about 1 in 15,000 individuals [Cassidy, 1984; Butler, 1990; Butler and Thompson, 2000]. Subjects with PWS have hyperphagia, decreased metabolic rate, and decreased muscle mass—all contributing to the obesity as a consequence of the chromosome 15 abnormalities [Butler and Thompson, 2000]. In addition, growth hormone deficiency has been documented in the majority of patients with PWS [Angulo et al., 1991; Carrel et al., 2000; Eiholzer et al., 2000]. Although resting metabolic rate and energy expenditure are decreased in PWS patients [Butler, 1990; Hill et al., 1990], the role of primary or secondary metabolic factors, such as coenzyme Q10 (CoQ10), which may play a role in energy balance, has not been studied in this condition. Currently, CoQ10 supplements are frequently given to individuals with PWS but the impact on energy expenditure and metabolism has not been clarified. The Prader-Willi Syndrome Association (USA) is aware of over 1,300 individuals with PWS, currently or in the past, who receive CoQ10 supplements.
CoQ10 is produced in all living organisms and in its oxidized form is an essential coenzyme for energy synthesis in the mitochondria and an important scavenger of reactive oxygen species [Mano et al., 1995; DiMauro, 1999; Keith et al., 2001]. As an antioxidant, it stabilizes cell membranes. Deficiencies of unknown cause have been found in individuals with low energy levels, reduced skeletal and cardiac muscle function and hypotonia (all seen in PWS) and in diseases associated with mitochondrial dysfunction.
Herein, we report the first preliminary study of baseline plasma CoQ10 levels in subjects with PWS and in two groups of similarly aged subjects; those with simple obesity and mild mental retardation and those without obesity.
MATERIALS AND METHODS
We studied 16 subjects with PWS without a previous history of growth hormone therapy on CoQ10 supplementation (9 males, 7 females; mean age ± SD = 25.1 ± 9.6 years; age range = 13–44 years; 12 subjects with 15q11-q13 deletion confirmed by FISH and 4 with maternal disomy 15); 13 subjects with simple obesity and mild mental retardation (4 males, 9 females; mean age ± SD = 27.1 ± 13.4 years; age range = 13–46 years), and 15 non-obese subjects with no mental retardation (6 males, 9 females; mean age ± SD = 22.5 ± 8.7 years; age range = 12–43 years). Obesity was defined as body mass index (kg/m2) greater than or equal to 27 for subjects 15 years of age or older and greater than or equal to 23 for ages 10–14 years [Butler et al., 1998]. Subjects with PWS or obesity were examined by one of us (M.G.B.) and the clinical diagnosis of PWS made and confirmed by genetic testing (e.g., chromosome studies with FISH, microsatellite studies, methylation testing). The subjects with obesity and mild mental retardation, comparable to the subjects with PWS, were nonsyndromic and had no known cause of their obesity. They had normal genetic testing results (e.g., chromosome studies and methylation analysis). Dual energy X-ray absorptiometry (DEXA) was performed on the subjects with obesity or PWS to determine body composition (e.g., fat and lean mass). Subjects with PWS or obesity had extensive endocrine evaluations including insulin, C peptide, glucose, cortisol, cholesterol, triglyceride, creatinine, sex hormone, thyroid, and leptin levels. Written and informed consent was obtained from these subjects and/or from their parents or legal guardians prior to subject participation.
Height and weight were measured on each subject and plasma samples from heparinized peripheral blood were collected and stored at −70°C until analyzed for Coenzyme Q10 levels by HPLC using established laboratory protocols [Lang et al., 1986]. Descriptive statistics, parametric and non-parametric tests, data transformation, and correlation analysis were performed.
RESULTS
There were no significant differences in the mean age or range among the three groups of subjects in our study. The mean ± SD for plasma CoQ10 levels for the 16 subjects with PWS was 0.45 ± 0.16 μg/ml; 0.73 ± 0.53 for the 13 subjects with obesity; and 0.93 ± 0.56 for the 15 non-obese subjects (Table I). The mean plasma CoQ10 level established by the laboratory for controls was 0.83 ± 0.22 μg/ml. Log transformation of the plasma concentration data was undertaken and standard bar graphs and box plots produced for each of the three subject groups (Fig. 1). Figure 2 shows the scatter plots of the individual CoQ10 data with age for each subject in the three subject groups. No difference was found in the plasma CoQ10 level in the subjects with PWS and the 15q11-q13 deletion and those with maternal disomy 15.
TABLE I.
Subject and Coenzyme Q10 Data
| Sex ratio | CoQ10 (μg/ml) | Log CoQ10 | ||
|---|---|---|---|---|
| Groups | M/F | Mean ± SD (age range) | Mean ± SD | Mean ± SD |
| PWS (N = 16) | 9/7 | 25.1y ± 9.6 (13–44) | 0.45 ± 0.16* | −0.38 ± 0.18** |
| Obese (N = 13) | 4/9 | 27.1y ± 13.4 (13–46) | 0.73 ± 0.53 | −0.22 ± 0.28 |
| Nonobese (N = 15) | 6/9 | 22.5y ± 8.7 (12–43) | 0.93 ± 0.56* | −0.10 ± 0.25** |
Significant, P = 0.018, Kruskal-Wallis ANOVA.
Significant, P = 0.010, Univariate ANOVA.
Fig. 1.
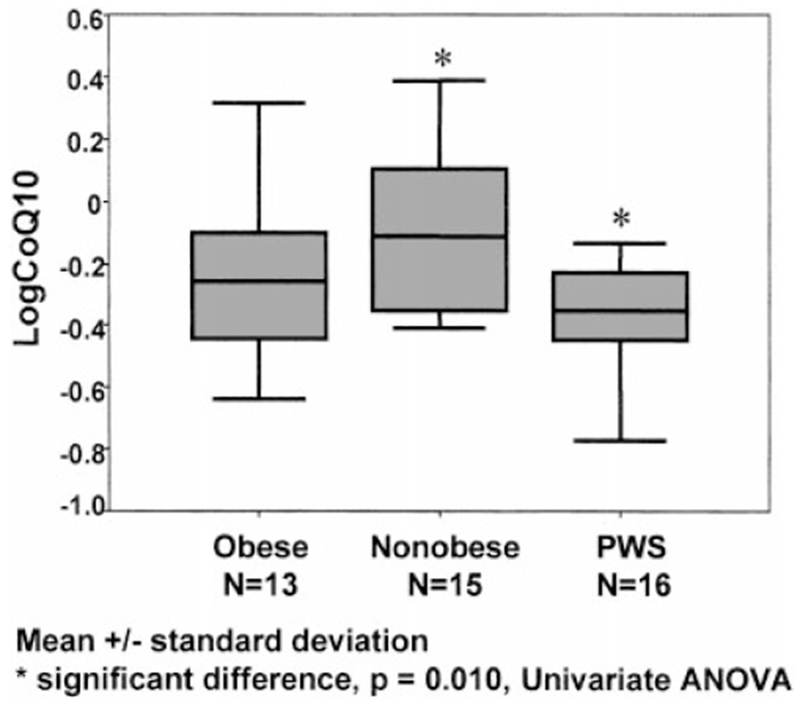
Box plots showing the log coenzyme Q10 data for each of the three groups of subjects.
Fig. 2.
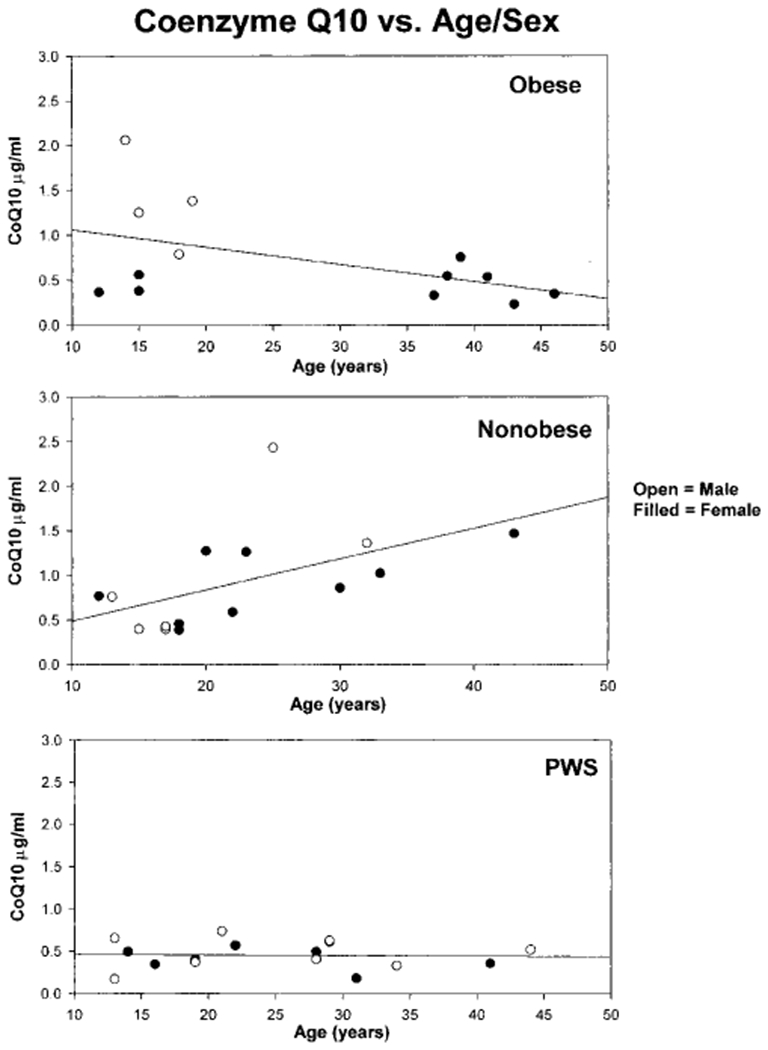
A scatter plot of the coenzyme Q10 data with age for each subject in the three groups. The Spearman rank order correlation value for the non-obese group was 0.68 (P < 0.05); for the obese group was −0.45 (P > 0.05) and for the PWS group was −0.05 (P > 0.05).
Plasma CoQ10 levels were significantly lower in our subjects with PWS than in non-obese subjects (P < 0.05 using the Kruskal-Wallis ANOVA test), but were not significantly different from CoQ10 levels found in obese subjects of comparable ages. The univariate ANOVA test on the original concentration data and on the log transformed data showed the same pattern as the non-parametric Kruskal-Wallis ANOVA test. Furthermore, although the sample size was relatively small, it still provided sufficient precision to draw reasonable conclusions. Using multiple comparison statistical analysis of observed means (e.g., Tukey HSD) and 95% confidence intervals (CI) of plasma CoQ10 levels (and transformed plasma log CoQ10 levels), a significant difference was again found (P < 0.05) between the subjects with PWS and those without obesity (95% CI = −0.86 to −0.08 for CoQ10 concentration: −48% to −6% for log CoQ10 concentration transformed back to geometric means), but not between the subjects with obesity or without obesity (95% CI = −0.61 to 0.22 for CoQ10 concentration; −34% to 10% for log CoQ10 concentration) or between subjects with PWS and those with obesity (95% CI = −0.69 to 0.13 for CoQ10 concentration; −37% to 6% for log CoQ10 concentration).
We found no significant relationship between age and CoQ10 levels except for one subject group. The Spearman rank order correlation of CoQ10 (standard and/or transformed data) with age was significant (P < 0.05) for the non-obese subjects, but not for the subjects with PWS or with obesity (P > 0.05). There was no significant correlation of CoQ10 levels with weight in the three study groups (PWS, obese, non-obese). In addition, sensitivity analysis was undertaken by removing outliers (i.e., CoQ10 levels in PWS were significantly lower than in non-obese subjects). Therefore, stray data points did not appear to influence our data analysis and interpretation of results.
To further determine whether plasma CoQ10 levels could be influenced by other factors such as reduced muscle mass or higher fat mass in PWS patients compared to obese subjects, we normalized CoQ10 levels to total cholesterol, triglyceride, and creatinine levels. If the regulation of tissue CoQ levels occurs locally within mitochondria-rich tissue such as muscle, low plasma CoQ levels could represent a dilutional effect caused by a large adipose mass acting as a reservoir for CoQ. A low ratio of muscle mass to plasma volume, particularly in subjects with PWS, could produce lower than expected plasma CoQ10 levels. In addition, obesity-related markers (e.g., plasma cholesterol, triglycerides) or biochemical muscle markers (e.g., plasma creatinine) could impact on the plasma CoQ levels and were, therefore used as references. The average ± SD for total cholesterol was 185 ± 33 mg/dl for PWS and 213 ± 70 mg/dl for obese subjects; triglycerides were 147 ± 63 mg/dl for PWS and 195 ± 117 mg/dl for obese subjects; and plasma creatinine was 0.62 ± 0.12 mg/dl for PWS and 0.71 ± 0.18 mg/dl for obese subjects. There were no significant differences in these plasma measures. In addition, the greater scatter in CoQ10 levels in the obese subjects without PWS seen in Figure 2 could reflect body composition differences, e.g., low muscle mass (as seen in PWS) or high muscle mass. However, there was no difference in body composition determined by DEXA between subjects with higher and lower CoQ10 levels. After normalizing plasma CoQ levels to plasma creatinine (a marker of lean tissue) and total cholesterol and triglycerides (a marker of fat tissue) in our subjects with PWS or with obesity, no statistical differences were identified, suggesting that the fat-to-lean mass ratio in our subjects did not impact on the plasma CoQ10 levels using this approach. Also, no significant differences were seen in PWS or obese subjects in CoQ10/creatinine ratios (P = 0.148); CoQ10/cholesterol (P = 0.130) or CoQ10/trigylceride (P = 0.962), whereas a significant difference (t-test; P = 0.048) was observed when CoQ10 was normalized against fat/lean mass, a lower ratio in PWS reflecting the lower lean mass (i.e., muscle) in these subjects. Conversely, the percentage of fat was 48.3 ± 5.6 in PWS and 44.3 ± 8.7 in obese subjects, while the percentage of lean mass was 46.6 ± 6.3 in PWS and 49.9 ± 8.6 for obese subjects as determined by DEXA. However, the average ± SD for fat (in kilograms) was 39.4 ± 15.0 in PWS subjects and 42.3 ± 15.4 in obese subjects of comparable ages (P = 0.622). The average (±SD) for lean tissue (in kilograms) was 36.0 ± 7.3 for PWS and 42.7 ± 8.1 for obese subjects (P = 0.031). However, there was no difference in CoQ10/lean mass ratio in PWS and obese subjects (P = 0.318). The CoQ10/fat ratio between PWS and obese subjects was also not significant (P = 0.190).
DISCUSSION
PWS can be divided into two distinct clinical stages. The first stage is characterized by neonatal hypotonia, hypogenitalism, and feeding difficulties. The second stage occurs between 1 and 3 years of age and is characterized by psychomotor retardation and onset of obesity. Food foraging, behavioral problems, and physical inactivity also occur during the second stage [Butler, 1990]. The marked weight gain and life-threatening obesity have been the focus of several investigations [Butler, 1990; Butler and Thompson, 2000]. Although the role of energy expenditure in the causation of obesity is not clearly understood, a combination of excessive caloric intake, decreased energy expenditure, and/or decreased physical activity can lead to morbid obesity. Growth hormone deficiency may also contribute to body composition abnormalities, as shown by growth hormone therapy in subjects with PWS, which increases stature and muscle mass while decreasing obesity [Carrel et al., 2000; Eiholzer et al., 2000]. However, CoQ10 has not been measured systematically in muscle from PWS subjects. For this reason, two children with PWS were studied by (S. DiMauro, unpublished observation) and CoQ10 levels were within or close to the normal range (PWS subject 1–30 μg/g muscle tissue; PWS subject 2–18 μg/g muscle tissue: mean ± SD in 121 control subjects was 27.64 ± 4.43 μg/g). Future studies are proposed to compare CoQ10 levels in muscle tissue and plasma to further characterize the CoQ10 status in PWS.
In summary, plasma CoQ10 levels in our subjects with PWS were lower than in similarly aged non-obese subjects but no different than in subjects with obesity when evaluated using several statistical approaches. Subjects with PWS have lower muscle mass (as supported by our study), strength, and reduced energy expenditure compared to the general population, including obese comparison subjects. The lower CoQ10 levels in subjects with PWS may reflect a lower muscle mass and therefore lower energy expenditure. We would encourage additional studies on CoQ10, including the use of animal models for PWS, to assess its impact on subjects with PWS.
ACKNOWLEDGMENTS
We thank Lisa Herdman and Deborah Moore for expert preparation of the manuscript and Steve Simon, Ph.D. for statistical advice. This work was partially funded by the National Institute of Child Health and Human Development (grant P01HD30329), Hall Family Foundation and through the Physician Scientist Award (Children’s Mercy Hospital).
Grant sponsor: National Institute of Child Health and Human Development; Grant number: P01HD30329; Grant sponsor: Hall Family Foundation; Grant sponsor: Physician Scientist Award (Children’s Mercy Hospital).
REFERENCES
- Angulo M, Castro-Magana M, Uy J. 1991. Pituitary evaluation and growth hormone treatment in Prader-Willi syndrome. J Pediatr Endocrinol 4:167–173. [Google Scholar]
- Bray GA, Dahms WT, Swerdloff RS, Fiser RH, Atkinson RL, Carrel RE. 1983. The Prader-Willi syndrome: A study of 40 patients and a review of literature. Medicine 62:59–80. [PubMed] [Google Scholar]
- Butler MG. 1990. Prader-Willi syndrome: Current understanding of cause and diagnosis. Am J Med Genet 35:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Thompson T. 2000. Prader-Willi syndrome: Clinical and genetic findings. The Endocrinol 10:3S–16S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Meaney JF, Palmer CG. 1986. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet 23:793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Hedges L, Hovis CL, Feurer ID. 1998. Genetic variants of the human obesity (OB) gene in subjects with and without Prader-Willi syndrome: Comparison with body mass index and weight. Clin Genet 54:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel AL, Myers SE, Whitman BY, Allen DB. 2000. Prader-Willi syndrome: The effect of growth hormone on childhood body composition. The Endocrinol 10:43S–49S. [Google Scholar]
- Cassidy SB. 1984. Prader-Willi syndrome. Curr Prob Ped 14:1–55. [DOI] [PubMed] [Google Scholar]
- DiMauro S 1999. Exercise intolerance and the mitochondrial respiratory chain. Int J Neurol Sci 20:387–393. [DOI] [PubMed] [Google Scholar]
- Eiholzer U, Bachmann S, L’Allemand D. 2000. Growth hormone deficiency in Prader-Willi syndrome. The Endocrinol 10:50S–56S. [DOI] [PubMed] [Google Scholar]
- Hill JO, Kaler M, Spetalnick B, Reed G, Butler MG. 1990. Resting metabolic rate in Prader-Willi syndrome. Dysmorph & Clin Genet 4:27–32. [PMC free article] [PubMed] [Google Scholar]
- Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, Greenberg F. 1993. Prader-Willi syndrome: Consensus diagnostic criteria. Pediatr 91:398–402. [PMC free article] [PubMed] [Google Scholar]
- Keith ME, Ball A, Jeejeebhoy KN, Kurian R, Butany J, Dawood F, We WH, Madapallimattam A, Sole MJ. 2001. Conditioned nutritional deficiencies in the cardiomyopathic hamster heart. Can J Cardiol 17:449–458. [PubMed] [Google Scholar]
- Lang JK, Gohil K, Packer L. 1986. Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Anal Biochem 157:106–116. [DOI] [PubMed] [Google Scholar]
- Mano T, Sinohara R, Sawai Y, Oda N, Nishida Y, Mokuno T, Kotake M, Hamada M, Masunaga R, Nakai A. 1995. Effects of thyroid hormone on coenzyme Q and other free radical scavengers in rat heart muscle. J Endrocinol 145:131–136. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Maeda H, Goto Y, Nonaka I. 1992. Muscle coenzyme Q10 in mitochondrial encephalomyopathies. Neuromuscul Disord 1:443–447. [DOI] [PubMed] [Google Scholar]


