Abstract
Background
The persistent the inflammatory condition in multiple sclerosis (MS) may due to the aberrant regulation of the elimination of the pathogenic autoreactive lymphocytes through apoptosis. Survivin, encoded by the BIRC5 gene, has been indicated to be involved in the regulation of apoptosis. This survey intended to investigate the genetic and microRNA mediated regulation of survivin in relapsing-remitting MS (RRMS) disease.
Results
It was observed that the C allele (OR = 1.38, 95% CI = 1.05–1.348, P = 0.022) and CC genotype (OR = 1.84, 95% CI = 1.06–3.19; P = 0.029) in the rs9904341 polymorphism increased the disease risk. Furthermore, miR-34a was significantly downregulated (Fold change = 0.41, P = 0.001) in the PBMCs from RRMS subjects. Survivin mRNA expression in PBMCs and serum survivin level were increased in RRMS patients in comparison to the controls. Downregulation of miR-34a was negatively correlated with increased survivin level.
Conclusion
Although the genetic polymorphism of BIRC5 gene was associated with the disease risk, miR-34a was suggested to be involved in the regulation of survivin in the RRMS patients.
Keywords: Multiple sclerosis, BIRC5 gene, Survivin, Apoptosis, microRNA
Background
Multiple sclerosis (MS) is considered as a complex, multifactorial, and demyelinating disease that is related to a damage to the axonal myelin sheaths and is accompanied by neuronal degeneration. During MS, there is a loss in oligodendrocyte alongside with axonal damage, blood-brain barrier (BBB) leakage, and finally recruitment of immune cells that finally leads to central nervous system (CNS) inflammation [1]. MS is markedly prevalent among young adults, in which the mean age of the patients range from 20 to 45 [2]. MS is typically classified into four main groups according to the disease duration, including relapsing remitting MS (RRMS), secondary progressive MS (SPMS), primary progressive MS (PPMS), and progressive relapsing MS (PRMS) [3]. MS oftentimes represent a relapsing and remitting picture in its early stages, and as the disease course advances, symptoms are intensified for a while and then an amelioration occurs, which is named as RRMS, being reported in 80–85% of subjects [4, 5]. The exact etiology and pathogenesis of MS is not clear; nonetheless, a bulk of surveys indicate that the disease is the result of interactions between the immune system, inflammatory and apoptotic responses, which might be occurred at the periphery or the CNS [6]. Results from microarray studies conducted on the peripheral blood mononuclear cells (PBMC) obtained from MS patients in the relapsing phase indicated suppression of cell death through impaired apoptosis mechanisms [7].
Baculoviral IAP repeat containing 5 (BIRC5) gene encodes a 16.5 kDa protein called survivin, which belongs to inhibitor of apoptosis gene family (IAP) and is involved in the apoptosis and cell proliferation process [8]. Survivin has been reported to regulate the immune system, in which the development and differentiation of effector CD4+ T cells, hemostasis of CD8+ memory T cells, as well as the proliferation of activated T cells are controlled by survivin [9].
It has been shown that single nucleotide polymorphisms (SNPs) located in the promoter of the BIRC5 gene might be involve in the alteration of expression level of survivin [10]. The rs9904341 SNP is found in regions encoding for the cell cycle dependent elements as well as the repressor binding site of the cell cycle homology regions and was associated with increased mRNA and protein expressions of survivin in cancers [11, 12]. Furthermore, rs17878467 and rs8073069 SNPs in the BIRC5 promoter region have been reported to modulate the expression of survivin. While rs17878467 increased BIRC5 promoter activity in HeLa cell lines [12], rs8073069 was associated with overexpression of survivin in the esophageal cancer [13].
microRNAs (miRNAs) are short and single stranded, non-coding RNA molecules composed of approximately 22 nucleotides that are involved in controlling the gene expression. It has been reported that almost 60% of human genes are attributed to at least one miRNA binding site. miRNAs primarily suppress the translation of target genes and eventuate in downregulation of genes. During the biogenesis of miRNAs, RNA polymerase II transcribes the gene encoding miRNA to a long RNA strand named as primary miRNAs (pri-miRNAs). Then Drosha, an endonuclease, catalyzes the development of pri-miRNA to precursor miRNAs (pre-miRNAs). Aftermath, the exportin-5 mediated the transportation of pre-miRNA to cytoplasm, where it is cleaved to a double strand miRNA by Dicer. The active miRNA strand in the ribonucleoprotein complex, namely RNA-induced silencing complexes (RISCs) binds to 3′-untranslated region (UTR) of a mRNA, culminating in gene repression [14].
On the one hand, it has been reported that survivin was highly expressed in PPMS patients [15]. As well, an overexpression of survivin in T cells from active MS patients compared with stable MS patients has been reported that correlated with cellular resistance to apoptosis as well as with disease activity manifestations, including the number of enhanced lesions and disease duration [16]. On the other hand, numerous studies have indicated a dysregulated miRNA in various cell types in MS patients [17] and testified the involvement of genetic contribution in the etiology of this disease [18]. Considering these facts, this study aimed to evaluate the role of genetic implications (rs9904341, rs17878467, and rs8073069 SNPs in BIRC5 gene) in the regulation survivin level. Additionally, important previously confirmed miRNAs (miR-16, miR-34a, miR-150, and miR-203a) in targeting the survivin mRNA was surveyed in the PBMCs of MS patients and investigated their involvement in the regulation of survivin mRNA expression in PBMCs as well as survivin serum concentration.
Methods
Patients and control subjects
This case-control study was carried out on 200 RRMS patients referred to Fars Multiple Sclerosis Society and 200 healthy controls. Patients who had chronic inflammatory disorders, cancer, autoimmune disease, drug intake etc. were excluded. All the patients were in the relapsing state. Furthermore, healthy controls had no autoimmune disease in themselves as well as their family members. Individuals in the case and control groups were age- and sex- matched. Patients were diagnosed as having MS based on the modified McDonald criteria [19, 20] and the disability score of patients was determined by Expanded Disability Status Scale (EDSS) [21]. In this study, MS subjects were selected from those in the remitting state that had received no immunomodulating medications for at least 3 months before sampling. The protocol of this study was approved by the Human Research Ethics Committee from the Shiraz University of Medical Sciences, Fars, Iran and written informed consent forms was taken by all subjects. Blood samples from MS patients were collected when the disease was clinically diagnosed. To perform experiments, 10 ml of venus blood from all participants was collected in EDTA tubes via venipuncture.
Real-time PCR genotyping of SNPs
Using Real-time allelic discrimination Taq-Man assays (Applied Biosystems, Foster City, USA), patients and controls were genotyped for rs9904341, rs17878467, and rs8073069 SNPs in BIRC5 gene. The reactions mixture contained 5 μl of genomic DNA (200 ng/μl), 5 μl of Taq-Man Master Mix (Applied Biosystems, Foster City, USA), 0.5 μl of Taq-Man Genotyping Assay mix containing FAM or VIC labeled probes and primers (Applied Biosystems, Foster City, USA), and H2O to reach a final volume of 25 μl. Real-Time allelic discrimination PCR condition that was performed via StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, USA) was: initially 60 °C for 30 s and then 95 °C for 10 min, and then 40 amplification cycles in 95 °C for 15 s and 60 °C for 1 min, and ultimately 60 °C for 30 s.
PBMC separation, RNA extraction, cDNA synthesis
To attain PBMCs from 50 RRMS patients and 50 healthy individuals, the Ficoll-Hypaque density gradient approach was done. To isolate the RNA, the MiRNeasy Mini kit (Qiagen, Germany) was used. For determination of the yield and purity of isolated RNAs, a Nano Drop spectrophotometer at 260/280 nm (Nano Drop ND-2000C Spectrophotometer, Thermo Fisher Scientific, USA) was used. The first strand complementary DNA (cDNA) was synthesized using the miScript II RT Kit (Qiagen, Germany) based on the manufacturer’s protocol.
Real-time PCR quantification of miRNA and survivin expression
Measurement of the miRNA expression levels (including miR-16, miR-34a, miR-150, and miR-203a) was carried out by miScript SYBR Green PCR Kit (Qiagen, Germany) and StepOne Plus Real-Time PCR (Applied Biosystems, Foster City, CA, USA). Each reaction mixture contained 6 μl cDNA template, 10 μl of SYBR Green PCR Master mix, 2 μl primers each, and RNase free water to a total volume of 25 μl. The Real-time quantitative PCR conditions were: initial 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 55 °C for 30 s, and finally 65 °C for 30 s.
Real-time PCR quantification of survivin mRNA level in PBMCs from RRMS patients and healthy controls was performed using RealQ Plus Master Mix Green High ROX (AMPLIQON, Denmark) and StepOne Plus Real-time PCR machine (Applied Biosystems, Foster City, CA, USA). The components of the mixture were 10 μl SYBR Green Master Mix, 8 μl cDNA, 0.4 μl primers each (forward primer of 5′-CCACCGCATCTCTACATTCA-3′ and reverse primer of 5′-GTCTGGCTCGTTCTCAGTGG-3′; adopted from Primer Bank; https://pga.mgh.harvard.edu/primerbank/), and RNase free H2O for a final volume of 25 μl. The thermocyclic conditions of Real-time PCR were: the holding step of 95 °C for 15 min, 50 cycles of 95 °C for 15 s, and 63 °C for 30 s, and then 70 °C for 1 min.
Comparative CT method was exploited to calculate relative miRNA and survivin expressions as previously described by Livak and Schmittgen [22]. For normalizing the expression levels of target genes, the transcript levels of RUN6 (for miRNAs) and GAPDH (for survivin), as the housekeeping genes, were determined.
Survivin concentration
To determine the survivin level, the serum samples were isolated from the peripheral blood of 50 patients and 50 control subjects. Survivin level was determined using enzyme-linked immunosorbent assay (ELISA) and a commercial kit (Human Survivin ELISA Kit, OriGene Technologies, Inc., Rockville, MD, USA).
Statistical analysis
Genotype and allelic distribution between case and control groups were implemented by Chi-Square test. Pearson’s χ2-tests were applied to test for significance differences of both genotype and allele frequencies between two groups. The odds ratio (OR) and 95% confidence interval (CI) were calculated. The genotype distributions of chosen SNPs were tested for deviation from Hardy-Weinberg equilibrium in case and control. Determination of normality of scale data distribution was conducted using the Kolmogorov-Smirnov test. The independent t-test or ANOVA was used to compare the groups. The GraphPad Prism v. 6.00 (GraphPad Software, Inc., San Diego, CA, USA, www.graphpad.com) was exploited for plotting. SPSS software v. 21 (SPSS, Chicago, IL, USA) was used for data analysis. Data were presented as number and percentage or mean ± standard deviation (SD) with statistical significance at P < 0.05.
Results
Baseline characteristic of the study population
RRMS patients and healthy controls had the mean age of 43.60 ± 11.33 and 41.54 ± 15.74, respectively. The RRMS group and healthy control group were comprised of female/male ratio of 144 (72%)/56 (28%) and 145 (72.5%)/55 (27.5%). Therefore, the patients and controls were age- and sex-matched. In the patient group, the age of onset of RRMS was 27.65 ± 10.88 and duration of the diseases was 7.41 ± 3.15. RRMS patients demonstrated to have EDSS score of 3.89 ± 1.45 (Table 1).
Table 1.
Baseline characteristics of the study participants
| Characteristic | RRMS Patients (n = 200) | Healthy Controls (n = 200) |
|---|---|---|
| Female/Male | 144 (72%)/56 (28%) | 145 (72.5%)/55 (27.5%) |
| Age | 43.60 ± 11.33 | 41.54 ± 15.74 |
| Onset age | 27.65 ± 10.88 | – |
| Duration of the disease | 7.41 ± 3.15 | – |
| EDSS | 3.89 ± 1.45 |
RRMS Relapsing Remitting Multiple Sclerosis, EDSS Expanded Disability Status Scale
Genotyping findings
According to the Table 1, the distribution of the genotypes in all three SNPs (rs9904341, rs17878467, and rs8073069) in the control group disclosed to have no significant distortion (P = 0.28, 0.21, and 0.52, respectively) from the Hardy–Weinberg equilibrium.
Among the genotyped SNPs, only rs9904341 demonstrated statistically significant difference with respect to the frequency of alleles and genotypes between the case and control groups. For this SNP, the major G allele was considered as the reference allele and the C allele was the minor allele, which was significantly prevalent in the patient group (Table 2). It was observed that the C allele had 47% frequency in the patient group and 39% in the control group. This allele significantly increased the risk of MS (OR = 1.38, 95% CI = 1.05–1.348, P = 0.022). on the other side, the GG genotype was the reference genotype. The CC genotype was detected in 24.5% of RRMS patients and 17% of the healthy subjects, and significantly increased the risk of the disease (OR = 1.84, 95% CI = 1.06–3.19; P = 0.029). However, the CG genotype had no significant difference between patient and control groups.
Table 2.
The allele and genotype frequencies of the genotyped SNPs in the BIRC5 gene in MS patients and healthy individuals (HWE value is a probability)
| SNP | Alleles/Genotypes | MS Patients (n = 200) N (%) | Controls (n = 200) N (%) | P value | OR (95%CI) |
|---|---|---|---|---|---|
| rs9904341 | C | 188 (47) | 156 (39) | 0.022 | 1.38 (1.05–1.84) |
| G | 212 (53) | 244 (61) | Reference | ||
| CC | 49 (24.5) | 34 (17) | 0.029 | 1.84 (1.06–3.19) | |
| CG | 90 (45) | 88 (44) | 0.238 | 1.31 (0.83–2.04) | |
| GG | 61 (30.5) | 78 (39) | Reference | ||
| HWE | 0.287 | ||||
| rs17878467 | T | 68 (17) | 73 (18.25) | 0.642 | 0.91 (0.63–1.32) |
| C | 332 (83) | 327 (81.75) | Reference | ||
| TT | 9 (4.5) | 4 (2) | 0.242 | 2.05 (0.62–6.80) | |
| CT | 50 (25) | 65 (32.5) | 0.110 | 0.70 (0.45–1.08) | |
| CC | 144 (72) | 131 (65.5) | Reference | ||
| HWE | 0.21 | ||||
| rs8073069 | C | 163 (40.75) | 150 (37.5) | 0.346 | 1.15 (0.86–1.52) |
| G | 237 (59.25) | 250 (62.5) | Reference | ||
| CC | 35 (17.5) | 26 (13) | 0.249 | 1.42 (0.78–2.59) | |
| GC | 93 (46.5) | 98 (49) | 0.993 | 1.00 (0.65–1.54) | |
| GG | 72 (36) | 76 (38) | Reference | ||
| HWE | 0.52 | ||||
OR odds ratio, CI confidence interval, HWE Hardy-Weinberg equilibrium
Values in bold shows significant P values
Expression level of miRNAs
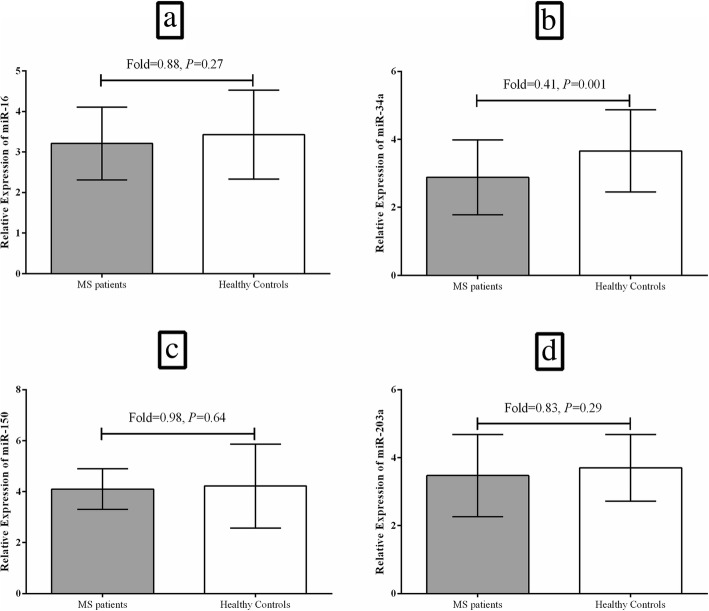
Among the evaluated miRNAs, only miR-34a indicated statistically significant difference in expression inside the PBMCs between the patients and controls. This miRNA was significantly downregulated in the PBMCs of the RRMS patients in comparison to the controls (Fold change = 0.41, P = 0.001, Fig. 1.b). Other miRNAs, including miR-16 (Fold change = 0.88, P = 0.27, Fig. 1.a), miR-150 (Fold change = 0.98, P = 0.64, Fig. 1.c), and miR-203a (Fold change = 0.83, P = 0.29, Fig. 1.d) were downregulated in the PBMCs of RRMS patients in comparison to healthy controls, but the differences were not statistically different. It was revealed that miR-34a transcript level had no statistically significant difference (P = 0.215) in the PBMCs from RRMS patients with the three genotypes of CC, CG, and GG for the rs9904341 SNP that had significantly different genotype frequencies between patients and controls (Fig. 2).
Fig. 1.
Representation of the expression of a; miR-16, b; miR-34a, c; miR-150, and d; miR-203a in the PBMCs from RRMS patients and the healthy subjects
Fig. 2.
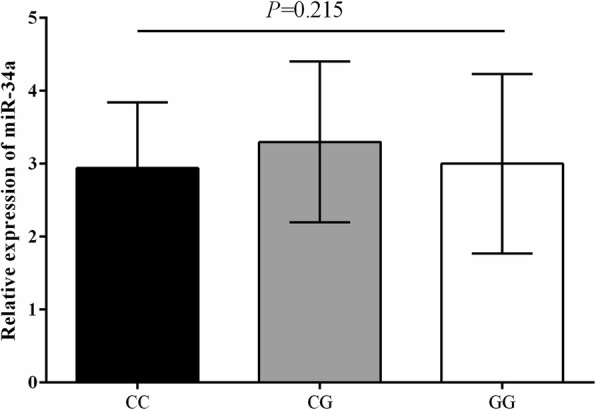
Bar graphs illustrates the relative miR-34a expression in the PBMCs from RRMS patients with the three genotypes of CC, CG, and GG for the rs9904341 SNP that had significantly different genotype frequencies between patients and controls
mRNA expression and serum concentration of survivin
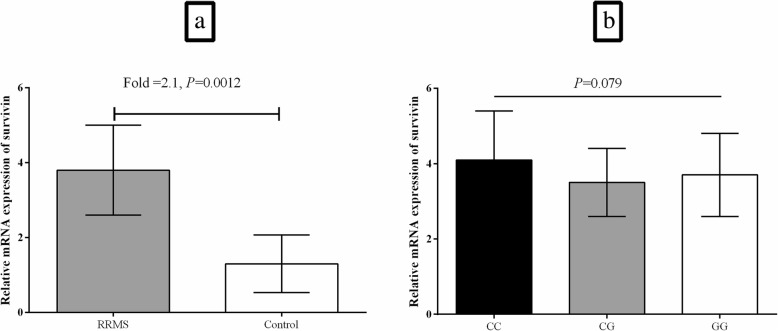
It was observed that mRNA expression of survivin was upregulated significantly in the PBMCs from RRMS patients compared with healthy control group (Fold change = 2.1, P = 0.0012; Fig. 3.a). Moreover, there was no statistically significant difference (P = 0.079) in the mRNA expression of survivin in the PBMCs from RRMS patients with the three genotypes of CC, CG, and GG for the rs9904341 SNP that had significantly different genotype frequencies between patients and controls (Fig. 3.b).
Fig. 3.
Bar graphs illustrates the relative mRNA expression of survivin in the PBMCs from RRMS patients in comparison to healthy controls (a) and in the RRMS patients with the three genotypes of CC, CG, and GG for the rs9904341 SNP (b) that had significantly different genotype frequencies between patients and controls
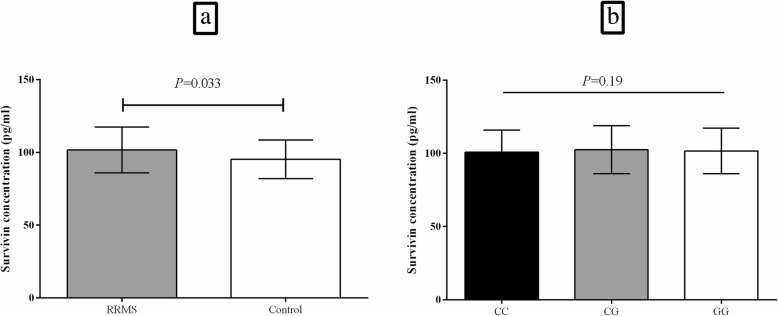
The serum level of survivin was increased significantly in the RRMS patients compared with healthy subjects (101.66 ± 15.68 pg/ml vs. 95.27 ± 13.28 pg/ml, P = 0.033; Fig. 4.a). However, the serum level of survivin had no statistically significant difference (P = 0.19) among the RRMS patients with the three genotypes of CC (100.78 ± 15.14 pg/ml), CG (102.49 ± 16.43 pg/ml), and GG (101.63 ± 15.52 pg/ml) for the rs9904341 SNP (Fig. 4.b).
Fig. 4.
Bar graphs illustrates the survivin serum concentration in RRMS patients in comparison to healthy controls (a) and in the RRMS patients with the three genotypes of CC, CG, and GG for the rs9904341 SNP (b) that had significantly different genotype frequencies between patients and controls
Correlation analyses
Among the miRNAs, downregulation of the miR-34a demonstrated statistically significant negative correlation with the mRNA expression of survivin in PBMCs (r = − 0.23, P = 0.0012) as well as with the serum level of survivin (r = − 0.29, P = 0.0027). However, the correlation analysis indicated no relation between the expression of miRNAs and the EDSS score of RRMS patients.
mRNA expression of survivin in PBMCs as well as serum survivin level did not show statistically significant correlation with the EDSS score in RRMS patients (r = 0.29, P = 0.088 and r = 0.41, P = 0.37, respectively). Furthermore, the EDSS score did not have statistically significant difference among the RRMS patients with the three genotypes of rs9904341 SNP.
Discussion
This study intended to survey the role of genetic variations (rs9904341, rs17878467, and rs8073069 SNPs located in BIRC5 gene) as well as important miRNAs (miR-16, miR-34a, miR-150, and miR-203a) in the regulation of survivin. It was observed that both genetic variations and miRNA dysregulation might be involved in the disease pathogenesis. However, only miR-34a was shown to regulate the survivin mRNA expression and survivin serum level in RRMS patients.
Survivin play a role in the inhibition of apoptosis and is involved in the regulation of both cell proliferation and survival [8]. During malignancies and immune tolerance failure in the autoimmune settings, mitosis and resistance to apoptosis are involved in the pathogenesis of the disease. It has been reported that upregulation of survivin in various tumor cells eventuates in the tumor progression, resistance to drug, and decreased survival [9, 23]. Survivin modulates important mechanisms of the immune system, including promoted proliferation of T cells upon activation, differentiation of CD4+ T cells, and hemostasis of CD8+ memory T cells. Upregulation of survivin has been associated with increased expression of the vital molecules during antigen presentation to T cells and required co-stimulatory molecules, including major histocompatibility complex (MHC) class II and CD80/86 molecules [9]. With respect to such critical roles of survivin in the context of immune system, survivin has been investigated in the autoimmunity including, MS [9]. That notwithstanding, there is a paucity of understandings regarding the regulatory mechanism of survivin in the pathogenesis of autoimmune diseases.
It has been reported that removal of autoreactive lymphocytes, which might be regulated by apoptosis through the regulation of survivin, is aberrantly modulated in MS [24–26]. It was reported that survivin level was overexpressed in the resting T cells from PPMS patients [15]. Moreover, decreased expression of survivin level was disclosed in the peripheral T cells stimulated by interferon-β-1a (IFN-β-1a) ex vivo, inducing the apoptosis in the T cells [27]. Ex vivo overexpression of survivin induced T cells from patients with active MS [16]. Upregulated expression of survivin was detected in the mitogen stimulated resting lymphocytes isolated from RRMS patients [28]. It seems that survivin increases the survival and proliferation of autoreactive T cells in MS patients. In the current study, the serum level of survivin was higher that control subjects. Moreover, mRNA expression of survivin was upregulated in the PBMCs (that contain predominantly the lymphocytes) from RRMS patients.
Genetic polymorphisms of BIRC5 gene has been associated with the modulation of expression level of survivin [10]. For example, the rs9904341 SNP was associated with increased mRNA and protein expressions of survivin in cancers [11, 12]. The rs17878467 SNP was associated with increased BIRC5 promoter activity in HeLa cell lines [12], and the rs8073069 SNP in the BIRC5 gene was associated with overexpression of survivin in esophageal cancer [13]. These polymorphisms impressed the response to treatment and the disease activity in rheumatoid arthritis patients [29]. This study for the first time evaluated the genetic polymorphisms in MS patients and indicated that the C allele and CC genotype in the rs9904341 SNP increased the disease risk. Nonetheless, this polymorphism was associated with neither survivin level in serum nor EDSS score in the RRMS patients.
Survivin expression is under the regulation of miRNAs. In the current study, important miRNAs regulating the survivin expression was selected miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/php/index.php), the experimentally validated microRNA-target interactions database (http://www.microrna.org/microrna/home.do), miRDB (http://mirdb.org/cgi-bin/search.cgi), and already confirmed miRNAs by the published works. To the best of our knowledge, this was the first investigation of the survivin regulation through miRNAs in multiple sclerosis. We observed that miR-34a was downregulated in the PBMCs from RRMS patients that was inversely correlated with overexpression of survivin in PBMCs from RRMS patients as well as with the increased survivin level in the serum samples. Nonetheless, decreased levels of miR-34a did not impress the disease activity based on EDSS score. It seems that miR-34a regulates the expression of survivin in PBMCs, regardless of its involvement in the clinical presentation of RRMS patients with respect to EDSS.
Conclusions
In consideration of all the findings, this was the first study, to the best of our knowledge, that intended to search for regulatory mechanisms of survivin expression in the MS disease. The genetic variations (rs9904341, rs17878467, and rs8073069 SNPs located in BIRC5 gene) was studied and was detected that the C allele and CC genotype in the rs9904341 SNP increased the disease risk. Furthermore, miR-34a was downregulated in the PBMCs from RRMS subjects that was negatively correlated with the mRNA expression of survivin in the PBMCs as well as with the survivin level in the serum. The findings here could be considered preliminary insight into the survivin regulator mechanisms in MS disease that still needs to be enrichened in the future works.
Acknowledgements
The authors are grateful of the patients and the healthy individuals for their participation in the study.
Abbreviations
- BBB
Blood-brain barrier
- BIRC5
Baculoviral IAP repeat containing 5
- CI
Confidence interval
- CNS
Central nervous system
- EDSS
Expanded disability status scale
- ELISA
Enzyme-linked immunosorbent assay
- IFN-β-1a
Interferon-β-1a
- MHC
Major histocompatibility complex
- miRNAs
microRNAs
- MS
Multiple sclerosis
- OR
odds ratio
- PBMC
peripheral blood mononuclear cells
- PPMS
Primary progressive MS
- pre-miRNAs
precursor miRNAs
- pri-miRNAs
primary miRNAs
- PRMS
Progressive relapsing MS
- RISCs
RNA-induced silencing complexes
- RRMS
Relapsing-remitting MS
- SD
Standard deviation
- SNPs
single nucleotide polymorphisms
- SPMS
Secondary progressive MS
- UTR
3′-Untranslated region
Authors’ contributions
DR: Performed the experiments, developed the main idea, participated in manuscript drafting, and read the manuscript critically. FM; Performed the statistical analysis, participated in manuscript drafting, and read the manuscript critically. MA; Participated in conducting the experiments, participated in manuscript drafting, and read the manuscript critically. TG: Performed the statistical analysis, participated in manuscript drafting, and read the manuscript critically. PAGK; Performed the sampling, participated in manuscript drafting, and read the manuscript critically. MA; Developed the main idea, participated in manuscript drafting, and read the manuscript critically. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
The protocol of this study was approved by the Human Research Ethics Committee from the Shiraz University of Medical Sciences, Fars, Iran and written informed consent forms was taken by all subjects.
Research involving human subjects and/or animals;
Research carried out here were in compliance with the Helsinki Declaration. The protocol of this study was approved by the Human Research Ethics Committee from the Shiraz University of Medical Sciences, Fars, Iran (Reference No. 97–05-451). Written informed consent forms were obtained from patients and healthy controls before blood taking.
Consent for publication
All authors read the manuscript and consent for its publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dariush Rahban, Phone: +98-930-278-0206, Email: rahbandariush@gmail.com.
Majid Ahmadi, Phone: +98-914-494-4668, Email: ahmadi.m@tbzmed.ac.ir.
References
- 1.Goldenberg MM. Multiple sclerosis review. Pharmacy and Therapeutics. 2012;37(3):175. [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson S, Blizzard L, Otahal P, Van der Mei I, Taylor B: Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry 2011:jnnp. 2011. 240432. [DOI] [PubMed]
- 3.Khondkarian O, Zavalishin I, Nevskaia O: [Classification of multiple sclerosis]. Zhurnal nevropatologii i psikhiatrii imeni SS Korsakova (Moscow, Russia: 1952) 1982, 83(2):164–166. [PubMed]
- 4.Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999;399:A40–A47. doi: 10.1038/399a040. [DOI] [PubMed] [Google Scholar]
- 5.Compston A, McDonald I, Noseworthy J, Lassmann H, Miller D, Smith K, Wekerle H, Confavreux C. McAlpine’s multiple sclerosis. 2006. [Google Scholar]
- 6.Macchi B, Marino-Merlo F, Nocentini U, Pisani V, Cuzzocrea S, Grelli S, AJJon M. Role of inflammation and apoptosis in multiple sclerosis: comparative analysis between the periphery and the central nervous system. 2015. pp. 80–87. [DOI] [PubMed] [Google Scholar]
- 7.Gurevich M, Achiron A. The switch between relapse and remission in multiple sclerosis: continuous inflammatory response balanced by Th1 suppression and neurotrophic factors. J Neuroimmunol. 2012;252(1–2):83–88. doi: 10.1016/j.jneuroim.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3(1):46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 9.Ebrahimiyan H, Aslani S, Rezaei N, Jamshidi A, Mahmoudi M. Survivin and autoimmunity; the ins and outs. Immunol Lett. 2018;193:14–24. doi: 10.1016/j.imlet.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Li F. Survivin study: what is the next wave? J Cell Physiol. 2003;197(1):8–29. doi: 10.1002/jcp.10327. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Fang F, Ludewig G, Iones G, Jones D. A mutation found in the promoter region of the human survivin gene is correlated to overexpression of survivin in Cancer Cells. DNA Cell Biol. 2004;23(9):527–537. doi: 10.1089/dna.2004.23.527. [DOI] [PubMed] [Google Scholar]
- 12.Jang JS, Kim KM, Kang KH, Choi JE, Lee WK, Kim CH, Kang YM, Kam S, Kim I-S, Jun JE. Polymorphisms in the survivin gene and the risk of lung cancer. Lung Cancer. 2008;60(1):31–39. doi: 10.1016/j.lungcan.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Xiong G, Chen X, Xu X, Wang K, Fu Y, Yang K, Bai Y. Polymorphisms of survivin promoter are associated with risk of esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2009;135(10):1341–1349. doi: 10.1007/s00432-009-0575-7. [DOI] [PubMed] [Google Scholar]
- 14.Koppers-Lalic D, Hackenberg M, Bijnsdorp IV, van Eijndhoven MA, Sadek P, Sie D, Zini N, Middeldorp JM, Ylstra B, de Menezes RX. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8(6):1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Hebb A, Moore C, Bhan V, Campbell T, Fisk J, Robertson H, Thorne M, Lacasse E, Holcik M, Gillard J. Expression of the inhibitor of apoptosis protein family in multiple sclerosis reveals a potential immunomodulatory role during autoimmune mediated demyelination. Mult Scler. 2008;14(5):577–594. doi: 10.1177/1352458507087468. [DOI] [PubMed] [Google Scholar]
- 16.Sharief M, Noori M, Douglas M, Semra YK. Upregulated survivin expression in activated T lymphocytes correlates with disease activity in multiple sclerosis. Eur J Neurol. 2002;9(5):503–510. doi: 10.1046/j.1468-1331.2002.00454.x. [DOI] [PubMed] [Google Scholar]
- 17.Aslani S, Jafari N, Javan MR, Karami J, Ahmadi M, Jafarnejad M. Epigenetic modifications and therapy in multiple sclerosis. NeuroMolecular Med. 2017;19(1):11–23. doi: 10.1007/s12017-016-8422-x. [DOI] [PubMed] [Google Scholar]
- 18.Patsopoulos NA. Genetics of multiple sclerosis: an overview and new directions. Cold Spring Harb Perspect Med. 2018;8(7):a028951. doi: 10.1101/cshperspect.a028951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtzke JF. Rating neurologic impairment in multiple sclerosis an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Garg H, Suri P, Gupta JC, Talwar G, Dubey S. Survivin: a unique target for tumor therapy. Cancer Cell Int. 2016;16(1):49. [DOI] [PMC free article] [PubMed]
- 24.Chervonsky AV. Apoptotic and effector pathways in autoimmunity. Curr Opin Immunol. 1999;11(6):684–688. doi: 10.1016/s0952-7915(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 25.Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407(6805):789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 26.Segal BM, Cross AH. Fas(t) track to apoptosis in MS: TNF receptors may suppress or potentiate CNS demyelination. Neurology. 2000;55(7):906–907. doi: 10.1212/wnl.55.7.906. [DOI] [PubMed] [Google Scholar]
- 27.Sharief MK, Semra YK. Down-regulation of survivin expression in T lymphocytes after interferon beta-1a treatment in patients with multiple sclerosis. Arch Neurol. 2002;59(7):1115–1121. doi: 10.1001/archneur.59.7.1115. [DOI] [PubMed] [Google Scholar]
- 28.Sharief MK, Semra YK. Heightened expression of survivin in activated T lymphocytes from patients with multiple sclerosis. J Neuroimmunol. 2001;119(2):358–364. doi: 10.1016/s0165-5728(01)00389-7. [DOI] [PubMed] [Google Scholar]
- 29.Jenko B, Praprotnik S, Čučnik S, Rotar Ž, Tomšič M, Dolžan V. Survivin polymorphism is associated with disease activity in rheumatoid arthritis patients. Pharmacogenomics. 2016;17(37):45–49. doi: 10.2217/pgs.15.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. All data generated or analyzed during this study are included in this published article.