Abstract
Prader–Willi syndrome (PWS), the most common genetic cause of marked obesity in humans, is usually due to a de novo paternally derived chromosome 15q11–q13 deletion or maternal disomy 15 [(uniparental disomy (UPD)]. Obesity is due to energy imbalance, but few studies have examined fat patterning and obesity-related factors in subjects with PWS (deletions and UPD) compared with subjects with simple obesity. We examined for differences in fatness patterning and lipid, leptin, and glucose and insulin levels in subjects with simple obesity and PWS and adjusted for gender, age, and body mass index (BMI). Fasting peripheral blood samples and cross-sectional magnetic resonance image scans at the level of the umbilicus were obtained in 55 subjects ranging in age from 10.4 to 49 years: 20 PWS deletion, 17 PWS UPD, and 18 obese controls. Subcutaneous fat area (SFA) and intra-abdominal visceral fat area (VFA) were calculated. No significant difference was seen between the PWS deletion subjects or PWS UPD subjects for fatness measurements or leptin levels. Twenty-three of 37 PWS subjects met the criteria for obesity (BMI > 95th percentile). No significant differences were observed for SFA and VFA between the PWS subjects judged to be obese and control subjects with simple obesity. There was an overall trend for decreased VFA in the PWS subjects but not significantly different. VFA was significantly positively correlated with both fasting insulin and total cholesterol in PWS deletion subjects but not in PWS UPD subjects or obese controls. Fasting insulin level was significantly lower in the obese PWS subjects compared with subjects with simple obesity, and insulin sensitivity (QUICKI) was significantly higher in PWS subjects with obesity. Homeostasis model assessment (HOMA) and QUICKI values were correlated and in opposite directions with triglycerides in the obese PWS subjects but not in the obese controls. Subjects in each group were stratified according to published criteria on the basis of their level of visceral fat (e.g. ≥ 130 cm2) to assess the influence of VFA on metabolic abnormalities. In the obese PWS subjects, the fasting triglyceride, glucose, and insulin levels, and HOMA value were significantly elevated, while the QUICKI value was significantly lower in those with VFA ≥ 130 cm2. Such significant differences were not seen in the obese control group. Our results indicate that VFA may be regulated differently in PWS subjects compared to individuals with simple obesity. Insulin resistance is lower in PWS subjects and insulin sensitivity is higher compared with obese controls. PWS subjects with increased VFA may be at a higher risk of obesity-related complications compared to PWS subjects without increased VFA.
Keywords: body mass index, HOMA, insulin resistance and sensitivity, leptin, Prader–Willi syndrome, QUICKI, simple obesity, subcutaneous and visceral fat areas
Prader–Willi syndrome (PWS) was first described by Prader et al. in 1956 (1). This syndrome is characterized by mental deficiency, infantile hypotonia, hypogonadism, short stature, small hands and feet, obesity, and minor facial anomalies (2–7). Individuals with PWS usually present with feeding difficulties during infancy followed by hyperphagia and early childhood obesity. PWS is considered the most common genetic cause of marked obesity in humans (4), and understanding obesity in this syndrome may yield useful information for those in the general population with obesity. Approximately, 70% of subjects with PWS have a de novo paternally derived chromosome 15q11–q13 deletion, while 25% have maternal disomy 15 [(uniparental disomy (UPD)], and the remaining subjects have an imprinting defect.
The development of obesity requires an energy imbalance with the rate of triglyceride synthesis and fat storage exceeding that of fat mobilization and utilization. The massive accumulation of adipose tissue observed in PWS and the unusual fat patterning (8) suggest abnormalities in fat mobilization and oxidation or triglyceride synthesis and storage. Furthermore, the unusual distribution of body fat observed in PWS subjects with marked obesity remains after weight loss (9), but excess fat continues even though normal weight is achieved. Sex reversal fat pattern is seen in PWS subjects with males having greater subcutaneous fat area (SFA) compared with females, beginning at an early age (8).
Plasma lipid profiles are reported to be similar in subjects with PWS compared with obese controls (10, 11); however, circulating free fatty acid levels are elevated in PWS (10, 12–14). In addition, fatty acid composition of adipose tissue triglyceride is atypical in PWS individuals compared to obese controls (15, 16). An early study of fat utilization and transport suggested no irregularities in PWS (13), although adipose tissue lipoprotein lipase activity was reported to be increased in PWS suggesting an increased efficiency of triglyceride storage (17). Furthermore, individuals with PWS have smaller fat cell numbers but greater fat cell size compared to individuals with simple obesity (18, 19). Hence, the excessive fat accumulation along with an unusual fat patterning in PWS may result from defects in fat metabolism or nutrient partitioning.
The health risks of obesity (such as the predisposition to diabetes, hypertension, musculoskeletal, and cardiovascular diseases) are related not only to the amount of total body fat but also to regional fat distribution. Specifically, individuals with a significant accumulation of intra-abdominal visceral fat are particularly at risk of obesity-related complications (20). The increase of visceral fat plays an integral role in the development of insulin resistance, glucose intolerance, and hyperlipidemia in obese subjects without PWS (21). Additionally, other studies have found that visceral adipocytes, which express higher glucocorticoid-binding capacity and chronic stress, may contribute to the deposition of intra-abdominal fat and insulin–glucose homeostasis (20). Because of an unusual fat patterning and the known predisposition to type 2 diabetes in subjects with PWS (7), we sought to determine whether individuals with PWS differ from subjects with simple obesity in terms of body composition, absolute or relative amounts of intra-abdominal visceral fat compared with peripheral fat, insulin resistance, leptin, and lipid data. In addition, few previous studies have examined fat patterning and insulin resistance in PWS individuals of all ages with the 15q deletion or UPD.
Subjects and methods
Subjects
Fifty-five subjects were studied including 20 PWS deletion [nine males (eight Caucasian, one African-American), 11 females (all Caucasian), average age of 22 years], 17 PWS UPD [nine males (eight Caucasian, one Hispanic), eight females (seven Caucasian, one Hispanic), average age of 24 years], and 18 non-syndromic subjects with obesity of unknown cause [eight males (six Caucasian, two African-American), 10 females (nine Caucasian, one African-American), average age of 26 years with an age range of 11–49 years] compared with an average range of 10.4–44 years for all PWS subjects. The subjects were recruited for genotype–phenotype studies in PWS following informed written consent. All subjects were examined by a clinical geneticist (Merlin G Butler). No subject was on growth or thyroid hormone treatment. Three of the 18 subjects with simple obesity and five of the 37 PWS subjects had a history of diabetes mellitus but were not currently on insulin or oral hypoglycemic agents.
The presence of the 15q11–q13 deletion was identified by fluorescence in situ hybridization using 15q11–q13 probes (e.g. SNRPN). All PWS subjects showed abnormal methylation testing with polymerase chain reaction (PCR) analysis consistent with the diagnosis of PWS. The presence of maternal disomy 15 or UPD was determined by PCR using established methods with polymorphic DNA microsatellites from the chromosome 15q11–q13 region (22, 23). Height to the nearest 0.1 cm and weight to the nearest 0.1 kg were obtained for each subject in the clinical setting. Waist circumference was obtained to the nearest 0.1 cm with a steel tape measure at the umbilicus level in the standing position. The hip circumference was obtained to the nearest 0.1 cm at the greater trochanter level. The body mass index (BMI), used to define obesity, which is equal to weight in kilograms divided by height in meters squared, was calculated for each subject. For adult subjects (≥18 years of age), obesity was defined as BMI ≥ 30. For subjects less than 18 years, obesity was defined as BMI > 95th percentile using published standardized growth charts for each sex (24).
Fat determination
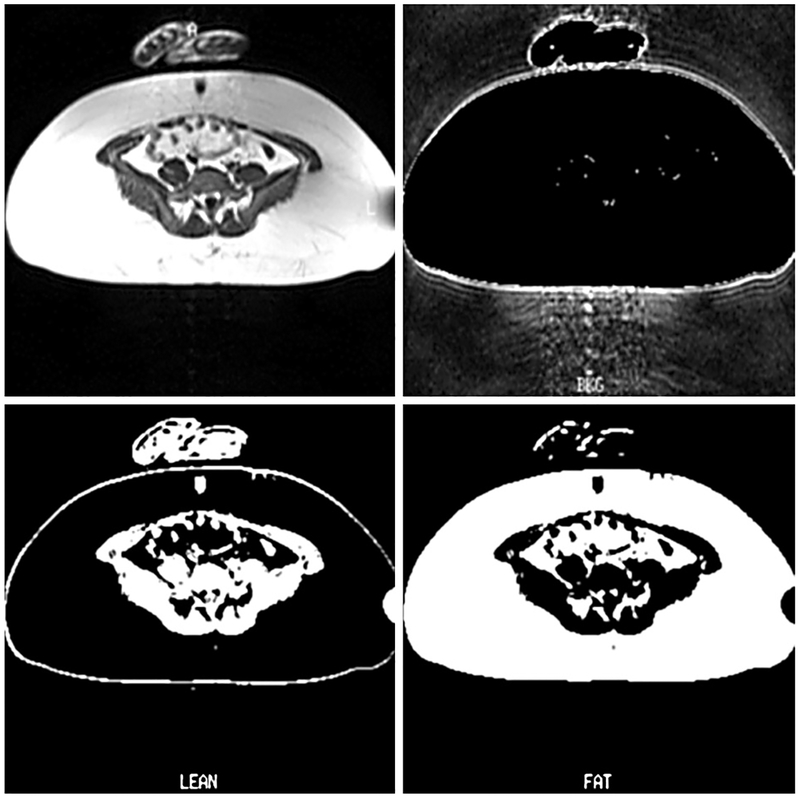
Regional fat distribution (fat patterning) was determined using T1-weighted magnetic resonance images (MRIs) acquired on a commercial clinical MRI scanner (SP63, Siemens Medical Systems, Erlanger, Germany). The quantity of abdominal subcutaneous and visceral adipose tissue was determined from cross-sectional abdominal MRI scans (Fig. 1) at the level of the umbilicus (fourth lumbar vertebra) and converted to pixels representing background, lean and fat tissues using established protocols (25, 26). Cross-sectional SFA and visceral fat area (VFA) in the supine position were calculated by the number of fat pixels divided by the sum of fat and lean pixels providing the fractional volume of fat tissue determined within the MR slice (25, 26). The fat (SFA : VFA) ratio was calculated on each subject. Furthermore, obese subjects in both groups (PWS and controls) were stratified based on their measurement for VFA using VFA value of 130 cm2 as a cutoff, as reported by Despres (27). An area of approximately 130 cm2 was found as a critical level for visceral adipose tissue, above which an increased risk of metabolic abnormalities was detected in both Caucasian men and women from the general population (27).
Fig. 1.
Magnetic resonance images through the umbilicus level with segmented background, lean and fat.
Laboratory testing
Fasting (6–12 h) peripheral blood samples were collected from the subjects in ethylenediaminetetraacetic acid vacutainer tubes and the plasma stored at −70 °C for assay at a later time. Standard enzymatic assay kits (Sigma Chemical Co., St. Louis, MO) were used to measure total plasma cholesterol and triglyceride levels. Fasting plasma glucose levels were calculated by the oxidase method (Glucose Analyzer II, Beckman Instruments, Fullerton, CA). Plasma insulin and leptin levels were measured utilizing double-antibody radioimmunoassay methods (28, 29). Assessment of insulin resistance using the homeostasis model assessment (HOMA) was calculated as described elsewhere (30) using the following formula: HOMA [fasting glucose (mg/dl) × fasting insulin (μU/ml)/405]. The insulin sensitivity index or QUICKI, defined as 1/[log (I0) + log (G0)], where I0 is fasting insulin and G0 is fasting glucose (31), was also calculated for each subject.
Statistical analysis
Overall differences in clinical, fatness patterning, and obesity-related variables were identified among the subject groups by utilizing the univariate analysis of variance (anova) to examine for the impact of age, gender, and BMI as confounders. Parametric independent t-test was used when no adjustment was needed for specific variables (e.g. age). The Pearsonian correlation coefficient (r) was calculated to determine relationships between the variables. Correlation data were plotted using a simple scatter plot to observe for differences and pattern of association among the variables in the two subject groups. The SPSS statistical software version 10.1 was used throughout (32). All p values were taken as significant at <0.05.
Results
Statistical comparison between subject groups
Table 1 presents clinical, anthropometric, fatness, and laboratory variables for the two studied groups (PWS and obese control) and includes means, standard deviations, and sample size. Twenty-three of the 37 PWS subjects (62%) were obese based on BMI calculations (Table 2). Clinical characteristics of the PWS group determined to be obese were compared with control subjects with simple obesity and BMI calculations in the obese range. No statistical differences were detected in the average measures for BMI, triglyceride, cholesterol, leptin, glucose, SFA, VFA, fat (SFA : VFA) ratio, waist or hip circumferences, and waist-to-hip ratio in the obese PWS group compared with obese controls. However, subjects with PWS were shorter as expected and weighed less than the obese controls (anova, p < 0.01). In addition, fasting insulin values were significantly lower for all PWS subjects (obese and non-obese) or the obese PWS subjects alone compared with obese controls (anova, p < 0.01). Insulin sensitivity (QUICKI) index was significantly higher in all PWS subjects (obese and non-obese) or the obese PWS subjects alone compared with obese controls (anova, p < 0.05 and p < 0.01, respectively). Fasting triglycerides were also lower in the total PWS group (obese and non-obese) compared with obese controls (anova, p < 0.05) but did not reach significance when comparing only obese PWS subjects with obese controls (anova, p = 0.10).
Table 1.
Clinical, anthropometric, fatness, and laboratory variables for Prader–Willi syndrome (PWS) subjects (deletion and UPD) and obese controls: mean, SD, and number of subjects (in parentheses)
| Variables | PWS deletion | PWS UPD | Obese (n = 23) and non-obese (n = 14) | Obese control |
|---|---|---|---|---|
| Age (years) | 22.4 ± 7.4 (20) | 24.2 ± 9.7 (17) | 23.2 ± 8.5 (37) | 25.9 ± 13.3 (18) |
| Gender (female/male) | 11/9 | 8/9 | 19/18 | 10/8 |
| Body mass index (kg/m2) | 32.9 ± 5.3 (20) | 31.8 ± 7.8 (17) | 32.4 ± 6.5 (37)a | 38.1 ± 5.8 (18)a |
| Following variables were analyzed by univariate analysis of variance (anova) and were adjusted for age, gender, and BMI | ||||
| Height (cm) | 151.2 ± 7.9 (20) | 149.9 ± 8.3 (17) | 150.6 ± 8.0 (37)b | 162.1 ± 10.2 (18)b |
| Weight (kg) | 75.4 ± 15.0 (20) | 71.2 ± 16.7 (17) | 73.5 ± 15.7 (37)b | 100.6 ± 20.6 (18)b |
| Waist circumference at umbilicus (in) | 41.3 ± 5.8 (18) | 43.0 ± 7.4 (11) | 42.0 ± 6.4 (29) | 46.6 ± 6.5 (18) |
| Hip circumference at greater trochanter (in) | 44.7 ± 5.0 (18)c | 42.6 ± 7.0 (12)c | 43.9 ± 5.9 (30) | 48.4 ± 5.6 (18) |
| Waist-to-hip ratio | 0.93 ± 0.11 (18)c | 1.02 ± 0.11 (11)c | 0.96 ± 0.12 (29) | 0.96 ± 0.08 (18) |
| SFA at umbilicus (cm2) | 465.1 ± 152.6 (20) | 419.1 ± 177.2 (17) | 444.0 ± 163.7 (37) | 547.0 ± 159.8 (18) |
| VFA at umbilicus (cm2) | 104.5 ± 59.8 (20) | 100.8 ± 52.1 (17) | 102.8 ± 55.7 (37) | 126.7 ± 60.1 (18) |
| Fat ratio (SFA : VFA) | 5.7 ± 2.8 (20) | 4.8 ± 2.2 (17) | 5.3 ± 2.6 (37) | 5.2 ± 2.9 (18) |
| Fasting leptin (ng/ml) | 38.6 ± 19.2 (14) | 43.1 ± 12.8 (5) | 39.8 ± 17.5 (19) | 41.7 ± 19.5 (11) |
| Fasting triglycerides (mg/dl) | 141.1 ± 82.6 (19) | 144.6 ± 86.0 (16) | 142.7 ± 83.0 (35)d | 252.4 ± 186.7 (16)d |
| Fasting total cholesterol (mg/dl) | 176.9 ± 43.1 (19) | 174.8 ± 36.4 (16) | 175.9 ± 39.6 (35) | 190.8 ± 37.7 (16) |
| Fasting glucose (mg/dl) | 95.5 ± 25.2 (19) | 81.9 ± 11.7 (16) | 89.3 ± 21.1 (35) | 105.9 ± 41.9 (16) |
| Fasting insulin (μU/ml) | 14.2 ± 8.8 (17) | 14.3 ± 10.1 (7) | 14.2 ± 8.9 (24)b | 24.8 ± 10.5 (17)b |
| HOMA (insulin resistance): (mg/dl × μU/ml)/405 | 3.8 ± 3.2 (17) | 3.3 ± 2.7 (6) | 3.7 ± 3.0 (23) | 6.8 ± 4.4 (16) |
| QUICKI (insulin sensitivity): 1/[log(I0) + log(G0)] | 0.33 ± 0.04 (17) | 0.34 ± 0.05 (6) | 0.34 ± 0.05 (23)d | 0.30 ± 0.02 (16)d |
SFA, subcutaneous fat area; VFA, visceral fat area.
Significant difference was found between all PWS (obese and non-obese) and obese control subjects (t-test, p < 0.01).
Significant difference was found between all PWS (obese and non-obese) and obese control subjects (anova, p < 0.01).
Significant difference was found between PWS deletion and PWS UPD groups (anova, p < 0.05).
Significant difference was found between all PWS (obese and non-obese) and obese control subjects (anova, p < 0.05).
Table 2.
Clinical, anthropometric, fatness, and laboratory variables for obese Prader–Willi syndrome (PWS) and obese control groups: mean, SD, and number of subjects (in parentheses)
| Variables | Obese PWS | Obese control |
|---|---|---|
| Age (years) | 22.7 ± 9.5 (23) | 25.9 ± 13.3 (18) |
| Gender (female/male) | 11/12 | 10/8 |
| Body mass index (kg/m2) | 36.5 ± 4.3 (23) | 38.1 ± 5.8 (18) |
| Following variables were analyzed by univariate analysis of variance (anova) and were adjusted for age, gender, and BMI | ||
| Height (cm) | 149.5 ± 8.3 (23)a | 162.1 ± 10.2 (18)a |
| Weight (kg) | 81.9 ± 12.9 (23)a | 100.6 ± 20.6 (18)a |
| Waist circumference at umbilicus (in) | 44.2 ± 5.2 (22) | 46.6 ± 6.5 (18) |
| Hip circumference at greater trochanter (in) | 45.9 ± 4.8 (23) | 48.4 ± 5.6 (18) |
| Waist-to-hip ratio | 0.97 ± 0.12 (22) | 0.96 ± 0.08 (18) |
| SFA at umbilicus (cm2) | 537.2 ± 134.9 (23) | 547.0 ± 159.8 (18) |
| VFA at umbilicus (cm2) | 120.7 ± 57.9 (23) | 126.7 ± 60.1 (18) |
| Fat ratio (SFA : VFA) | 5.4 ± 2.4 (23) | 5.2 ± 2.9 (18) |
| Fasting leptin (ng/ml) | 42.9 ± 18.7 (13) | 41.7 ± 19.5 (11) |
| Fasting triglycerides (mg/dl) | 158.4 ± 90.3 (21) | 252.4 ± 186.7 (16) |
| Fasting total cholesterol (mg/dl) | 182.6 ± 36.1 (21) | 190.8 ± 37.7 (16) |
| Fasting glucose (mg/dl) | 92.5 ± 21.1 (22) | 105.9 ± 41.9 (16) |
| Fasting insulin (μU/ml) | 14.7 ± 9.9 (18)a | 24.8 ± 10.5 (17)a |
| HOMA (insulin resistance): (mg/dl × μU/ml)/405 | 3.9 ± 3.4 (17) | 6.8 ± 4.4 (16) |
| QUICKI (insulin sensitivity): 1/[log(I0) + log(G0)] | 0.34 ± 0.05 (17)a | 0.30 ± 0.02 (16)a |
SFA, subcutaneous fat area; VFA, visceral fat area.
Significant difference was found between obese PWS and obese control groups (p < 0.01).
When only adult subjects were compared between the two groups (PWS subjects with obesity, n = 13; obese controls, n = 10), lower triglyceride and insulin levels as well as higher QUICKI values were found in the obese PWS adults compared with obese control adults (anova, p = 0.04, p = 0.02, and p = 0.04, respectively). Such differences were not detected for subjects less than 18 years of age (PWS subadults with obesity, n = 10; obese subadult controls, n = 8).
In addition, subjects in both obese groups (PWS and controls at all ages) were grouped separately based on their VFA level: ≥130 and <130 cm2 as described elsewhere (27). The subject distribution in each group after applying VFA stratification was as follows: obese control (VFA ≥ 130 cm2, n = 7; VFA < 130 cm2, n = 11) and obese PWS (VFA ≥ 130 cm2, n = 7; VFA < 130 cm2, n = 16). The presence of a cluster of metabolic abnormalities was found within each stratified group. In the control group, no significant differences were detected in the subjects with VFA ≥ 130 cm2 compared with the subjects with VFA < 130 cm2. However, in the obese PWS group, fasting triglyceride, glucose, insulin, and HOMA measurements were significantly increased in the subjects with VFA ≥ 130 cm2 compared with the PWS subjects with VFA < 130 cm2. As expected, the insulin sensitivity index or QUICKI was significantly lower in the PWS subjects with VFA ≥ 130 cm2, but no significant differences were found for leptin and total cholesterol (Table 3).
Table 3.
Laboratory variables analyzed in two obese groups [23 Prader–Willi syndrome (PWS) subjects and 18 controls] stratified by degree of visceral fat area (VFA)
| Obese PWS | Obese control | |||||
|---|---|---|---|---|---|---|
| Variables | VFA ≥ 130 cm2 | VFA <130 cm2 | p value | VFA ≥ 130 cm2 | VFA < 130 cm2 | p value |
| Fasting leptin (ng/ml) | 40.7 ± 16.2 (6) | 44.8 ± 21.8 (7) | 0.454 | 38.5 ± 27.0 (5) | 44.3 ± 12.6 (6) | 0.688 |
| Fasting triglycerides (mg/dl) | 226.3 ± 98.1 (7) | 124.5 ± 66.3 (14) | 0.006a | 368.3 ± 228.2 (7) | 162.2 ± 73.5 (9) | 0.317 |
| Fasting total cholesterol (mg/dl) | 203.1 ± 30.1 (7) | 172.3 ± 35.3 (14) | 0.100 | 188.7 ± 39.4 (7) | 192.4 ± 38.6 (9) | 0.363 |
| Fasting glucose (mg/dl) | 113.7 ± 24.2 (7) | 82.5 ± 9.3 (15) | 0.000a | 125.0 ± 58.1 (7) | 91.0 ± 13.7 (9) | 0.144 |
| Fasting insulin (μU/ml) | 24.9 ± 9.6 (6) | 9.6 ± 5.0 (12) | 0.000a | 21.6 ± 8.8 (7) | 26.9 ± 11.4 (10) | 0.671 |
| HOMA (insulin resistance): (mg/dl × μU/ml)/405 | 7.35 ± 3.49 (6) | 2.04 ± 1.25 (11) | 0.000a | 7.47 ± 6.23 (7) | 6.20 ± 2.58 (9) | 0.165 |
| QUICKI (insulin sensitivity): 1/[log(I0) + log(G0)] | 0.30 ± 0.03 (6) | 0.36 ± 0.05 (11) | 0.007a | 0.30 ± 0.03 (7) | 0.30 ± 0.02 (9) | 0.417 |
VFA stratification based on published study (27).
Obesity status determined by BMI.
Significant p values calculated using univariate anova and adjusting for age, gender, and BMI.
All 37 subjects with PWS were classified into the two genetic subtypes: deletion and UPD (Table 1). Waist-to-hip ratios were significantly lower and hip circumferences were higher in the PWS deletion subjects compared with the PWS UPD group (anova, p = 0.04 and p = 0.03, respectively). When only PWS adult subjects (PWS deletion, n = 13; PWS UPD, n = 12) were compared, a trend was detected for waist-to-hip ratio, but hip circumferences were still significantly higher in the PWS deletion subjects (anova, p = 0.06 and p = 0.01, respectively).
Correlation analysis in obese subjects with PWS and obese controls
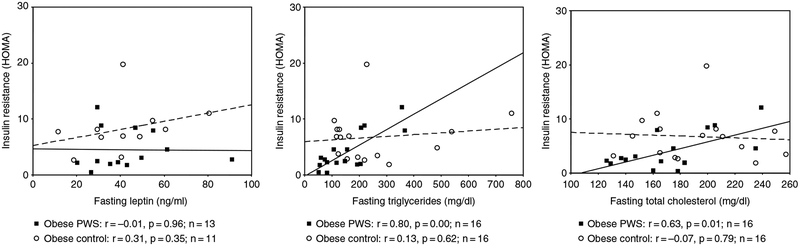
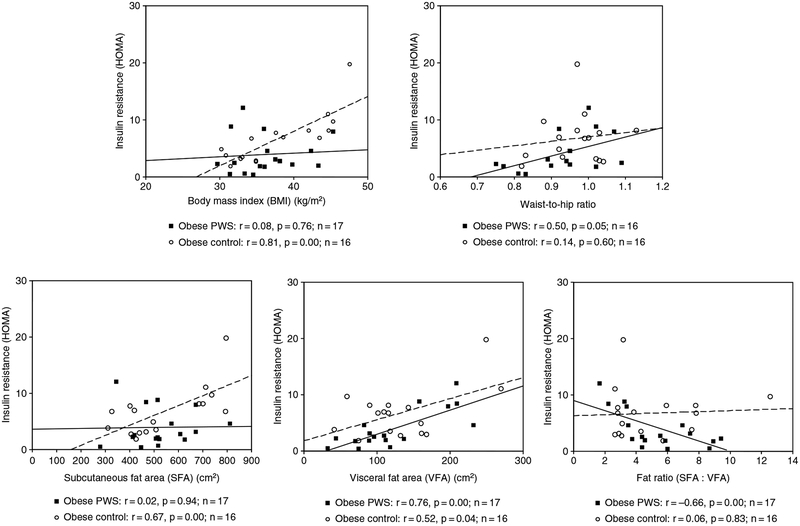
The HOMA value was positively correlated with both triglyceride and cholesterol levels in the obese PWS subjects, but these correlations were not significant for the obese controls. No significant correlations were observed when comparing HOMA with leptin in either obese group (Fig. 2). In addition, HOMA was positively correlated with VFA and negatively correlated with fat ratio (SFA : VFA) in the obese PWS group, but no significant correlation was seen between HOMA and SFA or with BMI in the obese PWS group (Fig. 3). The p value was equal to0.05 for the correlation between HOMA and waist-to-hip ratio in the obese PWS group.
Fig. 2.
Scatter plot and correlation data for insulin resistance [(homeostasis model assessment (HOMA)] and triglycerides, cholesterol, and leptin for both obese subjects with Prader–Willi syndrome (PWS) (solid line) and obese controls (dashed line). Obesity was determined for each subject using body mass index (BMI). For adult subjects (≥18 years of age), obesity was defined as BMI ≥ 30. For subjects less than 18 years, obesity was defined as BMI > 95th percentile using published standardized growth charts for each sex (24).
Fig. 3.
Scatter plot and correlation data for insulin resistance [(homeostasis model assessment (HOMA)] and body mass index (BMI), waist-to-hip ratio, subcutaneous fat area (SFA), visceral fat area (VFA), and fat ratio (SFA : VFA) for both obese subjects with Prader–Willi syndrome (PWS) (solid line) and obese controls (dashed line).
These correlations were also analyzed in the obese control group, and positive correlations were found between HOMA and both SFA and VFA but absent when insulin resistance or the HOMA value was correlated with fat ratio. Furthermore, HOMA was positively correlated with BMI in the obese controls. No significant association was noticed between HOMA and waist-to-hip ratio in the obese control group (Fig. 3).
Correlation analysis in PWS genetic subgroups (deletion and UPD)
Correlation values for obesity-related factors in PWS genetic subgroups are summarized in Table 4. Total cholesterol levels were positively correlated with glucose, triglyceride, and VFA in the PWS deletion group only. Strong positive associations were seen in the controls between insulin level and weight, BMI, and SFA, but no associations were identified for either PWS genetic subgroup. In contrast, strong correlations were found between insulin level and both VFA and SFA : VFA ratio in only the PWS deletion subject group but not in obese controls and UPD. Both VFA and triglyceride levels showed positive correlations with age of subject in the obese control group but not in the PWS subject groups. PWS deletion and PWS UPD subjects showed a significant positive correlation for glucose and triglyceride levels but not for the obese subjects. There was a significant positive correlation between insulin and glucose for the PWS deletion group only but not for the PWS UPD or obese control subjects. Insulin and triglyceride levels were significantly positively correlated for PWS deletion subjects but not for PWS UPD or obese subjects. The PWS deletion group showed a significantly positive correlation for insulin and total cholesterol levels, but this association was not found in the PWS UPD or obese subjects.
Table 4.
Correlation values for obesity-related factors in PWS genetic subgroups and obese controls
| PWS deletion | PWS UPD | Obese control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | r | p | n | r | p | n | r | p | n |
| Age and VFA | −0.12 | 0.62 | 20 | 0.26 | 0.31 | 17 | 0.51 | 0.03a | 18 |
| Age and triglycerides | −0.14 | 0.57 | 19 | −0.28 | 0.29 | 16 | 0.55 | 0.03a | 16 |
| Glucose and triglycerides | 0.72 | 0.00a | 18 | 0.70 | 0.00a | 16 | 0.26 | 0.33 | 16 |
| Cholesterol and glucose | 0.74 | 0.00a | 18 | 0.40 | 0.13 | 16 | 0.13 | 0.63 | 16 |
| Cholesterol and triglycerides | 0.79 | 0.00a | 19 | 0.37 | 0.16 | 16 | 0.31 | 0.24 | 16 |
| Cholesterol and VFA | 0.55 | 0.01a | 19 | 0.10 | 0.71 | 16 | −0.00 | 0.99 | 16 |
| Insulin and weight | −0.04 | 0.87 | 17 | 0.27 | 0.56 | 7 | 0.61 | 0.01a | 17 |
| Insulin and BMI | 0.24 | 0.35 | 17 | 0.21 | 0.65 | 7 | 0.84 | 0.00a | 17 |
| Insulin and SFA | 0.16 | 0.54 | 17 | 0.17 | 0.72 | 7 | 0.68 | 0.00a | 17 |
| Insulin and VFA | 0.74 | 0.00a | 17 | 0.70 | 0.08 | 7 | 0.01 | 0.95 | 17 |
| Insulin and fat ratio (SFA : VFA) | −0.62 | 0.01a | 17 | −0.57 | 0.18 | 7 | 0.47 | 0.06 | 17 |
| Insulin and triglycerides | 0.80 | 0.00a | 16 | 0.78 | 0.07 | 6 | −0.10 | 0.71 | 16 |
| Insulin and cholesterol | 0.59 | 0.02a | 16 | −0.04 | 0.94 | 6 | −0.31 | 0.24 | 16 |
| Insulin and glucose | 0.75 | 0.00a | 17 | 0.78 | 0.07 | 6 | 0.20 | 0.46 | 16 |
n, number of subjects; p, p value (asignificant values); r, Pearson’s correlation coefficient; SFA, subcutaneous fat area; VFA, visceral fat area.
Gender differences
The impact of gender differences on each studied variable was also examined in each obese subject group. In the control group, as expected, males were taller than females. No significant differences were seen between obese PWS males and obese PWS females for the analyzed variables. We also sought differences in body composition and obesity-related variables within and between the two obese groups (PWS and control) based on gender. Overall, obese PWS males (n = 12) were significantly shorter and weighed less than the obese control males (n = 8) (anova, p < 0.01). In addition, obese PWS males (n = 12) had significantly lower triglyceride levels than the obese control males (n = 8) (anova, p = 0.02). Height was significantly shorter in the obese PWS females compared with obese control females. In addition, insulin levels were significantly lower while insulin sensitivity (QUICKI) was higher in the obese PWS females compared with obese control males (anova, p = 0.01 and p = 0.02, respectively).
Discussion
Abdominal obesity has emerged as a strong predictor for non-insulin-dependent diabetes mellitus. Adiposity, localized in the abdominal region, specifically visceral fat compared with subcutaneous fat, is associated with high lipid levels (20). Elevated very low-density lipoprotein levels and decreased high-density lipoproteins are also influenced by fatness patterning in the general population. However, there is a paucity of data on fatness relationships with lipid and other obesity-related variables in PWS, particularly those classified with the 15q11–q13 deletion or UPD. Moreover, an unusual fatness pattern has been reported in PWS (8, 33), further supporting our study.
In addition to investigating differences in obesity-related factors between PWS subjects with obesity at the time of evaluation and obese controls, we examined general differences between all PWS subjects (regardless of their obesity status). These comparisons were performed to determine whether a relationship exists between the obesity status in PWS and obesity-related variables. However, no significant differences were detected when comparing these variables [i.e. leptin, triglycerides, cholesterol, glucose, insulin, HOMA, and QUICKI as well as fat (SFA : VFA), and waist-to-hip ratios] between obese and non-obese PWS subjects (data not shown).
The fasting insulin level was significantly lower and insulin sensitivity was higher in obese PWS subjects compared with obese controls. A significantly positive correlation was detected between HOMA and triglycerides in obese subjects with PWS but not observed in obese controls. Butler et al. (11) reported previously fasting plasma lipid (triglycerides, total cholesterol, HDL cholesterol, and LDL cholesterol), glucose, and insulin levels from 16 PWS deletion, 10 PWS non-deletion or UPD patients (age range of 7–39 years with a male/female ratio of 14 : 12), and 32 obese subjects, ranging in age from 8 to 33 years with a 6 : 26 male-to-female ratio, and found no significant difference in the levels of both insulin and triglycerides in PWS and obese individuals (11).
HOMA was also positively correlated with VFA but not correlated with SFA in the obese PWS group, which resulted in a negative correlation between HOMA and fat (SFA : VFA) ratio. A positive correlation was seen between HOMA and both VFA and SFA in the obese controls. In addition, stratified VFA data showed differences in the cluster of metabolic abnormalities in relation to VFA between the two obese groups. The observed differences between obese subjects with PWS and obese controls further suggest that fat metabolism might be regulated differently in PWS compared to simple obesity.
Moreover, previous studies have found that peripheral fat distribution is more prevalent in PWS subjects (34), which may also impact on insulin resistance or insulin sensitivity. Earlier studies on PWS adult females have shown a selective reduction in visceral fat, which may explain the observed reductions in insulin via increased hepatic insulin extraction and triglyceride levels (26). We did not identify significantly reduced visceral adiposity in PWS adult females as noted in the literature (26), but a trend did exist for a lower quantity of visceral adiposity in PWS compared to obese controls. For example, the level of visceral adipose was lower (136 vs 154 cm2, respectively) in our adult females with PWS (n = 6) compared with adult female controls (n = 6), but not statistically different. The amount of VFA or SFA (or fat ratio) did not differ significantly between obese control and obese PWS groups. However, our obese control group showed a higher percentage (39%) with VFA ≥ 130 cm2 compared with our obese PWS group (30%). In our study, visceral fat was measured at the umbilicus level (at fourth lumbar vertebra), while Goldstone et al. (26) calculated visceral fat at the fourth and fifth lumbar area. In addition, we found lower insulin levels in obese PWS females compared with obese control females and higher QUICKI indices in the PWS females. Additionally, lower triglyceride levels were found in obese PWS males compared with obese control males. These observations support fewer health-risk factors in the PWS population.
We assessed for differences between the two subgroups of PWS (deletion and UPD) and found that waist-to-hip ratios were significantly lower and hip circumferences were higher in subjects with PWS deletion compared with PWS UPD. Furthermore, correlation analyses for obesity-related factors revealed differences between the two genetic subgroups. For example, significant positive correlations were seen between cholesterol and glucose, triglycerides, and VFA in the PWS deletion subjects, but these correlations were absent in the PWS UPD group (with relatively similar sample size). Therefore, the results may suggest differences in mechanisms relating to fatness patterning between the two genetic subgroups of PWS (deletion and UPD).
Although the average age for the PWS subjects in our study was 22 years, a previous study demonstrated significantly low-insulin levels in pediatric PWS subjects, which may be attributed to differences in insulin metabolism in obese PWS subjects compared with obese non-PWS controls (11, 34). Additionally, the same study found that the adult PWS group had lower insulin levels, even though not statistically significant, compared with both non-obese and obese controls (34). In the current study, lower triglycerides and insulin levels as well as higher QUICKI values were detected in the obese PWS adults compared with obese control adults. However, these differences were not evident for subjects less than 18 years of age. A small group size or effects of aging could be a factor in these observations.
Fatness patterning and quantity of fat or fat ratio may impact differently on insulin and lipid profile in each subject group and may be a protective factor with lower insulin resistance and lower insulin and lipid levels in PWS. Insulin resistance and sensitivity measurements and their relationships to obesity-related variables were more favorable in the PWS group compared to obese controls. Thus, the chronic, long-term status of obesity in PWS seems to not impact as negatively on obesity-related factors as found in subjects with simple obesity. Following the VFA stratification reported by Despres (27), individuals with PWS with VFA ≥ 130 cm2 were found to have similar levels of insulin resistance and insulin sensitivity compared to obese controls with the same level of VFA. However, those PWS subjects (males and females) with lower VFA (<130 cm2) had significantly less insulin resistance and more insulin sensitivity than in the obese subject group. This observation would suggest that those PWS subjects with increased VFA may be at a higher risk of obesity-related complications compared to PWS subjects without increased VFA and should be monitored accordingly.
The results from our study were interpreted after adjusting for age, gender, and BMI to reduce the probability of skewed results generated by these factors which may contribute to differences in metabolic traits. Small sample size and multiple statistical tests may impact on the interpretation of results; therefore, additional studies with larger sample sizes would be recommended to further address possible factors identified contributing to obesity and obesity-related variables in PWS and obese subjects from the general population.
Acknowledgements
We thank the subjects with PWS and simple obesity and their families for participating in the study. This study was partially funded by NICHD P01HD30329, The Hall Foundation, NICHD R01HD41672, and Physician Scientist Award to Merlin G. Butler (Children’s Mercy Hospitals and Clinics). We acknowledge Dr Ron Price and Dr William Riddle for their assistance with MR imaging and fatness analyses.
References
- 1.Prader A, Labhart A, Willi H. Ein syndrome von adiposites, klienwuchs, kryptorchisumus und oligophrenia nach myatonieartigem zustand im neugeborenenalter. Schweiz Med Wochenschr 1956: 86: 1260–1261. [Google Scholar]
- 2.Cassidy SB. Prader-Willi syndrome. Curr Probl Pediatr 1984: 14: 1–55. [DOI] [PubMed] [Google Scholar]
- 3.Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet 1986: 23: 793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet 1990: 35: 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holm VA, Cassidy SB, Butler MG et al. Prader-Willi syndrome: consensus diagnostic criteria. Pediatrics 1993: 91: 398–402. [PMC free article] [PubMed] [Google Scholar]
- 6.Cassidy SB. Prader-Willi syndrome. J Med Genet 1997: 34 (11): 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler MG, Thompson T. Prader-Willi syndrome: clinical and genetic findings. The Endocrinologist 2000: 10: 3S–16S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meaney FJ, Butler MG. Characterization of obesity in Prader-Willi syndrome. Med Anthropol Q 1989: 5: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holm VA, Pipes PL. Food and children with Prader-Willi syndrome. Am J Dis Child 1976: 130: 1063–1067. [DOI] [PubMed] [Google Scholar]
- 10.Theodoridis CG, Albutt EC, Chance GW. Blood lipids in children with the Prader-Willi syndrome: a comparison with simple obesity. Aust Paediatr J 1971: 7: 20–23. [DOI] [PubMed] [Google Scholar]
- 11.Butler MG, Swift LL, Hill JO. Fasting plasma lipid, glucose, and insulin levels in Prader-Willi syndrome and obese individuals. Dysmorphol Clin Genet 1990: 4 (1): 23–26. [PMC free article] [PubMed] [Google Scholar]
- 12.Parra A, Cervantes C, Schultz RB. Immunoreactive insulin and growth hormone responses in patients with Prader-Willi syndrome. J Pediatr 1973: 83: 587–593. [DOI] [PubMed] [Google Scholar]
- 13.Bier DM, Kaplan SL, Havel RJ. The Prader-Willi syndrome: regulation of fat transport. Diabetes 1997: 26: 874–881. [DOI] [PubMed] [Google Scholar]
- 14.Butler MG, Carlson MG, Schmidt DE, Feurer ID, Thompson T. Plasma cholecystokinin (CCK) levels in Prader-Willi syndrome and obese subjects. Am J Med Genet 2000: 95: 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnsen S, Crawford JD, Haessler HA. Fasting hyperlipogenesis. An inborn error of energy metabolism in Prader-Willi syndrome. Pediatr Res 1967: 1: 291. [Google Scholar]
- 16.Kimbrough BO, Holman RT, Nelson RA, Callaway CW, Hayes AB. Triglyceride analysis of adipose tissue in Prader-Willi syndrome. Fed Proc 1976: 35: 344. [Google Scholar]
- 17.Schwartz RS, Brunzell JD, Bierman EL. Elevated adipose tissue lipoprotein lipase in the pathogenesis of obesity in Prader-Willi syndrome In: Prader-Willi Syndrome (Holm VA, Sulzbacher S, Pipes PL, eds). Baltimore: University Park Press, 1981. [Google Scholar]
- 18.Ginsberg-Fellner F, Knittle JL. Adipose tissue cellularity in the Prader-Willi Syndrome. Pediatr Res 1976: 10: 409. [Google Scholar]
- 19.Gurr MI, Jung RT, Robinson MP, James WPT. Adipose tissue cellularity in man: the relationship between fat cell size and number, the mass and distribution of body fat and the history of weight gain and loss. Int J Obes 1982: 6: 419–436. [PubMed] [Google Scholar]
- 20.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz D. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982: 54: 254–260. [DOI] [PubMed] [Google Scholar]
- 21.Anthony P, Goldstone E, Thomas L et al. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader-Willi syndrome female adults: evidence for novel influences on body fat distribution. Metabolism 2001: 86: 4330–4338. [DOI] [PubMed] [Google Scholar]
- 22.Butler MG, Christian SL, Kubota T, Ledbetter DH. Brief clinical report: a 5-year-old white with Prader-Willi syndrome and a submicroscopic deletion of chromosome 15q11q13. Am J Med Genet 1996: 65: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler MG, Bittel D, Talebizadeh Z. Prader-Willi syndrome and a deletion/duplication within the 15q11-q13 region. J Med Genet 2002: 39 (3): 202–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM et al. CDC growth charts. United States Adv Data 2000: 8 (314): 1–27. [PubMed] [Google Scholar]
- 25.Thomas EL, Brynes AE, McCarthy J et al. Preferential loss of visceral fat following aerobic exercise, measured by magnetic resonance imaging. Lipids 2000: 35 (7): 769–776. [DOI] [PubMed] [Google Scholar]
- 26.Goldstone AP, Thomas EL, Brynes AE et al. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader-Willi syndrome female adults: evidence for novel influences on body fat distribution. J Clin Endocrinol Metab 2001: 86 (9): 4330–4338. [DOI] [PubMed] [Google Scholar]
- 27.Despres JP. Waist circumference as a clinical assessment of visceral obesity, a risk factor for type 2 diabetes and cardiovascular disease. Can J Diabetes 1998: 22 (2): 32–37. [Google Scholar]
- 28.Morgan CR, Lazarow A. Immuno assay of insulin: two antibody system. Diabetes 1963: 12: 115–126. [Google Scholar]
- 29.Carlson MG, Snead WL, Oeser AM, Butler MG. Plasma leptin concentrations in lean and obese human subjects and Prader-Willi syndrome: comparison of RIA and ELISA methods. J Lab Clin Med 1999: 133 (1): 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985: 28 (7): 412–419. [DOI] [PubMed] [Google Scholar]
- 31.Katz A, Nambi SS, Mather K et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000: 85 (7): 2402–2410. [DOI] [PubMed] [Google Scholar]
- 32.SPSS Inc. SPSS Statistical Software System, Version 10.1. IL: Chicago, 2000. [Google Scholar]
- 33.Brambilla P, Bosio L, Manzoni P, Pietrobelli A, Beccaria L, Chiumello G. Peculiar body composition in patients with Prader-Labhart-Willi syndrome. Am J Clin Nutr 1997: 65 (5): 1369–1374. [DOI] [PubMed] [Google Scholar]
- 34.Schuster DP, Osei K, Zipf WB. Characterization of alterations in glucose and insulin metabolism in Prader-Willi subjects. Metabolism 1996: 45 (12): 1514–1520. [DOI] [PubMed] [Google Scholar]