Abstract
Abnormal β-adrenergic signaling plays a central role in human heart failure. In mice, chronic β-adrenergic receptor (βAR) stimulation elicits cardiac hypertrophy. It has been reported that cultured cardiac fibroblasts express βAR; however, the functional in vivo requirement of βAR signaling in cardiac fibroblasts during the development of cardiac hypertrophy remains elusive. β2AR null mice exhibited attenuated hypertrophic responses to chronic βAR stimulation upon continuous infusion of an agonist, isoprenaline (ISO), compared to those in wildtype controls, suggesting that β2AR activation in the heart induces pro-hypertrophic effects in mice. Since β2AR signaling is protective in cardiomyocytes, we focused on β2AR signaling in cardiac myofibroblasts. To determine whether β2AR signaling in myofibroblasts affects cardiac hypertrophy, we generated myofibroblast-specific transgenic mice (TG) with the catalytic subunit of protein kinase A (PKAcα) using Cre-loxP system. Myofibroblast-specific PKAcα overexpression resulted in enhanced heart weight normalized to body weight ratio, associated with an enlargement of cardiomyocytes at 12 weeks of age, indicating that myofibroblast-specific activation of PKA mediates cardiac hypertrophy in mice. Neonatal rat cardiomyocytes stimulated with conditioned media from TG cardiac fibroblasts likewise exhibited significantly more growth than those from controls. Thus, β2AR signaling in myofibroblasts plays a substantial role in ISO-induced cardiac hypertrophy, possibly due to a paracrine effect. β2AR signaling in cardiac myofibroblasts may represent a promising target for development of novel therapies for cardiac hypertrophy.
Keywords: Cardiac hypertrophy, cardiac fibroblast, β-adrenergic receptor, paracrine effect, protein kinase A
Introduction
Heart failure remains a leading cause of death and disability worldwide. Cardiac hypertrophy is a major risk factor for heart failure and is induced by various pathological and physiological stresses, including excessive neurohumoral stimuli. Among them, the role of catecholamines appears to be complicated, since β-adrenergic stimulation often leads to cardiomyocyte death instead of induction of hypertrophy in vitro 1. In contrast, chronic β-adrenergic stimulation consistently induces cardiac hypertrophy in mice 2. Thus, controversy between in vivo and in vitro results often leads to confusion in understanding the precise molecular mechanism of heart failure.
β-adrenergic signaling plays a central role in heart failure progression. Elevated sympathetic activity in heart failure patients is associated with poor survival 3. The sustained activation of β-adrenergic receptors (βARs) promotes contractile dysfunction, ventricular arrhythmias, and congestive heart failure, and βAR blockade has become a standard therapeutic treatment for heart failure, since multiple lines of evidence have shown improvements in prognosis 4. Mechanistically, the pathological roles of βARs in the development of heart failure are exceptionally complicated, since they are expressed in multiple cell types including cardiomyocytes, fibroblasts, endothelial cells, vascular smooth muscle cells, and migrated immune cells 5, 6. To complicate the matter further, there are four subtypes of βARs, some of which couple to multiple types of G-proteins and regulate multiple signaling pathways (PKA, CaMKII, etc) 7, 8. This complexity is one of the main reasons that the precise mechanism by which βAR blockade improve prognosis in heart failure patients remains elusive, despite its substantial effectiveness in clinical use.
Cardiac fibroblasts comprise one of the most abundant non-myocyte cell populations in the heart, accounting for approximately 20–70% of cardiac cells, depending on the species 9, 10. They play central roles in maintaining extracellular matrix homeostasis, normal cardiac architecture 11, and once activated in response to injury, mainly contribute to the development of myocardial fibrosis once activated in response injury. However, there is increasing evidence that activated cardiac myofibroblasts may also play an important role in mediating cardiac hypertrophy and remodeling through paracrine effects with adjacent myocytes 12, 13. While adult rat cultured cardiac fibroblasts are known to express βARs 14, 15, deciphering the precise role of βARs in cardiac fibroblasts in the in vivo development of cardiac hypertrophy is required.
In the present study, we elucidated the role of β2AR in the development of cardiac hypertrophy induced by chronic β-adrenergic stimulation using isoprenaline (ISO). Systemic deletion of β2AR attenuated ISO-induced hypertrophic responses in mice, and myofibroblast-specific activation of PKA induced cardiac hypertrophy under physiological conditions and promoted myocyte hypertrophy upon fibroblast-conditioned media stimulation. Accordingly, our results indicate that β2AR signaling in myofibroblasts plays a major role in ISO-induced cardiac hypertrophy due to a paracrine-mediated effect.
Materials and Methods
Additional detailed Materials and Methods are provided in the Supplemental Materials.
Genetically engineered mice
β2AR null mice (β2ARKO) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Periostin promoter-regulated Cre-recombinase-expressing mice (Pn-Cre) were used for myofibroblast-specific expression of the target gene, as previously described 16. A transgenic system expressing the chloramphenicol acetyltransferase (CAT) gene, which was floxed under the CAG promoter (the plasmid was kindly gifted by Professor Miyazaki, Osaka University) was used to generate responder mice by subcloning the cDNA of PKAcα downstream of the floxed-CAT gene (Accession No. CDB0533T: http://www2.clst.riken.jp/arg/TG%20mutant%20mice%20list.html). Periostin promoter-regulated PKAcα transgenic mice were obtained by crossbreeding Pn-Cre with the responder mice (CAG/CAT/PKAcα). The PCR primers used in this study for genotyping are shown in Supplemental Table1.
Statistical analysis
Data are shown as mean ± standard deviation (SD). Comparisons between the two groups were performed with Student’s t-tests. One-way ANOVA followed by Tukey-Kramer test was used for multiple comparisons. Differences were considered to be statistically significant at P<0.05.
Results
Deletion of β2AR attenuates cardiac hypertrophy after chronic β2AR stimulation in mice
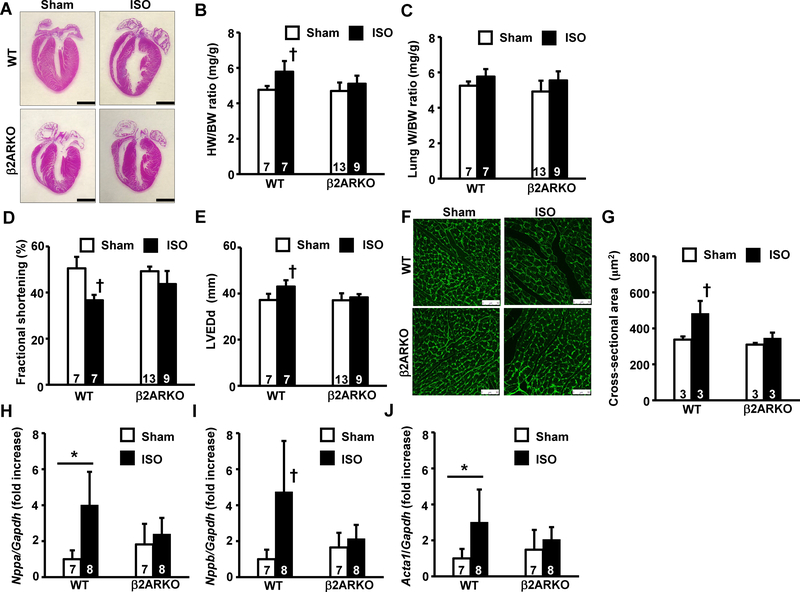
First, we examined the hypertrophic responses of β2ARKO and wildtype control (WT) mice 2 weeks after chronic βAR stimulation using ISO osmotic mini-pumps. Control mice showed overt cardiac hypertrophy, whereas this response was abolished in β2ARKO mice after chronic ISO stimulation (Fig 1A). In WT mice, there was a significant increase in the heart weight normalized to body weight ratio (HW/BW) compared to that in the control sham group (Fig 1B). In contrast, no significant difference in HW/BW ratios was observed between the ISO-stimulated and sham groups in the β2ARKO cohort. While the lung weight to body weight ratio (LW/BW), a marker of lung congestion, was similar among the four groups (Fig 1C), cardiac systolic function, as assessed by fractional shortening in echocardiographic analysis, showed a moderate decrease in ISO-stimulated WT mice, which was attenuated in the β2AR null back-ground (Fig 1D). Cardiac dilatation, as assessed by left ventricular dimension at end-diastole (LVEDd), was attenuated in β2ARKO mice compared to that in WT after chronic ISO stimulation (Fig 4E). An increase in cross-sectional area, a marker of cardiomyocyte hypertrophy, was significantly attenuated in β2ARKO mice compared to that in WT mice after chronic ISO stimulation (Fig 1F and 1G). Finally, hypertrophic marker gene expression was significantly upregulated in WT mice, and significantly attenuated in β2ARKO mice (Fig 1H–J). Taken together, these results indicate that β2AR deletion attenuates cardiac hypertrophy in response to chronic β-adrenergic stimulation in mice.
Fig 1. Deletion of β2AR attenuates cardiac hypertrophy after chronic β2AR stimulation in mice.
(A) Representative images of hematoxylin-eosin staining of whole hearts from wildtype control or β2ARKO mice 2 weeks after isoproterenol (ISO) stimulation or sham operation. The bar indicates 1 mm. (B) Gravimetric analysis. Heart weight (HW) normalized to body weight (BW) ratios are shown. (C) Lung weight normalized to BW (LW/BW) ratios. (D) Fractional shortening (%) obtained 2 weeks after sham operation or ISO-pump implantation by echocardiographic analysis. (E) Left ventricular dimension at end-diastole (LVEDd) obtained 2 weeks after sham operation or ISO-pump implantation by echocardiographic analysis. (F) Representative image of WGA-FITC conjugate staining of heart tissue. The bar indicates 50 μm. (G) Cross-sectional area measurements in WGA-FITC conjugate-stained hearts. (H, I, J) Expression levels of cardiac hypertrophic marker genes, Nppa (H), Nppb (I), and Acta1 (J), in ventricles from mouse cohorts determined by real-time RT-PCR. Values were normalized to that of GAPDH and are represented as fold increases relative to that in the wildtype sham group. Values are shown as mean ± SD, and the number displayed on each column indicates the number of samples. † P<0.05 vs. all other groups, *P<0.05 between two indicated groups by one-way ANOVA followed by Tukey–Kramer test.
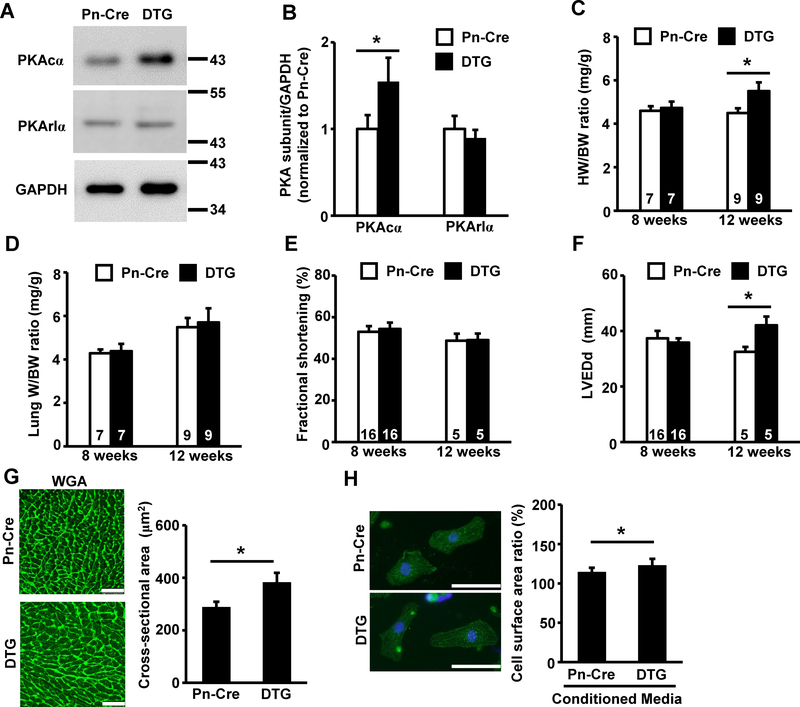
Fig 4. Fibroblast-specific activation of PKA mediates cardiac hypertrophy in mice.
(A) Representative image of immunoblot probed with indicated primary antibodies. Protein samples were prepared from adult cardiac fibroblasts from indicated genotypes at passage 2. The number on the right side of each panel indicates the size of the molecular marker. (B) Quantification of immunoblot bands using protein samples obtained from cardiac fibroblasts of Pn-Cre or DTG mice based on three independent experiments. (C, D) Gravimetric analysis of Pn-Cre and DTG mice. HW/BW ratio (C) or LW/BW ratio (D) at 8 weeks or 12 weeks of age are shown. (E) Fractional shortening (%) in Pn-Cre and DTG mice at 8 weeks or 12 weeks of age determined by echocardiographic analysis. Values are mean ± SD, and numbers displayed on the column indicate the number of samples. (F) Echocardiographic analyses of LVEDd from Pn-Cre and DTG mice at 8 weeks or 12 weeks of age. (G) Representative images of WGA-FITC conjugate staining of heart tissue at 12 weeks of age (left panels). Cross-sectional area measurements in WGA-FITC conjugate stained hearts from Pn-Cre and DTG at 12 weeks of age (right panel). The bar indicates 50 μm. (H) Representative images of actinin staining of NRCMs stimulated with media conditioned by cardiac fibroblasts from Pn-Cre or DTG mice (left panels). Cell surface area ratio normalized to that in non-treated NRCMs from six independent experiments are shown in right panel. The bar indicates 50 μm. Values are shown as mean ± SD, and numbers displayed on the columns indicate numbers of samples. *P<0.05 between two indicated groups by unpaired Student’s t-test.
Deletion of β2AR attenuates ISO-induced cardiac fibrosis as well as migration of cardiac fibroblasts
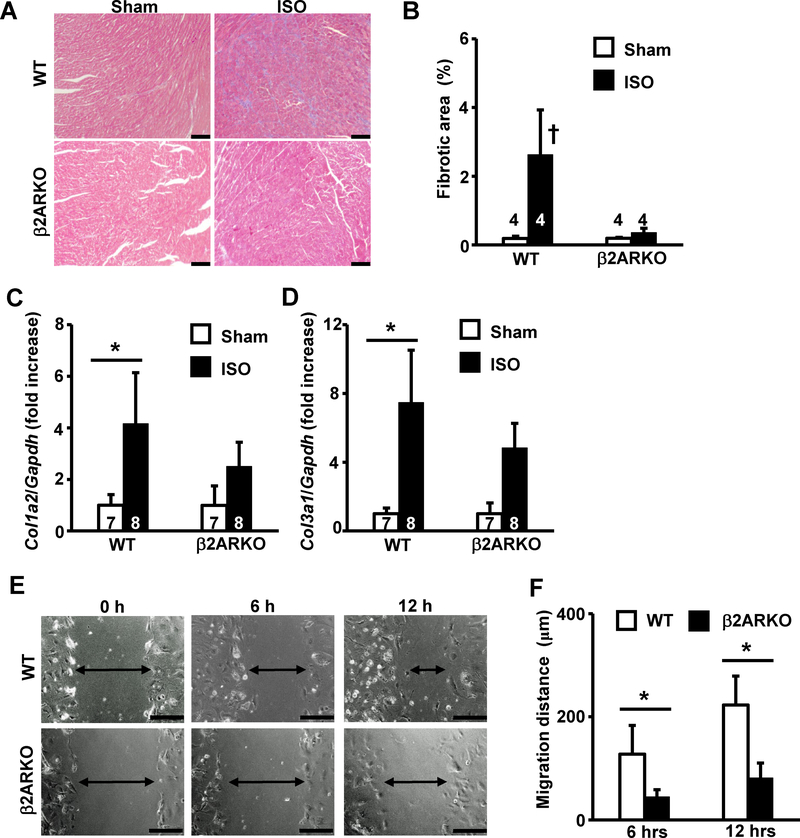
Next, we examined cardiac fibrosis in β2ARKO and WT mice. Chronic β-adrenergic stimulation led to development of cardiac fibrosis in WT mice, which was attenuated in β2ARKO hearts (Fig 2A). While the fibrotic area assessed in Masson’s trichrome (M-T) stained slides was significantly greater in ISO-stimulated WT mice, this was significantly attenuated in β2ARKO hearts (Fig 2B). Upon ISO stimulation, the expression of fibrotic marker genes, Col1a2 and Col3a1, was significantly upregulated compared to that in sham mice, but this increase was significantly attenuated in β2ARKO mice (Fig 2C and 2D). Moreover, the migration of β2ARKO-derived fibroblasts was significantly attenuated compared to that in WT mice, as assessed by scratch assays (Fig 2E and 2F). Taken together, these results suggest that β2AR in cardiac fibroblasts plays significant roles in the development of cardiac fibrosis after chronic β-adrenergic stimulation.
Fig 2. Deletion of β2AR attenuates ISO-induced cardiac fibrosis and migration of cardiac fibroblasts.
(A) Representative images of M-T staining of heart tissue obtained from wildtype or β2ARKO mice at 2 weeks after sham-operation or ISO-stimulation. The bar indicates 100 μm. (B) Quantification of fibrotic area assessed by M-T staining. (C, D) Expression levels of cardiac fibrotic marker gene, Col1a2 (C) and Col3a1 (D) in ventricles determined by real-time RT-PCR. Values were normalized to that of GAPDH and are represented as fold increases relative to that in the wildtype sham group. (E) Representative images of cell migration as assessed by scratch assay. Images of scratched confluent cardiac fibroblasts from wildtype control (upper panels) or β2ARKO (lower panels) mice were taken at indicated time points. Arrows indicate the width of cell-free areas. The bar indicates 200 μm. (F) Quantitation of migration distance of cardiac fibroblasts at 6 h and 12 h. Values are mean ± SD, and the number displayed on each column indicates the number of samples. †P<0.05 vs. all other groups, *P<0.05 between two indicated groups by one-way ANOVA followed by Tukey-Kramer test.
β2AR in cardiac fibroblasts affects cardiomyocyte hypertrophy
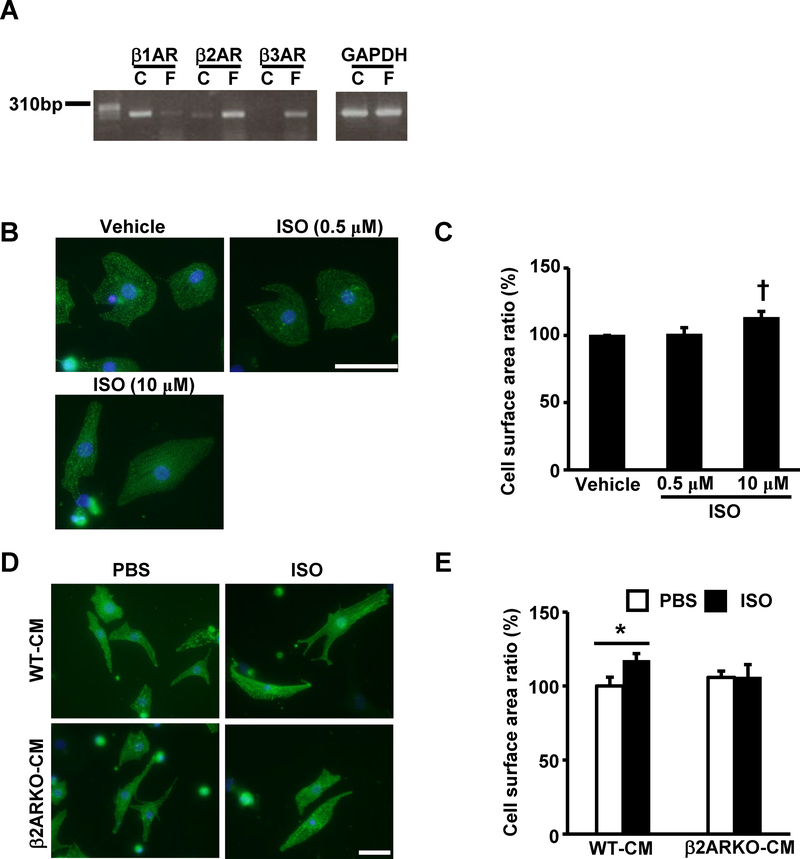
Next, we deciphered the underlying mechanism preventing of cardiac hypertrophy in β2ARKO mice. In previous reports, β2AR couples to Gαi and attenuates myocyte hypertrophy, suggesting that β2AR signaling is cardioprotective 17. Thus, we hypothesized that β2AR in cardiac fibroblasts plays a role in the development of cardiac hypertrophy after chronic β-adrenergic stimulation and examined hypertrophic responses caused by fibroblast-conditioned media. We examined the expression profiles of βARs in cultured cardiac myocytes and fibroblasts in normal adult mouse hearts; adult cardiomyocytes expressed mainly β1AR and β2AR, whereas adult cardiac fibroblasts showed dominant expression of β2AR and modest expression of β3AR (Fig 3A). While ISO stimulation with 10 μM induced myocyte hypertrophy, lower concentrations of ISO (0.5 μM) failed to mediate myocyte hypertrophy (Fig 3B and C). Treatment with conditioned media from WT cardiac myofibroblasts treated with ISO, with a concentration corresponding to the lower concentration (0.5 μM), mediated hypertrophy in neonatal rat cardiomyocytes (NRCMs), whereas media from β2ARKO cardiac myofibroblasts treated with ISO failed to enhance myocyte hypertrophy (Fig 3D). Cell surface area significantly increased in NRCMs treated with conditioned media from ISO-treated WT cardiac fibroblasts compared to that in vehicle-treated WT cardiac fibroblasts. However, this was abrogated in NRCMs treated with conditioned media from β2ARKO cardiac fibroblasts (Fig 3E). Collectively, these results indicate that β2AR in cardiac myofibroblasts plays substantial roles in the development of cardiac hypertrophy after β-adrenergic stimulation via a putative paracrine effect.
Fig 3. β2AR in cardiac fibroblasts regulates cardiomyocyte hypertrophy.
(A) Representative image of βAR RT-PCR products obtained from adult mouse cardiomyocytes or fibroblasts. (B) Representative images of neonatal rat cardiomyocytes (NRCMs) with actinin staining 24 h after stimulation with ISO (0.5 μM or 10 μM) or vehicle. (C) Cell surface area measurements of NRCMs 24 h after stimulation with low or high dose of ISO (0.5 μM or 10 μM) normalized to those of NRCMs treated with vehicle. Values are mean ± SD of three independent experiments. † P<0.05 vs. other groups by Student’s t-test. (D) Representative images of actinin staining of NRCMs stimulated with media conditioned by cardiac fibroblasts from wildtype or β2ARKO mice with or without ISO treatment. (E) Cell surface area measurements of NRCMs stimulated with media conditioned by cardiac fibroblasts from wildtype or β2ARKO mice with or without ISO treatment. The values were normalized to those of NRCMs treated with media conditioned by wildtype without ISO treatment. Values are mean ± SD of four independent experiments. P<0.05 between two indicated groups by one-way ANOVA followed by Tukey-Kramer test. The bars indicate 50 μm.
Myofibroblast-specific activation of PKA mediates cardiac hypertrophy in mice
To further examine the roles of fibroblast-specific β-adrenergic signaling in the development of cardiac hypertrophy, we generated genetically engineered mice with fibroblast-specific overexpression of the PKA catalytic subunit (PKAcα) using a periostin promoter-regulated Cre-loxP system. Double transgenic mice (DTG) were obtained by crossbreeding transgenic line with Periostin promoter regulated Cre-recombinase and transgenic line with CAG promoter regulated floxed CAT/PKAcα. Among the littermates, single transgenic periostin promoter-regulated Cre-recombinase (Pn-Cre) mice were used as controls. Adult cardiac fibroblasts from DTG mice showed increased expression of the PKAcα protein at passage 2 compared to that in Pn-Cre mice. Protein expression levels of the PKA regulatory subunit (PKArIα) were similar between the two genotypes of cardiac fibroblasts (Fig 4A). Following densitometric analysis, DTG cardiac fibroblasts showed a 50% increase in PKAcα protein levels compared to those from Pn-Cre mice, whereas there was no significant difference in PKArIα between the two genotypes (Fig 4B). While DTG mice showed no overt cardiac hypertrophy at 8 weeks of age, there was a significant increase in the HW/BW ratio compared to that in Pn-Cre mice at 12 weeks of age (Fig 4C). In contrast, there were no significant differences in LW/BW ratio or fractional shortening between the two genotypes, indicating no cardiac dysfunction in DTG mice (Fig 4D and 4E). Cardiac dilatation (LVEDd) was significantly promoted in DTG mice compared to that in Pn-Cre (Fig 4F), and cross-sectional area was significantly higher in DTG than Pn-Cre mice at 12 weeks of age (Fig 4G). Finally, treatment with conditioned media from DTG fibroblasts promoted increased growth compared to that from control mice fibroblasts (Fig 4H). Taken together, these results indicate that fibroblast-specific activation of PKA mediates cardiac hypertrophy in vivo.
Discussion
In the present study, we showed that deletion of β2AR attenuates cardiac hypertrophy and fibrosis induced by chronic β-adrenergic stimulation in mice. We also demonstrated that fibroblast-specific activation of PKA induces cardiac hypertrophy in mice via a putative paracrine mechanism. To the best of our knowledge, this study provides the first evidence demonstrating that PKA signaling in fibroblasts independently mediates cardiac hypertrophy.
Multiple reports have indicated that β2AR activation is involved in anti-apoptotic or cardioprotective effects in cardiomyocytes in vitro 18, 19. Morisco et al. reported that pre-treatment with ICI118551, a β2AR-specific antagonist, failed to attenuate ISO-induced hypertrophy in neonatal cardiomyocytes 20, indicating that β2AR is not involved in cardiomyocyte hypertrophy. Thus, we speculate that prevention of cardiac hypertrophy in β2ARKO mice is attributed to non-myocytes such as cardiac fibroblasts.
In contrast, several lines of evidence indicate that non-selective βAR agonists induce cardiomyocyte hypertrophy. However, the agonist-concentrations used in these experiments may be substantially higher than those observed in heart failure patients. For example, plasma concentration of norepinephrine in heart failure patients is approximately 4 nM, while 20 μM is required to induce myocyte hypertrophy 21, 22. While this is often attributed to the tissue concentration, which may be much higher based on the observation of catecholamine spill over 23, the precise mechanism remains elusive. The presented data suggest that cardiac fibroblasts at least partially contribute to the development of cardiac hypertrophy via βAR stimulation as an amplifier of hypertrophic signaling.
The hypertrophic responses to pathological stimuli in β2ARKO are controversial. In a previous report 24, β2ARKO mice with an FVB background showed exaggerated hypertrophic responses 3 weeks after pressure overload induced by transverse aortic constriction (TAC). We also performed TAC surgery on β2ARKO and WT mice and observed similar hypertrophic responses in both genotypes (data not shown). In any case, β2ARKO mice demonstrated differential hypertrophic responses to pressure overload compared to the responses to chronic β-adrenergic stimulation presented in this study. We believe that the latter model is more suitable for examining the effect of β2AR activation in the heart.
βARs are expressed in various cardiac cells. In cardiac fibroblasts, functional β2AR expression has been reported based on a pharmacological inhibition study 14, 15. The production of cAMP and its involvement in fibroblast growth has been also reported 25, 26. Thus, β-adrenergic signaling in fibroblasts is involved in the regulation of cellular properties. Consistent with this, we found that β2AR-deficient cardiac fibroblasts showed reduced migration. Collectively, we consider that β2AR plays functional roles in cardiac fibroblasts in mice.
While our results indicate that secretion of pro-hypertrophic factors from cardiac fibroblasts contributes to the development of hypertrophy upon β–adrenergic stimulation, the precise molecular mechanism remains undetermined. Some of candidate molecules have been previously reported to be secreted from cardiac fibroblasts to induce cardiomyocyte or cardiac hypertrophy 13, 27. For instance, catecholamines or isoproterenol reportedly stimulate interleukin-6 synthesis in both mouse and rat cardiac fibroblasts 28, 29. In addition, Bageghni et al. has demonstrated that fibroblast-specific activation of p38 MAPK signaling promotes cardiac hypertrophy via IL-6 signaling 30. Moreover, prevention of cardiac hypertrophy has been reported in IL-6KO mice 31. Thus, IL-6 appears to be a redundant candidate as a pro-hypertrophic factor secreted from cardiac fibroblasts during chronic β-adrenergic stimulation. Accordingly, we examined IL-6 expression in hearts from β2ARKO or WT mice 2 h after intraperitoneal ISO administration. However, contrary to our expectation, expression levels of IL-6 were similar between the two genotypes (data not shown). Therefore, it seems that IL-6 is not responsible for fibroblast-induced cardiac hypertrophy upon β-adrenergic stimulation.
Limitations: In the presented study, we utilized a Cre-recombinase transgenic model regulated by the periostin promoter. As this drives gene expression in both neonatal cardiac fibroblasts and adult myofibroblasts, this represents a limitation of our study. However, as myofibroblasts play a role in the process of cardiac remodeling and heart failure 32, this indicates that the significance of the PKA signaling presented here could be even more pronounced in the development of heart failure.
In summary, we demonstrated that β2AR in cardiac myofibroblasts plays a substantial role in the development of cardiac hypertrophy and fibrosis induced by chronic β-adrenergic stimulation in mice. We also showed that PKA in cardiac fibroblasts is involved in the induction of cardiac hypertrophy downstream of β2AR via a putative paracrine mechanism. Our results suggest that treatment with β2AR antagonists represents a promising novel approach for updating current β-blocker therapy, at least partially, by preventing fibroblast activation. Finally, determination of the secreted molecules responsible for fibroblast-mediated cardiomyocyte hypertrophy is needed for further development of novel therapeutics for heart failure.
Supplementary Material
Acknowledgments
The authors would like to thank Chiharu Tottori for their excellent secretarial work. This work is supported by the Terumo Foundation for Life Sciences and Arts and by MEXT/JSPS KAKENHI (Grant Number 17K09576) to H.N and via National Institutes of Health grant HL135657 to S.J.C.
References
- 1.Singh K, Xiao L, Remondino A, et al. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol. 2001;189:257–265 [DOI] [PubMed] [Google Scholar]
- 2.Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: Pathophysiological aspects. Heart Fail Rev. 2007;12:66–86 [DOI] [PubMed] [Google Scholar]
- 3.Packer M Neurohormonal interactions and adaptations in congestive heart failure. Circulation. 1988;77:721–730 [DOI] [PubMed] [Google Scholar]
- 4.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: Accf/aha guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:1977–2016 [DOI] [PubMed] [Google Scholar]
- 5.Balligand JL. Cardiac salvage by tweaking with beta-3-adrenergic receptors. Cardiovasc Res. 2016;111:128–133 [DOI] [PubMed] [Google Scholar]
- 6.Grisanti LA, Gumpert AM, Traynham CJ, et al. Leukocyte-expressed beta2adrenergic receptors are essential for survival after acute myocardial injury. Circulation. 2016;134:153–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najafi A, Sequeira V, Kuster DW, et al. Beta-adrenergic receptor signalling and its functional consequences in the diseased heart. Eur J Clin Invest. 2016;46:362–374 [DOI] [PubMed] [Google Scholar]
- 8.Grimm M, Brown JH. Beta-adrenergic receptor signaling in the heart: Role of camkii. J Mol Cell Cardiol. 2010;48:322–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee I, Fuseler JW, Price RL, et al. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–1891 [DOI] [PubMed] [Google Scholar]
- 10.Pinto AR, Ilinykh A, Ivey MJ, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: The renaissance cell. Circ Res. 2009;105:1164–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frieler RA, Mortensen RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation. 2015;131:1019–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamo T, Akazawa H, Komuro I. Cardiac nonmyocytes in the hub of cardiac hypertrophy. Circ Res. 2015;117:89–98 [DOI] [PubMed] [Google Scholar]
- 14.Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, et al. Identification of g protein-coupled signaling pathways in cardiac fibroblasts: Cross talk between g(q) and g(s). Am J Physiol Cell Physiol. 2000;278:C154–162 [DOI] [PubMed] [Google Scholar]
- 15.Aranguiz-Urroz P, Canales J, Copaja M, et al. Beta(2)-adrenergic receptor regulates cardiac fibroblast autophagy and collagen degradation. Biochim Biophys Acta. 2011;1812:23–31 [DOI] [PubMed] [Google Scholar]
- 16.Takeda N, Manabe I, Uchino Y, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang MH, Poh KK, Tan HC, et al. Therapeutic synergy and complementarity for ischemia/reperfusion injury: Beta1-adrenergic blockade and phosphodiesterase-3 inhibition. Int J Cardiol. 2016;214:374–380 [DOI] [PubMed] [Google Scholar]
- 18.Zhu WZ, Zheng M, Koch WJ, et al. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98:1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Communal C, Singh K, Sawyer DB, et al. Opposing effects of beta(1)- and beta(2)adrenergic receptors on cardiac myocyte apoptosis : Role of a pertussis toxin-sensitive g protein. Circulation. 1999;100:2210–2212 [DOI] [PubMed] [Google Scholar]
- 20.Morisco C, Zebrowski DC, Vatner DE, et al. Beta-adrenergic cardiac hypertrophy is mediated primarily by the beta(1)-subtype in the rat heart. J Mol Cell Cardiol. 2001;33:561–573 [DOI] [PubMed] [Google Scholar]
- 21.Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823 [DOI] [PubMed] [Google Scholar]
- 22.Luo JD, Xie F, Zhang WW, et al. Simvastatin inhibits noradrenaline-induced hypertrophy of cultured neonatal rat cardiomyocytes. Br J Pharmacol. 2001;132:159–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramchandra R, Hood SG, Denton DA, et al. Basis for the preferential activation of cardiac sympathetic nerve activity in heart failure. Proc Natl Acad Sci U S A. 2009;106:924–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao M, Fajardo G, Urashima T, et al. Cardiac pressure overload hypertrophy is differentially regulated by beta-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2011;301:H1461–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubey RK, Gillespie DG, Mi Z, et al. Cardiac fibroblasts express the camp-adenosine pathway. Hypertension. 2000;36:337–342 [DOI] [PubMed] [Google Scholar]
- 26.Dubey RK, Gillespie DG, Mi Z, et al. Endogenous cyclic amp-adenosine pathway regulates cardiac fibroblast growth. Hypertension. 2001;37:1095–1100 [DOI] [PubMed] [Google Scholar]
- 27.Fujiu K, Nagai R. Fibroblast-mediated pathways in cardiac hypertrophy. J Mol Cell Cardiol. 2014;70:64–73 [DOI] [PubMed] [Google Scholar]
- 28.Burger A, Benicke M, Deten A, et al. Catecholamines stimulate interleukin-6 synthesis in rat cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2001;281:H14–21 [DOI] [PubMed] [Google Scholar]
- 29.Chen C, Du J, Feng W, et al. Beta-adrenergic receptors stimulate interleukin-6 production through epac-dependent activation of pkcdelta/p38 mapk signalling in neonatal mouse cardiac fibroblasts. Br J Pharmacol. 2012;166:676–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bageghni SA, Hemmings KE, Zava N, et al. Cardiac fibroblast-specific p38alpha map kinase promotes cardiac hypertrophy via a putative paracrine interleukin-6 signaling mechanism. FASEB J. 2018:fj201701455RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meier H, Bullinger J, Marx G, et al. Crucial role of interleukin-6 in the development of norepinephrine-induced left ventricular remodeling in mice. Cell Physiol Biochem. 2009;23:327–334 [DOI] [PubMed] [Google Scholar]
- 32.Tarbit E, Singh I, Peart JN, et al. Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Heart Fail Rev. 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.