Serine proteases and serine protease homologs form the second largest gene family in the Drosophila melanogaster genome. Certain genes in the Jonah multigene family encoding serine proteases have been implicated in the fly antiviral immune response. Here, we report the involvement of Jonah66Ci in the Drosophila immune defense against Steinernema carpocapsae nematode infection.
KEYWORDS: Drosophila, Steinernema, innate immunity, parasitism, proteases
ABSTRACT
Serine proteases and serine protease homologs form the second largest gene family in the Drosophila melanogaster genome. Certain genes in the Jonah multigene family encoding serine proteases have been implicated in the fly antiviral immune response. Here, we report the involvement of Jonah66Ci in the Drosophila immune defense against Steinernema carpocapsae nematode infection. We find that Drosophila Jonah66Ci is upregulated in response to symbiotic (carrying the mutualistic bacterium Xenorhabdus nematophila) or axenic (lacking Xenorhabdus) Steinernema nematodes and is expressed exclusively in the gut of Drosophila larvae. Inactivation of Jonah66Ci provides a survival advantage to larvae against axenic nematodes and results in differential expression of Toll and Imd pathway effector genes, specifically in the gut. Also, inactivation of Jonah66Ci increases the numbers of enteroendocrine and mitotic cells in the gut of uninfected larvae, and infection with Steinernema nematodes reduces their numbers, whereas the numbers of intestinal stem cells are unaffected by nematode infection. Jonah66Ci knockdown further reduces nitric oxide levels in response to infection with symbiotic Steinernema nematodes. Finally, we show that Jonah66Ci knockdown does not alter the feeding rates of uninfected Drosophila larvae; however, infection with axenic Steinernema nematodes lowers larval feeding. In conclusion, we report that Jonah66Ci participates in maintaining homeostasis of certain physiological processes in Drosophila larvae in the context of Steinernema nematode infection. Similar findings will take us a step further toward understanding the molecular and physiological mechanisms that take place during parasitic nematode infection in insects.
INTRODUCTION
Drosophila melanogaster is an established model for dissecting the molecular and cellular basis of host-pathogen interactions (1). Extensive studies have led to the identification and understanding of evolutionarily conserved signaling pathways that are activated in response to different types of microbial infections (2–4). Drosophila has been employed recently to dissect the molecular mechanisms that occur in insects responding to parasitic nematode infections (5–8). The Drosophila immune system shares significant homology to the mammalian innate immune system, which facilitates modeling parasitic processes and antinematode immune reactions in humans (9–11).
Entomopathogenic nematodes of the genera Steinernema and Heterorhabditis are emerging as excellent models for studying insect-nematode interactions (9, 11, 12). They are natural obligate parasites of a wide range of insects that they infect to complete their life cycle. These nematode parasites infect susceptible insects as infective juveniles, a developmentally arrested stage analogous to the Caenorhabditis elegans dauer stage (12). A distinct feature of entomopathogenic nematodes is the presence of mutualistic bacteria that are localized to their intestines (13, 14). Steinernema carpocapsae forms a mutualistic relationship with the Gram-negative bacterium Xenorhabdus nematophila (symbiotic nematodes), and together they form potent pathogenic complexes that infect insects (7, 12). The nematodes enter the insect cavity through the cuticle or natural openings and subsequently expel their bacteria into the insect open circulatory system (15). The bacteria secrete toxins, virulence factors, and degradative enzymes that target several insect tissues and interfere with the insect immune response, which eventually leads to rapid insect death (15). The bacteria also provide nutrients to the nematodes that promote the completion of their reproductive cycle (16). Once the food source is depleted, the nematodes reacquire the bacteria and exit the insect cadaver in search of new insect hosts (12).
The use of Drosophila and Steinernema to unravel the insect antinematode immune response has certain advantages. Symbiotic and axenic Steinernema nematodes are pathogenic to Drosophila, and, interestingly, they are capable of killing larvae at similar rates (17). In addition, Steinernema nematode infection activates the expression of antimicrobial peptide (AMP) genes and the melanization pathway, and mutualistic Xenorhabdus bacteria suppress the latter response (7). The imaginal disc growth factor-3 (Idgf3) and two clotting factors (gp150 and fondue) have been found to participate specifically in the Drosophila antinematode immune response. Knockdown of Idgf3, gp150, or fondue increases the susceptibility of larvae responding to Heterorhabditis bacteriophora nematodes, whereas inactivation of Idgf2 provides a survival advantage to larvae responding to axenic Steinernema nematodes (18–20).
The Jonah multigene family consists of approximately 20 genes organized in small clusters on different chromosomal sites and exhibits complex expression patterns (21–23). In situ hybridization identified the expression of Jonah25Bi, Jonah65Ai, and Jonah99Cα in the Drosophila midgut (24). These Jonah genes are expressed during the larval and adult stages of Drosophila but not during the pupal stage (21). Low-level Jonah expression is also detected in the presumptive midgut from 18-h embryos (23). Because Jonah genes are exclusively expressed in the Drosophila gut, Jonah proteases are implicated in the breakdown of dietary proteins due to their homology to mammalian serine proteases, trypsin and chymotrypsin (25). More recently, transcriptomic studies have identified the induction of several Jonah genes in Drosophila responding to viral or nematode infections (8, 26, 27).
In this study, we have investigated the transcriptional regulation of Jonah66Ci in Drosophila larvae infected with symbiotic or axenic Steinernema nematodes. Jonah66Ci was selected from a previous transcriptomic study based on its high transcriptional induction in Drosophila larvae during Steinernema nematode infection (8). In uninfected and nematode-infected larvae, Jonah66Ci is solely expressed in the gut (22). To this end, we monitored the survival response, induction of immune signaling pathway effector genes, mitotic rates and numbers of gut cells, levels of nitric oxide (NO) and reactive oxygen species (ROS), and feeding rates in background control and Jonah66Ci knockdown larvae. We discuss how inactivation of Jonah66Ci in Drosophila alters different aspects of the immune response to Steinernema and how Jonah66Ci is involved in regulating gut physiology against entomopathogenic nematode infection. Identification and functional characterization of genes that are involved in the interaction of Drosophila with parasitic nematodes set the stage for uncovering conserved mechanisms in other insects of agricultural or medical importance.
RESULTS
Steinernema nematode infection upregulates Jonah66Ci in Drosophila.
To investigate the transcriptional induction of Jonah66Ci in Drosophila during nematode infection, we exposed larvae to 100 symbiotic or axenic Steinernema nematodes and estimated the relative transcript levels of Jonah66Ci at 6 and 24 h postinfection. We compared the transcript levels (as reads per kilobase per million [RPKM]) of Jonah66Ci from a recent transcriptomic study (8) and those from quantitative reverse transcription-PCR (qRT-PCR) analysis (ΔCT method, where CT is threshold cycle) (Fig. 1A; see also Table S2 in the supplemental material). We have found comparable transcript levels of Jonah66Ci by transcriptome sequencing (RNA-seq) and qRT-PCR analyses.
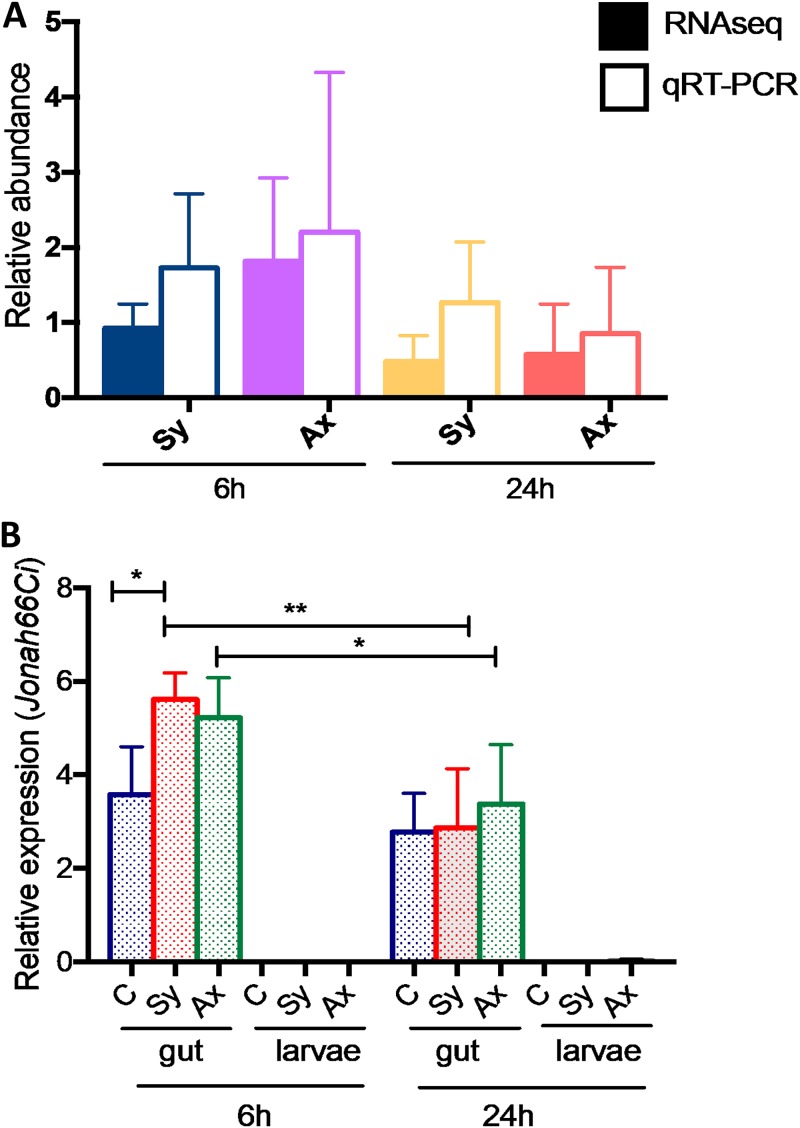
FIG 1.
Relative gene transcript levels of Jonah66Ci in Drosophila larvae upon infection with Steinernema nematodes. (A) Relative transcript levels of Jonah66Ci using RNA-seq and qRT-PCR analysis were estimated in Drosophila melanogaster late-second- or early-third-instar larvae (Oregon line) at 6 and 24 h postinfection with 10 symbiotic (Sy) or axenic (Ax) infective Steinernema carpocapsae juveniles. (B) Relative transcript levels for Jonah66Ci were estimated in the gut only and in the rest of the larvae in Drosophila infected with symbiotic (Sy) or axenic (Ax) Steinernema nematodes. Application of water served as a negative-control (C) treatment. Relative gene transcript levels for Jonah66Ci were measured as a ratio to the level of the uninfected control samples. Values represent the means from three separate experiments, and error bars represent standard deviations. Data analysis was performed using one-way analysis of variance (ANOVA) with Tukey’s post hoc test on GraphPad Prism, version 7, software. *, P < 0.05; **, P < 0.01; nonsignificant differences are not shown.
A previous study identified members of the Jonah gene family, Jonah25Bi, Jonah65Ai, and Jonah99Cα, that were expressed in the Drosophila gut (21). To determine whether Jonah66Ci is also expressed in the gut of Drosophila larvae during nematode infection, we estimated the transcript levels of Jonah66Ci in Drosophila larvae with a gut and without a gut (gutless larvae) at 6 and 24 h postinfection with symbiotic or axenic Steinernema nematodes. We detected no mRNA levels of Jonah66Ci in the body of gutless nematode-infected or uninfected control larvae (Fig. 1B; Table S2). At 6 h, Jonah66Ci transcript levels were significantly higher in the gut of larvae infected with symbiotic nematodes than in uninfected controls (P = 0.0190) (Fig. 1B). At 24 h, there were no differences in Jonah66Ci transcript levels in the gut of nematode-infected and uninfected larvae. We also found that Jonah66Ci transcript levels were significantly reduced from 6 h to 24 h in the gut of larvae infected with symbiotic (P = 0.0073) or axenic (P = 0.0452) nematodes (Fig. 1B; Table S2). These results indicate that Jonah66Ci is expressed at detectable levels in the gut of uninfected Drosophila larvae and that challenge with Steinernema nematodes leads to upregulation during the early stages of infection.
Drosophila Jonah66Ci knockdown larvae display enhanced survival in response to axenic Steinernema nematode infection.
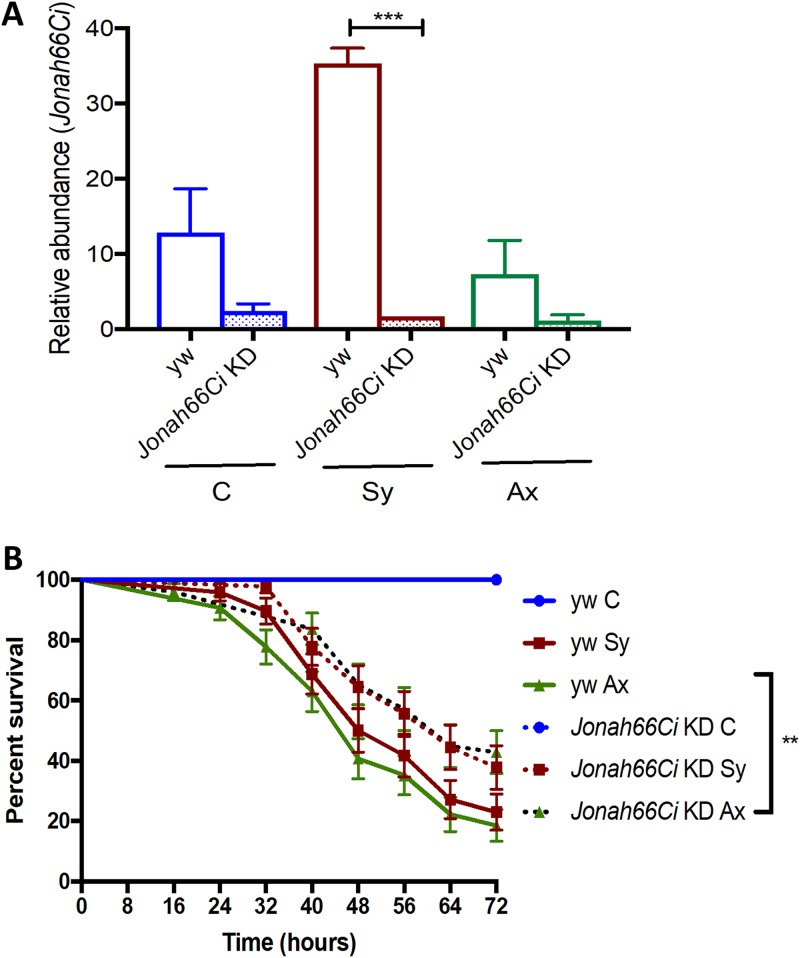
To investigate whether inactivation of Jonah66Ci affects the survival ability of Drosophila in the context of nematode infection, we challenged Jonah66Ci knockdown and yw control larvae with symbiotic or axenic Steinernema nematodes and assessed larval survival every 8 h for 3 days (Fig. 2B; Table S3). We found that upon infection with axenic nematodes, yw control larvae succumbed faster to infection than Jonah66Ci knockdown larvae (P = 0.0028) (Fig. 2B; Table S3). There were no differences in survival rates between Jonah66Ci knockdown and yw control larvae infected with symbiotic nematodes (P = 0.0801) (Fig. 2B; Table S3). These results indicate that loss of Jonah66Ci promotes the survival ability of Drosophila larvae in response to axenic Steinernema nematode infection.
FIG 2.
Drosophila Jonah66Ci knockdown validation and survival response of Drosophila Jonah66Ci knockdown larvae upon infection with Steinernema nematodes. (A) Representative figure depicting the relative expression of Jonah66Ci in control and Jonah66Ci knockdown (KD) larvae following infection with symbiotic (Sy) or axenic (Ax) Steinernema carpocapsae nematodes. Water-treated larvae served as negative controls (C). Larval progeny were obtained by crosses involving either female virgin flies from the yw background line or from the Jonah66Ci RNAi knockdown (KD) line with males from the Esg-Gal4 line. Relative gene transcript levels for Jonah66Ci were estimated as a ratio to the level of the uninfected control samples. Values represent the means from three independent experiments, and error bars represent standard deviations. Data analysis was performed using one-way analysis of variance (ANOVA) with Tukey’s post hoc test on GraphPad Prism, version 7, software (***, P < 0.001). (B) Survival rates of Drosophila melanogaster late-second- or early-third-instar yw control and Jonah66Ci knockdown larvae following infection with 10 symbiotic (Sy) or axenic (Ax) infective Steinernema carpocapsae juveniles. Larval progeny were generated by crossing either female virgin flies from the yw background line or Jonah66Ci RNAi line with males from the Esg-Gal4 line. Application of water served as a control (C) treatment. Survival results were monitored every 8 h and up to 72 h postinfection. Values are shown as percent survival of infected larvae, and data analysis was performed using a log rank (Mantel-Cox) test (GraphPad Prism, version 7 software). The means from three independent experiments are shown, and bars represent standard errors. **, P < 0.01; nonsignificant differences are not shown.
Imd pathway activation decreases in Drosophila Jonah66Ci knockdown larvae responding to axenic Steinernema nematodes.
To determine whether inactivation of Jonah66Ci in Drosophila has an effect on signaling pathway activation in response to nematode infection, we infected Jonah66Ci knockdown and background control larvae with symbiotic or axenic Steinernema nematodes and estimated transcript levels of Attacin (Imd pathway), Drosomycin (Toll pathway), Puckered (JNK pathway), and TotA (Turandot-A, Jak/Stat pathway) at two time points postinfection (Fig. 3; Table S4) (28–31).
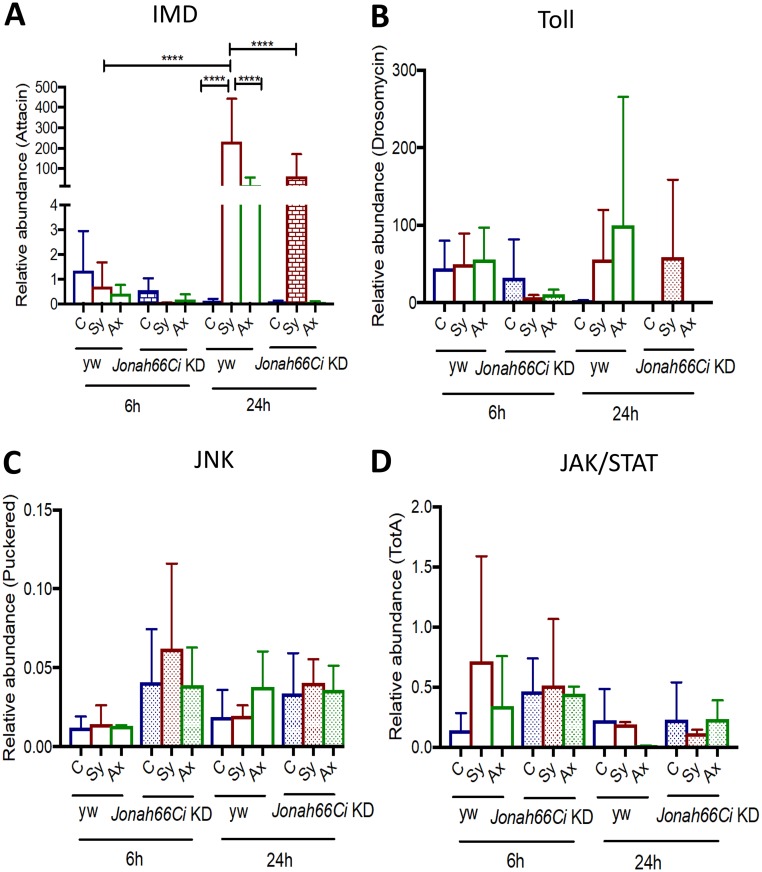
FIG 3.
Transcript levels of immune pathway readout genes in Drosophila Jonah66Ci knockdown larvae infected with Steinernema nematodes. (A to D) Shown are transcript levels of Attacin (IMD pathway), Drosomycin (Toll pathway), Puckered (JNK pathway), and TotA (JAK/STAT pathway), as indicated, in Drosophila melanogaster yw control and Jonah66Ci knockdown larvae 6 and 24 h after being infected with 10 symbiotic (Sy) or axenic (Ax) infective Steinernema carpocapsae juveniles or treated with water (control, C). Drosophila yw background control and Jonah66Ci knockdown (KD) virgin female flies were crossed with Esg-Gal4 males, and the resulting larval progeny were used for experiments. Gene transcript values were calculated relative to value for the housekeeping gene, RpL32, and expressed as a ratio to the level of the uninfected controls. Samples were run as technical duplicates, and three biological replicates were performed. Bars represent standard deviations. Data analysis was performed using one-way analysis of variance (ANOVA) with a Tukey’s post hoc test on GraphPad Prism, version 7, software. ****, P < 0.0001; nonsignificant differences are not shown.
At 6 h, we found low transcript levels of Attacin in both yw controls and Jonah66Ci knockdown larvae infected with symbiotic or axenic nematodes. At 24 h, infection with symbiotic nematodes significantly upregulated Attacin in yw control larvae compared to levels in uninfected individuals (P < 0.0001) (Fig. 3A and Table S4). However, we found no differences in Attacin mRNA levels in yw control larvae infected with axenic nematodes compared to levels in uninfected controls. Attacin transcript levels were lower in yw control larvae infected with axenic nematodes than in those infected with symbiotic nematodes (P < 0.0001) (Fig. 3A and Table S4). We also found that in yw control larvae, Attacin transcript levels increased significantly from 6 to 24 h after symbiotic nematode infection (P < 0.0001) (Fig. 3A and Table S4). At 24 h, Attacin transcript levels in uninfected Jonah66Ci knockdown larvae and in those infected with axenic nematodes were hardly detectable. Interestingly, upon symbiotic nematode infections, Attacin transcript levels were significantly higher in yw control larvae than in Jonah66Ci knockdown larvae (P < 0.0001) (Fig. 3A and Table S4).
There were no significant differences in transcript levels of Drosomycin, Puckered, or Tot-A between yw controls and Jonah66Ci knockdown larvae upon infection with symbiotic or axenic nematodes at any of the time points (Fig. 3B to D, respectively; Table S4). These results indicate that the absence of Jonah66Ci in Drosophila larvae reduces the induction of Imd signaling in response to axenic Steinernema nematodes.
Toll and Imd pathways are differentially activated in Drosophila Jonah66Ci knockdown larvae responding to symbiotic Steinernema nematodes.
Toll and Imd pathways regulate antimicrobial peptide production in the anterior midgut of Drosophila (32). Restricted expression of Jonah66Ci in the Drosophila gut (Fig. 1B) prompted us to investigate whether its inactivation would affect Toll or Imd signaling in the context of nematode infection. For this, we infected Jonah66Ci knockdown and their background control larvae with symbiotic or axenic Steinernema nematodes, and 24 h later we estimated transcript levels of antimicrobial peptide-encoding genes in the gut and the rest of the larva (Fig. 4; Table S5).
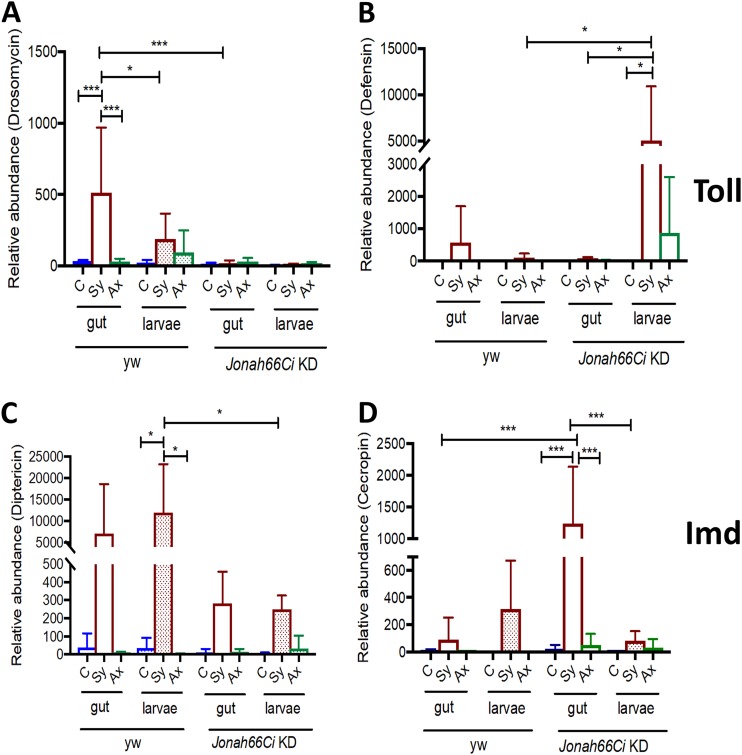
FIG 4.
Transcript levels of Toll and Imd pathway readout genes in the gut of Drosophila Jonah66Ci knockdown larvae infected with Steinernema nematodes. Shown are transcript levels of Drosomycin (A), Defensin (Toll pathway) (B), Diptericin (C), and Cecropin (IMD pathway) (D) in Drosophila yw control and Jonah66Ci knockdown larvae 24 h after being infected with 10 symbiotic (Sy) or axenic (Ax) infective Steinernema carpocapsae juveniles or treated with water (control, C). Drosophila yw background control and Jonah66Ci knockdown (KD) virgin female flies were crossed with Esg-Gal4 males, and the resulting larval progeny were used for experiments. Gene transcript levels are shown in gut tissue only and the rest of the larvae. Transcript level values are calculated relative to the level of the housekeeping gene, RpL32, and are expressed as a ratio to level of uninfected control samples. Three independent experiments were performed, and bars represent standard deviations. Data analysis was performed using one-way analysis of variance (ANOVA) with a Tukey’s post hoc test on GraphPad Prism, version 7, software. *, P < 0.05; ***, P < 0.001; nonsignificant differences are not shown.
Low transcript levels of Drosomycin were detected in Jonah66Ci knockdown larvae, with or without nematode infection. We found significantly elevated levels of Drosomycin in the gut of yw control larvae infected with symbiotic nematodes compared to levels in those infected with axenic nematodes (P = 0.005) and in uninfected controls (P = 0.0006) (Fig. 4A and Table S5). In yw control larvae infected with symbiotic Steinernema nematodes, Drosomycin transcript levels were significantly higher in the gut than in gutless larvae (P = 0.0391) (Fig. 4A and Table S5). Most importantly, Drosomycin transcript levels were higher in the gut of yw control larvae than in the gut of Jonah66Ci knockdown larvae infected with symbiotic nematodes (P = 0.0004) (Fig. 4A and Table S5).
In contrast, we found significantly higher levels of Defensin in gutless Jonah66Ci knockdown larvae responding to symbiotic nematodes than in uninfected larvae (P = 0.0152) (Fig. 4B and Table S5). Defensin in gutless Jonah66Ci larvae was also significantly higher than in the gut of the knockdown larvae (P = 0.171) and the control gutless larvae (P = 0.0188) in response to symbiotic nematode infection (Fig. 4B; Table S5).
Infection with symbiotic nematodes consistently increased Diptericin in the gut and in gutless yw control and Jonah66Ci knockdown larvae (Fig. 4C; Table S5). Diptericin was significantly higher in control gutless larvae responding to symbiotic nematode infection than in uninfected gutless larvae (P = 0.0203), in those infected with axenic nematodes (P = 0.0198), or in gutless Jonah66Ci knockdown larvae infected with symbiotic nematodes (P = 0.0441) (Fig. 4C and Table S5).
Interestingly, Cecropin was significantly upregulated in the gut of Jonah66Ci knockdown larvae infected with symbiotic nematodes compared to levels in those infected with axenic nematodes (P = 0.0001) and in uninfected control larval gut (P = 0.0002) (Fig. 4D and Table S5). This increase was also statistically significant compared to Cecropin levels in gutless Jonah66Ci knockdown larvae infected with symbiotic nematodes (P = 0.0005) as well as to levels in the gut of yw control larvae infected with symbiotic nematodes (P = 0.0002) (Fig. 4D and Table S5). Cecropin was upregulated in control gutless larvae upon infection with symbiotic nematodes, but this increase was not statistically significant (Fig. 4D; Table S5). These results demonstrate that the absence of Jonah66Ci in Drosophila larvae leads to differential expression of the Toll and Imd pathway-regulated antimicrobial peptide genes in the gut and the rest of the larval body in response to infection with Steinernema nematodes.
Mitosis is reduced in Drosophila Jonah66Ci knockdown larvae in response to symbiotic Steinernema nematodes.
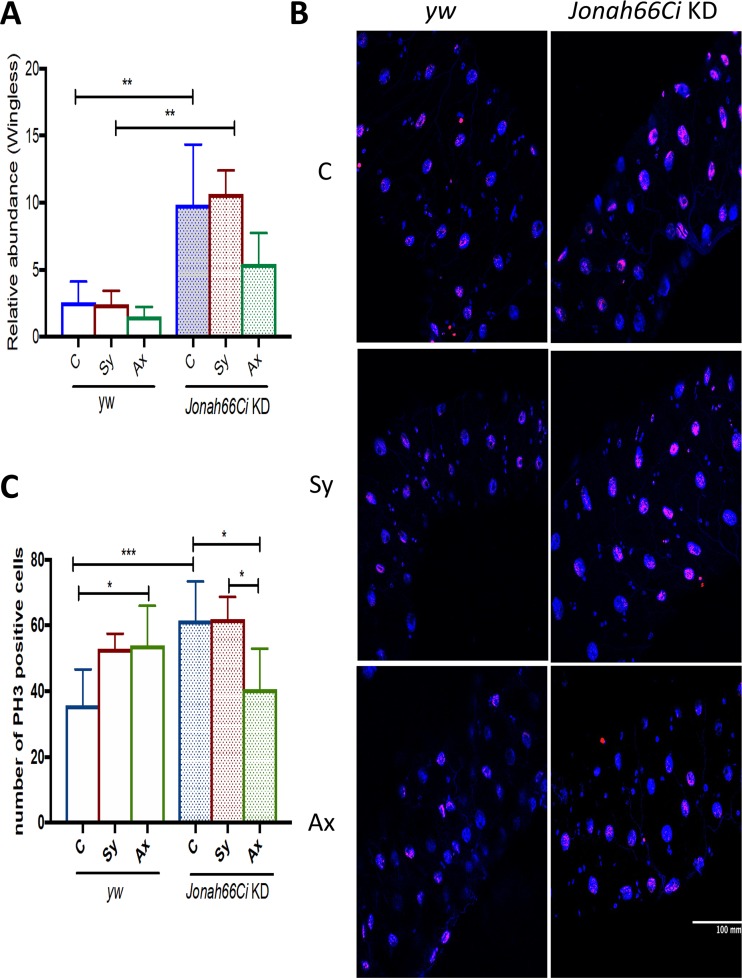
Because Jonah66Ci is entirely expressed in the gut of Drosophila larvae, we explored whether the absence of Jonah66Ci influences the activation of the gut-specific Wnt/Wg signaling pathway, which regulates gut tissue homeostasis during development (33, 34) (Fig. 5). For this, we infected yw background control and Jonah66Ci knockdown larvae with symbiotic or axenic Steinernema nematodes and estimated transcript levels of wingless, encoding a ligand of the Wnt/Wg signaling pathway, in the gut 24 h postinfection. We found that wingless was significantly upregulated in the gut of uninfected Jonah66Ci knockdown larvae compared to the level in the control line (Fig. 5A; Table S6). We also found that upon symbiotic nematode infection, wingless was upregulated in the gut of Jonah66Ci knockdown larvae compared to the level in yw control larvae (Fig. 5A; Table S6). However, we found no significant differences in wingless transcript levels between Jonah66Ci larvae infected with symbiotic and axenic nematodes or between nematode-infected and control larvae. Additionally, infection with symbiotic or axenic nematodes had no effect on wingless transcript levels in the gut of yw control larvae. Thus, these results suggest that inactivation of Jonah66Ci upregulates Wnt/Wg signaling in the gut of Drosophila larvae in the presence or absence of nematode infection.
FIG 5.
Mitosis in the intestinal cells of Drosophila Jonah66Ci knockdown larvae infected with Steinernema nematodes. (A) Relative wingless transcript levels. (B) Representative images of gut cells labeled with phospho-histone 3 (PH3; red) and DAPI (blue) at ×40 magnification. (C) Number of mitotic cells in the gut of Drosophila melanogaster yw control and Jonah66Ci knockdown larvae at 24 h postinfection with 10 symbiotic (Sy) or axenic (Ax) infective Steinernema carpocapsae juveniles. Water-treated larvae served as controls (C). Drosophila yw background control and Jonah66Ci knockdown (KD) virgin female flies were crossed with Esg-Gal4 males, and the resulting larval progeny were used for experiments. Transcript levels were estimated relative to the levels of the housekeeping gene, RpL32, and as a ratio to the level of uninfected control larvae. All experiments were repeated three times, and data analysis was performed using one-way analysis of variance (ANOVA) with Tukey’s post hoc test on GraphPad Prism, version 7. *, P < 0.05; **, P < 0.01; ***, P < 0.001; nonsignificant differences are not shown.
Wnt/Wg signaling promotes tissue regeneration in the Drosophila gut after injury (35). To investigate whether inactivation of Jonah66Ci in the gut affects tissue regeneration in response to nematode infection, we infected yw background control and Jonah66Ci knockdown larvae with symbiotic or axenic Steinernema nematodes and measured the number of mitotic cells (phospho-histone H3 [PH3] labeled) in the gut of infected and uninfected individuals (Fig. 5B). In uninfected guts, the numbers of PH3-labeled cells significantly increased in Jonah66Ci knockdown larvae compared to levels in the yw controls (P = 0.008) (Fig. 5B and C and Table S6). Interestingly, infection of yw controls with axenic nematodes significantly increased the numbers of PH3-labeled cells compared to the levels in uninfected control larvae (P = 0.0278) (Fig. 5B and C and Table S6). Also, inactivation of Jonah66Ci significantly reduced the numbers of PH3-labeled cells in the gut of larvae infected with axenic nematodes compared to levels in uninfected individuals (P = 0.0131) (Fig. 5B and C and Table S6). Additionally, the numbers of PH3-labeled cells were significantly lower in the gut of Jonah66Ci knockdown larvae infected with axenic nematodes than in those infected with symbiotic nematodes (P = 0.0148) (Fig. 5B and C and Table S6). Thus, under normal conditions, the absence of Jonah66Ci in Drosophila larvae increases the numbers of gut cells undergoing mitosis, and this effect is reduced in response to infection with axenic Steinernema nematodes.
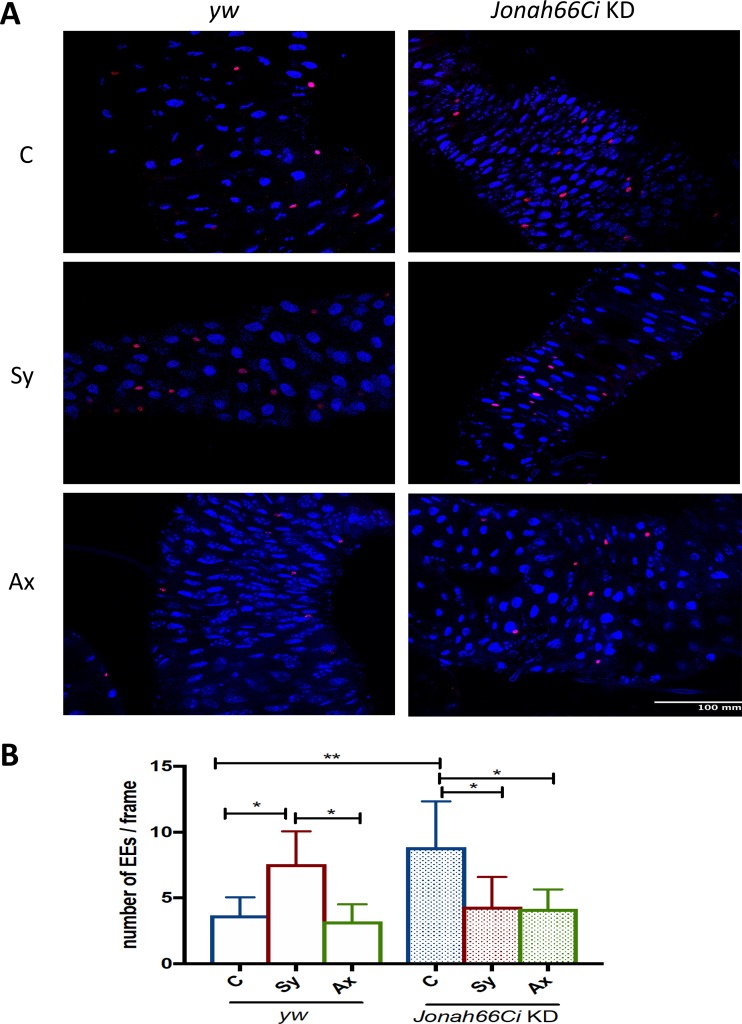
Enteroendocrine cell numbers are reduced in Drosophila Jonah66Ci knockdown larvae in response to Steinernema nematode infection.
Because inactivation of Jonah66Ci increases the numbers of mitotic cells in the gut of uninfected Drosophila larvae, we investigated whether Jonah66Ci inactivation also affects the specific cell types of the larval gut in the presence or absence of nematode infection. For this, we infected yw-background control and Jonah66Ci knockdown larvae with symbiotic or axenic Steinernema nematodes and estimated the numbers of enteroendocrine (EE) cells (Prospero labeled) in the gut of infected and uninfected individuals (Fig. 6; Table S7). In uninfected guts, the numbers of EE cells were significantly higher in Jonah66Ci knockdown larvae than in yw control larvae (P = 0.0048) (Fig. 6B and Table S7). Interestingly, infection of yw control larvae with symbiotic nematodes increased significantly the numbers of EE cells compared to levels in uninfected control larvae (P = 0.0286) (Fig. 6B and Table S7) and in yw control larvae infected with axenic nematodes (P = 0.0179) (Fig. 6B and Table S7). Conversely, inactivation of Jonah66Ci reduced significantly the numbers of EE cells in the gut of larvae infected with symbiotic or axenic nematodes compared to levels in uninfected controls (P = 0.0182 or P = 0.0131, respectively) (Fig. 6B and Table S7). Thus, under normal conditions, inactivation of Jonah66Ci in Drosophila larvae increases the numbers EE cells, which are conversely reduced in response to Steinernema nematode infection.
FIG 6.
Enteroendocrine cell numbers in Drosophila Jonah66Ci knockdown larvae infected with Steinernema nematodes. (A) Representative images of gut cells labeled with Prospero (red) and DAPI (blue) at ×40 magnification. (B) Number of enteroendocrine cells in the gut of Drosophila melanogaster yw control and Jonah66Ci knockdown (KD) larvae at 24 h postinfection with 10 symbiotic (Sy) or axenic (Ax) infective Steinernema carpocapsae juveniles. Water-treated larvae served as controls (C). Drosophila yw background control and Jonah66Ci knockdown virgin female flies were crossed with Esg-Gal4 males, and the resulting larval progeny were used for experiments. All experiments were repeated three times, and data analysis was performed using one-way analysis of variance (27) with Tukey’s post hoc test on GraphPad Prism, version 7. *, P < 0.05; **, P < 0.01; nonsignificant differences are not shown.
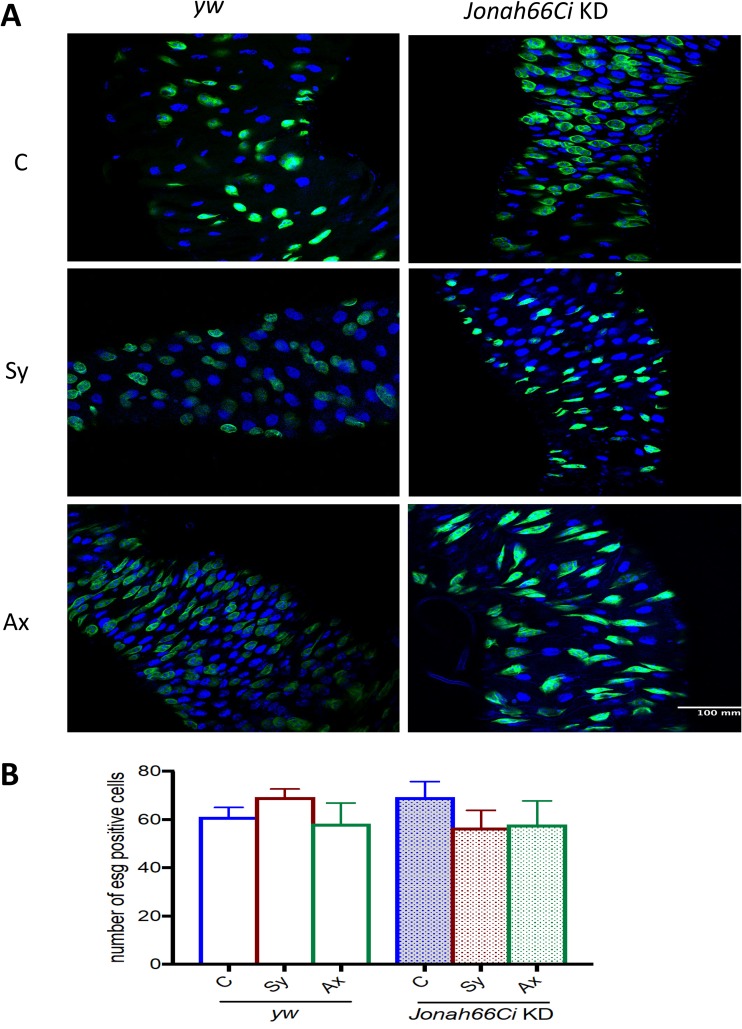
Intestinal stem cell numbers are unaffected in Drosophila Jonah66Ci knockdown larvae in response to Steinernema nematode infection.
Because inactivation of Jonah66Ci increases the numbers of EE cells as well as the numbers of cells undergoing mitosis in uninfected larvae, we investigated whether inactivation of Jonah66Ci in the gut affects the stem cell population in the presence or absence of nematode infection. For this, we infected yw background control and Jonah66Ci knockdown larvae with symbiotic or axenic Steinernema nematodes and assessed the number of intestinal stem cells (ISCs; escargot labeled) in the gut of infected and uninfected individuals (Fig. 7; Table S8). Interestingly, we found no changes in the ISC populations in the gut of yw control larvae and Jonah66Ci knockdown larvae, with or without nematode infection. These results suggest that inactivation of Jonah66Ci in Drosophila larvae infected with Steinernema nematodes has no effect on the numbers of ISCs.
FIG 7.
Intestinal stem cell numbers in Drosophila Jonah66Ci knockdown larvae infected with Steinernema nematodes. (A) Representative images of larval guts expressing the esg→gfp driver (green) and labeled with DAPI (blue) at ×40 magnification. (B) Number of intestinal stem cells in the gut of Drosophila melanogaster yw control and Jonah66Ci knockdown larvae at 24 h postinfection with 10 symbiotic (Sy) or axenic (Ax) infective Steinernema carpocapsae juveniles. Water-treated larvae served as controls (C). Drosophila yw background control and Jonah66Ci knockdown (KD) virgin female flies were crossed with Esg-Gal4 males, and the resulting larval progeny were used for experiments. All experiments were repeated three times, and data analysis was performed using one-way analysis of variance (ANOVA) with Tukey’s post hoc test on GraphPad Prism, version 7. Differences were nonsignificant.
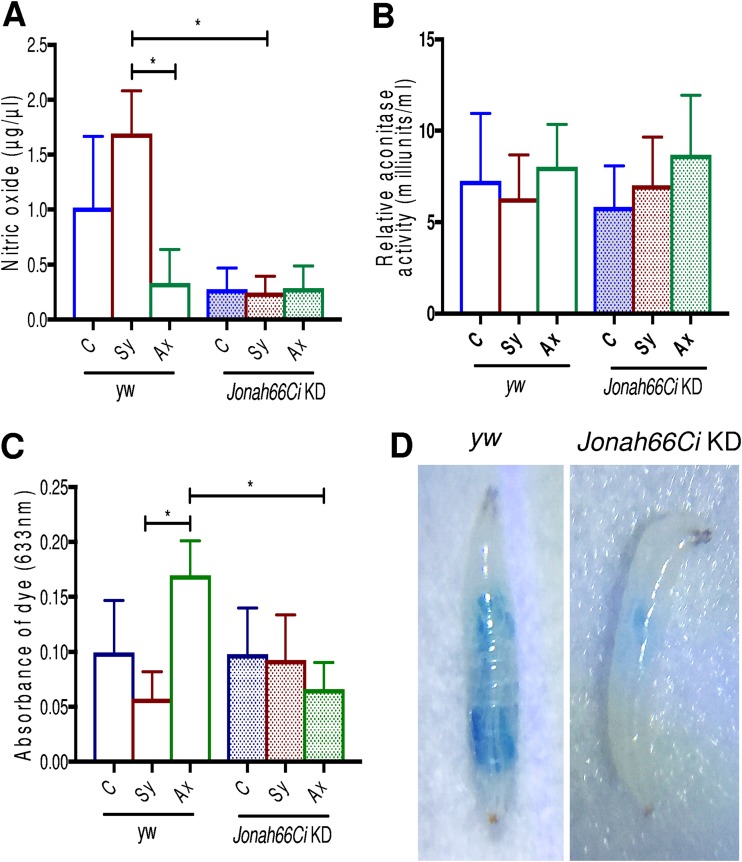
NO, but not ROS or feeding, is reduced in Drosophila Jonah66Ci knockdown larvae in response to symbiotic Steinernema nematodes.
To determine whether certain physiological processes in Drosophila are affected by the absence of Jonah66Ci in the context of nematode infection, we measured nitric oxide (NO) and reactive oxygen species (ROS) levels as well as feeding rates at 24 h postinfection of larvae with symbiotic or axenic Steinernema nematodes (Fig. 8; Table S9). We found that nitric oxide levels increased in yw control larvae infected with symbiotic nematodes compared to levels in those infected with axenic nematodes (P = 0.0320) and to those in Jonah66Ci knockdown larvae infected with symbiotic worms (P = 0.0123) (Fig. 8A and Table S9). There were no changes in nitric oxide in Jonah66Ci knockdown larvae in the presence or absence of nematode infection. We also measured ROS levels by estimating the relative aconitase activity in yw control and Jonah66Ci knockdown larvae at 24 h post-nematode infection. We found no changes in aconitase activity levels between yw control and Jonah66Ci knockdown larvae upon nematode infection or under normal conditions (P > 0.05) (Fig. 8B and Table S9). We also measured the feeding rates in yw control and Jonah66Ci knockdown larvae at 24 h post-nematode infection. We found that feeding rates increased in background control larvae upon infection with axenic Steinernema and that this increase was significantly higher than that in yw control larvae upon infection with symbiotic nematodes (P = 0.0204) and that in Jonah66Ci knockdown larvae upon infection with axenic worms (P = 0.0249) (Fig. 8C and D and Table S9). These results indicate that inactivation of Jonah66Ci decreases NO levels in Drosophila larvae upon symbiotic Steinernema nematode infection.
FIG 8.
Nitric oxide and aconitase activity levels and feeding rates in Drosophila Jonah66Ci knockdown larvae infected with Steinernema nematodes. (A and B) Relative nitric oxide (NO) and aconitase activity levels in the gut. (C) Spectrophotometric analysis of food intake in Drosophila melanogaster yw control and Jonah66Ci knockdown larvae at 24 h postinfection with symbiotic (Sy) or axenic (Ax) infective Steinernema carpocapsae juveniles. Water-treated larvae served as controls (C). (D) Feeding rate of yw control and Jonah66Ci knockdown larvae at 24 h postinfection with axenic S. carpocapsae nematodes. Drosophila yw background control and Jonah66Ci knockdown (KD) virgin female flies were crossed with Esg-Gal4 males, and the resulting larval progeny were used for experiments. NO and aconitase activity levels were measured relative to total protein. Experiments were repeated three times and analyzed using one-way analysis of variance (ANOVA) with a Tukey’s post hoc test on GraphPad Prism, version 7. *, P < 0.05; nonsignificant differences are not shown.
DISCUSSION
In this study, we investigated the immune and pathophysiological effects of Jonah66Ci in Drosophila larvae in the context of nematode infection. First, we showed that Jonah66Ci is expressed in the gut of Drosophila larvae in the presence or absence of Steinernema nematode infection. Then, we monitored the survival ability of wild-type and Jonah66Ci knockdown larvae in response to Steinernema nematode infection. We also evaluated the differential induction of the Toll and Imd pathway effector genes, quantified the mitotic cells in the gut, estimated the numbers of EE cells and ISCs, and measured the NO and ROS activity levels as well as feeding rates of wild-type and Jonah66Ci knockdown larvae during symbiotic or axenic nematode infection or under normal conditions. We report that the serine protease-encoding gene Jonah66Ci plays an essential role in maintaining homeostasis in the gut of Drosophila larvae upon infection with a potent nematode parasite.
Because we detected expression of Jonah66Ci only in the gut of uninfected and nematode-infected Drosophila larvae, we hypothesized that Jonah66Ci controls physiological processes and signaling pathways specific to this tissue. We found that inactivation of Jonah66Ci in the gut of Drosophila larvae responding to symbiotic or axenic Steinernema nematode infection resulted in differential expression of the Toll pathway readout genes Drosomycin and Defensin and of the Imd pathway genes Diptericin and Cecropin. A previous study reported an increase in expression of Drosomycin and Diptericin in Drosophila flies injected with Xenorhabdus, the mutualistic bacterium of Steinernema nematodes (36). Our data are in agreement with this study since we found upregulation of these two AMP-encoding genes in the gut of Drosophila yw control larvae responding to Steinernema nematodes carrying mutualistic Xenorhabdus bacteria compared to levels in larvae responding to axenic nematodes. In contrast, we found reduced expression of Drosomycin and Diptericin in the gut of Jonah66Ci knockdown larvae infected with the same type of nematode, implying that Jonah66Ci regulation of the Toll and Imd signaling activities in the gut of Drosophila infected with Steinernema nematodes is closely associated with the presence of Xenorhabdus bacteria. This finding is in agreement with a previous report indicating that expression of Toll and Imd effector genes in Drosophila is higher in the case of symbiotic nematode infections than in axenic nematode infections, which is probably due to the effect of Xenorhabdus bacteria on the insect host (7, 37). We also found that Defensin is upregulated in the Jonah66Ci gutless knockdown larvae infected with axenic Steinernema and is found at even higher levels in infection with symbiotic nematodes. However, both types of nematodes fail to upregulate this AMP in guts of wild-type larvae or gutless individuals (7). This suggests that Jonah66Ci interacts closely with Toll signaling in larvae responding to Steinernema nematode infections. Interestingly, Defensin is upregulated in thioester-containing protein-4 mutant flies responding to Photorhabdus luminescens or Photorhabdus asymbiotica bacterial infection, and this correlates with resistance of the mutant flies to infection (38). Our findings are in agreement with the results in this previous study as we found upregulation of Defensin in Jonah66Ci knockdown larvae following axenic Steinernema nematode infection, which was accompanied by higher survival of the knockdown larvae. The differential induction of Toll and Imd pathway effector genes suggests that inactivation of Jonah66Ci regulates immune signaling not only in the gut of Drosophila larvae but also in other immune tissues, probably the fat body or hemolymph, which might alter the survival response against parasitic nematode infection.
The Drosophila midgut is lined with approximately 2,000 ISCs. These multipotent ISCs give rise to two types of differentiated daughter cells, the secretory enteroendocrine cells and the absorptive enterocytes. Collectively these cells form the monolayer that line the Drosophila midgut (39–41). In the case of gut epithelial damage or stress such as bacterial infection, ISCs are able to produce new cells to replace the damaged epithelial cells and regenerate the gut (42). A previous study using Drosophila adult flies has indicated that inactivation of adenomatous polyposis coli (Apc), a tumor suppressor gene found in the intestinal epithelium, results in a significant increase in the numbers of cells undergoing mitosis (39). This finding agrees with our data since we also found that, in the absence of Steinernema nematode infection, the numbers of cells undergoing mitosis are significantly increased in the gut of Jonah66Ci knockdown larvae (Fig. 5B and C; see also Table S6 in the supplemental material). Thus, this suggests that Jonah66Ci, similar to Apc in Drosophila adults, is essential in maintaining homeostasis in the gut of Drosophila larvae responding to Steinernema nematodes. The upregulation of wingless in uninfected Jonah66Ci knockdown larvae also suggests that Jonah66Ci expression in the gut interferes with Wnt/Wg pathway activity in regulating cell proliferation (43). Additionally, in the absence of nematode infection, we found that the EE cell numbers were significantly increased in Jonah66Ci knockdown larvae compared to levels in background control larvae. This finding agrees with a previous report that loss of catalase function in Drosophila adult flies, Catn1 mutants, resulted in higher EE cell numbers (44). Thus, this suggests that the serine protease-encoding gene Jonah66Ci, similar to catalase in adult midguts, is responsible for maintaining gut integrity in Drosophila larvae.
Contrary to the changes in cell numbers observed above, the numbers of ISCs remained unchanged between control and Jonah66Ci knockdown larvae in the presence or absence of nematode infection. This led us to speculate that Jonah66Ci likely functions downstream of the ISCs. Further studies will have to be performed to identify the specific role of Jonah66Ci in stem cell signaling events. A previous study reported that Pseudomonas entomophila secretes hemolysin that targets and lyses the enterocytes in the gut epithelium of Drosophila adults and larvae (45, 46). Similarly, Steinernema nematodes secrete a serine protease, sc-sp-1, that functions as a virulence factor that disarms the immune system by destroying the gut lumen (47). Hence, we speculate that reduction in the numbers of mitotic and EE cells in the gut of Jonah66Ci knockdown larvae responding to Steinernema nematodes could be attributed to the virulence factors produced by Steinernema nematodes.
A previous study has reported the crucial role of NO in eliminating the eggs of the endoparasitic wasp Leptopilina heterotoma in Drosophila paramelanica larvae (48). Also, NO is essential for the survival of Drosophila flies responding to Gram-negative bacterial infection (49). We found increased NO levels in yw control larvae responding to symbiotic, but not axenic, Steinernema nematodes. This suggests that Drosophila larvae are capable of inducing a NO response against the mutualistic Xenorhabdus bacteria. We also found a reduction in NO levels in the gut of uninfected and nematode-infected Jonah66Ci knockdown larvae, suggesting a role for Jonah66Ci in regulating the NO antinematode response in Drosophila larvae.
In addition to NO, ROS has long been recognized to defend hosts against pathogen infection due to its cytotoxicity (50). Ecc15 oral infection has been shown to induce ROS stress in Drosophila adult flies, and ROS is known to control microbial growth in the host (50, 51). Altogether, ROS has been attributed to playing an important role in initiating immunological communications from gut to fat body in Drosophila. However, in the case of nematode infections, we observed no changes in ROS levels in background control or Jonah66Ci knockdown larvae. This finding demonstrates that Jonah66Ci plays no role in regulating ROS activity in the gut of Drosophila larvae in the context of Steinernema nematode infection.
We further found reduced feeding rates in Jonah66Ci knockdown larvae responding to axenic Steinernema nematodes. Infection of adult Drosophila with Drosophila C virus (DCV) increases the feeding rate of flies (27). Our data agree with this finding since we also found that yw control larvae responding to axenic Steinernema nematodes ingest significantly larger amounts of food. Because this effect is reduced in Jonah66Ci knockdown larvae upon axenic nematode infection, we postulate that Jonah66Ci is essential in regulating the food uptake of larvae during infection with parasitic nematodes.
In conclusion, we found that Jonah66Ci regulates certain gut-specific responses in Drosophila larvae responding to Steinernema infection. We showed that the absence of Jonah66Ci confers partial protection to larvae against axenic nematodes. We also showed that Jonah66Ci differentially induces the effector genes of Drosophila Toll and Imd signaling in the gut of larvae responding to symbiotic or axenic Steinernema nematodes. Finally, we showed that Jonah66Ci regulates gut-specific processes, including immune signaling, the numbers of mitotic and EE cells, and nitric oxide levels in response to nematode attack. Our findings demonstrate a novel function for the Drosophila serine protease Jonah66Ci in regulating the insect immune response to potent nematode parasites. Similar findings will pave the way toward a better understanding of the tissue-specific molecular players that modulate the insect immune response against parasitic nematodes.
MATERIALS AND METHODS
Fly lines.
Drosophila melanogaster yellow white (yw) and Jonah66Ci (v103008, FBst0474871; Vienna Drosophila Resource Centre) lines were used. Female flies from the Jonah66Ci RNA interference (RNAi) line were crossed with males from the Escargot (Esg)-Gal4 driver (w*; P{enG}esgG66/CyO, P{GAL4-Kr.C}DC3, P{UAS-GFP.S65T}DC7) (where UAS is upstream activation sequence and GFP is green fluorescent protein) (52). The knockdown of Jonah66Ci was validated using the Esg-Gal4 line (Fig. 2A). All lines were reared on Drosophila medium (Meidi Laboratories) and sprinkled with approximately 10 g of Saccharomyces cerevisiae (baker’s yeast). Stocks were maintained in a 12/12-h light/dark cycle at 25°C. Late-second- to early-third-instar larvae were used for all experiments.
Nematodes stocks.
Infective juveniles of Steinernema carpocapsae nematodes were used for all experiments. Symbiotic nematodes carrying Xenorhabdus nematophila bacteria were reared in larvae of the wax moth Galleria mellonella, as described previously (53). Axenic nematodes lacking Xenorhabdus were generated according to a previously established protocol (17). Axenic nematodes were washed in 1% bleach solution to remove bacteria from the nematode surface and rinsed five times with water to remove the bleach residue. Infective juveniles 2 to 5 weeks old were used for all experiments.
Gene transcript analysis with RNA-sequencing.
The number of reads per kilobase per million mapped reads (RPKM) for Jonah66Ci (locus CG7118) were obtained from a recent RNA sequencing study (8). The reads were obtained at 6 and 24 h postinfection of D. melanogaster Oregon larvae with 100 symbiotic or axenic infective S. carpocapsae juveniles. The RPKM values for nematode-infected larvae are shown relative to the RPKM values of uninfected control larvae at each time point.
Gene transcript analysis with quantitative RT-PCR.
Four larvae, each infected with 100 symbiotic or axenic Steinernema nematodes, were collected at 6 and 24 h postinfection for analyzing gene transcript levels using qRT-PCR. For estimating Jonah66Ci transcript levels in the gut, 10 larvae infected with 10 symbiotic or axenic nematodes were dissected at 6 and 24 h postinfection to separate the gut tissues from the rest of the larvae. In all cases, larvae treated with sterile distilled water served as the uninfected controls. Total RNA extraction was performed using Invitrogen/Ambion TRIzol reagent according to the manufacturer’s instructions. RNA extraction, cDNA synthesis, and qRT-PCR protocols were performed as described before (54). All primer sets used for qRT-PCR analyses and their respective annealing temperatures are listed in Table 1. Data were measured from technical duplicates, expressed as the ΔCT of 2CT(RpL32)/2CT(gene) and presented as a ratio of the value for infected larvae to that of the uninfected controls. Results depict mean and standard deviations from three biological replicates representing three independent experiments.
TABLE 1.
Primers used for quantitative RT-PCR
| Gene | Locus | Primer name | Sequence (5′–3′) | Tm (°C)a |
|---|---|---|---|---|
| Jonah66Ci | CG7118 | Forward | TTCATCACCCACGGATCTGC | 57 |
| Reverse | GCACTCGGAGTTGTGGATGA | |||
| Attacin-A | CG10146 | Forward | CAATGGCAGACACAATCTGG | 60 |
| Reverse | ATTCCTGGGAAGTTGCTGTG | |||
| Drosomycin | CG10810 | Forward | TGAGAACCTTTTCCAATATGATG | 60 |
| Reverse | CCAGGACCACCAGCAT | |||
| Puckered | CG7850 | Forward | GGCCTACAAGCTGGTGAAAG | 60 |
| Reverse | AGTTCAGATTGGGCGAGATG | |||
| Turandot-A | CG31509 | Forward | AGATCGTGAGGCTGACAAC | 60 |
| Reverse | CCTGGGCGTTTTTGATAA | |||
| Defensin | CG1385 | Forward | CGCATAGAAGCGAGCCACATG | 60 |
| Reverse | GCAGTAGCCGCCTTTGAACC | |||
| Diptericin | CG12763 | Forward | ACCGCAGTACCCACTCAATC | 60 |
| Reverse | CCCAAGTGCTGTCCATATCC | |||
| Cecropin-A1 | CG1365 | Forward | TCTTCGTTTTCGTCGCTCTC | 60 |
| Reverse | CTTGTTGAGCGATTCCCAGT | |||
| Wingless | CG4889 | Forward | GATTATTCCGCAGTCTGGTC | 60 |
| Reverse | CTATTATGCTTGCGTCCCTG | |||
| RpL32 | CG7939 | Forward | GATGACCATCCGCCCAGCA | 60 |
| Reverse | CGGACCGACAGCTGCTTGGC |
Tm, melting temperature.
Survival experiments.
A 96-well microtiter plate (Corning) was prepared by addition of 100 μl of 1.5% agarose gel (in 1× Tris-acetate-EDTA [TAE] buffer) to each well. A suspension (10 μl) containing 10 symbiotic or axenic Steinernema nematodes was added to each well. Application of sterile distilled water (10 μl) to larvae was used as an uninfected control treatment. An individual Drosophila larva was then added to each well, as described previously (8). For each experiment, 20 larvae per line per treatment were used, and each survival assay was repeated three times.
Immunohistochemistry.
Drosophila yw background and Jonah66Ci knockdown larvae were collected from five separate vials for each experiment. Gut samples from 10 larvae infected with 10 symbiotic or axenic Steinernema nematodes were dissected at 24 h postinfection. Gut samples from larvae treated with sterile distilled water served as uninfected controls. Gut tissues were fixed in 4% formaldehyde (Sigma) in 1× phosphate-buffered saline (PBS) for 30 min and then washed in 1× PBS containing 0.1% Triton X-100. Samples were incubated with primary and secondary antibodies in a solution consisting of 1× PBS, 0.1% Triton X-100, and 0.5% bovine serum albumin (BSA). The following primary antibodies were used: 1:500 rabbit anti-PH3 (Developmental Studies Hybridoma Bank [DSHB]) and mouse anti-Prospero (DSHB). Fluorescently labeled tissues were mounted in ProLong Diamond antifade mountant containing 4′,6′-diamidino-2-phenylindole (DAPI) nuclear stain (Life Technologies). Data were collected from gut tissues from each individual larva. Fluorescent images were obtained using an LSCM-510 Meta confocal microscope (Carl Zeiss) at ×40 magnification. Images were assembled using Adobe Photoshop (2018 release), and numbers of specific cell types (PH3 for mitotic cells, Prospero for EEs, and Gal4-UAS-GFP for ISCs) were estimated. The experiment was repeated two times.
NO and aconitase activity estimation.
Gut samples from 10 Drosophila larvae infected with 10 symbiotic or axenic Steinernema nematodes were dissected 24 h postinfection. Gut samples from larvae treated with sterile distilled water served as uninfected controls. For nitric oxide estimation, gut samples were homogenized in PBS by grinding with a sterile plastic pestle and then centrifuged at 10,000 × g for 10 min at 4°C. The resultant supernatant was mixed 1:1 with Griess reagent (Sigma), and absorbance was measured at 595 nm using a plate reader (BioTek). Nitric oxide (NO) levels were calculated from a silver nitrite standard curve. For aconitase activity estimation, gut samples were homogenized in aconitase assay buffer and processed according to the manufacturer’s instructions (MAK051-1KT; Sigma), and absorbance was measured at 450 nm. Aconitase activity levels were calculated from an isocitrate standard curve. Both NO and aconitase activity levels were represented relative to total protein content in each sample. Protein quantification was performed as described previously (55). The experiment was repeated three times.
Feeding rate.
Ten Drosophila larvae from each line were infected with 10 symbiotic or axenic Steinernema nematodes or treated with sterile distilled water and then collected at 24 h postinfection. All larvae were fed on yeast paste containing 0.16% erioglaucine disodium salt (FD&C blue no. 1; Sigma) for 15 min. Larvae from each line were starved for 24 h and served as background controls. The protocol for spectrophotometric detection of the food dye has been described previously (56). Sample supernatants (200 μl each), obtained from centrifuging the larval homogenates, were loaded into a 96-well plate (Corning) and measured as the optical density at 633 nm (OD633) using a plate reader (BioTek). The experiment was repeated three times.
Statistical analysis.
For gene transcript level analysis, immunohistochemistry, nitric oxide estimation, and feeding rate, data analysis was performed using one-way analysis of variance (ANOVA) with a Tukey post hoc test for multiple comparisons and an unpaired two-tailed t test. For survival experiments, a log rank (Mantel-Cox) and chi-square tests were performed. P values lower than 0.05 were considered statistically significant. All figures were generated using GraphPad Prism, version 7, software.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kyle Devine and Sonali Gupta for maintaining the Drosophila stocks and members of the Department of Biological Sciences at The George Washington University for critical reading of the manuscript. We also thank Sneh Harsh for assistance with microscopy and imaging.
Research in the Eleftherianos lab is supported by the National Institute of Allergy and Infectious Diseases.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00094-19.
REFERENCES
- 1.Kounatidis I, Ligoxygakis P. 2012. Drosophila as a model system to unravel the layers of innate immunity to infection. Open Biol 2:120075. doi: 10.1098/rsob.120075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hetru C, Hoffmann JA. 2009. NF-κB in the immune response of Drosophila. Cold Spring Harb Perspect Biol 1:a000232. doi: 10.1101/cshperspect.a000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann JA, Reichhart JM. 2002. Drosophila innate immunity: an evolutionary perspective. Nat Immunol 3:121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 4.Tanji T, Hu X, Weber AN, Ip YT. 2007. Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol Cell Biol 27:4578–4588. doi: 10.1128/MCB.01814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arefin B, Kucerova L, Dobes P, Markus R, Strnad H, Wang Z, Hyrsl P, Zurovec M, Theopold U. 2014. Genome-wide transcriptional analysis of Drosophila larvae infected by entomopathogenic nematodes shows involvement of complement, recognition and extracellular matrix proteins. J Innate Immun 6:192–204. doi: 10.1159/000353734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallem EA, Rengarajan M, Ciche TA, Sternberg PW. 2007. Nematodes, bacteria, and flies: a tripartite model for nematode parasitism. Curr Biol 17:898–904. doi: 10.1016/j.cub.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Pena JM, Carrillo MA, Hallem EA. 2015. Variation in the susceptibility of Drosophila to different entomopathogenic nematodes. Infect Immun 83:1130–1138. doi: 10.1128/IAI.02740-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav S, Daugherty S, Shetty AC, Eleftherianos I. 2017. RNAseq analysis of the Drosophila response to the entomopathogenic nematode Steinernema. G3 (Bethesda) 7:1955–1967. doi: 10.1534/g3.117.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo JC, Reynolds SE, Eleftherianos I. 2011. Insect immune responses to nematode parasites. Trends Parasitol 27:537–547. doi: 10.1016/j.pt.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Silverman N, Maniatis T. 2001. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev 15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- 11.Stock SP. 2005. Insect-parasitic nematodes: from lab curiosities to model organisms. J Invertebr Pathol 89:57–66. doi: 10.1016/j.jip.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Goodrich-Blair H. 2007. They've got a ticket to ride: Xenorhabdus nematophila-Steinernema carpocapsae symbiosis. Curr Opin Microbiol 10:225–230. doi: 10.1016/j.mib.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Ciche TA, Ensign JC. 2003. For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl Environ Microbiol 63:1890–1897. doi: 10.1128/AEM.69.4.1890-1897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens EC, Goodrich-Blair H. 2005. The Steinernema carpocapsae intestinal vesicle contains a subcellular structure with which Xenorhabdus nematophila associates during colonization initiation. Cell Microbiol 7:1723–1735. doi: 10.1111/j.1462-5822.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- 15.Goodrich-Blair H, Clarke DJ. 2007. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Mol Microbiol 64:260–268. doi: 10.1111/j.1365-2958.2007.05671.x. [DOI] [PubMed] [Google Scholar]
- 16.Richards GR, Goodrich-Blair H. 2009. Masters of conquest and pillage: Xenorhabdus nematophila global regulators control transitions from virulence to nutrient acquisition. Cell Microbiol 11:1025–1033. doi: 10.1111/j.1462-5822.2009.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav S, Shokal U, Forst S, Eleftherianos I. 2015. An improved method for generating axenic entomopathogenic nematodes. BMC Res Notes 8:461. doi: 10.1186/s13104-015-1443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyrsl P, Dobes P, Wang Z, Hauling T, Wilhelmsson C, Theopold U. 2011. Clotting factors and eicosanoids protect against nematode infections. J Innate Immun 3:65–70. doi: 10.1159/000320634. [DOI] [PubMed] [Google Scholar]
- 19.Kucerova L, Broz V, Arefin B, Maaroufi HO, Hurychova J, Strnad H, Zurovec M, Theopold U. 2016. The Drosophila chitinase-like protein IDGF3 is involved in protection against nematodes and in wound healing. J Innate Immun 8:199–210. doi: 10.1159/000442351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav S, Eleftherianos I. 2018. The imaginal disc growth factors 2 and 3 participate in the Drosophila response to nematode infection. Parasite Immunol 40:e12581. doi: 10.1111/pim.12581. [DOI] [PubMed] [Google Scholar]
- 21.Carlson JR, Hogness DS. 1985. The Jonah genes: a new multigene family in Drosophila melanogaster. Dev Biol 108:341–354. doi: 10.1016/0012-1606(85)90038-7. [DOI] [PubMed] [Google Scholar]
- 22.Carlson JR, Hogness DS. 1985. Developmental and functional analysis of Jonah gene expression. Dev Biol 108:355–368. doi: 10.1016/0012-1606(85)90039-9. [DOI] [PubMed] [Google Scholar]
- 23.Hafen E, Levine M, Garber RL, Gehring WJ. 1983. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J 2:617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akam ME, Carlson JR. 1985. The detection of Jonah gene transcripts in Drosophila by in situ hybridization. EMBO J 4:155–161. doi: 10.1002/j.1460-2075.1985.tb02330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Ayala DJ, Chen S, Kemppainen E, O'Dell KM, Jacobs HT. 2010. Gene expression in a Drosophila model of mitochondrial disease. PLoS One 5:e8549. doi: 10.1371/journal.pone.0008549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpenter J, Hutter S, Baines JF, Roller J, Saminadin-Peter SS, Parsch J, Jiggins FM. 2009. The transcriptional response of Drosophila melanogaster to infection with the sigma virus (Rhabdoviridae). PLoS One 4:e6838. doi: 10.1371/journal.pone.0006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chtarbanova S, Lamiable O, Lee KZ, Galiana D, Troxler L, Meignin C, Hetru C, Hoffmann JA, Daeffler L, Imler JL. 2014. Drosophila C virus systemic infection leads to intestinal obstruction. J Virol 88:14057–14069. doi: 10.1128/JVI.02320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brun S, Vidal S, Spellman P, Takahashi K, Tricoire H, Lemaitre B. 2006. The MAPKKK Mekk1 regulates the expression of Turandot stress genes in response to septic injury in Drosophila. Genes Cells 11:397–407. doi: 10.1111/j.1365-2443.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- 29.Imler JL, Bulet P. 2005. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem Immunol Allergy 86:1–21. doi: 10.1159/000086648. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko T, Silverman N. 2005. Bacterial recognition and signalling by the Drosophila IMD pathway. Cell Microbiol 7:461–469. doi: 10.1111/j.1462-5822.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- 31.McEwen DG, Peifer M. 2005. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development 132:3935–3946. doi: 10.1242/dev.01949. [DOI] [PubMed] [Google Scholar]
- 32.Buchon N, Silverman N, Cherry S. 2014. Immunity in Drosophila melanogaster—from microbial recognition to whole-organism physiology. Nat Rev Immunol 14:796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perochon J, Carroll LR, Cordero JB. 2018. Wnt signaling in intestinal stem cells: lessons from mice and flies. Genes (Basel) 4:E138. doi: 10.3390/genes9030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinhart Z, Angers S. 2018. Wnt signaling in development and tissue homeostasis. Development 145:dev146589. doi: 10.1242/dev.146589. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Hodgson JJ, Buchon N. 2017. Drosophila as a model for homeostatic, antibacterial, and antiviral mechanisms in the gut. PLoS Pathog 13:e1006277. doi: 10.1371/journal.ppat.1006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aymeric JL, Givaudan A, Duvic B. 2010. Imd pathway is involved in the interaction of Drosophila melanogaster with the entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus luminescens. Mol Immunol 47:2342–2348. doi: 10.1016/j.molimm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Castillo JC, Shokal U, Eleftherianos I. 2013. Immune gene transcription in Drosophila adult flies infected by entomopathogenic nematodes and their mutualistic bacteria. J Insect Physiol 59:179–185. doi: 10.1016/j.jinsphys.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Shokal U, Eleftherianos I. 2017. Thioester-containing protein-4 regulates the Drosophila immune signaling and function against the pathogen Photorhabdus. J Innate Immun 9:83–93. doi: 10.1159/000450610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee WC, Beebe K, Sudmeier L, Micchelli CA. 2009. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development 136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 40.Micchelli CA, Perrimon N. 2006. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 41.Ohlstein B, Spradling A. 2006. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 42.Buchon N, Poidevin M, Kwon HM, Guillou A, Sottas V, Lee BL, Lemaitre B. 2009. A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci U S A 106:12442–12447. doi: 10.1073/pnas.0901924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bejsovec A. 2013. Wingless/Wnt signaling in Drosophila: the pattern and the pathway. Mol Reprod Dev 80:882–894. doi: 10.1002/mrd.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. 2008. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell 7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liehl P, Blight M, Vodovar N, Boccard F, Lemaitre B. 2006. Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog 2:e56. doi: 10.1371/journal.ppat.0020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang J, Bandura J, Zhang P, Jin Y, Reuter H, Edgar BA. 2017. EGFR-dependent TOR-independent endocycles support Drosophila gut epithelial regeneration. Nat Commun 8:15125. doi: 10.1038/ncomms15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toubarro D, Lucena-Robles M, Nascimento G, Santos R, Montiel R, Veríssimo P, Pires E, Faro C, Coelho AV, Simões N. 2010. Serine protease-mediated host invasion by the parasitic nematode Steinernema carpocapsae. J Biol Chem 285:30666–30675. doi: 10.1074/jbc.M110.129346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carton Y, Frey F, Nappi AJ. 2009. Parasite-induced changes in nitric oxide levels in Drosophila paramelanica. J Parasitol 95:1134–1141. doi: 10.1645/GE-2091.1. [DOI] [PubMed] [Google Scholar]
- 49.Eleftherianos I, More K, Spivack S, Paulin E, Khojandi A, Shukla S. 2014. Nitric oxide levels regulate the immune response of Drosophila melanogaster reference laboratory strains to bacterial infections. Infect Immun 82:4169–4181. doi: 10.1128/IAI.02318-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu SC, Liao CW, Pan RL, Juang JL. 2012. Infection-induced intestinal oxidative stress triggers organ-to-organ immunological communication in Drosophila. Cell Host Microbe 11:410–417. doi: 10.1016/j.chom.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Lemaitre B, Hoffmann J. 2007. The host defense of Drosophila melanogaster. Annu Rev Immunol 25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 52.Le Bras S, Van Doren M. 2006. Development of the male germline stem cell niche in Drosophila. Dev Biol 294:92–103. doi: 10.1016/j.ydbio.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 53.White GF. 1927. A method for obtaining infective nematode larvae from cultures. Science 66:302–303. doi: 10.1126/science.66.1709.302-a. [DOI] [PubMed] [Google Scholar]
- 54.Shokal U, Yadav S, Atri J, Accetta J, Kenney E, Banks K, Katakam A, Jaenike J, Eleftherianos I. 2016. Effects of co-occurring Wolbachia and Spiroplasma endosymbionts on the Drosophila immune response against insect pathogenic and non-pathogenic bacteria. BMC Microbiol 16:16. doi: 10.1186/s12866-016-0634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yadav S, Frazer J, Banga A, Pruitt K, Harsh S, Jaenike J, Eleftherianos I. 2018. Endosymbiont-based immunity in Drosophila melanogaster against parasitic nematode infection. PLoS One 13:e0192183. doi: 10.1371/journal.pone.0192183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaun KR, Riedl CA, Chakaborty-Chatterjee M, Belay AT, Douglas SJ, Gibbs AG, Sokolowski MB. 2007. Natural variation in food acquisition mediated via a Drosophila cGMP-dependent protein kinase. J Exp Biol 210:3547–3558. doi: 10.1242/jeb.006924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.