Key Points
Question
For patients presenting to the emergency department with an acute intracerebral hemorrhage (ICH) and a spot sign on computed tomography angiography, a marker of hemorrhage expansion, does recombinant activated coagulation factor VII (rFVIIa) reduce hematoma expansion more than placebo?
Findings
In this pooled analysis including 69 patients from randomized clinical trials targeting patients with spot sign–positive ICH, rFVIIa did not significantly reduce hemorrhage expansion when administered up to 6.5 hours from stroke onset, although nearly all patients were treated more than 2 hours after stroke onset.
Meaning
These data do not support the clinical use of rFVIIa in patients with spot sign–positive ICH, and future trials to limit ICH expansion should test an earlier treatment window.
This randomized clinical trial investigates whether recombinant activated coagulation factor VII reduces hemorrhage expansion among patients with spot sign–positive intracerebral hemorrhage (ICH).
Abstract
Importance
Intracerebral hemorrhage (ICH) is a devastating stroke type that lacks effective treatments. An imaging biomarker of ICH expansion—the computed tomography (CT) angiography spot sign—may identify a subgroup that could benefit from hemostatic therapy.
Objective
To investigate whether recombinant activated coagulation factor VII (rFVIIa) reduces hemorrhage expansion among patients with spot sign–positive ICH.
Design, Setting, and Participants
In parallel investigator-initiated, multicenter, double-blind, placebo-controlled randomized clinical trials in Canada (“Spot Sign” Selection of Intracerebral Hemorrhage to Guide Hemostatic Therapy [SPOTLIGHT]) and the United States (The Spot Sign for Predicting and Treating ICH Growth Study [STOP-IT]) with harmonized protocols and a preplanned individual patient–level pooled analysis, patients presenting to the emergency department with an acute primary spontaneous ICH and a spot sign on CT angiography were recruited. Data were collected from November 2010 to May 2016. Data were analyzed from November 2016 to May 2017.
Interventions
Eligible patients were randomly assigned 80 μg/kg of intravenous rFVIIa or placebo as soon as possible within 6.5 hours of stroke onset.
Main Outcomes and Measures
Head CT at 24 hours assessed parenchymal ICH volume expansion from baseline (primary outcome) and total (ie, parenchymal plus intraventricular) hemorrhage volume expansion (secondary outcome). The pooled analysis compared hemorrhage expansion between groups by analyzing 24-hour volumes in a linear regression model adjusted for baseline volumes, time from stroke onset to treatment, and trial.
Results
Of the 69 included patients, 35 (51%) were male, and the median (interquartile range [IQR]) age was 70 (59-80) years. Baseline median (IQR) ICH volumes were 16.3 (9.6-39.2) mL in the rFVIIa group and 20.4 (8.6-32.6) mL in the placebo group. Median (IQR) time from CT to treatment was 71 (57-96) minutes, and the median (IQR) time from stroke onset to treatment was 178 (138-197) minutes. The median (IQR) increase in ICH volume from baseline to 24 hours was small in both the rFVIIa group (2.5 [0-10.2] mL) and placebo group (2.6 [0-6.6] mL). After adjustment, there was no difference between groups on measures of ICH or total hemorrhage expansion. At 90 days, 9 of 30 patients in the rFVIIa group and 13 of 34 in the placebo group had died or were severely disabled (P = .60).
Conclusions and Relevance
Among patients with spot sign–positive ICH treated a median of about 3 hours from stroke onset, rFVIIa did not significantly improve radiographic or clinical outcomes.
Trial Registration
ClinicalTrials.gov identifier: NCT01359202 and NCT00810888
Introduction
Intracerebral hemorrhage (ICH) has the highest case fatality rate of any stroke type and the fewest effective treatments, and most survivors are left with severe long-term disability.1 Because ICH prognosis directly relates to hemorrhage volume and expansion,2,3 treatments to arrest bleeding and limit ICH size are needed. Early ICH expansion by more than 33% occurs in one-third of patients with ICH undergoing imaging within 3 hours of onset.3 For every 10% increase in ICH size, mortality increases by 5%, and patients are 16% more likely to worsen by 1 disability level on the 7-point modified Rankin Scale (mRS).3
A promising treatment is recombinant activated coagulation factor VII (rFVIIa), a rapid procoagulant developed for hemophilia-related bleeding. In previous studies, rFVIIa reduced ICH expansion by about 50% compared with placebo4,5 but did not improve clinical outcomes in a phase III randomized clinical trial.5 However, as previous trials did not select patients based on any predictors of active bleeding other than time from stroke onset, the inclusion of patients who no longer had ongoing ICH expansion may have reduced the measured treatment effect size.
We designed 2 phase II randomized clinical trials to test whether rFVIIa could benefit a subgroup of patients with ICH at elevated risk of ICH expansion, defined by the presence of an imaging biomarker—the computed tomography (CT) angiography spot sign.6 The spot sign, which appears as bright foci of contrast enhancement within a parenchymal hematoma,7 is present in about one-quarter of patients with acute ICH and predicts ICH expansion and poor clinical outcomes.6,8,9,10,11,12,13 By randomizing only patients with spot sign–positive ICH, we aimed to enrich the cohort with active bleeders. We excluded from randomization patients with spot sign–negative ICH who are at low risk for ongoing bleeding because hemostatic therapy would entail risk14 and cost without expected benefit.
Methods
Study Design, Setting, and Participants
The “Spot Sign” Selection of Intracerebral Hemorrhage to Guide Hemostatic Therapy (SPOTLIGHT) and The Spot Sign for Predicting and Treating ICH Growth Study (STOP-IT) trials are investigator-initiated, phase II, multicenter, double-blind, placebo-controlled randomized clinical trials that ran in parallel as independent studies with harmonized protocols (Supplement 1 and Supplement 2). The SPOTLIGHT trial recruited patients from 14 stroke centers within the Canadian Stroke Consortium. The STOP-IT trial recruited patients from 10 stroke centers in the United States and 2 in Canada. Enrolling investigators were stroke neurologists or emergency physicians. See eAppendix in Supplement 3 for a list of participating sites, investigators, and coordinators. The trials were approved by national regulatory agencies (Health Canada and US Food and Drug Administration) and institutional research boards at all sites and registered on ClinicalTrials.gov (SPOTLIGHT: NCT01359202; STOP-IT: NCT00810888). Site monitoring was performed. Written informed consent was obtained for all participants, either from patients with capacity to consent or legally authorized representatives. In the SPOTLIGHT trial, a deferral of informed consent process was approved by research ethics boards at 7 of 14 sites. This option was intended to enable study participation and avoid delayed treatment for eligible but incapacitated patients for whom a legally authorized representative was not immediately available. Ethical justification for deferred consent is detailed in the protocol (Supplement 1) in accordance with Canadian federal guidance criteria.15
We recruited eligible adult patients presenting to the emergency department with an acute primary spontaneous ICH (ie, nontraumatic and non–anticoagulant-related ICH) who could undergo CT angiography and had a spot sign. Patients with spot sign–negative ICH were not randomized but were entered into a separate observational study. See eTable 1 in Supplement 3 for the main eligibility criteria. We excluded brainstem ICH, known secondary causes of ICH (eg, vascular malformation, tumor), planned surgery for ICH evacuation (but intraventricular drains were allowed), and planned withdrawal of care. To maximize safety of rFVIIa, we excluded patients with cardiac or cerebral ischemia or elevated risk of myocardial infarction or arterial or venous thromboembolism. Owing to slow recruitment, some eligibility criteria were modified in the SPOTLIGHT trial in a protocol amendment after the first 22 patients were enrolled, removing the upper age limit, increasing the maximum baseline ICH volume, and removing exclusions for Glasgow Coma Scale score, preexisting disability, and baseline troponin concentration.
Baseline head CT and CT angiography were assessed for eligibility in real time by enrolling investigators who determined the presence or absence of a spot sign and estimated ICH volumes using the ABC/2 method.16A spot sign was defined as foci of contrast enhancement within a parenchymal ICH identified by visual inspection of CT angiography source images without connection to a vessel outside the hematoma margin and without a corresponding hyperdensity on the noncontrast CT indicative of calcification.7,17 Enrolling investigators completed online imaging training and certification on spot sign identification and the ABC/2 method. Results of investigator performance on the training module have been reported.18 A central study neuroradiologist (R.I.A.) provided blinded adjudication of CT angiograms. The spot sign status assigned by site investigators was used for all analyses.
Randomization, Masking, and Study Drug
Eligible patients were randomly assigned 1:1 to a single weight-adjusted dose of either 80 μg/kg of rFVIIa (NiaStase RT or NovoSeven; Novo Nordisk) or saline placebo by intravenous bolus. The 80 μg/kg dose was chosen because it had the best balance of efficacy and safety in dose-escalation trials in patients with ICH.5,6 The study drug was to be administered as soon as possible after randomization, within 6 hours from stroke onset in the SPOTLIGHT trial or 6.5 hours in the STOP-IT trial.
The SPOTLIGHT trial used a computer-generated randomization schedule created by an independent statistician; randomization was stratified by site using a variable block randomization scheme. The STOP-IT trial used web-based randomization with an adaptive randomization scheme to improve balance in variables known to influence ICH expansion, including baseline ICH volume (<30 mL; 30-60 mL; >60 mL) and time from stroke onset to CT (0-3 hours; >3 hours).
At each site, a designated unblinded individual (pharmacist, blood bank technician, or nurse not involved in patient enrollment or follow-up) prepared the study drug in a blinded syringe ready for injection (out of sight of the patient, investigators, and members of the blinded study team). Both saline and reconstituted rFVIIa are clear, colorless solutions identical in appearance and texture.
Investigators were advised to follow American Heart Association/American Stroke Association ICH guidelines for blood pressure and general ICH management.19 Patients were admitted to intensive care or stroke units for standard stroke care and rehabilitation.
Outcomes
Final ICH volume was assessed by a noncontrast head CT scan at 24 hours (an earlier CT was allowed if a patient with neurological deterioration was expected to die or require surgical intervention before 24 hours). A subset of patients also had a CT repeated right after study drug administration to assess early ICH expansion.
Patients were observed for 90 days for adverse events and clinical outcomes using standard rating scales. Study assessments took place at 24 hours, day 2, day 3, day of hospital discharge, day 30 (by telephone), and day 90 (in person in the SPOTLIGHT trial; by telephone in the STOP-IT trial). Assessments also took place at day 4 in the SPOTLIGHT trial.
The primary outcome was ICH volume expansion measured on CT as the difference in parenchymal ICH volume from baseline to 24 hours. The secondary outcome was total (ie, parenchymal hemorrhage plus intraventricular hemorrhage [IVH]) hemorrhage volume expansion from baseline to 24 hours. The imaging core laboratory at the University of Calgary, Calgary, Alberta, Canada, performed central blinded volumetric measurements on all deidentified scans with Quantomo software (Cybertrial Inc) using a user-assisted neighborhood-connected region-growing threshold-segmentation method implemented in the Insight Segmentation and Registration Toolkit (ITK; National Library of Medicine) in conjunction with freehand drawing tools.20
The clinical outcome was the proportion of patients with an mRS score of 5 or 6 at 90 days. The primary safety outcome was the rate of myocardial infarction, ischemic stroke, or pulmonary embolism within 4 days. Daily electrocardiography, troponin concentration measurements, and clinical assessments were performed on days 1 to 4 (the SPOTLIGHT trial) or days 1 to 3 (the STOP-IT trial). All electrocardiograms were adjudicated by a medical monitor who was blinded to study group assignment (M.D.H. and D.G.). Serious adverse events were collected through day 90. Secondary safety outcomes included isolated troponin elevation (without clinical symptoms or electrocardiography evidence of acute coronary syndrome), deep venous thrombosis, and other arterial or venous thromboembolic events. Each trial had an independent safety monitoring committee and stopping rules in case of an excess of thromboembolic events.
Statistical Analysis
We conducted a prespecified, intention-to-treat, individual patient–level pooled analysis of the 2 trials (Supplement 4). There was no interim analysis. Patient characteristics and hemorrhage volumes were summarized for each group using descriptive statistics. The primary analysis compared the amount of hemorrhage expansion between the rFVIIa and placebo groups. To do this, we analyzed 24-hour hemorrhage volumes adjusted for baseline hemorrhage volumes in a linear regression model that also adjusted for time from stroke onset to treatment and trial. Since the residuals exhibited extreme nonnormality, the analysis was performed using log-transformed hemorrhage volumes; natural logarithms were used throughout. The P values for the between-group comparisons were obtained from the regression models. A covariate representing the study was retained in all models to account for any underlying study differences. The estimated sample size requirement was 106 patients to detect a 20-mL difference between groups in final ICH volume (adjusted for baseline volume) with 80% power, acknowledging that the minimum clinically important difference is unclear.13 To allow comparison with other studies, we reported the proportion of patients with an ICH volume increase of greater than 33% or greater than 6 mL from baseline to 24 hours using the χ2 test. Secondary analyses compared the 2 treatment groups in terms of the proportion of patients who died or were severely disabled at 90 days and safety outcomes using Fisher Exact test. All P values were 2-tailed, and significance was set at a P value less than .05. Analysis was conducted using R statistical software version 3.5.2 (The R Foundation).
Results
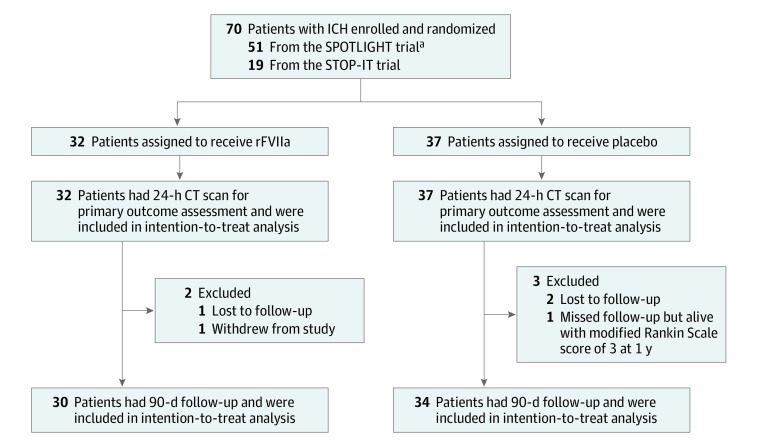
Between November 23, 2010, and May 31, 2016, we enrolled and randomly allocated 70 patients with spot sign–positive ICH (51 from the SPOTLIGHT trial; 19 from the STOP-IT trial), of whom 69 were eligible for study inclusion and received study drug and were analyzed. A total of 32 patients were assigned to the rFVIIa group, and 37 were assigned to the placebo group (Figure 1). Recruitment was slow, and the target sample size was not reached. The SPOTLIGHT trial was stopped because of slow enrollment after 2 extensions of its grant funding period, and the STOP-IT trial ended at the conclusion of its study funding. Main reasons for ineligibility were spot sign–negative ICH, age older than 80 years, history of thromboembolism, ICH volume too large, Glasgow Coma Scale score less than 8, neurosurgical hematoma evacuation, secondary causes of ICH, infratentorial ICH, and anticoagulation. All patients remained in their assigned treatment groups. All had follow-up CT volumetric data available and adverse event assessments within the first 4 days of randomization. A total of 64 patients had 90-day outcomes available; 3 were lost to follow-up (1 in the rFVIIa group and 2 in the placebo group), 1 withdrew from the study (rFVIIa group), and 1 missed the 90-day mRS score assessment (placebo group).
Figure 1. Flow Diagram.
CT indicates computed tomography; ICH, intracerebral hemorrhage; rFVIIa, recombinant activated coagulation factor VII.
aOne enrolled patient did not meet eligibility criteria, was not treated, and was not included in the analysis.
Baseline patient characteristics are presented in Table 1. All ICHs were supratentorial, including deep cerebral ICH in 57 of 69 patients (83%) and lobar ICH in 12 of 69 patients (17%), and IVH was present in 28 of 69 patients (41%). The median (interquartile range [IQR]) baseline ICH volume was 16.3 (9.6-39.2) mL in the rFVIIa group and 20.4 (8.6-32.6) mL in the placebo group. Median (IQR) baseline total (ie, IVH plus ICH) hemorrhage volume was 23.8 (15.3-40.0) mL in the rFVIIa group and 24.5 (9.6-46.2) mL in the placebo group.
Table 1. Patient and Baseline Imaging Characteristics and Process Measures.
| Characteristic | No. (%) | |
|---|---|---|
| rFVIIa (n = 32) | Placebo (n = 37) | |
| Age, mean (SD), y | 70.7 (13.7) | 66.7 (12.4) |
| Male | 15 (47) | 20 (54) |
| White | 21 (66) | 20 (54) |
| Hypertension | 23 (72) | 26 (70) |
| Diabetes | 8 (25) | 5 (14) |
| Preadmission antiplatelet use | 8 (25) | 7 (19) |
| Systolic blood pressure, mean (SD), mm Hg | 159.9 (28.9) | 167.2 (27.5) |
| GCS score, median (IQR) | 14 (14-15) | 13 (12-15) |
| NIHSS score, median (IQR) | 16.0 (11.0-18.5) | 16.0 (13.0-20.0) |
| ICH volume, median (IQR), mL | 16.3 (9.6-39.2) | 20.4 (8.6-32.6) |
| IVH present | 14 (44) | 14 (38) |
| IVH volume, median (IQR), mL | 9.8 (5.5-14.7) | 11.8 (3.7-16.7) |
| ICH plus IVH volume, median (IQR), mL | 23.8 (15.3-40.0) | 24.5 (9.6-46.2) |
| Stroke onset to CT, median (IQR), min | 89 (71-187) | 83 (66-153) |
| Stroke onset to CT angiography, median (IQR), min | 106 (77-188) | 94 (78-162) |
| CT to study drug administration, median (IQR), min | 79 (61-99) | 64 (56-86) |
| Stroke onset to study drug administration, median (IQR), min | 195 (157-266) | 161 (137-224) |
| Treatment less than 3 h from onset | 9 (37) | 24 (65) |
Abbreviations: CT, computed tomography; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; IQR, interquartile range; IVH, intraventricular hemorrhage; NIHSS, National Institutes of Health Stroke Scale; rFVIIa, recombinant activated coagulation factor VII.
The median (IQR) time from stroke onset to baseline CT was 84 (70-161) minutes, and median (IQR) time from baseline CT to drug administration was 70 (57-95) minutes. The median (IQR) time from stroke onset to treatment was 195 (157-266) minutes in the rFVIIa group and 161 (137-224) minutes in the placebo group (P = .14).
Table 2 shows the primary and secondary outcomes. The median (IQR) change in parenchymal ICH volume from baseline to 24 hours was 2.5 (0-10.2) mL in the rFVIIa group and 2.6 (0-6.6) mL in the placebo group. The median (IQR) change in total (ie, parenchymal hemorrhage plus IVH) hemorrhage volume was 3.2 (0.1-11.5) mL in the rFVIIa group and 4.8 (0-7.2) mL in the placebo group.
Table 2. Radiographic Outcomes.
| Outcome | Median (IQR) | P Valuea | |
|---|---|---|---|
| rFVIIa (n = 32) | Placebo (n = 37) | ||
| Primary outcome | |||
| ICH volume expansion from baseline to 24 h, mL | 2.5 (0 to 10.2) | 2.6 (0 to 6.6) | .89 |
| Secondary outcome | |||
| ICH plus IVH volume expansion from baseline to 24 h, mL | 3.2 (0.1 to 11.5) | 4.8 (0 to 7.2) | .91 |
| Additional radiographic outcomes | |||
| ICH volume at 24 h, mL | 22.0 (10.0 to 53.0) | 29.0 (14.0 to 52.0) | .89 |
| IVH present at 24 h, No. (%) | 17 (53) | 20 (54) | .94b |
| IVH volume at 24 h, mL | 10.2 (5.3 to 14.9) | 7.1 (2.1 to 14.2) | .18 |
| IVH volume expansion from baseline to 24 h, mL | −0.2 (−1.2 to 1.6) | 1.0 (0 to 4.4) | .18 |
| ICH plus IVH volume at 24 h, mL | 25.7 (18.5 to 55.5) | 31.0 (15.9 to 59.6) | .91 |
| ICH volume expansion >6 mL or >33% from baseline to 24 h, No. (%) | 13 (41) | 16 (43) | .83b |
Abbreviations: ICH, intracerebral hemorrhage; IQR, interquartile range; IVH, intraventricular hemorrhage; rFVIIa, recombinant activated coagulation factor VII.
P value for the between-group comparison is from the linear regression model with log-transformed hemorrhage volume, adjusted for baseline hemorrhage volume, time from stroke onset to treatment, and trial.
P value for the between-group comparison is from the χ2 test.
In the primary analysis evaluating the effect of rFVIIa on the primary outcome of parenchymal ICH expansion by linear regression, there was very little statistical evidence of a difference between the 2 treatment groups in 24-hour ICH volumes, adjusted for baseline ICH volumes, times from stroke onset to treatment, and trial. The adjusted treatment effect was estimated to reduce final ICH volume by 1.8% (95% CI, 27% reduction to 23% increase) (eTable 2 in Supplement 3). Similar findings were observed for the secondary outcome of 24-hour total (parenchymal hemorrhage plus IVH) hemorrhage volume in the adjusted model, where the adjusted treatment effect was estimated to reduce total hemorrhage volume by 1.5% (95% CI, 27% reduction to 24% increase) (eTable 2 in Supplement 3).
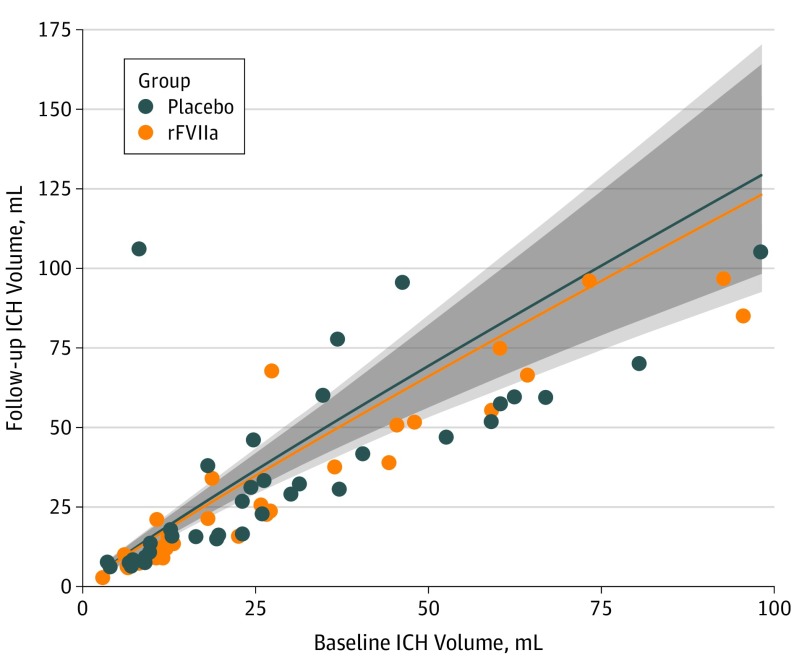
Figure 2 plots the individual patient changes in ICH volume in relation to time from stroke onset to baseline CT. Figure 3 shows the expected relationship between baseline ICH volume and final ICH volume; there is no significant between-group difference.
Figure 2. Distribution of Intracerebral Hemorrhage (ICH) Expansion Volumes for Individual Patients by Time From Stroke Onset to Baseline Computed Tomography (CT) Scan.
rFVIIa indicates recombinant activated coagulation factor VII.
Figure 3. 24-Hour Intracerebral Hemorrhage (ICH) Volumes as a Function of Baseline ICH Volumes and Treatment.
The shaded regions indicate 95% CIs. rFVIIa indicates recombinant activated coagulation factor VII.
In an exploratory subgroup analysis of 36 patients who received the study drug within 3 hours from onset, the median (IQR) ICH volume change from baseline to 24 hours was 0.9 (−0.02 to 6.2) mL in the rFVIIa group and 4.3 (0.5-12.4) mL in the placebo group. The median (IQR) total hemorrhage volume change was 1.6 (0-11.2) mL for the rFVIIa group and 4.9 (0.9-12.4) mL for the placebo group.
The eFigure in Supplement 3 shows the distribution of mRS scores at 90 days. There was no treatment effect on 90-day mRS score, with 9 of 30 patients in the rFVIIa group (30%) and 13 of 34 in the placebo group (38%) scoring 5 to 6 (P = .60). Ninety-day mortality was 20% (6 of 30) in the rFVIIa group and 21% (7 of 34) in the placebo group (P = .98).
There was agreement on the presence of a spot sign between enrolling investigators and the blinded study neuroradiologist in 64 of 69 patients (93%; 5 patients had spot sign–negative ICH according to the neuroradiologist). A per-protocol analysis excluding these 5 patients did not significantly alter the results for the primary or secondary outcomes.
There were no significant safety concerns (eTable 3 in Supplement 3). The composite outcome of myocardial infarction, ischemic stroke, or pulmonary embolism within 4 days occurred in 2 of 32 patients (6%) in the rFVIIa group and 4 of 37 patients (11%) in the placebo group (P = .68). There were no cases of ST-elevation myocardial infarction, deep vein thrombosis, or pulmonary embolism within 4 days. Non–ST-elevation myocardial ischemia or infarction within 4 days occurred in 1 of 32 patients (3%) in the rFVIIa group and 3 of 37 patients (8%) in the placebo group (P = .62), and none were clinically significant; 3 of these patients died of the index ICH. The incidence of isolated troponin elevation within 4 days was 9 of 32 patients (28%) in the rFVIIa group and 10 of 37 patients (27%) in the placebo group (P = .92); most were mild (only 8 of 69 patients [12%] had troponin levels greater than 0.1 ng/mL [to convert to micrograms per liter, multiply by 1]). There was 1 asymptomatic cerebral infarct on brain magnetic resonance imaging in the rFVIIa group and 1 ischemic stroke in the placebo group within 4 days. There were 3 cases of asymptomatic diffusion-weighted imaging–positive brain magnetic resonance imaging lesions of uncertain significance21 within the first 7 days (1 patient in the rFVIIa group and 2 patients in the placebo group).
Discussion
We used an imaging biomarker, the CT angiography spot sign, to target hemostatic therapy for patients with acute ICH. Our trials were designed on the premise that image-guided patient selection could identify a subgroup of patients with ICH who may derive greater benefit from rFVIIa than previous trials that did not assess spot sign status. However, in patients who had a spot sign on CT angiography and were treated within 6.5 hours from stroke onset, we found no benefit of rFVIIa on final hemorrhage volumes (adjusted for baseline volumes) or on clinical outcomes. Our findings reinforce the high morbidity of ICH and provide lessons for future treatment trials.
The neutral results likely relate to several factors: (1) limitations of the spot sign, (2) long times from stroke onset to CT angiography, (3) long times from stroke onset to treatment, (4) restrictive eligibility criteria, and (5) underpowered statistical analysis. We had anticipated that image-guided treatment, a strategy successful in ischemic stroke, could be effective for ICH. Our trials were predicated on the notion that the presence of a spot sign would be associated with significant ongoing ICH expansion. However, in contrast to observational studies, the spot sign was a poor predictor of significant ICH expansion in these trial patients. Most patients had surprisingly little ICH expansion after their baseline CT; the median ICH volume increase from baseline to 24 hours was only 2.5 mL, which is lower than expected from observational studies of patients with spot sign–positive ICH. For example, in the PREDICT study,6 the median ICH expansion volume was 8.6 mL (12.7 mL for ICH plus IVH expansion), and 61% of patients had ICH expansion of greater than 6 mL or greater than 33% in spite of similar baseline ICH volumes as patients in this trial and a longer median time from stroke onset to CT of 117 minutes. In the Tranexamic Acid for Hyperacute Primary Intracerebral Haemorrhage trial22 of tranexamic acid, which randomized patients up to 8 hours after stroke onset, there was no signal of efficacy in the small spot sign–positive subgroup.
The spot sign appears to be a more complex biomarker than previously thought. There is high between-patient variability in its imaging characteristics (eg, size, density, number of spots), ranging from a single faint punctate density to multiple large serpiginous densities. Differences in CT angiography acquisition (single-pass vs multiphase or dynamic) and timing of image acquisition in relation to contrast bolus injection results in variable detection and appearance of spots in early or late arterial or venous phases, with different potentials for ICH expansion.9,23,24 Importantly, only about half of spot signs are actively expanding at initial presentation, and this cannot be determined without delayed-phase images.8 Thus, the spot sign is not a binary predictor of ICH expansion but rather a heterogeneous marker, and different spot signs are associated with different rates of bleeding, ranging from a slow ooze to rapid expanders, akin to the variability in acute ischemic stroke (slow and fast progressors). A pooled analysis of observational studies25 has demonstrated that the prognostic value of the spot sign for predicting significant ICH expansion decreases as the time from stroke onset to CT angiography increases. Late-imaged spot signs are more likely to be associated with small oozing, whereas early-imaged spot signs are more likely to be associated with robust expansion. In that analysis, the sensitivity of the spot sign for ICH expansion of greater than 6 mL or greater than 33% from baseline CT to final CT declined from 0.60 when imaged less than 2 hours after stroke onset to 0.30 when imaged more than 8 hours after stroke onset.25
In our trials, despite best efforts, most patients were imaged, enrolled, and treated too late, after most of their ICH expansion had already occurred. Most patients (94%) were treated more than 2 hours after stroke onset, and 62% of rFVIIa-treated patients received treatment more than 3 hours after onset. We had hypothesized that the presence of a spot sign might allow a longer treatment window, but our results imply that should hemostatic therapy ultimately prove to be of any benefit, the time window for effective intervention, even with a spot sign, must be very short.
Our population differed from prior natural history studies that included all consecutive patients with spot sign–positive ICH. For example, we excluded patients presenting with already large hemorrhages or low Glasgow Coma Scale scores or taking anticoagulants. The rapid expanders, who are most likely to have large-magnitude ICH expansion and are most in need of hemostatic therapy, are paradoxically the ones least likely to be able to be enrolled in a clinical trial and treated in time because their condition deteriorates too quickly or they require emergency surgery. This treatment-risk paradox poses major challenges for ICH trials and emphasizes the need for prehospital treatment.
This work has implications for the design of ICH trials. Future trials are urged to treat much earlier after stroke onset, as the presence of a spot sign alone does not appear to be able to replace time for patient selection. It is time now for ultrafast ICH trials using prehospital randomization, deferred consent, and mobile stroke unit treatment. Additional independent predictors of ICH expansion are baseline ICH volume and antiplatelet or anticoagulant therapy,26 and noncontrast CT predictors (eg, hypodensities, blend sign) are worth exploring in the quest to define optimal treatment candidates,27,28 recognizing that early treatment will still likely be the most critical factor.
Limitations
Our study had limitations. The main study limitation is that the trials did not meet recruitment goals and the analysis is underpowered. The small sample size was a consequence of multiple eligibility criteria, exclusion of anticoagulated patients (who require reversal and would confound study results), low frequency of ICH compared with ischemic stroke in North America, and a lower-than-anticipated prevalence of spot signs (21%). Narrow eligibility criteria were imposed to define a more homogeneous and treatable ideal target population and maximize patient safety given the thrombotic risks of rFVIIa.14 However, the eligibility criteria were likely overly stringent, which hampered recruitment and limits generalizability. The times from stroke onset to treatment and from CT to treatment were substantially longer than our goals, reflecting delays waiting for spot sign interpretation, troponin results, consent, and study drug reconstitution. Many of the delays are modifiable and must be overcome in future trials.
Conclusions
In conclusion, in our trials, rFVIIa did not significantly reduce hemorrhage expansion in patients with spot sign–positive ICH when treated within 6.5 hours of onset. Future trials aiming to limit ICH expansion should test a much earlier treatment window.
The SPOTLIGHT trial protocol.
The STOP-IT trial protocol.
eAppendix. List of participating sites, investigators, and coordinators.
eTable 1. Main trial inclusion and exclusion criteria.
eTable 2. Coefficient and effect estimates from adjusted models.
eTable 3. Ischemic adverse events within the first 4 days after randomization.
eFigure. 90-Day modified Rankin Scale scores.
Statistical analysis plan for the pooled analysis.
Data sharing statement.
References
- 1.Flaherty ML, Haverbusch M, Sekar P, et al. . Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66(8):1182-1186. doi: 10.1212/01.wnl.0000208400.08722.7c [DOI] [PubMed] [Google Scholar]
- 2.Brott T, Broderick J, Kothari R, et al. . Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28(1):1-5. doi: 10.1161/01.STR.28.1.1 [DOI] [PubMed] [Google Scholar]
- 3.Davis SM, Broderick J, Hennerici M, et al. ; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators . Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66(8):1175-1181. doi: 10.1212/01.wnl.0000208408.98482.99 [DOI] [PubMed] [Google Scholar]
- 4.Mayer SA, Brun NC, Begtrup K, et al. ; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators . Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352(8):777-785. doi: 10.1056/NEJMoa042991 [DOI] [PubMed] [Google Scholar]
- 5.Mayer SA, Brun NC, Begtrup K, et al. ; FAST Trial Investigators . Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127-2137. doi: 10.1056/NEJMoa0707534 [DOI] [PubMed] [Google Scholar]
- 6.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. ; PREDICT/Sunnybrook ICH CTA study group . Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11(4):307-314. doi: 10.1016/S1474-4422(12)70038-8 [DOI] [PubMed] [Google Scholar]
- 7.Thompson AL, Kosior JC, Gladstone DJ, et al. ; PREDICTS/Sunnybrook ICH CTA Study Group . Defining the CT angiography ‘spot sign’ in primary intracerebral hemorrhage. Can J Neurol Sci. 2009;36(4):456-461. doi: 10.1017/S0317167100007782 [DOI] [PubMed] [Google Scholar]
- 8.Wada R, Aviv RI, Fox AJ, et al. . CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38(4):1257-1262. doi: 10.1161/01.STR.0000259633.59404.f3 [DOI] [PubMed] [Google Scholar]
- 9.Goldstein JN, Fazen LE, Snider R, et al. . Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68(12):889-894. doi: 10.1212/01.wnl.0000257087.22852.21 [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Smith A, Hemphill JC III, et al. . Contrast extravasation on CT predicts mortality in primary intracerebral hemorrhage. AJNR Am J Neuroradiol. 2008;29(3):520-525. doi: 10.3174/ajnr.A0859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado Almandoz JE, Yoo AJ, Stone MJ, et al. . Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40(9):2994-3000. doi: 10.1161/STROKEAHA.109.554667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado Almandoz JE, Yoo AJ, Stone MJ, et al. . The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke. 2010;41(1):54-60. doi: 10.1161/STROKEAHA.109.565382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE; VISTA Collaboration . Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76(14):1238-1244. doi: 10.1212/WNL.0b013e3182143317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diringer MN, Skolnick BE, Mayer SA, et al. . Thromboembolic events with recombinant activated factor VII in spontaneous intracerebral hemorrhage: results from the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke. 2010;41(1):48-53. doi: 10.1161/STROKEAHA.109.561712 [DOI] [PubMed] [Google Scholar]
- 15.Canadian Institutes of Health Research; Natural Sciences and Engineering Research Council of Canada; Social Sciences and Humanities Research Council of Canada Ethical conduct for research involving humans. http://www.pre.ethics.gc.ca/pdf/eng/tcps2-2014/TCPS_2_FINAL_Web.pdf. Accessed July 17, 2019.
- 16.Kothari RU, Brott T, Broderick JP, et al. . The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304-1305. doi: 10.1161/01.STR.27.8.1304 [DOI] [PubMed] [Google Scholar]
- 17.Gazzola S, Aviv RI, Gladstone DJ, et al. . Vascular and nonvascular mimics of the CT angiography “spot sign” in patients with secondary intracerebral hemorrhage. Stroke. 2008;39(4):1177-1183. doi: 10.1161/STROKEAHA.107.499442 [DOI] [PubMed] [Google Scholar]
- 18.Huynh TJ, Flaherty ML, Gladstone DJ, et al. . Multicenter accuracy and interobserver agreement of spot sign identification in acute intracerebral hemorrhage. Stroke. 2014;45(1):107-112. doi: 10.1161/STROKEAHA.113.002502 [DOI] [PubMed] [Google Scholar]
- 19.Morgenstern LB, Hemphill JC III, Anderson C, et al. ; American Heart Association Stroke Council and Council on Cardiovascular Nursing . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129. doi: 10.1161/STR.0b013e3181ec611b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kosior JC, Idris S, Dowlatshahi D, et al. ; PREDICT/Sunnybrook CTA ICH study investigators . Quantomo: validation of a computer-assisted methodology for the volumetric analysis of intracerebral haemorrhage. Int J Stroke. 2011;6(4):302-305. doi: 10.1111/j.1747-4949.2010.00579.x [DOI] [PubMed] [Google Scholar]
- 21.Menon RS, Burgess RE, Wing JJ, et al. . Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71(2):199-205. doi: 10.1002/ana.22668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprigg N, Flaherty K, Appleton JP, et al. ; TICH-2 Investigators . Tranexamic Acid for Hyperacute Primary Intracerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391(10135):2107-2115. doi: 10.1016/S0140-6736(18)31033-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Luna D, Coscojuela P, Rodriguez-Villatoro N, et al. . Multiphase CT angiography improves prediction of intracerebral hemorrhage expansion. Radiology. 2017;285(3):932-940. doi: 10.1148/radiol.2017162839 [DOI] [PubMed] [Google Scholar]
- 24.Koculym A, Huynh TJ, Jakubovic R, Zhang L, Aviv RI. CT perfusion spot sign improves sensitivity for prediction of outcome compared with CTA and postcontrast CT. AJNR Am J Neuroradiol. 2013;34(5):965-970, S1. doi: 10.3174/ajnr.A3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowlatshahi D, Brouwers HB, Demchuk AM, et al. . Predicting intracerebral hemorrhage growth with the spot sign: the effect of onset-to-scan time. Stroke. 2016;47(3):695-700. doi: 10.1161/STROKEAHA.115.012012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Shahi Salman R, Frantzias J, Lee RJ, et al. ; VISTA-ICH Collaboration; ICH Growth Individual Patient Data Meta-analysis Collaborators . Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol. 2018;17(10):885-894. doi: 10.1016/S1474-4422(18)30253-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulouis G, Morotti A, Brouwers HB, et al. . Association between hypodensities detected by computed tomography and hematoma expansion in patients with intracerebral hemorrhage. JAMA Neurol. 2016;73(8):961-968. doi: 10.1001/jamaneurol.2016.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morotti A, Dowlatshahi D, Boulouis G, et al. ; ATACH-II, NETT, and PREDICT Investigators . Predicting intracerebral hemorrhage expansion with noncontrast computed tomography: the BAT score. Stroke. 2018;49(5):1163-1169. doi: 10.1161/STROKEAHA.117.020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The SPOTLIGHT trial protocol.
The STOP-IT trial protocol.
eAppendix. List of participating sites, investigators, and coordinators.
eTable 1. Main trial inclusion and exclusion criteria.
eTable 2. Coefficient and effect estimates from adjusted models.
eTable 3. Ischemic adverse events within the first 4 days after randomization.
eFigure. 90-Day modified Rankin Scale scores.
Statistical analysis plan for the pooled analysis.
Data sharing statement.