Abstract
Background
Although the incidence of tuberculosis (TB) has decreased in South Korea, the mortality rate remains high. TB mortality is a key indicator for TB control interventions. The purpose of this study was to assess early and TB-related mortality during anti-TB treatment and describe the associated clinical characteristics.
Methods
A multicenter cross-sectional study was performed across South Korea. Patients with pulmonary TB who died during anti-TB treatment and whose records were submitted to the national TB surveillance system between 2015 and 2017 were enrolled. All TB deaths were categorized based on cause (TB-related or non-TB-related) and timing (early or late). We identified statistical associations using the frequency table, chi-square test, and binary logistic regression.
Results
Of 5595 notifiable mortality cases, 3735 patients with pulmonary TB were included in the analysis. There were 2541 (68.0%) male patients, and 2935 (78.6%) mortality cases were observed in patients older than 65 years. There were 944 (25.3%) cases of TB-related death and 2545 (68.1%) cases of early death. Of all cases, 187 (5.0%) patients were diagnosed post-mortem and 38 (1.0%) patients died on the first day of treatment. Low body mass index (adjusted odds ratio (aOR) = 1.26; 95% confidence interval (CI) = 1.08–1.48), no reported illness (aOR = 1.36; 95% CI = 1.10–1.68), bilateral disease on chest X-ray (aOR = 1.30; 95% CI = 1.11–1.52), and positive acid-fast bacilli smear result (aOR = 1.30; 95% CI = 1.11–1.52) were significantly associated with early death, as well as TB-related death. Acute respiratory failure was the most common mode of non-TB-related death. Malignancy was associated with both late (aOR = 0.71; 95% CI = 0.59–0.89) and non-TB-related (aOR = 0.35; 95% CI = 0.26–0.46) death.
Conclusions
A high proportion of TB death was observed in elderly patients and attributed to non-TB-related causes. Many TB-related deaths occurred during the intensive phase, particularly within the first month. Further studies identifying risk factors for different causes of TB death at different phases of anti-TB treatment are warranted for early targeted intervention in order to reduce TB mortality.
Keywords: Private-public mix, PPM, Death, Elderly, Korea
Background
An estimated 1.3 million people died due to tuberculosis (TB) in 2017, making TB one of the leading causes of death due to an infectious agent worldwide [1]. The World Health Organization’s (WHO) End TB target is a 95% reduction in the number of deaths due to active TB between 2015 and 2035 [2]. In South Korea, the TB rate has decreased significantly, with a 5.2% annual reduction in the incidence of newly reported TB cases from 2011 to 2016; however, South Korea has the highest TB incidence and mortality rates among the high-income countries [3]. In 2016, the total number of reported cases was 39,245, with an incidence rate of 78.8 persons per 100,000; the mortality rate was 5.1 persons per 100,000 [4]. As South Korea becomes an older-aged society, TB mortality and incidence are rapidly increasing among those over 60 years old; this is a huge obstacle to national TB control [5].
A review [6] of the risk factors associated with death during anti-TB treatment, which include human immunodeficiency virus (HIV) positivity, old age, comorbidities, and use of alcohol and drugs, indicates that there are differences in risk factors among regions with low and high incidence of TB. Due to a low prevalence of HIV infection [7, 8] and an intermediate TB burden, South Korea requires a different strategy to control TB mortality. In addition, the causes of TB mortality may differ depending on the phase of anti-TB treatment; however, there are only few studies investigating early deaths, defined as death occurring within the first 2 months of anti-TB treatment [9–12]. TB mortality is a key indicator for the national TB control program in South Korea. Further studies using nationwide data are required to better understand TB mortality, hence leading to opportunities for public health intervention that can decrease TB mortality and improve treatment outcomes.
With the introduction of the nationwide health insurance system in 1989, TB control in South Korea began to transition from a public health center-based program to a private hospital-based program [13]. In 2011, a public–private mix (PPM) collaboration model was implemented as a national TB control strategy. We collected data of TB mortality cases at PPM-participating hospitals for monitoring and evaluation. The purpose of this study was to assess early and TB-related mortality during anti-TB treatment and describe the associated clinical characteristics.
Methods
Study population
We conducted a multicenter cross-sectional study of TB mortality during anti-TB treatment within the PPM collaboration model in South Korea. Patients with pulmonary TB who died during anti-TB treatment and whose data were entered into the Korean National TB Surveillance System (KNTSS) [14] at PPM hospitals across South Korea between 2015 and 2017 were enrolled. The government implemented a PPM collaboration model in 2011 [3]. Through PPM collaboration, comprehensive management of TB patients is provided by TB specialist nurses dispatched to private PPM hospitals; this management includes cases studies, administration of medication during the infectious period, management of side effects until completion of the treatment, and contact examination among family members. More than 210 TB specialist nurses at 127 PPM hospitals and 236 public health officials at 254 public health centers across the country are working under the PPM projects. Sixty-six percent of new TB patients who were notified across the country were treated at PPM hospitals in 2016.
The inclusion criteria were as follows: adult patients over 18 years of age, patients diagnosed with pulmonary TB, patients who died from any cause during anti-TB treatment, and patients who started an initial standard anti-TB regimen. The exclusion criteria were as follows: patients with drug-resistant TB, patients with miliary TB or extrapulmonary TB, patients who did not receive initial standard anti-TB regimen, and patients with anti-TB treatment duration of more than one year.
Patients with drug-sensitive TB underwent a 6-month standard treatment regimen recommended by the Korean Guidelines for TB [15], which consists of a 2-month initial phase of isoniazid, rifampicin, ethambutol and pyrazinamide followed by a 4-month continuous phase of isoniazid, rifampicin, and ethambutol. Alternatively, a 9-month standard regimen with isoniazid, rifampicin, and ethambutol can be administered. Anti-TB drugs were self-administered with the support of TB specialist in the PPM project.
Data collection
In South Korea, TB notification is mandatory when a physician diagnoses or treats a patient with confirmed or suspected TB. All the TB patients are followed during the anti-TB treatment under the PPM project, and their follow-up is closed once a final treatment outcome, defined by the WHO, has been notified to the KNTSS. After identifying death as a final outcome, TB specialist nurses at each hospital complete a mortality case report form. We retrospectively collected the clinical, radiographic, and microbiological data for each mortality case. Since follow-up after completing treatment was not possible under the PPM project, we could not identify post-treatment mortality cases for TB survivors. We stratified age into 5 groups: ≤ 49, 50–59, 60–69, 70–79, and ≥ 80. Those who have smoked < 100 cigarettes per lifetime were defined as never smokers. Those who did not smoke and drink during the last one year were defined as ex-smokers and non-drinkers, respectively. Men and women who consumed at least five and four drinks, respectively, on a single occasion in the last month or had alcohol use disorder were defined as heavy drinkers.
Definition of death
The WHO definition of TB death was used in this study, and is defined as patients with TB who died from any cause during anti-TB treatment [16]. Each TB death was categorized by cause (TB-related or non-TB-related death) and timing (early or late death). TB-related death was confirmed by death certificate or medical records from the physician-in-charge. If another cause of death was determined, mortality was classified as non-TB-related. The mode of non-TB-related death was also recorded. In addition, mortalities were divided into groups of early and late death according to whether death occurred within the initial 2-month intensive phase or during the continuous phase of anti-TB treatment, respectively.
The mode of death for non-TB-related death was also collected. Malignant neoplasm included metastatic solid malignancy, leukemia, lymphoma, and chronic refractory hematologic diseases. Acute respiratory failure included acute exacerbation of chronic respiratory diseases. Patients who were diagnosed with pneumonia or aspiration pneumonia and complicated with respiratory failure or septic shock were categorized into pneumonia. Sudden cardiac death included ischemic heart disease, arrhythmia, pulmonary thromboembolism, acute hear failure, and aortic dissection. Senility included elderly patients with malnutrition, dementia, and poor general condition. Unknown category included death at home or another institution and death on arrival at emergency room.
Statistical analysis
Continuous variables are presented as mean and standard deviation, whereas discrete variables are presented as frequency and percentage. In order to compare the differences between TB-related and non-TB-related death, we performed univariate analysis using the chi-square test and binary logistic regression. We also compared early death with late death accordingly. Subsequently, we selected age, gender and other clinical variables with P values < 0.20 based on the univariate analysis and performed multivariate binary logistic regression to evaluate the possible association between variables and predefined TB mortality subset. The calibration of the prediction model was assessed using the Hosmer-Lemeshow goodness-of-fit test (P < 0.05 was considered as indicating a statistically significant lack of fit). For regression, unknown data were regarded as missing values. A P value of 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (Statistical Product and Service Solutions, Chicago, IL, USA).
Results
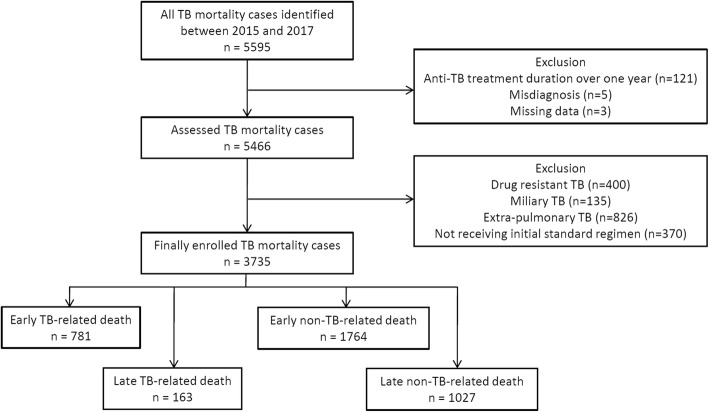
Of 5595 TB patients with a notifiable death, we excluded patients with anti-TB treatment duration of over 1 year (n = 121), patients with drug-resistant TB (n = 400), patients with miliary TB (n = 135), patients with extra-pulmonary TB (n = 826), patients not initially receiving the standard regimen (n = 370), and those with misdiagnosis and missing data (n = 8). Ultimately, 3735 patients with pulmonary TB were included in this study (Fig. 1). There were 944 (25.3%) TB-related deaths and 2791 (74.7%) non-TB-related deaths. The proportion of early TB-related deaths was significantly higher than that of early non-TB-related deaths (82.7% (781/944) vs. 63.2% (1764/2791), P = 0.000).
Fig. 1.
Flow chart of enrollment of tuberculosis mortality cases between 2015 and 2017, which were finally categorized based on cause (tuberculosis-related and non-tuberculosis related) and timing (early and late) TB, tuberculosis
The baselines characteristics of 3735 enrolled male and female patients (Tables 1 and 2). There were 2541 (68.0%) male patients, and 2935 (78.6%) mortality cases were observed in elderly patients over 65 years of age. The mean age of female patients was significantly higher than that of male patients (78.9 ± 11.7 vs. 72.1 ± 13.0, P = 0.000). The proportion of individuals with body mass index < 18.5 kg/m2 was similar between male and female patients. The proportion of prior TB history in male patients was significantly higher than that in female patients (21.5% vs. 10.9%, P = 0.000). Male patients were more likely to have chronic lung disease (8.0% vs. 4.7%, P = 0.000) and malignancy (24.8% vs. 12.7%, P = 0.000), and female patients had higher frequency of cardiovascular disease (8.1% vs. 13.1%, P = 0.000). The proportion of positive acid-fast bacilli (AFB) smear and culture test result were 46.9 and 69.7%, respectively.
Table 1.
Social and demographic characteristics of enrolled TB mortality cases categorized by genders
| Variablesa | All (n = 3735) |
Male (n = 2541) |
Female (n = 1194) |
p-value |
|---|---|---|---|---|
| Age group | ||||
| ≤ 49 years | 204 (5.5%) | 157 (6.2%) | 47 (3.9%) | 0.000 |
| 50–59 years | 365 (9.8%) | 324 (12.8%) | 41 (3.4%) | |
| 60–69 years | 539 (14.4%) | 437 (17.2%) | 102 (8.5%) | |
| 70–79 years | 1058 (28.3%) | 758 (29.8%) | 300 (25.1%) | |
| 80 years ≤ | 1569 (42.0%) | 865 (34.0%) | 705 (59.0%) | |
| Job | ||||
| Yes | 181 (4.8%) | 164 (6.5%) | 17 (1.4%) | 0.000 |
| No | 3545 (94.9%) | 2370 (93.3%) | 1175 (98.4%) | |
| Marriage | ||||
| Yes | 3244 (86.9%) | 2134 (84.0%) | 1110 (93.0%) | 0.000 |
| No | 251 (6.7%) | 218 (8.6%) | 33 (2.8%) | |
| Education | ||||
| Primary | 899 (24.1%) | 508 (20.0%) | 391 (32.7%) | 0.008 |
| Middle | 277 (7.4%) | 230 (9.1%) | 47 (3.9%) | |
| High | 385 (10.3%) | 330 (13.0%) | 55 (4.6%) | |
| University | 134 (3.6%) | 115 (4.5%) | 19 (1.6%) | |
| Smoking history | ||||
| Never smoker | 2413 (64.6%) | 1309 (51.5%) | 1104 (92.5%) | 0.000 |
| Ex-smoker | 872 (23.3%) | 820 (32.3%) | 52 (4.4%) | |
| Current smoker | 432 (11.6%) | 397 (15.6%) | 35 (2.9%) | |
| Alcohol history | ||||
| Non-drinker | 2562 (68.6%) | 1473 (58.0%) | 1089 (91.2%) | 0.000 |
| Social drinker | 817 (21.9%) | 743 (29.2%) | 74 (6.2%) | |
| Heavy drinker | 311 (8.3%) | 292 (11.5%) | 19 (1.6%) | |
| BMI | ||||
| ≥ 18.5 kg/m2 | 2130 (57.0%) | 1447 (56.9%) | 683 (57.2%) | 0.551 |
| < 18.5 kg/m2 | 1442 (38.6%) | 989 (38.9%) | 453 (37.9%) | |
BMI body mass index, HIV human immunodeficiency virus
a Missing data and values were not shown in this table
Table 2.
Clinical characteristics and laboratory findings of enrolled TB mortality cases categorized by genders
| Variablesa | All (n = 3735) |
Male (n = 2541) |
Female (n = 1194) |
p-value |
|---|---|---|---|---|
| Prior TB history | ||||
| No | 3033 (81.2%) | 1978 (77.8%) | 1055 (88.4%) | 0.000 |
| Yes | 677 (18.1%) | 547 (21.5%) | 130 (10.9%) | |
| Comorbidities | ||||
| Diabetes Mellitus | 947 (25.4%) | 647 (25.5%) | 300 (25.1%) | 0.863 |
| Chronic lung disease | 259 (6.9%) | 203 (8.0%) | 56 (4.7%) | 0.000 |
| Cardiovascular disease | 362 (9.7%) | 206 (8.1%) | 156 (13.1%) | 0.000 |
| Malignancy | 782 (20.9%) | 630 (24.8%) | 152 (12.7%) | 0.000 |
| HIV infection | 12 (0.3%) | 12 (0.5%) | 0 (0.0%) | 0.017 |
| No reported illness | 778 (20.8%) | 532 (20.9%) | 246 (20.6%) | 0.848 |
| Symptoms | ||||
| Cough | 1641 (43.9%) | 1129 (44.4%) | 512 (42.9%) | 0.369 |
| Dyspnea | 1250 (33.5%) | 861 (33.9%) | 389 (32.6%) | 0.427 |
| Haemoptysis | 132 (3.5%) | 94 (3.7%) | 38 (3.2%) | 0.424 |
| Fever | 509 (13.6%) | 342 (13.5%) | 167 (14.0%) | 0.664 |
| Cavitation on CXR | ||||
| Normal | 110 (2.9%) | 69 (2.7%) | 41 (3.4%) | 0.000 |
| Non-cavitary disease | 2714 (72.7%) | 1781 (70.1%) | 933 (78.1%) | |
| Cavitary disease | 873 (23.4%) | 666 (26.2%) | 207 (17.3%) | |
| Lesion extension on CXR | ||||
| Normal | 110 (2.9%) | 69 (7.1%) | 41 (3.4%) | 0.624 |
| Unilateral disease | 1998 (53.5%) | 1355 (53.3%) | 643 (53.9%) | |
| Bilateral disease | 1508 (40.4%) | 1035 (40.7%) | 473 (39.6%) | |
| AFB smear test | ||||
| Negative | 1889 (50.6%) | 1279 (50.3%) | 610 (51.1%) | 0.000 |
| Positive | 1753 (46.9%) | 1221 (48.1%) | 532 (44.6%) | |
| AFB culture test | ||||
| Negative | 989 (26.5%) | 693 (27.3%) | 296 (24.8%) | 0.021 |
| Positive | 2529 (67.7%) | 1717 (67.6%) | 812 (68.0%) | |
| Initial treatment regimen | ||||
| HERZ | 3042 (81.4%) | 2093 (82.4%) | 949 (79.5%) | 0.097 |
| HRE | 310 (8.3%) | 203 (8.0%) | 107 (9.0%) | |
| No treatment | 383 (10.3%) | 245 (9.6%) | 138 (11.6%) | |
TB tuberculosis, CXR chest X-ray, AFB acid-fast bacilli, HREZ combination regimen of isoniazid, rifampicin, ethambutol, and pyrazinamide, HRE combination regimen of isoniazid, rifampicin, and ethambutol
a Missing data and values were not shown in this table
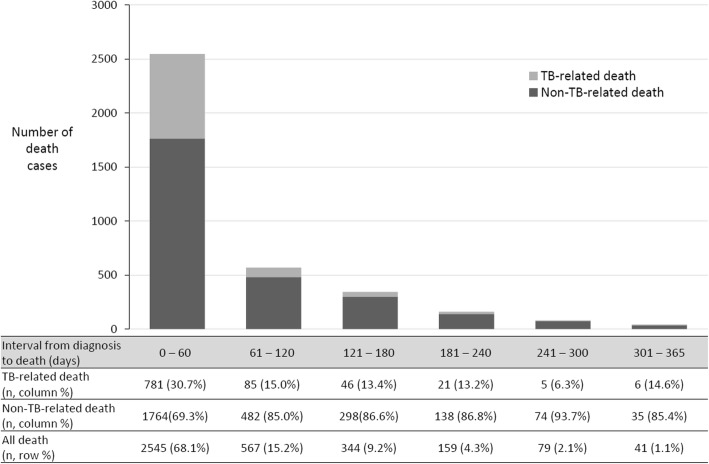
The cumulative number of deaths within 30 and 60 days were 1993 (53.4%) and 2545 (68.1%), respectively (Fig. 2). The median interval between diagnosis and death among all patients was 26.0 days (interquartile range 6.0–81.0 days). Among 383 (10.3%) patients who did not receive anti-TB treatment, 187 (5.0%) patients were diagnosed post-mortem. Thirty-eight (1.0%) patients died on the first day of treatment.
Fig. 2.
Number of tuberculosis-related, non-tuberculosis-related, and all death cases stratified by intervals between diagnosis and death. TB, tuberculosis
In multivariate analysis, current smoker (P = 0.023), body mass index less than 18.5 kg/m2 (P = 0.000), prior TB history (P = 0.002), no reported illness (P = 0.026), cavitary (P = 0.001) and bilateral disease (P = 0.000) on chest X-ray, positive AFB smear results (P = 0.000), cough (P = 0.002), and hemoptysis (P = 0.007) were significantly associated with TB-related death (Table 3). Cardiovascular disease, malignancy, and chest pain were associated with non-TB-related death. Calibration of this predictive model was good, as shown by a Hosmer-Lemeshow test (P = 0.590). Among 2791 patients who died of non-TB-related causes, acute respiratory failure (18.9%) was the most frequent cause of death, followed by pneumonia (18.7%) and malignant neoplasm (17.4%) (Table 4).
Table 3.
Comparison of profiles of tuberculosis patients categorized by cause of death using univariate and multivariate analysis
| Variables | TB-related death (n = 944) | Non-TB-related death (n = 2791) | OR (95% CI) | p-value | aORa(95% CI) | p-value |
|---|---|---|---|---|---|---|
| Age group | ||||||
| ≤ 49 years | 77 (8.2%) | 127 (4.6%) | 1 | 1 | ||
| 50–59 years | 107 (11.3%) | 258 (9.2%) | 0.68 (0.48–0.98) | 0.040 | 0.81 (0.52–1.26) | 0.350 |
| 60–69 years | 118 (12.5%) | 421 (15.1%) | 0.46 (0.33–0.66) | 0.000 | 0.86 (0.57–1.32) | 0.498 |
| 70–79 years | 214 (22.7%) | 844 (30.2%) | 0.42 (0.30–0.57) | 0.000 | 0.76 (0.51–1.14) | 0.183 |
| 80 years ≤ | 428 (45.3%) | 1141 (40.9%) | 0.62 (0.46–0.84) | 0.002 | 1.08 (0.74–1.60) | 0.686 |
| Female | 308 (32.6%) | 886 (31.7%) | 1.04 (0.89–1.22) | 0.615 | 1.03 (0.83–1.28) | 0.777 |
| Current smokerb | 161 (17.1%) | 271 (9.8%) | 1.89 (1.52–2.34) | 0.000 | 1.42 (1.05–1.92) | 0.023 |
| Heavy drinker | 116 (12.4%) | 195 (7.1%) | 0.51 (0.39–0.68) | 0.000 | 0.94 (0.67–1.33) | 0.730 |
| BMI < 18.5 kg/m2 | 441 (50.2%) | 1001 (37.2%) | 1.70 (1.46–1.98) | 0.000 | 1.38 (1.16–1.64) | 0.000 |
| Prior TB history | 210 (22.4%) | 467 (16.8%) | 1.42 (1.19–1.71) | 0.000 | 1.43 (1.14–1.79) | 0.002 |
| Comorbidities | ||||||
| Cardiovascular disease | 72 (7.7%) | 290 (10.5%) | 0.71 (0.55–0.93) | 0.014 | 0.69 (0.50–0.95) | 0.022 |
| Malignancy | 79 (8.4%) | 703 (25.3%) | 0.27 (0.21–0.35) | 0.000 | 0.35 (0.26–0.46) | 0.000 |
| No reported illness | 274 (29.2%) | 504 (18.2%) | 1.86 (1.57–2.21) | 0.000 | 1.27 (1.03–1.56) | 0.026 |
| Diagnostic tests | ||||||
| Cavitary disease on CXRc | 335 (36.7%) | 538 (20.1%) | 2.31 (1.96–2.72) | 0.001 | 1.43 (1.17–1.75) | 0.001 |
| Bilateral disease on CXRd | 516 (57.1%) | 992 (38.1%) | 2.17 (1.86–2.53) | 0.009 | 1.66 (1.39–1.99) | 0.000 |
| AFB smear test (+) | 595 (64.7%) | 1158 (42.5%) | 2.48 (2.13–2.90) | 0.000 | 1.85 (1.52–2.24) | 0.000 |
| AFB culture test (+) | 683 (77.8%) | 1846 (69.9%) | 1.51 (1.26–1.80) | 0.000 | 1.14 (0.91–1.43) | 0.246 |
| Symptoms | ||||||
| Cough | 492 (52.1%) | 1149 (41.2%) | 1.55 (1.34–1.80) | 0.000 | 1.32 (1.11–1.57) | 0.002 |
| Dyspnea | 349 (37.0%) | 901 (32.3%) | 1.23 (1.05–1.43) | 0.009 | 1.18 (0.99–1.42) | 0.072 |
| Hemoptysis | 48 (5.1%) | 84 (3.0%) | 1.72 (1.20–2.48) | 0.003 | 1.79 (1.17–2.73) | 0.007 |
| Chest pain | 32 (3.4%) | 136 (4.9%) | 0.68 (0.46–1.01) | 0.058 | 0.67 (0.42–1.06) | 0.086 |
TB, tuberculosis; OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; BMI, body mass index; CXR, chest x-ray; AFB, acid-fast bacillus
% = % of cases without missing data
aAll the variables listed in this table were used in the multivariate analysis
b Never smoker was used as reference
c Non-cavitary disease on chest X-ray was used as reference
d Unilateral disease on chest X-ray was used as reference
Table 4.
Modes of death among patients who died of non-tuberculosis-related cause
| Categories | Non-TB-related death (n = 2791) |
|---|---|
| Acute respiratory failure | 528 (18.9%) |
| Pneumonia | 521 (18.7%) |
| Malignant neoplasm | 487 (17.4%) |
| Septic shock | 303 (10.9%) |
| Sudden cardiac death | 242 (8.7%) |
| Multi-organ failure | 168 (6.0%) |
| Acute kidney injury | 60 (2.1%) |
| Acute hepatic failure | 44 (1.6%) |
| Cerebrovascular disease | 34 (1.2%) |
| Gastrointestinal bleeding | 19 (0.7%) |
| Others internal causes | 48 (1.7%) |
| External cause | 35 (1.3%) |
| Senility | 81 (2.9%) |
| Unknown | 221 (7.9%) |
TB tuberculosis
In comparison with individuals with late death, individuals with early death had significantly higher proportion of body mass index < 18.5 kg/m2 (P = 0.003), no reported illness (P = 0.005), bilateral disease on chest X-ray (P = 0.001), positive AFB smear result (P = 0.001), and dyspnea (P = 0.000) (Table 5). Current smoker status (P = 0.039) and malignancy (P = 0.000) were associated with late death. Model adequacy was checked using a Hosmer-Lemeshow test (P = 0.057).
Table 5.
Comparison of profiles of tuberculosis patients categorized by timing of death using univariate and multivariate analysis
| Variables | Early death (n = 2545) | Late death (n = 1190) | OR (95% CI) | p-value | aORa (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Age group | ||||||
| ≤ 49 years | 144 (5.7%) | 60 (5.0%) | 1 | 1 | ||
| 50–59 years | 248 (9.7%) | 117 (9.8%) | 0.88 (0.61–1.28) | 0.514 | 0.96 (0.64–1.44) | 0.835 |
| 60–69 years | 343 (13.5%) | 196 (16.5%) | 0.73 (0.52–1.03) | 0.076 | 0.90 (0.61–1.32) | 0.577 |
| 70–79 years | 711 (27.9%) | 347 (29.2%) | 0.85 (0.62–1.18) | 0.615 | 0.98 (0.69–1.41) | 0.929 |
| 80 years ≤ | 1099 (43.2%) | 470 (39.5%) | 0.97 (0.71–1.34) | 0.708 | 1.01 (0.71–1.44) | 0.968 |
| Female | 827 (32.5%) | 367 (30.8%) | 1.08 (0.93–1.25) | 0.312 | 0.90 (0.75–1.08) | 0.238 |
| Current smokerb | 723 (61.6%) | 1690 (66.7%) | 0.72 (0.62–0.85) | 0.000 | 0.76 (0.59–0.99) | 0.039 |
| Body mass index < 18.5 kg/m2 | 1030 (42.6%) | 412 (35.7%) | 1.34 (1.16–1.55) | 0.000 | 1.26 (1.08–1.48) | 0.003 |
| Comorbidities | ||||||
| Diabetes mellitus | 321 (27.1%) | 626 (24.8%) | 0.89 (0.76–1.04) | 0.128 | 1.01 (0.85–1.21) | 0.886 |
| Malignancy | 1030 (18.1%) | 412 (27.4%) | 0.58 (0.50–0.69) | 0.000 | 0.71 (0.59–0.89) | 0.000 |
| No reported illness | 580 (23.0%) | 198 (16.7%) | 1.48 (1.24–1.77) | 0.000 | 1.36 (1.10–1.68) | 0.005 |
| Diagnostic tests | ||||||
| Bilateral disease on CXRc | 414 (36.7%) | 1094 (46.0%) | 1.47 (1.27–1.70) | 0.000 | 1.30 (1.11–1.52) | 0.001 |
| AFB smear test (+) | 1254 (50.6%) | 499 (42.9%) | 1.36 (1.18–1.56) | 0.000 | 1.30 (1.11–1.52) | 0.001 |
| Symptoms | ||||||
| Dyspnea | 924 (36.3%) | 326 (27.4%) | 1.51 (1.30–1.76) | 0.000 | 1.52 (1.29–1.79) | 0.000 |
| Fever | 369 (14.5%) | 140 (11.8%) | 1.27 (1.03–1.57) | 0.023 | 1.21 (0.96–1.55) | 0.104 |
OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; CXR, chest X-ray; AFB, acid-fast bacillus
% = % of cases without missing data
aAll the variables listed in this table were used in the multivariate analysis
b Never smoker was used as reference
c Unilateral disease on chest X-ray was used as reference
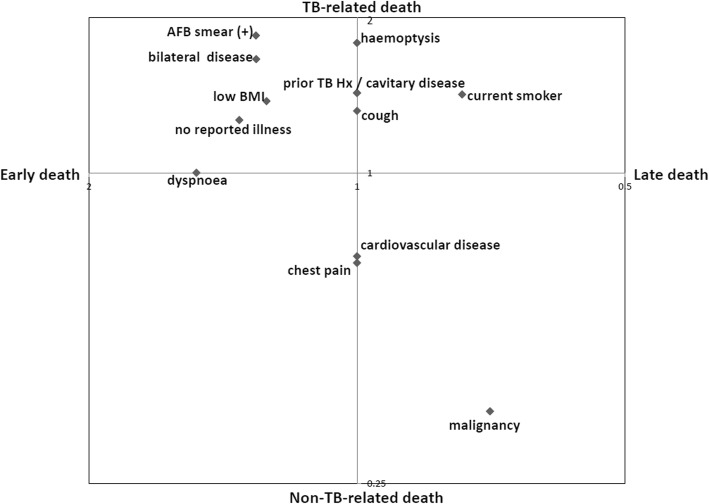
After identifying variables significantly associated with each predefined subsets of TB mortality, we plotted a log-log graph describing factors related to early TB-related and late non-TB-related deaths (Fig. 3). The factors in the right upper quadrant, such as low body mass index, no reported illness, bilateral disease on chest X-ray, and positive AFB smear result, were associated with both TB-related and early death. In contrast, malignancy was associated with both non-TB-related death and late death.
Fig. 3.
Log-Log plot describing variables, which were significantly associated with subsets of TB death (TB-related, non-TB-related, early and late deaths). TB, tuberculosis; AFB, acid-fast bacillus; BMI, body mass index; Hx, history
Discussion
This large cross-sectional study assessed 3735 TB mortality cases that occurred during anti-TB treatment among adult patients with pulmonary TB in South Korea. According to our study, three of every four deaths occurring during anti-TB treatment were attributed to non-TB-related causes such as acute respiratory failure and malignancy. We also observed a high number of TB mortality cases among elderly patients in this study. South Korea has become an aged society, and the epidemiologic pattern within the past few decades has changed from infectious to chronic non-communicable diseases that cause individuals (particularly elderly individuals) to be at a greater risk of developing TB [5]. More attentions to this elderly population are needed, and accordingly, South Korea is currently preparing a comprehensive management plan for its elderly population, which is a key vulnerable group [3].
There are several factors that contribute to the enormous TB death toll worldwide [17]. Although global initiatives address the importance of malnutrition [18] and smoking [19], which have been clearly linked with excess TB mortality, such key determinants of TB mortality are still under-emphasized at the country level. A weak and underinvested public health system, especially in low-income countries, is another important issue, and has led to a suboptimal cascade of care for TB patients [20]. Since 2011, South Korea has increased budgets and strengthened patient management policies together with a PPM collaboration model, which led to significant decreases in TB incidence [3]. However, a high and stagnant TB mortality rate poses a great challenge to the national TB control program in South Korea. Our study findings may allow for planning of effective interventions to strengthen patient-centered care and improve patient survival.
As illustrated in the results, low body mass index, no reported illness, bilateral disease on chest X-ray, and positive AFB smear result were significantly associated with early and TB-related death. Because we aimed to identify mortality cases that could be controlled and prevented through appropriate management, we excluded TB cases presumed to be hard to treat, such as cases involving immunocompromised host, drug resistance, and disseminated disease. Cases of spinal or miliary disease reflect compromised immunity and are difficult to manage even with proper intervention. Patients with drug resistance are prone to poor medication adherence and severe adverse reactions related to non-TB-related death [21]. Based on our results, we can propose that early interventions should target patients with severe TB disease and malnutrition, in order to improve TB mortality.
Proper diagnosis and rapid treatment of patients with infectious TB remains the cornerstone of a TB control program [22]; however, 10% of patients enrolled in our study died without receiving anti-TB drugs, and 5% of patients were diagnosed post-mortem. TB control is also complicated by post-mortem diagnosis, as these patients likely had multiple encounters with healthcare facilities while infected with unrecognized TB [23]. Automated nucleic acid amplification tests such as Xpert MTB/RIF assay are increasingly deployed in many countries as the point-of-care diagnostic test for TB [24]. Recently, South Korea has expanded the coverage of national health insurance for the Xpert MTB/RIF assay. Elderly patients who cannot produce adequate sputum specimen would benefit from the implementation of such a sensitive tool, which could reduce missed or delayed diagnosis.
Due to the difficulties inherent to differentiating TB-related death from non-TB-related death, only a small number of published studies have examined risk factors for TB-related death. A Taiwanese study [25] found that extrapulmonary, miliary, and pneumonic radiographic patterns were independent risk factors for TB-related death when compared to survivors. A single-center study from South Korea [11] suggested that early TB-related deaths were mainly attributable to delayed diagnosis. Another recent study that evaluated TB mortality revealed that an underlying chronic condition, lower hemoglobin level, and acute respiratory failure were independent risk factors for TB-related death among non-elderly patients with miliary TB [12]. In order to meet the target goal of reducing TB mortality in South Korea, clinicians should be educated on the identification of preventable risk factors for TB-related death. In addition, South Korea estimates for TB mortality are based on National Statistical Office data. These data reflect only TB-related death, because the cause of death on the death certificate is used to identify the mortality case in accordance with the WHO recommendations [26]. Accuracy and utility of cause of death data from death certificates are uncertain and often questionable. The current study is valuable in that it is the first attempt to assess TB mortality using a large nationwide database in South Korea. An accurate estimate of mortality enables a clearer understanding of the burden and cost of TB and can inform national TB control strategies to improve mortality [27].
Our study has several limitations. First, we only described and compared characteristics of predefined subsets of TB mortality cases, and data of TB survivors were unavailable for the statistical analysis. The WHO states that for country-specific purposes, deaths due to TB and deaths due to other causes can be separated in the treatment outcomes section [16]. In South Korea, both TB-related and non-TB-related deaths are reported in the KNTSS. Although our results cannot be extrapolated to the general TB population, they remain valuable for the purpose of assessing the national TB status and planning necessary interventions. Second, those who visited PPM hospitals before death were included for this analysis, which also limits the generalizability. More information regarding the characteristics and treatment outcomes of TB patients managed in non-PPM hospitals are essential to reducing overall TB mortality. Third, we extracted data regarding the causes of death from case report forms completed by TB nurses at PPM hospitals, who identify the causes by reviewing and comparing two available sources (medical charts and death certificates). However, in routine clinical practice, it is hard to identify the correct mode of death, especially for elderly individuals. We could not implement a robust verification method to validate the information regarding the mode of death, because of the large cross-sectional design of the study. This may have caused a potential misclassification bias. In addition, cases of out-of-hospital mortality are collected by interviewing the guardian. The mode of death was unknown or forgotten in 10% of cases and resulted in the underestimation of TB-related death. Fourth, because clinical data for this analysis were collected from a database designed for TB surveillance, we could not obtain all clinical data related to death, such as laboratory findings and vital signs. A detailed history of TB diagnosis and treatment were not captured. For example, non-adherence to anti-TB drugs and delays to diagnosis and treatment were known to be associated with TB death [28]. Because adverse reactions to anti-TB drugs are distinct concerns for elderly patients with TB [29], further analysis investigating adverse drug reactions and mortality could be helpful to elucidate the high proportion of mortality among the elderly population. Lastly, because of the cross-sectional study design and unavailability of data regarding TB survivors, we could not measure outcomes over time or apply time-to-event analysis, which might have provided a more complex understanding of TB mortality.
Conclusions
A high proportion of TB death was observed among elderly patients, indicating that special attention to this population is necessary. Our results revealed that most of the deaths during anti-TB treatment were attributed to non-TB related causes, such as acute respiratory failure, malignancy, and cardiovascular diseases. Although a timely diagnosis may not prevent most non-TB related deaths, early treatment could reduce transmission risk, which is valuable in the public health perspective. Many TB-related deaths occurred during the intensive phase, particularly within the first month. Further studies identifying risk factors for different causes of TB death at different phases of anti-TB treatment are warranted for early identification of risk factors and targeted intervention, which may reduce early TB-related deaths. Any such intervention requires the collaboration of public and private sectors to maximize its effect.
Acknowledgements
We are grateful to TB specialist nurses working at the PPM hospitals across the country for data collection.
Abbreviations
- AFB
Acid-fast bacilli
- PPM
Private-public mix
- SPSS
Statistical package for the social sciences
- TB
Tuberculosis
- WHO
World Health Organization
Authors’ contributions
Study design: JM, JSK, HYK, AYS, HKK, SSL, JSP. Data acquisition: JSK, SSL, YKK, KCS, JHC, GC, JL, MSP, JSP. Data analysis: JM, JSK, SSL, GC, JL, MSP, JSP. Manuscript drafting: JM, JSK, HWK, HKK, SSL, JSP. Critical manuscript revision: JM, JSK, SSL, YKK, KCS, JHC, JSP. All authors read and approved the final manuscript.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The ownership of the primary datasets lies with the Korea Centers for Disease Control and Prevention (KCDC). The datasets used and/or analyzed during the current study are available on reasonable request after obtaining permission from the KCDC in advance. Joosun Lee of the KCDC should be contacted for the request accessing the raw data.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki. The Korea Centers for Disease Control and Prevention (KCDC), South Korea, has authority to hold and analyse surveillance data for public health and research purpose. Mi Sun Park of the KCDC has granted permission to access the raw data.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Global Tuberculosis Report. Geneva: WHO; 2018. https://www.who.int/tb/publications/global_report/en/. Accessed 18 June 2019.
- 2.World Health Organization. Implementing the end TB strategy: the essentials. Geneva: WHO; 2015. https://www.who.int/tb/publications/2015/The_Essentials_to_End_TB/en/. Accessed 18 June 2019.
- 3.Go U, Park M, Kim U-N, Lee S, Han S, Lee J, Yang J, Kim J, Park S, Kim Y, et al. Tuberculosis prevention and care in Korea: evolution of policy and practice. J Clin Tuberc Other Mycobact Dis. 2018;11:28–36. doi: 10.1016/j.jctube.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korea Centers for Disease Control & Prevention. Annual Report on the Notified Tuberculosis in Korea. Osong: KCDC; 2016. http://www.cdc.go.kr/CDC/cms/content/mobile/78/73878_view.html. Accessed 20 Aug 2019.
- 5.Min J, Mi Shin Y, Lee WJ, Truong TT, Kang ES, An JY, Choe KH, Man Lee K. Clinical features of octogenarian patients with tuberculosis at a tertiary hospital in South Korea. J Int Med Res. 2018;47:271–280. doi: 10.1177/0300060518800597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15:871–885. doi: 10.5588/ijtld.10.0352. [DOI] [PubMed] [Google Scholar]
- 7.Lee YH, Bang JH, Park SM, Kang CR, Cho S-I, M-d O, Lee J-K. Cost-effectiveness of voluntary HIV testing strategies in a very low-prevalence country, the Republic of Korea. J Korean Med Sci. 2018;33:e304. doi: 10.3346/jkms.2018.33.e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suguimoto SP, Techasrivichien T, Musumari PM, El-saaidi C, Lukhele BW, Ono-Kihara M, Kihara M. Changing patterns of HIV epidemic in 30 years in East Asia. Current HIV/AIDS Rep. 2014;11:134–145. doi: 10.1007/s11904-014-0201-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim CW, Kim S-H, Lee SN, Lee SJ, Lee MK, Lee J-H, Shin KC, Yong SJ, Lee WY. Risk factors related with mortality in patient with pulmonary tuberculosis. Tuberc Respir Dis. 2012;73(1):38–47. doi: 10.4046/trd.2012.73.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon Y-S, Kim YH, Song J-U, Jeon K, Song J, Ryu YJ, Choi JC, Kim HC, Koh W-J. Risk factors for death during pulmonary tuberculosis treatment in Korea: a multicenter retrospective cohort study. J Korean Med Sci. 2014;29:1226–1231. doi: 10.3346/jkms.2014.29.9.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, Nam HW, Choi SH, Yoo SS, Lee SY, Cha SI, Park JY, Kim CH. Comparison of early and late tuberculosis deaths in Korea. J Korean Med Sci. 2017;32:700–703. doi: 10.3346/jkms.2017.32.4.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, Lim JK, Kim EJ, Lee DH, Kim YK, Yoo SS, Lee SY, Cha SI, Park JY, Kim CH. Comparison of clinical manifestations and treatment outcome according to age groups in adult patients with miliary tuberculosis. J Thorac Dis. 2018;10:2881–2889. doi: 10.21037/jtd.2018.04.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho KS. Tuberculosis control in the Republic of Korea. Epidemiol Health. 2018;40:e2018036. doi: 10.4178/epih.e2018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang H-Y, Yoo H, Park W, Go U, Jeong E, Jung K-S, Son H. Tuberculosis notification completeness and timeliness in the Republic of Korea during 2012–2014. Osong Public Health Res Perspect. 2016;7:320–326. doi: 10.1016/j.phrp.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joint Committee for the Revision of Korean guidelines for tuberculosis and Korea Centers for Disease Control and Prevention. Korean Guidelines For Tuberculosis 3rd. Osong: KCDC; 2017. http://cdc.go.kr/CDC/cms/content/mobile/01/74901_view.html. Accessed 20 Aug 2019.
- 16.World Health Organization. Definitions and reporting framework for tuberculosis – 2013 revision. Geneva: WHO; 2013. https://www.who.int/tb/publications/definitions/en/. Accessed 18 June 2019.
- 17.Pai M, Correa N, Mistry N, Jha P. Reducing global tuberculosis deaths-time for India to step up. Lancet. 2017;389:1174–1176. doi: 10.1016/S0140-6736(17)30790-0. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Guideline: Nutritional care and support for patients with tuberculosis. Geneva: WHO; 2013. https://apps.who.int/iris/bitstream/10665/94836/1/9789241506410_eng.pdf. Accessed 18 June 2019. [PubMed]
- 19.World Health Organization. A WHO / The Union monograph on TB and tobacco control: joining efforts to control two related global epidemics. Geneva: WHO; 2007. http://www.who.int/iris/handle/10665/43812. Accessed 18 June 2019.
- 20.Murray M, Subbaraman R, Nathavitharana RR, Satyanarayana S, Pai M, Thomas BE, Chadha VK, Rade K, Swaminathan S, Mayer KH. The tuberculosis Cascade of Care in India’s public sector: a systematic review and meta-analysis. PLoS Med. 2016;13:e1002149. doi: 10.1371/journal.pmed.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf. 2006;5:231–249. doi: 10.1517/14740338.5.2.231. [DOI] [PubMed] [Google Scholar]
- 22.Dowdy DW, Grant AD, Dheda K, Nardell E, Fielding K, Moore DAJ. Designing and evaluating interventions to halt the transmission of tuberculosis. J Infect Dis. 2017;216(suppl_6):S654–S661. doi: 10.1093/infdis/jix320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cadena J, Castro-Pena NA, Javeri H, Hernandez B, Michalek J, Arzola AF, Shroff M, Jinadatha C, Valero G, Bowling J, et al. Tuberculosis patients who are a potential source for unprotected exposure in health care systems: a multicenter case control study. Open Forum Infect Dis. 2017;4:ofx201. doi: 10.1093/ofid/ofx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albert H, Nathavitharana RR, Isaacs C, Pai M, Denkinger CM, Boehme CC. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J. 2016;48:516–525. doi: 10.1183/13993003.00543-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CH, Lin CJ, Kuo YW, Wang JY, Hsu CL, Chen JM, Cheng WC, Lee LN. Tuberculosis mortality: patient characteristics and causes. BMC Infect Dis. 2014;14:5. doi: 10.1186/1471-2334-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin H-Y, Lee S. How to write a death certificate: from a statistical point of view. J Korean Med Assoc. 2018;61:268–278. doi: 10.5124/jkma.2018.61.4.268. [DOI] [Google Scholar]
- 27.Lalor MK, Mohiyuddin T, Uddin T, Thomas HL, Lipman M, Campbell CNJ. The challenge of estimating tuberculosis mortality accurately in England and Wales. Int J Tuberc Lung Dis. 2018;22:572–578. doi: 10.5588/ijtld.17.0695. [DOI] [PubMed] [Google Scholar]
- 28.Nahid P, Jarlsberg LG, Rudoy I, de Jong BC, Unger A, Kawamura LM, Osmond DH, Hopewell PC, Daley CL. Factors associated with mortality in patients with drug-susceptible pulmonary tuberculosis. BMC Infect Dis. 2011;11(1). [DOI] [PMC free article] [PubMed]
- 29.Negin J, Abimbola S, Marais BJ. Tuberculosis among older adults – time to take notice. Int J Infect Dis. 2015;32:135–137. doi: 10.1016/j.ijid.2014.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The ownership of the primary datasets lies with the Korea Centers for Disease Control and Prevention (KCDC). The datasets used and/or analyzed during the current study are available on reasonable request after obtaining permission from the KCDC in advance. Joosun Lee of the KCDC should be contacted for the request accessing the raw data.