Abstract
Background:
People with obesity and/or the metabolic syndrome have an increased risk for developing diabetes and cardiovascular disease and may have low adiponectin levels. The obesity associated with Prader–Willi syndrome (PWS) would be expected to have similar complications. However, it was recently reported that, despite their adiposity, people with PWS have reduced visceral fat and are less likely to develop diabetes mellitus or the metabolic syndrome compared with people with simple obesity.
Objective:
To determine if plasma adiponectin levels and other variables relevant to diabetes and cardiovascular risk are different in a cohort of PWS subjects with known genetic subtypes compared with age-, sex- and weight-matched control subjects.
Results:
Fasting plasma glucose, C-peptide, triglycerides, leptin and cholesterol levels were similar in PWS and obese subjects. Our 20 PWS subjects (mean age = 27.7 years) had higher percent body fat (54.1 vs 48.5%) determined by DEXA measurements and lower percent lean mass (45.9 vs 51.5%) compared with 14 obese controls (mean age = 26.9 year). Plasma adiponectin levels were significantly higher in PWS (15.5±8.2 μg/ml) than in obese controls (7.5±2.7 μg/ml). A significant positive correlation was found with insulin sensitivity in PWS subjects (r=0.75, P=0.0003) but not in obese controls (r=0.36, P=0.20).
Discussion:
Our study confirmed an earlier observation of higher adiponectin levels in PWS subjects and less insulin resistance proportionate to their obesity status than found in subjects with simple obesity. Furthermore, no differences were seen in PWS subjects with the chromosome 15 deletion or maternal disomy 15. The reported excessive visceral adiposity in subjects with simple obesity compared with PWS may be associated with decreased production and lower circulating levels of adiponectin.
Keywords: adiponectin, simple obesity, Prader–Willi syndrome (PWS), body composition, obesity-related variables, insulin resistance and sensitivity
Introduction
Prader–Willi syndrome (PWS) is the most common syndromic cause of marked obesity.1 It is a congenital disorder characterized by short stature, muscular hypotonia, hypo-gonadism, mild mental retardation, diminished growth hormone secretion, hyperphagia and obesity which is extremely severe in some cases.1–3 Approximately 70% of PWS cases are due to a paternal deletion on chromosome 15 (15q11–q13 region), 25% of PWS cases have maternal uniparental disomy (UPD) of chromosome 15 and the remaining cases result from genetic imprinting defects.4–8
Much interest now revolves around the role of adipose tissue as an endocrine organ, capable of producing hormones and cytokines such as tumor necrosis factor-α, plasminogen activating inhibitor-1, leptin, interleukin-6, resistin and, more recently, adiponectin.9–12 Adiponectin is abundant in the circulation of nondiabetic humans, but is decreased in patients with obesity, type 2 diabetes and cardiovascular disease and higher in women than in men.13 In addition, treatment of obese type 2 diabetics with the peroxisome proliferator-activated receptor, gamma (PPARG) agonist troglitazone was associated with a three-fold increase in circulating adiponectin levels.13 Thus, adiponectin may play an important role in the links between obesity, insulin resistance, type 2 diabetes and risk of cardiovascular disease. Despite their adiposity, people with PWS may be at a lower risk to develop diabetes mellitus or the metabolic syndrome than people with simple obesity.14 Recently, Hoybye et al.15 reported that serum adiponectin levels were lower in adult PWS subjects compared with lean controls yet higher than in obese subjects. However, a paucity of data exists for adiponectin levels and obesity-related variables in PWS at any age. Hence, we report our experience with plasma adiponectin levels in individuals with PWS with both genetic subtypes (15q11–q13 deletion and maternal disomy 15) and similarly aged subjects with simple obesity and describe the relationship to body composition and obesity-related laboratory measures.
Subjects and methods
All individuals were recruited as part of a larger study on genotype/phenotype relationships and agreed to informed consent. All individuals were examined by a clinical geneticist (MGB) and genetic testing performed accordingly which included chromosome analysis, fluorescence in situ hybridization, DNA methylation and microsatellite studies using DNA probes from the 15q11–q13 region confirming the diagnosis of PWS and the genetic subtypes (deletion or maternal disomy). Nondysmorphic subjects with simple obesity having normal cytogenetic and molecular genetic testing results were recruited for comparison. The subjects had extensive obesity-related measures including insulin, C-peptide, glucose, cortisol, testosterone, estrogen, thyroid and leptin.
In total, 20 PWS subjects without diabetes were evaluated for this study including 12 female subjects and eight male subjects (18 Caucasian and two African American; 13 with 15q11–q13 deletion and 7 with maternal disomy 15) with an average age of 27.7±10.3 years with an age range of 16.9–48.4 years. In all, 14 nonsyndromic, obese control subjects (11 Caucasian and three African American; eight females and six males) were recruited for comparison with our PWS subjects. The comparison subjects had an average age of 26.9±11.4 years with an age range of 15.5–49.3 years. No PWS subject was currently or previously treated with growth hormone nor were any of our PWS or comparison subjects currently or previously treated with insulin or statins for hypercholesterolemia.
Peripheral blood samples were obtained after a 12 h overnight fast, immediately centrifuged and plasma was collected and stored at −70°C. Plasma adiponectin was measured in duplicate by a commercial radioimmunoassay kit (Linco Research Inc., St Charles, MO, USA) with an interassay coefficient of variation of 3.9% with no detectable crossreactivity. Plasma insulin was measured by enzyme immunoassay on the Abbott IMX system (Chicago, USA) with an interassay coefficient of variation of less than 5%. There was no measurable crossreactivity with C-peptide or proinsulin. Plasma leptin and C-peptide levels were similarly obtained. Plasma glucose was measured by a glucose oxidase technique using the Vitros DT 6011 System (Ortho-Clinical Diagnostics Inc, Rochester, NY, USA). Insulin sensitivity was calculated as the quantitative insulin sensitivity check index (QUICKI) according to the formula: 1/(log fasting insulin (mU/l) + log fasting glucose (mg/dl)).16 Assessment of insulin resistance using the homeostasis model assessment (HOMA) as described elsewhere17 was calculated using the following formula: HOMA (fasting glucose (mg/dl) × fasting insulin (μU/ml)/405). Plasma cholesterol and triglyceride levels were measured with the use of a standard multichannel analyzer.
Height was measured to the nearest 0.1 cm using a stadiometer. Weight was recorded routinely to the nearest0.1 kg using a balanced weight scale. Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters, kg/m2. Body composition (fat and lean mass in kg), percent body fat and percent lean mass were determined using total body dual energy X-ray absorptiometry-DEXA (Model DPX, Lunar Corporation, Madison, WI, USA) routinely performed in the radiology department while the subject was in supine position.18
To determine the degree of obesity and related complications, the criteria for the metabolic syndrome were applied to our subjects.19–21 The criteria used to distinguish the metabolic syndrome included waist circumference, fasting plasma lipid and glucose levels and blood pressure measurements. Three of our 34 subjects met the criteria (two PWS females and one obese female).
Differences in mean values of continuous variables between PWS and control subjects were compared by Student’s t-test. Linear regression and correlation analyses were performed to examine the relationship between plasma adiponectin and other variables in each of the cohorts. Significance of correlations was evaluated using Pearson product moment correlation coefficient. In addition, due to sample size and differences in variances, nonparametric tests were also used to evaluate the data. The Mann–Whitney U-test was used to evaluate differences in mean values and Spearman rank order correlation coefficient was used to evaluate correlations. Parametric and nonparametric analyses were in agreement in identifying significant relationships in the data. Statistical analyses were performed using the SPSS software package, version 11.
The original study protocol complied with Helsinki Declaration guidelines, and was approved by the local institutional review board at Vanderbilt University (previous affiliation for MGB). Permission to analyze stored samples for plasma adiponectin was also approved by the Institutional Review Board of the College of Medicine at the University of Florida, after it had the opportunity of reviewing the ethical approval of the original study. All subjects consented to study participation prior to sample collection.
Results
PWS and obese comparison subjects were matched for age, sex and BMI, as shown in Table 1. However, percent body fat was increased in the PWS individuals compared to the obese subjects, that is, 54.1±5.9 (mean±s.d.) vs 48.5±6.3 respectively, P<0.02, with a reciprocal decrease in lean body mass in the PWS subjects. Fasting plasma glucose, leptin, C-peptide, triglyceride and cholesterol levels were similar in PWS subjects and obese comparison subjects, as shown in Table 2. However, fasting plasma insulin was significantly higher and the QUICKI index of insulin sensitivity was significantly lower in obese comparison subjects than in PWS subjects. Plasma adiponectin levels were significantly higher in the PWS subjects compared to the control subjects(15.5 and 7.5 μg/ml, P=0.001, respectively), despite the fact that the PWS subjects had significantly greater percent body fat and lower lean mass. In addition, no significant differences were seen in adiponectin levels in male or female subjects within each subgroup. However, significant differences (P<0.05) in adiponectin levels were observed in female and male subjects between the two subject groups with higher levels in both female and male subjects in the PWS group compared to female and male subjects, respectively, in the obese group.
Table 1.
Age, BMI and body composition data in subjects with Prader–Willi syndrome and simple obesity
| Variable | Obese control | Prader–Willi syndrome | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | s.d. | N | Mean | s.d. | ||
| Age (years) | 14 | 26.9 | 11.4 | 20 | 27.7 | 10.3 | 0.818 |
| BMI (kg/m2) | 14 | 40.4 | 10.1 | 20 | 35.9 | 9.1 | 0.186 |
| Waist-to-hip ratio | 14 | 0.96 | 0.1 | 19 | 0.96 | 0.1 | 0.975 |
| % Body fat | 14 | 48.5 | 6.3 | 20 | 54.1 | 5.9 | 0.013* |
| Fat (kg) | 14 | 49.5 | 16.5 | 20 | 42.9 | 13.7 | 0.234 |
| % Lean | 14 | 51.5 | 6.3 | 20 | 45.9 | 5.9 | 0.013* |
| Lean (kg) | 14 | 51.4 | 11.7 | 20 | 35.3 | 7.5 | 0.001* |
Fat and lean measurements obtained by DEXA.
Significant difference between PWS and control determined by Student’s t-test.
Table 2.
Fasting plasma glucose, insulin, triglyceride, cholesterol, C-peptide, leptin, adiponectin and insulin response data in subjects with Prader–Willi syndrome and simple obesity
| Variable | Obese control | Prader-Willi syndrome | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | s.d. | N | Mean | s.d. | ||
| Glucose (mg/dl) | 14 | 89.6 | 16.0 | 20 | 95.3 | 42.8 | 0.637 |
| Insulin (μU/ml) | 14 | 25.1 | 14.6 | 18 | 9.2 | 5.4 | 0.001* |
| Triglycerides (mg/dl) | 13 | 186.2 | 114.6 | 16 | 137.3 | 82.6 | 0.192 |
| Total cholesterol (mg/dl) | 13 | 174.3 | 43.3 | 16 | 189.9 | 47.7 | 0.369 |
| Adiponectin (μg/ml) | 14 | 7.5 | 2.7 | 20 | 15.5 | 8.2 | 0.001* |
| Insulin sensitivity (QUICKI)a | 14 | 0.31 | 0.03 | 18 | 0.36 | 0.04 | 0.001* |
| Insulin resistance (HOMA)b | 14 | 5.7 | 3.7 | 18 | 2.4 | 2.6 | 0.005* |
| C-peptide (ng/ml) | 6 | 2.8 | 0.74 | 14 | 1.9 | 1.20 | 0.124 |
| Leptin (ng/dl) | 7 | 53.6 | 24.4 | 14 | 45.1 | 20.1 | 0.403 |
Significant difference between PWS and obese control determined by Student’s t-test.
Insulin sensitivity was calculated as the quantitative insulin sensitivity check index (QUICKI) according to the formula: (1/(log fasting insulin (mU/l)+log fasting glucose (mg/dl)) (Katz et al.16)).
Insulin resistance was calculated using the homeostasis model assessment (HOMA, Matthews et al.17) according to the following formula: (fasting glucose (mg/dl)×fasting insulin (μU/ml)/405).
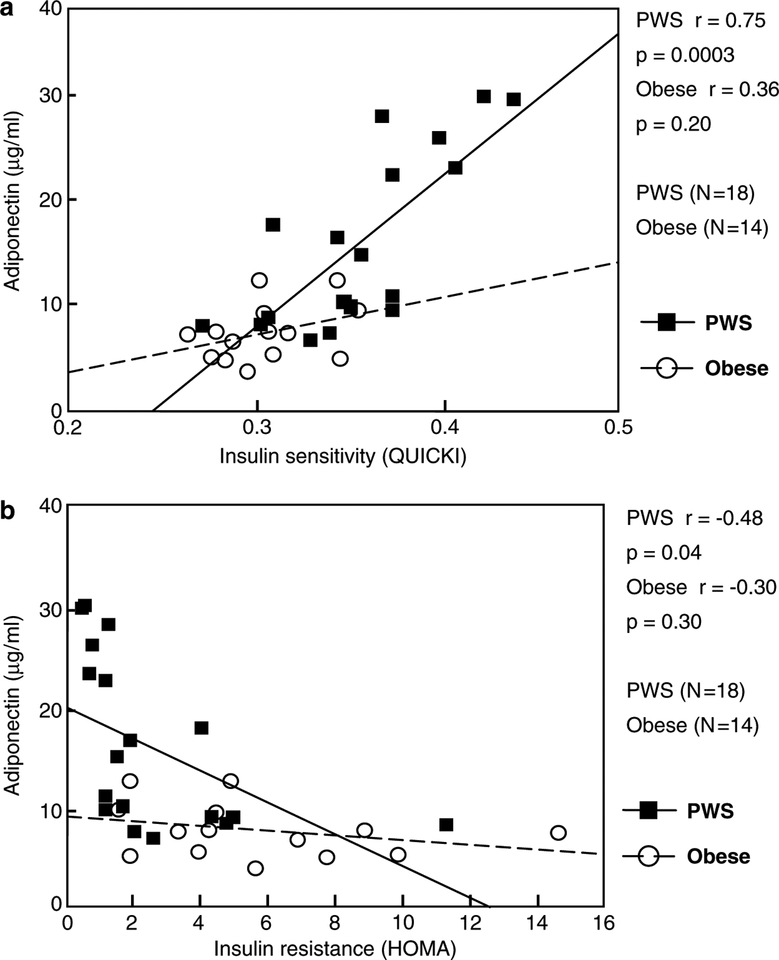
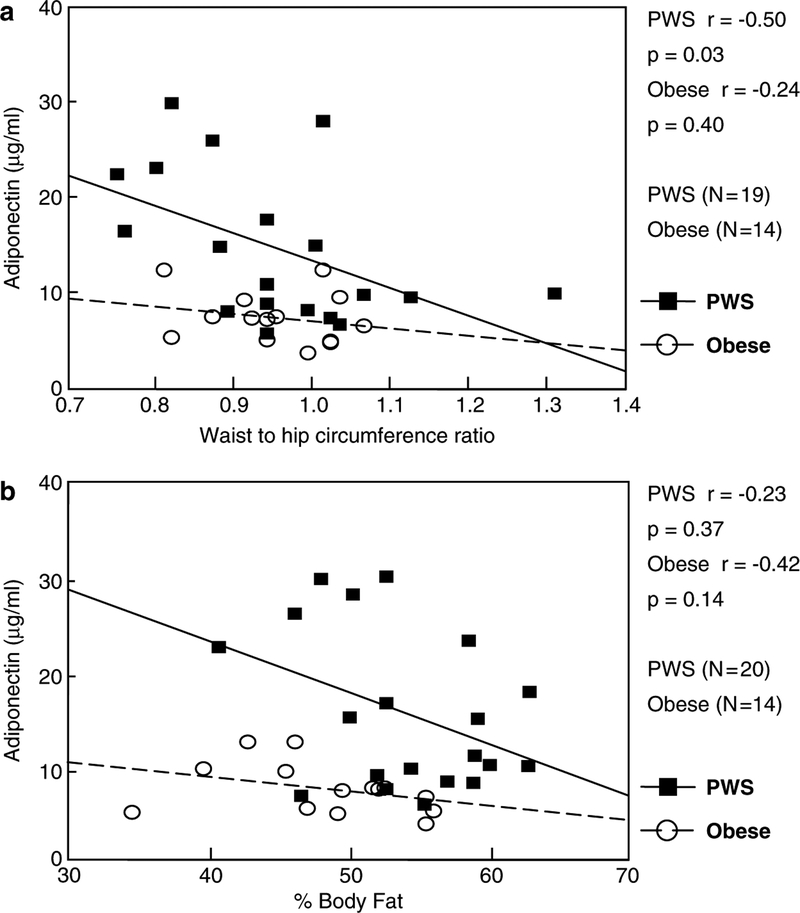
Fasting plasma adiponectin was significantly positively correlated with insulin sensitivity (QUICKI) in PWS subjects (r=0.75, P=0.0003, Figure 1a) but not in obese comparison subjects (r=0.36, P=0.20, Figure 1a). Conversely, insulin resistance (HOMA) was significantly negatively correlated with adiponectin levels in PWS subjects (r=−0.48, P=0.04, Figure 1b) but not in obese subjects (r=−0.30, P=0.30, Figure 1b). In subjects with PWS, but not in obese subjects, plasma adiponectin showed a significant negative correlation with waist-to-hip circumference ratio (r=−0.50, P=0.03, Figure 2a). However, adiponectin was not significantly correlated with percent body fat in either PWS or obese comparison subjects (Figure 2b). For several endocrine and obesity-related measurements, PWS subjects showed a significant negative correlation between fasting plasma adiponectin and fasting plasma: glucose (r=−0.45, P=0.049); insulin (r=−0.64, P=0.004); C-peptide(r=−0.55, P=0.04) and triglyceride (r=−0.52,P=0.04). A negative association seen between plasma adiponectin and leptin but not significant for either subject group. Furthermore, no significant differences were seen in adiponectin and other obesity-related variables reported in this study between our PWS-deletion and PWS-UPD subjects (data not shown).
Figure 1.
Correlation of adiponectin with measures of insulin resistance and sensitivity. (a) Pearson product moment correlation coefficient for fasting plasma adiponectin and insulin sensitivity as calculated by the QUICKI formula (Katz et al.16). (b) Pearson product moment correlation coefficient for fasting plasma adiponectin and insulin resistance as calculated by the HOMA formula (Matthews et al.17).
Figure 2.
Correlation of fasting plasma adiponectin with measures of body composition. (a) Pearson product moment correlation coefficient for fasting plasma adiponectin and waist-to-hip circumference ratio. (b) Pearson product moment correlation coefficient for fasting plasma adiponectin and percentage of body fat.
Discussion
Adipose tissue is not only the major source of energy storage, but also secretes hormones and metabolites that regulate energy metabolism and insulin sensitivity. Adiponectin is the most abundant adipose specific protein secreted by adipose tissue. Plasma adiponectin is decreased in obese subjects and in type 2 diabetics and is more closely related to insulin sensitivity than to adiposity. Adiponectin levels have been reported to be lower in obese adolescents and positively related to insulin sensitivity in all subjects.22 Strong inverse relationships have also been found between adiponectin and triglyceride levels in adolescent obesity.22 Triglyceride and intramyocellular lipid content are significant predictors of adiponectin levels explaining 62% of the variation.22 Thus, studies in adolescent obesity (non-PWS) have shown that plasma adiponectin levels are reduced and relate to insulin resistance, independent of total body fat and central adiposity. The antidiabetogenic and antiatherogenic properties of adiponectin are evident throughout life but, compromised in youth onset obesity. It was recently reported that adolescents with higher visceral adipose tissue (VAT) had lower adiponectin levels than those with lower VAT. Furthermore, hypoadiponectinemia was strongly correlated with insulin resistance and beta cell dysfunction.23
Serum adiponectin levels in adults with PWS were recently reported by Hoybye et al.15 They found that adiponectin levels in PWS subjects were significantly lower than in lean subjects but higher than in obese controls and were independent of anthropometrical parameters such as height, weight or body composition. They also found that correction of growth hormone deficiency in the PWS subjects had no effect on the serum adiponectin levels.
Central obesity is the most prevalent of the various abnormalities used to define the metabolic syndrome, the others being hypertriglyceridemia, low HDL-cholesterol, hypertension and raised fasting glucose.24 Evidence is accumulating that the distribution of fat, for example, visceral vs subcutaneous, is correlated with the propensity to develop diabetes. People with obesity and/or the metabolic syndrome have an increased risk for developing diabetes and cardiovascular disease.20,21 Increased insulin resistance is an underlying feature of the metabolic syndrome.19,25 The obesity associated with PWS would be expected to increase the degree of insulin resistance and along with it, the risk for type 2 diabetes and cardiovascular disease.26 People with PWS may be less likely to develop diabetes mellitus or the metabolic syndrome than nonsyndromic people with comparable obesity.14,27
Although our two groups of subjects (PWS and simple obesity) were matched for BMI, PWS subjects had a greater percentage body fat mass. The obesity in PWS affects both the trunk and the limbs,28 but a greater proportion of the abdominal fat is localized subcutaneously rather than around the visceral organs.14,27 Our results are consistent with the hypothesis that excessive visceral, rather than subcutaneous, adiposity is particularly likely to be associated with decreased production and circulating levels of adiponectin. This could explain why PWS subjects with a greater percentage body fat have higher adiponectin levels, and why, in PWS individuals, there is a significant negative correlation between plasma adiponectin and waist-to-hip ratio, although Hoybye et al.15 did not report a significant negative correlation between adiponectin levels and waist-to-hip ratios in their PWS subjects. The difference in fat distribution may result in increased adiponectin levels in PWS subjects resulting in reduced diabetes mellitus relative to individuals with simple obesity. The role fat distribution plays in maintaining adiponectin levels as well as the precise function of plasma and tissue adiponectin in PWS merits further investigation.
Our study further showed that PWS subjects have significantly less insulin resistance proportionate to their obesity than subjects with simple obesity. PWS individuals matched with obese controls for BMI had significantly greater insulin sensitivity as determined by the QUICKI calculation. In addition, plasma adiponectin levels showed positive correlations with insulin sensitivity in both subject groups but significant for only PWS subjects.
In conclusion, our study is confirmatory of the higher adiponectin levels in PWS compared with obese subjects reported by Hoybye et al.15 and further supports the role of adiponectin as protective against metabolic syndrome and complications related to obesity. It is recognized that adiponectin levels are decreased when there is an excess of visceral fat, as in general obesity and type 2 diabetes. It seems likely that the increased adiponectin we observed in our PWS subjects is a reflection of proportionately less visceral adiposity. A qualitative difference in the subcutaneous fat cells between PWS and obese controls might also contribute to the difference in adiponectin between the two groups. Further research is required to identify the mechanism responsible for higher adiponectin levels in PWS.
Acknowledgements
We thank Brian Sheridan at the Regional Biochemistry Laboratory, Royal Victoria Hospital, Belfast, Northern Ireland, for measuring plasma insulin and C-peptide and Karen Brezner for her assistance in processing the plasma samples. Support was provided by Grant PO1HD30329 and RO1HD41672 from the National Institutes of Health; Children’s Mercy Hospitals Physician Scientist Award(01.4871); and the Hall Family Foundation (01.3905) to MGB as well as Grant DK37373 from the National Institutes of Health to SPK.
References
- 1.Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet 1990; 35: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassidy SB. Prader-Willi syndrome. J Med Genet 1997; 34: 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler MG, Thompson T. Prader-Willi syndrome: clinical and genetic findings. Endocrinology 2000; 10: 35–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascari MJ, Gottlieb W, Rogan PK, Butler MG, Waller DA, Armour JA et al. The frequency of uniparental disomy in Prader-Willi syndrome. Implications for molecular diagnosis. N Engl J Med 1992; 326: 1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glenn CC, Driscoll DJ, Yang TP, Nicholls RD. Genomic imprinting: potential function and mechanisms revealed by the Prader-Willi and Angelman syndromes. Mol Hum Reprod 1997; 3: 321–332. [DOI] [PubMed] [Google Scholar]
- 6.Robinson WP, Kuchinka BD, Bernasconi F, Petersen MB, Schulze A, Brondum-Nielsen K et al. Maternal meiosis I non-disjunction of chromosome 15: dependence of the maternal age effect on level of recombination. Hum Mol Genet 1998; 7: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 7.Ohta T, Gray TA, Rogan PK, Buiting K, Gabriel JM, Saitoh S et al. Imprinting-mutation mechanisms in Prader-Willi syndrome. Am J Hum Genet 1999; 64: 397–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buiting K, Gross S, Lich C, Gillessen-Kaesbach G, el-Maarri O, Horsthemke B. Epimutations in Prader-Willi and Angelman syndromes: a molecular study of 136 patients with an imprinting defect. Am J Hum Genet 2003; 72: 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001; 86: 1930–1935. [DOI] [PubMed] [Google Scholar]
- 10.Argiles JM, Lopez-Soriano J, Almendro V, Busquets S, Lopez-Soriano FJ. Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med Res Rev 2004. [DOI] [PubMed] [Google Scholar]
- 11.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004; 89: 2548–2556. [DOI] [PubMed] [Google Scholar]
- 12.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem 2004; 50: 1511–1525. [DOI] [PubMed] [Google Scholar]
- 13.Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L et al. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes 2003; 52: 667–674. [DOI] [PubMed] [Google Scholar]
- 14.Goldstone AP, Thomas EL, Brynes AE, Bell JD, Frost G, Saeed N et al. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader-Willi syndrome female adults: evidence for novel influences on body fat distribution. J Clin Endocrinol Metab 2001; 86: 4330–4338. [DOI] [PubMed] [Google Scholar]
- 15.Hoybye C, Bruun JM, Richelsen B, Flyvbjerg A, Frystyk J. Serum adiponectin levels in adults with Prader-Willi syndrome are independent of anthropometrical parameters and do not change with GH treatment. Eur J Endocrinol 2004; 151: 457–461. [DOI] [PubMed] [Google Scholar]
- 16.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000; 85: 2402–2410. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 18.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy X-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 1990; 51: 1106–1112. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol 1999; 83: 25F–29F. [DOI] [PubMed] [Google Scholar]
- 20.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 1992; 41: 715–722. [DOI] [PubMed] [Google Scholar]
- 21.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001; 24: 683–689. [DOI] [PubMed] [Google Scholar]
- 22.Weiss R, Dufour S, Groszmann A, Petersen K, Dziura J, Taksali SE et al. Low adiponectin levels in adolescent obesity: a marker of increased intramyocellular lipid accumulation. J Clin Endocrinol Metab 2003; 88: 2014–2018. [DOI] [PubMed] [Google Scholar]
- 23.Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care 2004; 27: 547–552. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002; 287: 356–359. [DOI] [PubMed] [Google Scholar]
- 25.Lebovitz H Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes 2001; 109 (Suppl 2): S135–S148. [DOI] [PubMed] [Google Scholar]
- 26.Martin A, State M, Koenig K, Schultz R, Dykens EM, Cassidy SB et al. Prader-Willi syndrome. Am J Psychiatry 1998; 155: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 27.Talebizadeh Z, Butler M. Insulin resistance and obesity-related factors in Prader-Willi syndrome: Comparison with obese subjects. Clin Genet 2005; 67: 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meaney FJ, Butler MG. Characterization of obesity in the Prader-Labhart-Willi syndrome: Fatness patterning. Med Anthropo Quart 1989; 3: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]