Abstract
The present study investigated whether the Wnt/β-catenin pathway was involved in the protective effect of calcitonin (CT) in interleukin-1β (IL-1β)-injured rat chondrocytes. Chondrocytes were acquired from the articular cartilage of 4-week-old rats and treated with 10 ng/ml IL-1β to stimulate an in vitro osteoarthritis model. CT (10 and 50 nM) and 5 µm IWR-1-endo (a Wnt/β-catenin inhibitor) was used for treatment. The proliferation and apoptosis of rat articular chondrocytes were measured using a cell counting kit-8 assay and Annexin V/PI staining, respectively. Expression of matrix-metalloproteinases (MMP)-13 MMP3 and MMP9 and aggrecanases [metalloproteinase thrombospondin motifs (ADAMTS4 and ADAMTS5)] were measured to assess the degradation of the cartilage extracellular matrix. The results of the present study demonstrate that CT protected rat chondrocytes from IL-1β stimulation by enhancing cell viability, suppressing apoptosis and decreasing the expression of matrix metallopeptidase (MMP) MMP13, MMP3, MMP9, ADAMTS4 and ADAMTS5. CT treatment also upregulated dickkopf-1 and downregulated β-catenin. IWR-1-endo demonstrated similar effects to that of CT treatment. The administration of CT in addition to IWR-1-endo reinforced the change trends induced by CT or IWR-1-endo in the aforementioned events, indicating that CT possibly acted via the Wnt/β-catenin pathway to exert a protective effect on IL-1β-injured rat chondrocytes.
Keywords: osteoarthritis, rat chondrocytes, interleukin-1β, calcitonin, Wnt/β-catenin pathway
Introduction
Osteoarthritis (OA) is a degenerative joint disease, pathologically characterized by the progressive loss of cartilage, osteophyte formation, subchondral sclerosis, matrix degradation and matrix synthesis imbalance (1–3). Symptoms of OA include inflammation, stiffness and loss of mobility (1). Currently, OA treatment is challenging due to its multiple etiologies and complex pathological processes (2). New molecular targets are therefore urgently required to prevent this disease.
Calcitonin (CT), a naturally occurring peptide that provides benefits to articular cartilage and subchondral bone (3). CT improves cell viability and modulates inflammatory reactions in IL-1β- or lipopolysaccharide (LPS)-injured rat chondrocytes (4,5). Although CT is an ideal treatment for OA progression, the specific mechanism by which CT exerts its effects is yet to be fully elucidated.
Wnt/β-catenin signaling is critical for OA development and the expression of β-catenin is necessary for the maintenance of cartilage homeostasis (6). Furthermore, previous studies indicate that the Wnt/β-catenin pathway controls chondrocyte proliferation and is fundamental for differentiating stem cells into osteoblasts rather than chondrocytes (7). Following Wnt/β-catenin pathway activation, β-catenin is upregulated in the cytoplasm and transfers to the nucleus, where it binds to T-cell factor/lymphoid enhancer factor (8). This activates the transcription of target genes associated with cell proliferation, apoptosis, inflammation and matrix metabolism; including metallopeptidase (MMP) 13 and metalloproteinase with thrombospondin motifs 4 (ADAMTS4) (8). A report has demonstrated that CT inhibits β-catenin in interleukin (IL)-1β-injured rabbit chondrocytes (9). In the present study, an n in vitro OA model was established by isolating rat chondrocytes and injuring these with IL-1β. Following, the current study investigated whether the Wnt/β-catenin pathway was involved in the protective effect of CT treatment in OA and the mechanisms underlying this effect.
Materials and methods
Cell isolation
Male Sprague-Dawley (SD) rats (n=10; weight, 50±1.4 g; 4-week-old) were purchased from Shanghai SIPPR-Bk Lab Animal Co., Ltd. (Shanghai, China) with the certificate number 2008001682174. The animal license was provided by Shanghai Laboratory Animal Center (Shanghai, China) with the license number SCXK 2013–0016. Rats were housed at 25°C with a 12:12 h light/dark for a week, and given healthy atmosphere air, food and water ad libitum. Rats were sacrificed by cervical dislocation and cartilage tissue was harvested from knee joints, which was immersed in PBS, cut into slices (2–4 mm thick) and placed in 5 ml centrifuge tubes. The use and treatment of SD rats was approved by the Ethics Committee of the School of Medicine, Shandong University (Jinan, China). Chondrocytes were obtained by enzyme digestion using collagenase II and were then maintained in high glucose DMEM with 15% FBS (v/v; both GE Healthcare Life Sciences) at 37°C under 5% CO2 until a confluence of 50–60% was reached. Immunohistochemical staining (IHC) was subsequently performed for identification
Cell culture
Rat chondrocytes cultured at 37°C under 5% CO2 in DMEM containing 10% FBS, 0.1 mg/ml streptomycin and 100 U/ml penicillin (both P1400-100; Beijing Solarbio Science & Technology Co., Ltd.) until achieving the logarithmic growth phase. Cell morphology was observed by light microscopy (magnification, ×200).
Cell treatment
To establish IL-1β-injured rat chondrocytes, cells were exposed to various concentrations of 100 µl IL-1β (BioVision, Inc.; 0.5, 1, 2.5, 5, 10 and 20 ng/ml). To investigate the effect of CT (Signalway Antibody LLC, College Park, MD, USA) on IL-1β-injured rat chondrocytes, cells were exposed to 10 ng/ml IL-1β and treated with CT (10 and 50 nM). To assess the involvement of the Wnt/β-catenin pathway, cells were exposed to 10 ng/ml IL-1β and then treated with 5 µM of the β-catenin inhibitor, IWR-1-endo, (Selleck Chemicals) or CT (50 nM) with IWR-1-endo (5 µM). After treatment, cells were cultured as described above.
IHC
Primary cultured rat chondrocytes on cover slides were washed with 0.1 M PBS and then fixed with 4% paraformaldehyde (Sinopharm Chemical Reagent Co., Ltd) at 25°C for 30 min. Slides were treated with H2O2 (Sinopharm Chemical Reagent Co., Ltd.; 3% in methanol) to eliminate the activity of endogenous peroxide and then blocked with 1% BSA (Beijing Solarbio Science & Technology Co., Ltd.) at room temperature for 30 min. Slides were then incubated with rabbit polyclonal anti-collagen II (cat. no. ab34712) and anti-transcription factor SOX9 (cat. no. ab185966; both dilution 1:5,000; Abcam) antibodies at 25°C overnight at 4°C, followed by incubation with horseradish peroxidase (HRP)-labeled secondary antibodies (cat. no. D-3004; dilution 1:500; Shanghai Long Island Biotech Co., Ltd.) at room temperature for 1 h. A DAB-containing substrate kit (Shanghai Long Island Biotech. Co., Ltd.) was used to visualize collagen II and SOX9 signals, followed by hematoxylin nuclear counterstaining at 25°C for 3 min.
Cell proliferation
A cell counting kit-8 (CCK-8) was used to measure cell proliferation. Cells were seeded into 96-well plates (100 µl; 3.0×103 cells/well) and cultured overnight at room temperature prior to treatment. After treatment, cells were cultured as described in a previous subsection. At 0, 24, 48 and 72 h, cultured cells (suspended in 90 µl of the medium in the culture) were treated with CCK-8 solution (10 µl) for an additional 1 h. Optical density (450 nm) was recorded to assess cell proliferation. In the current study, proliferation of rat chondrocytes treated with IL-1β (0.5, 1, 2.5, 5, 10 and 20 ng/ml) was assessed at 0, 24, 48 and 72 h, and the viability of rat chondrocytes treated with IL-1β (10 ng/ml), IL-1β (10 ng/ml) + CT (10 and 50 nM), IL-1β (10 ng/ml) + IWR-1-endo and IL-1β (10 ng/ml) + CT (50 nM) + IWR-1-endo was assessed at 24 h.
Annexin V/PI staining
A FITC-labeled recombinant Annexin V Apoptosis Detection kit (Beyotime Institute of Biotechnology) was used to measure apoptosis. Cells were seeded in six-well plates (5.0×104 cells/well) and cultured at 25°C overnight prior to treatment. After treatment of IL-1β, IL-1β + CT, IL-1β + IWR-1- endo and IL-1β + CT + IWR-1-endo, for 48 h, cells were centrifuged at 1,000 × g for 5 min at 4°C. Supernatants were subsequently removed. Cells were incubated with 5 µl Annexin V-FITC at 4°C for 15 min in darkness, followed by 5 µl propidium iodide (PI) for a further 5 min. The rate of early apoptosis was detected using Accuri C6 FACScan flow cytometer with CFlow Plus® version 1.0 software (BD Bioscience). Early apoptotic cells were counted in the lower right quadrant of the display.
Reverse transcription-quantitative (RT)-qPCR
Total RNA from rat chondrocytes was isolated using TRIzol regent (cat. no. 1596-026; Invitrogen; Thermo Fisher Scientific, Inc.). First-strand cDNA was obtained using a Reverse Transcription kit (cat. no. K1622; Fermentas; Thermo Fisher Scientific, Inc.). A SYBR Green PCR kit (cat. no. K0223; Thermo Fisher Scientific, Inc.) and ABI Prism 7300 SDS Software (Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to perform qPCR by the 2−ΔΔCq method (10) with the following thermocycling conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 45 sec; 95°C for 15 sec; 60°C for 1 min; 95°C for 15 sec and 60°C for 15 sec. mRNA expression was normalized to that of GAPDH. The primer sequences used in RT-qPCR are listed in Table I.
Table I.
Primers used in RT-qPCR analysis.
| Name | GenBank | Primer (5′-3′) |
|---|---|---|
| DKK-1 | NM_001106350.1 at 164–389 position | Forward: CGGTTCTTGGTCGTGCTTTC; |
| Reverse: AAGGGTAGGGCTGGTAGTTG; 226 bps, 61% GC. | ||
| β-catenin | NM_053357.2 at 227–375 position | Forward: TCACGCAAGAGCAAGTAG; |
| Reverse: CTGGACATTAGTGGGATGAG; 149 bps, 55% GC. | ||
| MMP13 | NM_133530.1 at 2348–2572 position | Forward: CAGACAGCAAGAATAAAGAC; |
| Reverse: CAACATAAGCACAGTGTAAC; 225 bps; 41% GC. | ||
| ADAMTS4 | NM_023959.1at 714 - 955 position | Forward: GGTGGCAGATGACAAGATG; |
| Reverse: AGTCGTTCGGAGGGTTTAG; 242 bps; 59% GC. | ||
| GAPDH | NM_017008.4 at 357–593 position | Forward: GGAGTCTACTGGCGTCTTCAC; |
| Reverse: ATGAGCCCTTCCACGATGC; 237 bps; 55% GC. |
DKK-1, anti-dickkopf-1; MMP13, matrix metallopeptidase 13; ADAMTS4, ADAM metallopeptidase with thrombospondin type 1 motif 4.
Western blot analysis
Total protein from rat chondrocytes was obtained using RIPA lysis buffer (cat. no. R0010; Beijing Solarbio Science & Technology Co., Ltd.) and supernatant levels were measured using a BCA assay kit (cat. no. PICPI23223; Thermo Fisher Scientific, Inc.). Total protein (30 mg) was separated using 10% SDS-PAGE gel, followed by transfer to PVDF membranes. After blocking with 5% non-fat milk at 4°C overnight, PVDF membranes were subsequently incubated with the following primary antibodies at 4°C overnight: Anti-dickkopf-1 (Dkk-1; cat. no. ab109416; 1:1,000; Abcam), anti-β-catenin (cat. no. ab32572; 1:1,500; Abcam), anti-MMP13 (cat. no. ab39012; 1:3,000; Abcam), anti-ADAMTS4 (cat. no. ab185722; 1:500; Abcam), anti-Bax (cat. no. 5023; 1:1,000; Cell Signaling Technology Inc.), anti-Bcl-2 (cat. no. 15071; 1:1,000; Cell Signaling Technology, Inc.), anti-cleaved-caspase 3 (cat. no. 9664; 1:1,000; Cell Signaling Technology, Inc.), anti-MMP-3 (cat. no. ab53015; 1:1,000; Abcam), anti-MMP-9 (cat. no. ab38898; 1:500; Abcam), anti-ADAMTS5 (cat. no. ab41037; 1:1,000; Abcam) and anti-GAPDH (cat. no. 5174; 1:2,000; Cell Signaling Technology, Inc.). Samples were then incubated with HRP-conjugated secondary antibodies: Goat anti-rabbit (cat. no. A0208); donkey anti-goat (cat. no. A0181); and goat anti-mouse immunoglobulin G (cat. no. A0216; all dilution 1:1,000; Beyotime Institute of Biotechnology) for 1 h at room temperature. The HRP signal was assessed using ECL Plus (GE Healthcare) and a Tanon-5200 Imaging system (Tanon Science & Technology Co., Ltd.). ImageJ software version 1.8.0 (National Institutes of Health, Bethesda, MD, USA) was used for densitometry analysis.
Cleaved caspase 3 activity assay
Cleaved caspase 3 activity in cell supernatants was detected using a Caspase-3 Colorimetric Assay kit (cat. no. KGA203; Nanjing KeyGen Biotech Co., Ltd.) according to the manufacturer's protocol.
Statistical analyses
Graphs were generated using GraphPad Prism 7.0 software (GraphPad Software, Inc.). Data were expressed as the mean ± standard error of the mean (n=3). A student's t-test was performed for comparisons between two groups, while a post-hoc Tukey's test following ANOVA was used for comparisons between multiple groups. P<0.05 was considered to indicate a statistically significant result.
Results
Successful isolation of rat chondrocytes
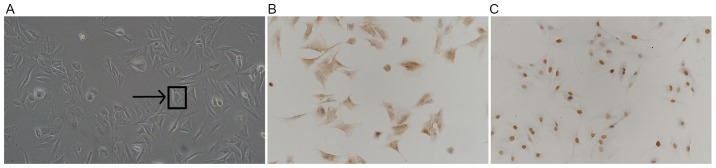
Under light microscopy, primary isolated rat chondrocytes were polygonal, spindle or irregularly shaped with copious cytoplasm and a large and round nuclei. (Fig. 1A). After IHC staining for type II collagens and transcription factor SOX9, brown-yellow granules were observed in the cytoplasm and nucleus, the color of which stained darker with increased cell density (Fig. 1B and C). Data collected from the current study indicates the successful isolation of rat chondrocytes.
Figure 1.
Successfully isolated rat chondrocytes. (A) Cell morphology observed using light microscopy (magnification, ×200). IHC staining for (B) collagen II-positive cells and (C) SOX9-positive cells (each magnification, ×200). IHC, immunohistochemistry; SOX9, transcription factor SOX9.
IL-1β inhibited the proliferation of rat chondrocytes
To assess the role of IL-1β on the proliferation of rat chondrocytes, cells were treated with IL-1β at 0.5, 1, 2.5, 5, 10 and 20 ng/ml and cell proliferation was measured using a CCK-8 assay at 0, 24, 48 and 72 h. As presented in Fig. 2, IL-1β significantly suppressed chondrocyte viability in a dose-and time-dependent manner, indicating the inhibitory effect of IL-1β on the proliferation of rat chondrocytes. In the present study, 10 ng/ml IL-1β had a relatively obvious anti-proliferation effect on chondrocytes and did not cause >50% inhibition on cell viability, thus this concentration was selected for the following study.
Figure 2.
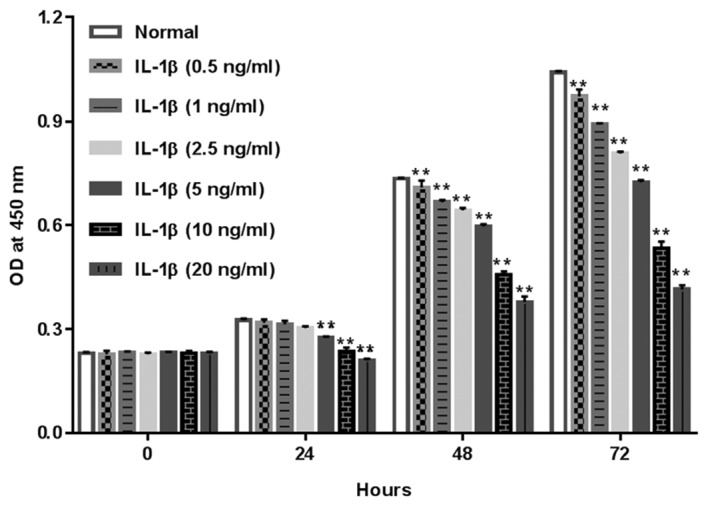
Role of IL-1β in the proliferation of rat chondrocytes. Rat chondrocytes were exposed to IL-1β (0.5, 1, 2.5, 5, 10 and 20 ng/ml), following which proliferation was assessed using a cell counting kit-8 assay at the indicated times. **P<0.01 vs. Normal group. IL-1β, interleukin-1β; OD, optical density.
CT promotes and inhibits the viability and apoptosis of IL-1β-injured rat chondrocytes, respectively, via the Wnt/β-catenin pathway
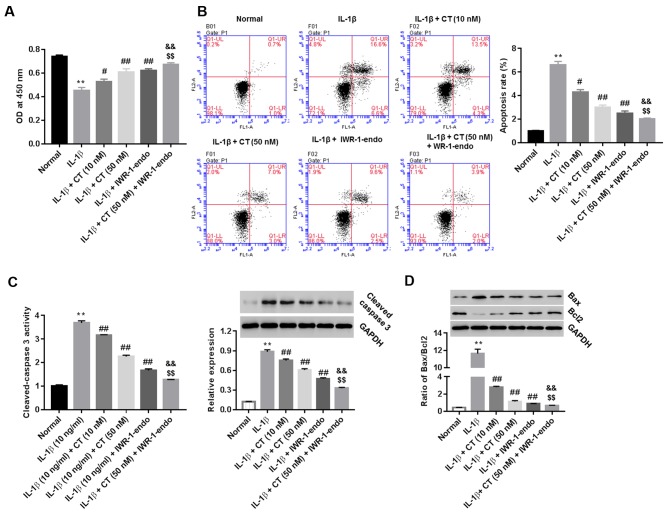
IL-1β-injured rat chondrocytes were treated with CT (10 and 50 nM), IWR-1-endo, or CT (50 nM) in addition to IWR-1-endo. Cell viability (at 24 h) and cell apoptosis (at 48 h) was measured using the CCK-8 assay and Annexin V/PI staining method, respectively. Furthermore, the activity of cleaved-caspase 3 in cell supernatants, as well as the protein expression of cleaved-caspase 3 and the ratio of pro-apoptotic Bax/anti-apoptotic Bcl2 within cells were also measured. As indicated in Fig. 3A and B, CT treatment enhanced cell viability and inhibited cell apoptosis compared with the IL-1β group. CT also inhibited the activity and expression of cleaved-caspase 3 (Fig. 3C) as well as the ratio of Bax/Bcl2 in a dose-dependent manner (Fig. 3D), with the most significant effect being observed at 50 nM CT when compared with the IL-1β group. However, pro-viability and anti-apoptotic effects were augmented in the CT (50 nM) plus IWR-1-endo group compared treated with CT (50 nM) or IWR-1-endo alone.
Figure 3.
CT acts on the Wnt/β-catenin pathway to regulate proliferation and apoptosis of IL-1β-injured rat chondrocytes. IL-1β (10 ng/ml)-injured cells were treated with CT (10 and 50 nM), IWR-1-endo (5 µM), or CT (50 nM) + IWR-1-endo (5 µM). (A) Cell proliferation at 24 h and (B) apoptosis at 48 h were assessed using a cell counting kit-8 assay and Annexin V/PI staining, respectively. (C) After 48 h of treatment, the activity of cleaved-caspase 3 in cell supernatant was detected using a Caspase-3 Colorimetric Assay kit and protein levels of cleaved-caspase 3 within cells was detected by western blot analysis. (D) Ratio of Bax/Bcl2 was assessed via western blot analysis. GAPDH was used as a loading control. **P<0.01 vs. Normal group; #P<0.05 and ##P<0.01 vs. IL-1β group; &&P<0.01 vs. IL-1β + CT (50 nM); $$P<0.01 vs. IL-1β + IWR-1-endo. CT, calcitonin; IL-1β, interleukin-1β.
CT inhibits cartilage extracellular matrix degradation via the Wnt/β-catenin pathway in IL-1β-injured rat chondrocytes
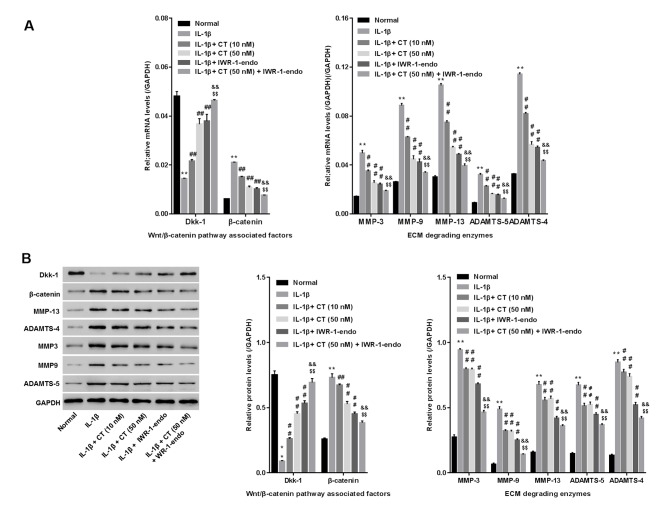
IL-1β-injured rat chondrocytes were treated with CT (10 and 50 nM), IWR-1-endo (a Wnt/β-catenin pathway inhibitor), or CT (50 nM) in addition to IWR-1-endo. The mRNA and protein levels of the cartilage extracellular matrix (ECM) degrading enzymes (MMP13, MMP3, MMP9, ADAMTS4 and ADAMTS5), Dkk-1 (a specific inhibitory protein for blocking Wnt/β-catenin) (11) and β-catenin (the core component of Wnt/β-catenin pathway) were then measured using RT-qPCR and western blot analysis, respectively. As presented in Fig. 4A and B, a significant increase in the mRNA and protein levels of MMP13, MMP3, MMP9, ADAMTS4 and ADAMTS5 and β-catenin, and decrease in Dkk-1 was observed in the IL-1β-treated group when compared with the normal group, indicating the severe degradation of ECM and the activation of the Wnt/β-catenin pathway in IL-1β-injured rat chondrocytes. However, in comparison with the IL-1β group, CT treatment reduced MMP13, MMP3, MMP9, ADAMTS4, ADAMTS5 and β-catenin while enhancing Dkk-1 in a dose-dependent manner. These results indicated that CT inhibited cartilage ECM degradation and during which, may have been associated with the inactivation of Wnt/β-catenin. Furthermore, similar to CT, IWR-1-endo consistently suppressed MMP13, MMP3, MMP9, ADAMTS4, ADAMTS5 and β-catenin while promoting Dkk-1, and produced an even greater effect than CT treatment. Yet, IWR-1-endo plus CT (50 nM) was more effective than IWR-1-endo treatment alone in inhibiting cartilage ECM degradation. However, CT treatment inhibited β-catenin but promoted Dkk-1. The results indicate that CT inhibits cartilage ECM degradation by blocking the activation of Wnt/β-catenin.
Figure 4.
Effect of CT on the expression of matrix degrading enzymes in IL-1β-injured rat chondrocytes. (A) mRNA and (B) protein levels of MMP-13, MMP-3, MMP-9, ADAMTS4 and ADAMTS5 were assessed using reverse transcription-quantitative PCR and western blot analysis. **P<0.01 vs. Normal group; ##P<0.01 vs. IL-1β group; &&P<0.01 vs. IL-1β + CT (50 nM); $$P<0.01 vs. IL-1β + IWR-1-endo. CT, calcitonin; IL-1β, interleukin-1β; MMP, matrix metallopeptidase; ADAMTS, ADAM metallopeptidase with thrombospondin type 1; Dkk-1, anti-dickkopf-1.
Discussion
IL-1β is a major contributor to OA pathology (12). To establish an in vitro model of IL-1β induced OA, well-characterized rat chondrocytes were isolated and stained using IHC for a set of marker proteins including collagen II and SOX9 (13). A previous report has indicated that proliferation and apoptosis are significantly inhibited in IL-1β (10 ng/ml)-induced OA chondrocytes (14). In the present study, IL-1β (0.5, 1, 2.5, 5, 10 and 20 ng/ml) was used to injure rat chondrocytes to assess its effect on cell proliferation. Relatively strong responses were observed with 10 ng/ml and 20 ng/ml IL-1β. As 20 ng/ml IL-1β exposure resulted in >50% inhibition of cell viability, IL-1β at 10 ng/ml was selected for the current study.
CT serves an important role in improving chondrocyte viability. Hypodermic injections of CT (5 U/kg) in OA rabbits have been demonstrated to substantially reduce apoptotic articular chondrocytes (15). Furthermore, in an in vitro OA model, CT exerted pro-proliferative and anti-apoptotic effects on LPS-injured chondrocytes (4). Moreover, CT (10−10−10−8 M) exerted anti-apoptotic effects in IL-1β-injured chondrocytes, (substantiated by a TUNEL assay) and reduced the ratios of Bax/Bcl2 and cleaved caspase-3 activity (16). In the present study, the data revealed the proliferative and anti-apoptotic effects of CT on IL-1β-injured rat chondrocytes. The downregulation of cleaved caspase-3 in supernatants and cells, and the decrease of Bax/BCL2 ratios, were also revealed to be involved in the process.
MMPs and ADAMTSs serve a key role in the degradation of ECM components, contributing to the destruction of articular cartilage (17). CT treatment has been previously demonstrated to inhibit MMP-13, MMP-3 and ADAMTS4 in a rat OA model (18,19), which was further confirmed in IL-1β-injured chondrocytes. To the best of our knowledge, the current study is the first to substantiate the inhibitory effect of CT on MMP9 and ADAMTS5 in an IL-1β-stimulated in vitro OA model. A previous study has revealed that growth differentiation factor 5 reduces MMP-13 expression in human chondrocytes via Wnt/β-catenin signaling, contributing to the homeostasis of cartilage ECM (20). However, whether Wnt/β-catenin signaling is involved in regulating the expression of these matrix-degrading enzymes in IL-1β-stimulated rat chondrocytes remains unclear.
It has been determined that CT downregulates β-catenin while upregulating secreted frizzled related protein 1 (a Wnt antagonist) in rat chondrocytes of the knee joint (21). To assess whether the Wnt/β-catenin pathway was the mechanism whereby CT functioned in OA in vitro, Wnt/β-catenin signaling was stimulated with IL-1β and blocked by IWR-1-endo. The proliferation, apoptosis and ECM degradation of rat chondrocytes was subsequently assessed after treatment with CT, IWR-1-endo or CT in combination with IWR-1-endo. The results demonstrated that CT counteracted IL-1β, as evidenced by the promotion of cell proliferation, the inhibition of apoptosis, the reduction in cleaved caspase-3 and Bax/Bcl2 ratios, the upregulation of Dkk-1, the downregulation of β-catenin and the suppression of matrix-degrading enzyme expression (MMP3, MMP9, MMP13, ADAMTS4 and ADAMTS5). This indicated that CT exerted a protective effect on IL-1β-injured rat chondrocytes, which was associated with the blockade of the Wnt/β-catenin pathway. The changes induced by IWR-1-endo in the aforementioned results were similar to those of CT (50 nM). However, changes were strengthened when these treatments were combined. Given the inhibitory effect of CT on β-catenin and the promoted effect of CT on Dkk-1, the data demonstrated that CT may protect rat chondrocytes against IL-1β injured via the Wnt/β-catenin pathway.
The results of the current study indicate that the Wnt/β-catenin pathway may be the mechanism by which CT exerts chondroprotective properties in rat chondrocytes injured by IL-1β. However, further investigation into human chondrocytes or rat OA models is required to confirm that CT acts via the Wnt/β-catenin pathway to improve OA pathological conditions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QHJ conceived and designed the study. MXB, LG and HC performed the experiments. MXB and QHJ wrote the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
The experimental protocols were approved by approved by the Ethics Committee of the School of Medicine, Shandong University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yimam M, Lee YC, Wright L, Jiao P, Horm T, Hong M, Brownell L, Jia Q. A botanical composition mitigates cartilage degradations and pain sensitivity in osteoarthritis disease model. J Med Food. 2017;20:568–576. doi: 10.1089/jmf.2016.0167. [DOI] [PubMed] [Google Scholar]
- 2.Ickinger C, Tikly M. Current approach to diagnosis and management of osteoarthritis. SA Fam Pract. 2010;52:382–390. [Google Scholar]
- 3.Karsdal MA, Sondergaard BC, Arnold M, Christiansen C. Calcitonin affects both bone and cartilage: A dual action treatment for osteoarthritis? Ann N Y Acad Sci. 2007;1117:181–195. doi: 10.1196/annals.1402.041. [DOI] [PubMed] [Google Scholar]
- 4.Zhang LB, Man ZT, Li W, Zhang W, Wang XQ, Sun S. Calcitonin protects chondrocytes from lipopolysaccharide-induced apoptosis and inflammatory response through MAPK/Wnt/NF-κB pathways. Mol Immunol. 2017;87:249–257. doi: 10.1016/j.molimm.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Yan XL, Wei LJ, Wang WY, Zhang L. Effect of calcitonin on the inflammatory reaction in IL-1β-induced chondrocytes in rats. Jie Fang Jun Yi Xue Za Zhi. 2015;24:47–51. (In Chinese) [Google Scholar]
- 6.Zhou Y, Wang T, Hamilton JL, Chen D. Wnt/β-catenin signaling in osteoarthritis and in other forms of arthritis. Curr Rheumatol Rep. 2017;19:53. doi: 10.1007/s11926-017-0679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossini M, Gatti D, Adami S. Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013;93:121–132. doi: 10.1007/s00223-013-9749-z. [DOI] [PubMed] [Google Scholar]
- 8.Liu HY, Zheng Y. A study on Wnt/β-catenin signal pathway in rabbit models with osteoarthritis. Feng Shi Bing Yu Guan Jie Yan. 2015;4:5–8. (In Chinese) [Google Scholar]
- 9.Zhang YN, Wang WY, Zhang L, Hu HY, Li B, Wang ZY. Effect of calcitonin on beta-catenin and secreted frizzled-related protein 1 in articular cartilages. Zhong Guo Zu Zhi Gong Cheng Yan Jiu Lin Chuang Kang Fu. 2010;14:7601–7604. (In Chinese) [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Alcaraz MJ, Megías J, García-Arnandis I, Clérigues V, Guillén MI. New molecular targets for the treatment of osteoarthritis. Biochem Pharmacol. 2010;80:13–21. doi: 10.1016/j.bcp.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 13.Jacques C, Recklies AD, Levy A, Berenbaum F. HC-gp39 contributes to chondrocyte differentiation by inducing SOX9 and type II collagen expressions. Osteoarthritis Cartilage. 2007;15:138–146. doi: 10.1016/j.joca.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Zou J, Li XL, Shi ZM, Xue JF. Effects of C-myc gene silencing on interleukin-1β-induced rat chondrocyte cell proliferation, apoptosis and cytokine expression. J Bone Miner Metab. 2018;36:286–296. doi: 10.1007/s00774-017-0845-4. [DOI] [PubMed] [Google Scholar]
- 15.You X. The experimental research of the effects of calcitonin on cytokines and apoptosis of articular chondrocytes during the development of knee osteoarthritis in rabbits. Chin J Osteoporosis. 2012;18:223–228. (In Chinese) [Google Scholar]
- 16.Greco KV, Nalesso G, Kaneva MK, Sherwood J, Iqbal AJ, Moradi-Bidhendi N, Dell'Accio F, Perretti M. Analyses on the mechanisms that underlie the chondroprotective properties of calcitonin. Biochem Pharmacol. 2014;91:348–358. doi: 10.1016/j.bcp.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 17.Sumer EU, Qvist P, Tankó LB. Matrix metalloproteinase and aggrecanase generated aggrecan fragments: Implications for the diagnostics and therapeutics of destructive joint diseases. Drug Dev Res. 2007;68:1–13. doi: 10.1002/ddr.20166. [DOI] [Google Scholar]
- 18.Cheng T, Zhang L, Fu X, Wang W, Xu H, Song H, Zhang Y. The potential protective effects of calcitonin involved in coordinating chondrocyte response, extracellular matrix, and subchondral trabecular bone in experimental osteoarthritis. Connect Tissue Res. 2013;54:139–146. doi: 10.3109/03008207.2012.760549. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Xie ZG, Xie Y, Dong QR. Calcitonin treatment is associated with less severe osteoarthritis and reduced toll-like receptor levels in a rat model. J Orthop Sci. 2014;19:1019–1027. doi: 10.1007/s00776-014-0629-9. [DOI] [PubMed] [Google Scholar]
- 20.Enochson L, Stenberg J, Brittberg M, Lindahl A. GDF5 reduces MMP13 expression in human chondrocytes via DKK1 mediated canonical Wnt signaling inhibition. Osteoarthritis Cartilage. 2014;22:566–577. doi: 10.1016/j.joca.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YN. Effect of calcitonin on articular cartilage chondrocytes of osteoarthritis. Journal. 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.