This cohort study examines whether clinically meaningful posttraumatic stress disorder symptom reduction is associated with lower risk of type 2 diabetes.
Key Points
Question
Is clinically meaningful posttraumatic stress disorder symptom decrease (≥20-point decrease on the Posttraumatic Stress Disorder Checklist score) associated with a lower risk of incident type 2 diabetes compared with less than a clinically meaningful or no improvement?
Findings
In this cohort study of medical records from 1598 patients, clinically meaningful posttraumatic stress disorder improvement compared with less than clinically meaningful or no improvement was associated with a 49% lower risk of incident type 2 diabetes.
Meaning
Long-term chronic health conditions associated with posttraumatic stress disorder may be less likely to occur among patients who experience clinically meaningful symptom reduction through treatment or spontaneous improvement.
Abstract
Importance
Posttraumatic stress disorder (PTSD) is associated with increased risk of type 2 diabetes (T2D). Improvement in PTSD has been associated with improved self-reported physical health and hypertension; however, there is no literature, to our knowledge, on whether PTSD improvement is associated with T2D risk.
Objective
To examine whether clinically meaningful PTSD symptom reduction is associated with lower risk of T2D.
Design, Setting, and Participants
This retrospective cohort study examined Veterans Health Affairs medical record data from 5916 patients who received PTSD specialty care between fiscal years 2008 and 2012 and were followed up through fiscal year 2015. Eligible patients had 1 or more PTSD Checklist (PCL) scores of 50 or higher between fiscal years 2008 and 2012 and a second PCL score within the following 12 months and at least 8 weeks after the first PCL score of 50 or higher. The index date was 12 months after the first PCL score. Patients were free of T2D diagnosis or an antidiabetic medication use for 12 months before the index date and had at least 1 visit after the index date. Data analyses were completed during January 2019.
Exposures
Reduction in PCL scores during a 12-month period was used to define patients as those with a clinically meaningful improvement (≥20-point PCL score decrease) and patients with less or no improvement (<20-point PCL score decrease).
Main Outcomes and Measures
Incident T2D diagnosed during a 2- to 6-year follow-up.
Results
Medical records from a total of 1598 patients (mean [SD] age, 42.1 [13.4] years; 1347 [84.3%] male; 1060 [66.3%] white) were studied. The age-adjusted cumulative incidence of T2D was 2.6% among patients with a clinically meaningful PCL score decrease and 5.9% among patients without a clinically meaningful PCL score decrease (P = .003). After control for confounding, patients with a clinically meaningful PCL score decrease were significantly less likely to develop T2DM compared with those without a clinically meaningful decrease (hazard ratio, 0.51; 95% CI, 0.26-0.98).
Conclusions and Relevance
The findings suggest that clinically meaningful reductions in PTSD symptoms are associated with a lower risk of T2D. A decrease in PCL score, whether through treatment or spontaneous improvement, may help mitigate the greater risk of T2D in patients with PTSD.
Introduction
Posttraumatic stress disorder (PTSD) is a chronic condition that affects up to 12% of civilians and up to 30% of the veteran population and is associated with increased risks for multicomorbidity.1,2,3,4 Completion of evidence-based psychotherapy is associated with clinically meaningful reductions in PTSD symptoms5,6 and can result in improvement in psychiatric comorbidities and perceived health.7,8,9 Improvement in PTSD is associated with parallel improvements in depression and general emotional well-being,7,8,10,11 sleep,12,13 blood pressure,9,14 general physical concerns (eg, back pain, headache, and cough), and perceived health.15
Posttraumatic stress disorder is associated with increased risk of type 2 diabetes (T2D),3,15,16,17,18,19,20 and this association may be partly explained by the high prevalence of obesity, glucose dysregulation, inflammation, the metabolic syndrome, depression, and other T2D risk factors among those with vs without PTSD.20,21,22 To our knowledge, no studies have examined whether a clinically meaningful reduction in PTSD symptoms is associated with lower risk of incident T2D.
We hypothesized that clinically meaningful PTSD symptom reduction would be associated with a decreased risk of incident T2D during a 2- to 6-year follow-up period. In addition, to explore potential mechanisms for the association between clinically meaningful PTSD symptom reduction and risk of T2D, we evaluated change in body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared), hemoglobin A1c values, and depression symptoms in patients with and without clinically meaningful PTSD symptom reduction.
Methods
This retrospective cohort study used medical record data from Veterans Health Affairs (VHA) patients who used a PTSD specialty clinic at 1 of 5 VHA medical centers across the United States between fiscal years (FYs) 2008 and 2012. Follow-up continued until FY2015. Medical record data included diagnostic codes, type of clinic encounter (eg, primary care, PTSD psychotherapy, and physical therapy), medications, laboratory results, vital signs, and demographic measures. The study was approved by the institutional review boards of Saint Louis University and the Harry S. Truman Veterans Administration Medical Center with a waiver of informed consent because data were deidentified.
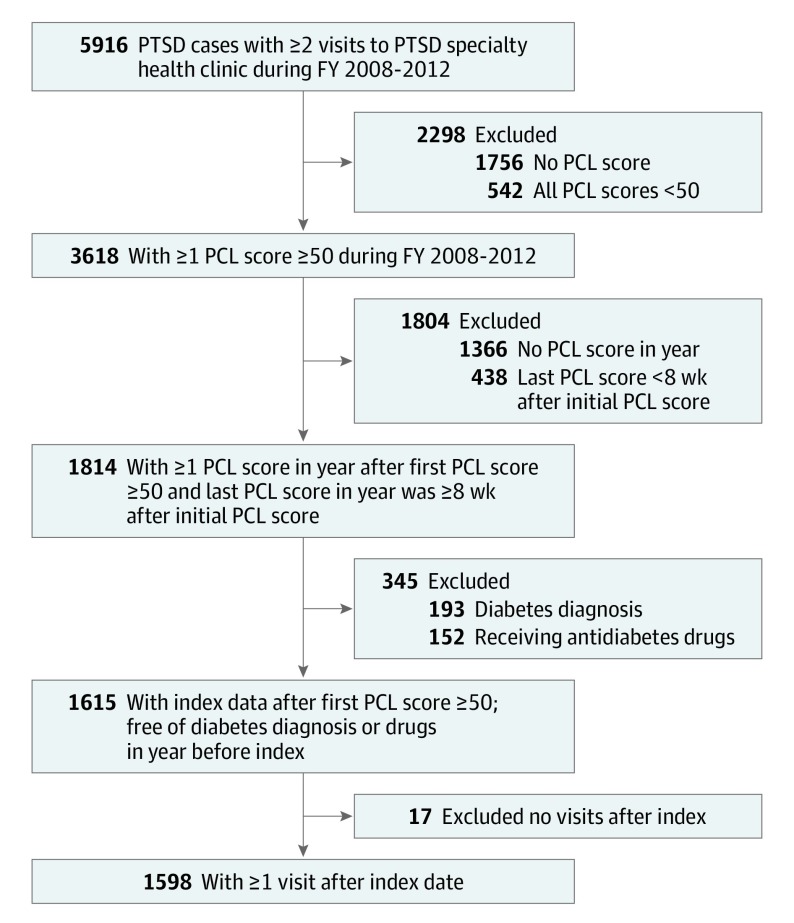
From patients 18 to 70 years of age who had 2 or more visits to PTSD specialty care, we randomly selected 5916 patients with PTSD. Eligible patients had a PTSD Checklist (PCL) score of 50 or higher (ie, above the threshold for probable PTSD)23,24 in FY2008 to FY2012. We required patients to have at least 1 PCL score within the following 12 months of their first PCL score of 50 or higher. The last PCL score in this 12-month period was required to be at least 8 weeks after the first PCL score of 50 or higher. The index date was 12 months after the first PCL score of 50 or higher. Index dates could occur from FY2009 to FY2013. Therefore, follow-up time ranged from 2 to 6 years. All patients had to be free of a T2D diagnosis or not receiving an antidiabetic medication for 12 months before the index date and have had at least 1 visit after the index date. After eligibility criteria were applied, 1598 patients with PTSD and free of diabetes at the index date were available for analysis. The sample selection is shown in the Figure, and the retrospective cohort design is shown in eFigure 1 in the Supplement.
Figure. Sample Selection.
FY indicates fiscal year; PCL, PTSD Checklist; PTSD, posttraumatic stress disorder.
Variable Definitions
Posttraumatic stress disorder was defined as 2 or more outpatient visits within a 12-month period or 1 inpatient stay with an International Classification of Diseases, Ninth Revision (ICD-9) code of 309.81. Requiring 2 or more PTSD diagnoses has good positive predictive value (82%) compared with a criterion standard PCL score of 50 or higher25 and has good agreement (79.4%) with lifetime diagnosis according to the Structured Clinical Interview for DSM-IV.26
Outcome
Incident T2D was defined as the first occurrence of ICD-9 codes 250.x0, 250.x2, 357.2, 362.0x, or 366.41 or a fill for an antidiabetic medication. Follow-up time was measured as days since the index date to T2D or censor date, which was the last clinical encounter in FY2008 to FY2015.
Exposure
The PCL scores were obtained from medical record abstraction and administrative data. Because VHA administrative medical record data do not capture all PCL scores (eg, those stored in physician notes), medical record abstraction was conducted by trained medical record abstractors from Abt Associates (https://www.abtassociates.com/). During 6 months, Abt Associates abstracted 22 287 valid PCL scores from eligible patients with encounters between FY2008 and FY2015. After administrative data were added and duplicate scores removed, 26 631 valid PCL values for 4441 patients with PTSD were available from encounters between FY2008 and FY2015.
The difference between the last PCL score in the exposure year and first PCL score of 50 or higher (PCL score range, 17-85) was used to classify patients as having a clinically meaningful decrease in PTSD symptoms, defined as a 20-point or greater decrease vs less than or no PTSD symptom decrease defined as a less than 20-point decrease. Research suggests that 10 points reflect clinically meaningful improvement.24 To increase the ability to detect an association with PTSD improvement, we used 20 points to define meaningful PCL score reduction.
Covariates
Detailed variable definitions are given in eTable 1 in the Supplement. Sociodemographic variables were measured at the start of the observation period. Some patients had missing data for race/ethnicity; therefore, we created a missing category to retain all cases in analyses. Other covariates were measured up to the index date. These covariates included depression, anxiety disorders, alcohol and drug abuse or dependence, smoking, sleep disorder, adequate acute-phase antidepressant treatment, receipt of any atypical antipsychotic medication, hypertension, hyperlipidemia, and obesity. We selected covariates that are correlated with PCL score change (eg, depression) and medications and comorbid metabolic conditions that are correlated with PTSD and T2D. Receipt of minimally adequate PTSD psychotherapy (≥9 visits in 15 weeks) was measured from the first PCL score of 50 or higher to the index date. We controlled for PTSD psychotherapy to reduce the risk of confounding treatment seeking, adherence, and treatment response with health prevention behaviors that would reduce the risk of T2D. Our goal was to measure the total association between PCL score decrease (whether attributable to treatment or spontaneous remission) and risk of T2D and not to specifically assess the effect of treatment response.
Propensity Score Methods
We used propensity scores and inverse probability of exposure weighting (IPEW) to balance the distribution of potential confounders between patients who did and did not have a clinically meaningful PCL score decrease. The propensity score is a binary logistic regression model that was used to measure the probability of a clinically meaningful PCL decrease vs less than a clinically meaningful decrease given covariates.27 The propensity score was used to compute a stabilized weight,28,29 which is the marginal probability of experiencing a clinically meaningful PCL score decrease divided by the propensity score for having a clinically meaningful PCL decrease for those with a decrease of 20 points or higher and for those with a less than 20-point decrease. Well-behaved weights have a mean close to 1 and a maximum value less than 10; therefore, weights of 10 or higher were trimmed.29,30,31 A pseudo-population was created after applying IPEW, and confounding was controlled when variables balanced between patients with and without a clinically meaningful PCL decrease. Balance is evidenced by a standardized mean difference less than 10%.29
Exploratory Analyses
Using a subsample of patients with available data, we computed exploratory analyses to evaluate whether decreased T2D risk after clinically meaningful PTSD improvement was associated with concurrent decreases in BMI (n = 1405), Patient Health Questionnaire 9 (PHQ-9) scores (n = 324), or hemoglobin A1c values (n = 585). We assessed changes in hemoglobin A1c and BMI values from the first available value in the exposure year to the first available value that occurred at least 12 months later. For PHQ-9, we compared changes from the first PHQ-9 score in the exposure year with the last in the exposure year that occurred at least 8 weeks after the first PHQ-9 score. A repeated-measures difference in differences analysis for each measure was conducted using mixed-effects regression models. The difference in differences analysis provided an estimation of the mean change (ie, slope) in each measure in the PCL groups and assesses whether the 2 slopes are significantly different.
Sensitivity Analysis
To determine whether an unmeasured confounder could completely explain our results, we computed the E-value.32 The E-value is the minimum magnitude of association that an unmeasured confounder would have with both the exposure and the outcome to completely explain the association between clinically meaningful PTSD improvement and incident T2D.32
Statistical Analysis
Descriptive analysis used χ2 tests for categorical variables and 2-tailed, independent-sample t tests for continuous variables to estimate the association between potential confounders and PCL score decrease. For unweighted data, we computed Poisson regression models to calculate T2D incidence rates per 1000 person-years. Bivariate Cox proportional hazards regression models were used to measure the association of each covariate with incident T2D.
For unweighted and weighted data, we used Cox proportional hazards regression models to test the association between clinically meaningful PCL score decrease and incident T2D. Weighted Cox proportional hazards regression models controlled for all measured confounders (Table 1 and eFigure 2 in the Supplement). An expanded Cox proportional hazards regression model included adjustment for hypertension, obesity, and hyperlipidemia that could be present after the index date. Results were expressed by hazard ratios (HRs) and 95% CIs. Robust, sandwich-type variance estimators were used to calculate 95% CIs and P values for weighted data. For all models, the proportional hazards assumption was tested and met. SAS statistical software, version 9.4 (SAS Institute Inc) was used for all analyses, and α = .05 indicated statistical significance. Data analyses were completed during January 2019.
Table 1. Sample Characteristics Overall and by Experiencing a Clinically Meaningful PCL Score Decrease for Patients With PTSDa .
| Characteristic | Overall (N = 1598) | Less Than Clinically Meaningful Decrease (n = 1259) | Clinically Meaningful PCL Score Decrease (n = 339) | P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 42.1 (13.4) | 41.7 (13.4) | 43.6 (13.5) | .02 |
| Male | 1347 (84.3) | 1071 (85.1) | 276 (81.4) | .10 |
| Race/ethnicity | ||||
| White | 1060 (66.3) | 819 (65.1) | 241 (71.1) | .12 |
| Black | 350 (21.9) | 281 (22.3) | 69 (20.4) | |
| Other | 122 (7.6) | 104 (8.3) | 18 (5.3) | |
| Missing | 66 (4.1) | 55 (4.4) | 11 (3.2) | |
| Married | 716 (44.8) | 558 (44.3) | 158 (46.6) | .45 |
| VHA-only insurance | 1035 (64.8) | 805 (63.9) | 230 (67.9) | .18 |
| High primary HCU | 399 (25.0) | 325 (25.8) | 74 (21.8) | .13 |
| First PCL score severe, ≥70 | 500 (31.3) | 380 (30.2) | 120 (35.4) | .07 |
| First PCL score, mean (SD) | 64.6 (9.0) | 64.2 (9.1) | 66.1 (8.6) | .001 |
| Last PCL score, mean (SD) | 56.1 (15.9) | 61.8 (11.9) | 35.0 (10.3) | <.001 |
| Psychiatric comorbidities and treatmentsb | ||||
| Depression | 1165 (72.9) | 921 (73.2) | 244 (72.0) | .67 |
| Other anxietyc | 433 (27.1) | 345 (27.4) | 88 (26.0) | .60 |
| Sleep disorder | 730 (45.7) | 580 (46.1) | 150 (44.2) | .55 |
| Alcohol abuse or dependence | 645 (40.4) | 512 (40.7) | 133 (39.2) | .63 |
| Drug abuse or dependence | 408 (25.5) | 310 (24.6) | 98 (28.9) | .11 |
| Smokingd | 832 (52.1) | 649 (51.6) | 183 (54.0) | .43 |
| Adequate PTSD psychotherapye | 737 (46.1) | 537 (42.6) | 200 (59.1) | <.001 |
| Adequate ADM treatmentf | 1191 (74.5) | 959 (76.2) | 232 (68.4) | .004 |
| Atypical antipsychotic | 468 (29.3) | 388 (30.8) | 80 (23.6) | .009 |
| Physical comorbiditiesb | ||||
| Hypertension | 560 (35.0) | 447 (35.5) | 113 (33.3) | .46 |
| Hyperlipidemia | 545 (34.1) | 426 (33.8) | 119 (35.1) | .66 |
| Obesityg | 805 (50.4) | 631 (50.1) | 174 (51.3) | .69 |
Abbreviations: ADM, antidepressant; HCU, health care utilization; PCL, PTSD Checklist (range, 17-85); PTSD, posttraumatic stress disorder; VHA, Veterans Health Administration.
Data are presented as number (percentage) of patients unless otherwise indicated. A PCL score decrease less than 20 was considered to be less than clinically meaningful; a PCL score decrease of less than or equal to 20 was considered to be clinically meaningful.
Comorbidities occur from the start of fiscal year 2008 to the index date.
Composite of panic disorder, obsessive compulsive disorder, social phobia, generalized anxiety disorder, and anxiety not otherwise specified.
Current smoker in health factors or International Classification of Disorders, Ninth Revision, Clinical Modification (ICD-9-CM) code.
Measured from the first PCL score to the index date (exposure year). Presence of at least 9 psychotherapy visits in any 15-week period.
At least 12 weeks of continuous ADM fills before the index date.
Body mass index (calculated as weight in kilograms divided by height in meters squared) of 30 or higher or ICD-9-CM code.
Results
Medical records from a total of 1598 patients (mean [SD] age, 42.1 [13.4] years; 1347 [84.3%] male; 1060 [66.3%] white) were studied (Table 1). Older age was associated with a clinically meaningful PCL score decrease (mean [SD], 43.6 [13.5] years in the group with PCL score decrease ≥20 vs 41.7 [13.4] years in the group with PCL score decrease <20, P = .02). Other demographic variables were not associated with PCL score decrease. Although statistically significant, the mean (SD) of the first PCL score was similar in both groups (66.1 [8.6] in the group with PCL score decrease ≥20 vs 64.2 [9.1] in the group with PCL score decrease <20; P < .001). The distribution of psychiatric disorders, substance use disorders, sleep disorders, smoking, hypertension, hyperlipidemia, and obesity did not significantly differ between groups. Minimally adequate duration of PTSD psychotherapy was significantly more prevalent among those who did (200 [59.0%]) vs did not (537 [42.6%]) experience a clinically meaningful PCL score decrease (P < .001). Receipt of acute-phase antidepressant medication (959 [76.2%] vs 232 [68.4%], P = .004) and receipt of any antipsychotic fill (388 [30.8%] vs 80 [23.6%], P < .001) were significantly more prevalent among patients with less than a clinically meaningful PCL score decrease.
Bivariate associations between potential confounders and incident T2D are given in Table 2. Older age (HR, 1.05; 95% CI, 1.04-1.07; P < .001) and black race (HR, 1.86; 95% CI, 1.23-2.83; P = .004) were significantly associated with incident T2D. High primary care utilization (HR, 1.55; 95% CI, 1.04-2.31; P = .03) and minimally adequate PTSD psychotherapy duration (HR, 1.73; 95% CI, 1.17-2.55; P = .006) were associated with incident T2D. Hypertension (HR, 3.46; 95% CI, 2.33-5.16), hyperlipidemia (HR, 2.82; 95% CI, 1.91-4.16), and obesity (HR, 3.32; 95% CI, 2.12-5.21) were significantly associated with incident T2D (P < .001).
Table 2. Bivariate Association of Each Covariate With Incident Type 2 Diabetes in the Study Patients.
| Covariate | Crude, HR (95% CI) | P Value |
|---|---|---|
| First PCL score severe, ≥70 | 1.38 (0.93-2.04) | .11 |
| Age | 1.05 (1.04-1.07) | <.001 |
| Male | 0.86 (0.52-1.42) | .55 |
| Race/ethnicity | ||
| White | 1 [Reference] | NA |
| Black | 1.86 (1.23-2.83) | .004 |
| Other | 1.18 (0.56-2.47) | .66 |
| Married | 0.87 (0.59-1.29) | .498 |
| VHA-only insurance | 0.66 (0.45-0.97) | .03 |
| High primary HCU | 1.55 (1.04-2.31) | .03 |
| Depression | 1.37 (0.86-2.19) | .19 |
| Other anxiety | 1.01 (0.66-1.56) | .96 |
| Sleep disorder | 1.44 (0.98-2.12) | .06 |
| Alcohol abuse or dependence | 0.91 (0.62-1.36) | .66 |
| Drug abuse or dependence | 0.98 (0.63-1.52) | .91 |
| Smoking | 0.80 (0.55-1.18) | .26 |
| Adequate PTSD psychotherapy | 1.73 (1.17-2.55) | .006 |
| Adequate ADM treatment | 1.31 (0.81-2.11) | .27 |
| Atypical antipsychotic | 1.13 (0.75-1.70) | .57 |
| Hypertension | 3.46 (2.33-5.16) | <.001 |
| Hyperlipidemia | 2.82 (1.91-4.16) | <.001 |
| Obesity | 3.32 (2.12-5.21) | <.001 |
Abbreviations: ADM, antidepressant; HCU, health care utilization; HR, hazard ratio; NA, not applicable; PCL, PTSD Checklist; PTSD, posttraumatic stress disorder; VHA, Veterans Health Administration.
The overall T2D incidence rate per 1000 person-years is given in eTable 2 in the Supplement. For the unweighted data, the T2D incidence rate was 18.0 per 1000 person-years. The age-adjusted T2D incidence rate was significantly lower among patients with a clinically meaningful PCL score decrease (7.3 per 1000 person-years) compared with those with less than a clinically meaningful PCL score decrease (16.0 per 1000 person-years) (P = .005).
The IPEW results are shown in eFigure 2 in the Supplement. The IPEW balanced all confounders (standardized mean difference <10%) between those who did and did not experience a clinically meaningful PCL score decrease. Stabilized weights ranged from 0.39 to 3.30 (mean [SD], 1.00 [0.25]), and there were no extreme weights of 10 or greater (ie, no weights were trimmed).
Results of the Cox proportional hazards regression models revealed that, before weighting in model 1, a clinically meaningful PCL score decrease vs less than a clinically meaningful PCL score decrease was associated with a significantly lower risk of T2D (HR, 0.50; 95% CI, 0.27-0.90; P = .02). This estimate remained largely unchanged after adjusting for age (HR, 0.45; 95% CI, 0.25-0.82; P = .01) and after computing the model in weighted data (HR, 0.51; 95% CI, 0.26-0.98; P = .04). Adding postindex hypertension, obesity, and hyperlipidemia to the model produced similar results (HR, 0.49; 95% CI, 0.26-0.95; P = .03).
Results of exploratory analyses, given in Table 3, revealed similar BMI values before and after a clinically meaningful PCL score decrease and before and after less than a clinically meaningful decrease. Among patients with a clinically meaningful PCL score decrease, the first mean (SD) BMI was 28.7 (5.4), and 12 months later, the mean (SD) BMI was 29.3 (5.4). Similar results were obtained among those with less than a clinically meaningful decrease. At measurements of the first and 12-month PCL scores, mean hemoglobin A1c values ranged from 5.4% to 5.5% (to convert to proportion of total hemoglobin, multiply by 0.01) in both groups. Among 324 patients with PHQ-9 scores, at the time of the first PCL score measurement, we observed a mean (SD) PHQ-9 score of 16.5 (5.4) among patients with less than a clinically meaningful PCL score decrease compared with a mean (SD) PHQ-9 score of 14.8 (6.5) among those with a clinically meaningful decrease. Twelve months later, patients who had less than a clinically meaningful PCL score decrease experienced a mean PHQ-9 decrease of 1.05 (95% CI, −1.74 to −0.37), which was significantly less than the decrease of 6.12 (95 CI, −7.71 to −4.52) among patients who had a clinically meaningful PCL decrease (P < .001).
Table 3. Change in BMI, Hemoglobin A1c Values, and PHQ-9 Score by Clinically Meaningful PCL Score Decrease.
| Variable | PCL Score Change | P Value | DID (95% CI) | P Value | |
|---|---|---|---|---|---|
| Group With <20 PCL Score Change | Group With ≥20 PCL Score Change | ||||
| BMI change, mean (SD) | |||||
| Score measurement 1 | 29.4 (5.4) | 28.7 (5.4) | .04 | NA | NA |
| Score measurement 2 | 29.9 (5.6) | 29.3 (5.4) | .07 | NA | NA |
| BMI change (95% CI) | 0.49 (0.36 to 0.62) | 0.57 (0.31 to 0.82) | NA | 0.08 (−0.21 to 0.37) | .58 |
| Hemoglobin A1c value change, mean (SD) | |||||
| Score measurement 1 | 5.5 (0.5) | 5.4 (0.4) | .005 | NA | NA |
| Score measurement 2 | 5.5 (0.6) | 5.5 (0.5) | .25 | NA | NA |
| Hemoglobin A1c value change (95% CI) | 0.021 (−0.018 to 0.060) | 0.084 (0.01 to 0.16) | NA | 0.063 (−0.02 to 0.14) | .14 |
| PHQ-9 change, mean (SD) | |||||
| Score measurement 1 | 16.5 (5.6) | 14.8 (6.5) | .05 | NA | NA |
| Score measurement 1 | 15.4 (5.8) | 8.7 (5.4) | <.001 | NA | NA |
| PHQ-9 change (95% CI) | −1.05 (−1.74 to −0.37) | −6.12 (−7.71 to −4.52) | NA | −5.06 (−6.80 to −3.33) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DID, difference in differences; NA, not applicable; PCL, PTSD Checklist; PHQ-9, Patient Health Questionnaire 9; PTSD, posttraumatic stress disorder.
To assess whether our results simply reflect improvement in comorbid depression or a clinically meaningful PCL score decrease is associated with lower T2D risk in patients without depression, we conducted a post hoc analysis limited to patients with only PTSD (n = 433). Among patients without depression, the age-adjusted T2D incidence rate was 2.0 per 1000 person-years among patients who experienced a clinically meaningful PCL score reduction and 12.4 per 1000 person-years among those who did not experience a clinically meaningful PCL score reduction.
The E-value was 3.33 for the association between a clinically meaningful PCL score decrease and incident T2D. An unmeasured confounder would require an association of 3.33 with a clinically meaningful PCL score decrease and incident T2D to explain our results.
Discussion
In this large cohort of VHA patients with PTSD, a clinically meaningful PCL score decrease (defined as a decrease in PCL score ≥20 points) compared with less than a clinically meaningful decrease was significantly associated with a lower risk of incident T2D. This result was independent of numerous demographics and psychiatric and physical comorbidities. The association was also independent of the number of PTSD psychotherapy sessions used, suggesting that a healthy adherer effect, or a general orientation to improve health, is unlikely to explain our observations.
With use of a subsample of patients with available data, exploratory analyses suggest that depression symptoms, not BMI and hemoglobin A1c values, decreased in patients who experienced clinically meaningful PTSD improvement. These results combined with findings from our expanded Cox proportional hazards regression models suggest that clinically meaningful PTSD improvement and lower T2D risk is not associated with comorbid hypertension, hyperlipidemia, obesity, and hemoglobin A1c levels. However, modeling change in depression, BMI, and hemoglobin A1c did not include multivariate adjustment, and the role of these factors in the association between meaningful PTSD improvement and risk of T2D should be considered preliminary.
Depression remission may contribute to lower T2D incidence. Decades of research have shown a bidirectional association between depression and T2D.33,34,35,36,37 In patients with PTSD alone, reduction in PCL scores were associated with lower risk of T2D. On the basis of our main analyses, among patients with PTSD who had comorbid depression, a reduction in PTSD symptoms and depression was associated with lower risk for T2D. These findings suggest that improved depression does not account for our results but may be a necessary component for an association with lower T2D risk among patients with PTSD and comorbid depression.
Depression and PTSD may be linked to incident T2D through hypothalamic-pituitary-adrenal axis and cortisol dysregulation.38 Evidence for lower cortisol levels after improvement in PTSD and depression is not consistent.39 However, 1 study40 found that cortisol levels decreased in patients who experienced decreased PTSD and depression symptoms after PTSD psychotherapy. On the basis of the existing literature, we cautiously speculate that normalization of hypothalamic-pituitary-adrenal axis and cortisol levels could be one mechanism behind our results. PTSD is associated with inflammation, which may in turn be associated with increased risk for T2D.21 Thus, another possible explanation for our results is through reduction in PTSD-related inflammation through PTSD improvement or in combination with selective serotonin reuptake inhibitor use.41
Anxiety and depression have long been known to contribute to the development of cardiometabolic disease.42,43 Numerous studies, but not all,44 suggest that decreased depression is associated with improvement in insulin resistance and glycemic control45,46,47,48,49 and adherence to selective serotonin reuptake inhibitor therapy may be associated with reduced proinflammatory markers.50 The current study expands this field to PTSD with and without comorbid depression and T2D risk.
Prospective intervention studies are warranted to measure these factors, which are not routinely available in administrative medical records. Such a study could determine whether large decreases in PCL scores are associated with improved insulin resistance and reduced inflammation. These measures may be more sensitive to improvements in PTSD and depression and complement hemoglobin A1c values.
Limitations
Unmeasured confounding could influence our results. For instance, we did not measure use of antihypertensives, some of which have potential beneficial effects on mood.51 Variables not in the medical record, such as social support, could help patients make lifestyle changes.32 However, unmeasured confounding is not likely to completely explain our findings because the E-value was 3.33. Such a value is unlikely given that the largest observed magnitude of association between measured confounders and incident T2D was hypertension. Our follow-up time is insufficient to conclude that PTSD improvement is associated with reduced T2D during the lifetime. We did not have data to link the qualifying traumatic event to PTSD diagnosis, and we were unable to draw inferences about the potential association of unique types of trauma with our results. The duration of PTSD and treatments used before the start of the data collection period could influence our results in unknown ways, but this confounding may be controlled because we balanced PTSD severity between groups. Patients with less than 2 PCL scores and not eligible for analyses had fewer PTSD psychotherapy sessions; therefore, results may not apply to younger patients because young age appears to be the only robust correlate of PTSD psychotherapy dropout.52 Also, results may not be generalizable to non-VHA patients; however, results have previously been replicated from analysis of VHA patient data in private sector cohorts on a range of topics including the association between opioids and depression53 and the association between metformin vs sulfonylurea and reduced risk for incident dementia.54
Conclusions
The findings suggest that clinically meaningful PTSD improvement is associated with a decreased risk of developing T2D. Patient education regarding potential health benefits of PTSD treatment may incentive psychotherapy use.
eTable 1. Variable Definitions
eTable 2. Type 2 Diabetes Outcome, Cumulative Incidence %, and Incidence Rate Per 1000 Person-Years (N = 1598)
eFigure 1. Retrospective Cohort Design
eFigure 2. Unweighted and Weighted SMD% for Covariates for Patients With <20 Point PCL Decrease vs ≥20 Point PCL Decrease
References
- 1.Goldstein RB, Smith SM, Chou SP, et al. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc Psychiatry Psychiatr Epidemiol. 2016;51(8):1137-1148. doi: 10.1007/s00127-016-1208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith SM, Goldstein RB, Grant BF. The association between post-traumatic stress disorder and lifetime DSM-5 psychiatric disorders among veterans: data from the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III). J Psychiatr Res. 2016;82:16-22. doi: 10.1016/j.jpsychires.2016.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnurr PP. Physical health and health services utilization In: Gold S, Cook J, Dalenberg CJ, eds. Handbook of Trauma Psychology. Vol 1 Washington, DC: American Psychological Association; 2017:349-370. [Google Scholar]
- 4.McFarlane AC. The long-term costs of traumatic stress: intertwined physical and psychological consequences. World Psychiatry. 2010;9(1):3-10. doi: 10.1002/j.2051-5545.2010.tb00254.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauch SAM, Defever E, Favorite T, et al. Prolonged exposure for PTSD in a Veterans Health Administration PTSD clinic. J Trauma Stress. 2009;22(1):60-64. doi: 10.1002/jts.20380 [DOI] [PubMed] [Google Scholar]
- 6.Lamp KE, Avallone KM, Maieritsch KP, Buchholz KR, Rauch SAM. Individual and group cognitive processing therapy: effectiveness across two Veterans Affairs posttraumatic stress disorder treatment clinics. Psychol Trauma. 2019;11(2):197-206. doi: 10.1037/tra0000370 [DOI] [PubMed] [Google Scholar]
- 7.Pacella ML, Hruska B, Delahanty DL. The physical health consequences of PTSD and PTSD symptoms: a meta-analytic review. J Anxiety Disord. 2013;27(1):33-46. doi: 10.1016/j.janxdis.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 8.Resick PA, Williams LF, Suvak MK, Monson CM, Gradus JL. Long-term outcomes of cognitive-behavioral treatments for posttraumatic stress disorder among female rape survivors. J Consult Clin Psychol. 2012;80(2):201-210. doi: 10.1037/a0026602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schubert CF, Schreckenbach M, Kirmeier T, et al. PTSD psychotherapy improves blood pressure but leaves HPA axis feedback sensitivity stable and unaffected: first evidence from a pre-post treatment study. Psychoneuroendocrinology. 2019;100:254-263. doi: 10.1016/j.psyneuen.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 10.Asukai N, Saito A, Tsuruta N, Kishimoto J, Nishikawa T. Efficacy of exposure therapy for Japanese patients with posttraumatic stress disorder due to mixed traumatic events: a randomized controlled study. J Trauma Stress. 2010;23(6):744-750. doi: 10.1002/jts.20589 [DOI] [PubMed] [Google Scholar]
- 11.Resick PA, Wachen JS, Dondanville KA, et al. ; and the STRONG STAR Consortium . Effect of group vs individual cognitive processing therapy in active-duty military seeking treatment for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(1):28-36. doi: 10.1001/jamapsychiatry.2016.2729 [DOI] [PubMed] [Google Scholar]
- 12.Gutner CA, Casement MD, Stavitsky Gilbert K, Resick PA. Change in sleep symptoms across cognitive processing therapy and prolonged exposure: a longitudinal perspective. Behav Res Ther. 2013;51(12):817-822. doi: 10.1016/j.brat.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galovski TE, Monson C, Bruce SE, Resick PA. Does cognitive-behavioral therapy for PTSD improve perceived health and sleep impairment? J Trauma Stress. 2009;22(3):197-204. doi: 10.1002/jts.20418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burg MM, Brandt C, Buta E, et al. Risk for incident hypertension associated with posttraumatic stress disorder in military veterans and the effect of posttraumatic stress disorder treatment. Psychosom Med. 2017;79(2):181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyko EJ, Jacobson IG, Smith B, et al. ; Millennium Cohort Study Team . Risk of diabetes in U.S. military service members in relation to combat deployment and mental health. Diabetes Care. 2010;33(8):1771-1777. doi: 10.2337/dc10-0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004;1032:141-153. doi: 10.1196/annals.1314.011 [DOI] [PubMed] [Google Scholar]
- 17.Roberts AL, Agnew-Blais JC, Spiegelman D, et al. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry. 2015;72(3):203-210. doi: 10.1001/jamapsychiatry.2014.2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agyemang C, Goosen S, Anujuo K, Ogedegbe G. Relationship between post-traumatic stress disorder and diabetes among 105,180 asylum seekers in the Netherlands. Eur J Public Health. 2012;22(5):658-662. doi: 10.1093/eurpub/ckr138 [DOI] [PubMed] [Google Scholar]
- 19.Miller-Archie SA, Jordan HT, Ruff RR, et al. Posttraumatic stress disorder and new-onset diabetes among adult survivors of the World Trade Center disaster. Prev Med. 2014;66:34-38. doi: 10.1016/j.ypmed.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 20.Scherrer JF, Salas J, Lustman PJ, et al. The role of obesity in the association between posttraumatic stress disorder and incident diabetes. JAMA Psychiatry. 2018;75(11):1189-1198. doi: 10.1001/jamapsychiatry.2018.2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kibler JL, Tursich M, Ma M, Malcolm L, Greenbarg R. Metabolic, autonomic and immune markers for cardiovascular disease in posttraumatic stress disorder. World J Cardiol. 2014;6(6):455-461. doi: 10.4330/wjc.v6.i6.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbaum S, Stubbs B, Ward PB, Steel Z, Lederman O, Vancampfort D. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism. 2015;64(8):926-933. doi: 10.1016/j.metabol.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 23.Using the PTSD Chechlist (PCL) 2012; https://sph.umd.edu/sites/default/files/files/PTSDChecklistScoring.pdf.
- 24.Monson CM, Gradus JL, Young-Xu Y, Schnurr PP, Price JL, Schumm JA. Change in posttraumatic stress disorder symptoms: do clinicians and patients agree? Psychol Assess. 2008;20(2):131-138. doi: 10.1037/1040-3590.20.2.131 [DOI] [PubMed] [Google Scholar]
- 25.Gravely AA, Cutting A, Nugent S, Grill J, Carlson K, Spoont M. Validity of PTSD diagnoses in VA administrative data: comparison of VA administrative PTSD diagnoses to self-reported PTSD Checklist scores. J Rehabil Res Dev. 2011;48(1):21-30. doi: 10.1682/JRRD.2009.08.0116 [DOI] [PubMed] [Google Scholar]
- 26.Holowka DW, Marx BP, Gates MA, et al. PTSD diagnostic validity in Veterans Affairs electronic records of Iraq and Afghanistan veterans. J Consult Clin Psychol. 2014;82(4):569-579. doi: 10.1037/a0036347 [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika Trust. 1983;70:41-55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 28.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(10)(suppl 2):S103-S107. doi: 10.1097/MLR.0b013e31806518ac [DOI] [PubMed] [Google Scholar]
- 29.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656-664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stürmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med. 2014;275(6):570-580. doi: 10.1111/joim.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321(6):602-603. doi: 10.1001/jama.2018.21554 [DOI] [PubMed] [Google Scholar]
- 33.Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes: a prospective population-based study. Diabetes Care. 1996;19(10):1097-1102. doi: 10.2337/diacare.19.10.1097 [DOI] [PubMed] [Google Scholar]
- 34.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus: a meta-analysis. Diabetologia. 2006;49(5):837-845. doi: 10.1007/s00125-006-0159-x [DOI] [PubMed] [Google Scholar]
- 35.Lustman PJ, Griffith LS, Clouse RE. Depression in adults with diabetes: results of 5-yr follow-up study. Diabetes Care. 1988;11(8):605-612. doi: 10.2337/diacare.11.8.605 [DOI] [PubMed] [Google Scholar]
- 36.Lustman PJ, Clouse RE. Treatment of depression in diabetes: impact on mood and medical outcome. J Psychosom Res. 2002;53(4):917-924. doi: 10.1016/S0022-3999(02)00416-6 [DOI] [PubMed] [Google Scholar]
- 37.Lustman PJ, Clouse RE. Depression in diabetes: the chicken or the egg? Psychosom Med. 2007;69(4):297-299. doi: 10.1097/PSY.0b013e318060cc2d [DOI] [PubMed] [Google Scholar]
- 38.Semenkovich K, Brown ME, Svrakic DM, Lustman PJ. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75(6):577-587. doi: 10.1007/s40265-015-0347-4 [DOI] [PubMed] [Google Scholar]
- 39.Pacella ML, Feeny N, Zoellner L, Delahanty DL. The impact of PTSD treatment on the cortisol awakening response. Depress Anxiety. 2014;31(10):862-869. doi: 10.1002/da.22298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerardi M, Rothbaum BO, Astin MC, Kelley M. Cortisol response following exposure treatment for PTSD in rape victims. J Aggress Maltreat Trauma. 2010;19(4):349-356. doi: 10.1080/10926771003781297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song H, Fang F, Tomasson G, et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA. 2018;319(23):2388-2400. doi: 10.1001/jama.2018.7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott KM. Depression, anxiety and incident cardiometabolic diseases. Curr Opin Psychiatry. 2014;27(4):289-293. doi: 10.1097/YCO.0000000000000067 [DOI] [PubMed] [Google Scholar]
- 43.Rotella F, Mannucci E. Depression as a risk factor for diabetes: a meta-analysis of longitudinal studies. J Clin Psychiatry. 2013;74(1):31-37. doi: 10.4088/JCP.12r07922 [DOI] [PubMed] [Google Scholar]
- 44.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042-1049. doi: 10.1001/archpsyc.61.10.1042 [DOI] [PubMed] [Google Scholar]
- 45.Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus: a randomized, controlled trial. Ann Intern Med. 1998;129(8):613-621. doi: 10.7326/0003-4819-129-8-199810150-00005 [DOI] [PubMed] [Google Scholar]
- 46.Lustman PJ, Penckofer SM, Clouse RE. Recent advances in understanding depression in adults with diabetes. Curr Psychiatry Rep. 2008;10(6):495-502. doi: 10.1007/s11920-008-0079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okamura F, Tashiro A, Utumi A, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism. 2000;49(10):1255-1260. doi: 10.1053/meta.2000.9515 [DOI] [PubMed] [Google Scholar]
- 48.Shomaker LB, Kelly NR, Pickworth CK, et al. A randomized controlled trial to prevent depression and ameliorate insulin resistance in adolescent girls at risk for type 2 diabetes. Ann Behav Med. 2016;50(5):762-774. doi: 10.1007/s12160-016-9801-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shomaker LB, Kelly NR, Radin RM, et al. Prevention of insulin resistance in adolescents at risk for type 2 diabetes with depressive symptoms: 1-year follow-up of a randomized trial. Depress Anxiety. 2017;34(10):866-876. doi: 10.1002/da.22617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Wang R, Liu L, Qiao D, Baldwin DS, Hou R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: a systematic review and meta-analysis. Brain Behav Immun. 2019;79(Feb):24-38. doi: 10.1016/j.bbi.2019.02.021 [DOI] [PubMed] [Google Scholar]
- 51.Boal AH, Smith DJ, McCallum L, et al. Monotherapy with major antihypertensive drug classes and risk of hospital admissions for mood disorders. Hypertension. 2016;68(5):1132-1138. doi: 10.1161/HYPERTENSIONAHA.116.08188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goetter EM, Bui E, Ojserkis RA, Zakarian RJ, Brendel RW, Simon NM. A systematic review of dropout from psychotherapy for posttraumatic stress disorder among Iraq and Afghanistan combat veterans. J Trauma Stress. 2015;28(5):401-409. doi: 10.1002/jts.22038 [DOI] [PubMed] [Google Scholar]
- 53.Scherrer JF, Salas J, Copeland LA, et al. Prescription opioid duration, dose, and increased risk of depression in 3 large patient populations. Ann Fam Med. 2016;14(1):54-62. doi: 10.1370/afm.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scherrer JF, Salas J, Floyd JS, Farr SA, Morley JE, Dublin S. Metformin and sulfonylurea use and risk of incident dementia. Mayo Clin Proc. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Variable Definitions
eTable 2. Type 2 Diabetes Outcome, Cumulative Incidence %, and Incidence Rate Per 1000 Person-Years (N = 1598)
eFigure 1. Retrospective Cohort Design
eFigure 2. Unweighted and Weighted SMD% for Covariates for Patients With <20 Point PCL Decrease vs ≥20 Point PCL Decrease