Abstract
We tested whether a single nucleotide polymorphism (SNP) that affects splicing of CD33 predicted response to treatment in adults with acute myeloid leukemia (AML) who received the novel CD33 antibody-drug conjugate SGN-CD33A. This genotype, for the CD33 splice site SNP rs12459419, was not associated with clinical response (30% CR/CRi in both groups), event-free survival, or overall survival.
Keywords: Acute myeloid leukemia, CD33, Antibody-drug conjugate
Main
Antibody-drug conjugates (ADCs) are among the most promising immunotherapies developed in the last few decades for patients with acute myeloid leukemia (AML) [1]. The CD33 antigen (SIGLEC-3) is highly expressed on AML blasts and has been a popular target for immunoconjugate drugs, as well as unconjugated antibodies and radioimmunotherapeutics [2]. Gemtuzumab ozogamicin (GO), a humanized anti-CD33 monoclonal antibody conjugated to the cytotoxic agent calicheamicin, first demonstrated the potential efficacy of targeting CD33; it was most effective in patients with favorable-risk cytogenetics [3, 4] and higher expression of CD33 [5–8]. In 2017, Lamba and colleagues found an association in pediatric AML patients between response to GO plus chemotherapy and genotype at a single nucleotide polymorphism (SNP) in CD33 [9]. This SNP (rs12459419) occurs at a splice site of the CD33 gene and affects the expression of the extracellular epitope recognized by GO. Variation from the common C allele to the rare T allele abrogates the splice site for inclusion of exon 2, which codes for the IgV domain of CD33; without the C allele, the exon is skipped during transcription. Thus, there is a plausible biological mechanism for altered response to GO. However, when Gale and colleagues performed a similar analysis in adult patients with AML treated with GO plus chemotherapy, they did not find an association between the SNP and outcomes [9, 10]. Furthermore, there is no data for CD33-targeted agents beyond GO. We aimed to assess the association between this SNP and the efficacy of CD33-targeting in a cohort of adults (age ≥ 18 years) with AML. Our patients were treated with an alternative ADC directed against CD33, SGN-CD33A, a monoclonal anti-CD33 antibody conjugated to a pyrrolobenzodiazepine (PBD) dimer.
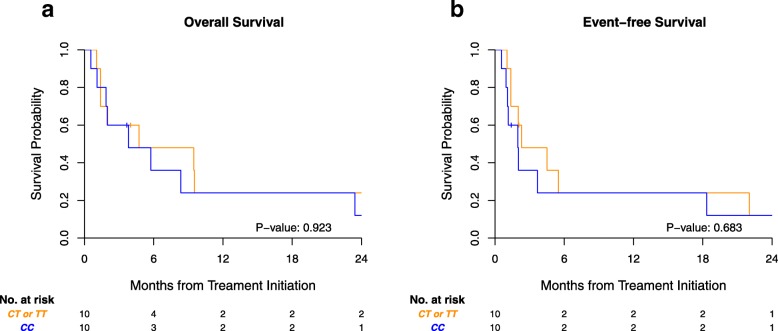
Twenty patients with CD33+ AML who received SGN-CD33A either as monotherapy (10–50 mcg/kg) or in combination with hypomethylating agents (10 mcg/kg SGN-CD33A and standard doses of hypomethylating agent) were tested for the CD33 SNP genotype (rs12459419) using TaqMan SNP genotyping (Applied Biosystems, CA). Clinical characteristics of disease, prior treatments, and outcome data were collected and analyzed for association of the SNP genotype with response rate, the primary objective. Event-free and overall survivals were secondary objectives assessed by the Kaplan-Meier estimator. We included adults with de novo and secondary AML who had either experienced disease relapse or declined intensive chemotherapy. Much as would be expected at the population level, we saw a 50%/40%/10% distribution of genotypes CC, CT, and TT, respectively. The CT and TT genotypes were combined in our analysis because of the low numbers of TT genotype (n = 2) and the previously reported decreased response to anti-CD33 ADCs in patients carrying even one T risk allele [9]. Baseline characteristics by genotype are shown in Table 1. There was no significant difference in response between patients carrying the most common genotype, CC (n = 10), and those carrying CT or TT genotypes (n = 10): both groups had 30% complete response (CR) or complete response with incomplete hematologic recovery (CRi). The genotype for the CD33 splice site SNP rs12459419 was also not associated with event-free survival or overall survival (Fig. 1).
Table 1.
Characteristics of patients treated with SGN-CD33A by genotype
| CC (n=10) | CT/TT (n=10) | Total (n=20) | p value | |
|---|---|---|---|---|
| Gender | 0.361 | |||
| Female | 5 (50.0%) | 7 (70.0%) | 12 (60.0%) | |
| Male | 5 (50.0%) | 3 (30.0%) | 8 (40.0%) | |
| Age | 0.418 | |||
| Mean (range) | 67.3 (27.5-80.0) | 72.3 (42.0 -82.6) | 69.8 (27.5-82.6) | |
| ECOG | 0.160 | |||
| 0 | 2 (20.0%) | 5 (50.0%) | 7 (35.0%) | |
| 1 | 8 (80.0%) | 5 (50.0%) | 13 (65.0%) | |
| Risk group | 0.148 | |||
| Adverse | 7 (70.0%) | 4 (40.0%) | 11 (55.0%) | |
| Favorable | 1 (10.0%) | 0 (0.0%) | 1 (5.0%) | |
| Intermed. | 2 (20.0%) | 6 (60.0%) | 8 (40.0%) | |
| BM blast % | 0.334 | |||
| Median (Q1, Q3) | 64.0 (43.2, 80.0) | 54.0 (24.0,66.0) | 56.0 (36.0,78.0) | |
| WBC | 0.200 | |||
| Mean (SD) | 13.6 (17.1) | 5.6 (8.1) | 9.6 (13.7) | |
| Median (Q1, Q3) | 5.3 (1.7, 16.5) | 1.7 (1.2, 3.8) | 2.5 (1.5, 16.4) | |
| Range | 0.8 - 51.7 | 0.4 - 23.1 | 0.4 - 51.7 | |
| IQR | 14.8 | 2.7 | 14.9 | |
| Platelets | 0.913 | |||
| Mean (SD) | 50.3 (41.2) | 52.4 (43.9) | 51.4 (41.5) | |
| Median (Q1, Q3) | 34.0 (22.0, 75.0) | 32.0 (21.8,90.8) | 34.0 (19.8,89.5) | |
| Range | 11.0 - 133.0 | 5.0 - 116.0 | 5.0 - 133.0 | |
| IQR | 53.0 | 69.0 | 69.8 | |
| De novo | 0.531 | |||
| Yes | 1 (10.0%) | 2 (20.0%) | 3 (15.0%) | |
| No | 9 (90.0%) | 8 (80.0%) | 17 (85.0%) | |
| Line of tx | 0.361 | |||
| 1st | 3 (30.0%) | 5 (50.0%) | 8 (40.0%) | |
| 2nd | 7 (70.0%) | 5 (50.0%) | 12 (60.0%) |
BM bone marrow, ECOG Eastern Cooperative Oncology Group, IQR interquartile range, tx treatment, WBC white blood cell
Fig. 1.
No difference in outcome according to CD33 splice site genotype for patients receiving SGN-CD33A. Kaplan-Meier survival estimates for a overall survival and b event-free survival
While limited by the small sample size of this study, our data show that genotype of the CD33 splice site SNP was not associated with outcomes of patients treated with an anti-CD33 drug conjugate, aligning with previously reported data for adult patients [10] and extending this finding to the novel ADC SGN-CD33A. The fragment variable (Fv) regions of SGN-CD33A and GO recognize the same epitope on CD33, so any effect of the CD33 splice site SNP is expected to be similar for both agents. While the drug payload conjugated to CD33 differs between GO and SGN-CD33A, both are very potent agents unlikely to produce significantly different efficacy. However, published studies of GO have included different patient populations and treatment combinations that may account for disparate results between pediatric and adult populations. Compared with patients in previous studies, our patients were generally older and were not treated in combination with chemotherapy. Notably, CD33 SNPs are germline mutations, so these could result in different expression of CD33 in off-target tissue. An alternative hypothesis, therefore, is that increased toxicity in the CC genotype would offset the drugs’ benefit; however, neither our study nor the other adult study showed any difference in response rates between genotypes. This lack of any benefit for the CC genotype in adult AML suggests that age-related or other biological differences between adult and pediatric AML may explain disparate results between these groups.
This study suggests that CD33 genotype is a poor biomarker for broad use in adults with AML to predict response to CD33-targeted ADCs. While these results are disappointing—because genotype is much more reliably measured, reported, and interpreted than CD33 expression by flow cytometry, another potential biomarker for anti-CD33 ADC response—larger studies may nonetheless show a benefit for genotype testing in specific patient subsets identified by age, disease risk, or mutational subtype.
Acknowledgements
Editorial support in the preparation of this paper was provided by Hannah Rice, ELS.
Abbreviations
- ADC
Antibody-drug conjugate
- AML
Acute myeloid leukemia
- CR
Complete response
- CRi
Complete response with incomplete hematologic recovery
- GO
Gemtuzumab ozogamicin
- SNP
Single nucleotide polymorphism
Authors’ contributions
MS, CF, ES, and JT collected the patient data and performed the genotyping. MS, AP, and SD analyzed the data. MS, ES, and JT wrote the manuscript. All authors read and approved the final manuscript.
Funding
J.T. is supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology, the American Association for Cancer Research, the American Society of Hematology (ASH), the Robert Wood Johnson Foundation, and the NIH/NCI (1K08CA230319-01). This work was also supported by P30 CA008748. These funding bodies had no role in the design or performance of the study nor in the writing or interpretation thereof.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This research was approved by the MSKCC Institutional Review Board.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rashidi A, Walter RB. Antigen-specific immunotherapy for acute myeloid leukemia: where are we now, and where do we go from here? Expert Rev Hematol. 2016;9(4):335–350. doi: 10.1586/17474086.2016.1142868. [DOI] [PubMed] [Google Scholar]
- 2.Walter RB. Investigational CD33-targeted therapeutics for acute myeloid leukemia. Expert Opin Investig Drugs. 2018;27(4):339–348. doi: 10.1080/13543784.2018.1452911. [DOI] [PubMed] [Google Scholar]
- 3.Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loke J, Khan JN, Wilson JS, Craddock C, Wheatley K. Mylotarg has potent anti-leukaemic effect: a systematic review and meta-analysis of anti-CD33 antibody treatment in acute myeloid leukaemia. Ann Hematol. 2015;94(3):361–373. doi: 10.1007/s00277-014-2218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter RB, Gooley TA, van der Velden VH, Loken MR, van Dongen JJ, Flowers DA, et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007;109(10):4168–4170. doi: 10.1182/blood-2006-09-047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollard JA, Loken M, Gerbing RB, Raimondi SC, Hirsch BA, Aplenc R, et al. CD33 expression and its association with gemtuzumab ozogamicin response: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2016;34(7):747–755. doi: 10.1200/JCO.2015.62.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olombel G, Guerin E, Guy J, Perrot JY, Dumezy F, de Labarthe A, et al. The level of blast CD33 expression positively impacts the effect of gemtuzumab ozogamicin in patients with acute myeloid leukemia. Blood. 2016;127(17):2157–2160. doi: 10.1182/blood-2016-01-689976. [DOI] [PubMed] [Google Scholar]
- 8.Khan N, Hills RK, Virgo P, Couzens S, Clark N, Gilkes A, et al. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia. 2017;31(5):1059–1068. doi: 10.1038/leu.2016.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamba JK, Chauhan L, Shin M, Loken MR, Pollard JA, Wang YC, et al. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in de novo acute myeloid leukemia: report from randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2017;35(23):2674–2682. doi: 10.1200/JCO.2016.71.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale RE, Popa T, Wright M, Khan N, Freeman SD, Burnett AK, et al. No evidence that CD33 splicing SNP impacts the response to GO in younger adults with AML treated on UK MRC/NCRI trials. Blood. 2018;131(4):468–471. doi: 10.1182/blood-2017-08-802157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.