Mycoplasma gallisepticum is an avian respiratory and reproductive tract pathogen that has a significant economic impact on the poultry industry worldwide. Although membrane proteins of Mycoplasma spp. are thought to play crucial roles in host interactions, very few have had their biochemical function defined. In this study, we found that the GroEL protein (heat shock protein 60) of Mycoplasma gallisepticum could induce apoptosis in peripheral blood mononuclear cells, and the underlying molecular mechanism was further determined.
KEYWORDS: Mycoplasma gallisepticum, host-pathogen interactions
ABSTRACT
Mycoplasma gallisepticum is an avian respiratory and reproductive tract pathogen that has a significant economic impact on the poultry industry worldwide. Although membrane proteins of Mycoplasma spp. are thought to play crucial roles in host interactions, very few have had their biochemical function defined. In this study, we found that the GroEL protein (heat shock protein 60) of Mycoplasma gallisepticum could induce apoptosis in peripheral blood mononuclear cells, and the underlying molecular mechanism was further determined. The GroEL gene from Mycoplasma gallisepticum was cloned and expressed in Escherichia coli to facilitate the functional analysis of recombinant protein. The purified GroEL protein was shown to adhere to peripheral blood mononuclear cells (PBMCs) and DF-1 cells and cause apoptosis in PBMCs. A protein pulldown assay coupled with mass spectrometry identified that annexin A2 possibly interacted with GroEL protein. Coimmunoprecipitation assays confirmed that GroEL proteins could bind to annexin A2, and confocal analysis further demonstrated that GroEL colocolized with annexin A2 in HEK293T cells and PBMCs. Moreover, annexin A2 expression was significantly induced by a recombinant GroEL protein in PBMCs, and knocking down annexin A2 expression resulted in significantly reduced apoptosis. Taken together, these data suggest that GroEL induces apoptosis in host cells by interacting with annexin A2, a novel virulence mechanism in Mycoplasma gallisepticum. Our findings lead to a better understanding of molecular pathogenesis in Mycoplasma gallisepticum.
INTRODUCTION
Mycoplasma gallisepticum, an avian respiratory and reproductive tract pathogen, has a significant economic impact on the poultry industry worldwide (1). M. gallisepticum affects the respiratory tract, causing significant inflammation in the air sacs, lungs, and trachea, as well as the reproductive tract, resulting in decreased weight gain and egg production (2). The M. gallisepticum genome of the Rlow strain contains 996,442 nucleotides with an overall G+C content of 31 mol% and 742 putative coding DNA sequences (3).
M. gallisepticum membrane proteins play important roles in adhesion, nutrient transport, and host colonization (4–7). Many M. gallisepticum membrane proteins are shown to be components of solute transport systems or involved in antigenic variation and cytoadherence (8–10). M. gallisepticum has developed a wide array of surface molecules that are involved in adherence to the host cells (11, 12). Some cytoadhesion proteins are essential for its virulence (4). The membrane surface proteins of Mycoplasma bovis undergo substantial antigenic variation involving high-frequency phenotypic switching, resulting in an increased ability to evade the host immune system (13–15). M. bovis is capable of invading bovine peripheral blood mononuclear cells (PBMCs) and inducing immune cell apoptosis (16). It was reported that M. gallisepticum can invade host cells and multiply intracellularly, and this cell invasion capacity contributes to the systemic spread of M. gallisepticum (17).
Heat shock proteins (HSPs) are a family of highly conserved proteins that stabilize cellular proteins under a variety of conditions, such as heat shock, infection, and inflammation (18, 19). Some HSPs located on the cell surface facilitate pathogen adherence to host cells and, therefore, play key roles in virulence (20). HSP60 (also known as GroEL) belongs to the HSP family. Kol et al. reported that chlamydial HSP60 can adhere to human endothelial cells and macrophages and induce inflammatory responses and host cell apoptosis (21). The M. gallisepticum R strain also carries the GroEL gene (MGA0152). The 1,605-bp open reading frame (ORF) encodes a protein with a molecular mass of 60 kDa that shares 66.5% sequence identity with chlamydial HSP60 by sequence analysis. Despite the importance of GroEL in the virulence of other bacterial pathogens (21, 22), there are no reports about the biological functions and/or virulence mechanisms of GroEL (HSP60) in M. gallisepticum. In this study, we investigated the characteristics of a recombinant form of GroEL (rGroEL) of M. gallisepticum in adhering to cells of the host cell line DF-1 and PBMCs and the mechanism of apoptosis induction. The results showed that recombinant GroEL protein can induce PBMC apoptosis by adhering to annexin A2 and inducing annexin A2 expression.
RESULTS
Adherence of recombinant GroEL (rGroEL) to DF-1 cells and PBMCs.
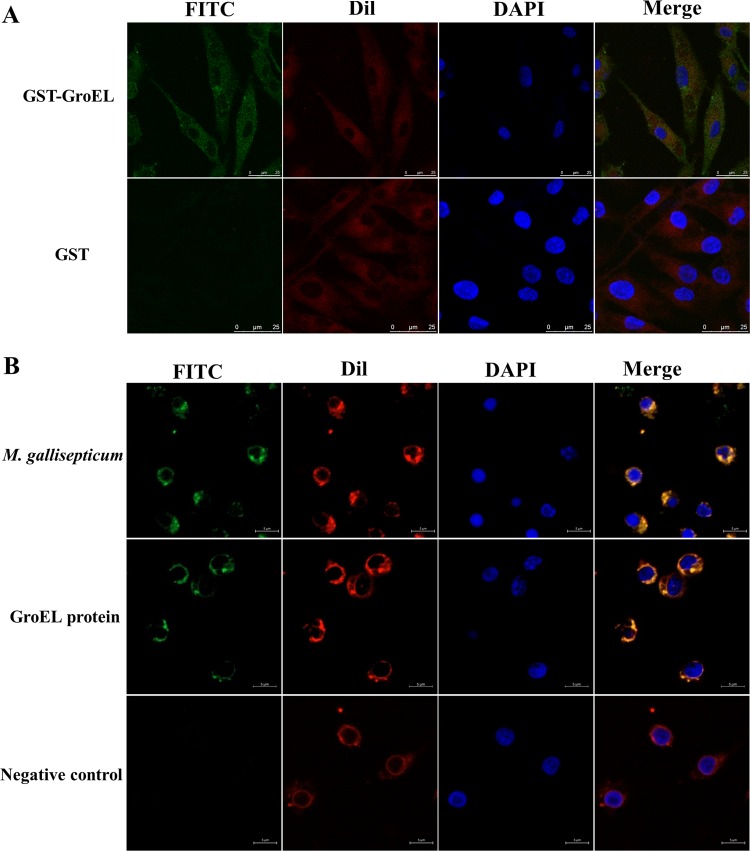
To explore the biological function of GroEL from M. gallisepticum, His-tagged (His-GroEL) and glutathione S-transferase (GST)-tagged (GST-GroEL) proteins were expressed in E. coli DE3 and purified using a high-affinity Ni-nitrilotriacetic acid (NTA) resin column (GE) or glutathione Sepharose 4B (GE). The adhesion of the GST-GroEL protein to DF-1 cells and His-GroEL to PBMCs was determined by incubation of purified GroEL proteins with cells from two cell lines and visualization by laser scanning confocal microscopy. As shown by the results in Fig. 1, M. gallisepticum GST-GroEL adhered to DF-1 cells (Fig. 1A) and His-GroEL adhered to PBMCs (Fig. 1B). These results indicated that the GroEL protein could interact with DF-1 cells and PBMCs by direct adherence to the cells. Additionally, when PBMCs cultured in 6-well plates were infected with 108 CFU M. gallisepticum Rlow for 12 h, M. gallisepticum could also adhere to the cells and was located on the cell surface of the PBMCs (Fig. 1B).
FIG 1.
Adherence characteristics of GST-GroEL or His-GroEL to DF-1 cells and PBMCs as detected by confocal laser scanning microscopy. (A) GST-GroEL adhering to DF-1 cells. (B) M. gallisepticum R strain cells and His-GroEL adhering to PBMCs. The attached GST-GroEL or His-GroEL protein and M. gallisepticum cells were immunostained with mouse anti-GroEL MAb and goat anti-mouse IgG-FITC (green). The DF-1 cell/PBMC membranes were labeled with DiI (19-dioctadecyl-3,3,39,39-tetramethylindocarbocyanine perchlorate) (red), and the cell nuclei were counterlabeled with DAPI (4′,6-diamidino-2-phenylindole) (blue). GST protein was added to DF-1 cells/PBMCs as the negative control; scale bars represent 25 μm and 5 μm for DF-1 cells (A) and PBMCs (B), respectively.
GroEL protein induces apoptosis in PBMCs.
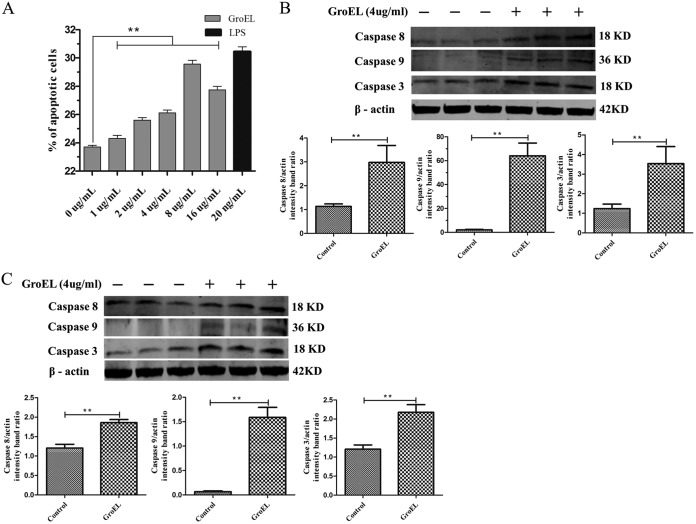
To determine if GroEL protein from M. gallisepticum causes any cytopathic effect on cells, PBMCs were treated with or without the His-GroEL protein for 24 h and stained with annexin V and PI to detect the rate of cell death by flow cytometry (Fig. 2A). The results showed significant differences in the proportions of apoptotic cells; namely, 23.2% ± 0.1% of the cells were apoptotic in the nontreated, negative-control group, while 28.8% ± 0.5% of the cells were apoptotic in the groups treated with 8 μg/ml His-GroEL protein. These results demonstrated a dose-dependent apoptosis induction effect of 2 to 8 μg/ml His-GroEL protein on PBMCs (P < 0.01).
FIG 2.
Apoptosis induced by His-GroEL protein in PBMCs as detected by flow cytometry and Western blotting. (A) Apoptosis induced by GroEL protein in PBMCs. PBMCs were treated with different concentrations of His-GroEL protein for 24 h in order to identify apoptotic cells using an annexin V-FITC apoptosis detection kit. Untreated cells were used as the negative control. All assays were performed with three independent experiments, and apoptosis was analyzed by flow cytometry. (B, C) Levels of proteins related to apoptosis in PBMCs induced by 4 μg/ml His-GroEL protein for 12 h or 24 h. His-GroEL protein induced changes in caspase-3, -8 and -9 in PBMCs treated for 12 h (B) and 24 h (C). The PBMCs were lysed, and Western blotting was performed. Western blotting results were analyzed digitally, and the optical density ratio was calculated. The data are presented as the mean values and standard deviations from three independent experiments. **, P < 0.01 compared with the results for the control (Wilcoxon rank sum test).
The activation of caspase-3, caspase-8, and caspase-9 is considered a hallmark of apoptosis; therefore, we used Western blot analysis to investigate the cleavage of caspase-3, caspase-8, and caspase-9 in PBMCs stimulated with 4 μg/ml His-GroEL protein for 12 h and 24 h. The results showed that, compared with the results for untreated control cells, His-GroEL-treated cells exhibited significant activation of caspase-3, caspase-8, and caspase-9 (Fig. 2B and C). At 12 h, His-GroEL protein stimulation had induced caspase-8 activation (P < 0.01) (Fig. 2B), and the activation of both caspase-3 and caspase-9 had occurred at 12 and 24 h (P < 0.01) (Fig. 2B and C). These results demonstrated that initiator caspases (mainly caspase-8 and caspase-9) and effector caspases (caspase-3) were activated during GroEL protein stimulation of PBMCs.
M. gallisepticum GroEL-interacting proteins identified by GST pulldown and mass spectrometry.
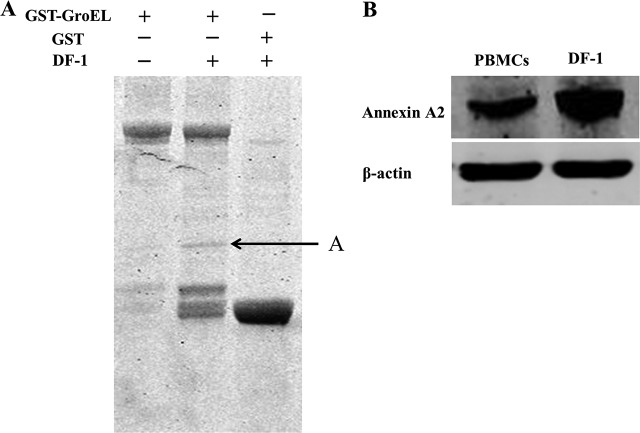
To identify cellular proteins that potentially associate with the M. gallisepticum GroEL protein, GST pulldown was coupled with mass spectrometry. The results showed that in DF-1 cells, several common bands were detected in both the GST and GST-GroEL pulldowns, and a specific band was detected in the GST-GroEL protein lane (Fig. 3A). The specific protein band was excised and subjected to mass spectrometry analysis. The cellular proteins that can possibly interact with GroEL protein were identified in this analysis (Table 1), and annexin A2 protein was chosen for further characterization as a potential protein that might interact with the GroEL protein. Using the anti-annexin A2 antibody, the Western blot analysis showed that annexin A2 was present in both DF-1 cells and PBMCs (Fig. 3B).
FIG 3.
Identification of GroEL-interacting protein by GST pulldown and of annexin A2 protein in PBMCs and DF-1 cells by Western blotting. (A) GroEL-interacting protein by GST pulldown. Lane 1, purified GroEL; lane 2, GST-GroEL protein produced in E. coli BL21(DE3) was used for the GST pulldown assay; lane 3, GST protein produced in E. coli BL21(DE3) was used as the negative control. The arrow indicates annexin A2. (B) The eluted protein complex was resolved on a 12% gel, followed by Coomassie staining and analysis by mass spectrometry. Annexin A2 protein exists in both PBMCs and DF-1 cells.
TABLE 1.
Proteins present in the specific band observed by GST-GroEL pulldown in the experiment whose results are shown in Fig. 3, as identified by mass spectrometry
| GenInfo identifier | Protein (approx mol mass [kDa]) | Mascot scorea |
|---|---|---|
| gi45382533 | Annexin A2 (38.616) | 256 |
| gi513211828 | Suppressor APC domain-containing protein 2 isoform X2 (38.006) | 60 |
Results were scored using the probability-based Mascot score. The protein score is −10×Log (P), where P is the probability that the observed match is a random event. Under the conditions of this experiment, a score greater than 60 indicated significant identification (P < 0.05).
GroEL interacts and colocalizes with annexin A2.
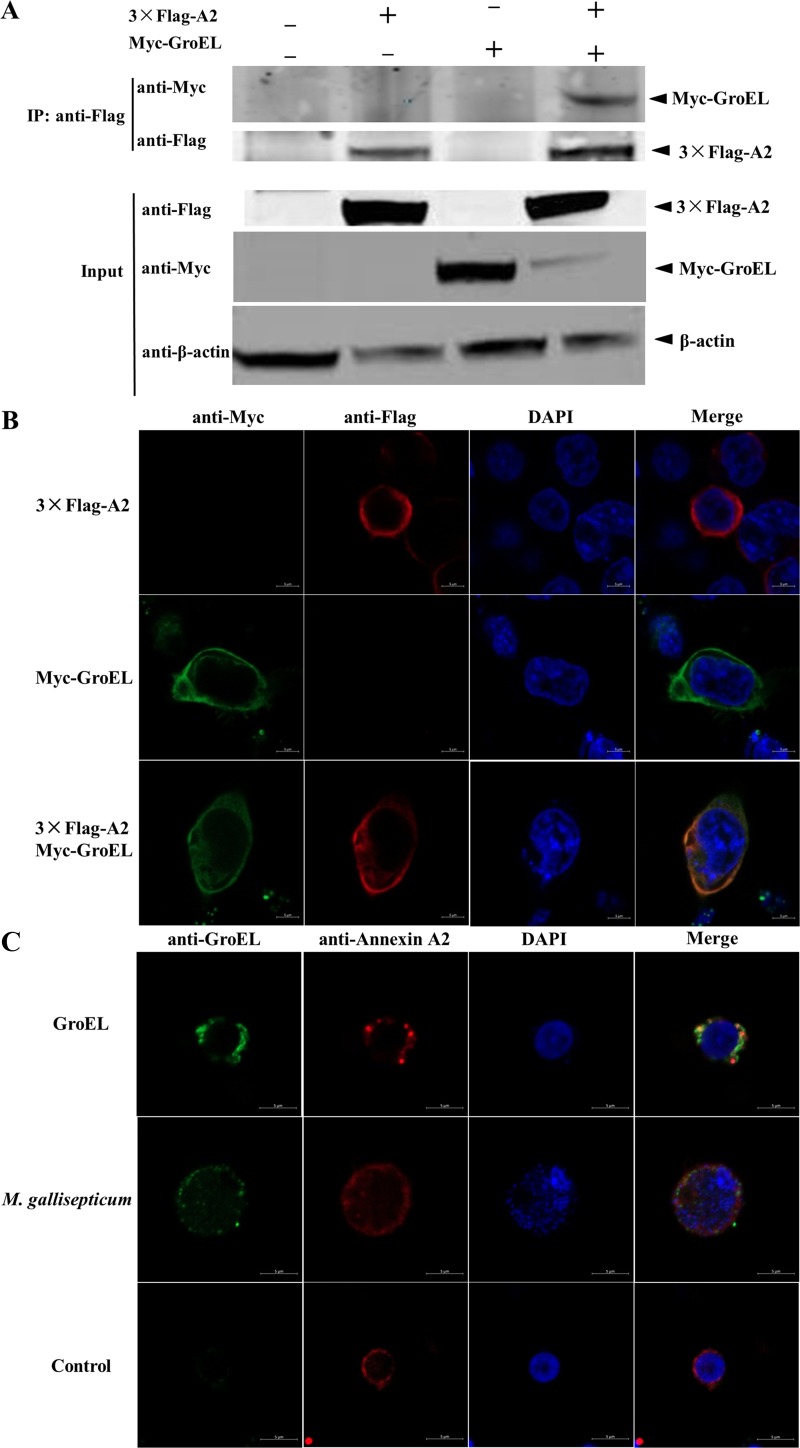
To further confirm the interaction between the GroEL and annexin A2 proteins, 3×Flag-tagged annexin A2 and Myc-tagged GroEL alone or both were transiently expressed in HEK293T cells. Cells coexpressing 3×Flag and the Myc-GroEL protein were used as a negative control. Coimmunoprecipitation (co-IP) with an anti-Flag monoclonal antibody (MAb) showed that the Myc-GroEL protein formed a complex with 3×Flag-annexin A2 but not with 3×Flag (Fig. 4A). The subcellular localizations of annexin A2 and the GroEL protein complex in HEK293T cells were also examined by confocal microscopy. Both Myc-GroEL and 3×Flag-annexin A2 were distributed throughout the cytomembrane, and annexin A2 colocalized extensively with GroEL (Fig. 4B).
FIG 4.
M. gallisepticum GroEL protein interacts with annexin A2. (A) Co-IP of GroEL protein with annexin A2 protein. HEK293T cells were cotransfected with the indicated plasmid (+) or empty vectors (−), and the whole-cell lysates obtained at 48 h posttransfection (hpt) were immunoprecipitated (IP) with anti-Flag MAb. After separation by SDS-PAGE, proteins were detected by immunoblotting with the indicated antibodies. The identities of the protein bands are indicated on the right. (B) Colocalization of GroEL protein and annexin A2. HEK 293T cells were cotransfected with P3×Flag-A2 and pMyc-GroEL. Cells were fixed at 48 hpt and subjected to indirect immunofluorescence to detect Myc-GroEL (green) and P3×Flag-A2 (red) with mouse anti-Myc and rabbit anti-Flag antibodies, respectively. The position of the nucleus is indicated by DAPI (blue) staining in the merged image. (C) Colocalization of GroEL protein with endogenous annexin A2. PBMCs were stimulated with 2 μg/ml GroEL protein. Cells were fixed 48 h after the cells were stimulated and subjected to indirect immunofluorescence to detect GroEL protein (green) and annexin A2 (red) with mouse anti-GroEL antibody and rabbit anti-annexin A2 antibodies. The position of the nucleus is indicated by DAPI (blue) staining in the merged image. Bars = 5 μm. Experiments were performed at least three times.
To confirm whether endogenous GroEL also colocalizes with annexin A2 in PBMCs, PBMCs cultured in 6-well plates were infected with 108 CFU M. gallisepticum Rlow or treated with1 μg His-GroEL for 12 h. Confocal images of the cells immunostained with anti-GroEL and anti-annexin A2 antibodies showed both GroEL protein and M. gallisepticum cells distributed throughout the cytomembrane and colocalized with annexin A2 protein (Fig. 4C). Cumulatively, these findings confirmed that the annexin A2 protein is an interaction partner of the M. gallisepticum GroEL protein.
GroEL induces annexin A2 expression, and depleting annexin A2 using small interfering RNA (siRNA) decreases apoptosis in PBMCs.
To determine whether GroEL stimulation affects annexin A2 expression in PBMCs, different concentrations of His-GroEL protein were used to treat PBMCs for 24 h, and the PBMCs were harvested for Western blotting to detect the expression levels of annexin A2. As shown by the results in Fig. 5, when the PBMCs were cultured with the His-GroEL protein, annexin A2 expression was induced significantly compared to its expression in the negative control.
FIG 5.
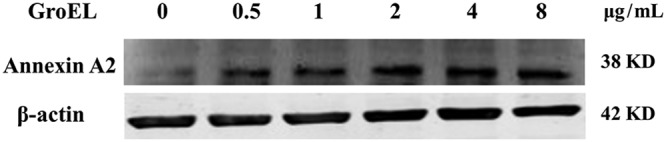
GroEL protein upregulated annexin A2 protein expression in chicken PBMCs. Chicken PBMCs were cultured with different concentrations of His-GroEL protein and harvested at 24 h. The cells were detected by immunoblotting with rabbit anti-annexin A2 antibody.
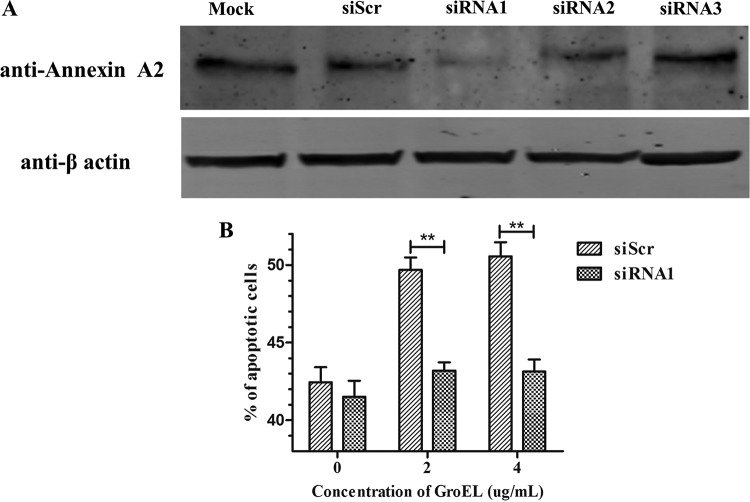
Whether GroEL induces apoptosis by interacting with endogenous annexin A2 in PBMCs was investigated by siRNA interference. Endogenous annexin A2 expression in PBMCs was knocked down by siRNAs targeting chicken annexin A2 (Fig. 6A). The levels of PBMC apoptosis and necrosis induced by the GroEL protein were evaluated by flow cytometry. Compared to the results of treatment with a scrambled siRNA, knocking down annexin A2 expression with 120 nM siRNA resulted in significantly decreased apoptosis induction by GroEL protein (P < 0.01) (Fig. 6B). Lipopolysaccharide (LPS)-treated (20 ng/ml) PBMCs were used as the positive control (data not shown). Taken together, these data suggested that GroEL induces apoptosis through interacting with annexin A2 and affecting its expression.
FIG 6.
Knockdown of annexin A2 decreased the apoptosis induced by GroEL protein. (A) Knockdown of annexin A2 protein levels by siRNA treatment. PBMCs transfected with no siRNA (Mock) or scrambled siRNA (siScr) or treated with siRNAs against annexin A2 (120 nM siRNA1, siRNA2, or siRNA3) were harvested at 24 hpt. Endogenous annexin A2 was detected by immunoblotting with antibodies directed against the indicated proteins. (B) The apoptosis levels of PBMCs were detected by flow cytometry. PBMCs treated with 120 nM siRNA1 or siScr for 3 h were induced with the indicated concentrations of GroEL protein for 24 h (PBS was used as a negative control). The data are presented as the mean values and standard deviations from three independent experiments. **, P < 0.01 compared with the results for siScr-treated PBMCs (Wilcoxon rank sum test).
GroEL upregulates Bax/Bcl2 in PBMCs.
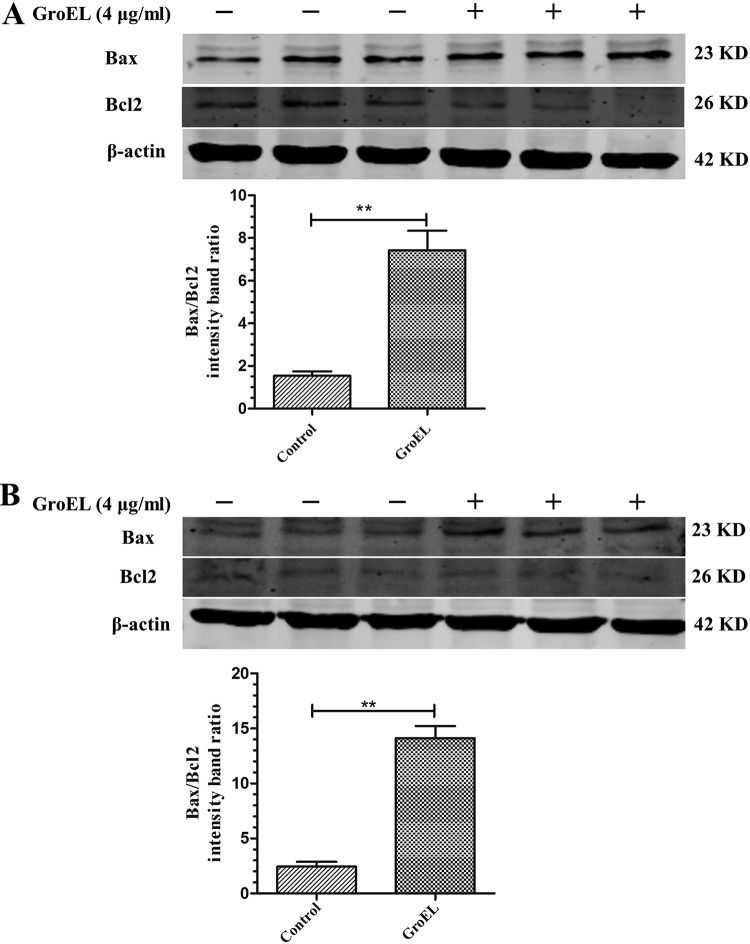
Bax and Bcl2 are two members of the Bcl2 family that play proapoptotic and antiapoptotic roles, respectively. To determine whether GroEL protein stimulation affects Bax and/or Bacl2 expression, PBMCs were stimulated with 4 μg/ml His-GroEL protein for 12 h or 24 h. After that, Western blot analysis was performed to compare the expression levels of Bax and Bcl2 with and without GroEL stimulation. As shown by the results in Fig. 7, His-GroEL protein treatment significantly increased Bax expression, while Bcl2 expression decreased compared to that of the control at 12 h and 24 h (P < 0.01). These results indicated that GroEL stimulation increased the Bax/Bcl2 ratio in PBMCs, thus inducing apoptosis.
FIG 7.
His-GroEL protein induced changes in Bax and Bcl2 in PBMCs. Further investigation demonstrated activation of apoptotic signaling pathways by upregulation of the proapoptotic protein Bax and downregulation of the antiapoptotic protein Bcl2. (A, B) His-GroEL protein-induced changes in Bax and Bcl2 in PBMCs at 12 h (A) and 24 h (B). Levels of proteins related to apoptosis in PBMCs induced by 4 μg/ml His-GroEL protein for 12 h or 24 h are shown. The PBMCs were lysed, and Western blotting was performed. Western blotting results were analyzed digitally, and the optical density ratios were calculated. The data are presented as the mean values and standard deviations from three independment experiments. **, P < 0.01 compared with the results for the control (Wilcoxon rank sum test).
DISCUSSION
M. gallisepticum impacts the poultry industry worldwide, and cytoadhesion to the respiratory epithelium is the most critical step of M. gallisepticum colonization and subsequent host infection (4, 23). The membrane and membrane-associated proteins of Mycoplasma spp. are believed to play significant roles in adhesion (10). The current study was undertaken to investigate the characteristics of the membrane-associated protein GroEL of M. gallisepticum in adhesion to cells of the host cell line DF-1 and to PBMCs. In this study, we cloned and expressed the GroEL gene of M. gallisepticum and determined the adhesion characteristics of its product as a consequence of interactions with host cells and the interacting protein. The results showed that GroEL of M. gallisepticum adheres to host cells and induces apoptosis. GroEL induces apoptosis by interacting with annexin A2 and upregulating annexin A2 expression. To the best of our knowledge, this is the first report that the GroEL protein of M. gallisepticum can induce apoptosis of host cells by interacting with the annexin A2 protein.
Heat shock proteins (HSPs) are a class of highly conserved proteins that have important functions in cell metabolism and aid cells in dealing with adverse environmental stimuli (18, 19). It has been documented that certain HSPs located on the cell surface facilitate pathogen adherence to host cells and, therefore, play key roles in virulence (20). GroEL (HSP60) is ubiquitous and highly conserved in eukaryotes and prokaryotes, including pathogens, and has an important role in maintaining the structural and functional integrity of many other proteins (24, 25). In both Mycoplasma pneumoniae and Mycoplasma genitalium infection, GroEL is associated with adherence, complementing adhesion proteins like HMW1 and P1. In addition, Chlamydophila pneumoniae Hsp60 was reported to have an association with the apoptotic signaling pathway, and in some patients with Hsp60-positive coronary artery disease, proapoptotic genes or proteins exhibit high expression (26). Similar results were observed in our study: GroEL of M. gallisepticum not only attached to the host cells but also upregulated the expression of apoptosis-related caspase proteins (Fig. 2).
Annexin A2, which belongs to the annexin superfamily, is predominantly expressed at the plasma membrane but is also present on intracellular vesicles and in the nucleus (27). Annexin A2 is involved in multiple cellular processes, including DNA replication, cell proliferation, cell adhesion, and apoptosis (28–31). Upregulation of annexin A2 expression can promote cell viability in human CaSki cervical cancer cells and others (31, 32). In addition, annexin A2 has been implicated as a host factor that regulates the growth of many different viruses and bacteria; e.g., annexin A2 supports virus assembly by interacting with hepatitis C virus (HCV) nonstructural protein 5A (NS5A) (33) and HIV Gap (34) and also acts as a potential receptor for respiratory syncytial virus in human epithelial cells (35).
Despite the fact that previous reports have indicated that the upregulation of annexin A2 expression during cell apoptosis is rare, some results have demonstrated that the overexpression of annexin A2 affects cell proliferation and apoptosis and that the most likely reason may be that annexin A2 serves as a ligand/proapoptotic protein on the surface of apoptotic cells when overexpressed (36–39). In our study, annexin A2 expression increased with caspase and Bax/Bcl2 activation during GroEL protein stimulation of PBMCs. Molecular markers of cell death, such as the Bax/Bcl2 ratio and caspase cleavage, showed increases in the GroEL-stimulated cells compared with the results for the controls (Fig. 2 and 7). Bax/Bcl2 and caspase-8 induce the intrinsic/extrinsic pathways of apoptosis, and caspase-3 is a final executioner in the extrinsic pathways (40, 41).
Bcl2 and Bcl-xL expression can be regulated by STAT3, an important factor that relays signals from cytokines and growth factors to regulate gene expression in biological processes, including apoptosis induced by the JAK/STAT pathway (42, 43). The relationship among GroEL, annexin A2, cell apoptosis, and the JAK/STAT pathway remains unknown and requires further studies.
In addition, GroEL of M. gallisepticum induced the apoptosis of PBMCs, which are important immune cells during the immune responses to a foreign antigen. Abnormal apoptosis in PBMCs might lead to immunomodulation, manifested by the disordered expression of cytokines and increased severity of diseases, e.g., inflammation and decreased weight gain and egg production, as well as an increased susceptibility to coinfection.
In conclusion, our study showed that the GroEL protein can adhere to DF-1 cells and PBMCs. The key finding is the identification of cellular annexin A2 as a novel interacting partner of the GroEL protein during the induction of apoptosis in PBMCs. Therefore, GroEL could additionally contribute to M. gallisepticum pathogenicity and tissue damage. The cell apoptosis occurring as a result of the interaction between the GroEL protein and annexin A2 involved the intrinsic/extrinsic pathways, and the relationship among GroEL, annexin A2, cell apoptosis, and the JAK/STAT pathway remains unknown and requires further studies.
MATERIALS AND METHODS
Mycoplasma strains, cells, and culture conditions.
The M. gallisepticum R strain was purchased from China General Microbiological Culture Collection Center (CGMCC), and M. gallisepticum Rlow (passage 29) was cultured in modified pleuropneumonia-like organism (PPLO) medium supplemented with 20% inactivated horse serum (HyClone, Logan, WV, USA), 10% yeast extract, thallium acetate (0.125 mg/ml), and penicillin (200 IU/ml) at 37°C until mid-log phase was reached (2 or 3 days), as indicated by a color shift from red to orange. To estimate the numbers of CFU in cultures, serial dilutions were plated on modified PPLO medium containing 1.5% agarose (catalog number V2111; Promega) and incubated at 37°C. CFU were counted 7 to 10 days later using a microscope (17). Bacteria were pelleted by centrifugation at 10,000 × g for 10 min and resuspended at 5 × 108 CFU/ml in phosphate-buffered saline (PBS). DF-1 cells and HEK293T cells were purchased from American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco; Thermo Fisher Scientific, China) with 10% fetal bovine serum (Gibco), 100 U penicillin/ml, and 100 μg streptomycin/ml at 37°C in an incubator with 5% CO2. Fresh chicken PBMCs were isolated from the heparinized wing venous blood of 6-week-old SPF White Leghorn chickens (Animal Breeding Center of Harbin Veterinary Research Institute) by density gradient centrifugation according to the instructions of a PBMC isolation kit (product number LTS1090C; TBD Science, China). After washing twice with PBS, the isolated PBMCs were resuspended in RPMI 1640 medium (Gibco) without the addition of any serum, and the cell viability exceeded 95%, as assessed by trypan blue staining and hemocytometer counting. PBMCs (5 × 106/ml) were cultured in RPMI 1640 medium with 10% fetal bovine serum, 100 U penicillin/ml, and 100 μg streptomycin/ml at 37°C in an incubator with 5% CO2. Chicken blood collection was conducted with approval from the Animal Ethics Committee of Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences (CAAS) and performed in accordance with the animal ethics guidelines and approved protocols (approval number SY-2016-CH-114-2).
Cloning, expression, and purification of a recombinant GroEL (rGroEL) protein.
The GroEL gene was amplified from the M. gallisepticum R strain using primer pair His-G-F/His-G-B or primer pair GST-G-F/GST-G-B (Table 2), with reference to the M. gallisepticum Rlow strain sequence (GenBank accession number NC_004829.2). The recombinant plasmids pET-GroEL and pGEX-GroEL were constructed by cloning the GroEL gene into the pET-32a vector or pGEX-6p-1 vector. The recombinant fusion proteins His-GroEL and GST-GroEL were obtained by transforming the corresponding plasmids into Escherichia coli BL21(DE3) cells. The His-GroEL protein was purified by using a high-affinity Ni-nitrilotriacetic acid (NTA) resin column (GE) according to the manufacturer’s instructions. Purified proteins were analyzed using SDS-PAGE. For the cellular viability assay, the endotoxin concentrations of the His-GroEL protein and GST-GroEL protein (<0.04 endotoxin units/ml) were detected by using the ToxinSensor chromogenic LAL endotoxin assay kit (GenScript, China).
TABLE 2.
Primers used in this study
| Primer | Sequence (5′–3′) (restriction enzyme)a |
|---|---|
| His-G-F | CGCGGATCCATGGCAAAAGAATTAAC (BamHI) |
| His-G-B | CCGGTCGACTTATAGGTGATTTAAGC (SalI) |
| GST-G-F | CGCGGATCCATGGCAAAAGAATTAAC (BamHI) |
| GST-G-B | CGGCTCGAGTTATAGGTGATTTAAGC (XhoI) |
| P3-F | CGCGAATTCATCGATAGATCTTATGTCTACTGTCCATGAAATTTTAAGC (BglII) |
| P3-B | CCTCTAGAGTCGACTGGTACCTCAGTCCTCTCCACCACACAGG (KpnI) |
| Myc-F | GAATTCGGTCGACCGAGATCTCTATGGCAAAAGAATTAACATTTGAACA (BglII) |
| Myc-B | CATGTCTGGATCCCCGCGGCCGCTTATAGGTGATTTAAGCTTGGTTTTTC (NotI) |
Restriction sites are underlined.
Localization of the rGroEL protein.
A monolayer of DF-1 cells cultured in a dish was fixed with 4% paraformaldehyde (PFA) for 30 min at room temperature and then blocked with 5% dry milk in PBS with 0.05% Tween 20 (PBS-T, pH 7.4) for 2 h at 37°C. After washing, 1 μg of GST-GroEL protein in PBS was added to the cells and incubated at 37°C for 12 h. After washing, anti-GST MAb (HT601; TransGen Biotech, Beijing, China) was added to the corresponding wells as the primary antibody and incubated at 37°C for 2 h. After washing, fluorescein isothiocyanate (FITC)-goat anti-mouse IgG (Jackson ImmunoResearch) was added and incubated at 37°C for 1 h. The membranes were labeled with DiI (19-dioctadecyl-3,3,39,39-tetramethylindocarbocyanine perchlorate) (C1036; Beyotime, China), and the cell nuclei were counterlabeled with DAPI (4′,6-diamidino-2-phenylindole) (F6057; Sigma-Aldrich). GST protein was used as the negative control.
PBMCs (5 × 106 cells/ml) were cultured in 6-well plates in RPMI 1640 medium with 10% fetal bovine serum, and 108 CFU M. gallisepticum Rlow or 1 μg His-GroEL was added to each well for 12 h. The cells were harvested, centrifuged at 250 × g for 10 min, and washed three times with ice-cold PBS. After resuspension in 100 μl PBS, the cells were attached to a dish, fixed with 4% PFA for 30 min at room temperature, and then blocked with 5% dry milk in PBS-T for 2 h at 37°C. After washing, anti-GroEL MAb 3G9 (GroEL specific, produced and purified in our laboratory) was added to the wells as the primary antibody and incubated at 37°C for 2 h. After washing, FITC-goat anti-mouse IgG (Jackson ImmunoResearch) was added and incubated at 37°C for 1 h. The membranes were labeled with DiI, and the cell nuclei were labeled with DAPI. GST protein (unrelated protein) was used as a negative control. The results were observed with a fluorescence microscope.
Flow cytometry.
PBMCs (5 × 106 cells/ml) in 6-well plates were treated with His-GroEL protein at a concentration of 1 μg/ml, 2 μg/ml, 4 μg/ml, 8 μg/ml, or 16 μg/ml for 24 h. LPS-treated (20 ng/ml, product number L4391; Sigma-Aldrich) and nontreated cells were used as the positive and negative control, respectively. Cell death was detected using the annexin V-FITC apoptosis detection kit I (556547; BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. Briefly, PBMCs were washed twice with cold PBS and resuspended in 1× binding buffer at a concentration of 1 × 106 cells/ml. Next, 100 μl of the solution (1 × 105 cells) was transferred into a 5-ml culture, and then 5 μl of FITC-conjugated annexin V and 5 μl of propidium iodide (PI) were added. The PBMCs were gently vortexed and incubated for 15 min at room temperature in the dark. Finally, 400 μl of 1× binding buffer was added to the PBMCs. PBMC apoptosis was evaluated using a BD Accuri C6 flow cytometer. At least 1 × 104 cells/event were evaluated for each analysis, and the annexin V+-plus-annexin V+ PI+ population was gated for apoptosis analysis.
Western blot analysis.
Fresh PBMCs treated with the indicated concentrations of His-GroEL (Fig. 2, 5, and 7) for 12 h/24 h were collected, washed with PBS three times, and then incubated on ice with a cell lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 0.1% SDS, and 5 mM sodium orthovanadate) containing a protease inhibitor cocktail (product number 04693132001; Roche, Bern, Switzerland) and 0.1 mM phenylmethylsulfonyl fluoride (PMSF) for 2 h. The cell lysates were centrifuged at 13,000 × g for 20 min at 4°C, and protein concentrations were determined using the Bradford assay (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of protein samples were loaded on 12% (wt/vol) SDS-PAGE gels, and the proteins were transferred from the gels to polyvinylidene difluoride (PVDF) membranes (product number ISEQ00010; Millipore, Billerica, MA, USA). Then, the PVDF membranes were blocked with 5% dry milk dissolved in PBS-T at 4°C overnight. The PVDF membranes were incubated with the following primary antibodies (1:1,000) for 1 h at room temperature and then at 4°C overnight: anti-caspase-3 antibody (ab90437), anti-caspase-8 antibody (ab25901), anti-caspase-9 antibody (ab69514), and anti-annexin A2 antibody (ab40943) (Abcam, Shanghai, China); anti-β-actin antibody (TransGen Biotech, Beijing, China); and anti-Bax antibody (sc-23959) and Bcl2 antibody (sc-23960) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing with PBS-T, either DyLight 800-labeled goat anti-rabbit antibody or DyLight 800-labeled goat anti-mouse antibody (1:5,000; Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA) was added and incubated for 2 h at room temperature. The blots were imaged at an appropriate excitation wavelength using a digital imaging system (Odyssey infrared imaging system; LI-COR Biosciences, Lincoln, NE, USA). Quantification of bands in Western blots was performed by densitometry using ImageJ software. The protein levels were normalized by a comparison with β-actin levels.
GST pulldown assays.
DF-1 cells cultured in flasks were harvested by centrifugation at 500 × g for 15 min at 4°C. The pellets were suspended in lysing buffer containing 50 mM Tris-HCl, (pH 7.4), 1.0 mM EDTA, 150 mM NaCl, 5 mM MgCl2, 10% glycerol, 1 mM cocktail, 1 mM PMSF, and 1% Triton X-100. The cells were lysed by three cycles of freezing and thawing. After incubating at 4°C overnight with gentle agitation, the lysed cells were centrifuged at 2,000 × g for 20 min at 4°C. The supernatant containing soluble cellular proteins was harvested for GST pulldown assays. GST and the GST-GroEL protein produced in E. coli BL21(DE3) cells were conjugated to glutathione beads (GE biosciences) and blocked in 5% bovine serum albumin for 1 h. The beads were then washed twice with TIF buffer, which contains 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM MgCl2, 0.1% NP-40, 10% glycerol, 0.1 mM dithiothreitol (DTT), and 1 mg/ml protease inhibitor, and incubated with the supernatant from the lysed DF-1 cells for 2 h at 4°C. The beads were washed six times with TIF buffer, followed by elution and detection of the proteins by SDS-PAGE and immunoblotting. The binding specificity to the GroEL protein was analyzed by mass spectrometry.
Plasmid construction and plasmid DNA transfection.
The P3×Flag-A2 plasmid encoding annexin A2 (GenBank accession number NM_205351.1) was constructed by cloning annexin A2 with the KpnI and SalI restriction enzymes. The GroEL gene was cloned into the pCMV-Myc vector (Clontech) with the KpnI and SalI restriction enzymes to generate the pMyc-GroEL plasmid. All plasmids were verified by sequencing. To further identify the interaction of GroEL protein and annexin A2 protein, HEK293T cells in 6- or 12-well plates cultured at 37°C in an incubator with 5% CO2 were transfected with the appropriate plasmids (1 μg/ml) with X-tremeGENE HP-DNA transfection reagent (Roche) according to the manufacturer’s instructions. At 4 h posttransfection, the transfection mixture was replaced with complete growth medium and incubated for another 48 h before being used for coimmunoprecipitation (co-IP) and confocal microscopy.
Confocal imaging.
HEK293T cells transfected with pMyc-GroEL or 3×Flag-annexin A2 or both were fixed with 4% paraformaldehyde in PBS for 30 min and permeabilized with 0.1% Triton X-100 for 5 min. The PBMCs were treated and fixed using the procedure described above in “Localization of the rGroEL protein.” After washing, HEK293T cells were incubated with mouse anti-Myc MAb (E022050; EarthOx, San Francisco, CA) or rabbit anti-Flag polyclonal antibodies (PAb) (F7425; Sigma-Aldrich), and PBMCs were incubated with rabbit anti-annexin A2 antibody (ab40943; Abcam) and anti-GroEL MAb (3G9) for 2 h. After washing, the cells were incubated with an FITC-goat anti-mouse IgG (Jackson ImmunoResearch) and tetramethyl rhodamine isocyanate (TRITC)-conjugated goat anti-rabbit antibody (T6778; Sigma-Aldrich). The membranes were labeled with DiI, and the cell nuclei were labeled with DAPI.
Co-IP.
HEK293T cells were transfected with the indicated constructs as described above in “Plasmid construction and plasmid DNA transfection.” The transfected cells were harvested at 48 h posttransfection. After washing with cold PBS (pH 7.4), the pellet was lysed with NP-40 buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 0.5% NP-40, and 0.5 mM EDTA) containing 1 mM PMSF and 1 mg/ml protease inhibitor cocktail (Roche) at 4°C for 1 h. Clarified extracts were precleared with protein A/G beads (SC2003; Santa Cruz) and then incubated with protein A/G beads plus an anti-Flag MAb (F7425; Sigma-Aldrich) for 4 h. The beads were then washed with NP-40 buffer and boiled in sample buffer, and the proteins were subjected to SDS-PAGE, followed by immunoblotting analysis with anti-Flag PAb (F7425; Sigma-Aldrich) and anti-Myc PAb (E022050; EarthOx).
siRNA interference.
The siRNA sequences targeting annexin A2 were designed and synthesized by Sigma-Aldrich (using the Rosetta algorithm in their pipeline to design the siRNAs; NCBI blast was used for off-target analysis). The siRNAs specific for annexin A2 were siRNA1, siRNA2, and siRNA3 (catalog numbers 72275/22558, 72273, and 72274, respectively). A nontargeting siRNA (siScr, catalog number 11065/180709) was used as the control siRNA. The siRNAs targeting annexin A2 were used at a final concentration of 120 nM. siRNA duplexes were transfected into PBMCs using X-tremeGENE siRNA transfection reagent according to the manufacturer’s protocol. PBMCs (5 × 106/ml) in 12-well plates were treated with 120 nM siRNA or siScr for 3 h and induced with different concentrations of the His-GroEL protein for 24 h. LPS-treated (20 ng/ml) PBMCs were used as the positive control. Flow cytometry was used to analyze apoptosis induced by the GroEL protein in the PBMCs using the procedure described above in “Flow cytometry.”
Statistical analyses.
The numerical data are expressed as the mean values ± standard deviation (SD) and were analyzed using the Wilcoxon rank sum test (nonparametric test for independent treatment groups). A P value of less than 0.05 was considered statistically significant.
ACKNOWLEDGMENT
This work was supported by a grant from the National Science Foundation for Young Scholars of China (grant number 31602084).
REFERENCES
- 1.Szczepanek SM, Tulman ER, Gorton TS, Liao X, Lu Z, Zinski J, Aziz F, Frasca S Jr, Kutish GF, Geary SJ. 2010. Comparative genomic analyses of attenuated strains of Mycoplasma gallisepticum. Infect Immun 78:1760–1771. doi: 10.1128/IAI.01172-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pflaum K, Tulman ER, Beaudet J, Canter J, Geary SJ. 2018. Variable lipoprotein hemagglutinin A gene (vlhA) expression in variant Mycoplasma gallisepticum strains in vivo. Infect Immun 86:e00524-18. doi: 10.1128/IAI.00524-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papazisi L, Gorton TS, Kutish G, Markham PF, Browning GF, Nguyen DK, Swartzell S, Madan A, Mahairas G, Geary SJ. 2003. The complete genome sequence of the avian pathogen Mycoplasma gallisepticum strain R(low). Microbiology 149:2307–2316. doi: 10.1099/mic.0.26427-0. [DOI] [PubMed] [Google Scholar]
- 4.Papazisi L, Frasca S Jr, Gladd M, Liao X, Yogev D, Geary SJ. 2002. GapA and CrmA coexpression is essential for Mycoplasma gallisepticum cytadherence and virulence. Infect Immun 70:6839–6845. doi: 10.1128/IAI.70.12.6839-6845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaunson JE, Philip CJ, Whithear KG, Browning GF. 2000. Lymphocytic infiltration in the chicken trachea in response to Mycoplasma gallisepticum infection. Microbiology 146:1223–1229. doi: 10.1099/00221287-146-5-1223. [DOI] [PubMed] [Google Scholar]
- 6.Gharaibeh S, Hailat A. 2011. Mycoplasma gallisepticum experimental infection and tissue distribution in chickens, sparrows and pigeons. Avian Pathol 40:349–354. doi: 10.1080/03079457.2011.582480. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Nonomura I, Shimizu F, Shoya S, Horiuchi T. 1970. Mixed infection with Mycoplasma gallisepticum and the B1 strain of Newcastle disease virus in chickens. Natl Inst Anim Health Q (Tokyo) 10:58–65. [PubMed] [Google Scholar]
- 8.Cleavinger CM, Kim MF, Wise KS. 1994. Processing and surface presentation of the Mycoplasma hyorhinis variant lipoprotein VlpC. J Bacteriol 176:2463–2467. doi: 10.1128/jb.176.8.2463-2467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panicker IS, Kanci A, Chiu CJ, Veith PD, Glew MD, Browning GF, Markham PF. 2012. A novel transposon construct expressing PhoA with potential for studying protein expression and translocation in Mycoplasma gallisepticum. BMC Microbiol 12:138. doi: 10.1186/1471-2180-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Indikova I, Much P, Stipkovits L, Siebert-Gulle K, Szostak MP, Rosengarten R, Citti C. 2013. Role of the GapA and CrmA cytadhesins of Mycoplasma gallisepticum in promoting virulence and host colonization. Infect Immun 81:1618–1624. doi: 10.1128/IAI.00112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boguslavsky S, Menaker D, Lysnyansky I, Liu T, Levisohn S, Rosengarten R, Garcia M, Yogev D. 2000. Molecular characterization of the Mycoplasma gallisepticum pvpA gene which encodes a putative variable cytadhesin protein. Infect Immun 68:3956–3964. doi: 10.1128/iai.68.7.3956-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh MS, Gorton TS, Forsyth MH, Troy KE, Geary SJ. 1998. Molecular and biochemical analysis of a 105 kDa Mycoplasma gallisepticum cytadhesin (GapA). Microbiology 144:2971–2978. doi: 10.1099/00221287-144-11-2971. [DOI] [PubMed] [Google Scholar]
- 13.Lysnyansky I, Rosengarten R, Yogev D. 1996. Phenotypic switching of variable surface lipoproteins in Mycoplasma bovis involves high-frequency chromosomal rearrangements. J Bacteriol 178:5395–5401. doi: 10.1128/jb.178.18.5395-5401.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lysnyansky I, Sachse K, Rosenbusch R, Levisohn S, Yogev D. 1999. The vsp locus of Mycoplasma bovis: gene organization and structural features. J Bacteriol 181:5734–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nussbaum S, Lysnyansky I, Sachse K, Levisohn S, Yogev D. 2002. Extended repertoire of genes encoding variable surface lipoproteins in Mycoplasma bovis strains. Infect Immun 70:2220–2225. doi: 10.1128/iai.70.4.2220-2225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Merwe J, Prysliak T, Perez-Casal J. 2010. Invasion of bovine peripheral blood mononuclear cells and erythrocytes by Mycoplasma bovis. Infect Immun 78:4570–4578. doi: 10.1128/IAI.00707-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogl G, Plaickner A, Szathmary S, Stipkovits L, Rosengarten R, Szostak MP. 2008. Mycoplasma gallisepticum invades chicken erythrocytes during infection. Infect Immun 76:71–77. doi: 10.1128/IAI.00871-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young RA, Elliott TJ. 1989. Stress proteins, infection, and immune surveillance. Cell 59:5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann SH. 1990. Heat shock proteins and the immune response. Immunol Today 11:129–136. doi: 10.1016/0167-5699(90)90050-J. [DOI] [PubMed] [Google Scholar]
- 20.Ensgraber M, Loos M. 1992. A 66-kilodalton heat shock protein of Salmonella typhimurium is responsible for binding of the bacterium to intestinal mucus. Infect Immun 60:3072–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kol A, Bourcier T, Lichtman AH, Libby P. 1999. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest 103:571–577. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kol A, Sukhova GK, Lichtman AH, Libby P. 1998. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation 98:300–307. doi: 10.1161/01.CIR.98.4.300. [DOI] [PubMed] [Google Scholar]
- 23.Dykstra MJ, Levisohn S, Fletcher OJ, Kleven SH. 1985. Evaluation of cytopathologic changes induced in chicken tracheal epithelium by Mycoplasma gallisepticum in vivo and in vitro. Am J Vet Res 46:116–122. [PubMed] [Google Scholar]
- 24.Sakamoto M, Ohkuma M. 2010. Usefulness of the hsp60 gene for the identification and classification of Gram-negative anaerobic rods. J Med Microbiol 59:1293–1302. doi: 10.1099/jmm.0.020420-0. [DOI] [PubMed] [Google Scholar]
- 25.Abdeen S, Salim N, Mammadova N, Summers CM, Frankson R, Ambrose AJ, Anderson GG, Schultz PG, Horwich AL, Chapman E, Johnson SM. 2016. GroEL/ES inhibitors as potential antibiotics. Bioorg Med Chem Lett 26:3127–3134. doi: 10.1016/j.bmcl.2016.04.089. [DOI] [PubMed] [Google Scholar]
- 26.Jha HC, Srivastava P, Vardhan H, Singh LC, Bhengraj AR, Prasad J, Mittal A. 2011. Chlamydia pneumoniae heat shock protein 60 is associated with apoptotic signaling pathway in human atheromatous plaques of coronary artery disease patients. J Cardiol 58:216–225. doi: 10.1016/j.jjcc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Koga R, Kubota M, Hashiguchi T, Yanagi Y, Ohno S. 2018. Annexin A2 mediates the localization of measles virus matrix protein at the plasma membrane. J Virol 92:e00181-18. doi: 10.1128/JVI.00181-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang Y, Schneiderman MH, Vishwanatha JK. 1993. Annexin II expression is regulated during mammalian cell cycle. Cancer Res 53:6017–6021. [PubMed] [Google Scholar]
- 29.Vishwanatha JK, Kumble S. 1993. Involvement of annexin II in DNA replication: evidence from cell-free extracts of Xenopus eggs. J Cell Sci 105:533–540. [DOI] [PubMed] [Google Scholar]
- 30.Rescher U, Ludwig C, Konietzko V, Kharitonenkov A, Gerke V. 2008. Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. J Cell Sci 121:2177–2185. doi: 10.1242/jcs.028415. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Jin Y, Yan CH, Yu Y, Bai J, Chen F, Zhao YZ, Fu SB. 2008. Involvement of annexin A2 in p53 induced apoptosis in lung cancer. Mol Cell Biochem 309:117–123. doi: 10.1007/s11010-007-9649-5. [DOI] [PubMed] [Google Scholar]
- 32.Janzen C, Sen S, Lei MY, Gagliardi de Assumpcao M, Challis J, Chaudhuri G. 2017. The role of epithelial to mesenchymal transition in human amniotic membrane rupture. J Clin Endocrinol Metab 102:1261–1269. doi: 10.1210/jc.2016-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solbak SMO, Abdurakhmanov E, Vedeler A, Danielson UH. 2017. Characterization of interactions between hepatitis C virus NS5B polymerase, annexin A2 and RNA: effects on NS5B catalysis and allosteric inhibition. Virol J 14:236. doi: 10.1186/s12985-017-0904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodham AW, Sanna AM, Taylor JR, Skeate JG, Da Silva DM, Dekker LV, Kast WM. 2016. Annexin A2 antibodies but not inhibitors of the annexin A2 heterotetramer impair productive HIV-1 infection of macrophages in vitro. Virol J 13:187. doi: 10.1186/s12985-016-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhotra R, Ward M, Bright H, Priest R, Foster MR, Hurle M, Blair E, Bird M. 2003. Isolation and characterisation of potential respiratory syncytial virus receptor(s) on epithelial cells. Microbes Infect 5:123–133. doi: 10.1016/S1286-4579(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Sánchez G, Jiménez A, Quezada-Ramírez MA, Estudillo E, Ayala-Sarmiento AE, Mendoza-Hernández G, Hernández-Soto J, Hernández-Hernández FC, Cázares-Raga FE, Segovia J. 2018. Annexin A1, annexin A2, and Dyrk 1B are upregulated during GAS1-induced cell cycle arrest. J Cell Physiol 233:4166–4182. doi: 10.1002/jcp.26226. [DOI] [PubMed] [Google Scholar]
- 37.Zhao D, Ge H, Ma B, Xue D, Zhang W, Li Z, Sun H. 26 November 2018. The interaction between ANXA2 and lncRNA Fendrr promotes cell apoptosis in caerulein-induced acute pancreatitis. J Cell Biochem doi: 10.1002/jcb.28097. [DOI] [PubMed] [Google Scholar]
- 38.Leffler J, Herbert AP, Norstrom E, Schmidt CQ, Barlow PN, Blom AM, Martin M. 2010. Annexin-II, DNA, and histones serve as factor H ligands on the surface of apoptotic cells. J Biol Chem 285:3766–3776. doi: 10.1074/jbc.M109.045427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin M, Leffler J, Blom AM. 2012. Annexin A2 and A5 serve as new ligands for C1q on apoptotic cells. J Biol Chem 287:33733–33744. doi: 10.1074/jbc.M112.341339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. 1997. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405–413. doi: 10.1016/S0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 41.Falschlehner C, Emmerich CH, Gerlach B, Walczak H. 2007. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol 39:1462–1475. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Xiu D, Liu L, Qiao F, Yang H, Cui L, Liu G. 2016. Annexin A2 coordinates STAT3 to regulate the invasion and migration of colorectal cancer cells in vitro. Gastroenterol Res Pract 2016:3521453. doi: 10.1155/2016/3521453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart JR, Liao L, Yates JR III, Vogt PK. 2011. Essential role of Stat3 in PI3K-induced oncogenic transformation. Proc Natl Acad Sci U S A 108:13247–13252. doi: 10.1073/pnas.1110486108. [DOI] [PMC free article] [PubMed] [Google Scholar]